Process for co-production of sodium carbonate and calcium sulfate from ammonium bicarbonate and glauberite tailings
A glauberite and calcium sulfate technology, applied in the direction of calcium/strontium/barium sulfate, carbonate preparations, etc., can solve the problems of high power consumption, difficult continuous operation, large labor consumption, etc. Reasonable process flow and good environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
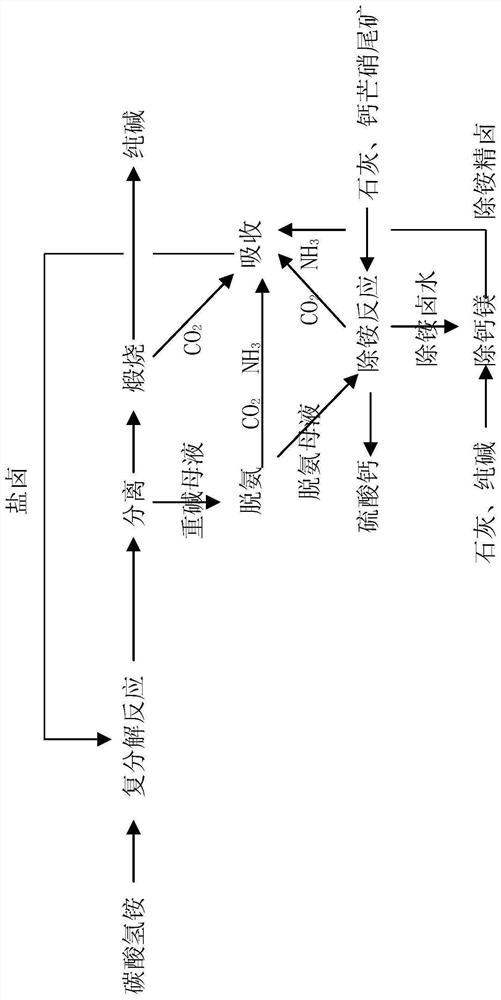
[0034]Example 1: 1) Take 158 tons of carbon ammonium (NH4HCO3) 380M in step 7)3Saline halide (NaCl 305g / L, NA2SO45.22g / l and NH4HCO3The et al decreases for the raw material (reaction temperature 30 ° C, reaction time 1.5 hours) to isolate 168 tons of solid sodium hydrogencarbonate (NaHCO3) And 380M3Heavy alkali - NaCl 71g / L, NA2SO4 5.22g / L, NH4CL179G / L and NH4HCO3(NH4)2CO3); 2) 168 tons of sodium bicarbonate (Nahco3) Calcination to obtain 106 tons of pure base (NA2CO3) Products and carbon dioxide (CO2); 3) 380M3Heavy alkali - NaCl 71g / L, NA2SO4 5.22g / L, NH4CL 179g / L and NH4HCO3(NH4)2CO3) Preheating high temperature (temperature 100 ° C) removes ammonium bicarbonate (NH4HCO3), Ammonium carbonate ((NH4)2CO3After getting ammonia (NH3), Carbon dioxide (CO2) And 380M3Detexamine (NaCl 71g / L, NA2SO4 5.22g / L, NH4CL 179g / L); 4) 380M3Detexamine (NaCl 71g / L, NA2SO4 5.22g / L, NH4CL 179g / L) with 37 tons of lime (CA (OH)2), 200 tons of calcium rice nitroids (NA2SO4 30%,...
Embodiment 2
[0035]Example 2: 1) Take 158 tons of carbon ammonium (NH4HCO3) 380M in step 7)3Saline halide (NaCl 305g / L, NA2SO45.22g / l and NH4HCO3The et al decreases for the raw material (reaction temperature 30 ° C, reaction time 1.5 hours) to isolate 168 tons of solid sodium hydrogencarbonate (NaHCO3) And 380M3Heavy alkali - NaCl 71g / L, NA2SO4 5.22g / L, NH4CL179G / L and NH4HCO3(NH4)2CO3); 2) 168 tons of sodium bicarbonate (Nahco3) Calcination to obtain 106 tons of pure base (NA2CO3) Products and carbon dioxide (CO2); 3) 380M3Heavy alkali - NaCl 71g / L, NA2SO4 5.22g / L, NH4CL 179g / L and NH4HCO3(NH4)2CO3) Preheating high temperature (temperature 100 ° C) removes ammonium bicarbonate (NH4HCO3), Ammonium carbonate ((NH4)2CO3After getting ammonia (NH3), Carbon dioxide (CO2) And 380M3Detexamine (NaCl 71g / L, NA2SO4 5.22g / L, NH4CL 179g / L); 4) 380M3Detexamine (NaCl 71g / L, NA2SO4 5.22g / L, NH4CL 179g / L) and 400 tons of calcium rice nitroidation (NA2SO430%, CASO4 30%, Caco3 25%, MGS...
Embodiment 3
[0036]Example 3: 1) Take 158 tons of carbon ammonium (NH4HCO3) 380M in step 7)3Saline halide (NaCl 305g / L, NA2SO45.22g / l and NH4HCO3The et al decreases for the raw material (reaction temperature 30 ° C, reaction time 1.5 hours) to isolate 168 tons of solid sodium hydrogencarbonate (NaHCO3) And 380M3Heavy alkali - NaCl 71g / L, NA2SO4 5.22g / L, NH4CL179G / L and NH4HCO3(NH4)2CO3); 2) 168 tons of sodium bicarbonate (Nahco3) Calcination to obtain 106 tons of pure base (NA2CO3) Products and carbon dioxide (CO2); 3) 380M3Heavy alkali - NaCl 71g / L, NA2SO4 5.22g / L, NH4CL 179g / L and NH4HCO3(NH4)2CO3) Preheating high temperature (temperature 100 ° C) removes ammonium bicarbonate (NH4HCO3), Ammonium carbonate ((NH4)2CO3After getting ammonia (NH3), Carbon dioxide (CO2) And 380M3Detexamine (NaCl 71g / L, NA2SO4 5.22g / L, NH4CL 179g / L); 4) 380M3Detexamine (NaCl 71g / L, NA2SO4 5.22g / L, NH4CL 179g / L) and 400 tons of calcium rice nitroidation (NA2SO430%, CASO4 30%, Caco3 25%, MGS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com