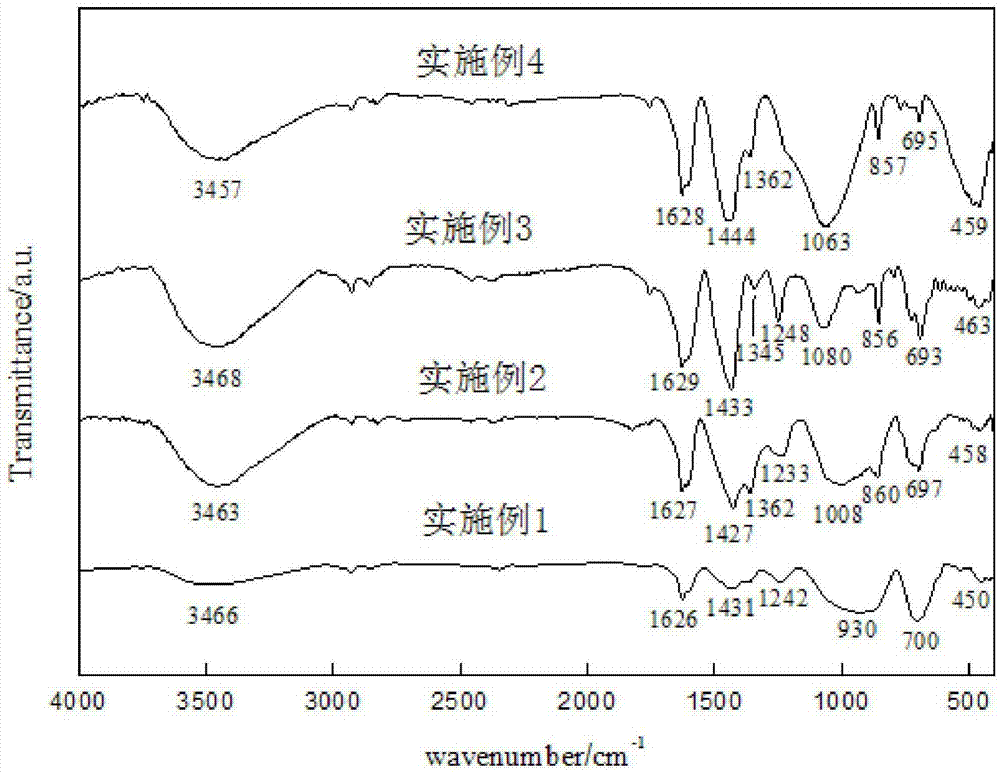
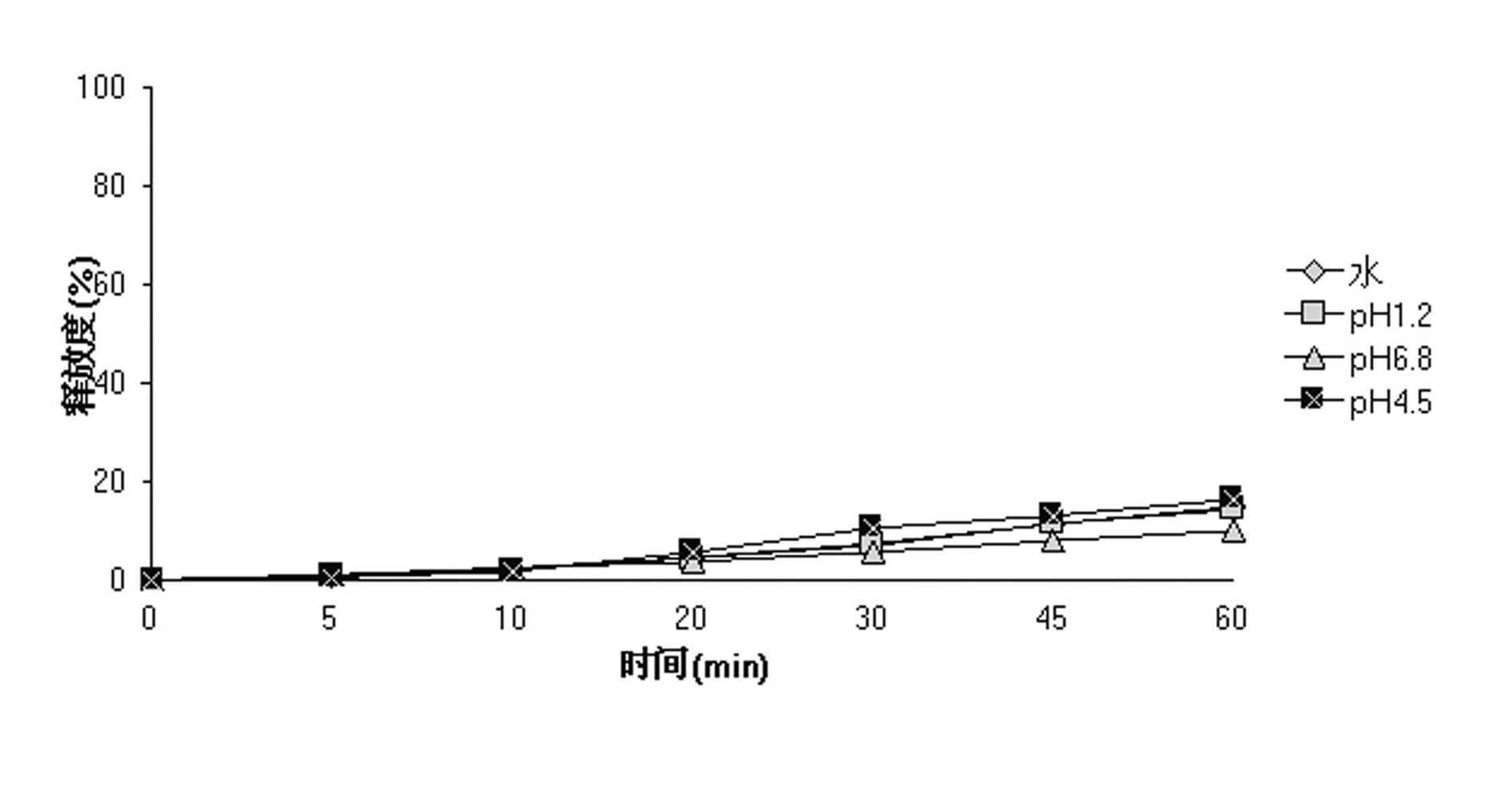
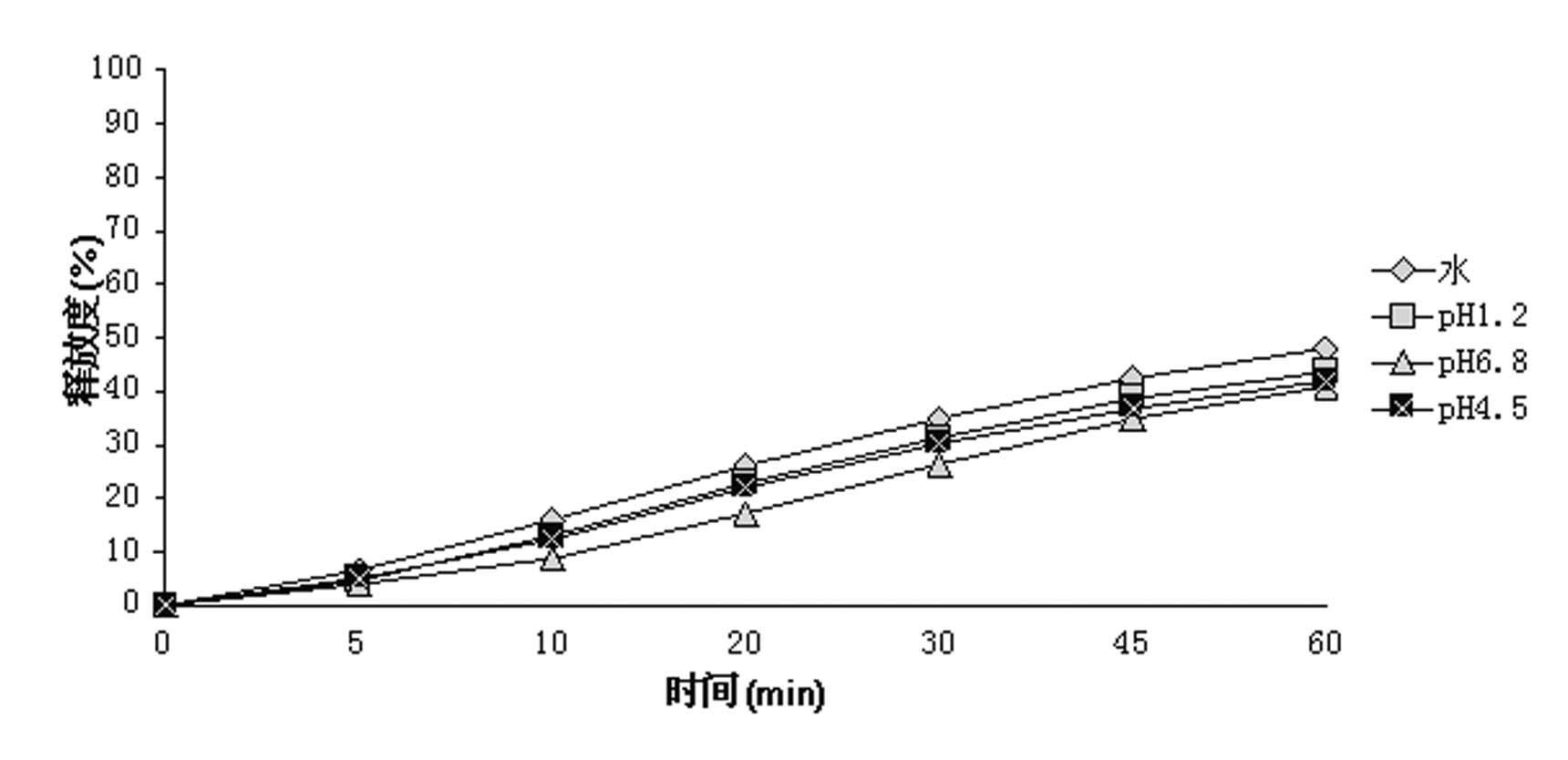
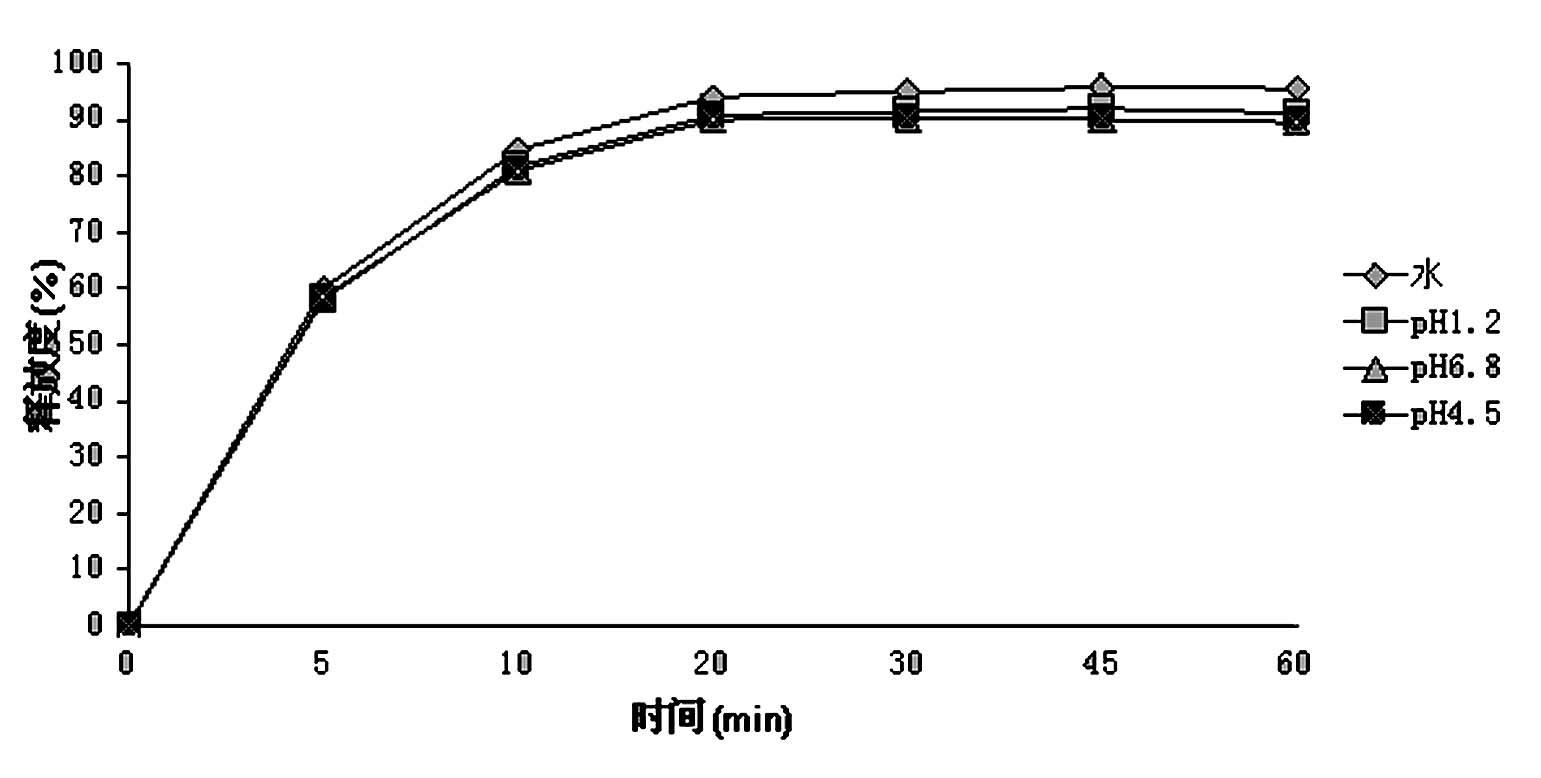
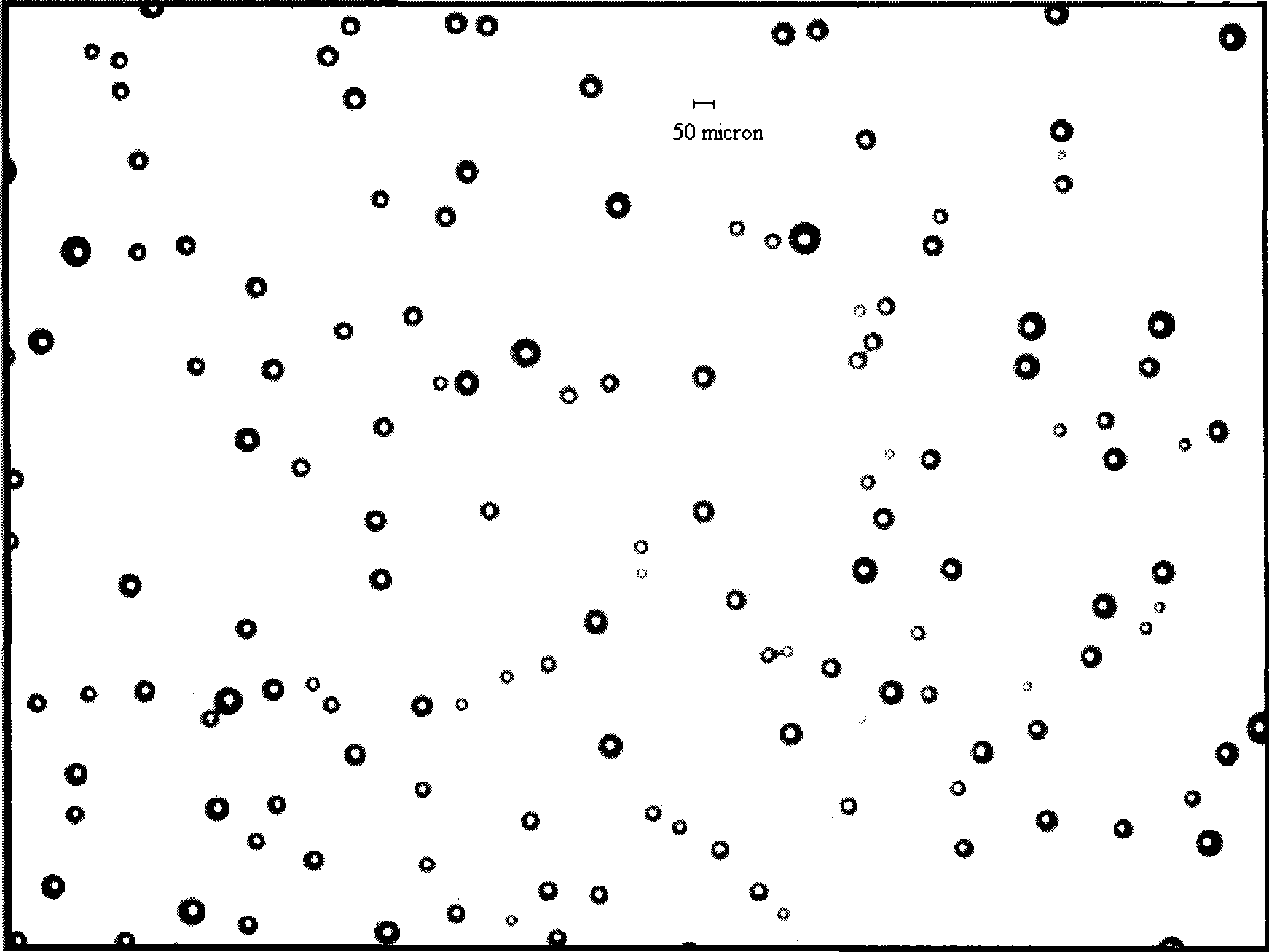
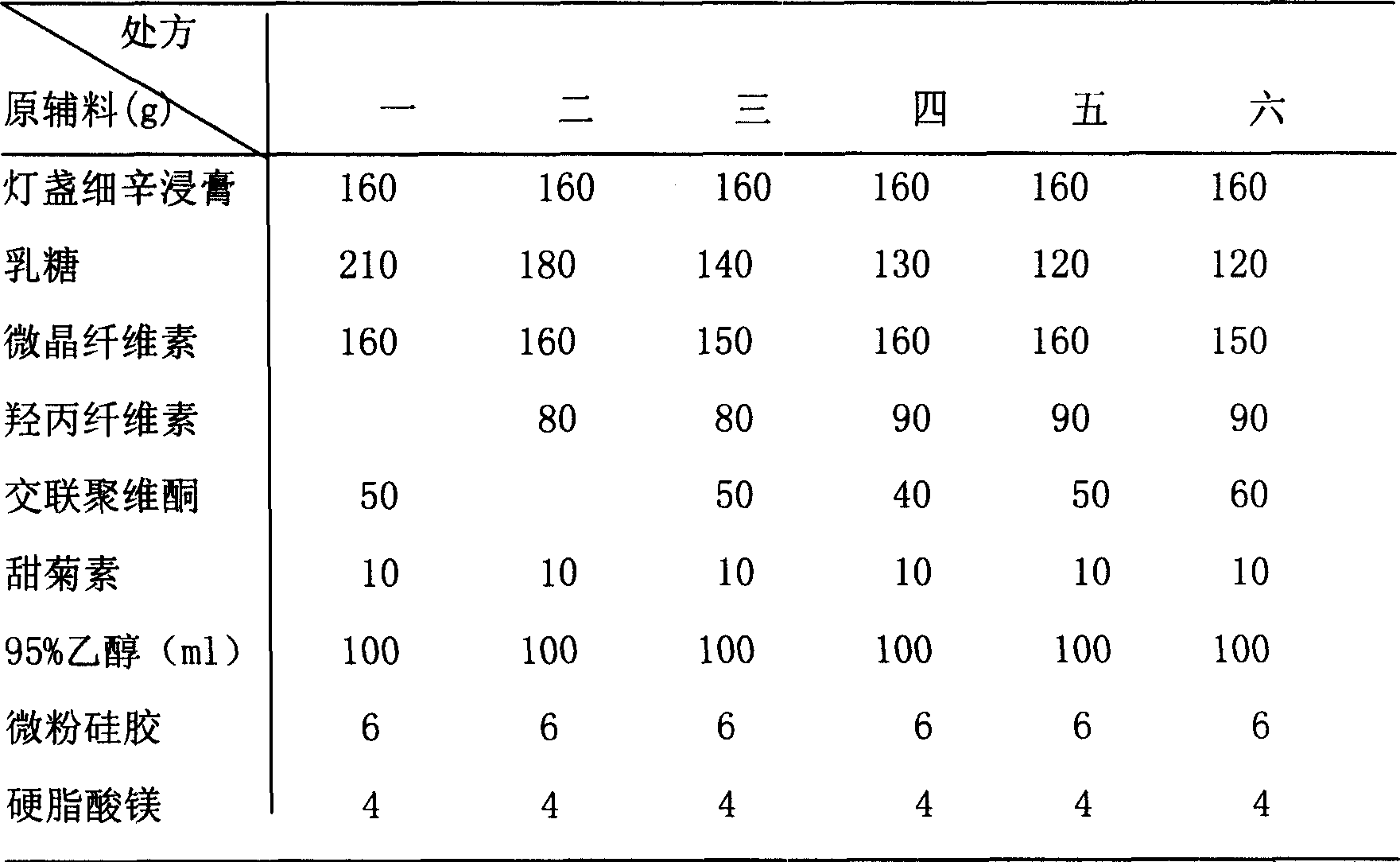
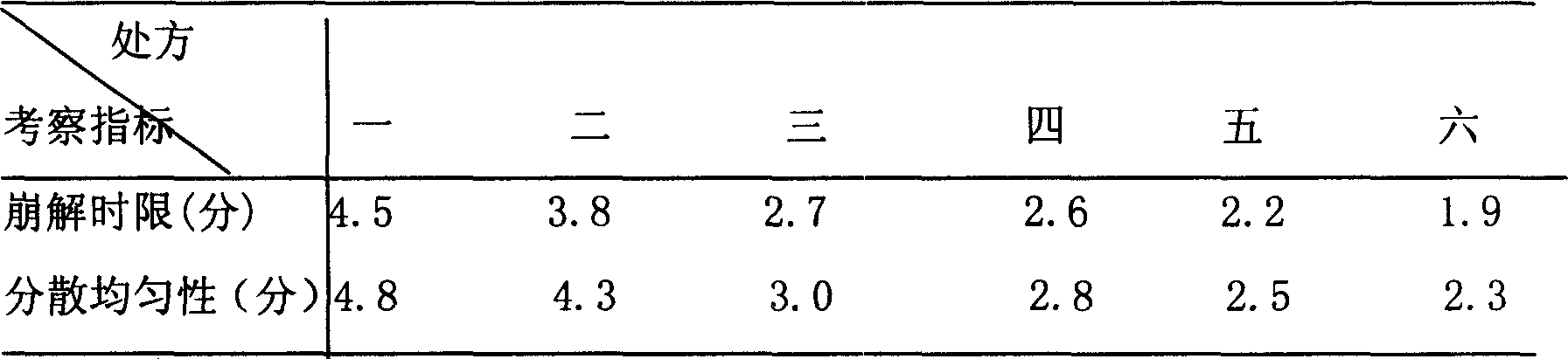
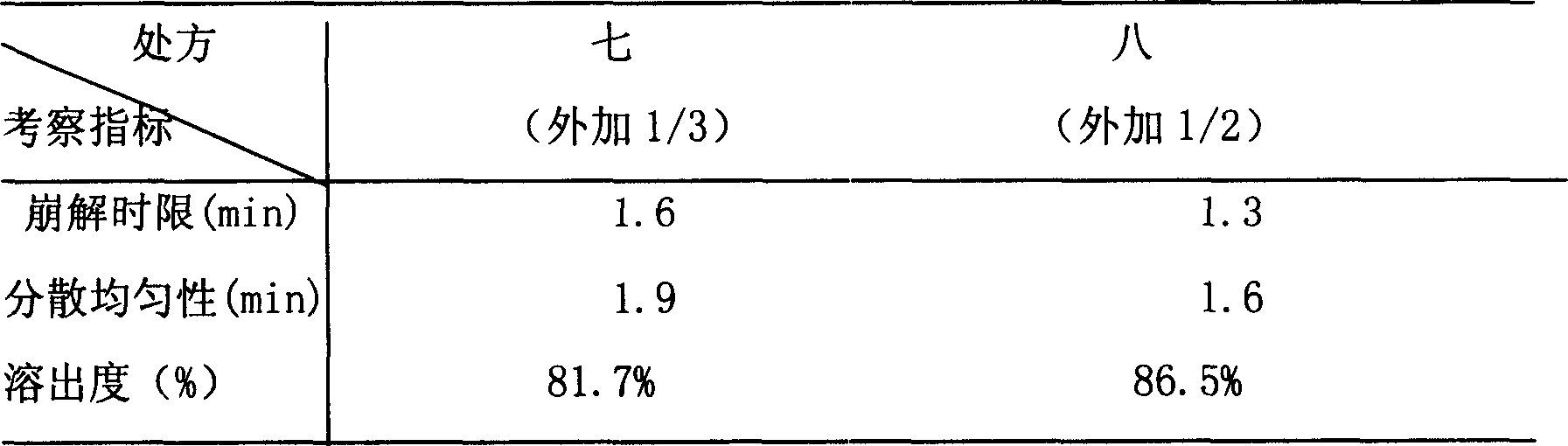
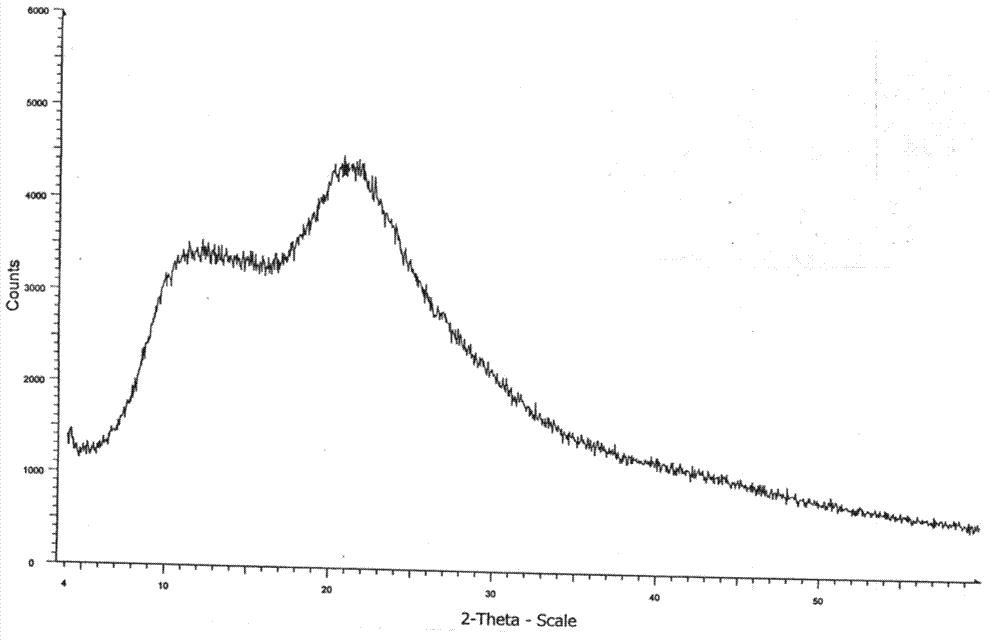
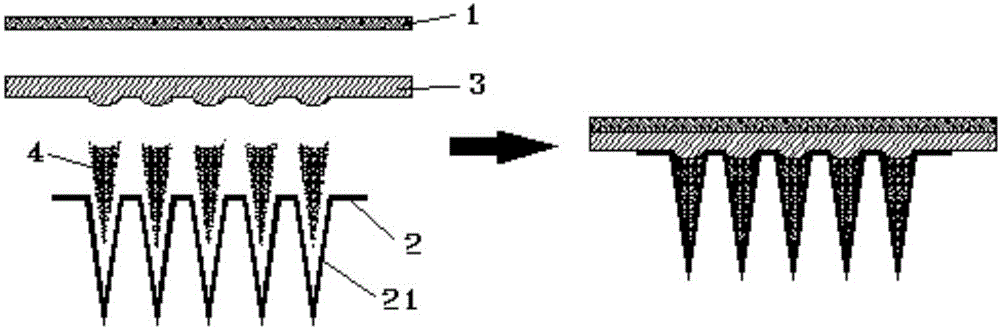
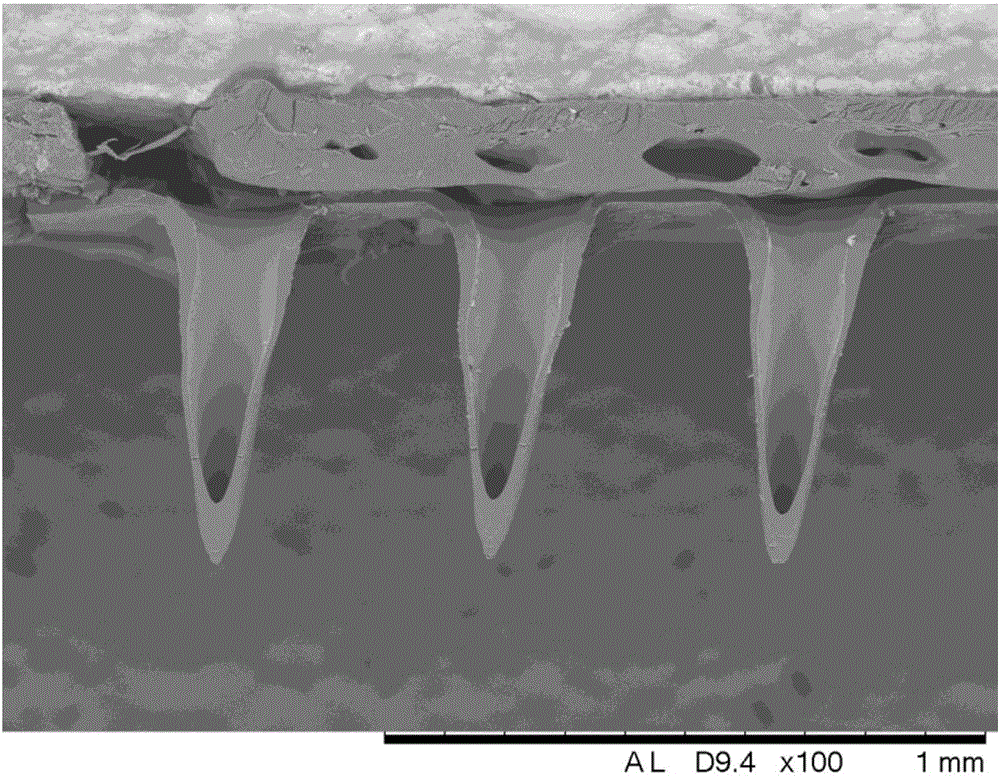
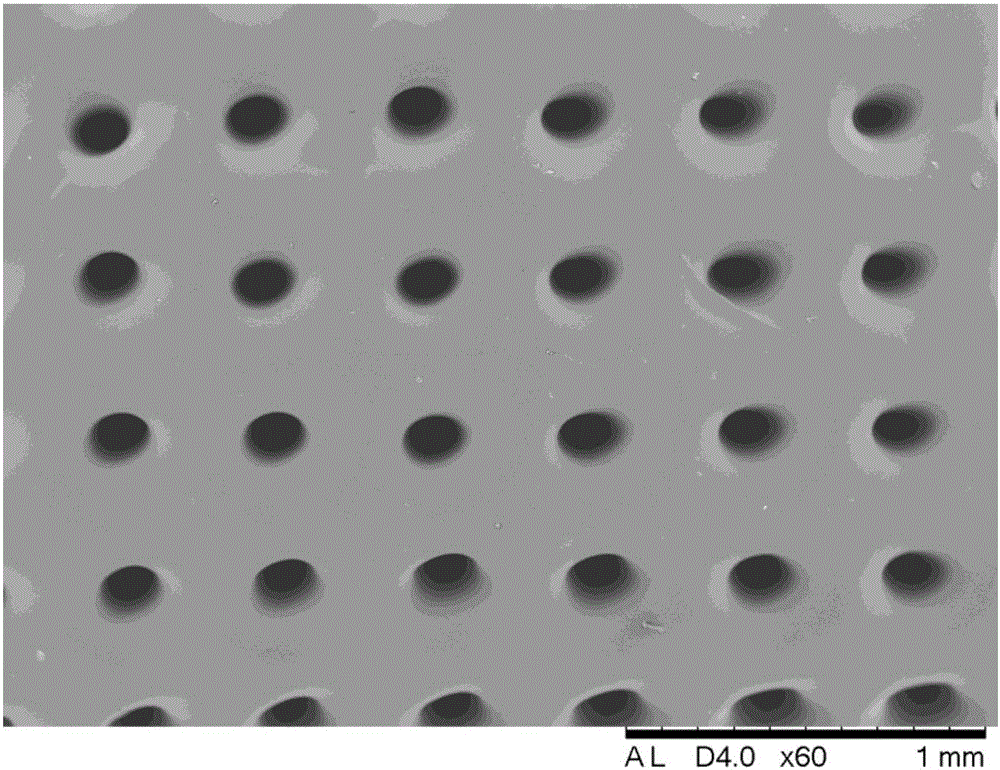
Patents
Literature
1180results about How to "Increase dissolution rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
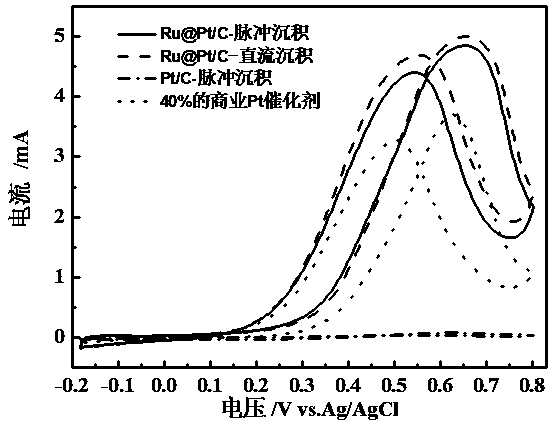
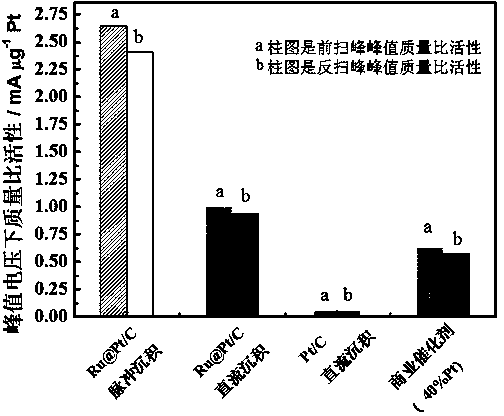
Core-shell structure catalyst for fuel cells and its pulse electrodeposition preparation method
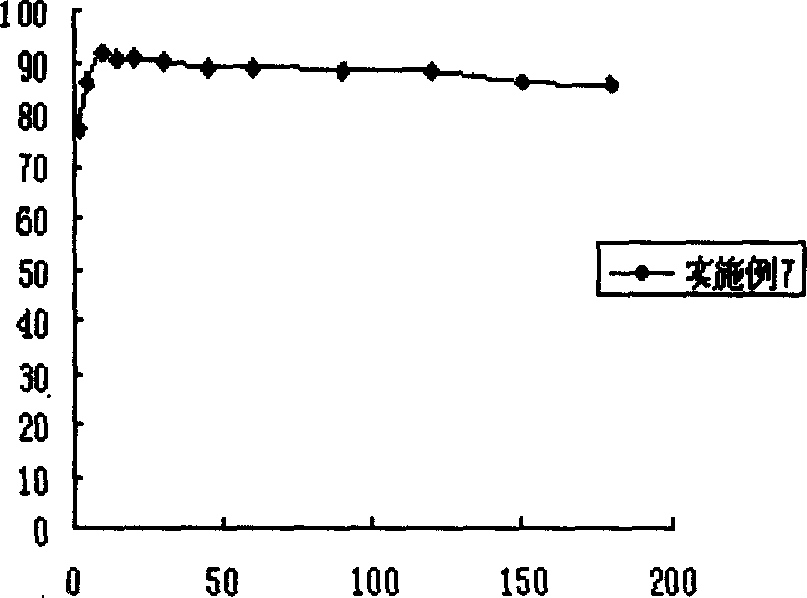
ActiveCN103638925AIncrease profitReduce usageCell electrodesMetal/metal-oxides/metal-hydroxide catalystsPlatinumPtru catalyst
The invention discloses a core-shell structure catalyst for fuel cells and its pulse electrodeposition preparation method. The active component of the catalyst is a nanoparticle with a core-shell structure, and an active metal is cladded in the form of an ultrathin shell on the surface of a carbon carrier loaded metal or alloy nanoparticle serving as a core. The catalyst takes a non-platinum noble metal or transition metal as the core, and adopts more than one of Pt, Ir or Au as the shell. The preparation method includes: preparation of the nanoparticle serving as the core, making of a working electrode for pulse electrodeposition, and preparation of the catalyst by pulse electrodeposition. The catalyst can be used as an anode or cathode catalyst of a low temperature fuel cell. The obtained catalyst has very high stability. Compared with underpotential deposition, the method is simple to operate, has no need for inert atmosphere protection, and is more suitable for large-scale industrial production, also can greatly reduce the noble metal consumption of fuel cells, and greatly reduce the cost of fuel cells, thus having great significance in promoting the commercialization process of fuel cells.
Owner:SOUTH CHINA UNIV OF TECH
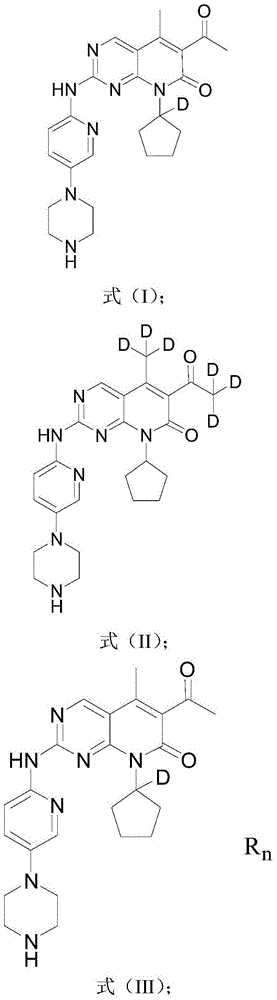
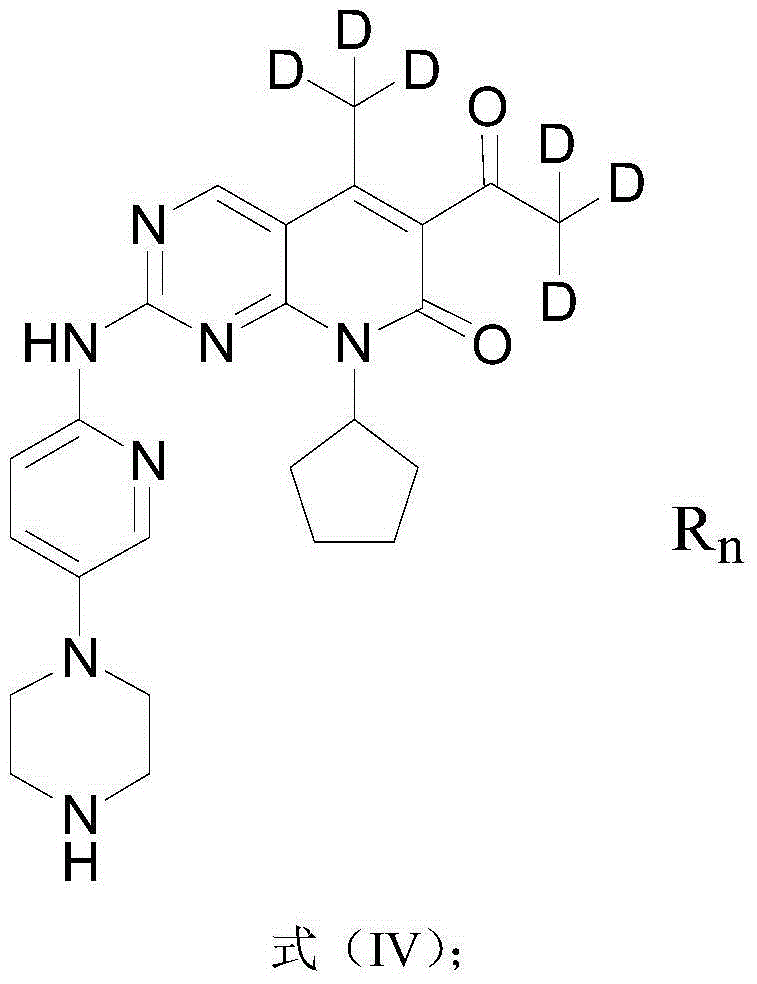
Deuterated palbociclib derivative, and preparation method and application thereof
ActiveCN104447739AImprove pharmacokinetic propertiesGood curative effectOrganic chemistry methodsAntineoplastic agentsSolubilityCurative effect
The invention discloses a deuterated palbociclib derivative, and a preparation method and an application thereof. A structural formula of the deuterated palbociclib derivative is as shown in a formula (I), a formula (II), a formula (III) or a formula (IV). According to the deuterated palbociclib derivative disclosed by the invention, through selective deuteration of palbociclib, the pharmacokinetic property of the medicine is improved, thus the curative effect, the safety and the tolerance of the medicine are improved. According to the deuterated palbociclib salt disclosed by the invention, the solubility and the dissolution rate of the medicine are improved; a new compound is provided for synthesis of a novel anti-tumor medicine through synthesis of the deuterated palbociclib derivative; and the deuterated palbociclib derivative has similar biologic activity to the palbociclib, and has a good medicine application prospect.
Owner:TYK MEDICINES INC
Method for preparing rivaroxaban solid composition
InactiveCN103705520ASolve the problem that the particle size cannot be reduced to 5μmIncrease dissolution rateOrganic active ingredientsRespiratory disorderMedicineRivaroxaban
The invention discloses a method for preparing a rivaroxaban solid composition. The method comprises the following steps: crushing rivaroxaban by adopting a wet crushing method, and simultaneously preparing a suspension solution; spraying the suspension solution into other auxiliaries to prepare grains with proper sizes; further preparing minimum medical dosage units. The method has the beneficial effects that: the problem that the grain diameter cannot be reduced to 5mu m when rivaroxaban particles are prepared by adopting a conventional crushing method can be solved, and the dissolution speed of the rivaroxaban preparation can be increased.
Owner:CHINA RESOURCES SAIKE PHARMA
Device and method for extracting effective components from natural plant by high-pressure spraying reverse-flow process
ActiveCN102772912AImprove extraction efficiencyIncreased contact surface areaSolid solvent extractionSolventChemistry
The invention discloses a device and a method for extracting effective components from a natural plant by a high-pressure spraying reverse-flow process. The method comprises the steps of: after the device is connected, removing impurities of a natural plant to be extracted and smashing, putting the smashed natural plant to be extracted in a stirring pot and adding a soak solution in the stirring pot to obtain material suspension, adding an extraction solvent into the extracting pot, spraying the prepared material suspension to be mist-state drops so as to perform spraying extraction, extracting by a high-pressure spraying reverse-flow, and finally, using a centrifugal machine to remove solid impurities, reducing pressure to concentrate to obtain a product by spraying drying, microwave drying or vacuum drying. With the adoption of the device and the method for extracting the effective components of the natural plant by the high-pressure spraying reverse-flow process, the production efficiency can be obviously improved, the use ratio of the device is improved, a plurality of devices can be used for extracting via a dynamic cycle mode, the extracting process does not need to be heated and is performed at a normal temperature, so that the damage of the effective components in the high-temperature heating process is avoided; and the device is low in investment and running costs.
Owner:XIAN HONSON BIOTECH
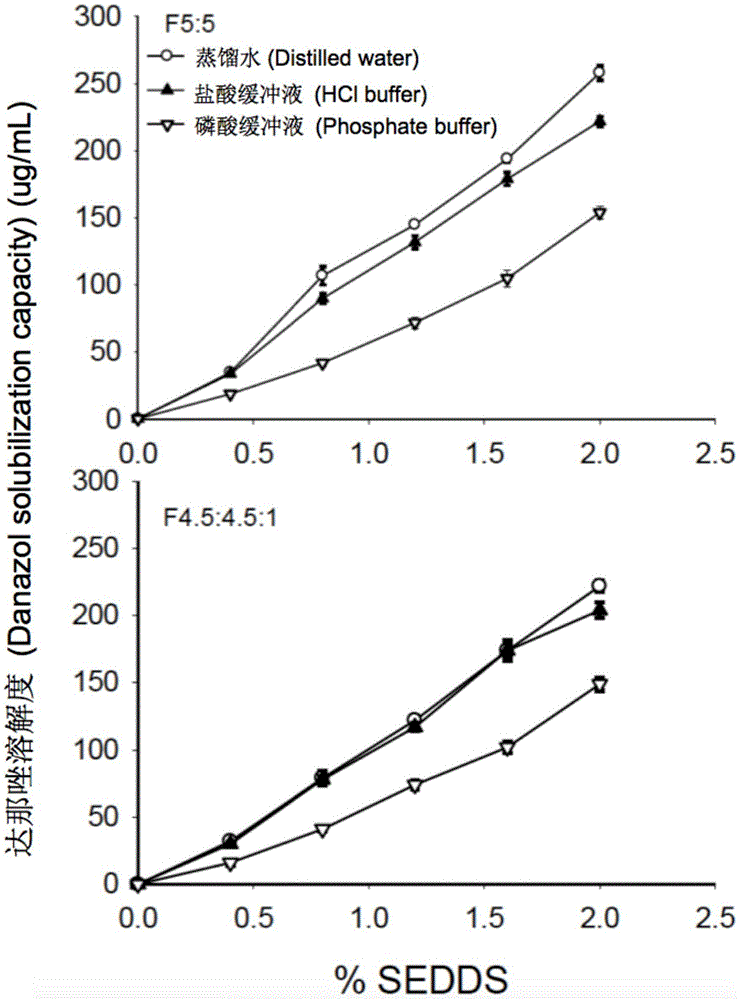
Self-emulsifying drug delivery system for improving bioavailability of insoluble medicine, and application thereof
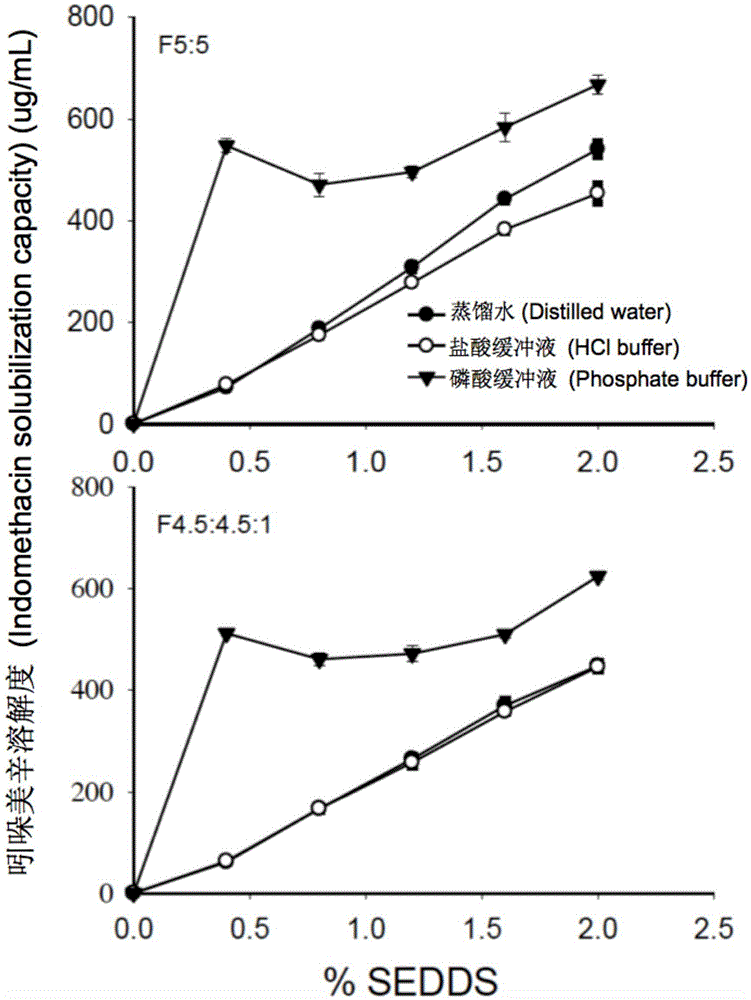
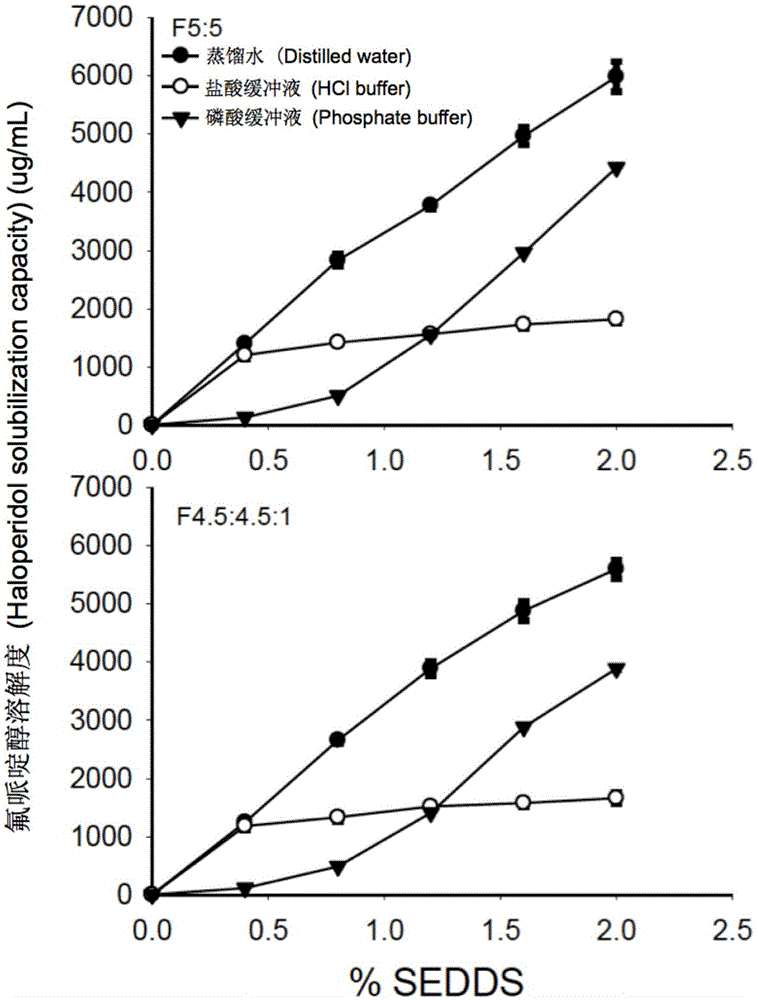
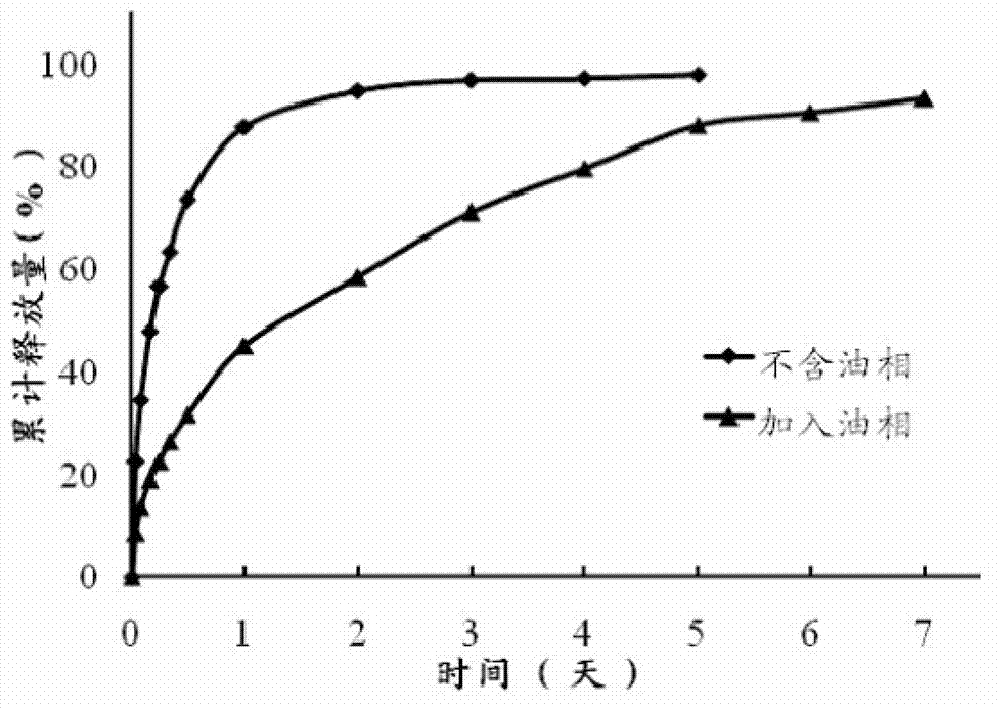
ActiveCN105535979AGood water solubilityIncrease dissolution rateOrganic active ingredientsCapsule deliverySolubilityOil phase
The invention provides a novel self-emulsifying drug delivery system (SEDDS). An SEDDS carrier material comprises a surfactant and an oil phase containing Capmul MCMs and medium chain fatty acids, is suitable for loading pH-dependent (weakly acidic and weakly alkaline) and a pH-independent (neutral) insoluble medicines, greatly improves the solubility of the medicines to realize optimum bioavailability, and has important application values in the development of preparations of the insoluble medicines.
Owner:李素华
Stripping solution for sintered neodymium-iron-boron surface aluminum coating
The invention discloses stripping solution for a sintered neodymium-iron-boron surface aluminum coating. The stripping solution comprises sodium hydroxide serving as a corroding agent, sodium carbonate serving as an auxiliary salt, a complexing agent capable of promoting the dissolution of metallic aluminum, a corrosion inhibitor for retarding the corrosion of a matrix, and a surfactant for uniformly dissolving an aluminum coating. Compared with the prior art, the stripping solution for the sintered neodymium-iron-boron surface aluminum coating has the advantages of quickly and uniformly stripping the sintered neodymium-iron-boron surface aluminum coating and simultaneously reducing the damage to the sintered neodymium-iron-boron matrix due to the reasonable design of components and content of the stripping solution; and besides, the stripping solution has the characteristics of low production cost, high stability and long service cycle.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI +4
Biological phosphatic fertilizer as well as preparation method thereof
ActiveCN104016795AAvoid fixationImprove phosphorus dissolution rateFertilizer mixturesSoil sciencePhosphate solubilizing bacteria
The invention discloses a biological phosphatic fertilizer. The preparation method of the phosphatic fertilizer comprises the following steps: culturing AM mycorrhiza, phosphate solubilizing bacteria and rhizobium respectively, mixing with a phosphatic fertilizer to react, then adding various nutritional elements required for plants, and mixing and granulating to obtain porous fertilizer particles, so that the contact area between the fertilizer and water is increased, and the dissolving velocity of the fertilizer in water is accelerated. The biological phosphatic fertilizer can activate the phosphatic fertilizer, and can improve the absorption of the phosphatic fertilizer by plants, increase the utilization ratio of the phosphatic fertilizer, reduce the using quantity of the fertilizer and reduce the pollution to the environment; the fertilizer can be rapidly dissolved in water for irrigation and fertilization together with water, and is suitable for modern agricultural water and fertilization integration.
Owner:内蒙古和盛生态科技研究院有限公司
Precursor suspension of lyotropic liquid crystal and preparation method thereof
ActiveCN103040741AHigh viscosityHigh strengthSolution deliveryEmulsion deliveryOrganic solventUltimate tensile strength
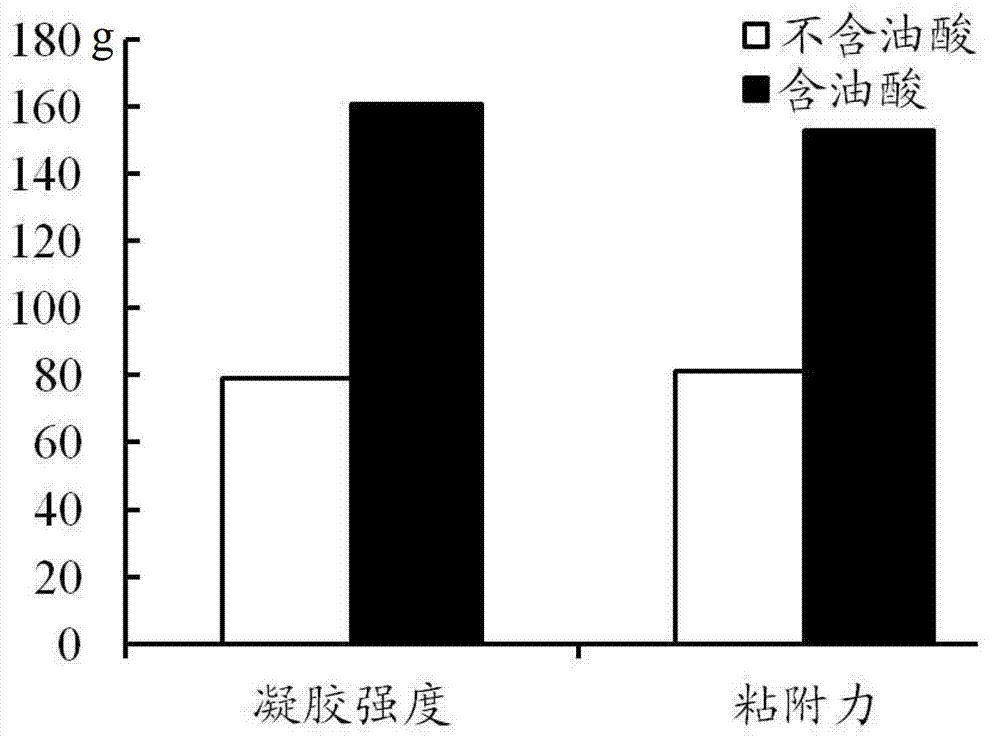
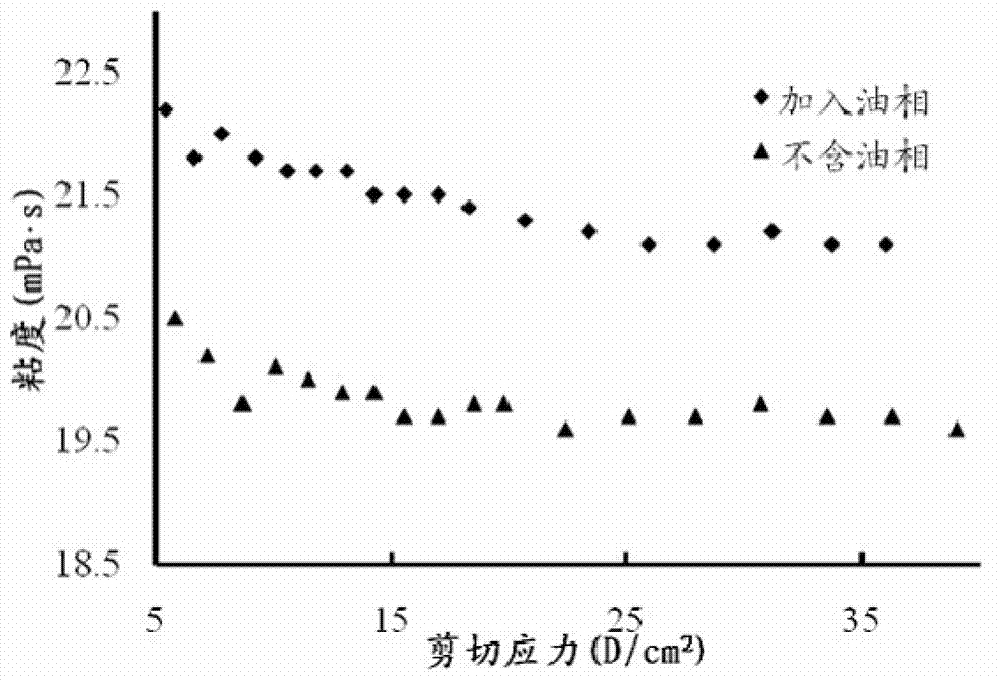
The invention discloses a precursor suspension of a lyotropic liquid crystal. The precursor suspension comprises lyotropic liquid crystal material, organic solvent, oil phase and a drug, wherein the weight percentage of the oil phase in the precursor suspension is 2-50 percent, the weight percentage of the drug in the precursor suspension is 1-30 percent, and the weight ratio of the lyotropic liquid crystal material and the organic solvent in the precursor suspension is 2-9:1. According to the invention, through the adding of the oil phase into the precursor suspension, the stability of the suspension is improved, the sedimentation rate is reduced, and the strength and the adhesive force of the gel formed are enhanced at the same time; the gel formed in the body is more liable to stay at a lesion location and less liable to be relocated and the shape is less liable to be damaged by the mechanical motion of the body, so that the drug therapy can be located effectively; and the preparation technology is simple and the precursor suspension of the lyotropic liquid crystal is a partial slow-release drug delivering system provided with a favorable perspective.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Lycium ruthenicum beverage and preparation method thereof
InactiveCN103960727AEasy to keepReduce the effects of oxidationFood ingredient functionsFood preparationFlavorLiver and kidney
The invention discloses a lycium ruthenicum beverage and a preparation method thereof and provides a beverage prepared by taking dried lycium ruthenicum fruit powder as a main raw material and a preparation method thereof. The lycium ruthenicum beverage is composed of following raw materials by weight percent: 0.1%-7% of the dried lycium ruthenicum fruit powder, 0.05%-3% of guar gum, 0.2%-7% of CMC-Na (Carboxyl Methyl Cellulose-Na), 0.05%-3% of xanthan gum, 3%-15% of erythritol, 0.006%-0.6% of stevioside, 0.01%-0.8% of citric acid, 0.01%-0.4% of sodium citrate, 0.01%-1.5% of tea polyphenol and the balance being water. The beverage is prepared by taking the dried lycium ruthenicum fruit powder as the main raw material; reasonable compatibility and synergistic effects of all the components are utilized and the color of a product is amaranth; the lycium ruthenicum beverage has a unique flavor, no astringent and mellow mouth feel, and has the effects of nourishing liver and kidney, replenishing vital essence to improve eyesight and nourishing blood, enhancing the immunity of people, beautifying, resisting oxidization, decaying aging, resisting fatigues, lowering blood pressure and lowering blood sugar.
Owner:TIANJIN UNIV OF COMMERCE
Laundry liquid and preparation method thereof
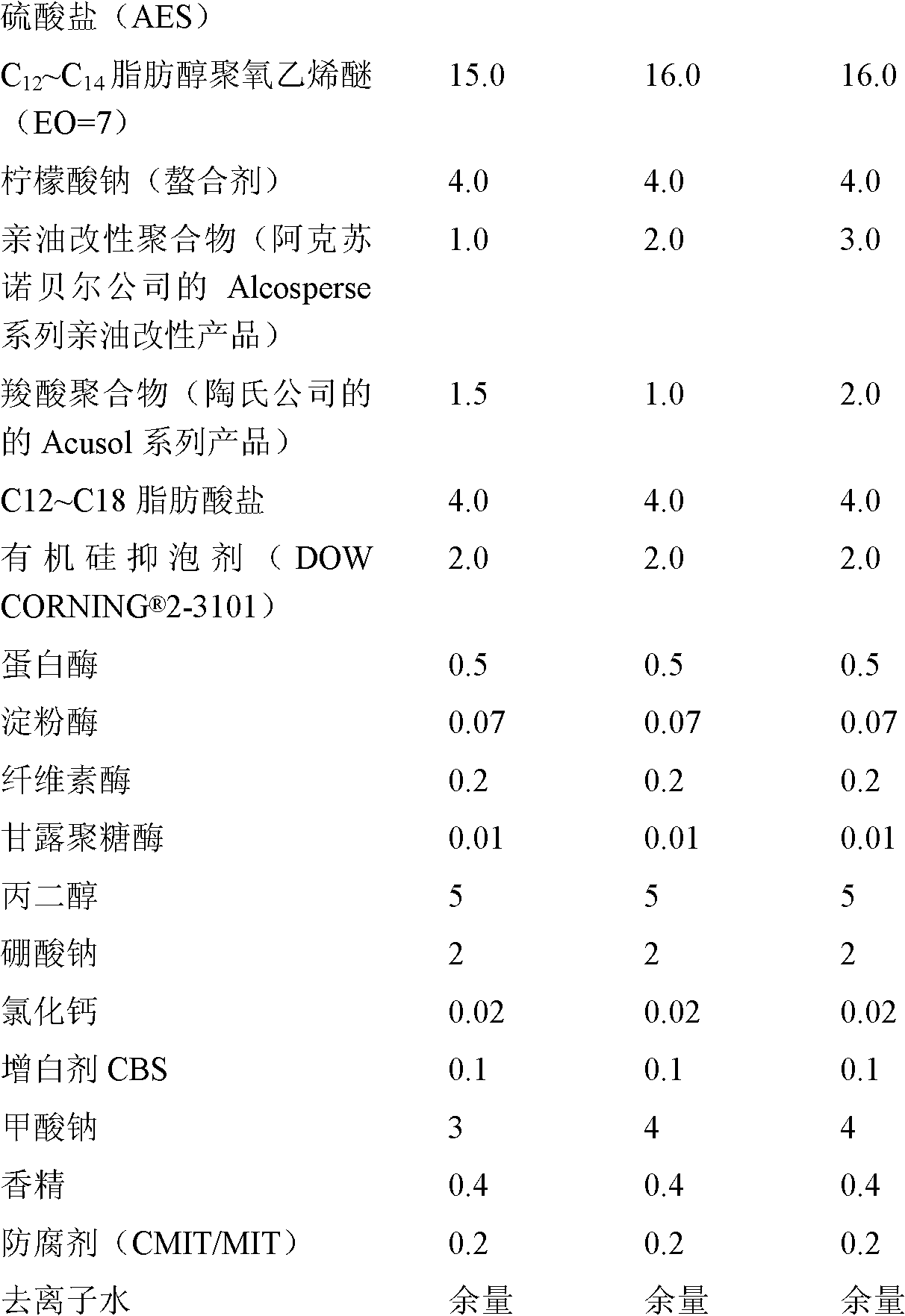
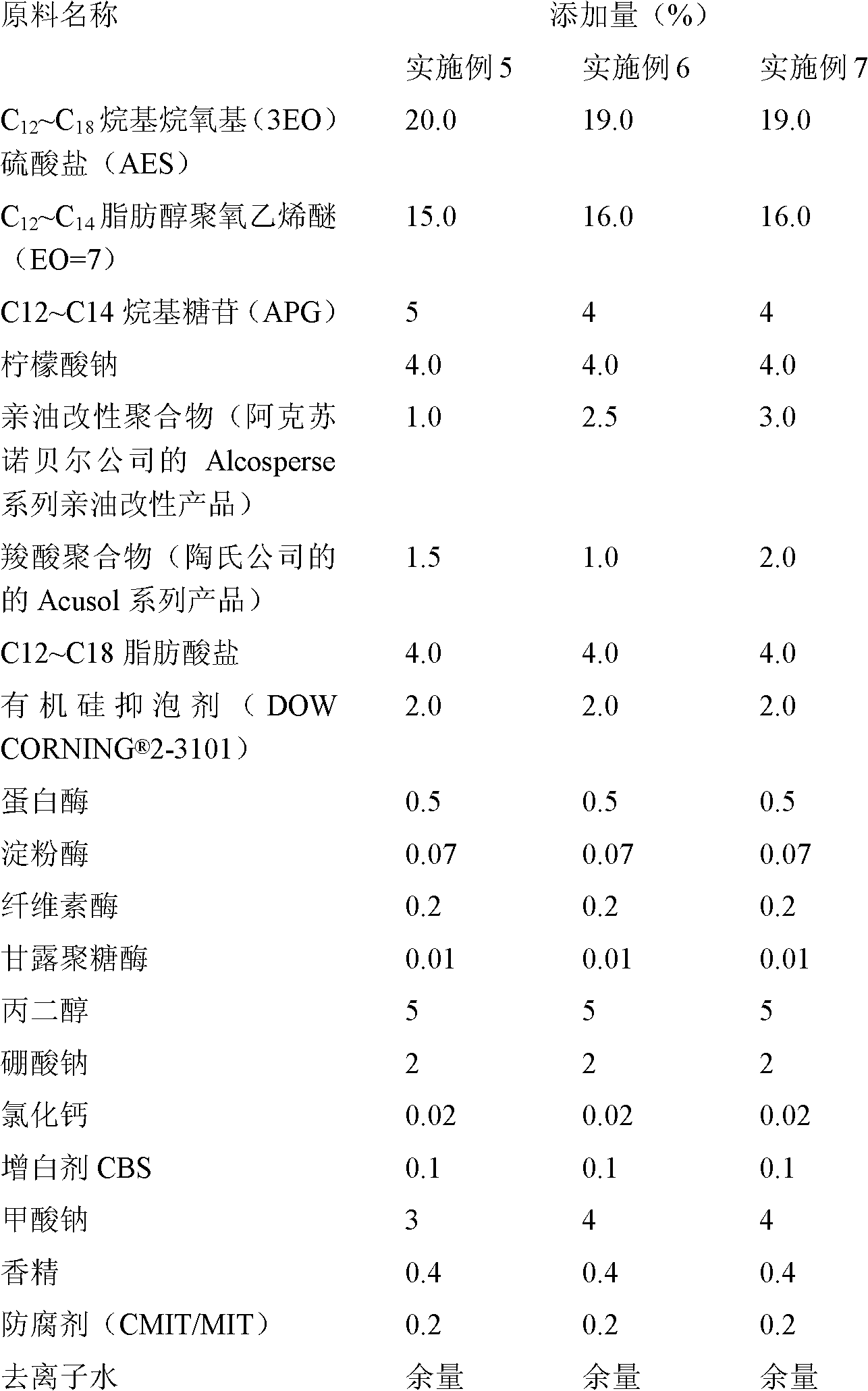
ActiveCN102071111AShorten the dissolution timeIncrease productivityNon-ionic surface-active compoundsOrganic detergent compounding agentsCarboxylic acidFOAM CONTROL
The invention discloses laundry liquid and a preparation method thereof. The laundry liquid is prepared by the following materials in percentage by mass: 0.1 to 5.0 percent of oleophylic modified polymer, 0.2 to 5.0 percent of carboxylic acid polymer, 1 to 25.0 percent of nonionic surfactant, 1 to 25.0 percent of anionic surfactant, 0.01 to 1.5 percent of enzyme, 0.1 to 15.0 percent of enzyme stabilizer, 0.5 to 8.0 percent of foam control agent, 0.5 to 10 percent of other auxiliary agents and the balance of water. By the preparation method of the laundry liquid, the production time can be reduced by about 30 percent, and the laundry liquid is efficiently produced; and the prepared laundry liquid has high detergency to mud and oil stains and high stain redeposition resistance.
Owner:GUANGZHOU LIBY
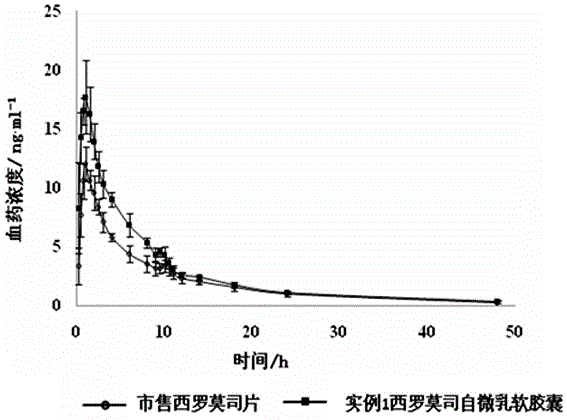
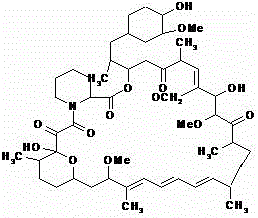
Sirolimus self-microemulsion preparation and preparation method thereof
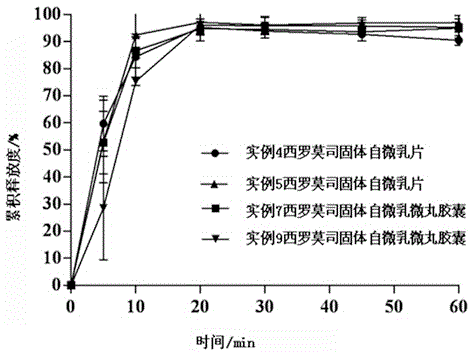
InactiveCN105640886AImprove solubilityIncrease dissolution rateOrganic active ingredientsAntipyreticBiomedical engineeringOil phase
The invention discloses a sirolimus self-microemulsion preparation and a preparation method thereof. Sirolimus, an oil phase, an emulgator and a co-emulsifier are compounded into a liquor self-microemulsion preparation, or the obtained liquor self-microemulsion preparation and an excipient are further prepared into a solid self-microemulsion preparation. According to the sirolimus self-microemulsion preparation and the preparation method thereof, a self-microemulsion technology is adopted, so that the solubility and the dissolution rate of the sirolimus are remarkably improved, and the sirolimus self-microemulsion preparation has the advantages that the bioavailability is higher, raw materials are easily obtained, the preparation technology is simple and practicable, the yield is high, the cost is low, the large-scale industrial production can be realized, and the remarkable economic benefit can be achieved.
Owner:FUZHOU GENERAL HOSPITAL OF NANJING MILITARY COMMAND P L A
Solid corrosion and scale inhibitor for oil field
InactiveCN101805597ALow cost of corrosion and scale inhibitionReduce filling operationsDrilling compositionBorehole/well accessoriesPolyethylene glycolTricarboxylic acid
The invention discloses a solid corrosion and scale inhibitor for an oil field, which relates to the technical field of chemical medicine reagents used for the oil field. The solid corrosion and scale inhibitor comprises the following ingredients: 2-phosphonic acid butane 1, 2, 4-tricarboxylic acid PBTCA, polyethyleneglycol, sodium hexametahposphate, span-60 and stearic acid, wherein each ingredient accounts for the mass percent of the sum of all the ingredients as follow: 30 percent to 40 percent of 2-phosphonic acid butane 1, 2, 4-tricarboxylic acid PBTCA, 10 percent to 15 percent of polyethyleneglycol, 10 percent to 15 percent of sodium hexametahposphate, 15 to 25 percent of span-60 and 20 percent to 30 percent of stearic acid. The invention is particularly suitable for well tubes of oil-gas wells with server corrosion and scale conditions, and has good corrosion and scale inhibition effects on underground pipe posts and equipment.
Owner:CHINA NAT PETROLEUM CORP CHUANQING DRILLING ENG CO LTD
Novel tabletting type smokeless tobacco product and preparation method thereof
InactiveCN103549648AIncrease dissolution rateImprove absorption rateTobacco preparationTobacco treatmentAdditive ingredientMultivitamin
The invention relates to a novel tabletting type smokeless tobacco product and a preparation method thereof and belongs to the technical field of smokeless tobacco processing. The novel tablet type smokeless tobacco product consists of main ingredients and auxiliary materials, wherein the main ingredient is any one or combination of tobacco ultramicro powder, tobacco extract and nicotine; the auxiliary materials include newtol, sorbitol, honey, adhesive, composite vitamin compound, plant extract, citric acid, edible essence and magnesium stearate. The preparation method comprises the steps that the ultramicro powder is processed, ingredients are mixed, soft material is prepared and dried, particles are processed, and the particles, the edible essence and the magnesium stearate are uniformly mixed and subjected to tabletting. The novel tabletting type smokeless tobacco product can provide consumers with smoking-alike satisfaction, is fresh and cool in taste and has no residues, and the content of the nicotine in the product can be accurately controlled. The novel tablet type smokeless tobacco product is simple and convenient to carry with, store and take.
Owner:YUNNAN RES INST OF TOBACCO SCI
Orally-disintegrating-tablet-type smokeless tobacco product containing tobacco ultra-micro powder and preparation method thereof
ActiveCN103549646AIncrease dissolution rateImprove absorption rateTobacco treatmentSodium bicarbonateOrally disintegrating tablet
The invention relates to an orally-disintegrating-tablet-type smokeless tobacco product containing tobacco ultra-micro powder and a preparation method thereof, belonging to the technical field of smokeless tobacco processing. The smokeless tobacco product is made of the following raw materials by weight percentage: 30-70 percent of tobacco ultra-micro powder with grain size being smaller than or equal to 10mum, 20-50 percent of xylitol, 1-10 percent of water-soluble starch, 1-10 percent of bonding agent, 0.5-10 percent of composite vitamin, 1-10 percent of plant extract, 0.01-2 percent of edible essence, 3-10 percent of sodium hydrogen carbonate, 2-5 percent of citric acid and 0.3-5 percent of magnesium stearate. The preparation method comprises the steps of micro powder processing, raw material component mixing, soft material preparation and drying, particle processing and tablet pressing after the particles are uniformly mixed with the citric acid, the sodium hydrogen carbonate, the edible essence and the magnesium stearate. After tobacco is micro-powdered, the dissolution rate of active components is improved; the product melts in the mouth, the taste is fine, the human body absorption rate is improved and the cost is saved; the product is naturally disintegrated in the mouth during chewing, a unique pleasant sense can be produced during disintegration and the taste is refreshing and fine.
Owner:YUNNAN RES INST OF TOBACCO SCI
Method for preparing copper/ceramic composite substrate on basis of low-melting-point glass powder
InactiveCN105439643ALow softening temperatureImprove adhesionGlass shaping apparatusSol-gelGel method
The invention provides a method for preparing a copper / ceramic composite substrate on the basis of low-melting-point glass powder. The method comprises the following steps: (1) preparing low-melting-point glass powder; (2) preparing an organic carrier; (3) preparing copper electronic paste; and (4) preparing a copper / ceramic composite substrate. According to the method, the BZBS glass powder which is prepared by employing a sol-gel method is used as a glass binder, and the copper powder is used as a functional phase, so that a copper thick film with good properties is obtained. The glass binder is good in wettability and relatively low in beginning wetting temperature, so that the thick film is sintered to be compacted, and good properties can be obtained. Meanwhile, the sintering temperature is kept for a period of time to melt the glass powder and to sufficiently wet the functional phase and the substrate, so that the increment of copper powder particles is facilitated, and the sintering is promoted.
Owner:SINOTENG SILICA MATERIALS TECH (JIANGSU) CO LTD
Andrographolide ground suspending liquid, preparation method thereof, and application of pharmaceutical preparation
InactiveCN102614133AImprove stabilityDissolution rate is fastAntibacterial agentsOrganic active ingredientsImmediate releaseDrugs preparations
The invention relates to andrographolide ground suspending liquid, a preparation method thereof, and the application of pharmaceutical preparation, which belongs to the field of pharmaceutical preparation. The preparation method comprises the steps of adding andrographolide into hydrophilic accessory solution with certain concentration, and grinding the andrographolide in a basket grinder to prepare suspending liquid with particle sizes smaller than 3000 nm. Liquid layers of pharmaceutical suspending liquid are laminated onto blank pellet cores with certain particle size range, to prepare andrographolide immediate-release pellets. After grinding, by reducing pharmaceutical particle sizes, increasing particle surface areas and improving the wettability of pharmaceutical particles, the dissolution in vitro of the drug is improved, and hydrophilic carriers are adopted to effectively prevent the aggregation of pharmaceutical particles so as to improve the stability of the pharmaceutical preparation. The preparation method is simple and easy for industrialized production, and the dissolving-out speed of the prepared andrographolide immediate-release pellets is high, so that the bioavailability is obviously improved.
Owner:SHENYANG PHARMA UNIVERSITY
Lansoprazole enteric coated tablet and preparing method thereof
ActiveCN101229142AImprove stabilitySimple production processOrganic active ingredientsDigestive systemPlasticizerPharmaceutical formulation
The invention belongs to the technical field of pharmaceutical preparation, in particular to a lansoprazole enteric-coated tablet; wherein, the enteric-coated tablet consists of the following components: a) tablet core consisting of lansoprazole and pharmaceutical excipient; b) a enteric coating layer consisting of castor oil, polyacrylic resin II and pharmaceutical excipient. The enteric coating layer of the invention contains castor oil which is water insoluble plasticizer and has good compatibility with the polyacrylic resin II, thereby effectively preventing the lansoprazole of the raw material from being damaged by the polyacrylic resin II which is the acidic and intestinal-lysis coating material; therefore, an isolated layer is not needed to be packed between an enteric-coated layer and a tablet core, and fatherly allowing the invention has the advantages of simplifying the preparation process, reducing production cost, high dissolution rate of the prepared enteric-coated tablet, high bioavailability and good stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
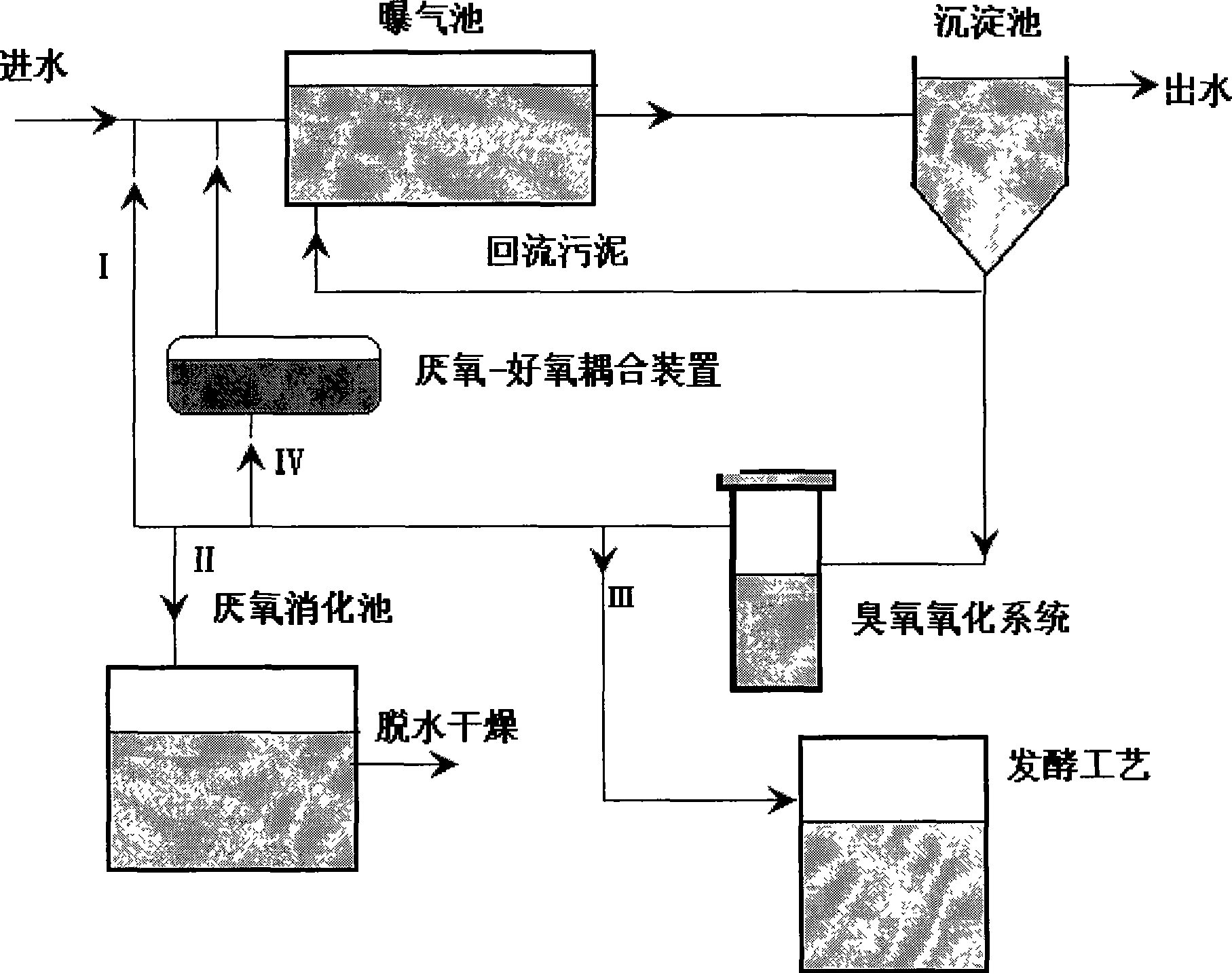
Method for dissolving biological cell in sludge and use thereof
InactiveCN101456655ASolve the bottleneck problem that the dissolution rate is difficult to increaseIncrease profitSludge treatment by oxidationBiological cellSludge
The invention discloses a method for dissolving biological cells in sludge and application thereof. The method for dissolving the biological cells in the sludge comprises: micron bubbles of ozone are introduced into the sludge to dissolve the biological cells in the sludge. The method for dissolving the biological cells in the sludge can be used for sludge reduction; the specific method comprises: the micron bubbles of the ozone are introduced into a sludge solution for sludge reduction. The method greatly improves the dissolution rate of the sludge; the initial sludge concentration is within the range of between 1,500 and 6,000 mg / L; when the addition amount of the ozone (the amount of the ozone introduced into the sludge solution) is between 0.06 and 0.16g O3 / g TSS, the COD dissolution rate and solid dissolution rate of the sludge can reach 25 to 50 percent and 15 to 35 percent respectively; the utilization rate of ozone gas is above 99 percent; and the ozone concentration in tail gas can be controlled within the range allowed by emission.
Owner:TSINGHUA UNIV +1
Yimaikang dispersion tablet and its preparing method
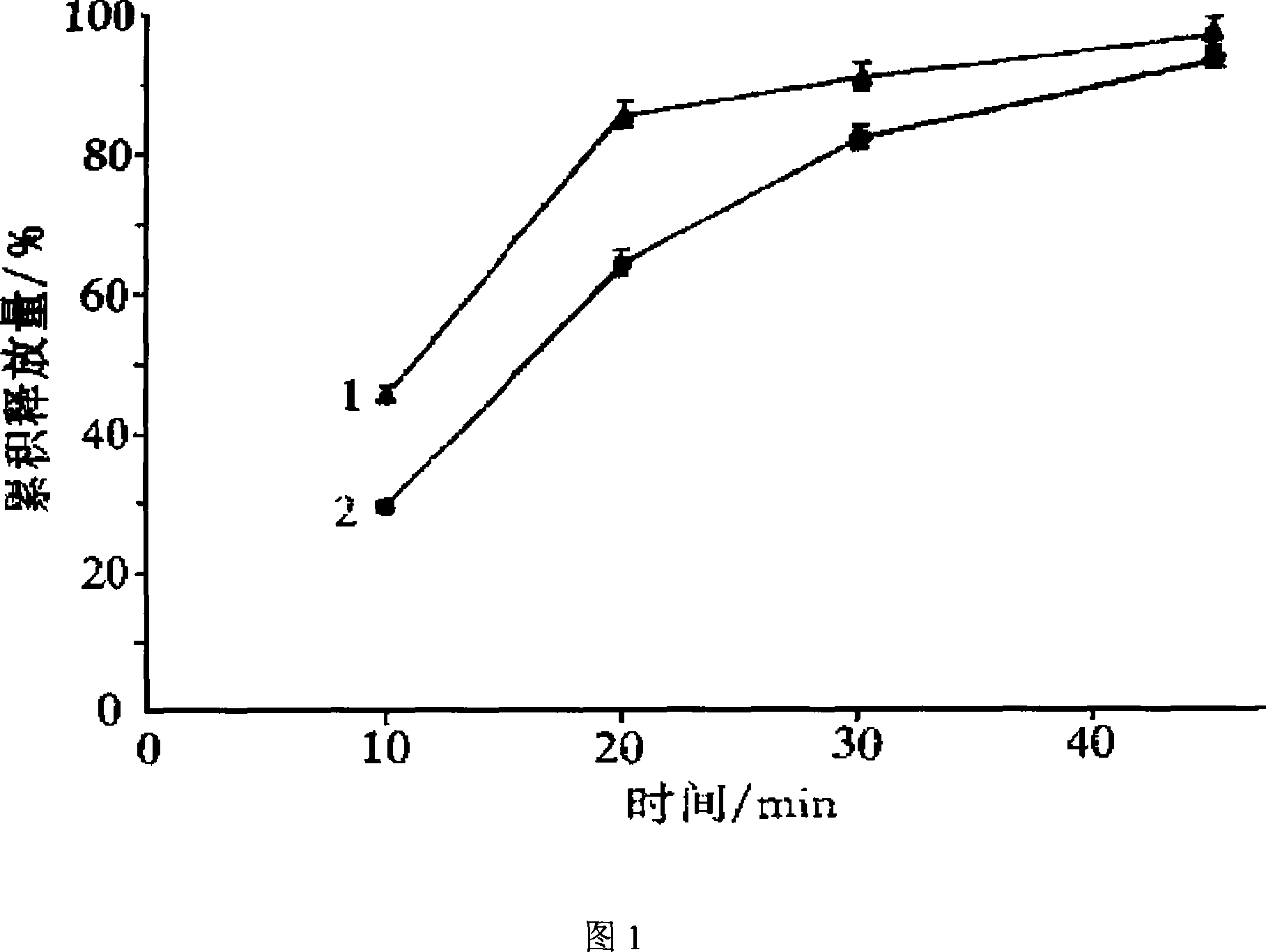
The Yimaikang dispersion tablet consists of fleabane extractum and medicine carrier. Its recipe includes fleabane extractum, lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, cross-linked polyvidon, steviosin, alcohol, fine silica gel powder and magnesium stearate. The dispersion tablet can disintegrate completely in 3 min to reach homogeneous dispersive state and its effective component andrographolide has dissolving rate reaching 60 % in 10 min. It is used in treating ischemic cerebral vascular diseases, cerebral hemorrhage sequela, vein obstruction, etc. and has fast acting, good taste and convenient taking.
Owner:云南白药集团大理药业有限责任公司
Method for efficiently extracting polysaccharide of lycium barbarum leaves
The invention relates to a method for extracting polysaccharide from lycium barbarum leaves and particularly relates to a method for efficiently extracting polysaccharide of lycium barbarum leaves. The method is characterized by comprising the following steps: (1) treating raw materials: drying lycium barbarum leaves and then crushing and sieving by a 40-80-mesh sieve; (2) adding petroleum ether into powder obtained, vibrating for 20-30 minutes, carrying out suction filtration, and drying for 1-3 hours in a drying box at a reduced box at 40-60 DEG C so as to obtain lycium barbarum leaf powder without fat; and (3) adding distilled water into the obtained lycium barbarum leaf powder, controlling the material-water ratio at 1g / 30-50ml, extracting, after suction filtration, extracting filter residues twice by virtue of the method, and mixing the extraction liquids obtained three times. The invention provides the method for efficiently extracting polysaccharide of lycium barbarum leaves. Based on a water extraction method in addition to a high-temperature high-pressure condition, the dissolution speed and the dissolution rate of polysaccharide of cells of the lycium barbarum leaves are increased. The yield is over 2 times that of other methods, and the raw materials are saved and the time is shortened.
Owner:森淼科技集团股份有限公司 +1
Method for separating and preparing corosolicacid in loquat leaf
InactiveCN101143887AImprove solubilityIncrease dissolution rateSteroidsPlant ingredientsSide effectCorosolic acid
The present invention provides a method of separating and preparing corosolic acid from loquat leaves, which includes the separation and preparation of the total triterpene acid loquat leaf, the purification of the resin concentration of the corosolic acid, the crystallization and purification of the corosolic acid, etc. In the preparation method, the loquat leaves are used as a material, and the total triterpene acid loquat leaf is extracted by applying the aqueous solution of the hydrophilic organic solvent; under the alkaline condition and before cooling, impurities are removed by filtration, and activated carbon is decolored; extracting solution applies macroporous absorptive resin concentration to purify the corosolic acid, and the crude corosolic acid is further crystallized and recrystallized by applying the hydrophilic organic solvent, so that the high-purity corosolic acid crystal is prepared. By measurement, the purity of the white corosolic acid crystal is larger than or equal to ninety eight percent. The preparation technique of the present invention has the advantages of simple technique, high efficiency of separation and purification, easy industrialized production, high purity of the prepared corosolic acid and low production cost, and moreover, the preparation technique of the present invention has the advantages of high efficiency, high availability, long effect, little dosage, little toxicity, little side effect, convenient storage, convenient carrying and convenient administration, etc. of the modern Chinese medicine.
Owner:FUZHOU UNIV
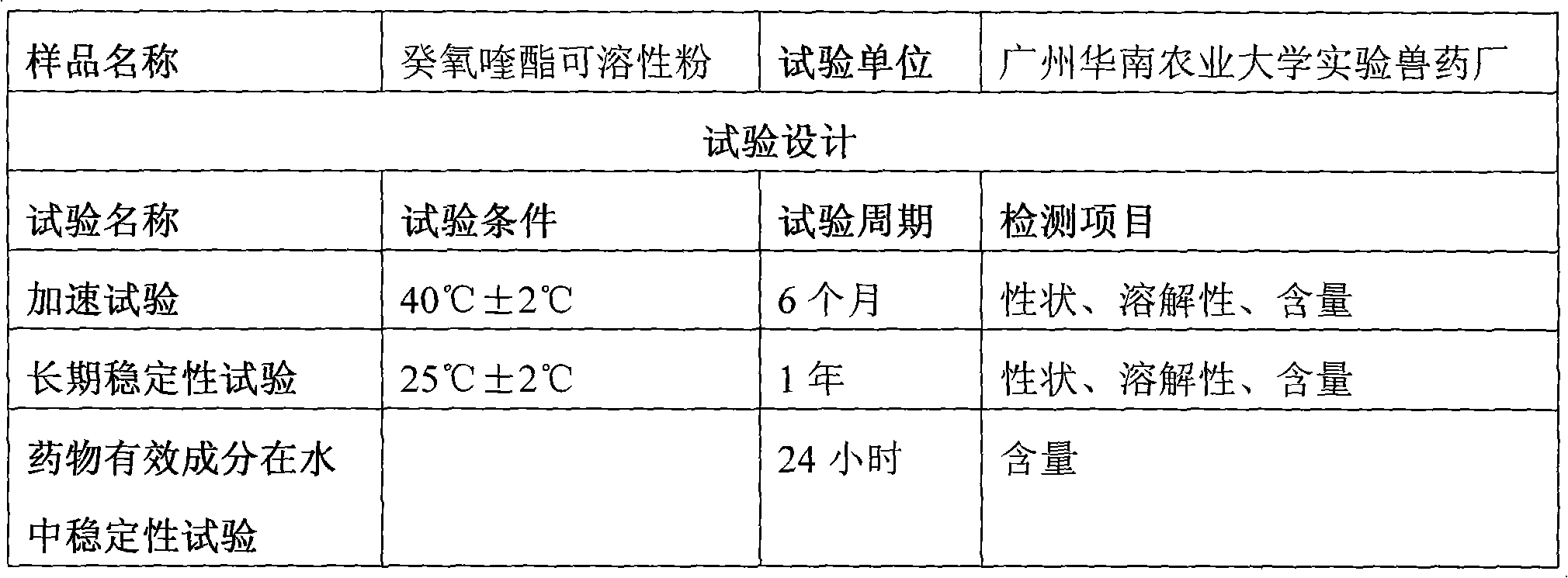
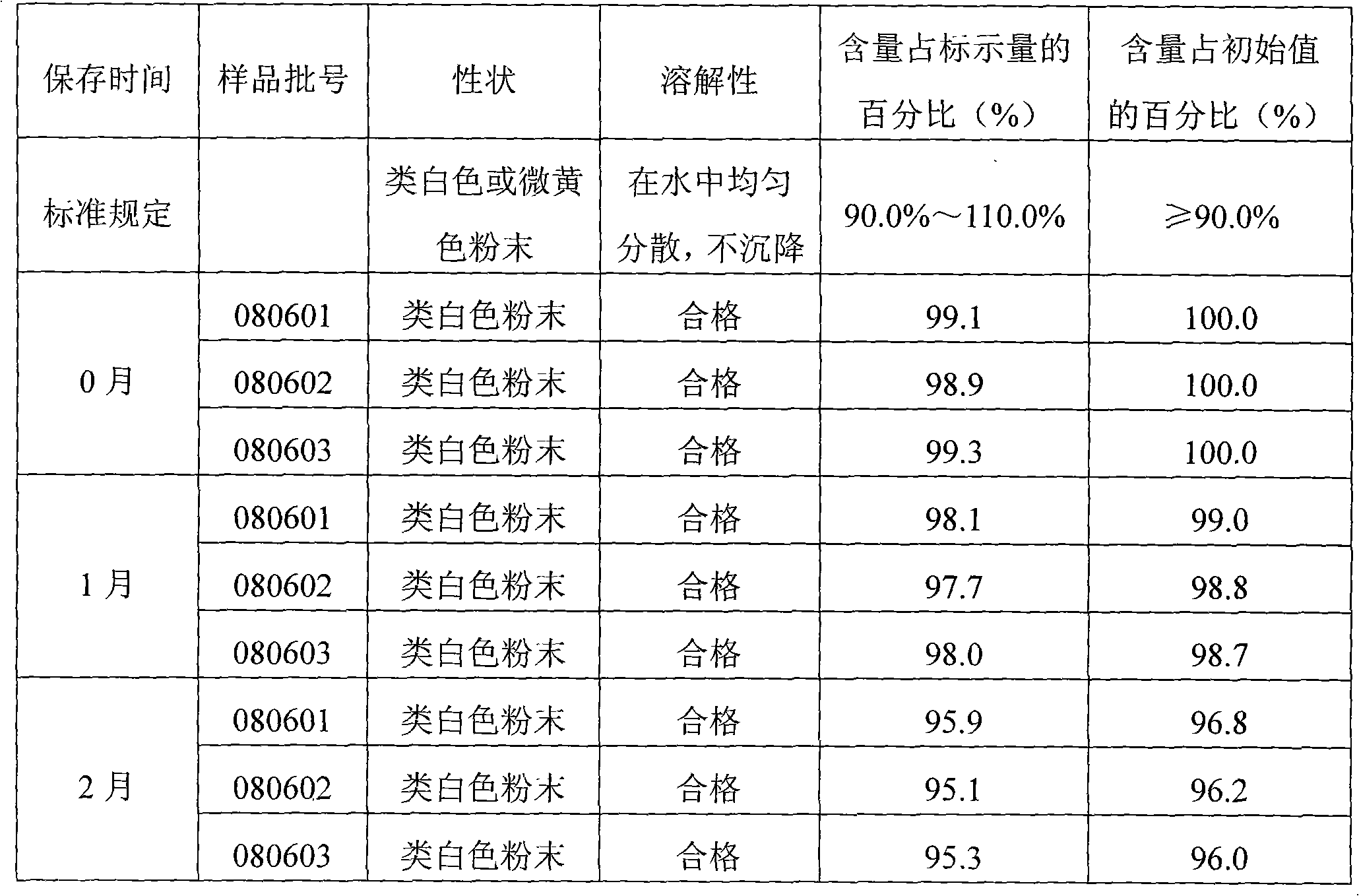
Decoquinate soluble powder and preparation method thereof
ActiveCN101606908AImprove solubilityIncrease dissolution ratePowder deliveryPharmaceutical non-active ingredientsSolubilityMolten state
The invention provides ecoquinate soluble powder and a preparation method thereof. The preparation method comprises the steps: evenly mixing ecoquinate and a solid dispersion carrier together; heating the mixture to a fusing state at the temperature of 120DEG C to 150 DEG C; then, airing and crashing the mixture at room temperature; adding anhydrous dextrose to obtain the ecoquinate soluble powder. The decoquinate soluble powder comprises 1 percent to 20 percent of ecoquinate, 4 percent to 80 percent of solid dispersion carrier and anhydrous dextrose. The prepared soluble powder has good solubility and high stability, the property and the solubility of the soluble powder are not changed after being stored for 6 mouths under an accelerating test condition (40DEG C+ / -2DEG C) or being stored for 1 year at room temperature (25DEG C+ / -2DEG C ), the content of the ecoquinate soluble powder is reduced and meets the requirements of galenic pharmacy, the effective period of the ecoquinate soluble powder can be as long as 3 years by calculation, the content reduction is not obvious under an illumination experiment condition, and the content of drug active ingredients can be maintained above 90 percent for four hours in water. A prepared drug preparation has good effect and convenient use and has good popularization and application value.
Owner:广东华农高科生物药业有限公司
Insoluble drug delivery system based on water-soluble cyclodextrin
InactiveCN1879887AMild reaction conditionsThe purification process is simplePill deliveryCapsule deliveryChemistryWater soluble
The invention relates to an insoluble medicine feeding system based on soluble cyclodextrin polymer, with high yield and the application in batch production, wherein said medicine compound is formed by insoluble medicine and cyclodextrin derivant, whose mass ratio is 0.1-100:1; and said insoluble medicine is the one whose solubility is less than 1% in room temperature; the soluble cyclodextrin polymer uses cyclodextrin as crosslink agent to be concentrated with cyclodextrin monomer. The inventive product is not shaped powder, whose average molecular weight can reach 10000.
Owner:SHENYANG PHARMA UNIVERSITY
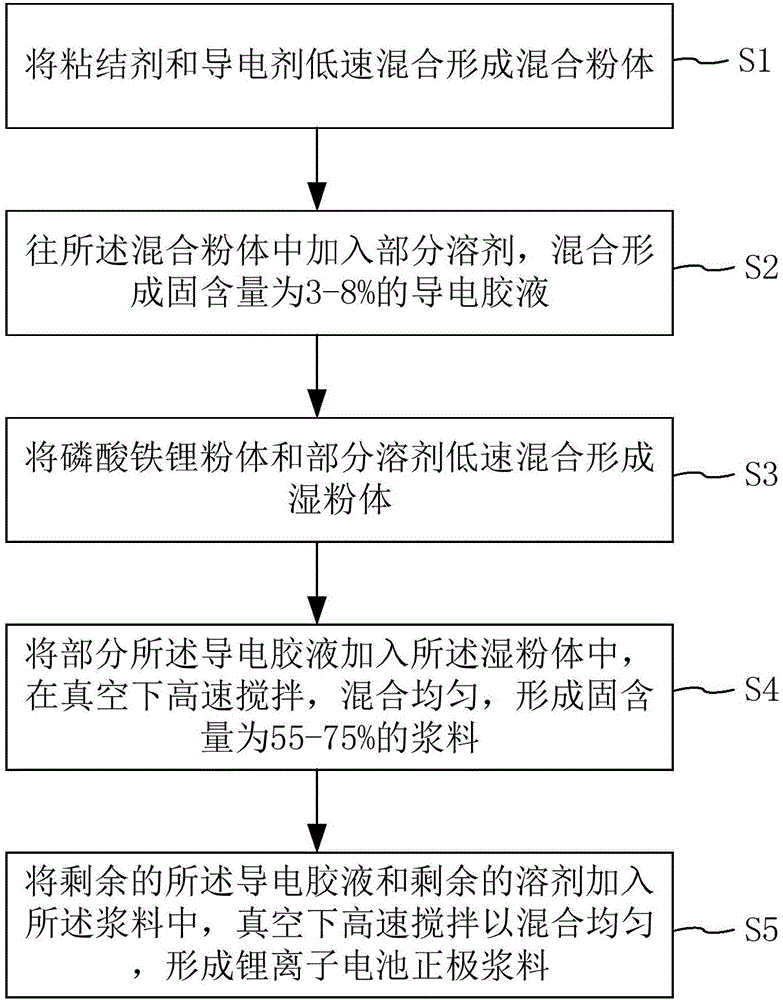
Lithium ion battery anode slurry as well as preparation method thereof, pole piece, and lithium ion battery
ActiveCN106450171AReduce group behaviorQuality improvementElectrode manufacturing processesSecondary cellsLithium iron phosphateAdhesive
The invention discloses lithium ion battery anode slurry as well as a preparation method thereof, a pole piece and a lithium ion battery. The preparation method comprises the following steps: S1, mixing an adhesive and a conductive agent at low speed to form mixed powder; S2, adding a part of a solvent in the mixed powder, and mixing to form a conductive glue solution; S3, mixing lithium iron phosphate powder and a part of the solvent at low speed to form wet powder; S4, adding a part of the conductive glue solution in the wet powder, stirring at high speed under vacuum, and uniformly mixing to form slurry; and S5, adding the residual conductive glue solution and the residual solvent in the slurry, stirring at high speed under vacuum, and uniformly mixing to form lithium iron phosphate lithium ion battery anode slurry. Powder mixing of the conductive agent and the adhesive is carried out in advance, and then the solvent is added to carry out dispersion, so that the aggregation behavior of the adhesive during dissolution is reduced, the dissolution rate of the adhesive is increased, the conductive agent is also dispersed together at the same time, the mixing time is shortened, the efficiency is improved, and the quality of the prepared slurry is improved.
Owner:SHENZHEN TOPBAND NEW ENERGY CO LTD
Isolation and purification method of geniposide
InactiveCN101037460AImprove solubilityIncrease dissolution rateSugar derivativesPlant ingredientsPurification methodsSolvent
The invention provides a separation method of the geniposide, including: extracting the geniposide from the Gardenia to produce a concrete with a hydrophilic organic water solution; removing the gardenia yellow pigment of the concrete by a large-hole adsorbed resin; depressed recovery of the solvent of the transudate, and concentrating to a coarse geniposide in vacuum; fully extracting the coarse product by hot ethylhexoate, cryogenic treatment of the extract, getting the geniposide crystal; crystallizing the ethylhexoate and acetone solvent and removing the impurities in room temperature; an obtaining the geniposide crystal with a purity >=98%. The invention separates the highly purified geniposide crystal. It has a cheap material, an easy and effective preparation craft, a broad extension and a novel design.
Owner:FUZHOU UNIV
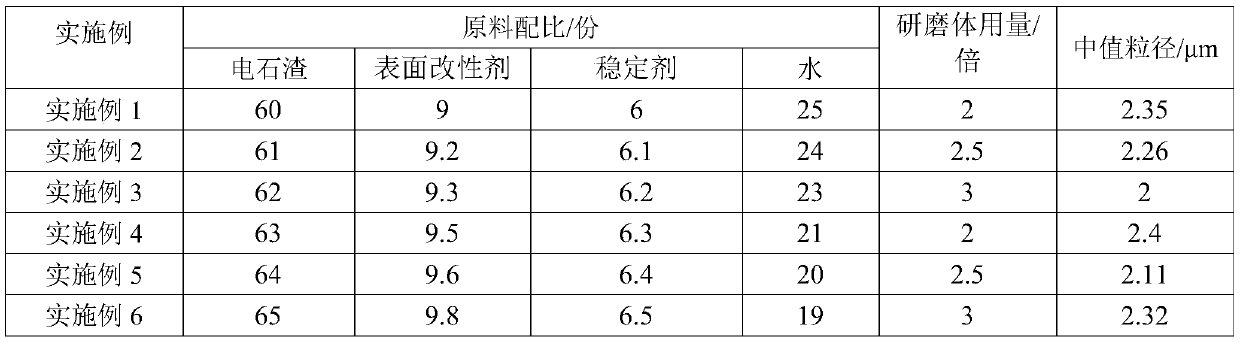
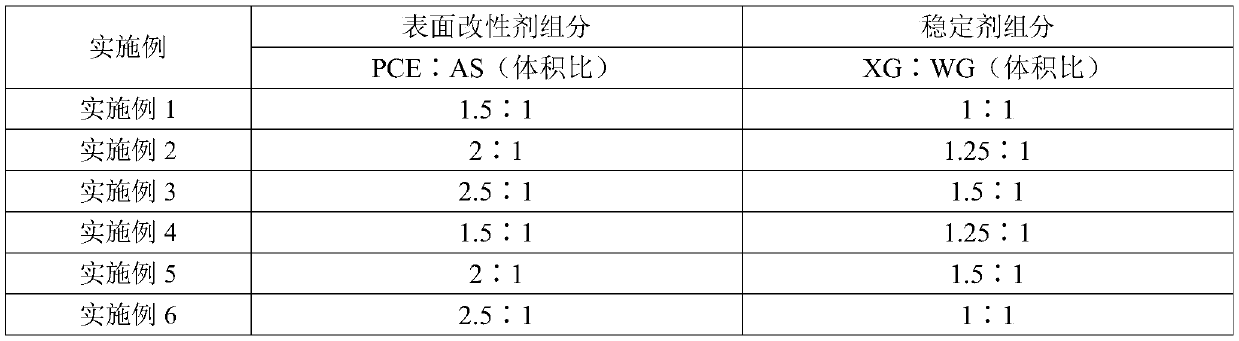
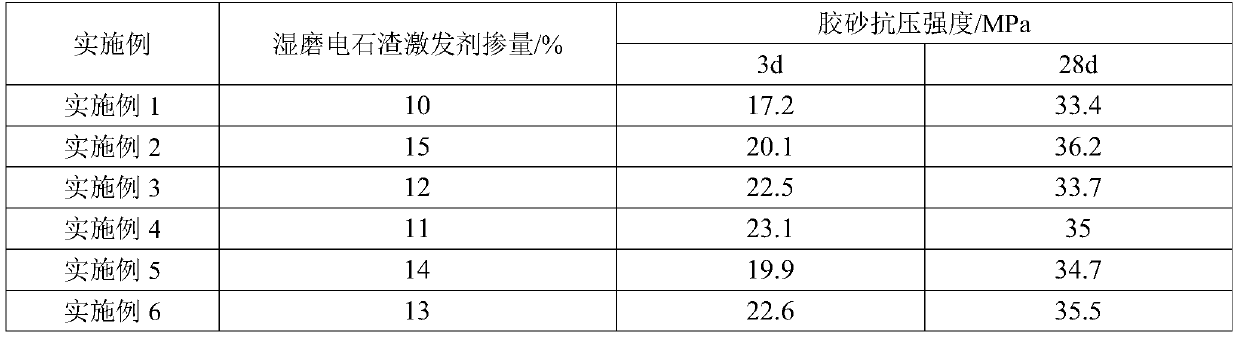
Wet-ground carbide-slag-containing activator and application
ActiveCN110304847ALarge specific surface areaImprove dissolution rate and dissolution volumeCement productionWet grindingCalcium hydroxide
The invention provides a wet-ground carbide-slag-containing activator and application. The wet-ground carbide-slag-containing activator is mainly prepared by subjecting 60-65 parts of carbide slag, 9-9.8 parts of surfactant, 6-6.5 parts of stabilizer and 10-30 parts of water to a wet grinding process. The wet-ground carbide-slag-containing activator has the advantages that the activator uses the carbide slag whose main components are calcium hydroxide and calcium carbonate as the main raw material, the wet grinding process is used to improve the fineness of the carbide slag and increase the specific surface area of the carbide slag so as to increase the dissolution rate and dissolution quantity of the calcium hydroxide and calcium carbonate in the carbide slag, high alkalinity is providedfor the pozzolanic reaction of slag glass, and accordingly slag pozzolanic reaction efficiency is increased, and a slag-based cementitious material is allowed to be high in compressive strength; whenthe mixing amount of the wet-ground carbide-slag-containing activator is 10-15%, the slag-based cementitious material can meet the national standard of 32.5 strength level of ordinary Portland cement.
Owner:WUHAN UNIV OF TECH
Itraconazole composite powder and preparation method thereof
ActiveCN101780046ASimple processEasy to operateOrganic active ingredientsPowder deliverySolubilityAdditive ingredient
The invention relates to itraconazole composite powder and a preparation method thereof. The method comprises the following steps: dissolving itraconazole medicine into an organic solvent; adding the mixture of the itraconazole medicine and the organic solvent into a water solution with hydrophilic accessory ingredients for in-situ precipitation to separate out medicine; obtaining the nanometer amorphous itraconazole medicine grain turbid liquor; carrying out spraying drying or freeze drying on the obtained itraconazole medicine turbid liquor to obtain micron level itraconazole high molecular accessory ingredient composite powder; and then, dispersing the powder into water to obtain the uniform nanometer amorphous itraconazole medicine grain turbid liquor. The itraconazole high molecular accessory ingredient composite powder has good water-solubility and high dissolution speed, in addition, the operation is simple, the amplification is easy, the production cost is low, and the invention lays the foundation for the industrial production of the itraconazole medicine and the development and the utilization of novel preparations of the itraconazole medicine.
Owner:BEIJING UNIV OF CHEM TECH
Chidamide solid dispersion and preparing method and application thereof
InactiveCN104771363AGood water solubilityIncrease dissolution ratePowder deliveryOrganic active ingredientsSolubilityWater soluble
The invention relates to the chemical pharmaceutical field, and discloses a chidamide solid dispersion and a preparing method and an application thereof. The invention also discloses an oral preparation containing the chidamide solid dispersion. The chidamide solid dispersion is composed of chidamide and a water soluble carrier material, and the weight ratio of chidamide to the water soluble carrier material is 1:1 to 1:20. Chidamide of the chidamide solid dispersion can be highly dispersed in the water soluble carrier material in a molecular form or an amorphous state, and thus the water solubility of chidamide is greatly improved, and the dissolution rate and bioavailability of chidamide are improved.
Owner:SHENZHEN CHIPSCREEN BIOSCIENCES CO LTD
Silk fibroin micro-needle patch and preparation method thereof
InactiveCN105833424AIncrease dissolution rateImprove mechanical propertiesMicroneedlesMedical devicesBody fluidUltimate tensile strength
The invention belongs to the field of medical beauty micro-needles, and in particular relates to a silk fibroin micro-needle patch and a preparation method thereof. The silk fibroin micro-needle patch comprises a backing layer, a micro-needle layer and a pressure-sensitive adhesive coating by which the backing layer and the micro-needle layer are stuck, wherein a plurality of needle-shaped bumps, which are uniformly distributed, are arranged on the micro-needle layer; needle-shaped cavities are formed in the needle-shaped bumps; the volume of the needle-shaped cavities is 40-90% of the volume of the needle-shaped bumps; medicines are kept in the needle-shaped cavities; the micro-needle layer is made from silk fibroin, and the molecular weight of the silk fibroin is lower than 100,000; the silk fibroin micro-needle patch is excellent in mechanical performance under a dry state, the silk fibroin micro-needle patch is sufficient in strength to penetrate into skin, and since the molecular weight of the silk fibroin is lower than 100,000, the micro-needle patch is high in dissolving rate, and as the micro-needle patch penetrates into the skin and gets into contact with body liquid, the micro-needle layer can rapidly dissolve, and subsequently, the medicines can get into contact with the body liquid and can dissolve in and disperse to the inner side of epidermis; therefore, the silk fibroin micro-needle patch is applicable to symptoms which require the rapid release of the medicines and have a relatively high requirement on a blood medicine dose.
Owner:PHARSUN MEDICAL BIOTECHNICS (SHANGHAI) CO LTD
Compound concrete anti-corrosion and rust-resistant agent
The invention discloses a compound concrete anti-corrosion and rust-resistant agent which comprises the following components in parts by weight: 15-30 parts of a polycarboxylic acid water reducing agent, 3-12 parts of sodium dodecyl sulfate, 15.7-20.6 parts of N-dimethylethanolamine, 1-5 parts of sodium molybdate, and 50-60 parts of water. The compound concrete anti-corrosion and rust-resistant agent can block capillary holes in the concrete, improves the chloride ion permeability resistance of concrete, can significantly alleviate the damage of chloride ions on a reinforcing steel bar passivation film, enhances the sulfate corrosion resistant ability of the concrete, has excellent rust-resistant performance and good repair performance, can enhance durability of the concrete and the comprehensive anti-corrosion ability of the concrete, and significantly improves the durability of buildings.
Owner:HUNAN CONSTR ENG GRP COR +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com