Self-emulsifying drug delivery system for improving bioavailability of insoluble medicine, and application thereof
A technology for drugs and derivatives, applied in the field of self-microemulsifying drug-carrying systems and their preparation, can solve problems such as different solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation of blank SEDDS prescription
[0054] The oil phase was mixed with CapmulMCM and caprylic acid (1:1w / w) on a shaker at the highest speed for 48h. The oil phase, CremophorRH40 or oil phase, CremophorRH40 and PEG400 were accurately weighed into a 20mL vial, vortexed for 2min, and then shaken on a shaker at room temperature at the highest speed for 48h to prepare a blank SEDDS formulation.
Embodiment 2
[0055] Embodiment 2: Preparation of drug-loaded SEDDS prescription
[0056] The excipients of drug-loaded SEDDS prescription and blank SEDDS prescription are the same. The drug loading is calculated based on the dissolution performance of the blank SEDDS after dispersion. The preparation method of the drug-loaded SEDDS dosage form is as follows: Weigh the various excipients and drugs of the blank SEDDS into a 20mL vial, vortex and mix for 2min, and then shake on a shaker at room temperature at the highest speed for 48h to prepare the drug-loaded SEDDS prescription.
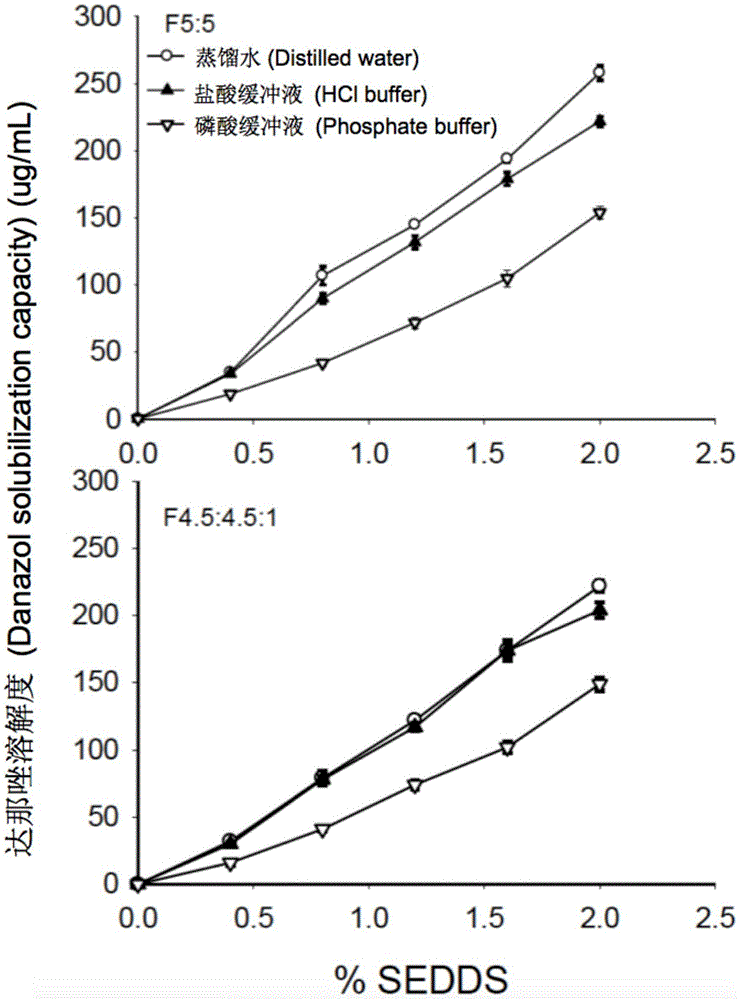
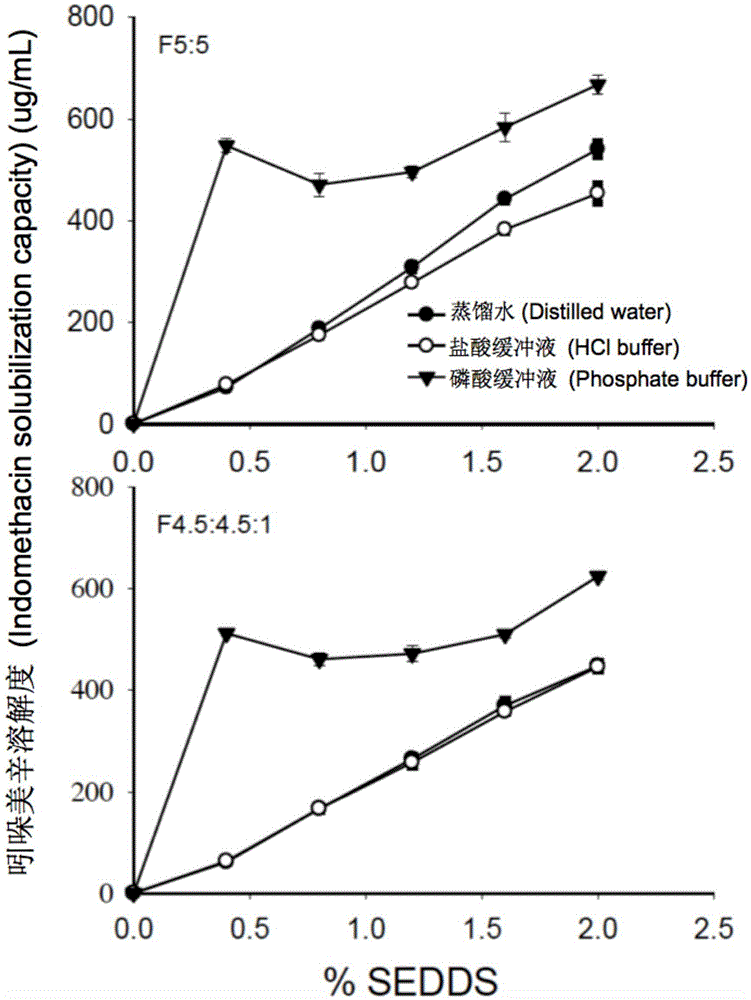
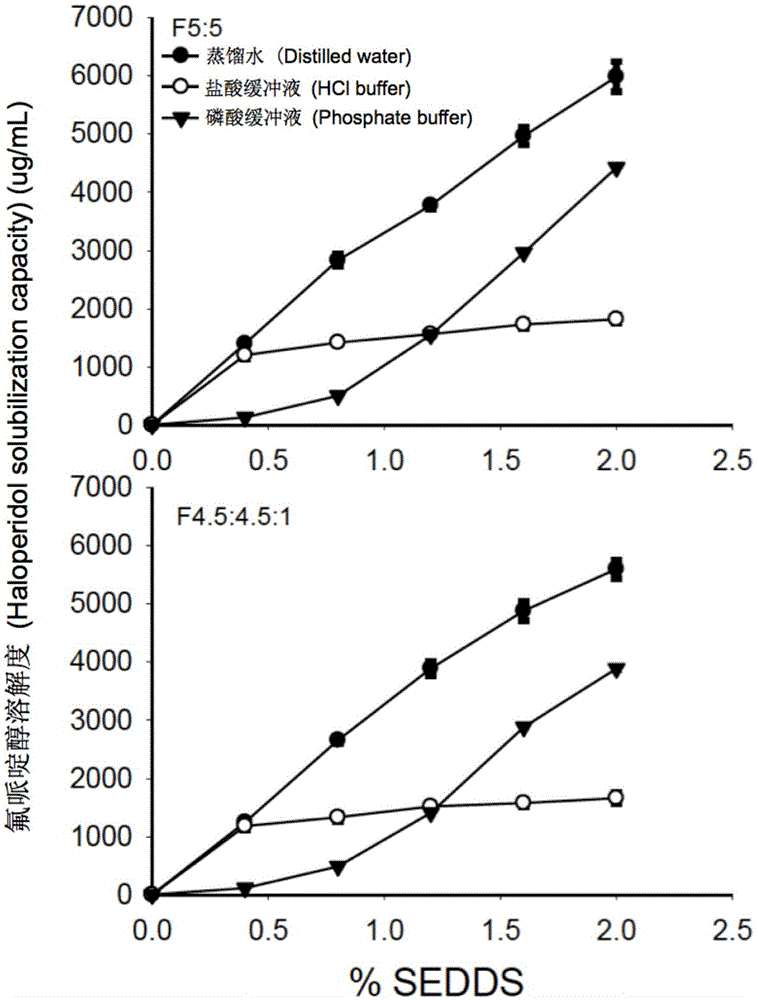
[0057] In order to prevent the drug from being precipitated in the gastrointestinal tract, the drug loading of SEDDS was calculated based on the dissolving ability of the drug after the blank SEDDS was dispersed. For example, 1 g of blank SEDDS formulation F5:5 dispersed in phosphoric acid solution exhibited the lowest solubility for danazol (19 μg / mL). Therefore, for danazol-loaded SEDDS formulation, 1 g of F5:5...
Embodiment 3
[0059] Embodiment 3: the research of solubility property
[0060] The study of solubility properties is to measure the equilibrium solubility of danazol, indomethacin and haloperidol in the microemulsion formed after dispersion in the blank SEDDS aqueous medium. Using method II (paddle method) in the United States Pharmacopoeia, disperse 1, 2, 3, 4, 5g blank SEDDS prescriptions in 250mL water (pH7.0), hydrochloric acid buffer (pH1.2), phosphate buffer ( pH6.8) to obtain blank SEDDS microemulsion. During the experiment, the temperature of the aqueous medium was maintained at 37°C, the rotation speed was 50 rpm, and the dispersion time was 60 minutes. The hydrochloric acid buffer and phosphate buffer were prepared according to the method of the United States Pharmacopoeia. Thereafter, take 5 mL of blank SEDDS microemulsion in a 20 mL vial, and add excess danazol, indomethacin or haloperidol, respectively. The vial was shaken at 37° C., 220 rpm in a water-bath shaker for 48 h t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com