Deuterated palbociclib derivative, and preparation method and application thereof
A derivative and deuterium technology, applied in the field of pharmaceutical compounds, can solve the problems of loose cell connections, life-threatening, easy to fall off, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
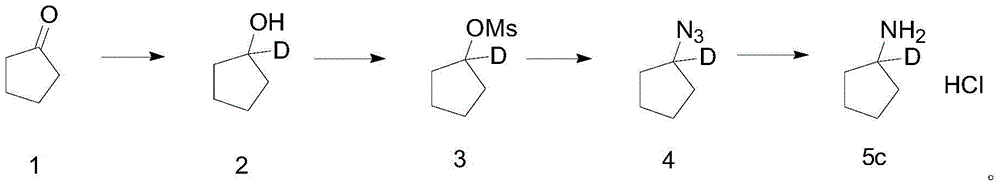
[0105] The synthetic route of deuterated cyclopentylamine hydrochloride involves chemical formula as:
[0106]
[0107] Deuterated cyclopentylamine hydrochloride is prepared by a method comprising the following steps:
[0108] a) Synthesis of compound 2: take 2.2g of LiAlD 4 Add 100ml of diethyl ether (Et 2(0), the system was cooled to 0°C; under the protection of nitrogen, slowly dropwise added an ether solution containing 8g of cyclopentanone (compound 1), after the dropwise addition, the system was warmed up to room temperature and continued to react for 1h; after the reaction, Add saturated NH 4 Cl solution quenched the reaction, extracted three times with ether, combined the organic phases, dried and concentrated to obtain 8 g of colorless transparent liquid compound 2;
[0109] The NMR information of compound 2 is: 1 H NMR (400MHz, CDCl 3 ): δ1.78-1.74(m,4H),1.58-1.55(m,4H);
[0110] b) Synthesis of Compound 3: Dissolve 5.2g of Compound 2 in 100ml of dichloromet...
Embodiment 2
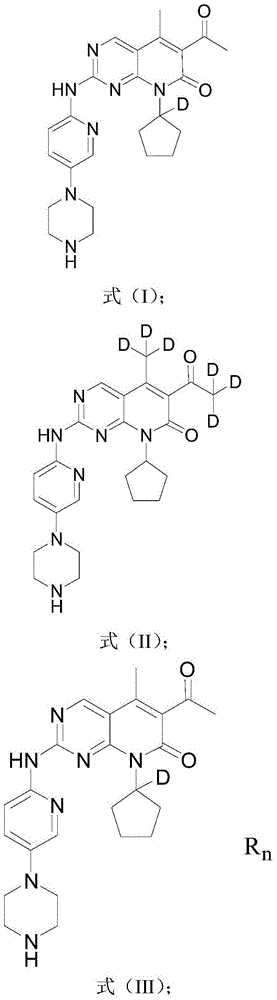
[0116] The deuterated Palbociclib derivative of the present embodiment has a structural formula as shown in formula (I):
[0117]
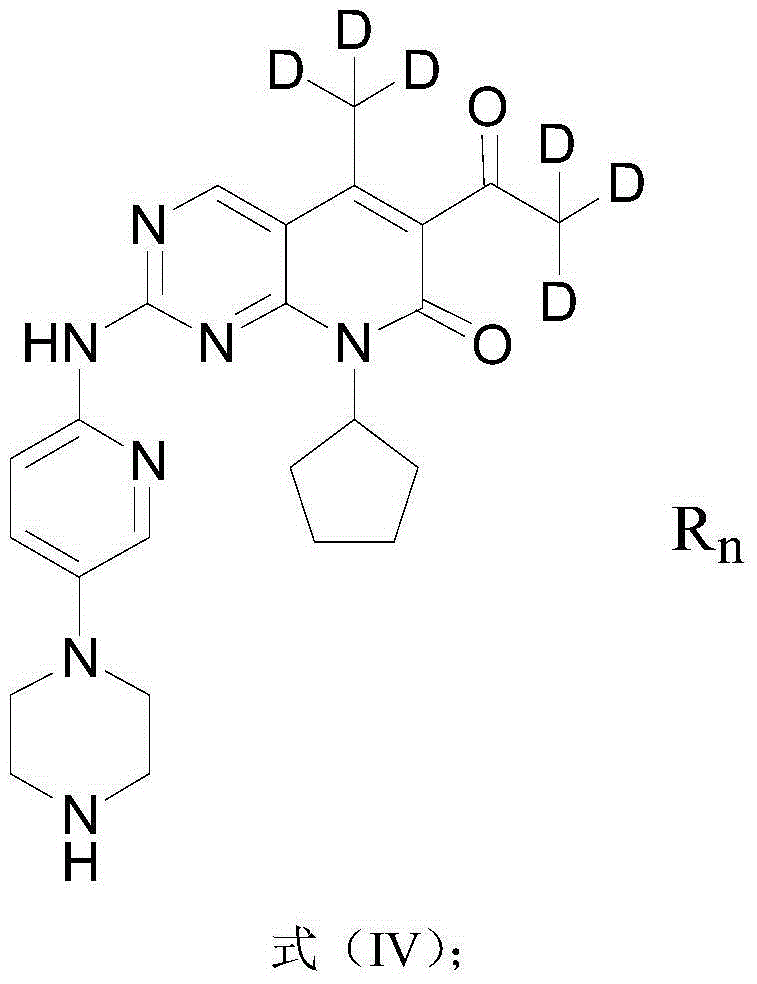
[0118] The chemical formula involved in the synthetic route of the deuterated Palbociclib derivative of the present embodiment is as follows:
[0119]
[0120] The preparation method of the deuterated Palbociclib derivative of the present embodiment comprises the following steps:
[0121] 1) Synthesis of Compound 6: Dissolve 7g of compound 5c (deuterated cyclopentylamine hydrochloride, deuterated cyclopentylamine (compound 5)) obtained in Example 1 in 100ml of absolute ethanol, and add 16ml Et 3 N, add 9 g of 2,4-dichloro-5-bromopyrimidine (compound 5a) in batches under stirring conditions (the molar ratio of 2,4-dichloro-5-bromopyrimidine to deuterated cyclopentylamine salt 0.7:1), continue to react for 2 hours after the addition; after the reaction, filter, concentrate the filtrate, add water to precipitate a white solid, add a small amo...
Embodiment 3
[0138] The deuterated Palbociclib derivative of the present embodiment has a structural formula as shown in formula (II):
[0139]
[0140] The chemical formula involved in the synthetic route of the deuterated Palbociclib derivative of the present embodiment is as follows:
[0141]
[0142] The preparation method of the deuterated Palbociclib derivative of the present embodiment comprises the following steps:
[0143] 1) Synthesis of compound 14: Dissolve 7g of cyclopentylamine (compound 5b) in 100ml of absolute ethanol, add 16ml of Et 3 N, under stirring conditions, add 9g of 2,4-dichloro-5-bromopyrimidine (compound 5a) in batches to the system, and continue to react for 2h after the addition; after the reaction, filter, concentrate the filtrate, add water to precipitate a white solid, A small amount of petroleum ether was added to the solid, stirred for 10 minutes and then filtered to obtain 8 g of white solid compound 14;
[0144] The NMR information of compound 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com