Salt of benzo-thiopyrone compound as well as preparation method and application of salt
A kind of technology of benzothiopyran and compound, applied in the field of medicine, can solve the problems such as undisclosed specific embodiment and experimental result
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0045] The present invention can be described in detail by the following examples, but it does not imply any adverse limitation on the present invention. The present invention has been described in detail herein, and its specific embodiments are also disclosed. For those skilled in the art, it is necessary to make various changes and improvements to the specific embodiments of the present invention without departing from the spirit and scope of the present invention. Obvious.
[0046] For all of the following examples, standard manipulations and purification methods known to those skilled in the art can be used. All temperatures are in °C (degrees Celsius) unless otherwise indicated. The structures of the compounds were determined by nuclear magnetic resonance spectroscopy (NMR).
[0047] Preparation of the Example section
[0048] The structure of the compound was obtained by H NMR spectroscopy ( 1 H NMR) to determine. Proton NMR chemical shifts (δ) are given in parts ...
Embodiment 1
[0072]
[0073]2-(4-(cyclohexylmethyl)piperazin-1-yl)-6-(trifluoromethyl)-8-nitro-benzothiopyran-4-one 1 maleate ( Compound 1)
[0074] synthetic route:
[0075]
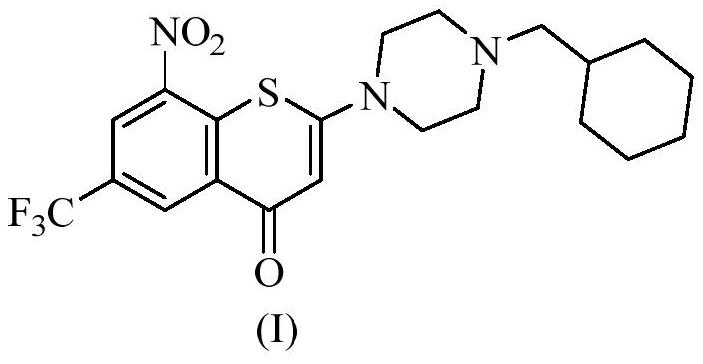
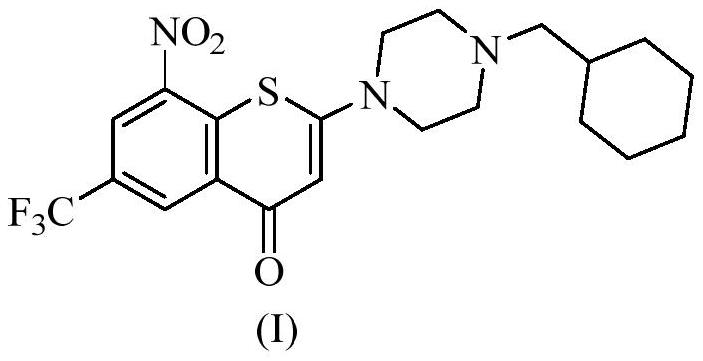
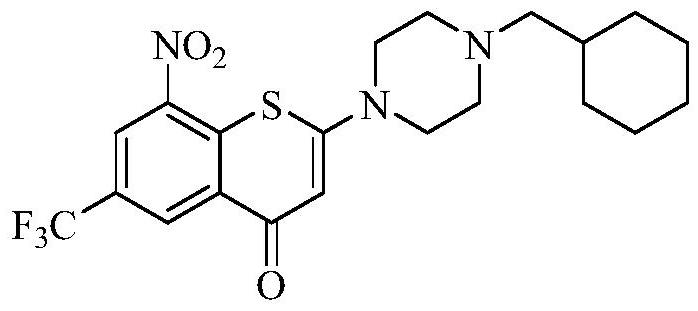
[0076] Add compound (I) (1.14g, 2.5mmol) into a 100mL three-neck flask, add 21mL of anhydrous methanol, stir well at room temperature, slowly add maleic acid (0.348g, 3.0mmol) at room temperature, and after 2-3min after the addition, the solution A yellow solid started to precipitate, kept stirring at room temperature for 3 hours, then suction filtered, the filter cake was rinsed with 5 mL of methanol, and dried to obtain 1.23 g of a yellow powdery solid, yield: 86%.
[0077] 1 H NMR (400MHz, CD 3 OD)δ:9.02(d,J=2.2Hz,1H),8.90(d,J=2.2Hz,1H),6.40(s,1H),6.27(s,2H),3.98(brs,4H),3.32 (brs,4H),2.94-2.92(m,2H),1.86-1.79(m,5H),1.76-1.72(m,1H),1.39-1.21(m,3H),1.12-1.03(m,2H) .
Embodiment 2
[0079]
[0080] 2-(4-(Cyclohexylmethyl)piperazin-1-yl)-6-(trifluoromethyl)-8-nitro-benzothiopyran-4-one·3 / 2 fumaric acid Salt (compound 2)
[0081] synthetic route:
[0082]
[0083] Add compound (I) (0.228g, 0.5mmol) into a 25mL single-necked bottle, add 6mL of anhydrous methanol, stir at room temperature, add fumaric acid (0.232g, 2.0mmol), after the addition, keep stirring at 80°C for 3 After 1 hour, it was naturally cooled to room temperature, ice-bathed for 10 min, and suction filtered. The filter cake was rinsed with 1 mL of methanol, and dried to obtain 0.25 g of a yellow powdery solid, yield: 79%.
[0084] 1 H NMR (400MHz, DMSO-d 6 )δ:13.08(brs,2H),8.85-8.83(m,2H),6.62(s,3H),6.30(s,1H),3.66-3.64(m,4H),2.48(brs,4H),2.16 -2.14(m,2H),1.76-1.65(m,5H),1.54-1.48(m,1H),1.27-1.12(m,3H),0.90-0.82(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com