A peptide targeting and recognizing immune cells and its application
An immune cell, targeted recognition technology, applied in applications, medical preparations with non-active ingredients, preparations for in vivo testing, etc. problem, to achieve the effect of good target specificity, low immunogenicity and excellent selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The preparation of embodiment 1.AK9 fluorescent probe
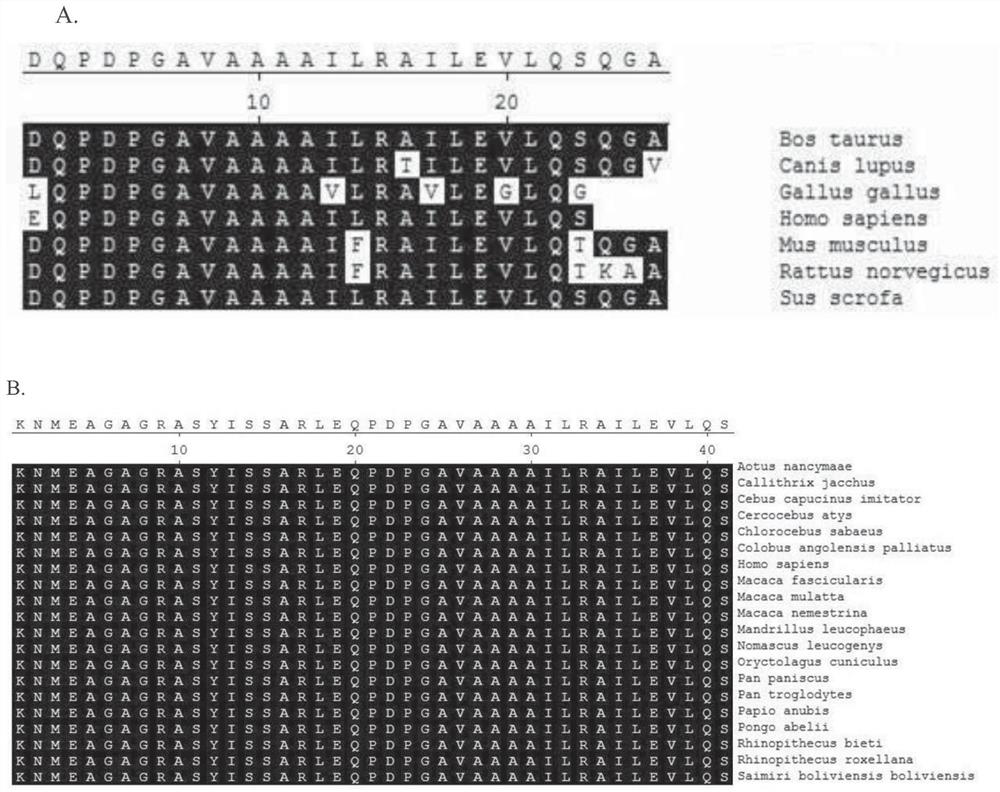
[0094] The polypeptide used in the present invention is obtained by conventional solid-phase polypeptide chemical synthesis using a CEM automatic microwave polypeptide synthesizer according to the instructions provided by the instrument supplier. The polypeptide used in the present invention is derived from the C-terminus of human or non-human Triokinase / FMN cyclase.
[0095] In this example, a polypeptide having the following amino acid sequence was synthesized: AILEVLQSK.
[0096] Combine the above synthetic peptides with HOOK TM Dye rhodamine labeling kit (Cat. #786-142, Biosciences) was mixed with reagents, adjusted the pH value according to the manufacturer's instructions, and reacted for 1-2 hours. The product was purified by HPLC to obtain the target AK9 fluorescent probe. The chemical structure of the obtained fluorescent probe is as follows:
[0097] AILEVLQS-Y (AK9),
[0098] Wherein Y is lysine+fluo...
Embodiment 2
[0099] The preparation of embodiment 2.AK9 fluorescent probe solution
[0100] Add 177 μL DMSO (dimethyl sulfoxide) to dissolve 1 mg AK9 fluorescent probe, then add 13981 μL serum-free DMEM (Hyclone) incomplete culture solution, and pipette thoroughly to obtain a 50 μM AK9 fluorescent probe solution. When needed, the 50 μM AK9 fluorescent probe solution can be diluted by adding serum-free DMEM incomplete culture solution.
Embodiment 3
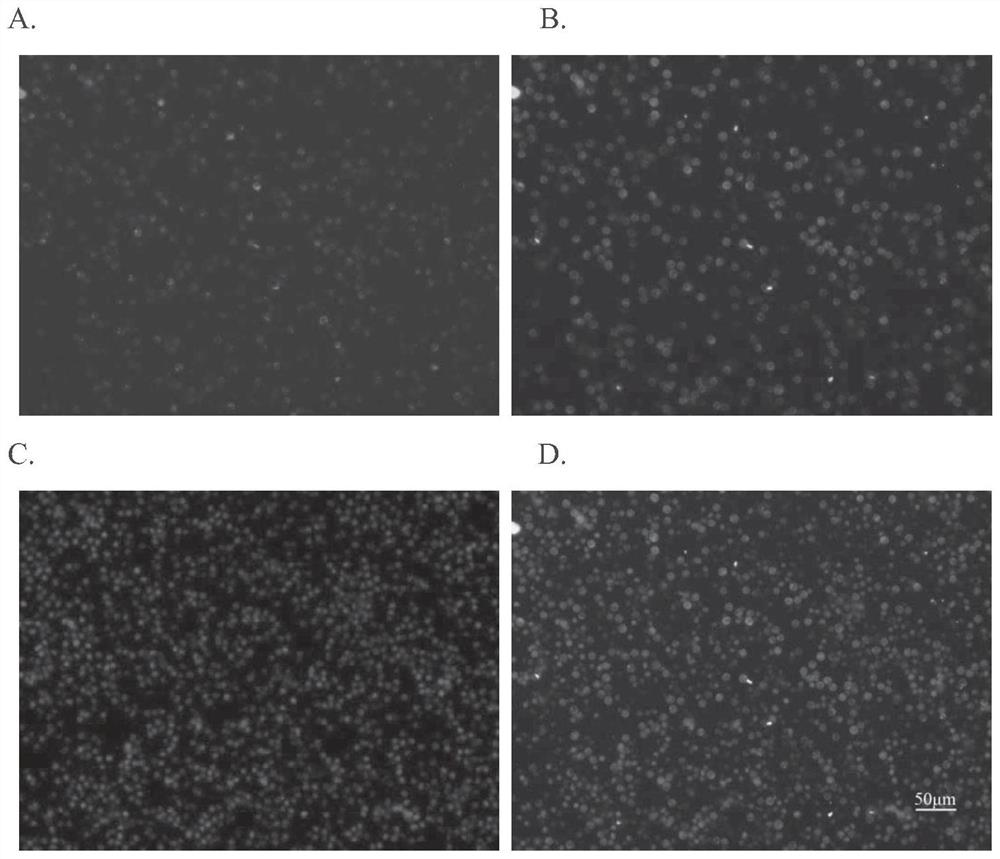
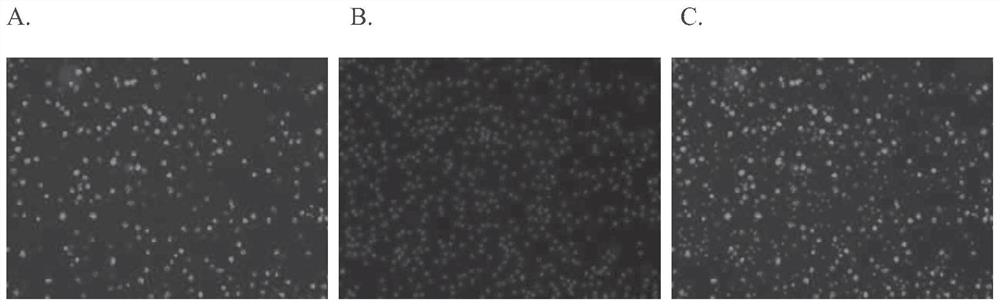
[0101] Example 3. In vivo fluorescent labeling of macrophages in the peritoneal cavity of mice using AK9 fluorescent probe
[0102] 300 μL of 50 μM AK9 fluorescent probe solution was diluted to 1 mL with serum-free DMEM incomplete culture medium, and then injected into the peritoneal cavity of Kunming mice aged 5-8 weeks. After 4 hours, 5 mL of 1×PBS was injected and rubbed. After 10 minutes, the mice were sacrificed by cervical dislocation. Cut the skin of the abdominal cavity of the mouse, pierce the abdominal wall muscle with a syringe, and extract the fluid in the abdominal cavity of the mouse. The extracted peritoneal fluid was centrifuged at 1000rpm for 5min and washed twice with 1×PBS. Apply the appropriate amount of cells to Alexa After counterstaining with 488 anti-mouse F4 / 80 antibody (Biolegend), add it to a 96-well plate, add nuclear dye Nucblue (Invitrogen) for counterstaining, and wait for the suspended cells to settle at the bottom of the plate, Observed un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com