Cabazitaxel-fatty acid conjugate and nano preparation thereof
A technology of cabazitaxel and fatty acids, which is applied in the field of medicine, can solve the problems of strong toxic and side effects, poor targeting, and poor stability, and achieve the effect of improving curative effect, optimal effect, and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
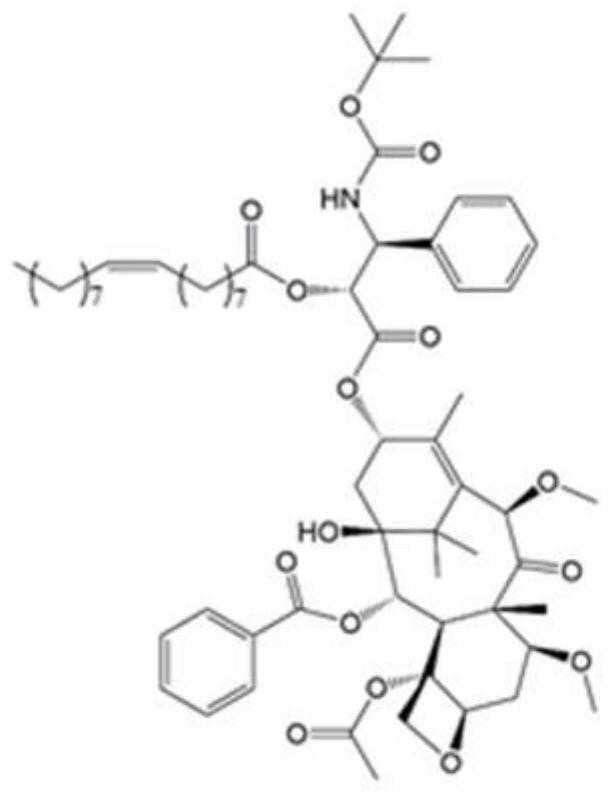
[0038] Example 1: Synthesis of Cabazitaxel-Oleic Acid Conjugate (CTX-OA) Directly Connected by Ester Bonds
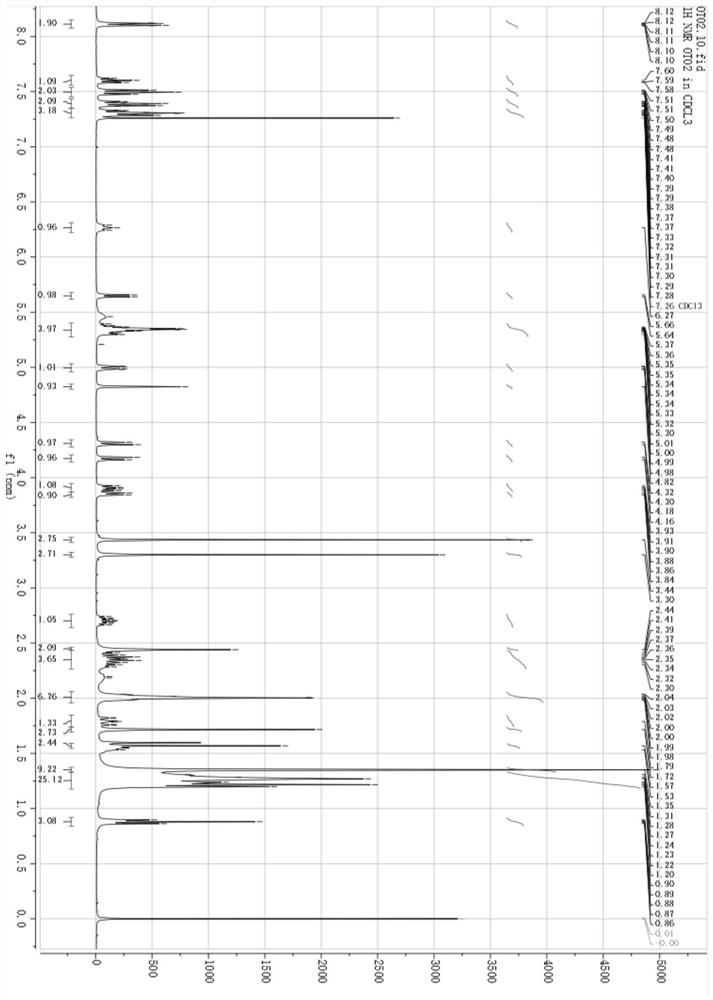
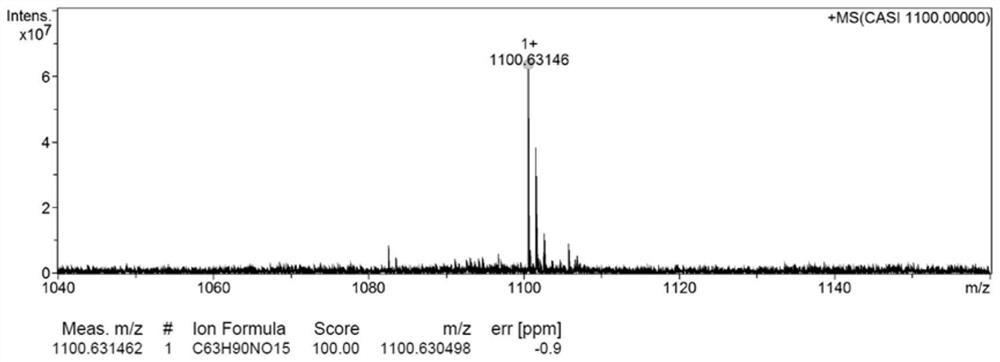
[0039] Accurately weigh OA (118.6 mg, 0.42 mmol), place DCC and DMAP in a 50 mL eggplant-shaped flask, add 10 mL of anhydrous CH 2 Cl 2 , in N 2Treated in an ice-water bath for 1 h under the protection of Subsequently, CTX (350 mg, 0.42 mmol) was accurately weighed and added to the above reaction solution, under N 2 Under the protection, the reaction was carried out at 25 °C for 48 h, and the reaction was monitored by thin-layer chromatography. After the reaction was completed, washed with saturated NaCl water for 3 times, and then washed with anhydrous Na 2 SO 4 Dry, filter to remove insolubles, and spin dry the filtrate to obtain a crude product. The crude product was purified with the preparation solution to obtain the final product, cabazitaxel oleate (CTX-OA), with a yield of 45.4% and a purity of 99.42%. The structure is shown in the figure. The high-resol...
Embodiment 2
[0041] Example 2: Synthesis of cabazitaxel-oleic acid conjugate (CTX-SS-OA) linked by disulfide bonds
[0042] Precisely measure ethylene glycol (90 mL, 1.61 mol) and place it in a 250 mL three-neck flask, weigh p-toluenesulfonic acid and place it in the three-neck flask, and raise the temperature of ethylene glycol to 110°C. OA (5000 mg, 17.7 mmol) was precisely weighed, dissolved in toluene, and the OA dissolved in toluene was added dropwise to a three-necked flask. After the reaction is complete, the crude product is purified by silica gel column chromatography, and the target product (Z)-oleic acid-2-hydroxyethyl ester is collected. Weigh EDCI, HOBt, DMAP and (Z)-oleic acid-2-hydroxyethyl ester into an eggplant-shaped bottle of dithioglycolic anhydride to obtain a crude product. The target product (2-oxo-2-(2-((Z)-oleoyloxy)ethoxy)ethanedithio)acetic acid was collected by purification. The reaction product (2-oxo-2-(2-((Z)-oleoyloxy)ethoxy)ethanedithio)acetic acid was pl...
Embodiment 3
[0044] Example 3: Synthesis of cabazitaxel-linoleic acid conjugate (CTX-SS-LA) linked by disulfide bonds
[0045] Precisely measure ethylene glycol (90 mL, 1.61 mol) and place it in a 250 mL three-neck flask, weigh p-toluenesulfonic acid and place it in the three-neck flask, and raise the temperature of ethylene glycol to 110°C. LA (5000 mg, 17.7 mmol) was accurately weighed, dissolved in toluene, and the LA dissolved in toluene was added dropwise to a three-necked flask. After the completion of the reaction, the crude product was purified by silica gel column chromatography, and the target product (9Z, 12Z)-linoleic acid-2-hydroxyethyl ester was collected. Weigh EDCI, HOBt, DMAP and (9Z, 12Z)-linoleic acid-2-hydroxyethyl ester in an eggplant-shaped bottle of dithioglycolic anhydride to obtain a crude product. The target product (2-oxo-2-(2-((9Z,12Z)-linoleoyloxy)ethoxy)ethanedithio)acetic acid was collected by purification. The reaction product (2-oxo-2-(2-((9Z,12Z)-linoleo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com