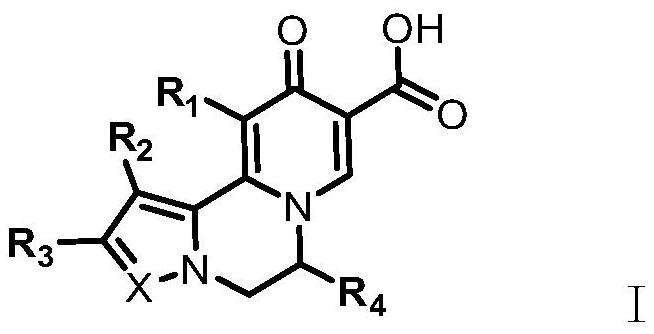
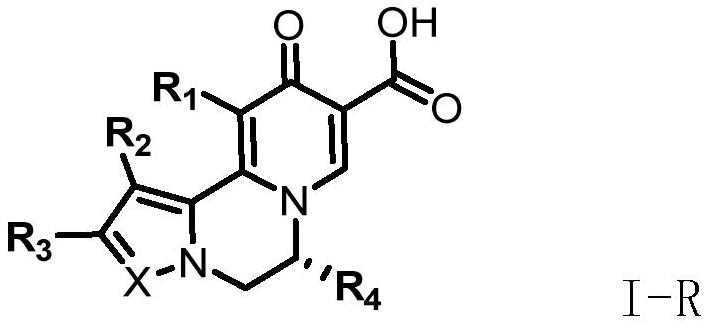
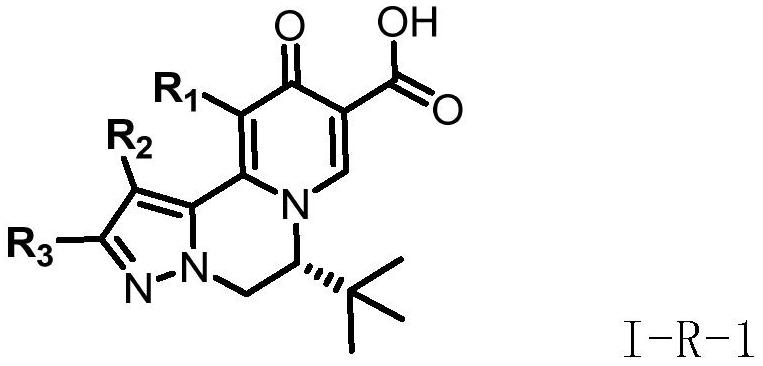
A kind of compound for treating and/or preventing hepatitis B virus infection and its preparation method and application
A compound and composition technology, applied in antiviral agents, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] The preparation of embodiment 1 compound EXP 1
[0176]
[0177] 1) Synthesis of compound 2
[0178] Compound 1 (10 g, 64.1 mmol, 1.0 eq) was dissolved in DMF (100 mL), then sodium carbonate (17 g, 160.2 mmol, 2.5 eq) and benzyl bromide (8.4 mL, 70.5 mmol, 1.1 eq) were added. The reaction solution was stirred at 60°C for 16 hours. After cooling to room temperature, add water (300mL) to the reaction solution and extract with EA (80mL x2), combine the organic phases, wash with saturated brine (150mL), then dry with anhydrous sodium sulfate, concentrate, and the residue is subjected to silica gel column chromatography Compound 2 (9.6 g, 61%) was obtained as a white solid after purification (petroleum ether / ethyl acetate=10 / 1). LCMS:[M+H] + = 247.1.
[0179] 2) Synthesis of compound 3
[0180] Compound 2 (9.6g, 39mmol, 1.0eq) was dissolved in DMF (100mL), then NCS (8.34g, 62.4mmol, 1.6eq) was added, and the reaction solution was stirred overnight at 80°C. After coo...
Embodiment 2
[0213] The preparation of embodiment 2 compound EXP 2
[0214]
[0215] Compound EXP 1 (200 mg) was dissolved in ethyl acetate (1 mL), n-hexane (10 mL) was added, the resulting solution was filtered to obtain a solid, and compound EXP 2 (150 mg, yield 75%) was obtained after drying under reduced pressure.
[0216] LCMS:[M+H] + =422.1
[0217] 1 H NMR (400MHz, DMSO) δ15.78(s,1H),8.94(s,1H),7.23(s,1H),5.10–5.03(m,1H),4.83-4.82(m,1H),4.65- 4.64(m,2H),4.10-4.07(m,1H),3.16(s,3H),2.41–2.31(m,4H),0.76(s,9H).
Embodiment 3
[0218] The preparation of embodiment 3 compound EXP 3
[0219]
[0220] 1) Synthesis of compound 2
[0221] Compound 1 (1.1g, 5.7mmol, 1.0eq) was dissolved in methanol (30mL), then palladium on carbon (10%, 500mg) was added, hydrogen was replaced and reacted at room temperature for 18 hours. After the reaction was complete, palladium carbon was removed by filtration, and concentrated to obtain compound 2 (500 mg, 86%) as a colorless oil.
[0222] 2) Synthesis of Compound 4
[0223] Compound 3 (500mg, 3.0mmol, 1.0eq) was dissolved in tetrahydrofuran (20mL), then compound 2 (306mg, 3.0mmol, 1.0eq) and triphenylphosphine (1.18g, 4.5mmol, 1.5eq) were added, ice The bath was cooled to zero degrees Celsius, and a solution of DIAD (909mg, 4.5mmol, 1.5eq) in tetrahydrofuran (5mL) was added slowly, followed by reaction at room temperature for 18 hours. After the reaction was complete, the reaction solution was concentrated, and the residue was purified by silica gel column chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com