Preparation method of composite solid electrolyte based on polyphosphazene carbide micro-nanometer material
A solid electrolyte, carbonized polyphosphazene technology, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of small surface area, few voids, limiting the electrical properties of solid composite polymer electrolytes, etc. The effect of good mechanical properties and high electrochemical stability window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1: Weigh 0.005g of carbonized polyphosphazene microspheres (the mass of carbonized polyphosphazene microspheres is 0.5% of the mass of PEO), add it to 30ml of acetonitrile, and ultrasonically disperse for 15 minutes;
[0028] Step 2: Weigh 1.0g PEO (Mw~300,000) and 0.242g LiClO respectively 4 (PEO and LiClO 4 O / Li molar ratio of 10:1) was added to the acetonitrile dispersion of carbonized polyphosphazene microspheres, and stirred and dissolved with a magnetic stirrer for 8 hours;
[0029] Step 3: Cast the obtained mixed solution onto a polytetrafluoroethylene template, and volatilize the solvent for 6 hours;
[0030] Step 4: Transfer the polymer electrolyte membrane obtained in step 3 to a vacuum drying oven, and continue to dry at 50°C for 24 hours to obtain a composite solid polymer electrolyte.
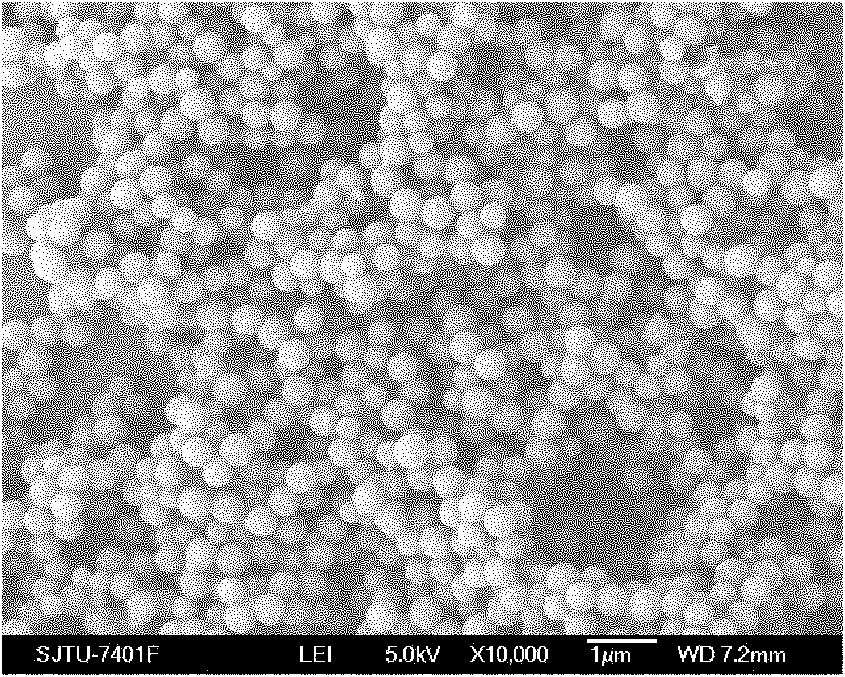
[0031] Implementation effects of this embodiment: figure 1 It is a scanning electron microscope SEM picture of the prepared carbonized polyphosphazene microspheres. It can be s...
Embodiment 2
[0033] Step 1: Weigh 0.0025g of carbonized polyphosphazene nanotubes (the mass of carbonized polyphosphazene nanotubes is 0.25% of the mass of PEO), add them to 40ml of acetonitrile, and ultrasonically disperse for 20 minutes;
[0034] Step 2: Weigh 1.0g PEO (Mw~400,000) and 0.3025g LiClO respectively 4 (PEO and LiClO 4 The molar ratio of O / Li is 8:1) was added to the acetonitrile dispersion of carbonized polyphosphazene nanotubes, and stirred and dissolved with a magnetic stirrer for 9 hours;
[0035] Step 3: Cast the obtained mixed solution onto a polytetrafluoroethylene template, and volatilize the solvent for 7 hours.
[0036] Step 4: Transfer the polymer electrolyte membrane obtained in step 3 to a vacuum drying oven, and continue drying at 50° C. for 28 hours to obtain a composite solid polymer electrolyte.
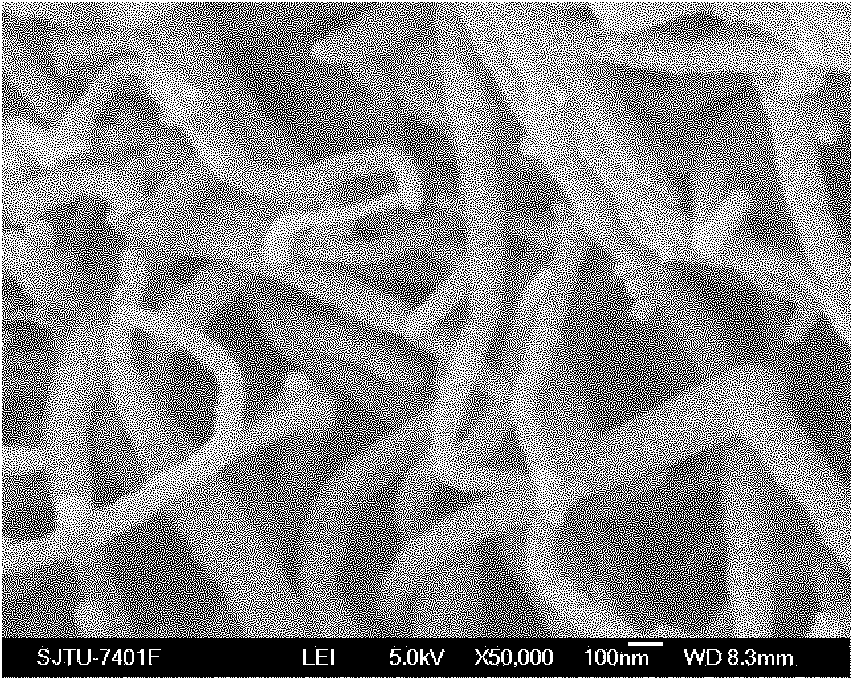
[0037] Implementation effect of this embodiment: The composite solid polymer electrolyte is characterized as in Example 1. figure 2 It is a scanning electron microscope SEM...
Embodiment 3
[0039] Step 1: Weigh 0.0075g of carbonized polyphosphazene nanofibers (the mass of carbonized polyphosphazene nanofibers is 0.75% of the mass of PEO), add them to 45ml of acetonitrile, and ultrasonically disperse for 30 minutes;
[0040] Step 2: Weigh 1.0g PEO (Mw~500,000) and 0.2017g LiClO respectively 4 (PEO and LiClO 4 O / Li molar ratio of 12:1) was added to the acetonitrile dispersion of carbonized polyphosphazene nanofibers, and stirred and dissolved with a magnetic stirrer for 10 hours;
[0041] Step 3: Cast the obtained mixed solution onto a polytetrafluoroethylene template, and volatilize the solvent for 8 hours.
[0042] Step 4: Transfer the polymer electrolyte membrane obtained in step 3 to a vacuum drying oven, and continue to dry at 50° C. for 32 hours to obtain a composite solid polymer electrolyte.
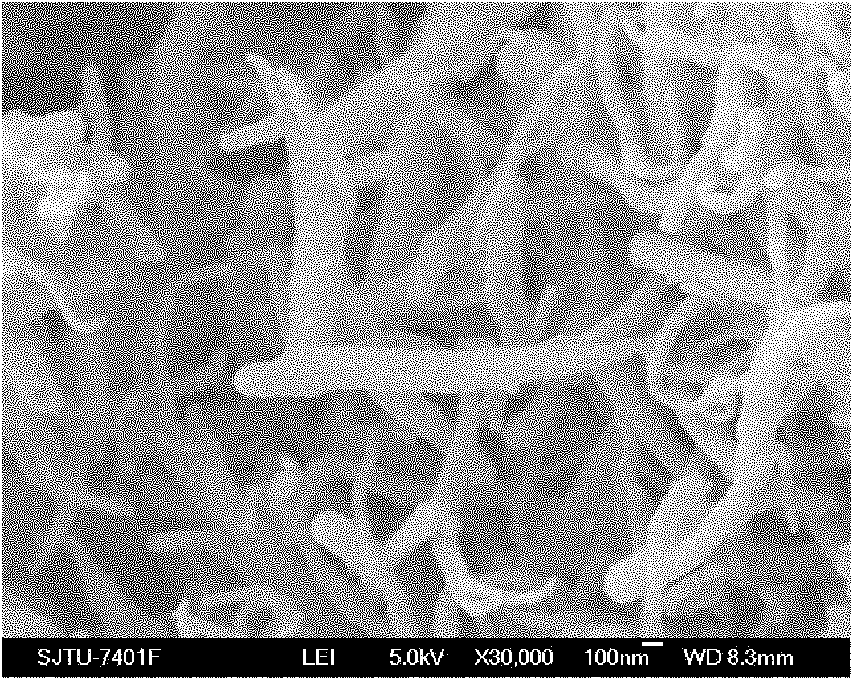
[0043] Implementation effect of this embodiment: The composite solid polymer electrolyte is characterized as in Example 1. image 3 It is the scanning electron microscope SEM ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity at room temperature | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com