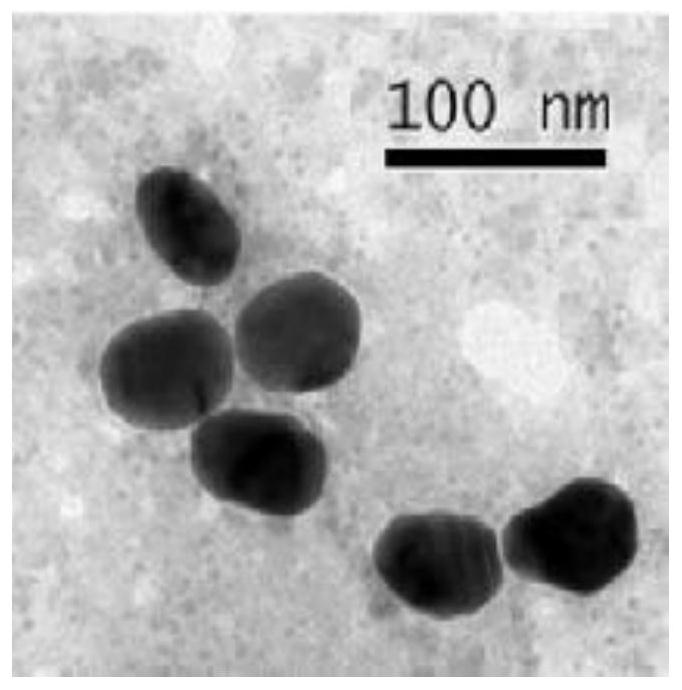
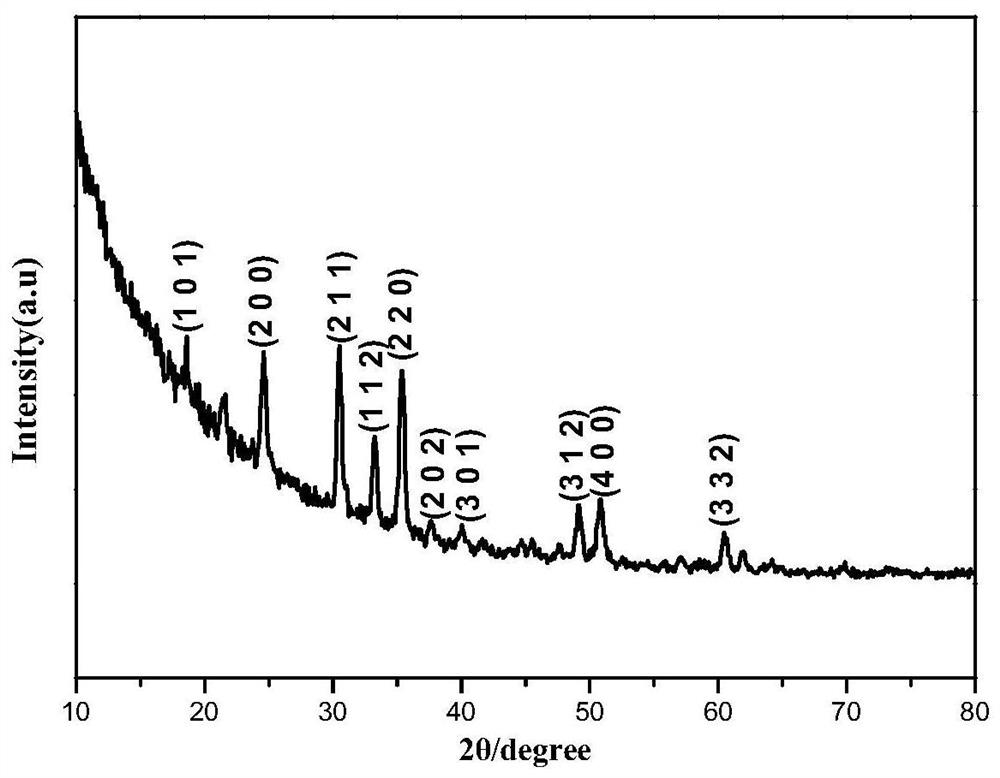
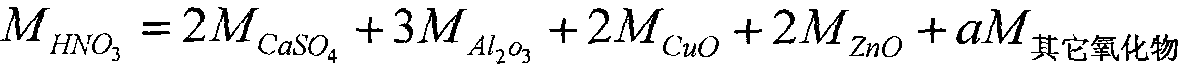Patents
Literature
47 results about "Nitratine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
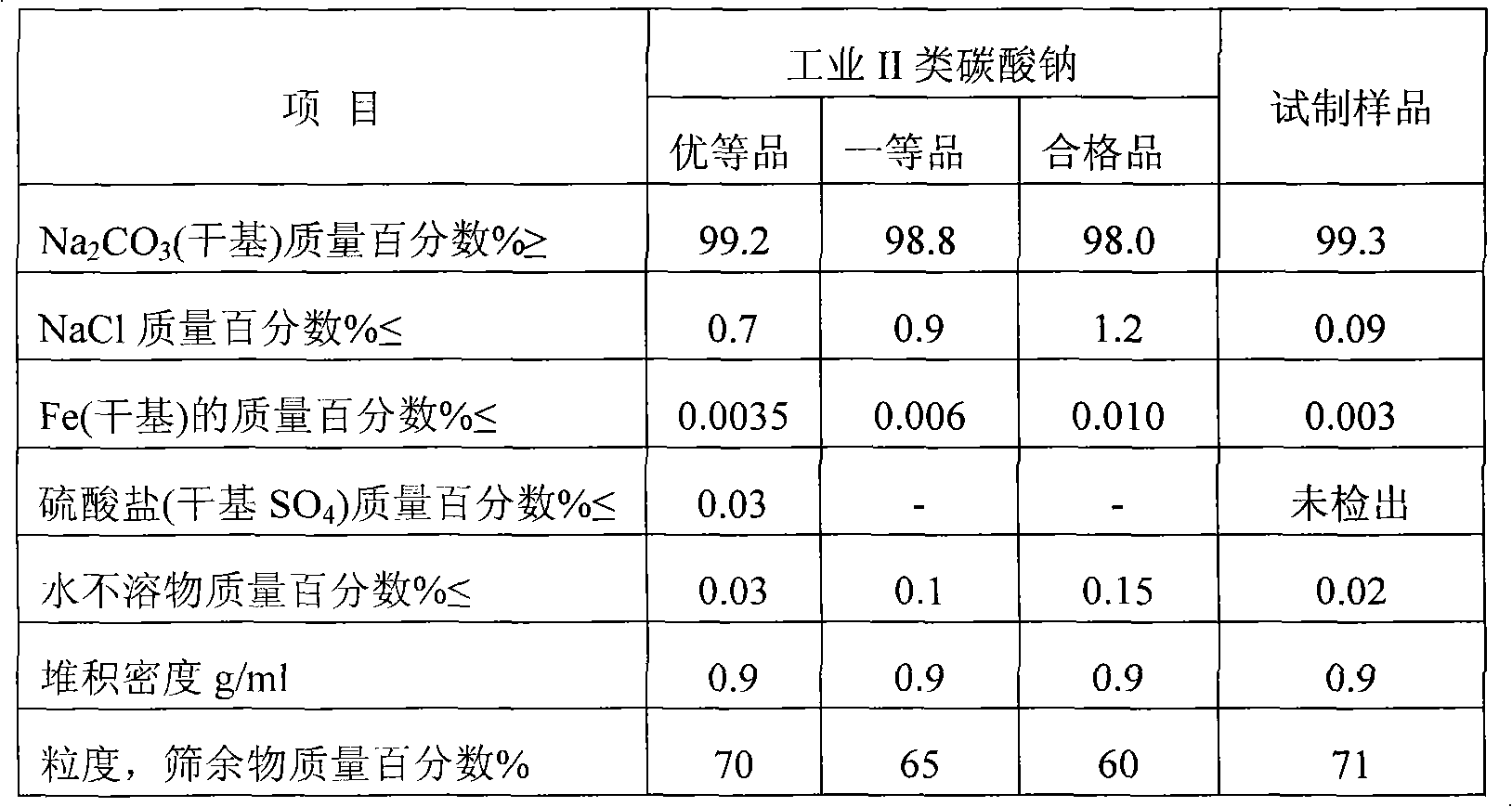
Nitratine or nitratite, also known as cubic niter (UK: nitre), soda niter or Chile saltpeter (UK: Chile saltpetre), is a mineral, the naturally occurring form of sodium nitrate, NaNO₃. Chemically it is the sodium analogue of saltpeter. Nitratine crystallizes in the trigonal system, but rarely occurs as well formed crystals. It is isostructural with calcite. It is quite soft and light with a Mohs hardness of 1.5 to 2 and a specific gravity of 2.24 to 2.29. Its refractive indices are nω=1.587 and nε=1.336.
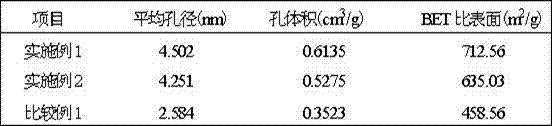
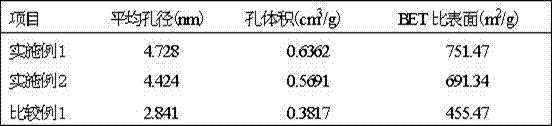
Method for preparing sodium nitrate with soda niter
ActiveCN101343072ABlocked mobilityGuaranteed liquidityEnergy inputAlkali metal nitrate preparationEvaporationNiter
The invention provides a method for using nitratine to produce sodium nitrate, which is characterized in that the method comprises the steps of: crushing a piece of nitratine raw ore, and sieving the raw ore to obtain nitratine ore and nitratine ore powder; adding water into the nitratine ore powder, and agitating and leaching the powder and then gaining a clear liquid by sedimentation and clarification; positioning the nitratine ore in a leaching pond, feeding a mother liquor for cold separation and / or a washing liquid, starting up a circulating pump to circulate the leaching solution, and leaching the ore under normal temperature; inputting the leaching solution to a solar pond after the leaching; heating the leaching solution to a temperature between 30 DEG C and 80 DEG C by utilizing the stored energy in the solar pond, and extracting high-temperature concentrated solution from the bottom of the solar pond after evaporation concentration; and separating out NaNO3 through crystallization by cooling. The method is applied to low grade nitratine resources, and locations of resources are areas short of water seriously and rich in solar energy resources, and the method has advantages of energy and water saving and easy realization.
Owner:BLUESTAR LEHIGH ENG INST CO LTD
Method for producing sodium nitrate by chilisaltpeter ore
InactiveCN101182013ASmall particle sizeLow grade requirementsAlkali metal nitratesWater savingCentrifugation
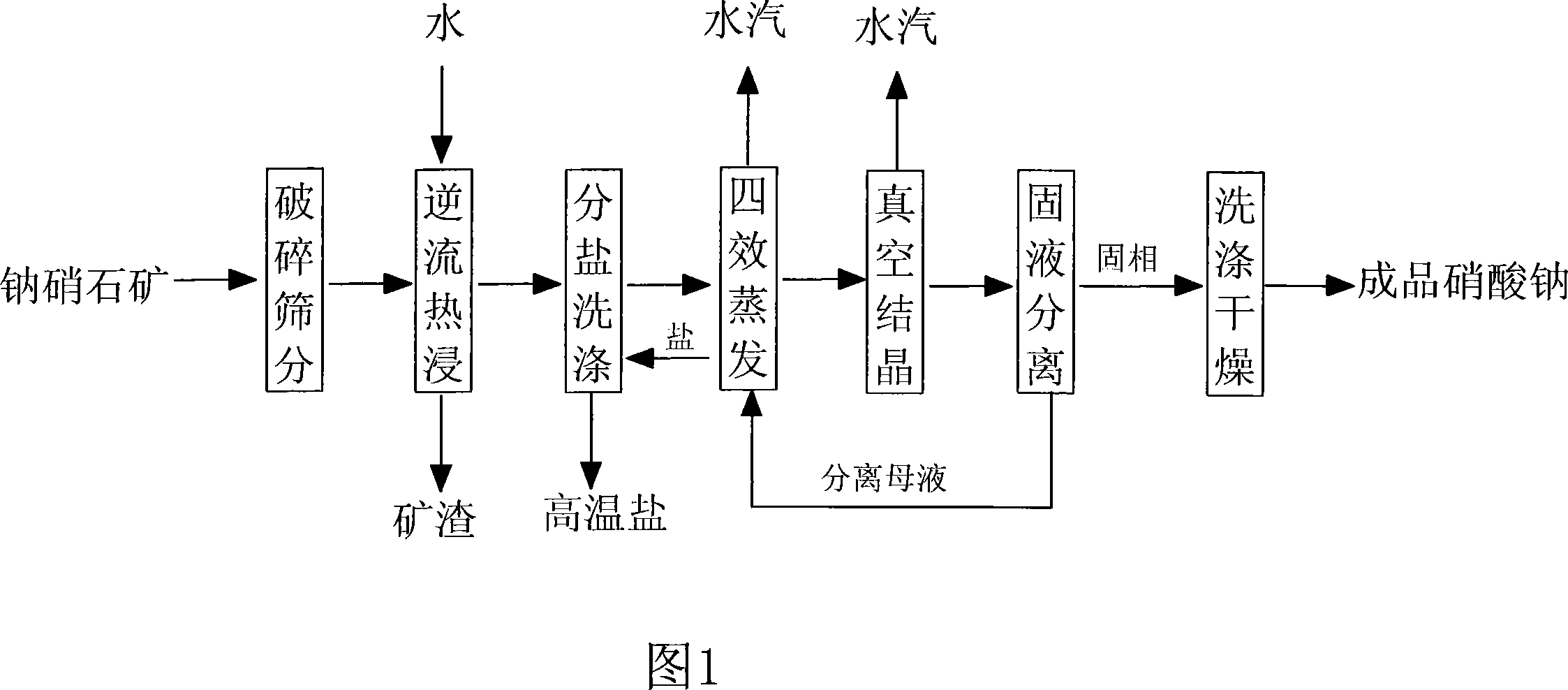
The invention relates to a production method for producing sodium nitrate with nitratine and the process steps thereof are that ore is crushed with the particle diameter below 2cm and is first treated with a multi-stage countercurrent hot soak so as to ensure that the content of NaNO3 in leaching solution is more than or equal to 450g / l, and then with a four-effect evaporation to remove NaCl and NaSO4 to ensure that the content of NaNO3 is more than or equal to 950g / l, and finally with centrifugation and drying after a three-stage vacuum cold separation crystallization to obtain the finished sodium nitrate. By adopting the method which has low dependency on natural conditions, water saving and low energy consumption and is suitable for low-grade nitratine mining, the overall yield of the sodium nitrate can reach more than 75 percent.
Owner:TURPAN BRANCH OF SINKIANG NITRATE MINERALS
Technique for producing sodium nitrate by chilisaltpeter adverse current circulation leaching
ActiveCN101168444AShort processEasy to operateAlkali metal nitrate preparationSingle processLower grade
The invention relates to a process for producing sodium nitrate by using nitratine reverse-flow circulation extraction, which comprises steps as follows: (1) breaking ores into the grains smaller not larger than 5cm, (2) reverse-flow circulation leaching: after 2-6 stages of reverse-flow circulation immerge, the sodium nitrate content of extraction solution is increased from 300-380g / l to 390-500g / l, (3) separating, washing and drying at low temperature that exchanges heat between the extraction solution and low-temperature separation mother liquid, to reduce temperature, mixing and reducing temperature to -10-20DEG C, and keeping temperature for 100-140min, separating solid and liquid, filtering and washing via a saturated sodium nitrate, according to the mass ratio between product and sodium nitrate solution as 6 / 1-5 / 1, then drying to obtain sodium nitrate product, and feeding the separated mother liquid into the step (2), to process next circulation. The invention can save water more than 40%, and save energy more than 25%, while the single-process collecting rate is more than 25% and the total collecting rate of sodium nitrate in ore is more than 95.0%. The product obtained by low-temperature separation contains sodium nitrate more than 92%, and the washed product contains sodium nitrate more than 98%. The invention can use low-grade nitratine mine to produce sodium nitrate.
Owner:TURPAN BRANCH OF SINKIANG NITRATE MINERALS +1
Technique for preparing iron concentrate from pyrite cinder by nitric acid-hydrochloric acid combined treatment
InactiveCN103725869AIncrease lossWill not cause secondary pollutionProcess efficiency improvementFertilizer mixturesPyriteSulfur
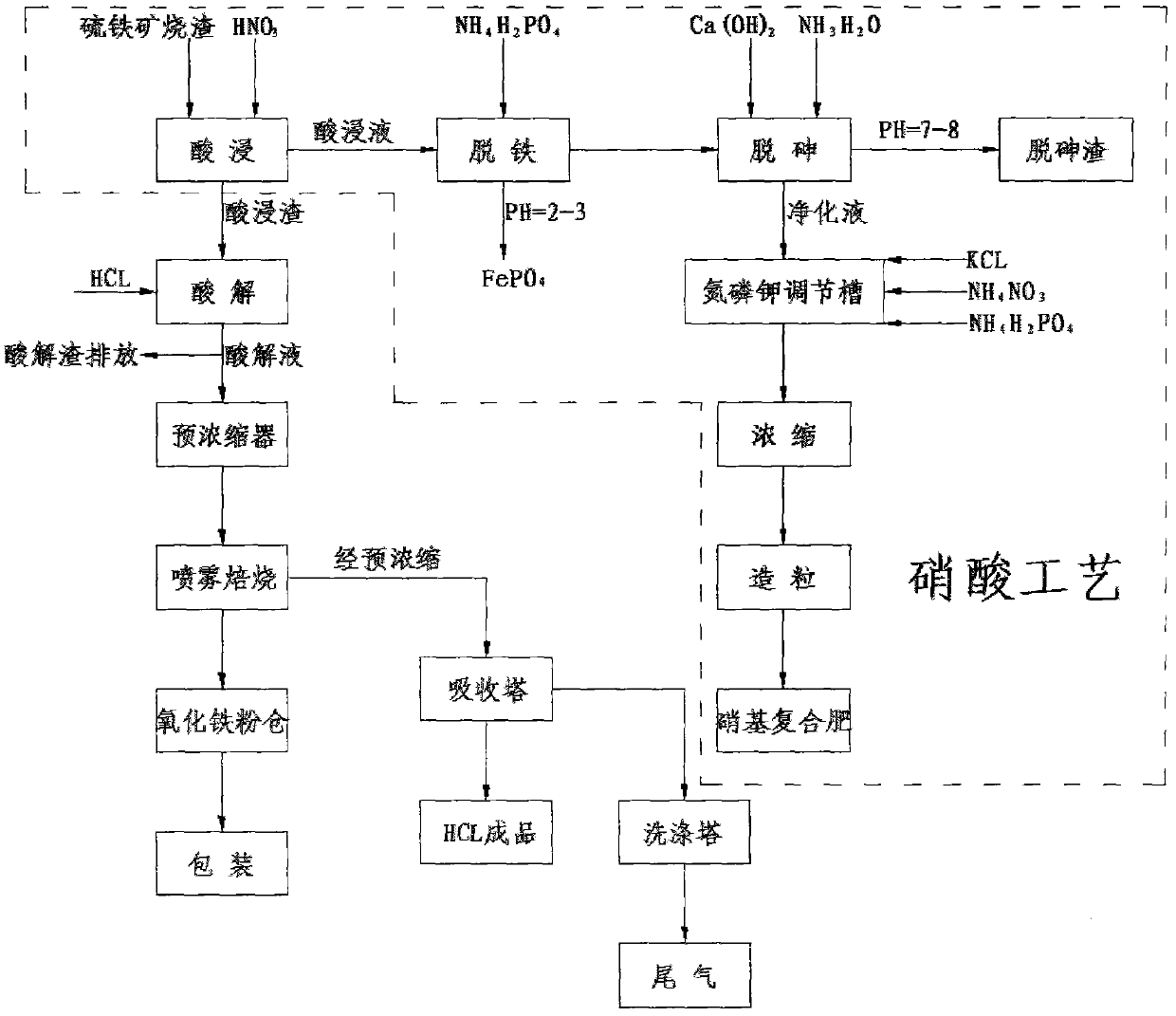
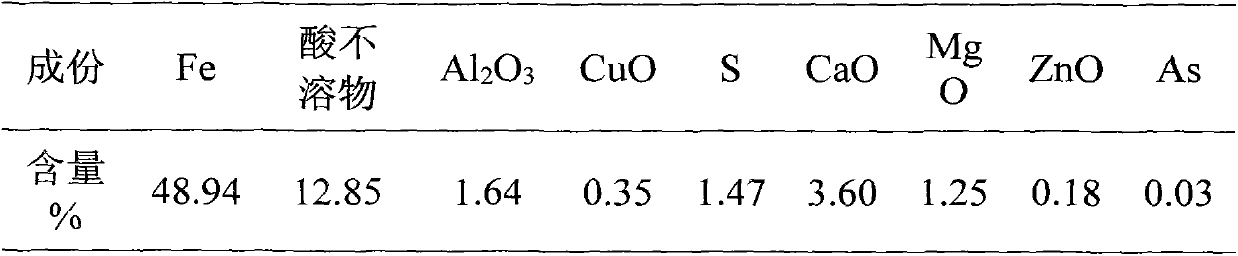
The invention discloses a technique for preparing iron concentrate from pyrite cinder by nitric acid-hydrochloric acid combined treatment, which is characterized by comprising the following steps: detecting the component content in the pyrite cinder; and if the SiO2 content in the pyrite cinder is greater than 10%, using a double-acid process, and if the SiO2 content in the pyrite cinder is smaller than 10%, adopting a nitric acid process. The technique can utilize the iron resource and eliminate pollution, thereby implementing clean production in pyrite acid making industry.
Owner:KAIFENG UNIV
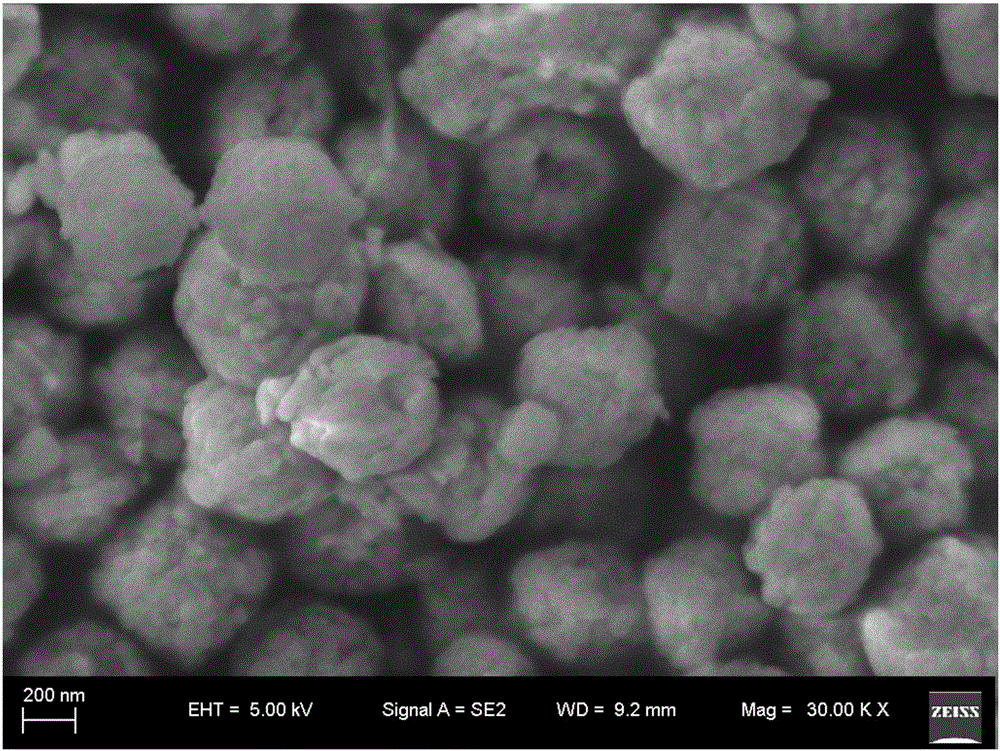
Doped-state spherical FeF<3>.0.33H<2>O positive electrode material and preparation method therefor
InactiveCN105845933AUniform particle sizeHigh purityCell electrodesSecondary cellsRough surfaceNitrate
The invention discloses a doped-state spherical FeF<3>.0.33H<2>O positive electrode material and a preparation method therefor. The chemical expression of the positive electrode material is Fe<1-x>M<x>F<3+(n-3)x>.0.33H<2>O, wherein M is doped elements Mg, Co, Ni or Zn; x is equal to 0.03-0.3; and n is the valance of the corresponding doped elements. The preparation method comprises the steps of putting Fe(NO<3>)<3>.9H<2>O into a reactor, adding nitrate of other doped metal; then adding alcohol, magnetically stirring for 10-20min to obtain a settled solution; then adding an HF water solution in a dropwise manner and stirring for 10-20min; moving the mixture to a hydrothermal reaction kettle to react, then carrying out solid-liquid separation; and carrying out vacuum drying at a temperature of 20-80 DEG C to obtain the doped-state spherical FeF<3>.0.33H<2>O particles. The doped state spherical ferric fluoride obtained by the invention has uniform particles, rough surface, high tap density, high repeatability and excellent electrochemical performance; and the reaction yield can reach greater than 80%.
Owner:XIANGTAN UNIV
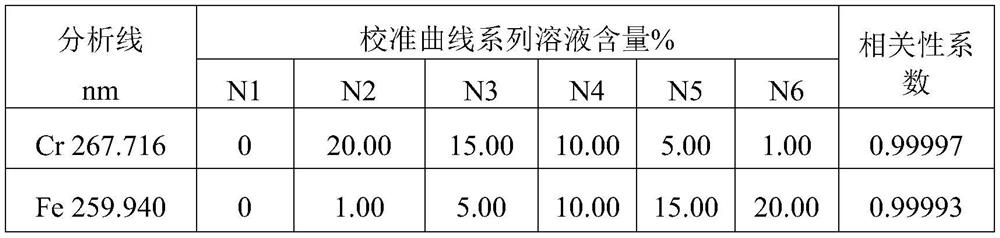
ICP-OES (Inductively Coupled Plasma-Optical Emission Spectrometer) rapid determination method for contents of chromium and iron in nickel-based superalloy
PendingCN112834487AEliminate the effects ofFully affectedPreparing sample for investigationAnalysis by thermal excitationOptical Emission SpectrometerElemental analysis
The invention relates to an ICP-OES rapid determination method for contents of chromium and iron in nickel-based superalloy, belongs to the technical field of chemical analysis test. The method comprises steps of dissolving pure nickel to serve as a matrix, adding different amounts of chromium and iron standard solutions to prepare a calibration solution, and adding an yttrium internal standard solution; simultaneously measuring the intensity ratio of the internal marking line of the internal standard element to the analysis line of the element to be measured in the calibration solution by using ICP-OES to make a calibration curve; dissolving a to-be-measured sample with nitric acid, hydrochloric acid and hydrofluoric acid, adding an yttrium internal standard solution with the same content as the standard solution, diluting with water to a fixed volume, introducing the solution into ICP-OES to measure the spectral line intensity of the to-be-measured element, and calculating the content of the to-be-measured element on the calibration curve. The calibration solution prepared by the method is similar to a sample solution in matrix, so that the matrix effect is eliminated; and a high-precision analysis instrument is used, so that the problems of complicated chemical analysis operation and low analysis speed are solved, and the method has the advantages of simplicity, rapidness, high accuracy, high precision and the like.
Owner:JIANGSU LONGDA SUPERALLOY MATERIAL CO LTD
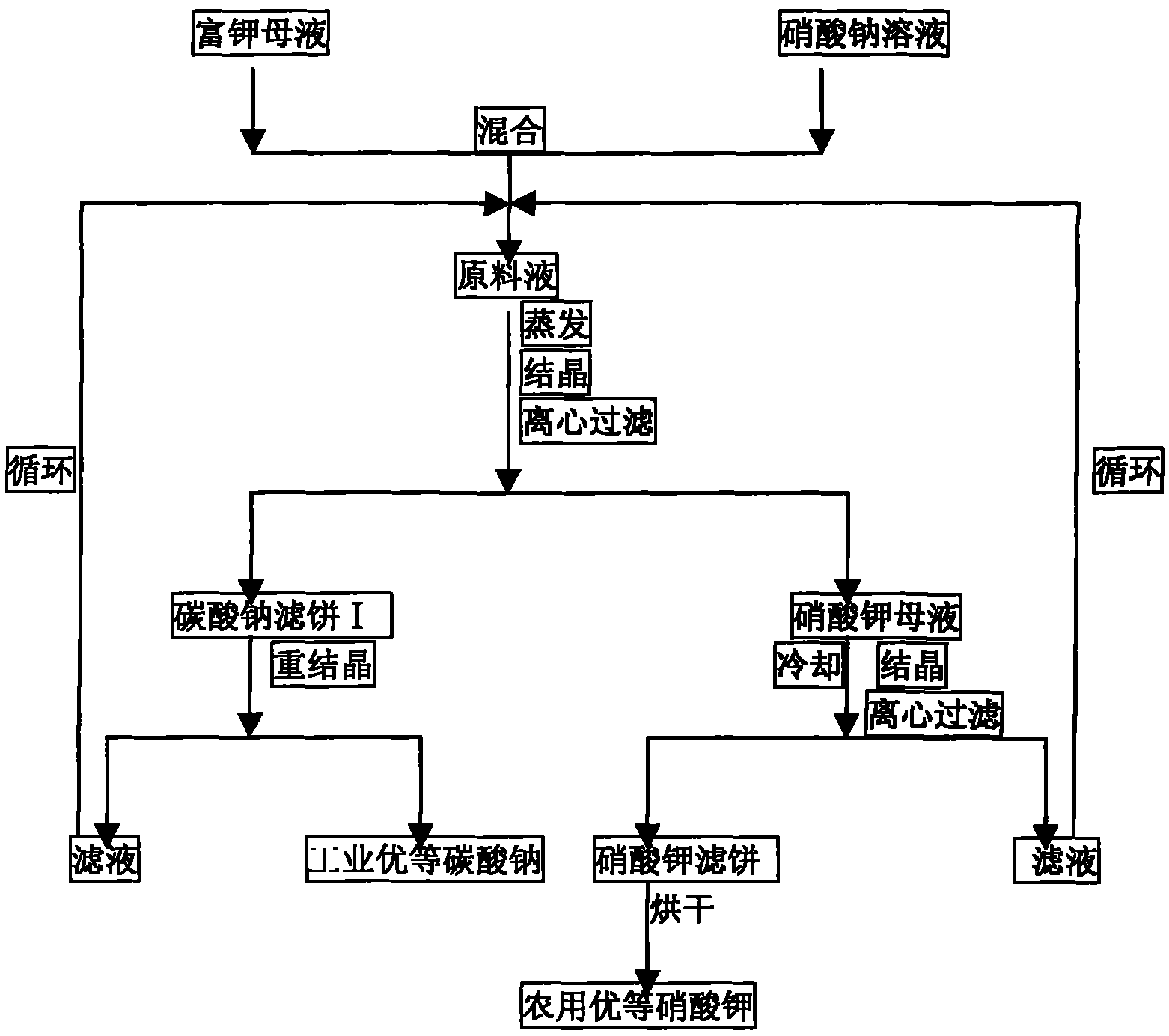
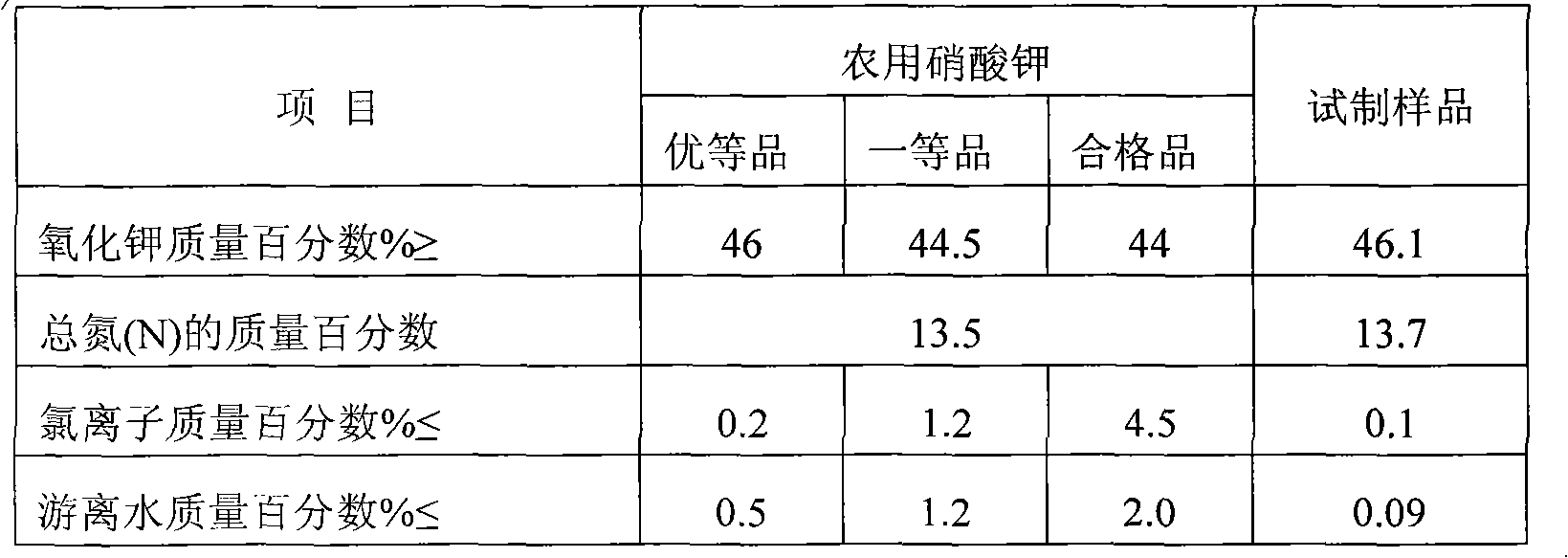
Method for preparing agricultural potassium nitrate by using nitratine and potassium-rich mother liquid
ActiveCN102372294AWide variety of sourcesExcellent chloride ion contentAlkali metal nitrate preparationPotassium nitratePotassium carbonate
The invention discloses a method for preparing agricultural potassium nitrate by using nitratine and potassium-rich mother liquid. The method comprises the following steps: crushing and grinding potassium-rich rocks to prepare raw ore powder, sintering, water leaching, silicon removing, sodium removing to obtain the potassium-rich mother liquor which takes potassium carbonate as a main component, mixing with a sodium nitrate solution obtained by extracting and concentrating nitratine ore powder through water to obtain raw material liquid, high temperature evaporating and crystallizing, forming a potassium-rich solution and a sodium carbonate filter cake, filtering and separating, recrystallizing the sodium carbonate filter cake, filtering deposition and drying to obtain an industrial superior sodium carbonate product, recrystallizing filtrate to raw material liquid for circular utilization. The potassium nitrate crystal is precipitated by cooling and crystallizing the potassium-rich solution, and is centrifuged and dried to an agricultural superior potassium nitrate product. The residual solution is cooled and crystallized to the raw material liquid for circular utilization.
Owner:CHINA UNIV OF GEOSCIENCES (BEIJING)
Adjustable morphology rare-earth ion codoped tungsten oxide nanoparticle and synthesis method thereof
InactiveCN110681376AReduce coagulationSmall particle sizeMetal/metal-oxides/metal-hydroxide catalystsPtru catalystN-Butyl Alcohol
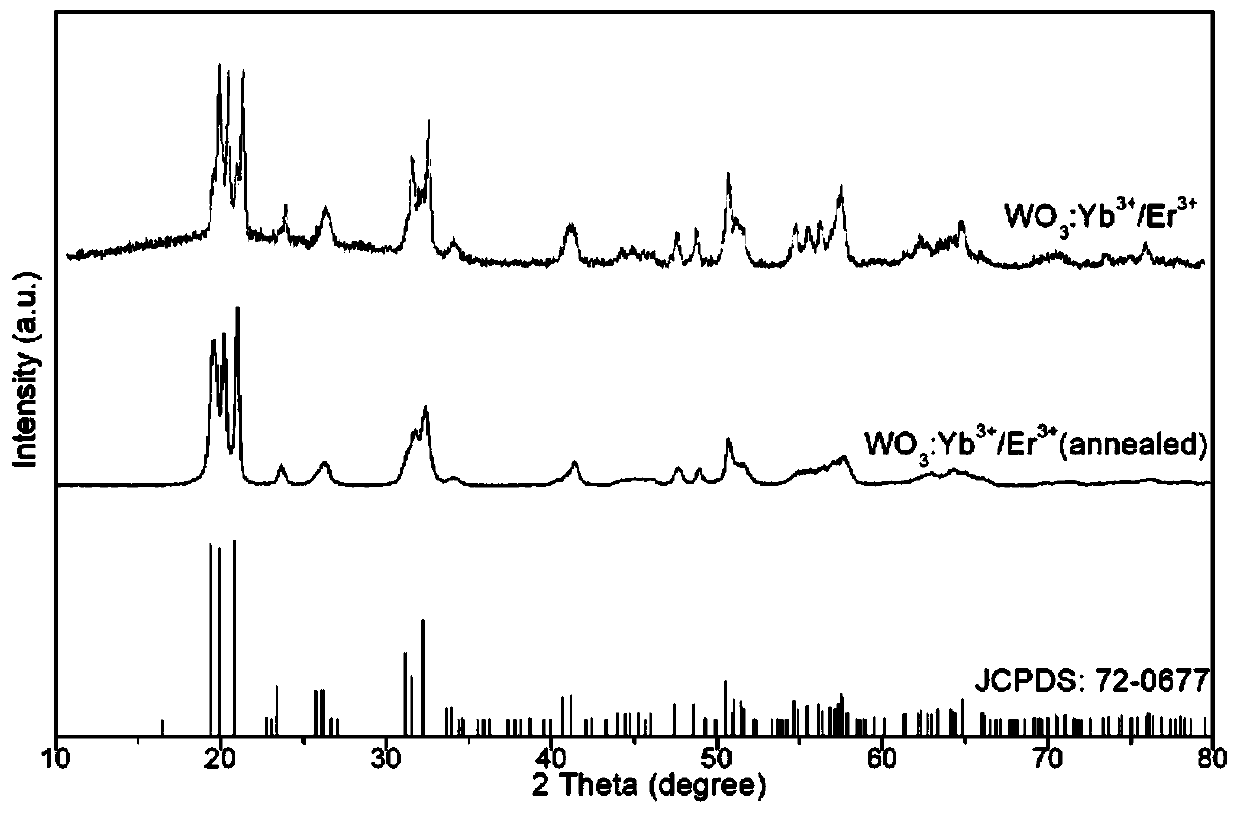
The invention belongs to the field of inorganic nano materials and in particular relates to an adjustable morphology rare-earth ion codoped tungsten oxide nanoparticle and a synthesis method thereof.The rare-earth ion codoped tungsten oxide nanoparticle has a chemical formula of WO3:RE<3+>, and has a crystal phase of a monoclinic crystal phase. The specific synthesis process comprises the following steps: adding a rare-earth ion nitrate solution into a sodium tungstate aqueous solution, further dropwise adding concentrated hydrochloric acid, n-butyl alcohol and EDTA-2Na, performing sufficientstirring, performing hydrothermal treatment on the mixed solution, naturally cooling the solution, performing centrifugation, washing and drying so as to obtain a cubic WO3:RE<3+> nanoparticle, and performing high-temperature annealing on the nanoparticle, so as to obtain an ellipsoid-like WO3:RE<3+> nanoparticle. The WO3:RE<3+> nanoparticle which is synthesized by using a mixed solvothermal method, which is provided by the invention, has the advantages of being uniform in particle size, small in size, good in dispersibility, high in purity, and the like, has the potential of being used as aphotocatalyst to widen the spectrum response range of conventional tungsten oxide, and is capable of effectively improving the catalysis efficiency of a catalyst.
Owner:TIANJIN UNIV
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107159198AEvenly dopedImprove adsorption capacityOther chemical processesCatalyst activation/preparationUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical field of environmentally-friendly and chemical catalysts. The preparation method comprises the following steps: by taking medical stone, wollastonite, dolomite, calcite, nitratine and dolomite as carriers, reaming the carriers with lithium hypochlorite and bis(acetylacetone) beryllium, then adding myristyl tributyl ammonia chloride serving as a surfactant for activation treatment under ultrasonic action, putting the carriers into a hydrothermal reaction kettle for hydrothermal reaction together with borax and potassium sulfate which serve as composite mineralizers, isopropyl scandium oxide (III), tri(4,4,4-trifluoro-1-(2-thiophene)-1,3-butanedione) europium, thulium trifluoromethane sulfonate (III) and lutetium carbonate hydrate which serve as catalytic active auxiliary precursors, a dicyclopentadienyl titanium cyclo-replaced salicylic acid complex, zinc lactate and a catechol ethanediamine tungsten complex which serve as catalytic active center precursors and iridium tetrachloride under the action of dimethyl hexadecyl ethyl sulfate serving as an emulsifier, drying a reaction product to remove water, and firing the reaction product in a muffle furnace, thus obtaining the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Counterflow circulation washing extraction method by using nitratine
InactiveCN102910651AEfficient use ofReduce wasteAlkali metal nitrate purificationAlkali metal sulfite/sulfate purificationSlagEvaporation
The invention relates to a counterflow circulation washing extraction method by using nitratine, comprising the following steps: (1) stacking nitratine ores with the lumpiness of smaller than 400mm in a dump leaching pool for soaking, and continuously stirring, wherein the soaking period is larger than or equal to 120h; (2) when the average content of NaNO3 in the soaking slag of ores is smaller than or equal to 0.6%, stopping soaking and stirring, discharging soaking solution, discharging the soaking slag in a spoil area after clear water is filtered and washed, then stacking the ores again and carrying out the next round of washing and stirring by using the soaking solution; (3) when the content of NaNO3 in leach liquor is larger than or equal to 12%, pumping in an evaporation pond for evaporation, and concentrating to precipitate sodium chloride crystal and sodium sulfate crystal; and (4) when the concentration of bittern is continuously improved till the content of NaNO3 is larger than or equal to 20%, concentrating the bittern into a half-finished product which is a mixed crystal of sodium nitrate, sodium sulfate, sodium chloride and darapskite. The method is high in leaching rate, low in cost, short in cycle and high in yield, and is capable of efficiently utilizing resources and reducing the waste of resources.
Owner:冯耀礼
Special iodine-enriched fertilizer for paddy rice and preparation method thereof
InactiveCN106565375AWide variety of sourcesIncrease productionAnimal corpse fertilisersAlkali orthophosphate fertiliserPlant diseaseFermentation
The invention discloses a special iodine-enriched fertilizer for paddy rice. The fertilizer is prepared from the following raw materials: animal dung, laver, laminaria japonica, mussel, sea shrimp shell, shell, nitratine, airpotato yam rhizome, Enteromorpha clathrata (Roth) Greville, an organic raw material, a composite trace element, animal amino acid, composite microbial strains, etc. The invention also discloses a preparation method of the fertilizer, comprising steps of crushing, fermentation, drying and pelletizing, etc. The nuisance-free fertilizer is prepared by fermentation of multiple iodine-enriched natural polymer organic matter. The fertilizer is absorbed by paddy rice, then converted and finally eaten by human, thus achieving the purpose of supplementing iodine. By the addition of straw, soybean meal, etc., contents of mineral and organic matter in soil are increased, soil permeability is enhanced, water retention is improved, soil fertility is boosted, negative effect of poor soil quality caused by long-time application of lots of inorganic fertilizers is prevented, soil disease can be effectively controlled, rice root development can be promoted, yield of rice is increased, and rice quality is enhanced.
Owner:张春
Special fertilizer for iodine-rich strontium-rich rice and preparation method for special fertilizer
InactiveCN106565318AIncrease productionImprove qualityAnimal corpse fertilisersAlkali orthophosphate fertiliserClaviceps purpureaLivestock manure
The invention discloses a special fertilizer for iodine-rich strontium-rich rice. The special fertilizer is prepared from raw materials such as porphyra, kelp, mussel, sea shrimp shells, shells, nitratine, airpotato yam rhizome, enteromorpha, organic raw materials, nitrohumic acid, coal ash, poultry and livestock manure, L-asparaginic acid srtrontium chelate, composite trace elements, compound microorganism species, a fertilizer adhesive and the like. The special fertilizer for iodine-rich strontium-rich rice disclosed by the invention contains a great deal of iodine and strontium, is eaten by people after being absorbed and converted by rice as a fertilizer, so that the purpose of supplementing an iodine element and a strontium element can be achieved; organic substances such as humic acid are added, so that minerals and organic substances in the soil are increased, soil permeability is improved, water-retaining property is improved, soil diseases can be effectively controlled, the root system development of rice can be promoted better, the rice yield is increased, and rice quality is improved. The preparation method for the special fertilizer for iodine-rich strontium-rich rice provided by the invention is simple and easy to implement, can reduce use of a chemical fertilizer, and has effects of improving economic benefits.
Owner:张春
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008446AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionPyrophyllite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environment-friendly and chemical engineering catalysts. The preparation method comprises the following steps: by taking medical stones, wollastonite, light shale ceramisite, pyrophyllite, nitratine and dolomite as carriers, after chambering and modifying the carriers through lithium hypochlorite and di(acetylacetone) beryllium, adding a surfactant, octadecyl trimethyl ammonium chloride, for surface activating treatment under the action of ultrasonic waves; then causing the carriers to generate hydrothermal reaction with borax and potassium sulfate which serve as compound mineralizers, isoproscandium oxide (III), 1,1,1-trifluoroacetyl acetone neodymium, tri(2,2,6,6-tetramethyl-3,5- heptylic diketone acid) gadolinium and holmium oxalate decahydrate which serve as catalytic active auxiliary agent precursors, and a titanocene ring substituted salicylic acid complex, nickel citrate, molybdenum L-aspartate and gold potassium tetrachloride which serve as catalytic active central compound precursors in a hydrothermal reaction kettle under the action of an emulsifier, N-octadecyl dimethyl-N'-propyl ammonium dichloride, drying a reaction product to remove water, and firing the reaction product in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method for solid catalyst for ozone heterogeneous oxidization
InactiveCN107008377AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionLithium hypochlorite
The invention relates to a preparation method for a solid catalyst for ozone heterogeneous oxidization and belongs to the technical field of an environment-friendly and chemical catalyst. The preparation method comprises the following steps: by taking activated carbon, carnallite, dolomite, calcite, nitratine and dolomite as carriers, modifying the carriers by broaching with lithium hypochlorite and bis(acetylacetone) beryllium, and then adding a surfactant, beta-ethoxy dimethyl dodecyl ammonium sulfate, and performing surface activating treatment under the effect of ultrasonic wave; performing hydrothermal reaction on the carriers, compound mineralizers including borax and potassium sulphate, catalytic activated assistant precursors including tricyclopentadiene promethium, tri(2,2,6,6-tetramethyl-3,5-heptanedionato) gadolinium, tri(6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-octene diketone) dysprosium (III) and thulium trifluoromethane sulfonate (III), and catalytic active core component precursors including cobalt gluconate, zinc lactate, L-aspartic acid molybdenum complex and terpyridyl ruthenium chloride hexahydrate in a hydrothermal reaction kettle under the effect of octadecyl dimethyl hydroxypropyl ammonium chloride used as an emulsifier; drying and dewatering the reaction product; burning in a muffle furnace under a certain temperature, thereby acquiring the solid catalyst for ozone heterogeneous oxidization.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method for solid catalyst for ozone heterogeneous oxidization
InactiveCN107008441AEvenly dopedImprove adsorption capacityOther chemical processesWater treatment compoundsUltrasound - actionIodide
The invention relates to a preparation method for a solid catalyst for ozone heterogeneous oxidization and belongs to the technical field of an environment-friendly and chemical catalyst. The preparation method comprises the following steps: taking gamma-aluminum oxide, barite, sepiolite, celsian, nitratine and dolomite as a carrier; modifying the carrier by broaching with lithium hypochlorite and bis(acetylacetone) beryllium, and then adding surfactant didodecyldimethylammonium bromide and performing surface activating treatment under the effect of ultrasonic wave; performing hydrothermal reaction on an ultrasonic surface activated carrier, a compound mineralizer including borax and potassium sulphate, catalytic activated assistant precursors, including 4(2, 2, 6, 6, 6-tetramethyl-3, 5-heptanedione) cerium (IV), tri(3-trifluoroacetyl-D-camphor) praseodymium (III), tri(2,2,6,6-tetramethyl-3,5-hydrochelidonic acid) gadolinium, thulium trifluoromethane sulfonate (III) and catalytic active core component precursors, including manganese lysine, cupric glutamate, L-aspartic acid molybdenum and dipotassium hexachloroosmium in a hydrothermal reaction kettle under the effect of trimethylamine stearate trimethylammonium iodide used as an emulsifier; drying and dewatering the reaction product; burning in a muffle furnace under a certain temperature, thereby acquiring the solid catalyst for ozone heterogeneous oxidization.
Owner:SICHUAN NORMAL UNIVERSITY
Method for preparing sodium nitrate with soda niter
ActiveCN101343072BBlocked mobilityGuaranteed liquidityEnergy inputAlkali metal nitrate preparationEvaporationNiter
The invention provides a method for using nitratine to produce sodium nitrate, which is characterized in that the method comprises the steps of: crushing a piece of nitratine raw ore, and sieving the raw ore to obtain nitratine ore and nitratine ore powder; adding water into the nitratine ore powder, and agitating and leaching the powder and then gaining a clear liquid by sedimentation and clarification; positioning the nitratine ore in a leaching pond, feeding a mother liquor for cold separation and / or a washing liquid, starting up a circulating pump to circulate the leaching solution, and leaching the ore under normal temperature; inputting the leaching solution to a solar pond after the leaching; heating the leaching solution to a temperature between 30 DEG C and 80 DEG C by utilizing the stored energy in the solar pond, and extracting high-temperature concentrated solution from the bottom of the solar pond after evaporation concentration; and separating out NaNO3 through crystallization by cooling. The method is applied to low grade nitratine resources, and locations of resources are areas short of water seriously and rich in solar energy resources, and the method has advantages of energy and water saving and easy realization.
Owner:BLUESTAR LEHIGH ENG INST CO LTD
Preparation method of ozone heterogeneous oxidized solid catalyst
InactiveCN107020125AImprove adsorption capacityBurning at high temperature makes the organic matter completely carbonized and strong adsorptionCatalyst carriersWater contaminantsUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidized solid catalyst, and belongs to the technical field of environmental protection and chemical catalyst. The preparation method comprises the following steps: taking perlite, albite, nitratine, dolomite, coal ash and coal gangue as carriers, performing lithium hypochlorite and bis(acetylacetonato) beryllium reaming, adding a surfactant N-decyl dimethyl-N'-trimethyl-2-hydroxypropyl ammonium dichloride to perform activating treatment under an ultrasonic effect; and then enabling the active carriers to perform hydrothermal reaction with the compound mineralizing agent borax and potassium sulfate, catalytic active promoter precursors tri(cyclopentadienyl) promethium, tris(4,4,4-trifluoro-1-(2-thienyl)-1,3-butanediono)europium (III), holmium oxalate decahydrate, lutetium carbonate hydrate, catalytic active center precursors cobalt gluconate, cupric glutamate, terpyridyl ruthenium chloride hexahydrate, dipotassium hexachloroosmium under the emulsifier N-oleyl-N'N'-diethyl ethylenediamine dihydrochloride, drying to remove the moisture, firing in a muffle furnace to obtain the ozone heterogeneous oxidized solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
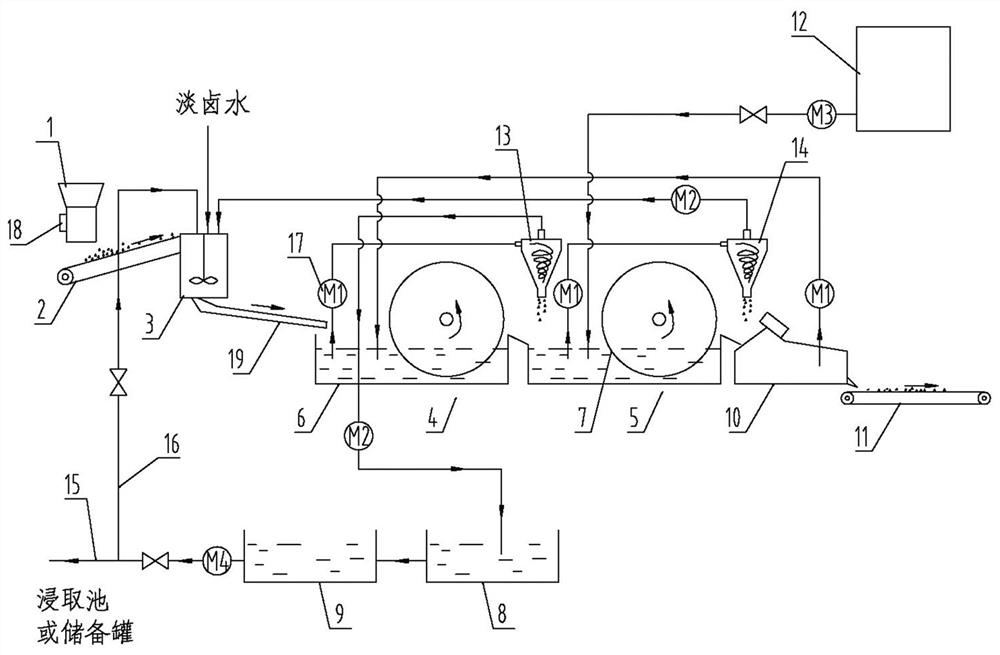
Continuous marinating system for powdery nitratine
PendingCN111892070AAvoid wastingCompact and reasonable structureAlkali metal nitrate preparationEngineeringWasher
The invention relates to the technical field of a nitratine mineral powder marinating system, which is a continuous marinating system for powdery nitratine. The system comprises a feeding bin, a material conveying belt, a stirring tank, a first wheel bucket type sand washer, a second wheel bucket type sand washer, a dewatering screen and a material slag conveying belt which are sequentially arranged from front to back, wherein the stirring tank is communicated with the first wheel bucket type sand washer through a chute, and a first cyclone and a second cyclone are respectively arranged at thefirst wheel bucket type sand washer and the second wheel bucket type sand washer. According to the invention, by arranging a first wheel bucket type sand washer, and a mortar pump and a first cyclonewhich correspond to the first wheel bucket type sand washer, sodium nitrate can be extracted for the first time, by arranging a second wheel bucket type sand washer and a mortar pump and a second cyclone which correspond to the second wheel bucket type sand washer, sodium nitrate can be extracted for the second time, and finally a mortar pump is used for sucking and conveying sieved liquid generated by a dehydration sieve to extract sodium nitrate for the third time, so that the sodium nitrate component in nitratine mineral powder is fully utilized.
Owner:TURPAN BRANCH OF SINKIANG NITRATE MINERALS
Technique for producing sodium nitrate by chilisaltpeter adverse current circulation leaching
ActiveCN100595149CShort processEasy to operateAlkali metal nitrate preparationSingle processLower grade
The invention relates to a process for producing sodium nitrate by using nitratine reverse-flow circulation extraction, which comprises steps as follows: (1) breaking ores into the grains smaller notlarger than 5cm, (2) reverse-flow circulation leaching: after 2-6 stages of reverse-flow circulation immerge, the sodium nitrate content of extraction solution is increased from 300-380g / l to 390-500g / l, (3) separating, washing and drying at low temperature that exchanges heat between the extraction solution and low-temperature separation mother liquid, to reduce temperature, mixing and reducing temperature to -10-20DEG C, and keeping temperature for 100-140min, separating solid and liquid, filtering and washing via a saturated sodium nitrate, according to the mass ratio between product and sodium nitrate solution as 6 / 1-5 / 1, then drying to obtain sodium nitrate product, and feeding the separated mother liquid into the step (2), to process next circulation. The invention can save water more than 40%, and save energy more than 25%, while the single-process collecting rate is more than 25% and the total collecting rate of sodium nitrate in ore is more than 95.0%. The product obtained bylow-temperature separation contains sodium nitrate more than 92%, and the washed product contains sodium nitrate more than 98%. The invention can use low-grade nitratine mine to produce sodium nitrate.
Owner:TURPAN BRANCH OF SINKIANG NITRATE MINERALS +1
Ozone heterogeneous oxidation solid catalyst preparation method
InactiveCN107051514AImprove adsorption capacityImprove bindingCatalyst carriersWater contaminantsUltrasound - actionSodium Bentonite
The invention belongs to the technical field of environment protection and chemical catalysts and relates to an ozone heterogeneous oxidation solid catalyst preparation method. The preparation method includes: taking porous mineral materials including attapulgite, diopside, bentonite, polyhalite, nitratine and dolomite as carriers; subjecting the carriers to lithium hypochlorite and bis(acetylacetone)beryllium broaching modification; adding surfactant trioctylmethyl ammonium chloride for activation under the action of ultrasonic waves; subjecting the carriers to hydrothermal reaction, with a complex mineralizer composed of borax and potassium sulfate, catalytic activity auxiliary agent precursors including tri(3-trifluoroacetyl-D-camphor)praseodymium (III), 1,1,1-neodymium trifluoroacetylacetonate, tris[N,N-bis(trimethylsilane)amine]erbium and lutetium carbonate hydrate and catalytic activity central precursors including ferrous fumarate, nickel citrate, zinc lactate and tetraammine dichloropalladium, in a hydrothermal reactor under the action of N-dimethyldodecyl-N'-dodecyl-dimethyl-2-hydroxypropyl ammonium chloride serving as an emulsifying agent; drying to remove moisture, and firing in a muffle furnace at a certain temperature to obtain an ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
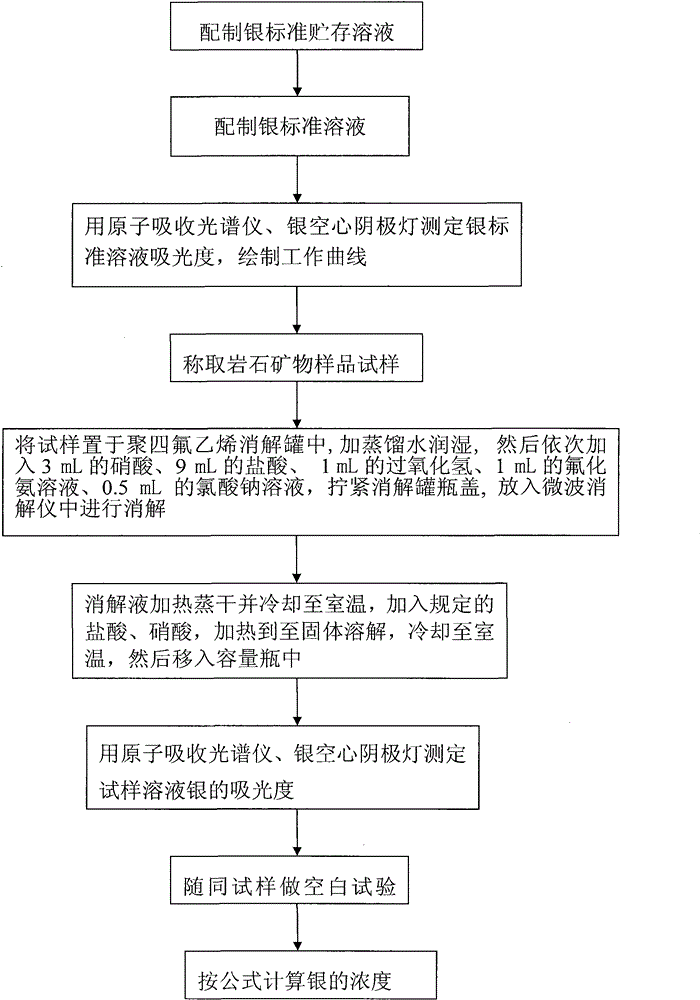
Method of determining silver content in rock minerals
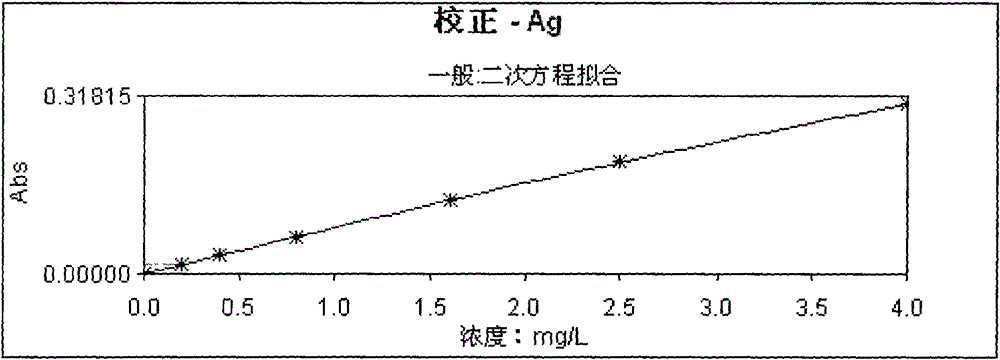
InactiveCN102353637BReduce dosageProtect your healthColor/spectral properties measurementsVolumetric flaskSpectrometer
Owner:山东正元地质资源勘查有限责任公司
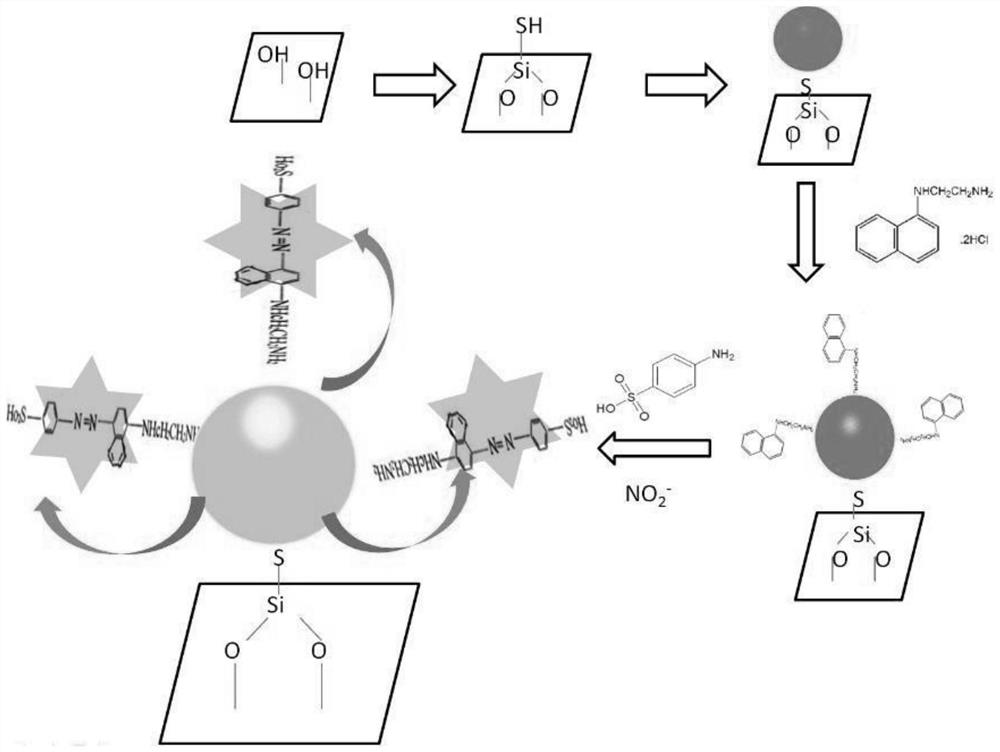
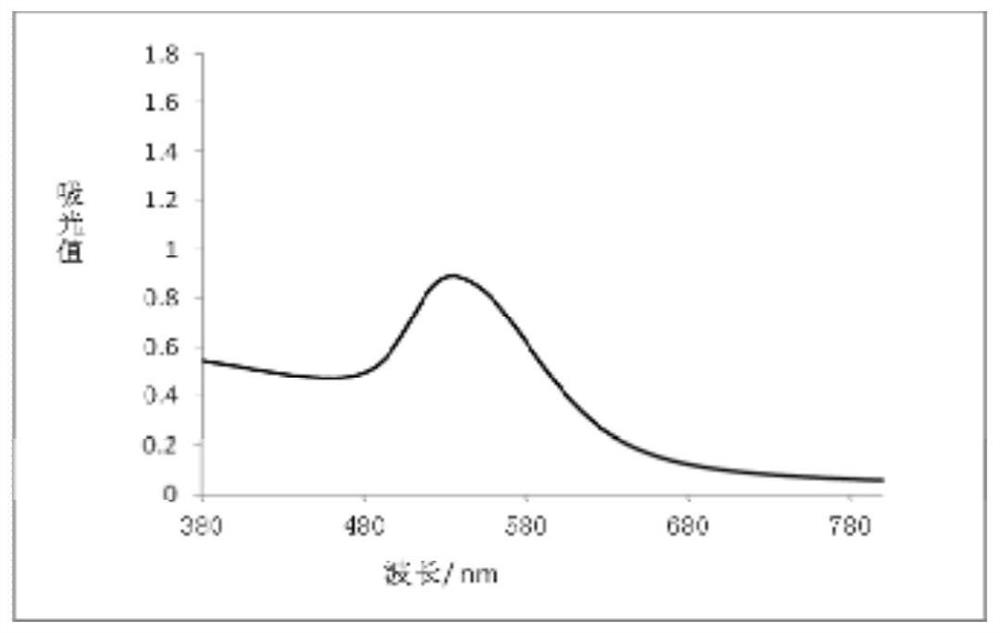
A method for detecting nitrite based on silver-coated gold nanoparticles
ActiveCN110702681BIncreased sensitivityLow detection limitMaterial analysis by optical meansMicroscopic observationEthylenediamine hydrochloride
Owner:CENTRAL SOUTH UNIVERSITY OF FORESTRY AND TECHNOLOGY
Method for preparing ozone heterogeneous oxidized solid catalyst
InactiveCN107042114AEvenly dopedImprove adsorption capacityCatalyst carriersWater contaminantsUltrasound - actionLithium hypochlorite
The invention relates to a method for preparing an ozone heterogeneous oxidized solid catalyst, which belongs to the technical fields of environmental protection and a chemical catalyst. The preparation method comprises the following steps: diatom pure, kyanite, hydrotalcite, alum stone, nitratine and dolostone are taken as carriers, the carriers are subjected to hole-reaming modification through lithium hypochlorite and dis(acetylacetone)beryllium, a surfactant dodecyl dimethyl hydroxyethyl ammonium chloride is added, surface activation treatment is carried out under supersonic wave effect, then the carriers after ultrasonic surface activation are subjected to a hydro-thermal reaction with a composite mineralizer borax and potassium sulfate, a catalytic activity auxiliary agent predecessor tri(hexafluoroacetylacetone)yttrium (III)dehydrate, tricyclopentadiene promethium, tri(6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-octylene dione)dysprosium (III), tri(trifluoromethanesulfonimide)ytterbium, a catalytic activity central composite predecessor common transition metallorganic compound pyruvate iso-nicotinoylhydrazone vanadium, cobalt gluconate and hexanitro rhodium trisodium, dipotassium hexachloroosmium in a hydro-thermal reaction vessel under effect of an emulsifier dioctadecalkyl ammonium bromide, the reaction products are dried to remove the moisture, in a muffle furnace, and the product is calcinated to obtain the ozone heterogeneous oxidized solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008273AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environmental-friendly and chemical engineering catalysts. The preparation method comprises the following steps: by taking erionite, garnet, diatom pure, kyanite, nitratine and dolomite as a carrier, after chambering and modifying the carrier through lithium hypochlorite and di(acetylacetone) beryllium, adding a surfactant dodecyl trimethyl ammonium chloride for surface activating treatment under the action of ultrasonic waves; then performing a reaction on the ultrasonic surface activated carrier in a hydrothermal reaction kettle and a compound mineralizer borax and potassium sulfate and catalytic active auxiliary agent precursors isoproscandium oxide (III), a tri(hexafluoroacetyl acetone) yttrium (III) dihydrate, 1,1,1-trifluoroacetyl acetone neodymium and tri[N,N-bis(trimethyl silyl)amide erbium; and performing a hydrothermal reaction with catalytic active central compound precursors a titanocene ring substituted salicylic acid complex, vanadium isonicotinoyl hydrazone pyruvate, zinc lactate and iridic tetrachloride dihydrate under the action of an emulsifier (2-(methacryloyloxy)ethyl) trimethyl ammonium chloride, drying a reaction product to remove water, and firing the reaction product in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
A method for preparing rare earth vanadate thin film by replacement reaction
ActiveCN110552036BEasy to prepareEasy to operateElectrolytic inorganic material coatingRare earth ionsCrystallinity
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Method for preparing ozone heterogeneous oxidation solid catalysts
InactiveCN107051493AImprove adsorption capacityBurning at high temperature makes the organic matter completely carbonized and strong adsorptionCatalyst carriersWater contaminantsUltrasound - actionLithium hypochlorite
The invention relates to a method for preparing ozone heterogeneous oxidation solid catalysts, and belongs to the technical field of environmental protection and chemical catalysts. The method includes carrying out pore expansion and modification on carriers which are purification diatom, kyanite, nitratine, dolomite, amazonite and lithium hydroxyapatite porous materials by the aid of lithium hypochlorite and bis (acetylacetone) beryllium; adding chlorinated dodecyl dimethyl hydroxyethyl ammonium chloride into the carriers and carrying surface activation treatment on the carriers under the effects of ultrasonic waves; carrying out hydrothermal reaction on the carriers which are subjected to ultrasonic surface activation, borax, potassium sulfate, tri (hexafluoroacetylacetone) yttrium (III) dihydrate, samarium acetylacetone, tri-[4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-butanedione] europium, holmium oxalate decahydrate rare earth metal organic compounds, vanadium pyruvic acid isonicotinyl hydrazone, nickel citrate, cupric glutamate and tetrachloro iridium dihydrate in hydrothermal reaction kettles under the effect of N-dimethyl dodecyl-N'-trimethyl-2-hydroxypropyl ammonium dichloride which is an emulsifier; drying reaction products to remove moisture; burning the reaction products in muffle furnaces at the certain temperatures to obtain the ozone heterogeneous oxidation solid catalysts. The chlorinated dodecyl dimethyl hydroxyethyl ammonium chloride is used as a surfactant. The borax and the potassium sulfate are used as composite mineralizers, the tri (hexafluoroacetylacetone) yttrium (III) dihydrate, the samarium acetylacetone, the tri-[4, 4, 4-trifluoro-1-(2-thiophene)-1, 3-butanedione] europium and the holmium oxalate decahydrate rare earth metal organic compounds are used as catalytic active auxiliary precursors, the vanadium pyruvic acid isonicotinyl hydrazone, the nickel citrate, the cupric glutamate and the tetrachloro iridium dihydrate are used as catalytic active central components, the vanadium pyruvic acid isonicotinyl hydrazone is a common transition metal organic compound, and the tetrachloro iridium dihydrate is a precious metal compound.
Owner:SICHUAN NORMAL UNIVERSITY
A kind of cerium, terbium co-doped activated aluminosilicate luminescent phosphor and preparation method thereof
ActiveCN108865122BImprove luminous performanceExcellent luminous propertiesLuminescent compositionsCeriumUltraviolet
A cerium and terbium co-doped activated aluminosilicate luminescent phosphor powder and its preparation method belong to the technical field of rare earth luminescent materials, and its chemical formula representing the composition and molar composition is Ca 19.96‑ 2x al 26 Mg 3 Si 3 o 68 :0.2Ce 3+ ,xTb 3+ ,(0.2+x)A + , where A + is a charge compensator, which is Li, Na or K; x represents the number of moles doped with terbium ions, 0≤x≤0.4. The present invention synthesizes a series of fluorescent powders by a high-temperature solid-phase method, and uses corresponding oxides, hydroxides, nitrates, carbonates, etc. as raw materials when synthesizing the above products. 2 、H 2 Calcining at 1350°C-1400°C for 2-3 hours in a reducing atmosphere of a mixed gas, cooling and treating to obtain cerium and terbium co-doped activated aluminosilicate luminescent phosphors. The phosphor powder prepared by the invention has wide absorption range, high emission intensity and strong thermal stability, can be combined with ultraviolet LED chips to prepare white light LEDs with high luminous performance, and has good application prospects.
Owner:JILIN UNIV
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107159200AImprove bindingImprove adsorption capacityOther chemical processesCatalyst activation/preparationUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical field of environmentally-friendly and chemical catalysts. The preparation method comprises the following steps: by taking medical stone, wollastonite, montmorillonoid, sylvine, nitratine and dolomite, reaming the carriers with lithium hypochlorite and bis(acetylacetone) beryllium, then adding octadecyl chloride trimethyl ammonium serving as a surfactant for activation treatment under ultrasonic action, putting the activated carriers into a hydrothermal reaction kettle for hydrothermal reaction together with borax and potassium sulfate which serve as composite mineralizers, isopropyl scandium oxide (III), tri(6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-octene diketone) dysprosium (III), tri[N,N-bis(trimethylsilane)amine] erbium and thulium trifluoromethane sulfonate (III) which serve as catalytic active auxiliary precursors and a dicyclopentadienyl titanium cyclo-replaced salicylic acid complex, L-aspartic acid molybdenum, a catechol ethanediamine tungsten complex and tetraammine dichloropalladium (II) which serve as catalytic active center component precursors under the action of bieicosyl dimethyl ammonium serving as an emulsifier, drying a reaction product to remove water, and firing the reaction product in a muffle furnace, thus obtaining the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107051497AEnhanced anti-toxicityHigh catalytic activityCatalyst carriersOther chemical processesUltrasound - actionChlorobenzene
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical field of environment protection and chemical engineering catalysts. The preparation method includes using perlite, albite, bentonite, polyhalite, nitratine and dolomite as carriers; subjecting the carriers to pore expanding through lithium hypochlorite and bis(acetylacetone)beryllium, and adding a surfactant 2, 6-di(diethylamino methyl)-4-nonyl phenol-chlorobenzene quaternary ammonium salt, activating under ultrasonic action; enabling the activated carriers to be in hydrothermal reaction with composite mineralizing agent borax and potassium sulfate, catalytic active auxiliary precursor 1, 1, 1-trifluoroacetylacetone neodymium, holmium oxalate decahydrate, tri[N, N-bis(trimethylsilane)amine]erbium, andtrifluoromethane thulium sulfonate (III) and catalytic activity central precursor cobalt gluconate, nickel citrate, potassium tetrachloroaurate and hexanitroso rhodium trisodium in a hydrothermal reaction kettle under action of an emulsifier diethyl maleate bis(chlorinated dodecyl dimethyl ammonium); drying for removing water; firing in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Chemiluminescence enhancer and its preparation method and application in detecting nitrite
ActiveCN111337479BEnhanced chemiluminescent intensityEasy to detectChemiluminescene/bioluminescenceFluorescence/phosphorescenceRare-earth elementNitrite
The embodiment of this specification relates to the technical field of nitrite detection, in particular to a chemiluminescent enhancer, its preparation method and its use in the detection of nitrite. Wherein, the chemiluminescence enhancer includes nano-sized rare earth fluoride, the rare earth fluoride is doped with cerium element, and the valence state of the cerium element is positive trivalent; wherein, the cerium element and the rare earth fluoride in the rare earth fluoride The rare earth elements are different elements, and the molar ratio of the cerium element to the rare earth element in the rare earth fluoride is (1:10)-(3:10). The chemiluminescence enhancer can enhance the chemiluminescence in the process of nitrite detection, thereby improving the detection effect of nitrite by chemiluminescence.
Owner:CHINA UNIV OF GEOSCIENCES (BEIJING)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com