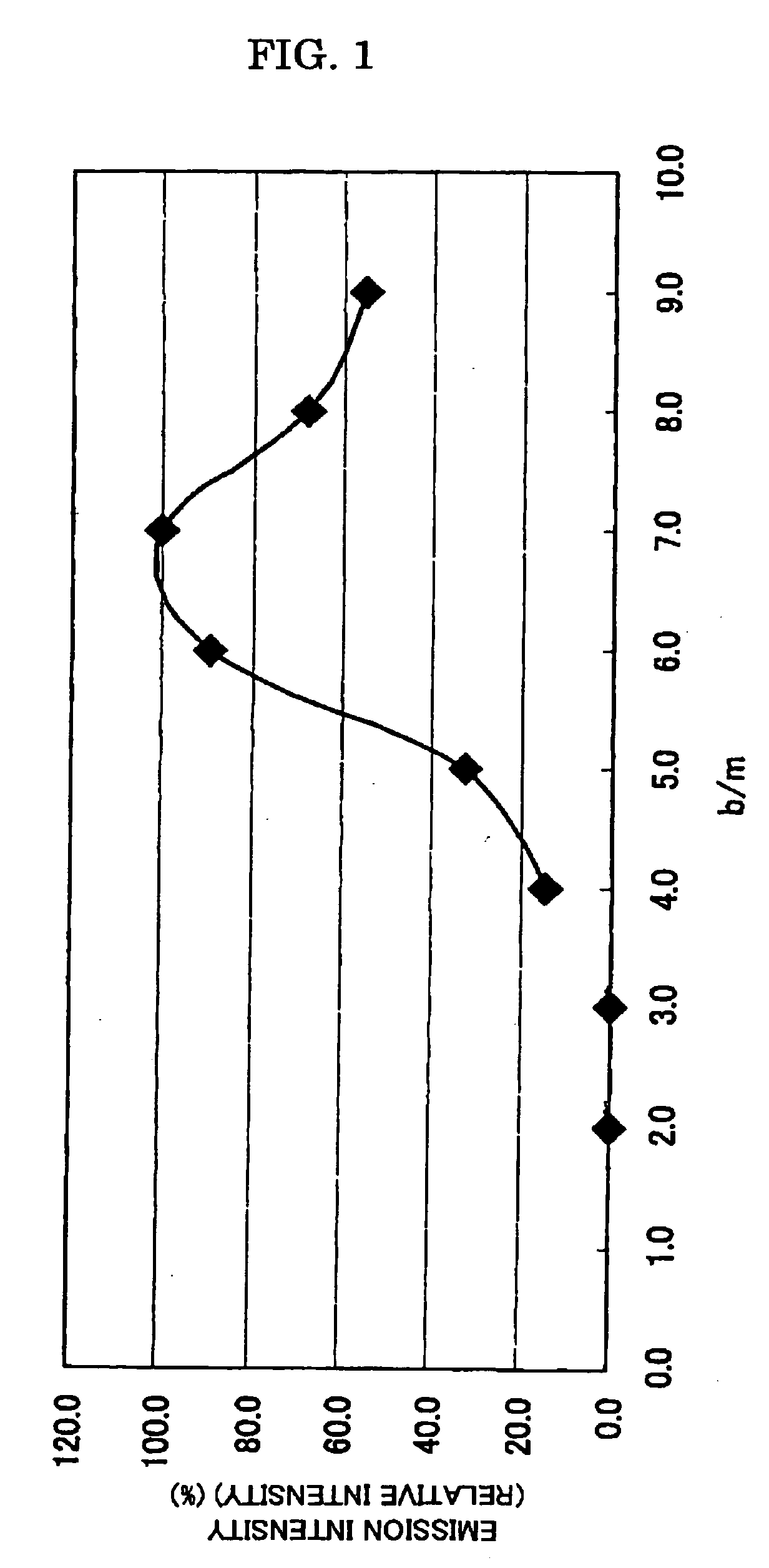
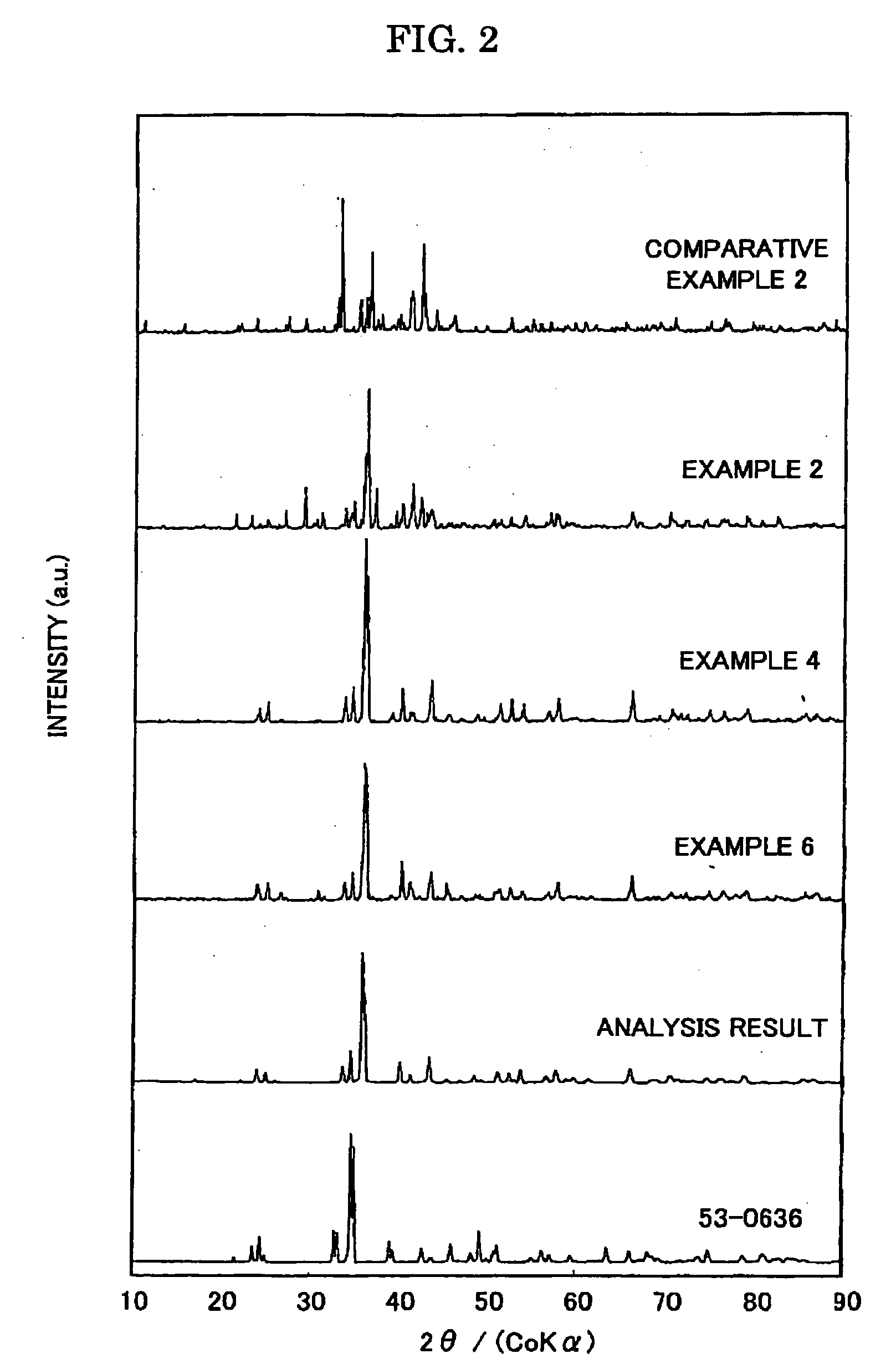
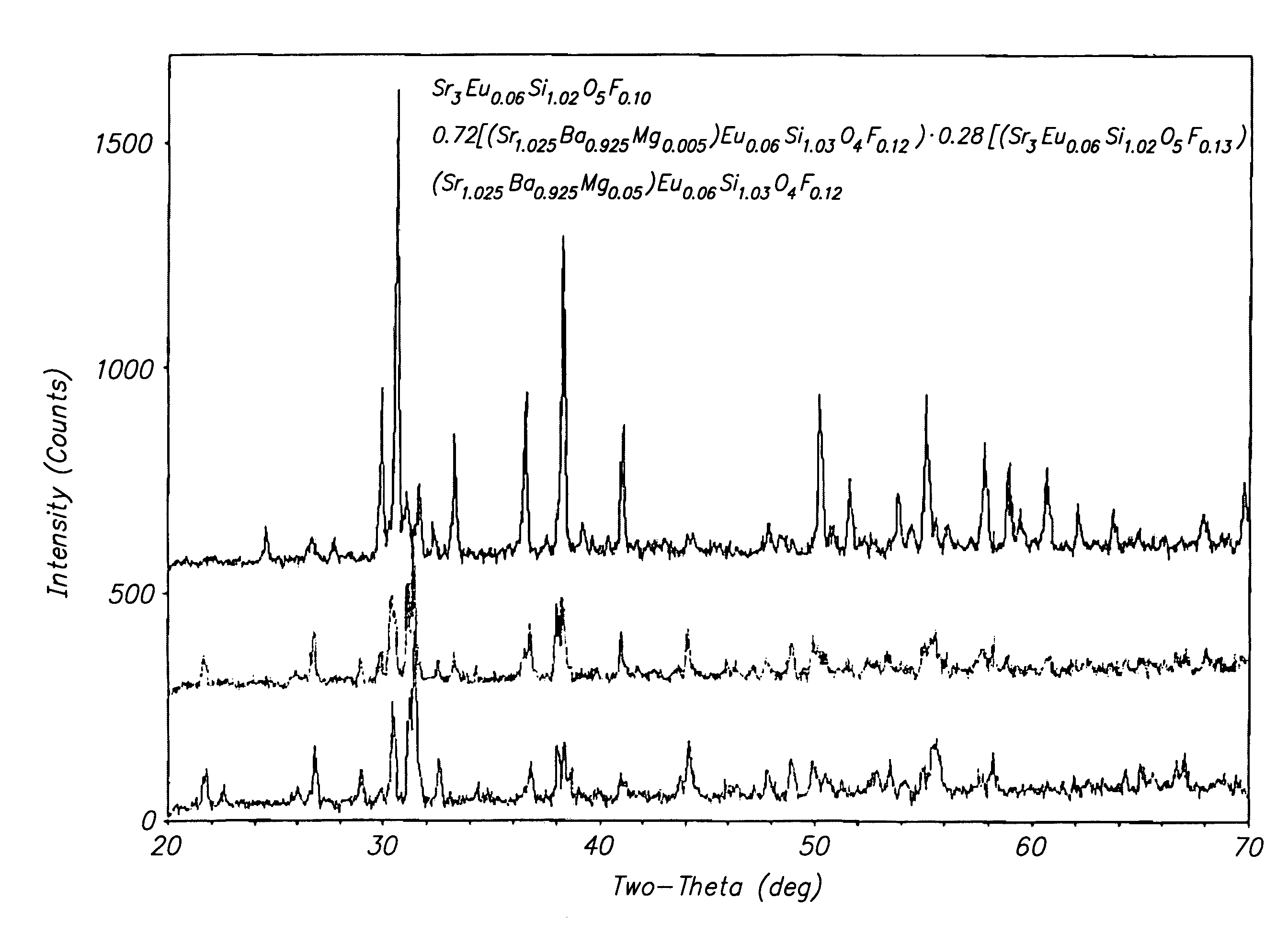
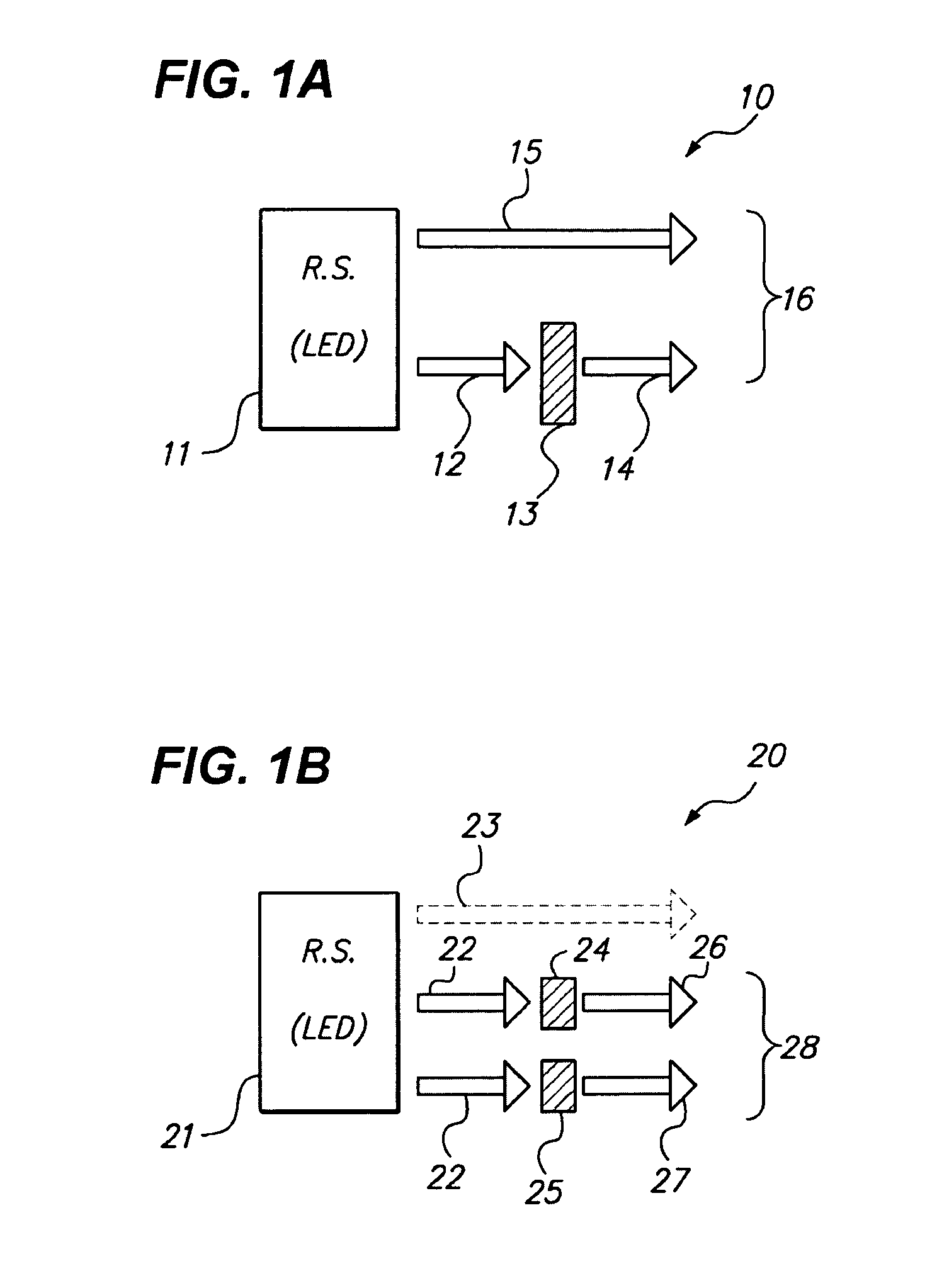
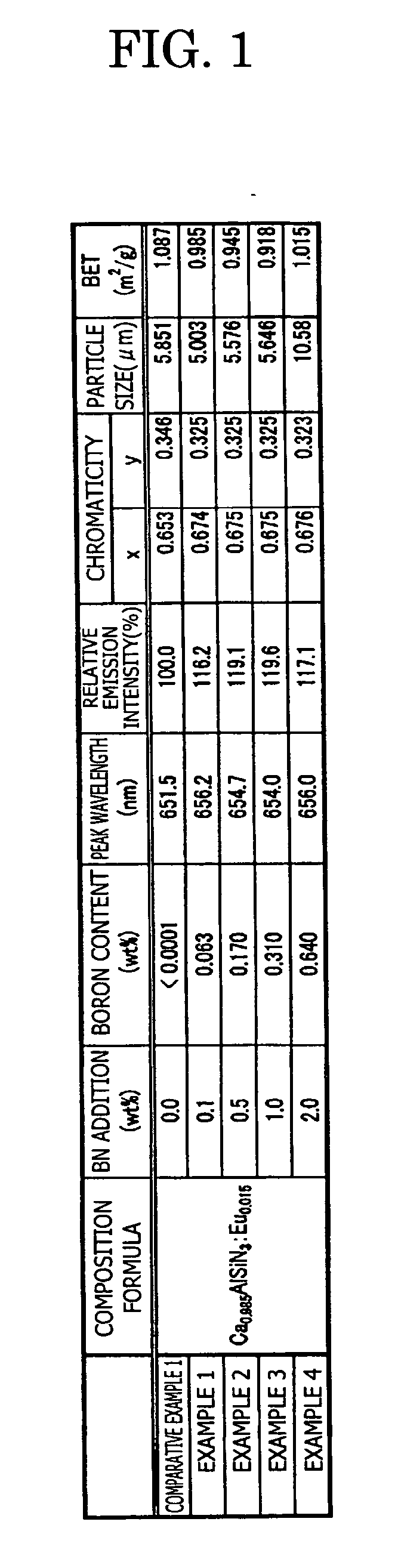
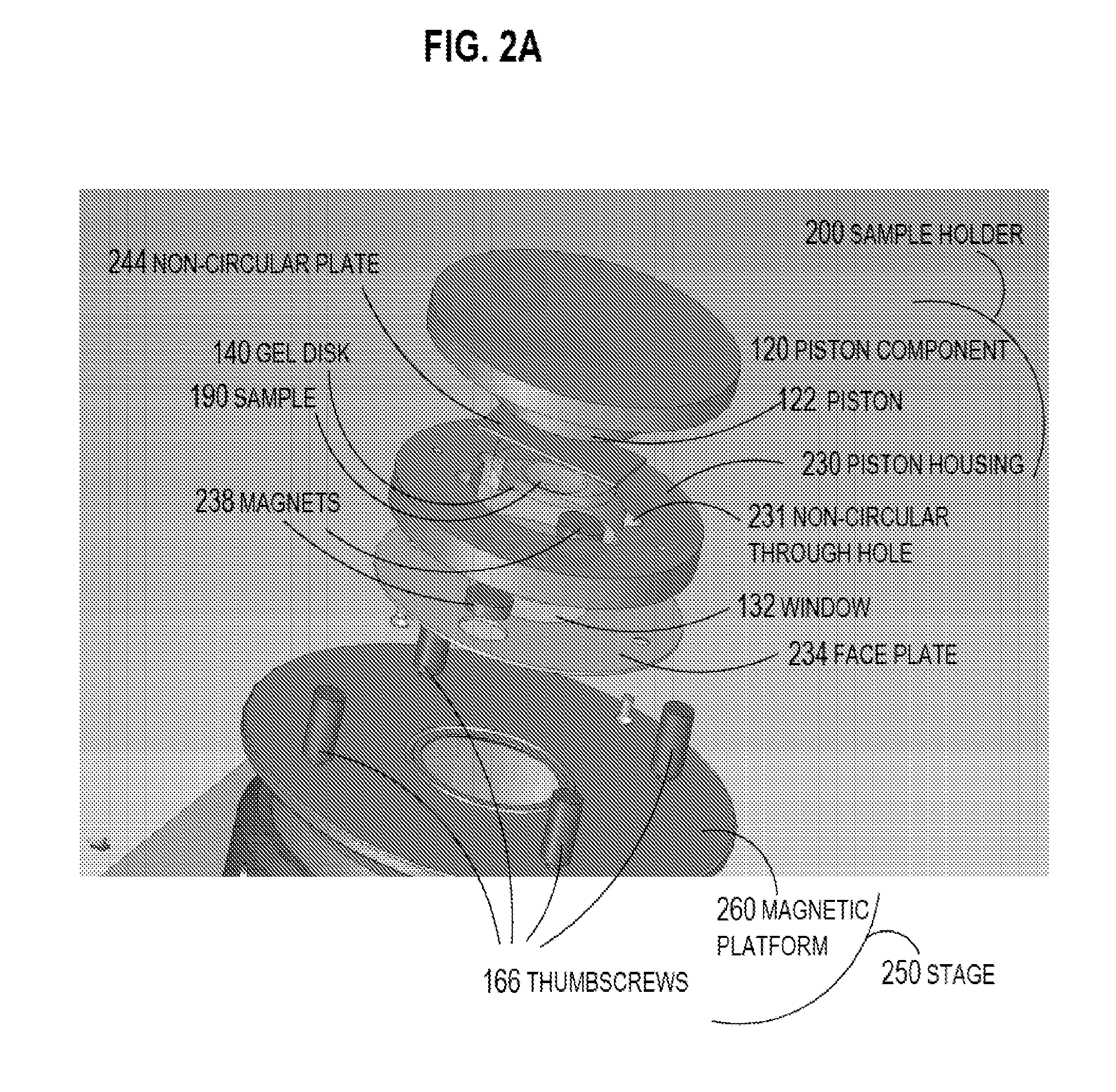
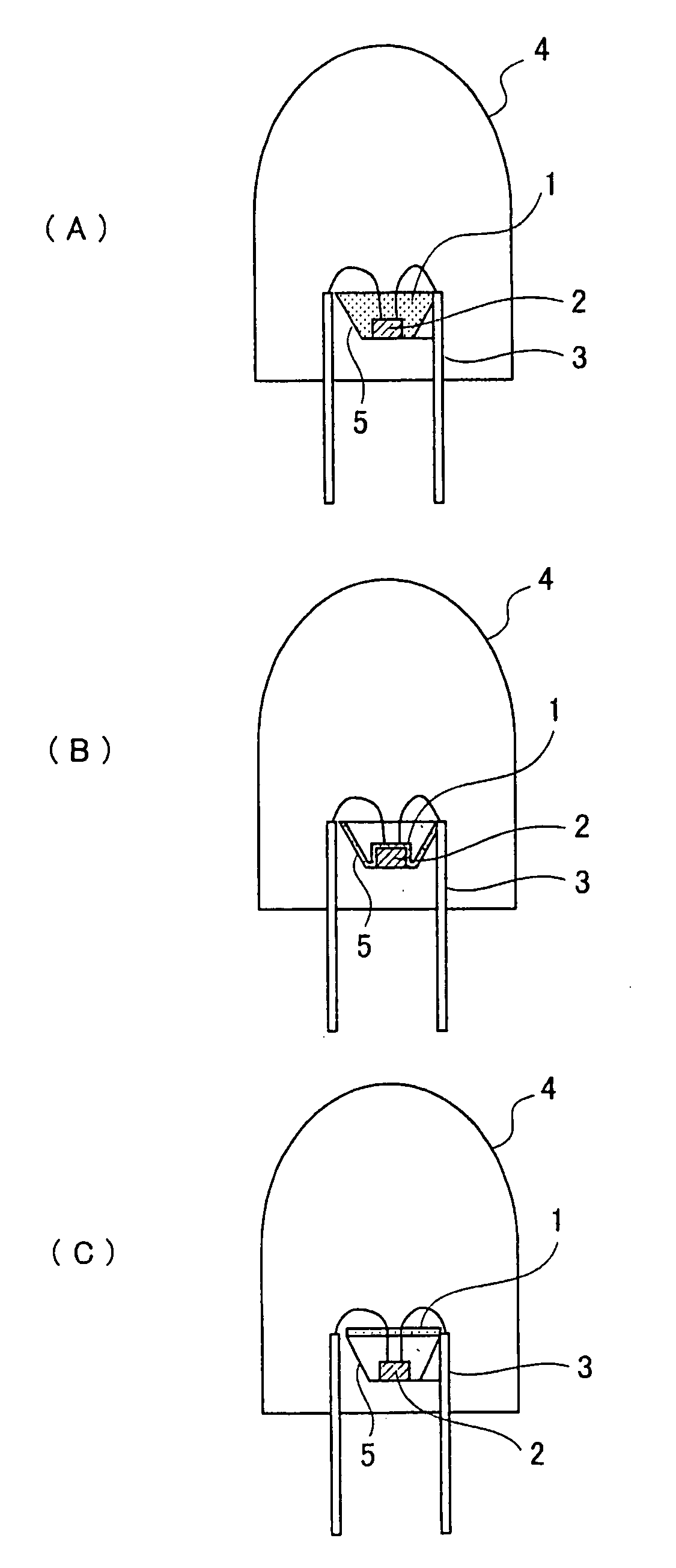
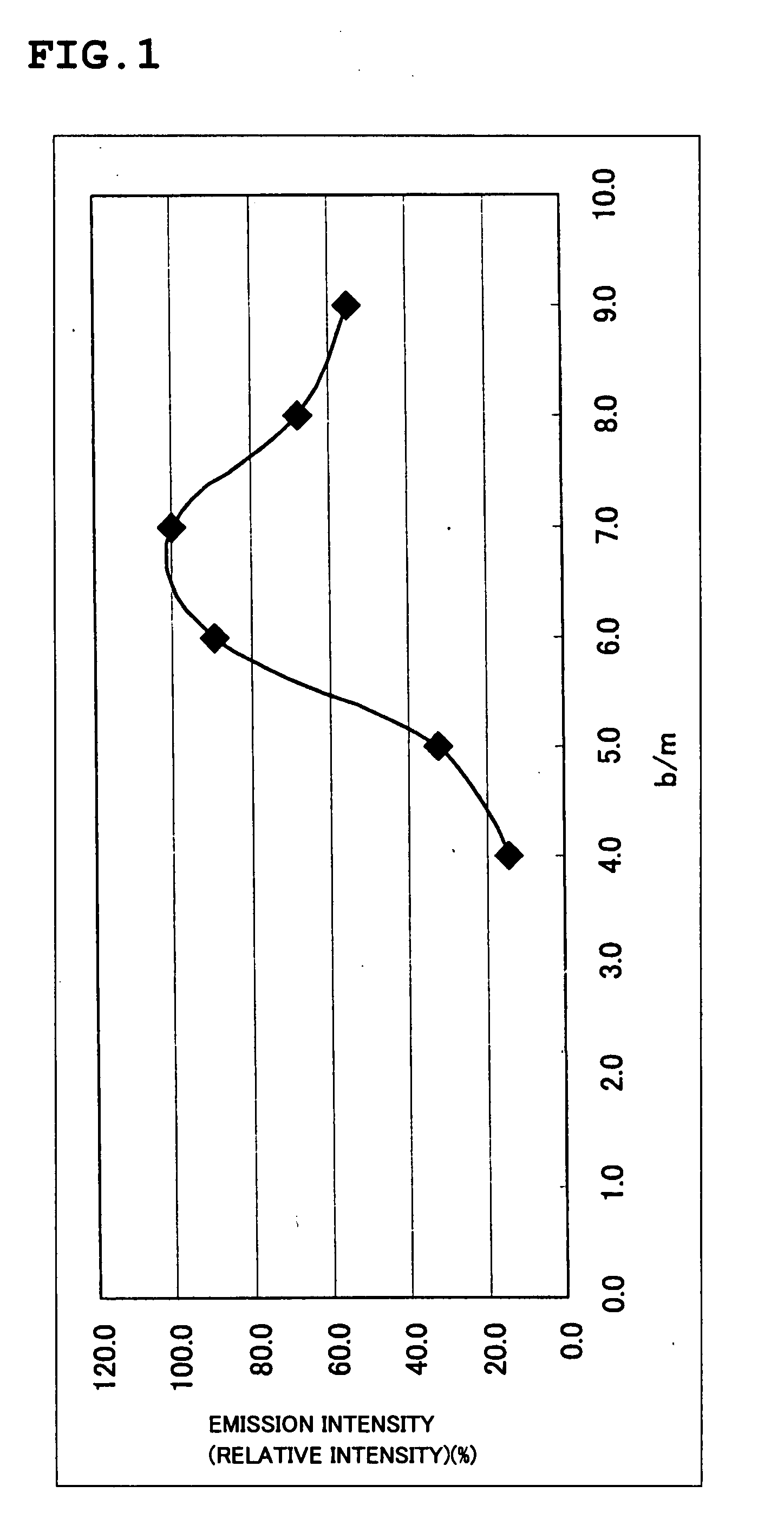
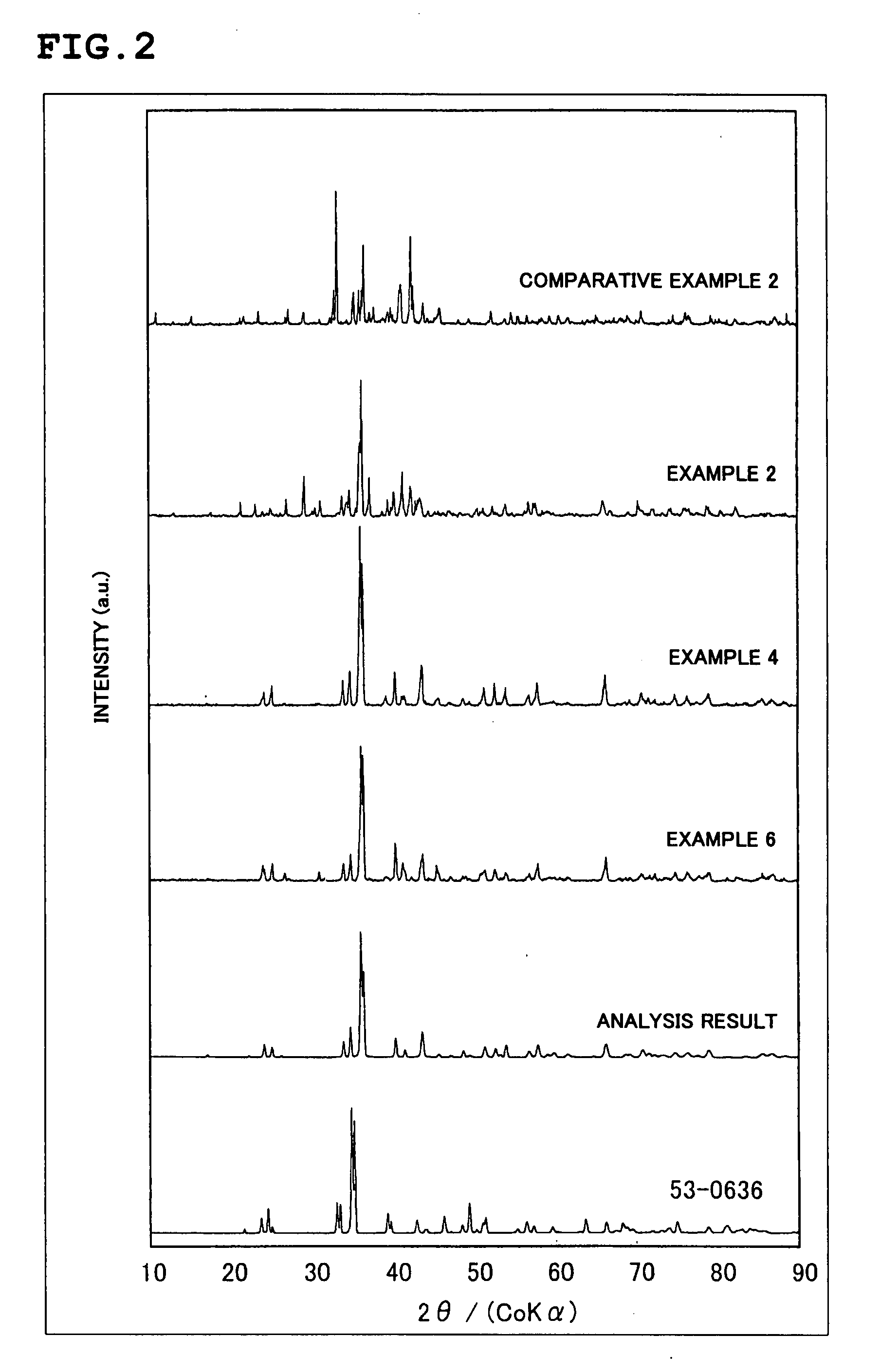
Patents
Literature
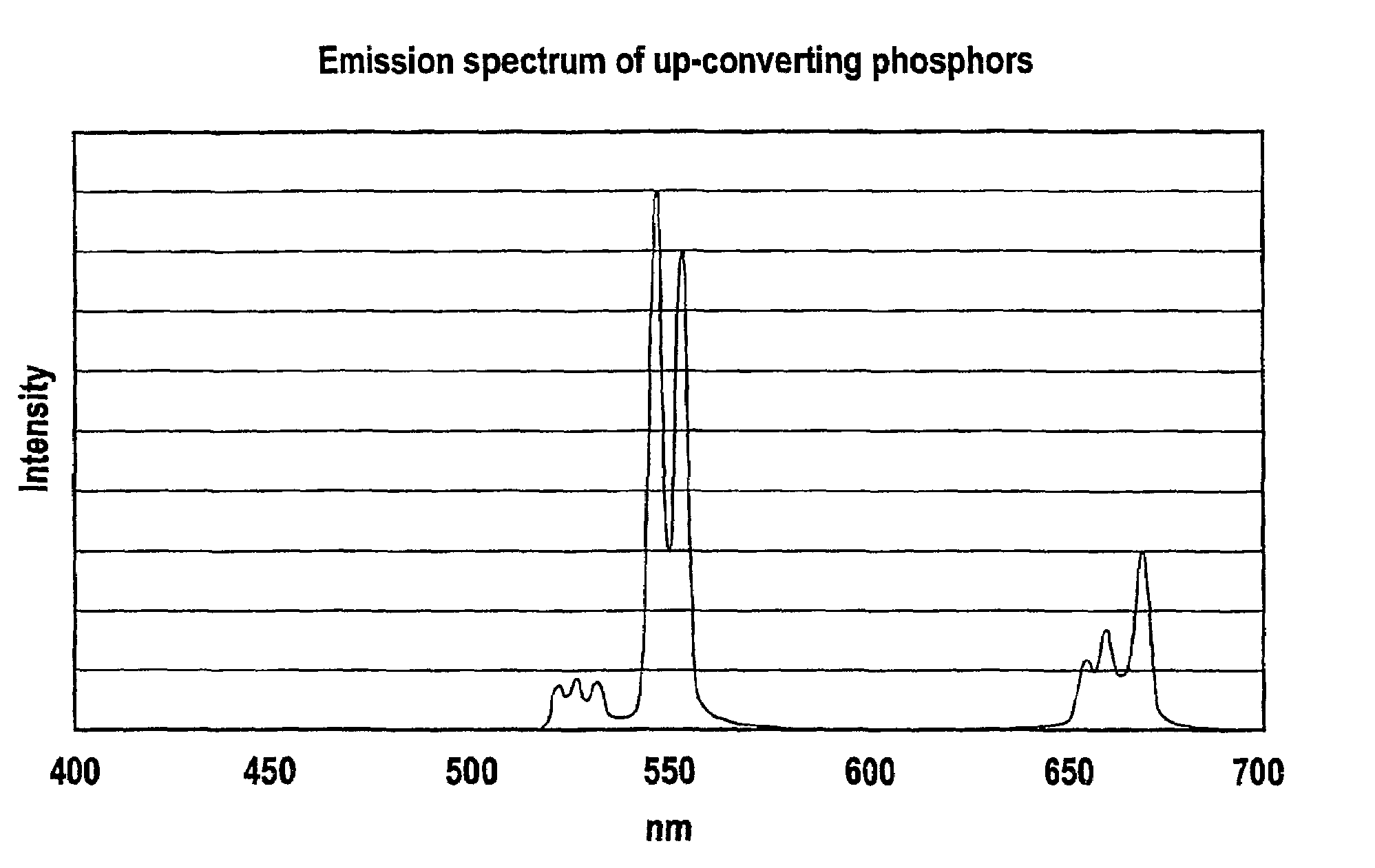
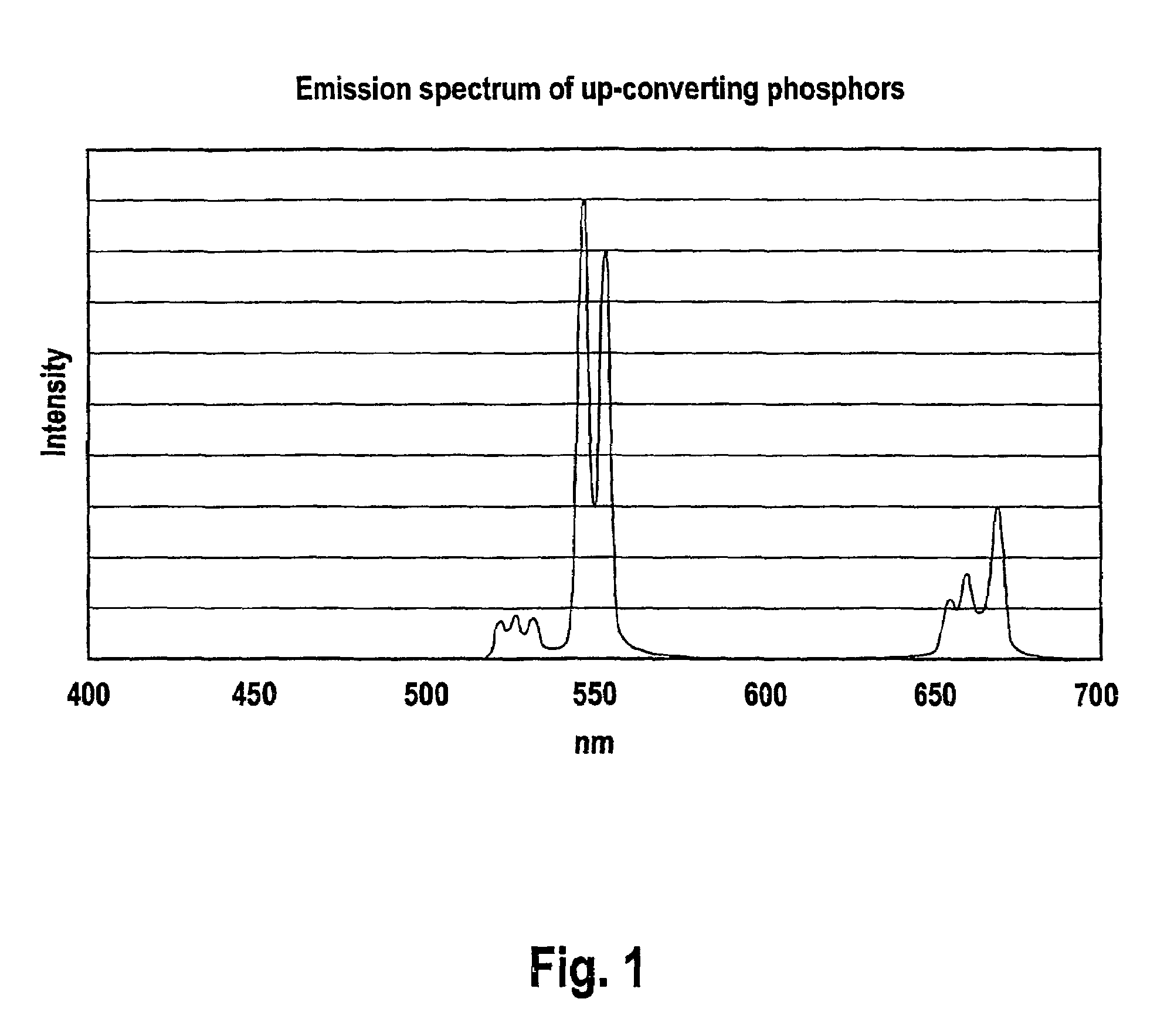
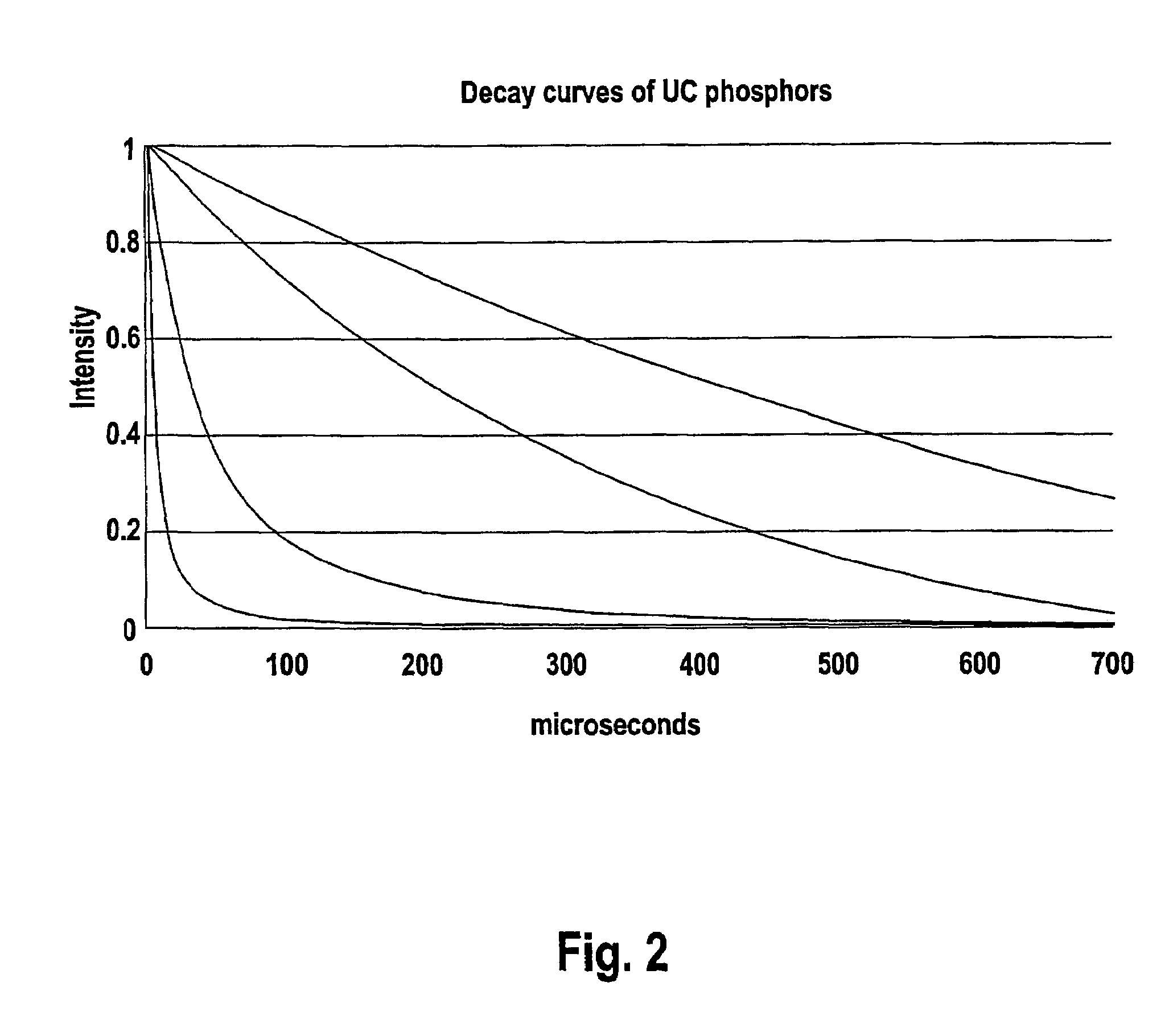
990 results about "Emission intensity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
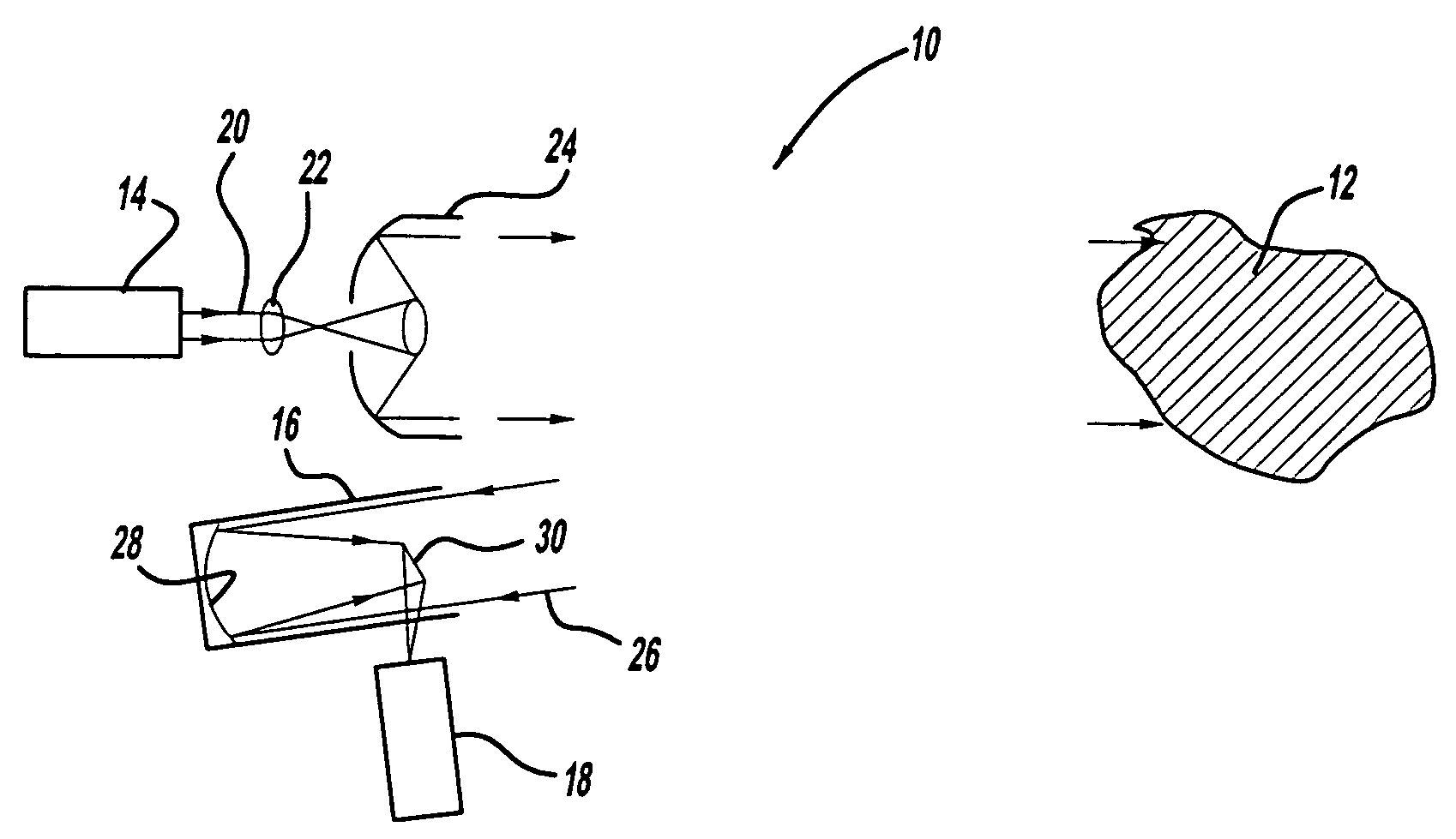
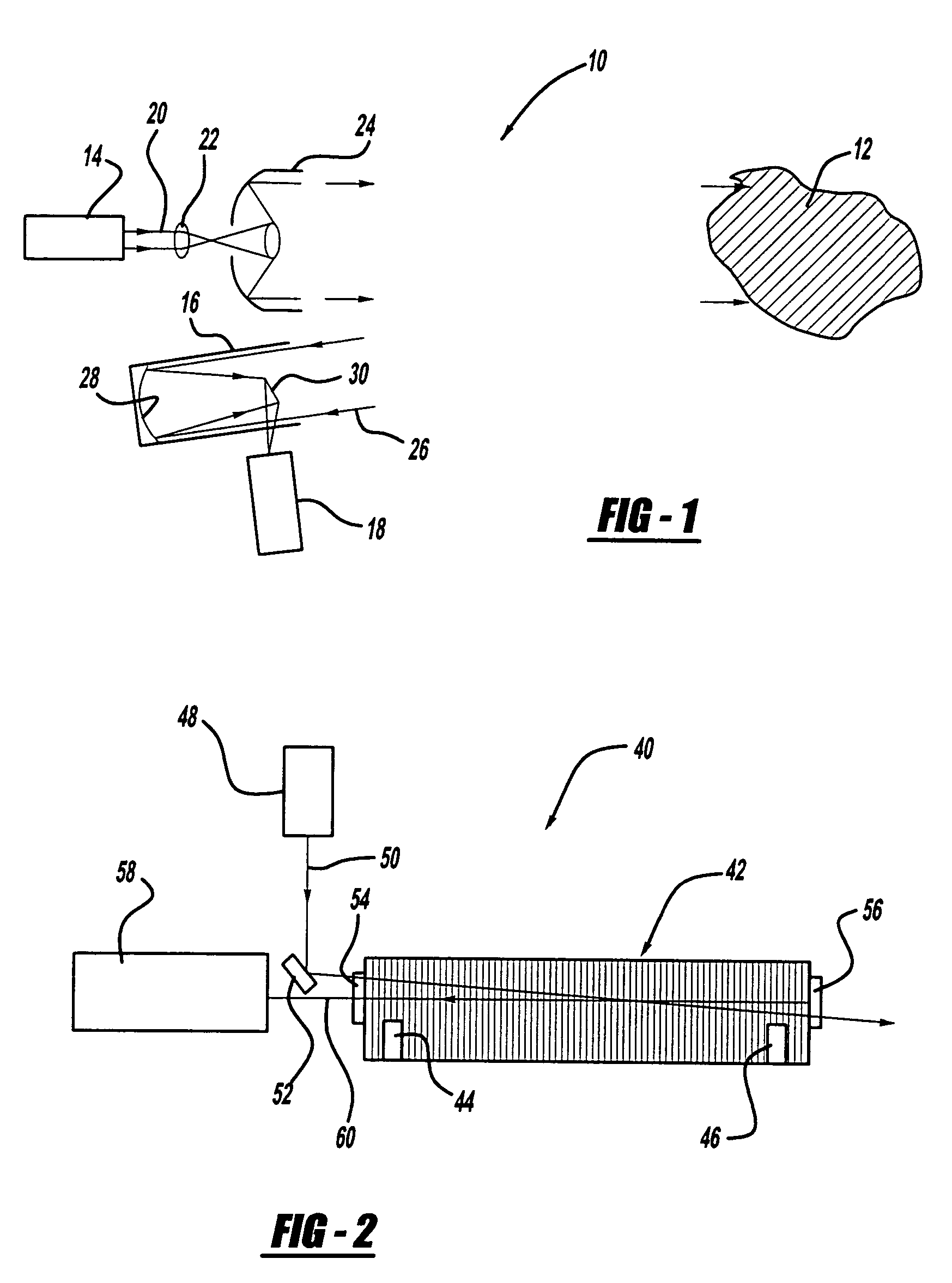
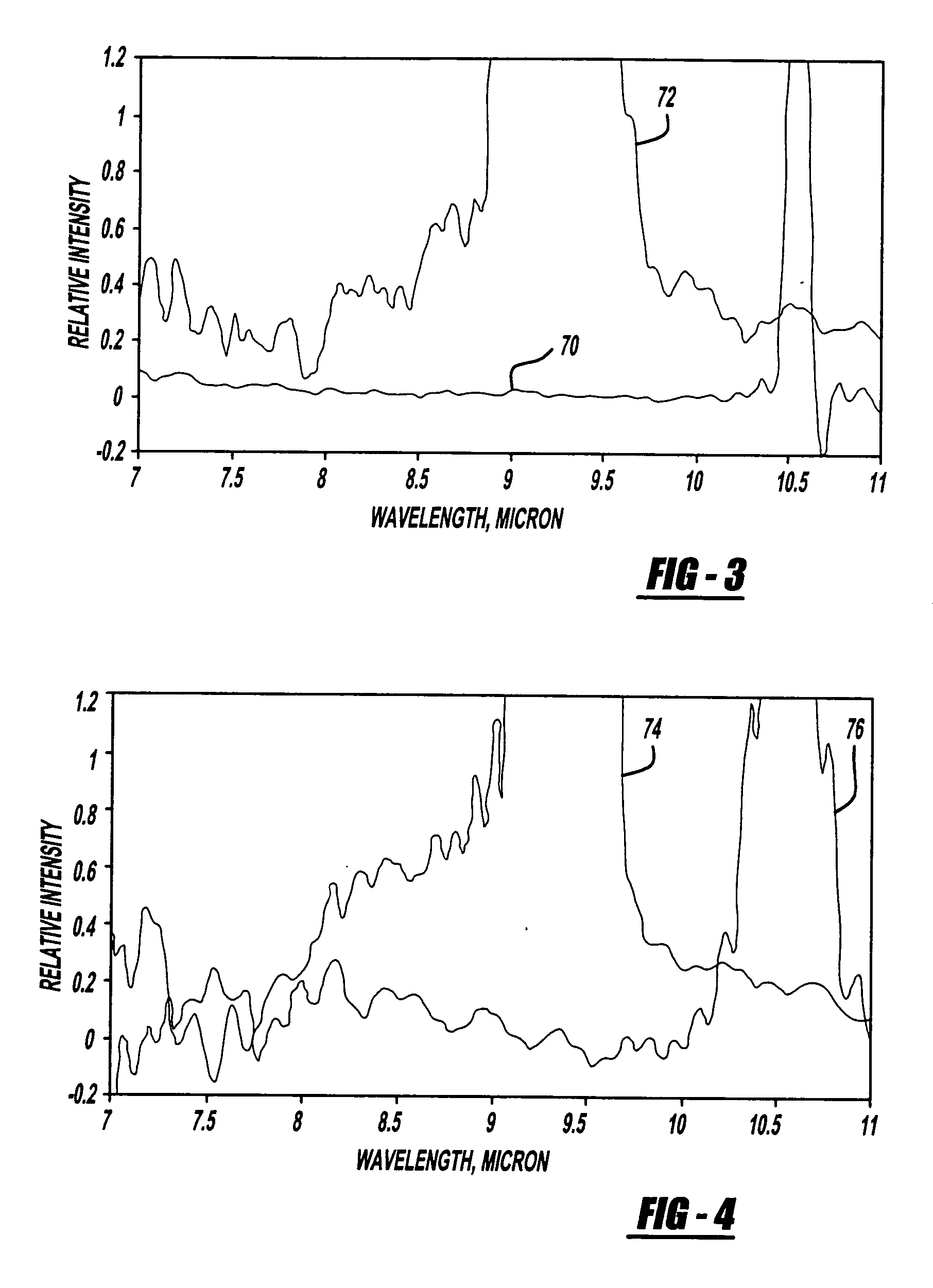
An emission intensity (also carbon intensity, C.I.) is the emission rate of a given pollutant relative to the intensity of a specific activity, or an industrial production process; for example grams of carbon dioxide released per megajoule of energy produced, or the ratio of greenhouse gas emissions produced to gross domestic product (GDP). Emission intensities are used to derive estimates of air pollutant or greenhouse gas emissions based on the amount of fuel combusted, the number of animals in animal husbandry, on industrial production levels, distances traveled or similar activity data. Emission intensities may also be used to compare the environmental impact of different fuels or activities. In some case the related terms emission factor and carbon intensity are used interchangeably. The jargon used can be different, for different fields/industrial sectors; normally the term "carbon" excludes other pollutants, such as particulate emissions. One commonly used figure is carbon intensity per kilowatt-hour (CIPK), which is used to compare emissions from different sources of electrical power.
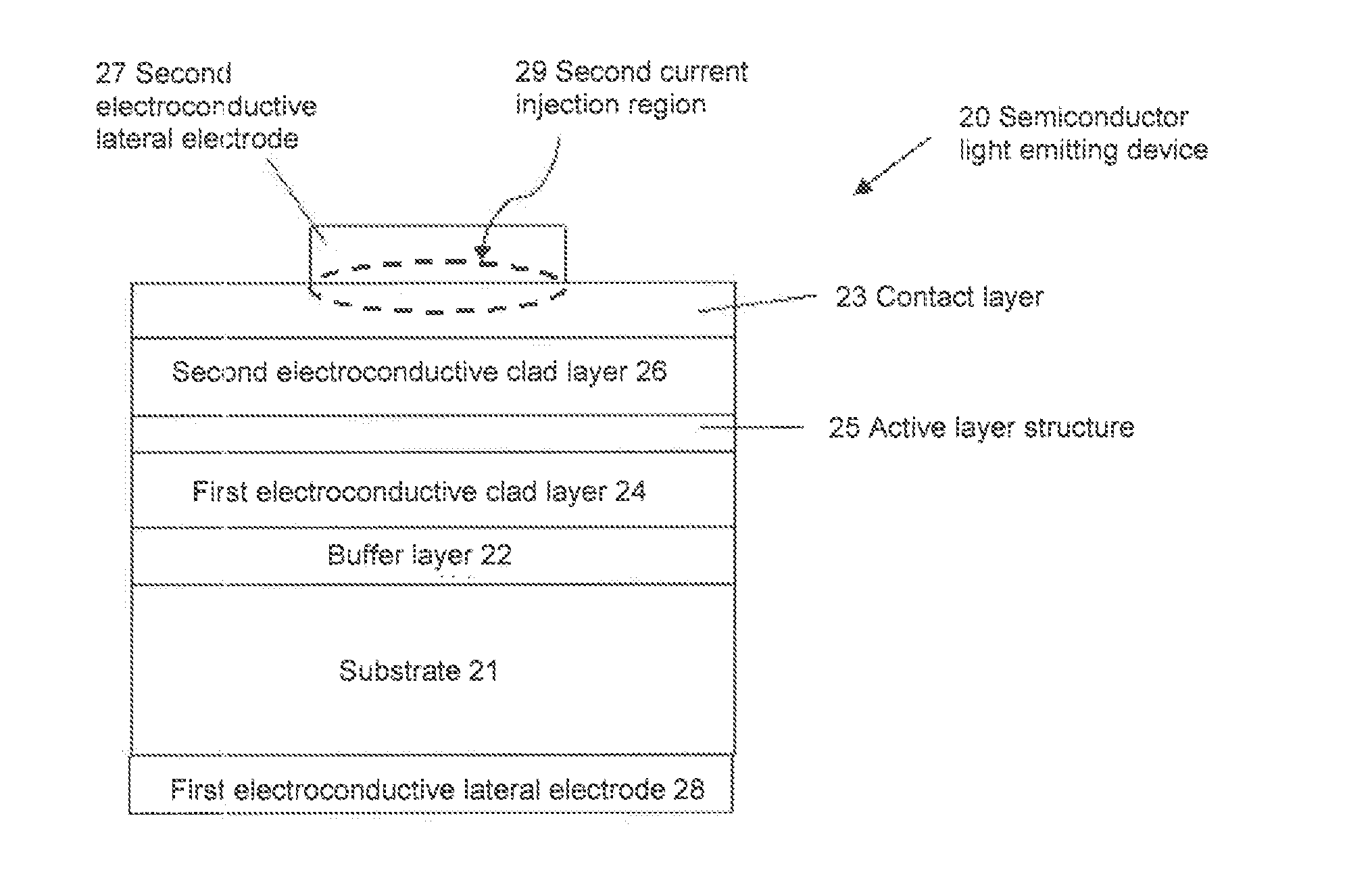
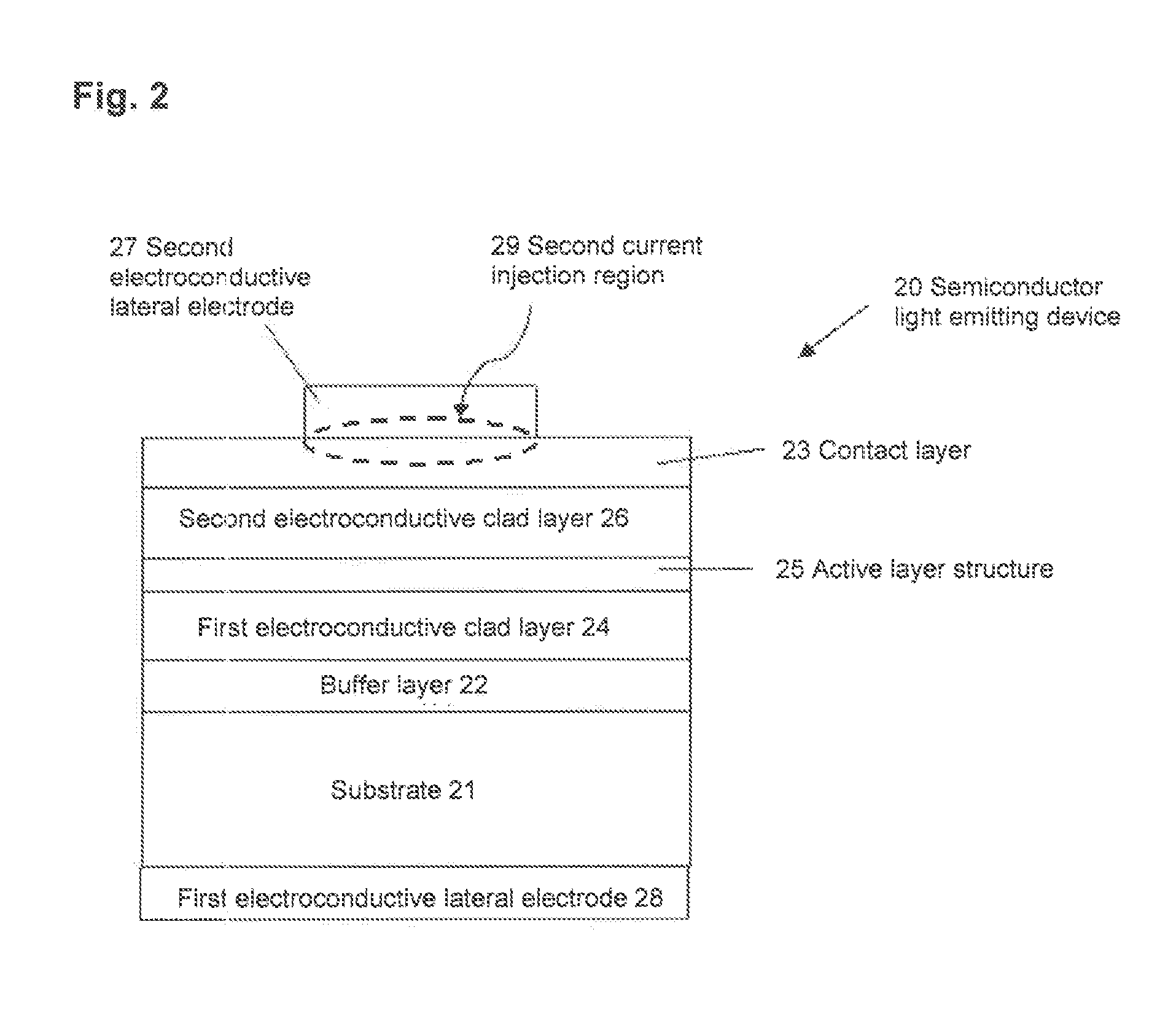
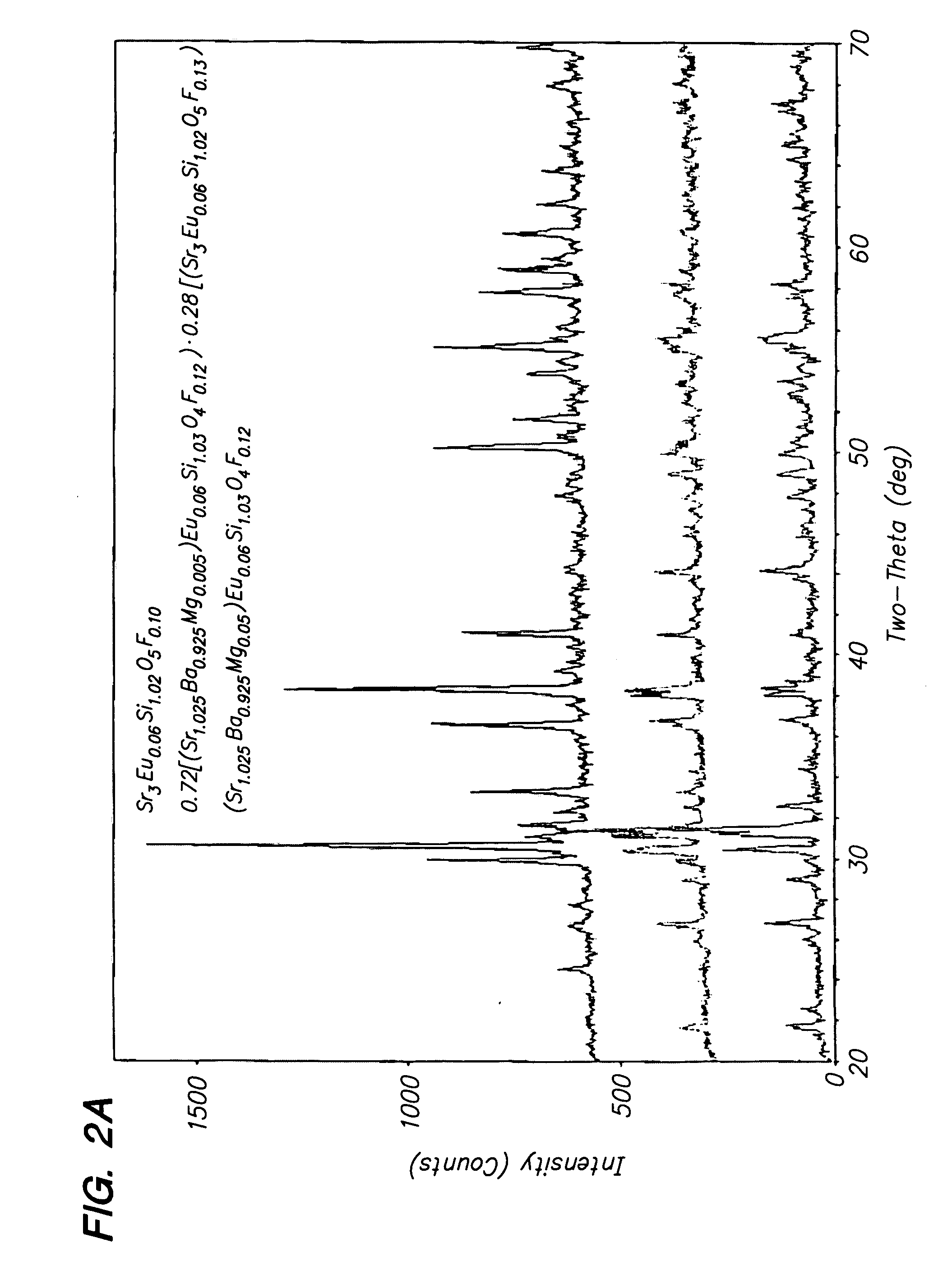
Semiconductor light emitting device, backlight, color image display device and phosphor to be used for them
ActiveUS20100142189A1Broad color reproducibilityImprove emission efficiencyPoint-like light sourceElectroluminescent light sourcesColor imageLuminous intensity
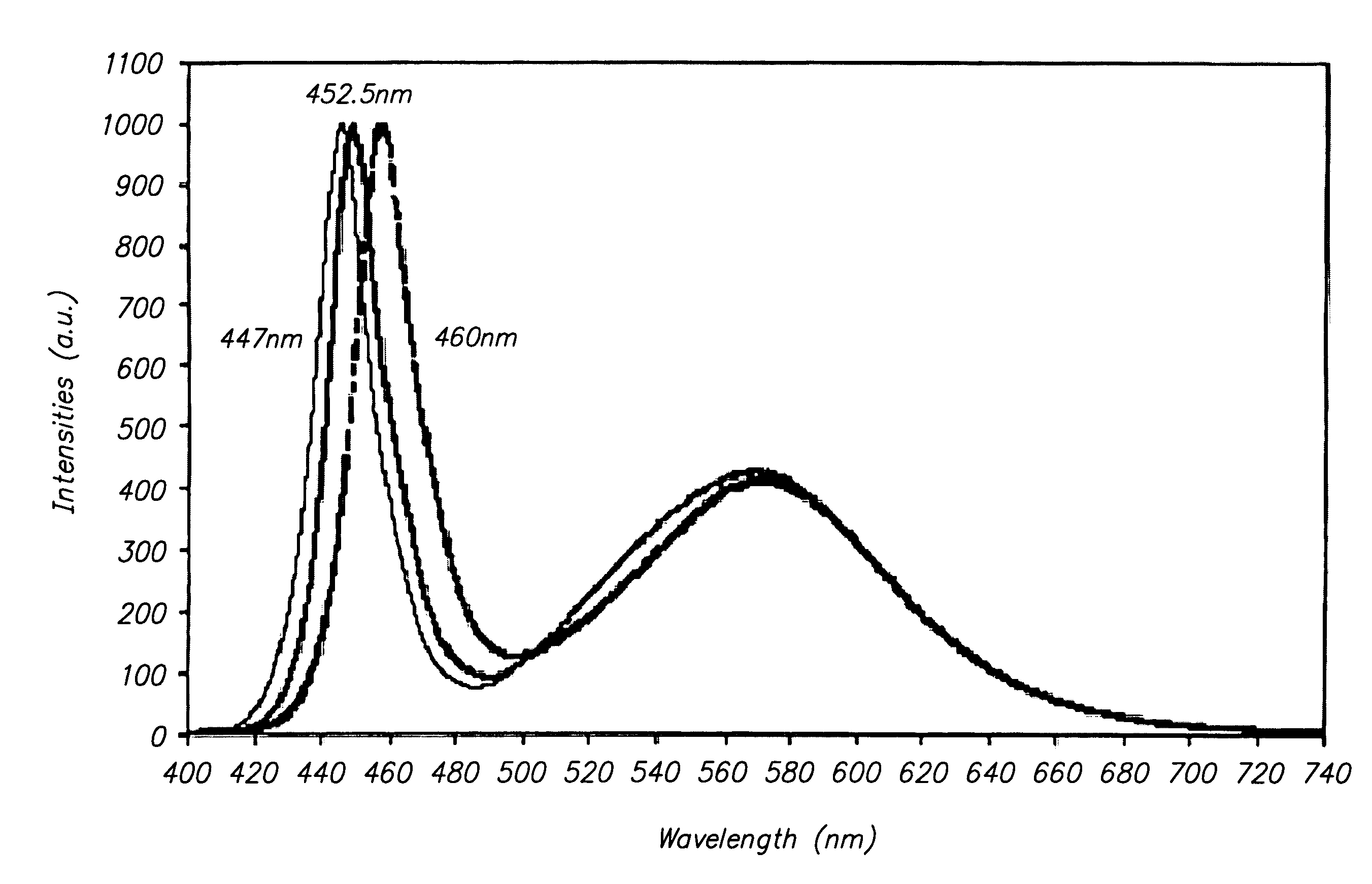
To provide a semiconductor light emitting device which is capable of accomplishing a broad color reproducibility for an entire image without losing brightness of the entire image.A light source provided on a backlight for a color image display device has a semiconductor light emitting device comprising a solid light emitting device to emit light in a blue or deep blue region or in an ultraviolet region and phosphors, in combination. The phosphors comprise a green emitting phosphor and a red emitting phosphor. The green emitting phosphor and the red emitting phosphor are ones, of which the rate of change of the emission peak intensity at 100° C. to the emission intensity at 25° C., when the wavelength of the excitation light is 400 nm or 455 nm, is at most 40%.
Owner:CITIZEN ELECTRONICS CO LTD +1
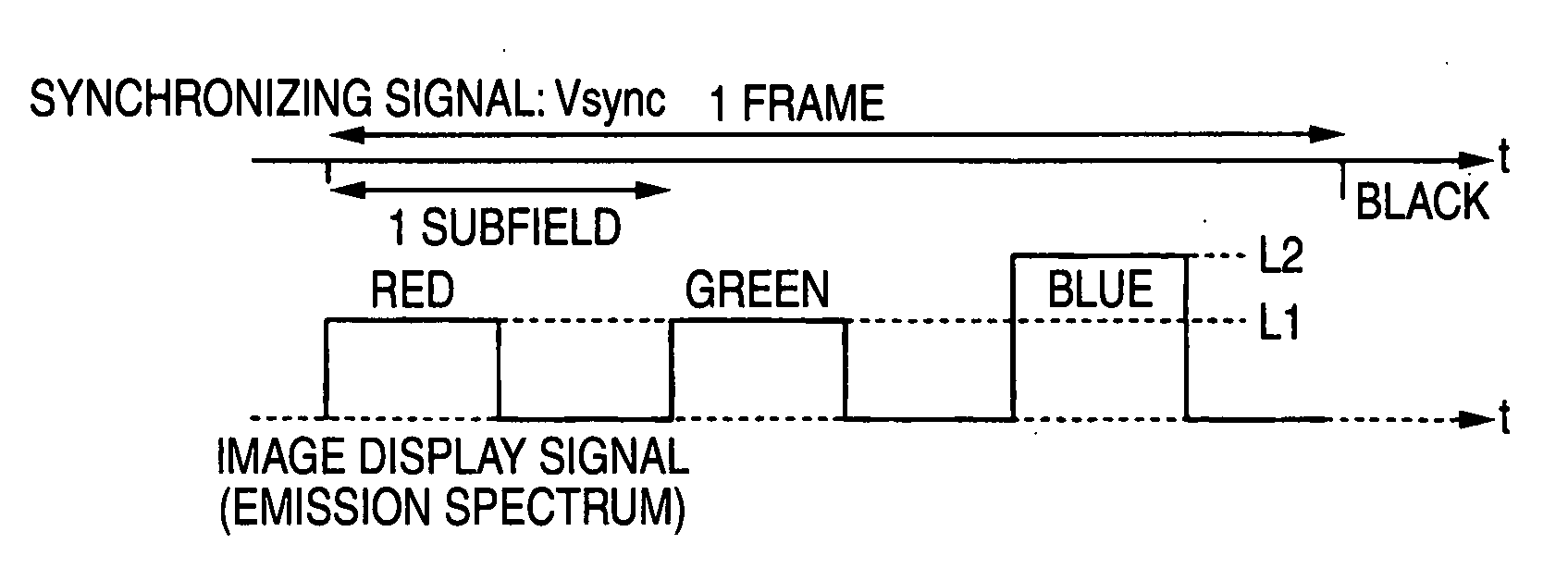
Liquid crystal display
InactiveUS20070222743A1Enhance displayed imageImprove image qualityStatic indicating devicesLuminous intensityLiquid-crystal display
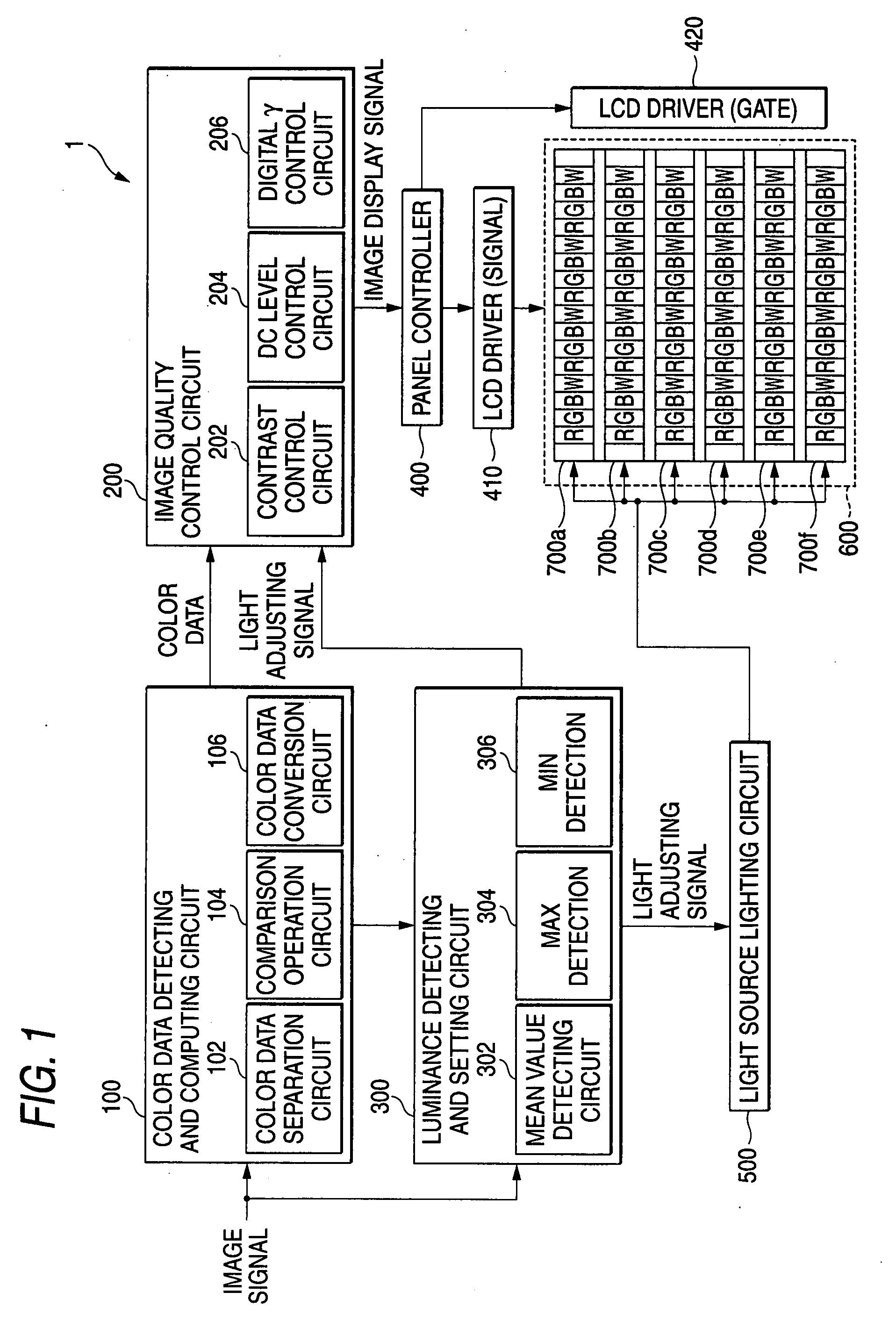
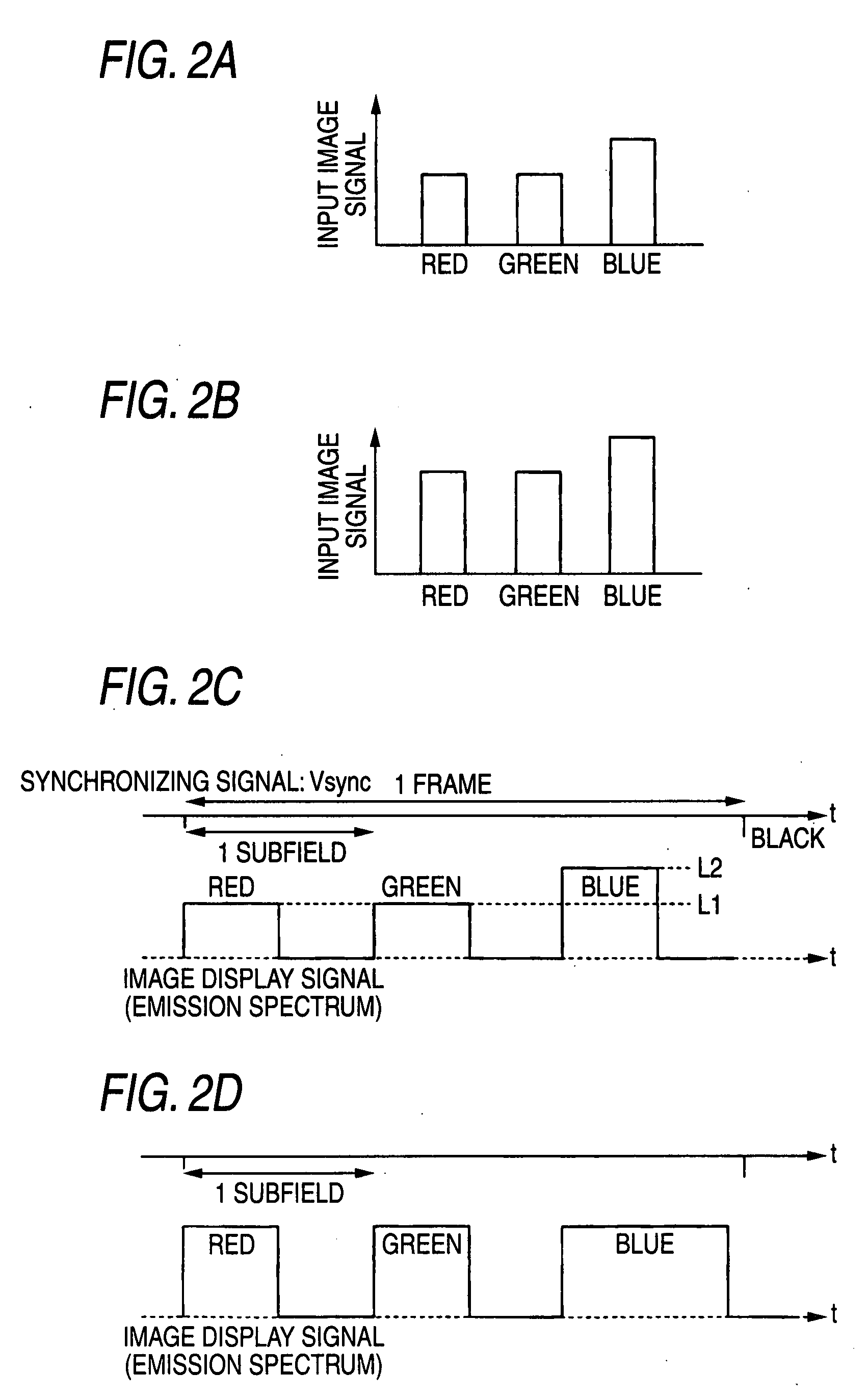
A liquid crystal display is provided and includes: a liquid crystal panel; light sources for illuminate light from M kinds of colors onto the liquid crystal panel; and a light source driving unit in which a one-frame period of an input image signal is divided into M or more subfields, and the light sources are sequentially driven in a time-sharing mode in correspondence with the subfields. The light source driving unit changes in correspondence with the input image signal at least one of an emission intensity and an emission period of a light source in a period of the subfield and the number of emission times of the light source during the one-frame period. Alternatively, a liquid crystal driving unit performs gradation control for changing a gradation characteristic independently with respect to each of the light sources, the gradation characteristic representing a relationship of emission intensity of each of the light sources with respect to the input image signal. The maximum luminance of a specific color in the subfield is thereby changed.
Owner:FUJIFILM CORP
Two-phase silicate-based yellow phosphor
InactiveUS20060261309A1Efficient at fluorescingSolve low luminous efficiencyDischarge tube luminescnet screensLamp detailsOxygenSilicon dioxide
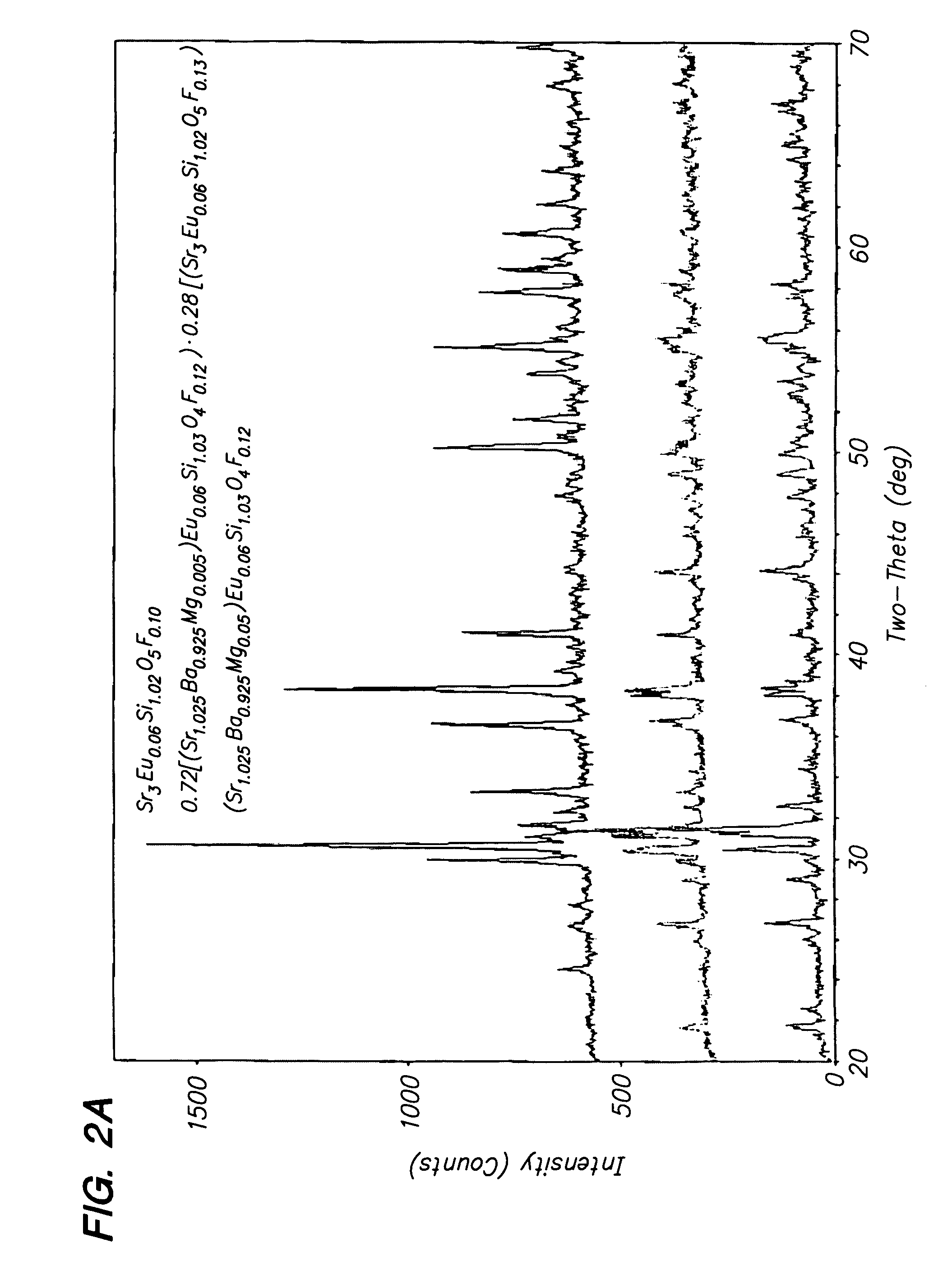
Novel two-phase yellow phosphors are disclosed having a peak emission intensity at wavelengths ranging from about 555 nm to about 580 nm when excited by a radiation source having a wavelength ranging from 220 nm to 530 nm. The present phosphors may be represented by the formula a[Srx(M1)1-x]zSiO4●(1-a)[Sry(M2)1-y]uSiO5:Eu2+D, wherein M1 and M2 are at least one of a divalent metal such as Ba, Mg, Ca, and Zn, the values of a, x, y, z and u follow the following relationships: 0.6≦a≦0.85; 0.3≦x≦0.6; 0.85≦y≦1; 1.5≦z≦2.5; 2.6≦u≦3.3; and Eu and D each range from 0.001 to about 0.5. D is an anion selected from the group consisting of F, Cl, Br, S, and N, and at least some of the D anion replaces oxygen in the host silicate lattice of the phosphor. The present yellow phosphors have applications in high brightness white LED illumination systems, LCD display panels, plasma display panels, and yellow LEDs and illumination systems.
Owner:INTEMATIX
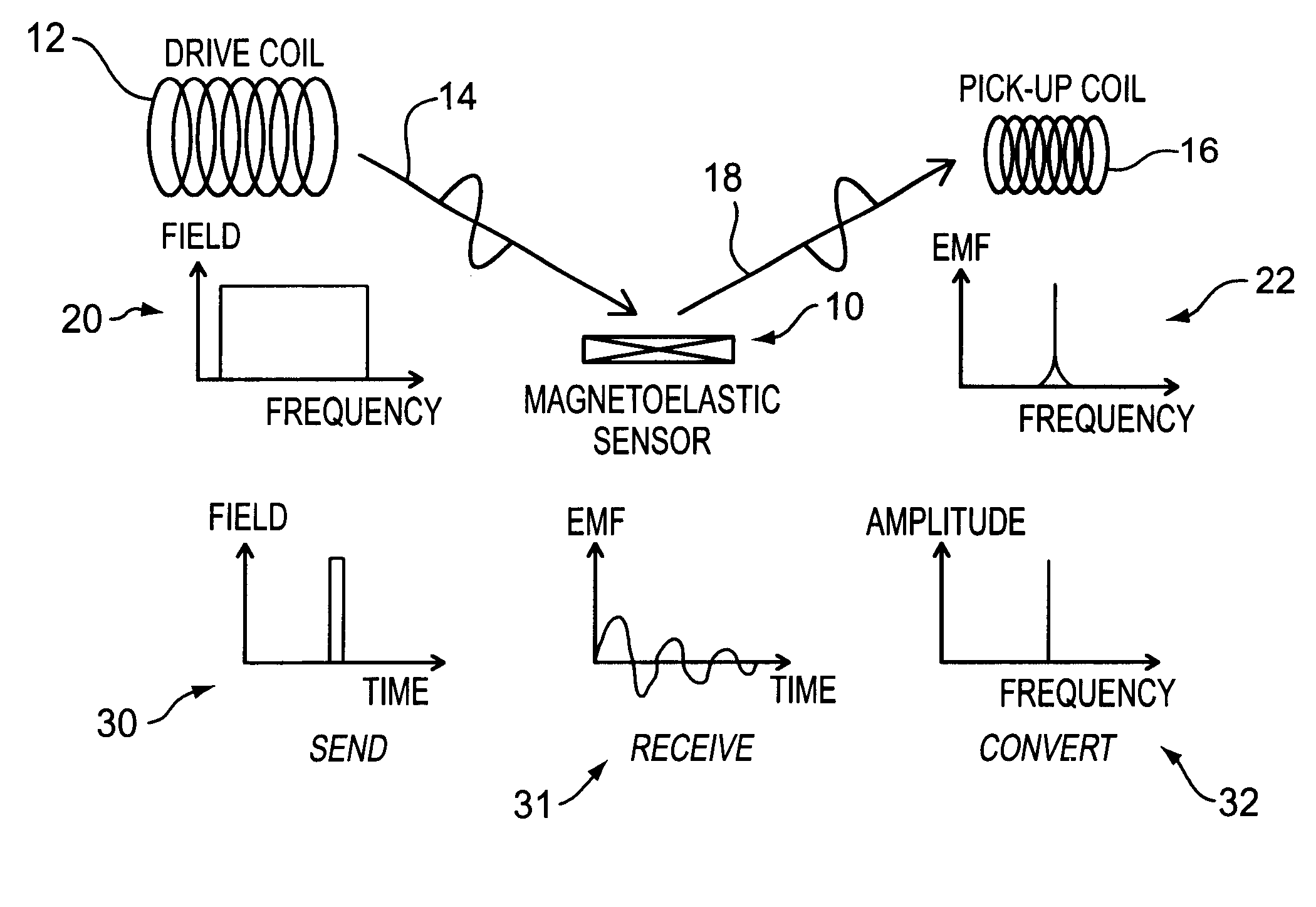
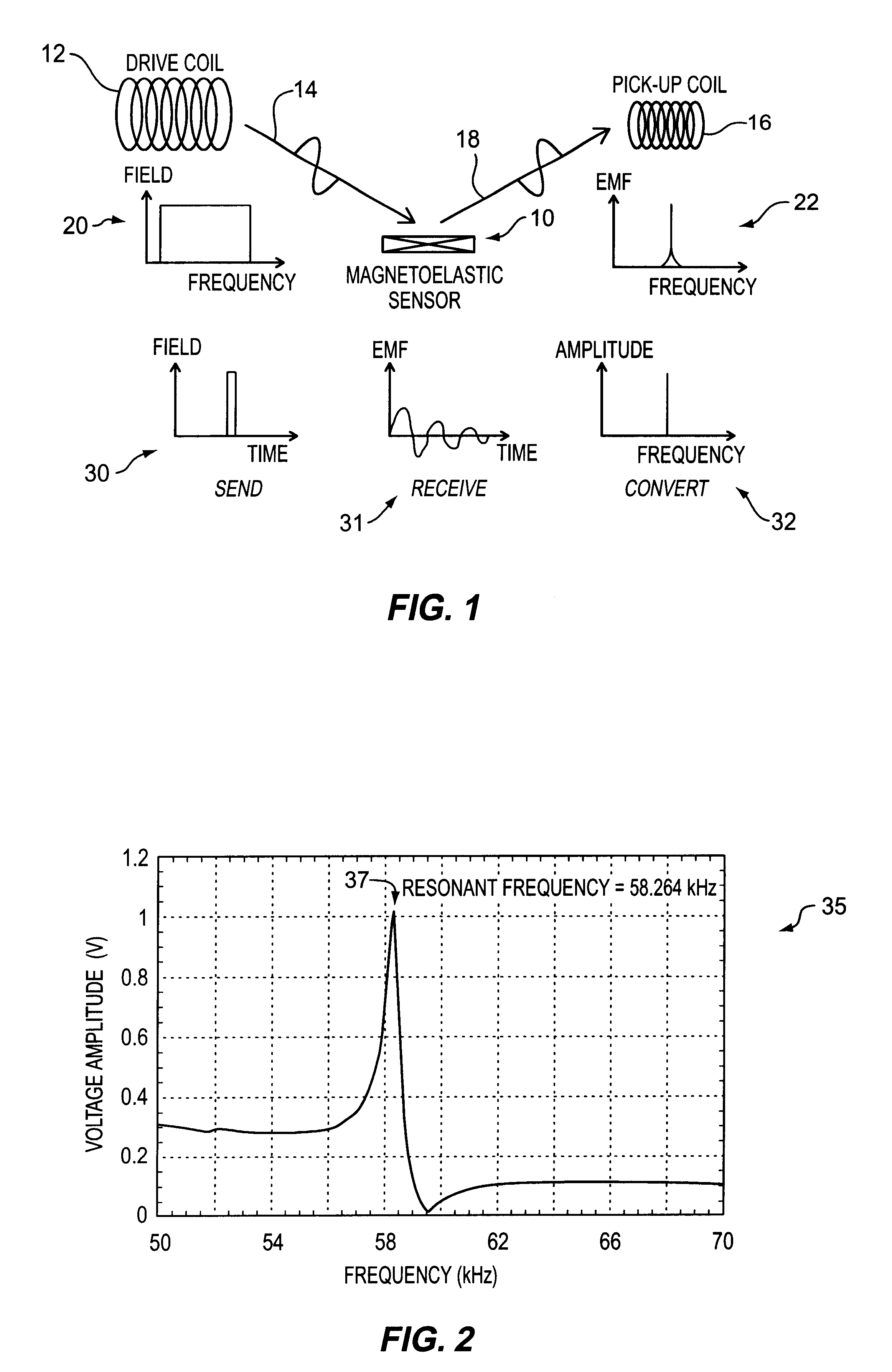
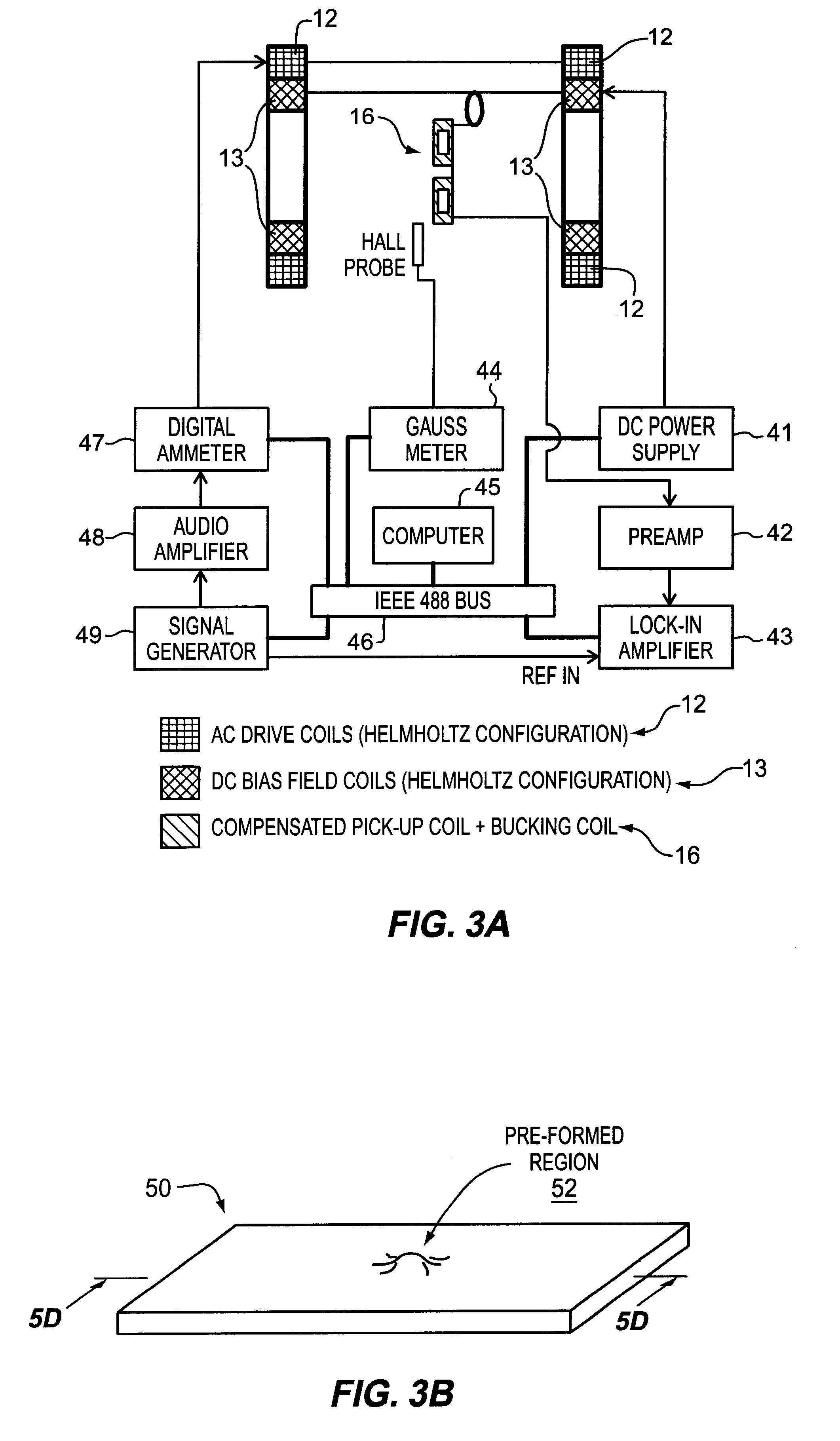
Magnetoelastic sensing apparatus and method for remote pressure query of an environment
InactiveUS6393921B1Fluid pressure measurement using inductance variationFluid pressure measurement using magnet displacementMagneto elasticPressure sense
A pressure sensing apparatus for operative arrangement within an environment, having: a sensor comprising a hermetically-sealed receptacle, at least one side of which has an flexible membrane to which a magnetically hard element is attached. Enclosed within the receptacle is a magnetostrictive element that vibrates in response to a time-varying magnetic field. Also included is a receiver to measure a plurality of successive values for magneto-elastic emission intensity of the sensor taken over an operating range of successive interrogation frequencies to identify a resonant frequency value for the sensor. Additional features include: (a) the magnetically hard element may be adhered to an inner or outer side of, or embedded within, the membrane; (b) the magnetostrictive element can include one or more of a variety of different pre-formed, hardened regions; (c) the magneto-elastic emission may be a primarily acoustic or electromagnetic emission; and (d) in the event the time-varying magnetic field is emitted as a single pulse or series of pulses, the receiver unit can detect a transitory time-response of the emission intensity of each pulse (detected after a threshold amplitude value for the transitory time-response is observed). A Fourier transform of the time-response can yield results in the frequency domain. Also, an associated method of sensing pressure of an environment is included that uses a sensor having a magnetostrictive element to identify a magneto-elastic resonant frequency value therefore. Using the magneto-elastic resonant frequency value identified, a value for the pressure of the environment can be identified.
Owner:UNIV OF KENTUCKY RES FOUND
Two-phase yellow phosphor with self-adjusting emission wavelength
InactiveUS20080036364A1Discharge tube luminescnet screensElectroluminescent light sourcesPhosphorLength wave
Disclosed herein are “smart” phosphor compositions capable of regulating the chromaticity of their emission to substantially constant values even with variations in the excitation radiation they receive to induce photoluminescence. One phosphor of the smart composition demonstrates an increase in emission intensity increases as the wavelength of the excitation radiation is increased. The other phosphor shows a decrease in emission intensity with increasing excitation wavelength. Constant chromaticity in this context is defined as a change in CIE x or y coordinate of less than about five percent over a 10 nm range of excitation wavelengths.
Owner:INTEMATIX
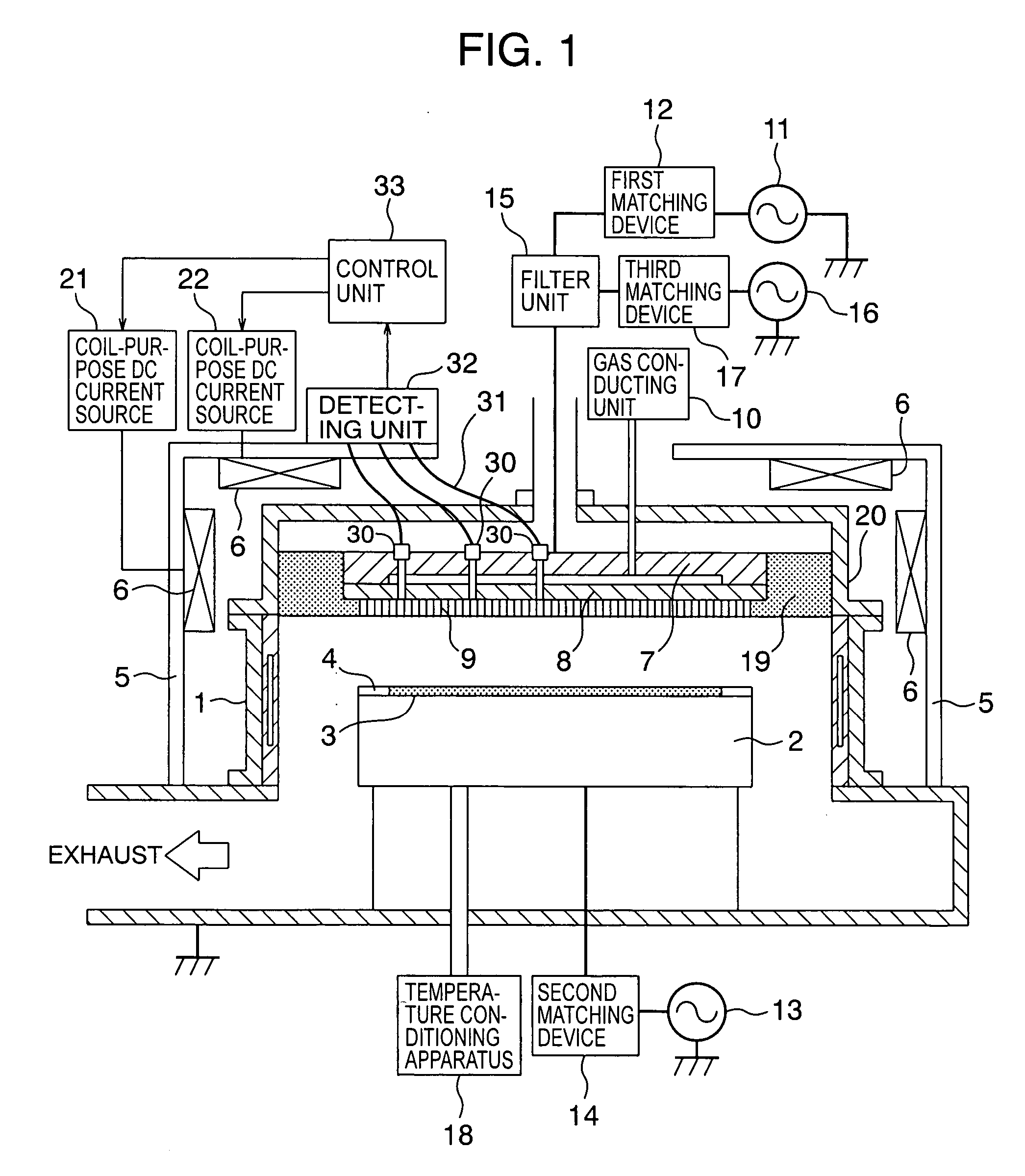
Plasma processing apparatus capable of controlling plasma emission intensity
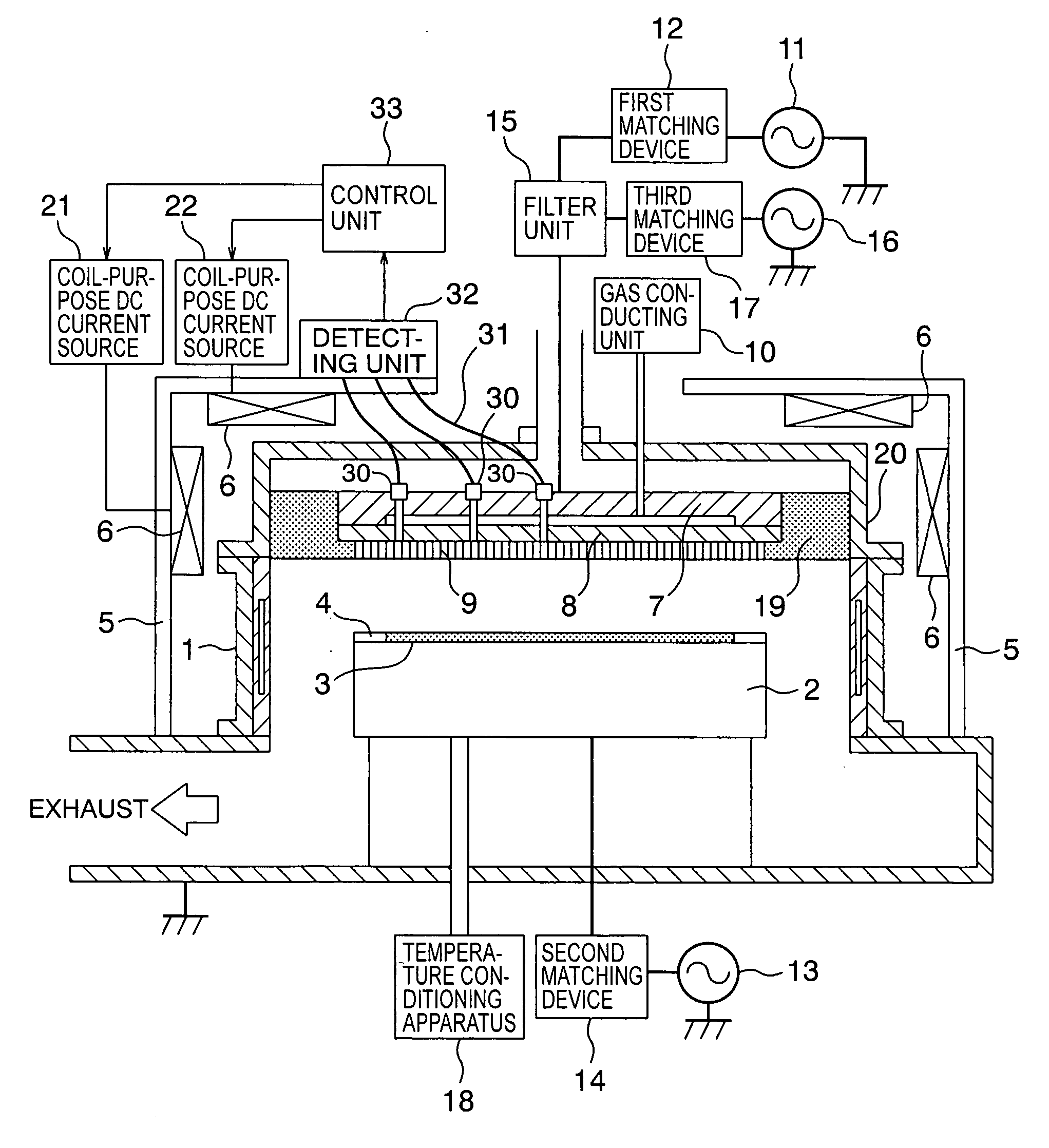
InactiveUS20060169410A1Simple equipmentImprove accuracyElectric discharge tubesSemiconductor/solid-state device manufacturingIntensity controlPlasma processing
An antenna electrode having a substantially circular shape, is arranged on a plane of a processing vessel, which is located opposite to a stage for mounting a sample within the processing vessel, and positioned parallel to the stage. An emission monitor monitors emission intensity of plasma present in at least 3 different points along a radial direction of the antenna electrode. A control unit adjusts an energizing current supplied to an external coil for forming a magnetic field within the processing vessel. The control unit adjusts the energizing current supplied to the external coil based upon the monitoring result obtained from the emission monitor so as to control the emission intensity of the plasma to become uniform emission intensity.
Owner:HITACHI HIGH-TECH CORP
Phosphor and manufacturing method for the same, and light source
ActiveUS20060033083A1Easy to manufactureReduce manufacturing costElectrical apparatusElectroluminescent light sourcesLuminous intensityPhosphor
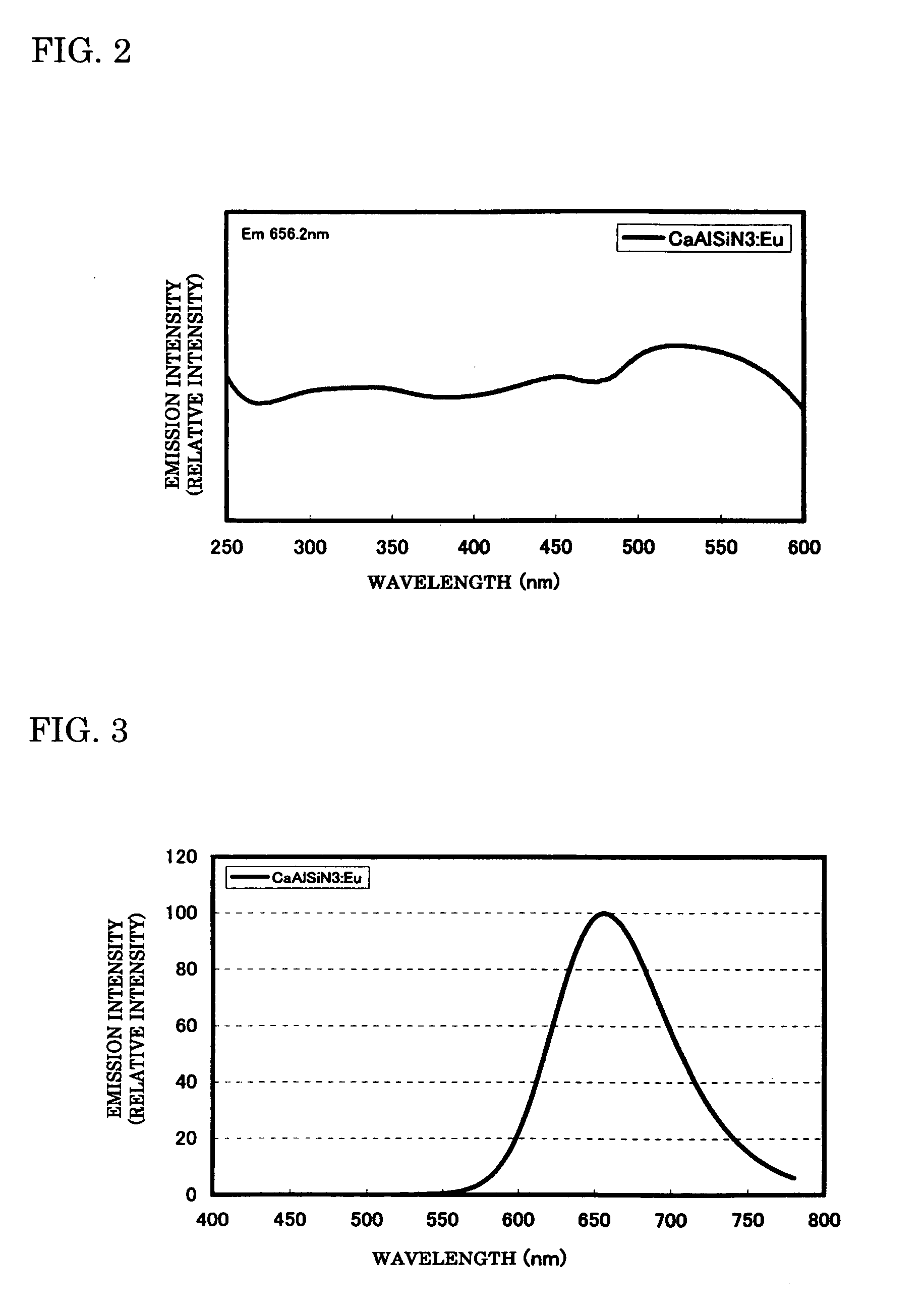
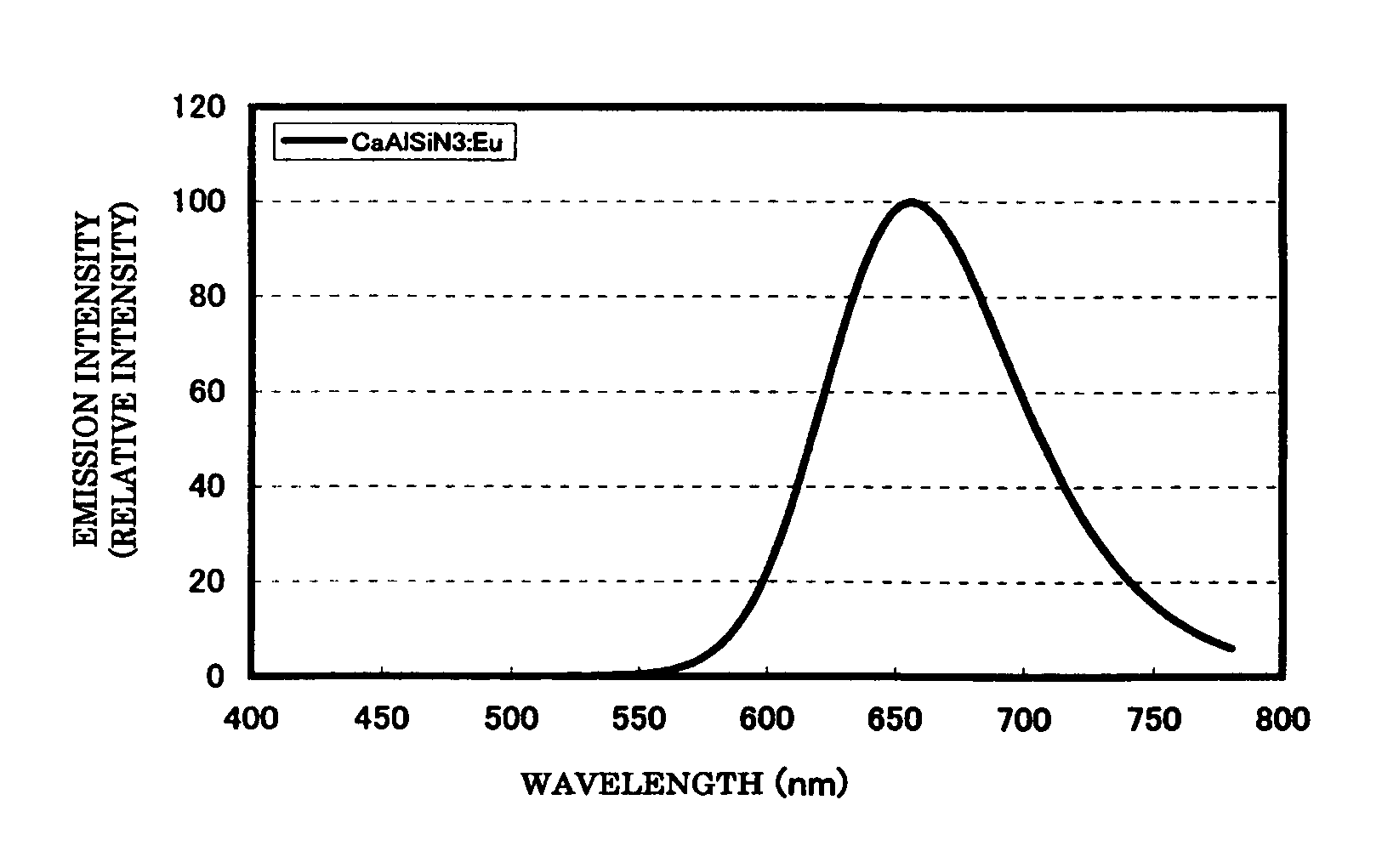
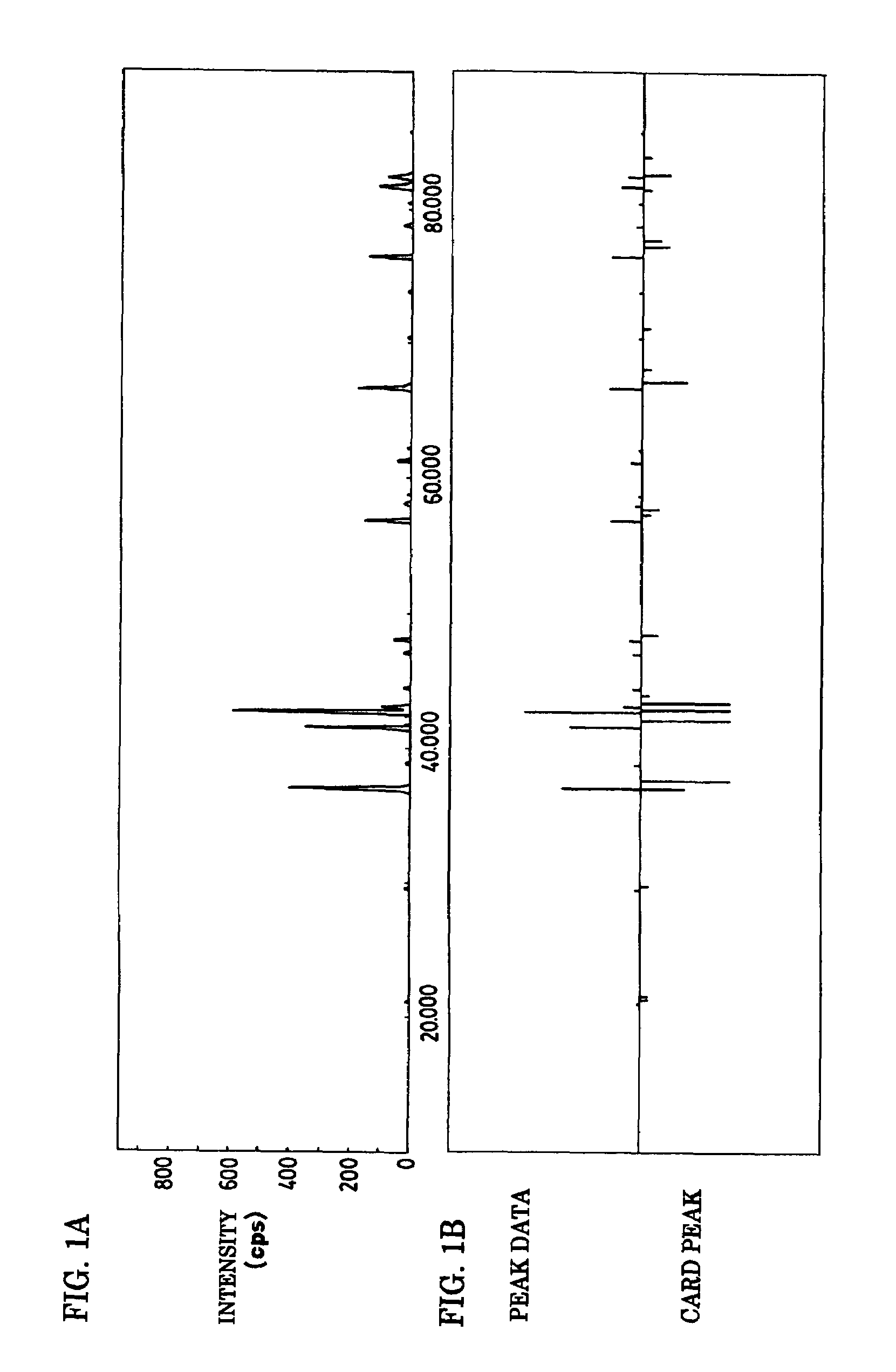
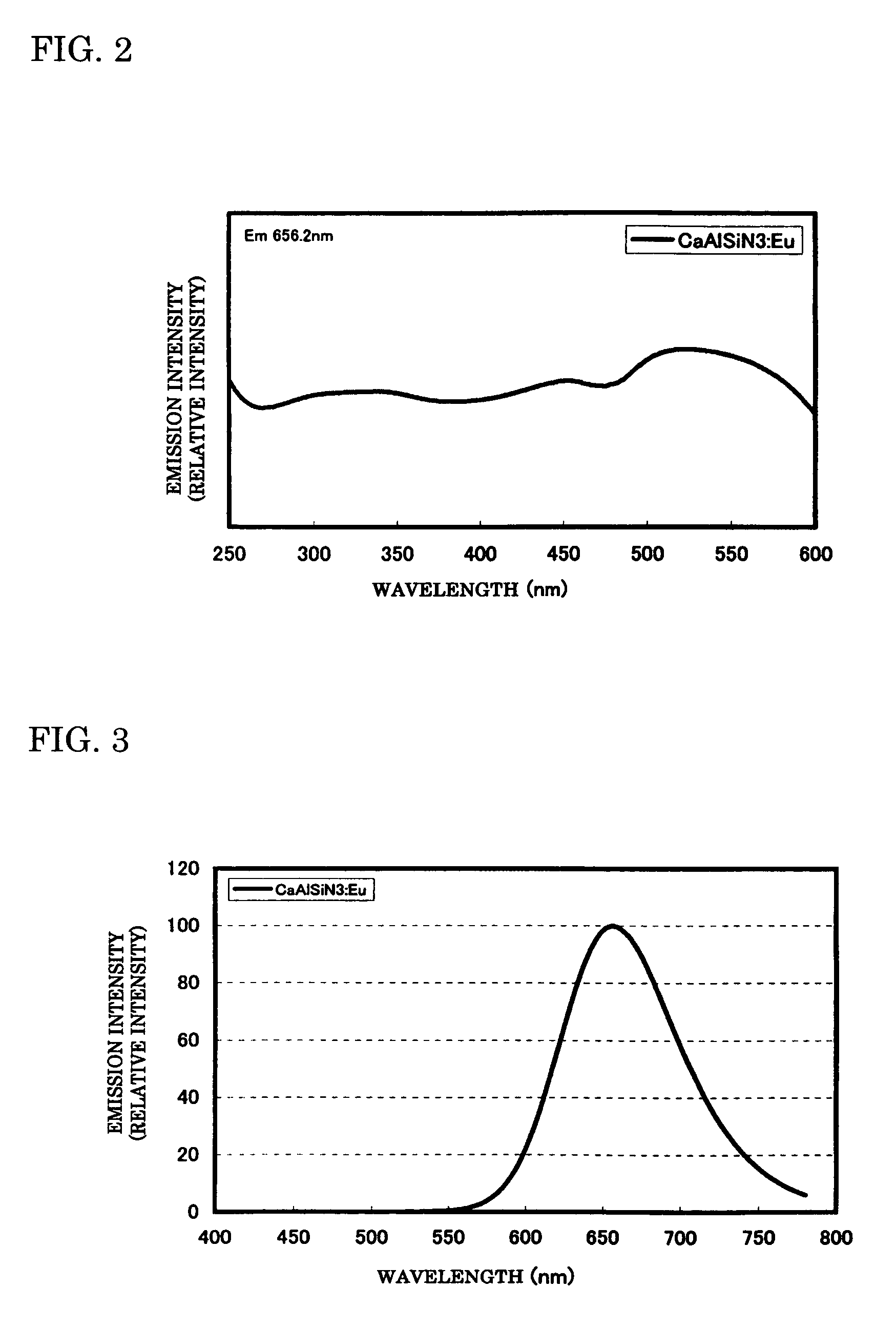
To provide a phosphor having an emission characteristic such that a peak wavelength of light emission is in a range from 580 to 680 nm, and having a high emission intensity, and having a flat excitation band with high efficiency for excitation light in a broad wavelength range from ultraviolet to visible light (wavelength range from 250 nm to 550 nm). For example, Ca3N2(2N), AlN(3N), Si3N4(3N), Eu2O3(3N) are prepared, and after weighing and mixing a predetermined amount of each raw material, raw materials are fired at 1500° C. for 6 hours, thus obtaining the phosphor including a product phase expressed by a composition formula CaAlSiN3:Eu and having an X-ray diffraction pattern satisfying a predetermined pattern.
Owner:CITIZEN ELECTRONICS CO LTD +1
Method and apparatus for detecting and counting platelets individually and in aggregate clumps
ActiveUS7929121B2Accurate countShorten the counting processBiological particle analysisCharacter and pattern recognitionFluorescenceGranularity
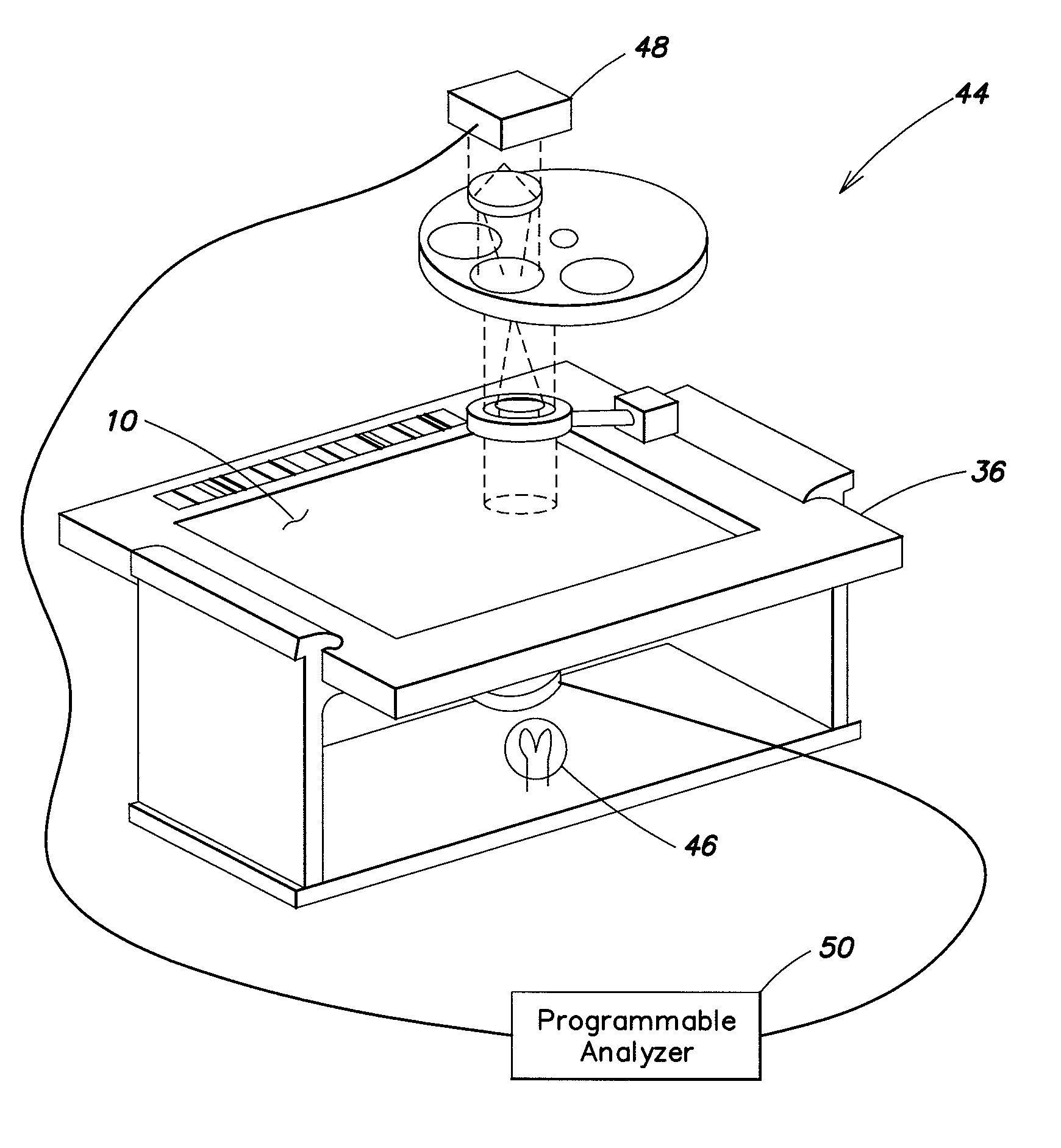
A method for enumerating platelets within a blood sample is provided. The method includes the steps of: 1) depositing the sample into an analysis chamber adapted to quiescently hold the sample for analysis, the chamber defined by a first panel and a second panel, both of which panels are transparent; 2) admixing a colorant with the sample, which colorant is operative to cause the platelets to fluoresce upon exposure to one or more predetermined first wavelengths of light; 3) illuminating at least a portion of the sample containing the platelets at the first wavelengths; 4) imaging the sample, including producing image signals indicative of fluorescent emissions from the platelets, which fluorescent emissions have an intensity; 5) identifying the platelets by their fluorescent emissions, using the image signals; 6) determining an average fluorescent emission intensity value for the individual platelets identified within the sample; 7) identifying clumps of platelets within the sample using one or more of their fluorescent emissions, area, shape, and granularity; and 8) enumerating platelets within each platelet clump using the average fluorescent emission intensity value determined for the individual platelets within the sample.
Owner:ABBOTT POINT CARE
Remote detection and analysis of chemical and biological aerosols
InactiveUS20050026276A1Quickly thermalizedEasy to detectBioreactor/fermenter combinationsEmission spectroscopyElectromagnetic radiationOxygen
A system for detecting and analyzing chemical and biological aerosols. A beam of radiation is used to radiate a target cloud including the aerosol. The radiation energy that is absorbed by the cloud is thermalized by collisional energy transfer between the molecules that absorb the radiation to generate heat. The wavelength of the electromagnetic radiation is selected to be in resonance with the absorption lines of water or oxygen molecules in the cloud, or to be in resonance with absorption lines of known target molecules in the cloud to generate the heat. An increase in the cloud temperature increases the emission intensity of the molecules against the background, resulting in improved detection of the target molecules in the aerosol. A tracking telescope collects the thermal emissions generated by the radiation beam. A spectrometer receives the emissions from the cloud and generates an emission spectrum.
Owner:NORTHROP GRUMAN CORP
Phosphor and manufacturing method of the same, and light emitting device using the phosphor
InactiveUS20060220047A1Improve efficiencyIncrease intensityDischarge tube luminescnet screensLamp detailsUltravioletPeak value
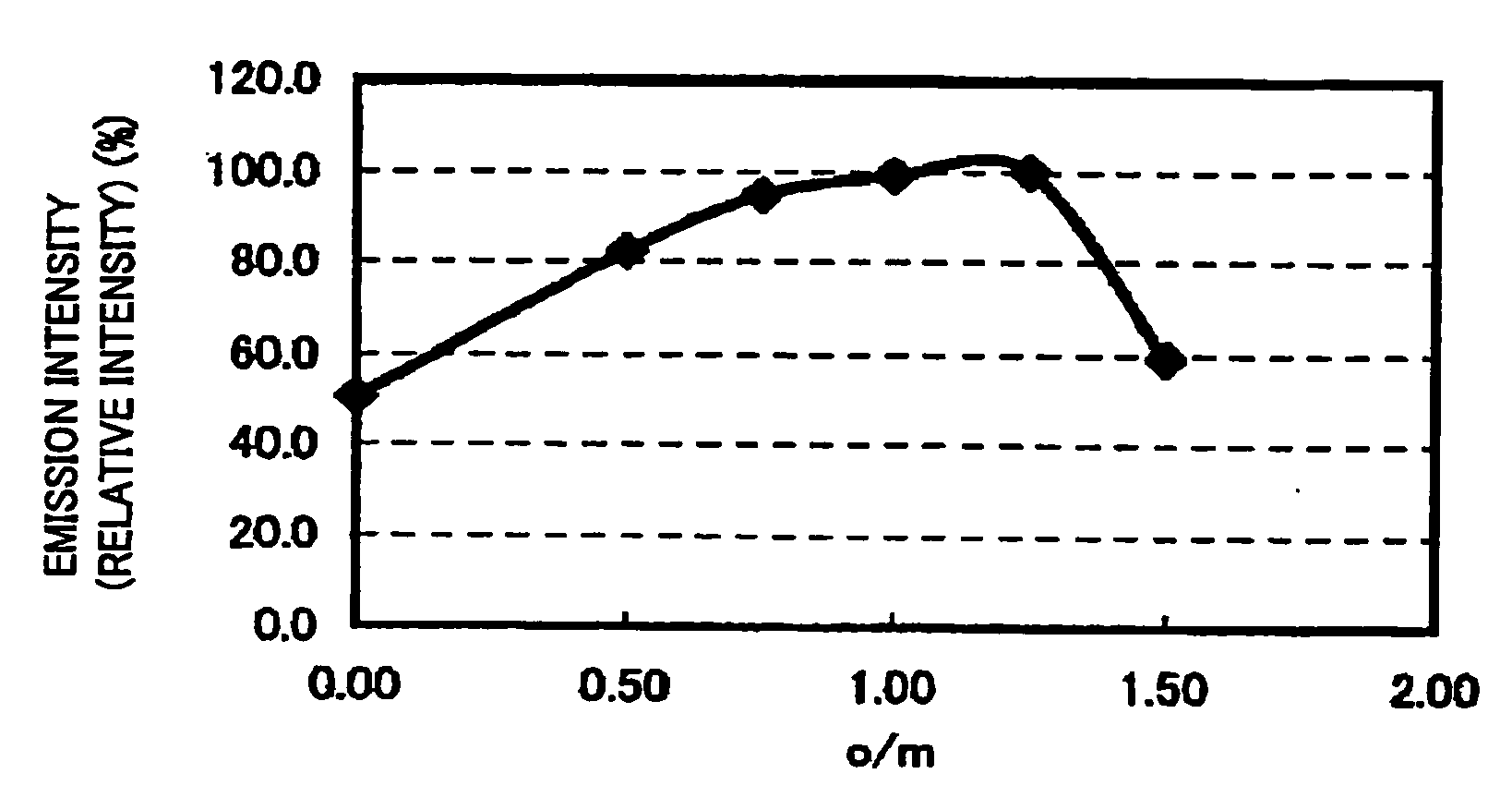
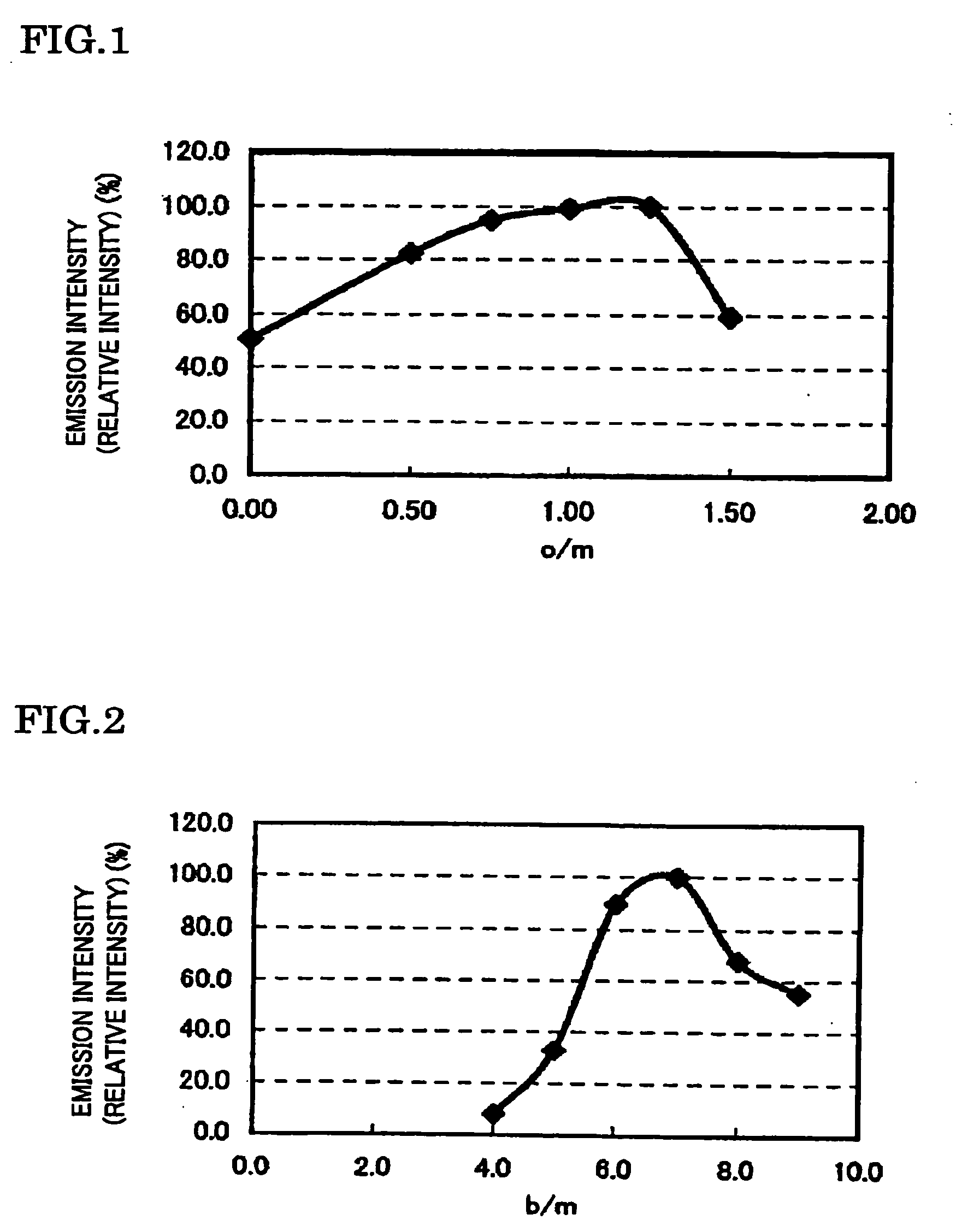
To provide a phosphor having a broad emission spectrum in a range of blue color (peak wavelength from 400 nm to 500 nm), having a broad and flat excitation band in the range of near ultraviolet / ultraviolet, and having excellent emission efficiency and emission intensity / luminance. The phosphor is expressed by a general composition formula MmAaBbOoNn:Z (where element M is more than one kind of element having bivalent valency, element A is more than one kind of element having tervalent valency, element B is more than one kind of element having tetravalent valency, O is oxygen, N is nitrogen, and element Z is more than one kind of element acting as an activator.), satisfying 5.0<(a+b) / m<9.0, 0≦a / m≦2.0, 0≦o<n, n=2 / 3m+a+4 / 3b−2 / 3o, and having an emission spectrum with a maximum peak wavelength from 400 nm to 500 nm under an excitation of the light in a wavelength range from 350 nm to 430 nm.
Owner:MITSUBISHI CHEM CORP
Two-phase silicate-based yellow phosphor
InactiveUS7601276B2High luminous efficiencyImprove temperature stabilityDischarge tube luminescnet screensLamp detailsDivalent metalLighting system
Novel two-phase yellow phosphors are disclosed having a peak emission intensity at wavelengths ranging from about 555 nm to about 580 nm when excited by a radiation source having a wavelength ranging from 220 nm to 530 nm. The present phosphors may be represented by the formula a[Srx(M1)1−x]zSiO4.(1-a)[Sry(M2)1−y]uSiO5:Eu2+D, wherein M1 and M2 are at least one of a divalent metal such as Ba, Mg, Ca, and Zn, the values of a, x, y, z and u follow the following relationships: 0.6≦a≦0.85; 0.3≦x≦0.6; 0.85≦y≦1; 1.5≦z≦2.5; 2.6≦u≦3.3; and Eu and D each range from 0.001 to about 0.5. D is an anion selected from the group consisting of F, Cl, Br, S, and N, and at least some of the D anion replaces oxygen in the host silicate lattice of the phosphor. The present yellow phosphors have applications in high brightness white LED illumination systems, LCD display panels, plasma display panels, and yellow LEDs and illumination systems.
Owner:INTEMATIX
Phosphor and manufacturing method therefore, and light source using the phosphor
ActiveUS20060043337A1Increase intensityHigh luminanceDischarge tube luminescnet screensLamp detailsLuminous intensityUltraviolet
To provide a phosphor having a broad emission spectrum with a peak in the range from yellow color to red color (wavelength from 570 nm to 620 nm), having a flat excitation band with large area on the long wavelength side from near ultraviolet / ultraviolet to green color (wavelength from 250 nm to 550 nm), and excellent in emission intensity and luminance, and a method of manufacturing the same, and also a light source such as white LED using the phosphor. As raw materials, Ca3N2(2N), AlN(3N), Si3N4(3N), and Eu2O3(3N) are prepared, and out of each raw material, 0.950 / 3 mol of Ca3N2. 2 mol of AlN, 4 / 3 mol of Si3N4, and 0.050 / 2 mol of Eu2O3 are weighed, and the raw materials thus weighed are mixed by using a mortar. The raw materials thus mixed are put in a BN crucible, and retained / fired for 3 hours at 1700° C. in a nitrogen atmosphere, and thereafter cooled from 1700° C. to 200° C., to thereby obtain the phosphor expressed by a composition formula Ca0.950Al2Si4O0.075N7.917:Eu0.050.
Owner:NICHIA CORP +1
Semiconductor light emitting device, backlight, color image display device and phosphor to be used for them
ActiveUS8491816B2Broad color reproducibilityImprove efficiencyElectroluminescent light sourcesSolid-state devicesColor imageLuminous intensity
To provide a semiconductor light emitting device which is capable of accomplishing a broad color reproducibility for an entire image without losing brightness of the entire image.A light source provided on a backlight for a color image display device has a semiconductor light emitting device comprising a solid light emitting device to emit light in a blue or deep blue region or in an ultraviolet region and phosphors, in combination. The phosphors comprise a green emitting phosphor and a red emitting phosphor. The green emitting phosphor and the red emitting phosphor are ones, of which the rate of change of the emission peak intensity at 100° C. to the emission intensity at 25° C., when the wavelength of the excitation light is 400 nm or 455 nm, is at most 40%.
Owner:CITIZEN ELECTRONICS CO LTD +1
Phosphor and manufacturing method for the same, and light source
ActiveUS20060065878A1Improve emission efficiencyImprove efficiencySynthetic resin layered productsCellulosic plastic layered productsRare-earth elementUltraviolet
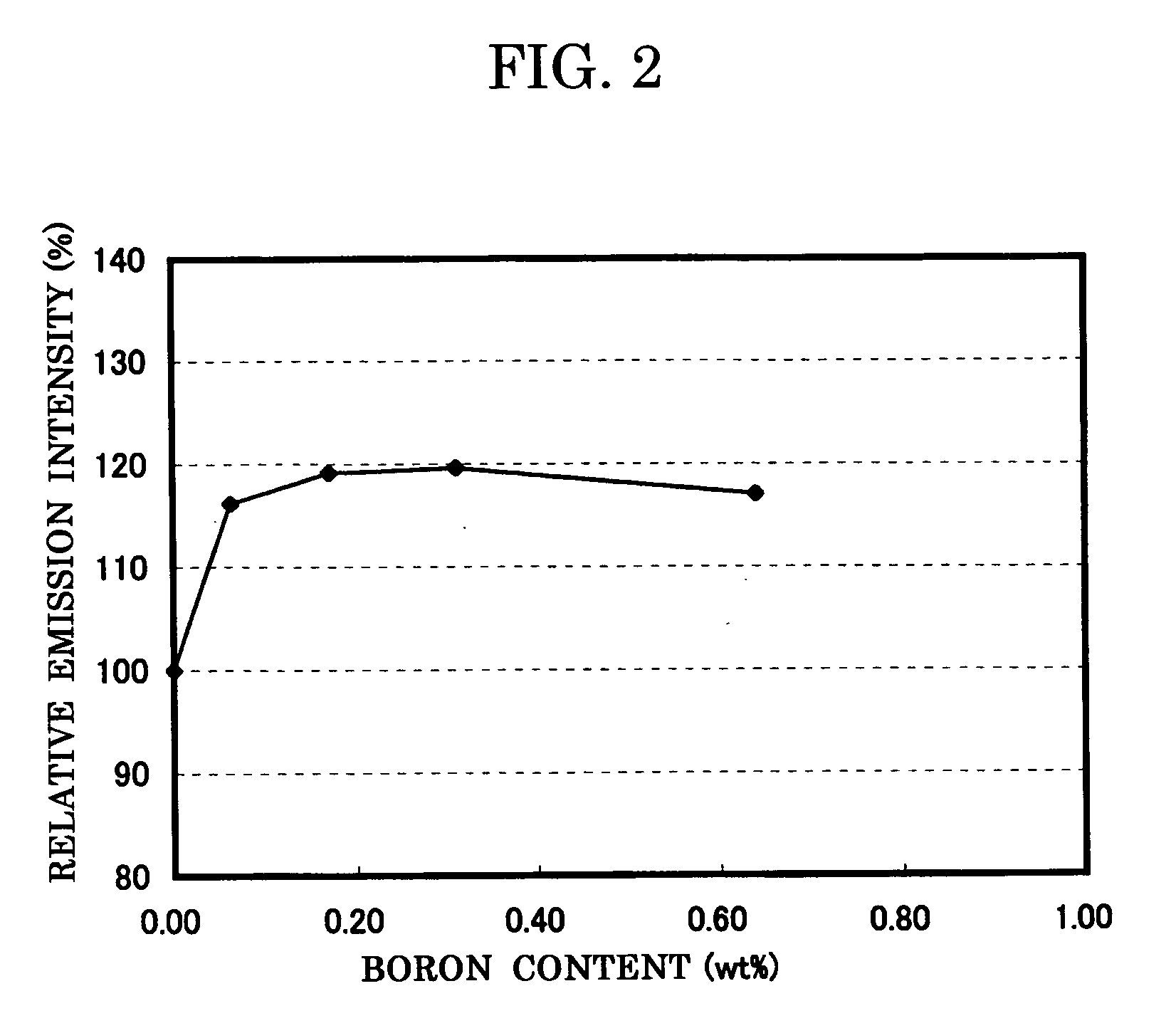
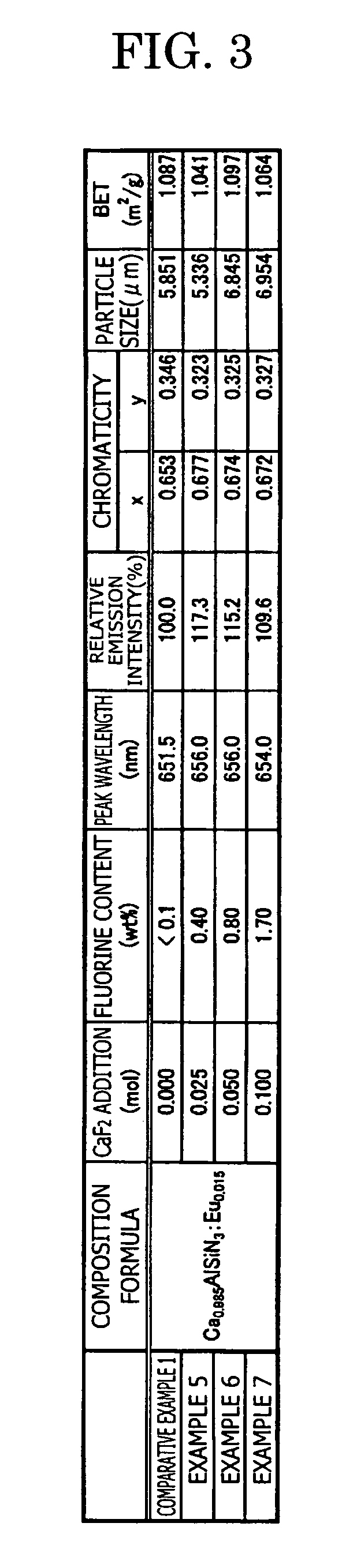
To provide a phosphor having an emission spectrum with a broad peak in a range from yellow color to red color (580 nm to 680 nm) and an excellent excitation band on the longer wavelength side from near ultraviolet / ultraviolet of excitation light to visible light (250 nm to 550 nm), and having an improved emission intensity. The phosphor is provided, which is given by a general composition formula expressed by MmAaBbOoNn:Z, (wherein element M is more than one kind of element having bivalent valency, element A is more than one kind of element having tervalent valency selected from the group consisting of Al, Ga, In, Tl, Y, Sc, P, As, Sb, and Bi, element B is more than one kind of element having tetravalent valency, O is oxygen, N is nitrogen, and element Z is more than one kind of element selected from rare earth elements or transitional metal elements, satisfying m>0, a>0, b>0 o≧0, and n=2 / 3m+a+4 / 3b−2 / 3o), and further containing boron and / or fluorine.
Owner:CITIZEN ELECTRONICS CO LTD +1
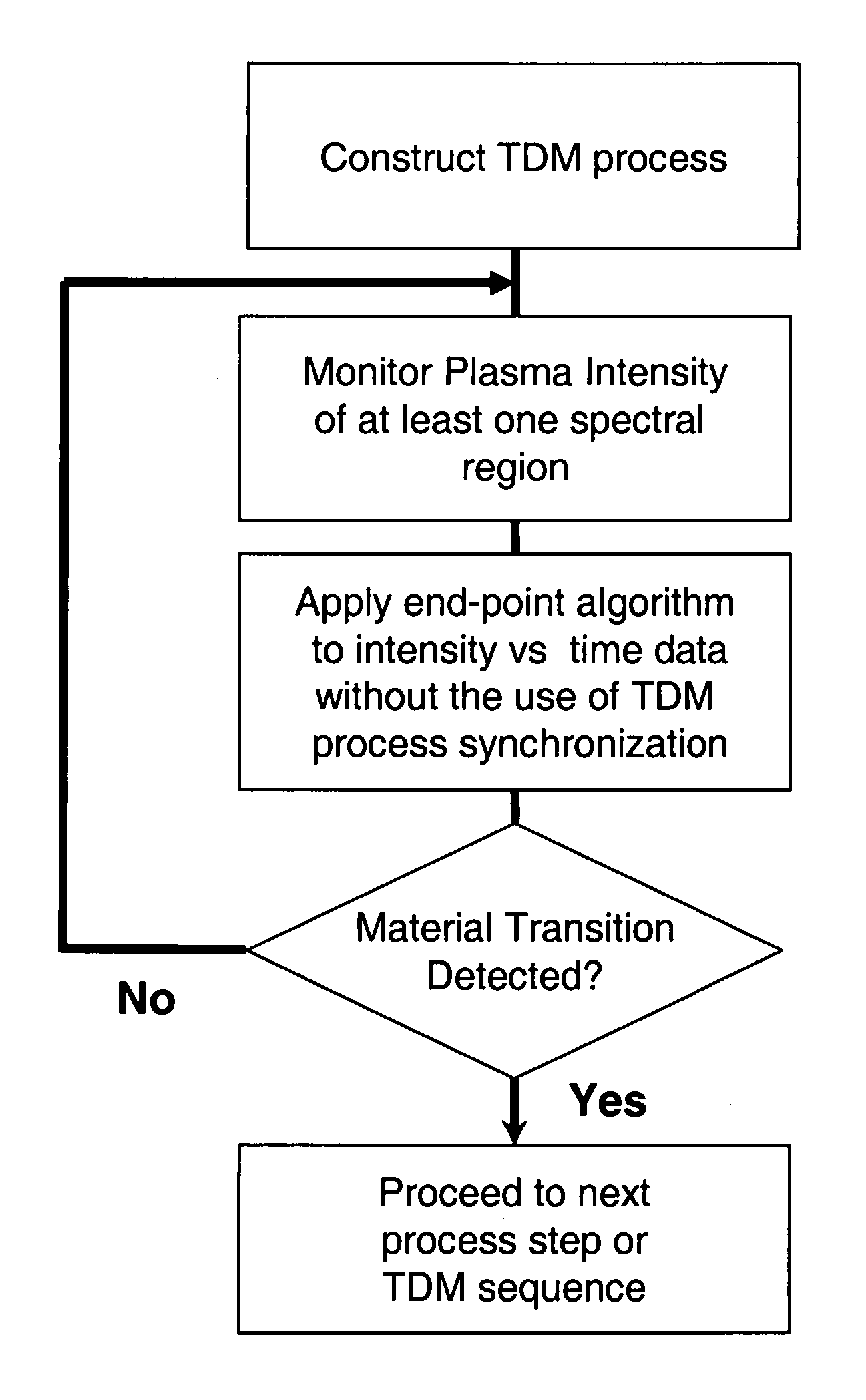
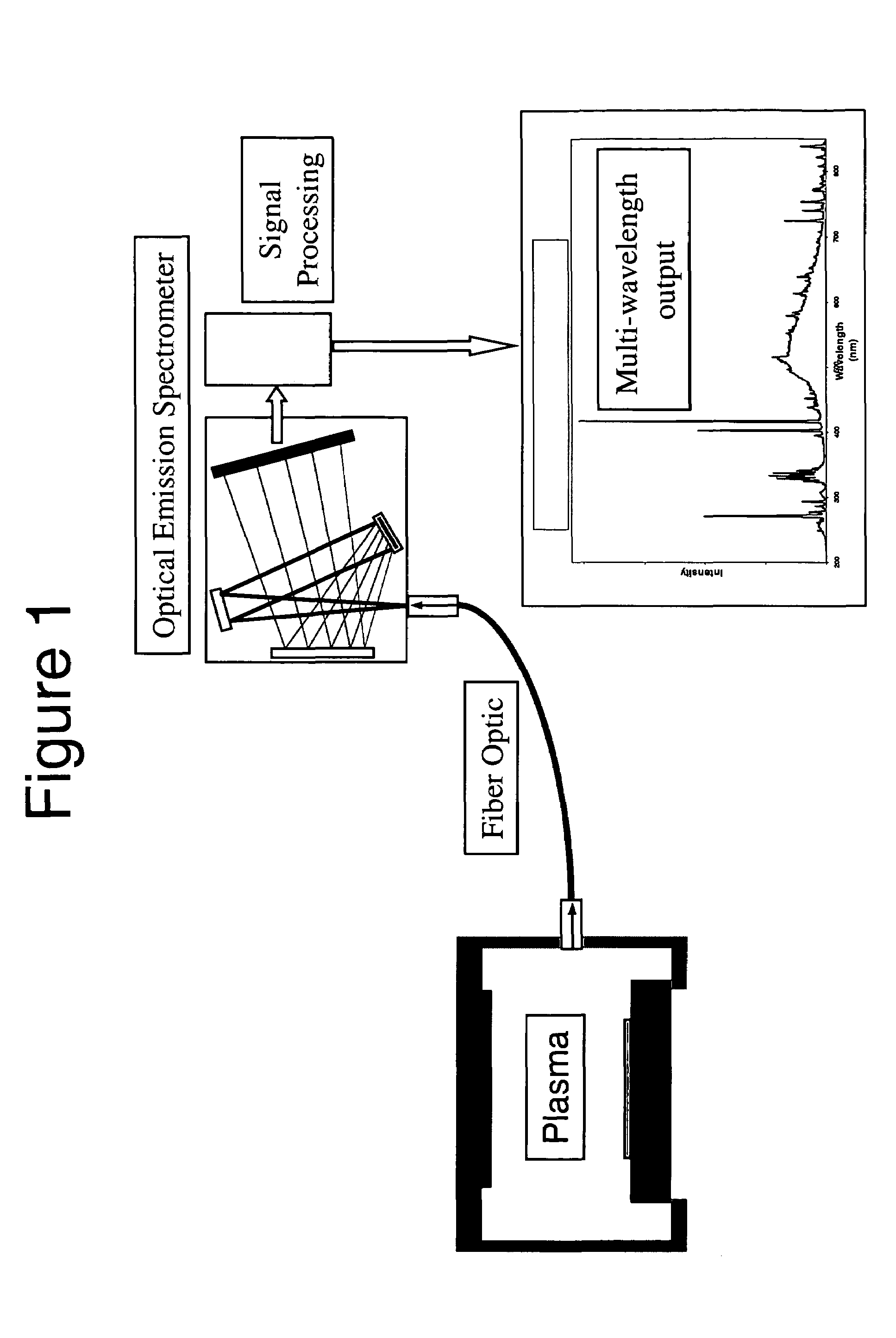
Envelope follower end point detection in time division multiplexed processes
ActiveUS7101805B2Semiconductor/solid-state device testing/measurementElectric discharge tubesOptical emission spectrometryPlasma chamber
The present invention provides a method and an apparatus for establishing endpoint during an alternating cyclical etch process or time division multiplexed process. A substrate is placed within a plasma chamber and subjected to an alternating cyclical process having an etching step and a deposition step. A variation in plasma emission intensity is monitored using known optical emission spectrometry techniques. An amplitude information is extracted from a complex waveform of the plasma emission intensity using an envelope follower algorithm. The alternating cyclical process is discontinued when endpoint is reached at a time that is based on the monitoring step.
Owner:PLASMA THERM
Ratiometric fluorescent pH sensor for non-invasive monitoring
InactiveUS20050090014A1Chemical analysis using titrationChemiluminescene/bioluminescenceNon invasiveLocal environment
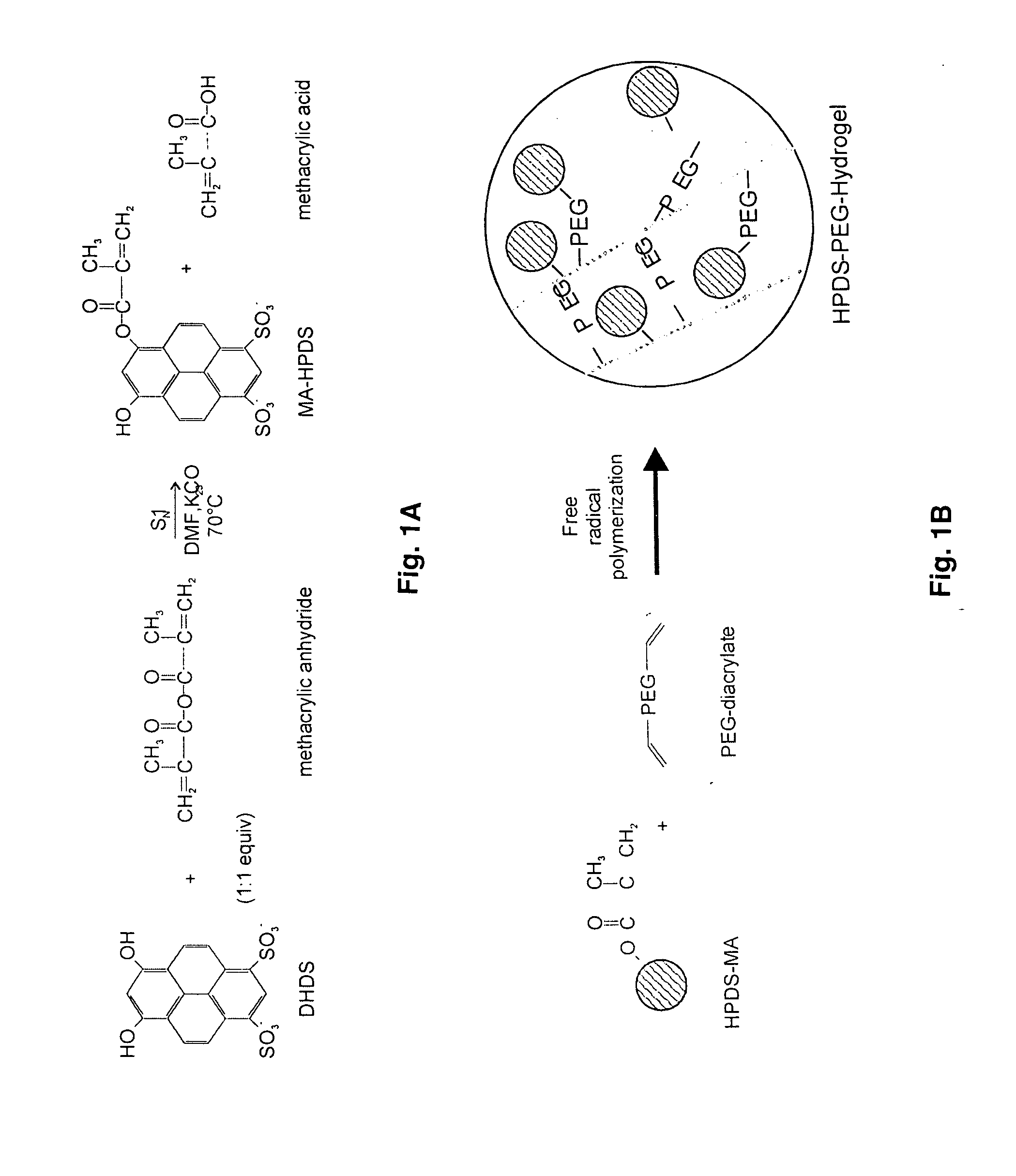
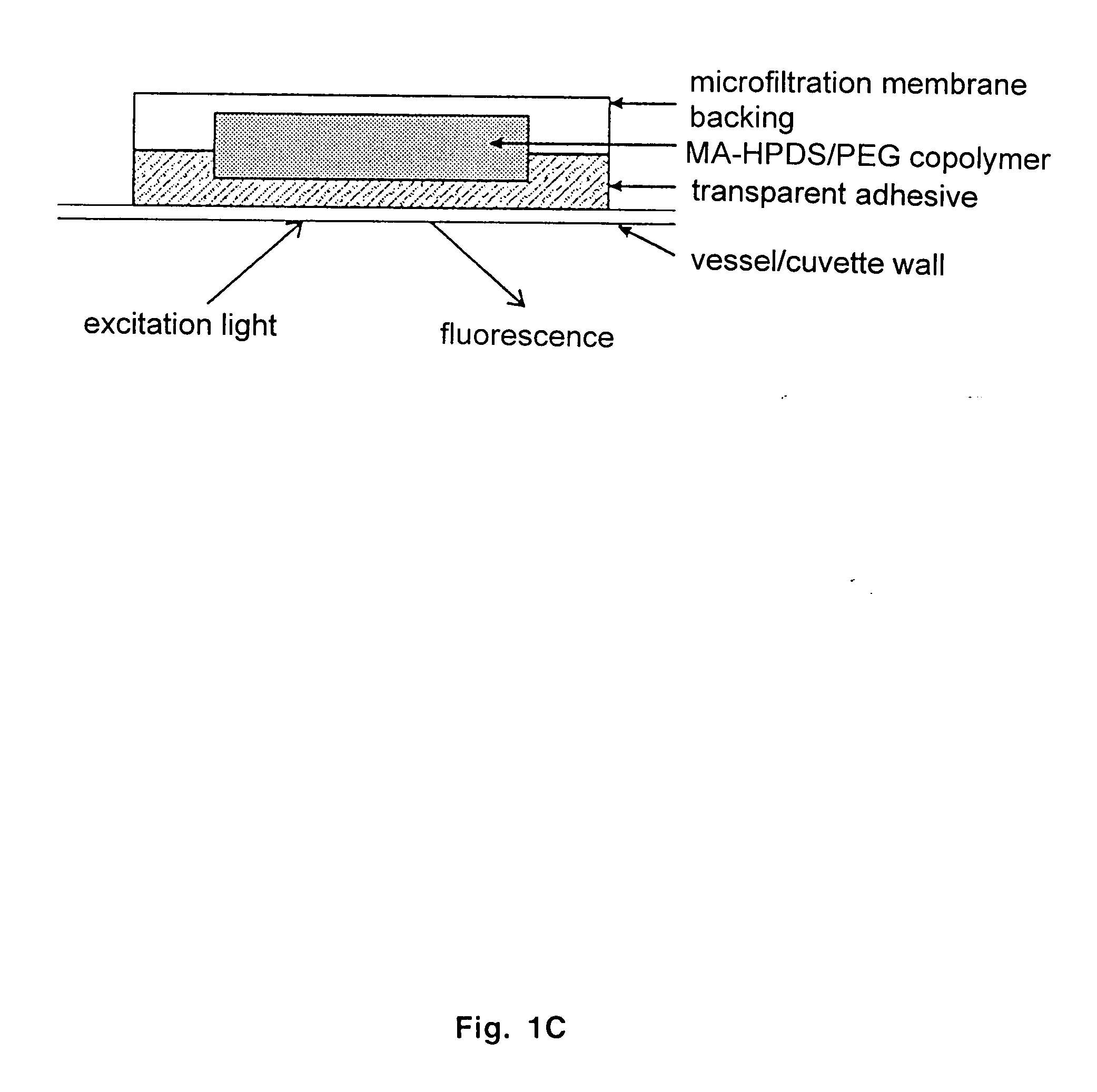
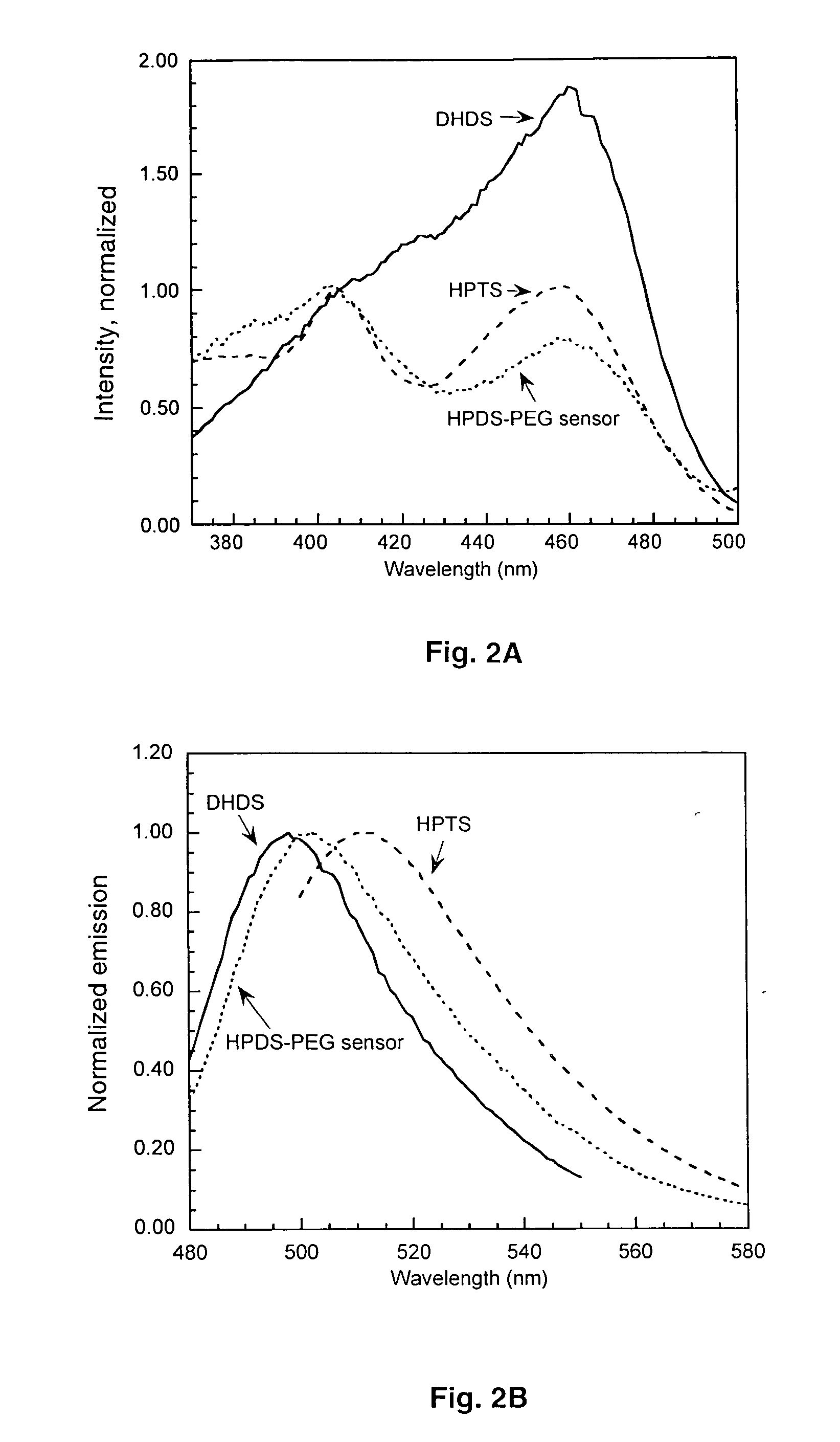
The present invention provides ratiometric fluorescent pH sensors for non-invasive, continuous monitoring of pH in such applications as fermentation processes. The ratiometric fluorescent pH sensors comprise a fluorescent dye that exhibits a shift in excitation wavelength with a corresponding shift in pH in the local environment of said fluorescent dye. Ratiometric measurements of the emission intensities at dual excitation maxima correlate to pH. Also provided is a fluorescent dye 6-methacryloyl-8-hydroxy-1,3-pyrene disulfonic acid (MA-HPDS). Further provided are systems and methods to non-invasively and continuously monitor pH.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY
Electroluminescent Device
InactiveUS20160359143A1Improve efficiencyElectroluminescent light sourcesSolid-state devicesHigh intensityLight emission
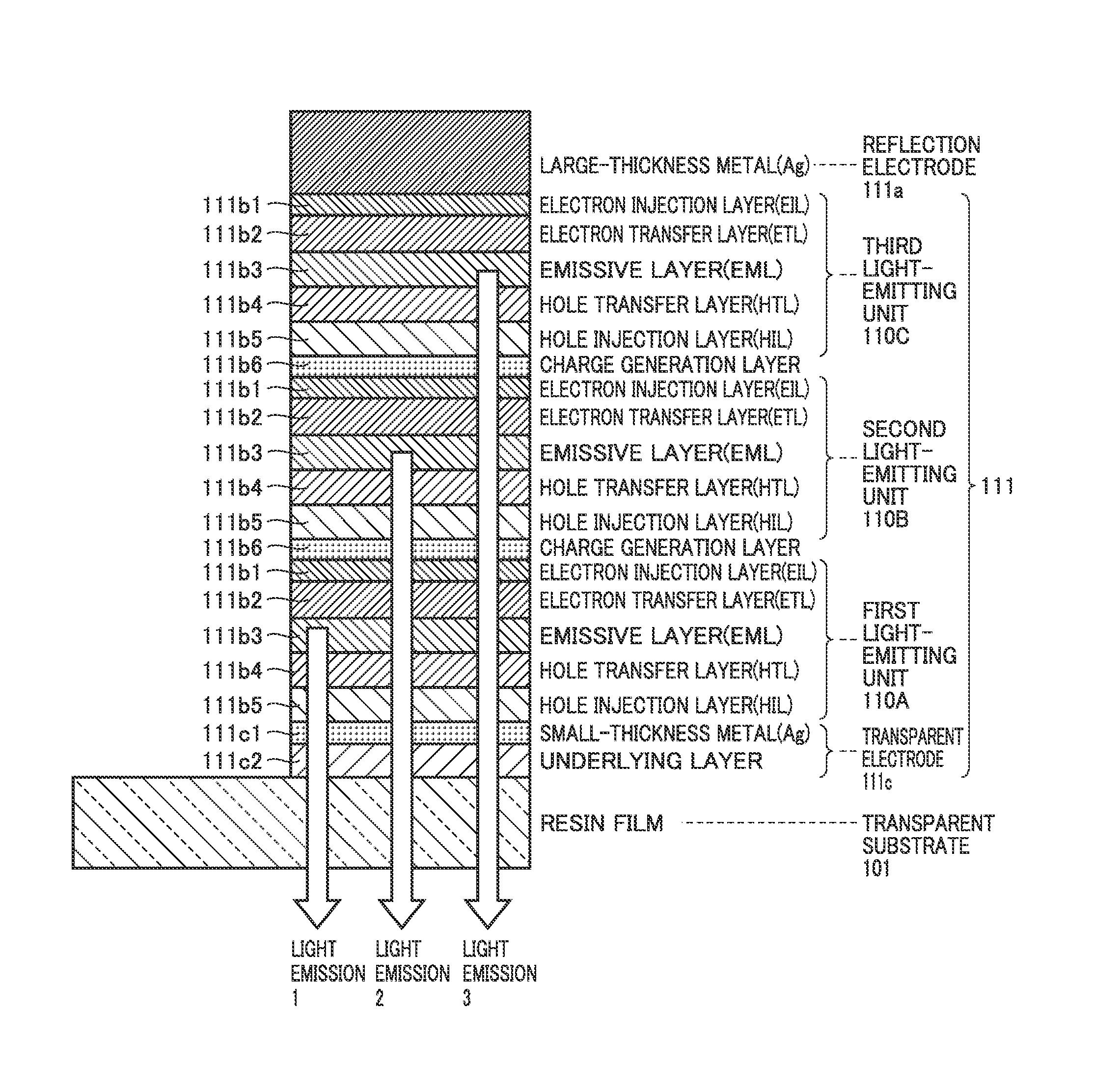
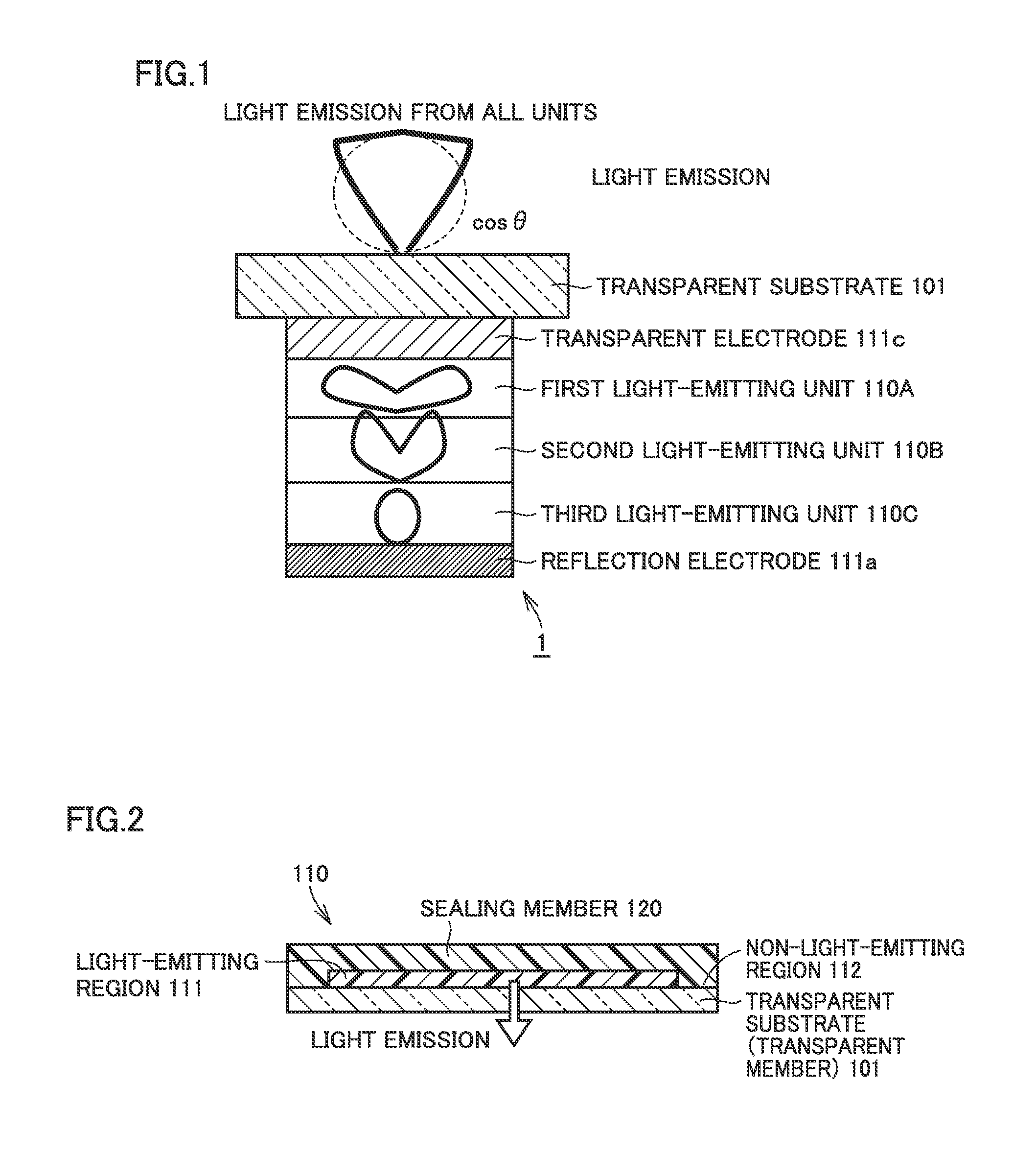
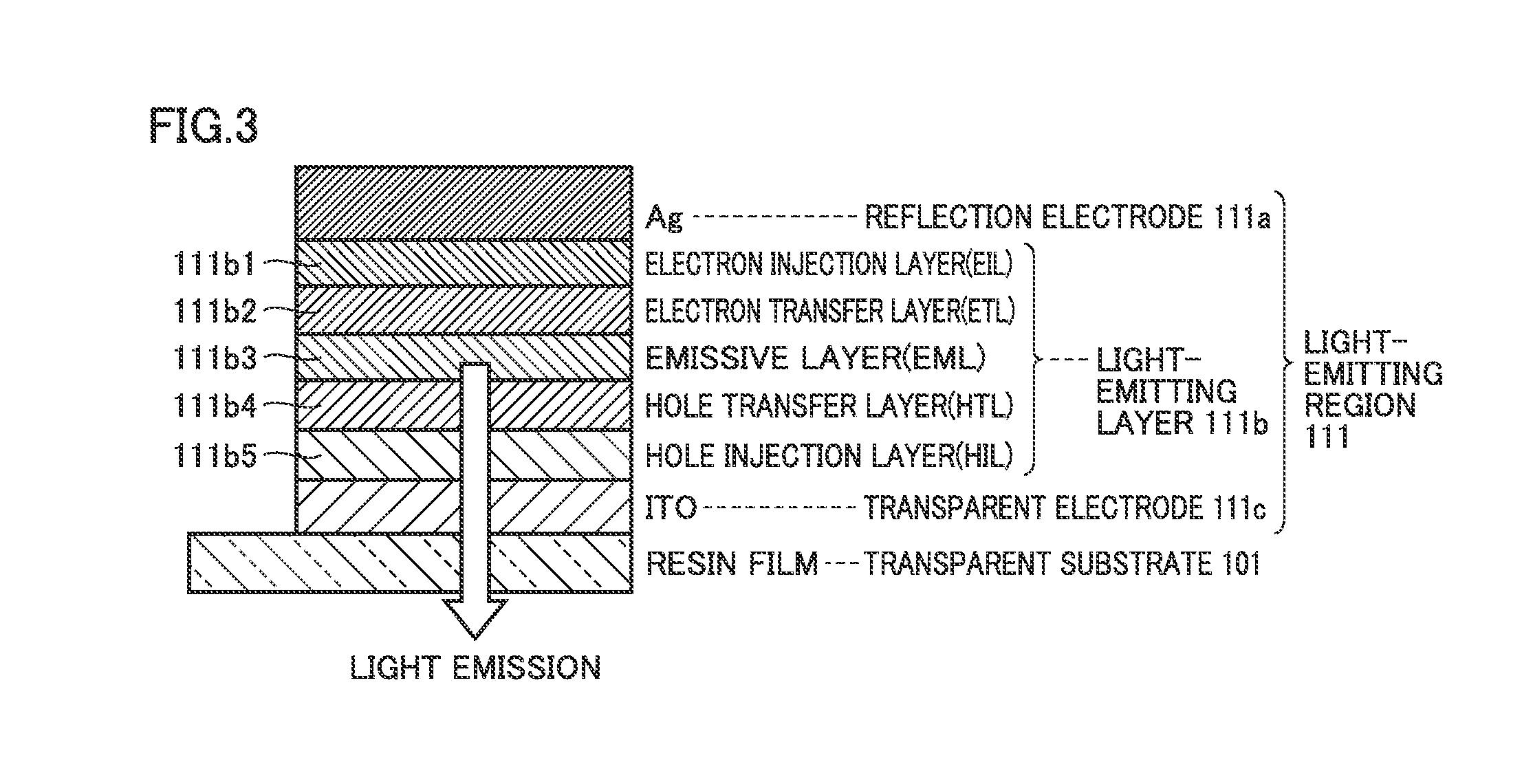
An electroluminescent device in which two or more light-emitting units which emit light identical in color are vertically stacked is configured such that a first relative maximal angle or a highest intensity angle viewed in a front direction of angular dependency of emission intensity in light emission from each light-emitting unit alone is different for each light-emitting unit, and D(θ)≧D(0)cos θ(0≦θ≦θD≦60 degrees) . . . Expression (1) is satisfied, where D(θ) represents angular dependency of emission intensity and θD represents a specific angle in simultaneous light emission from all light-emitting units.
Owner:KONICA MINOLTA INC
Method and device for monitoring oil oxidation in real time by measuring fluorescence
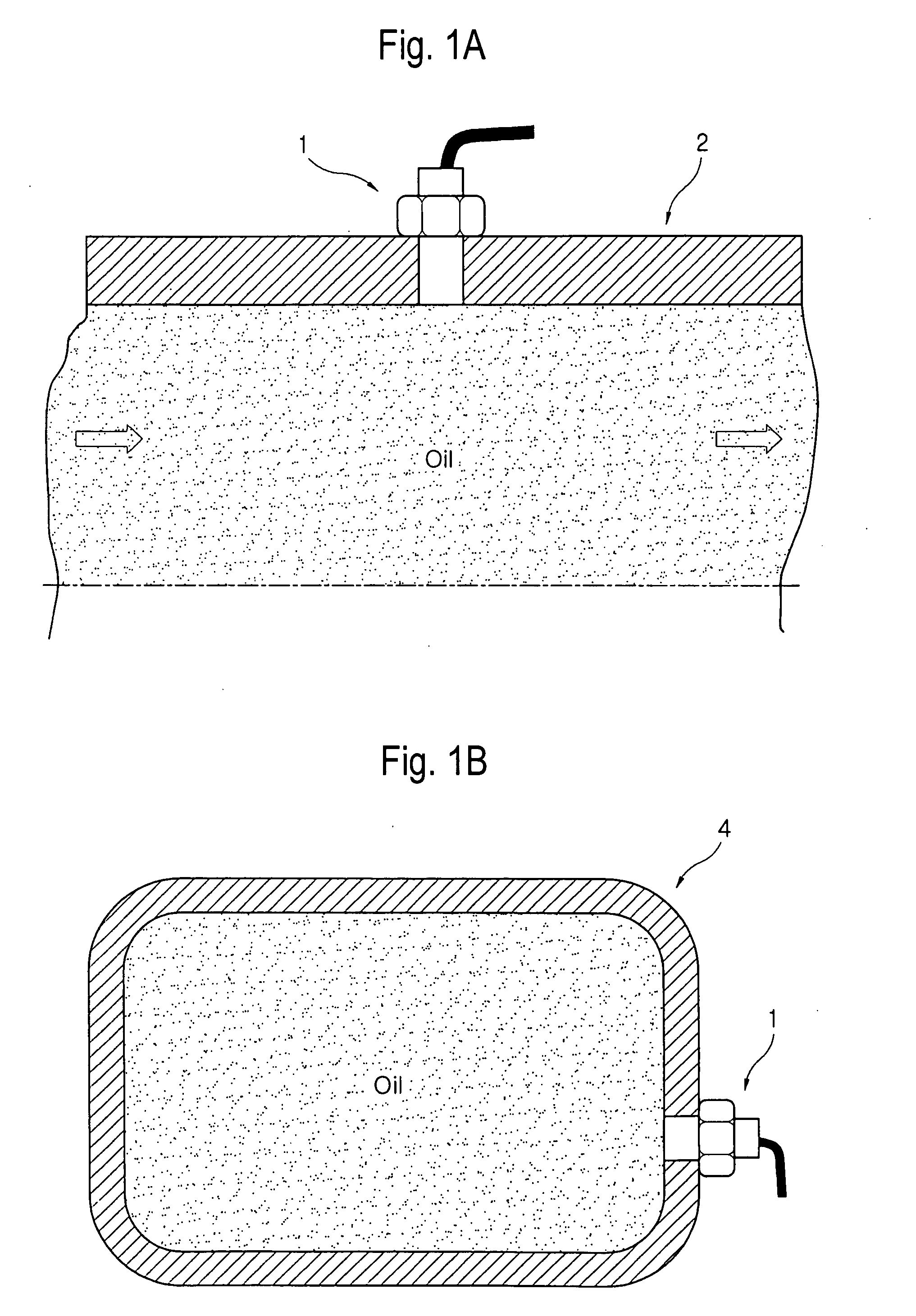
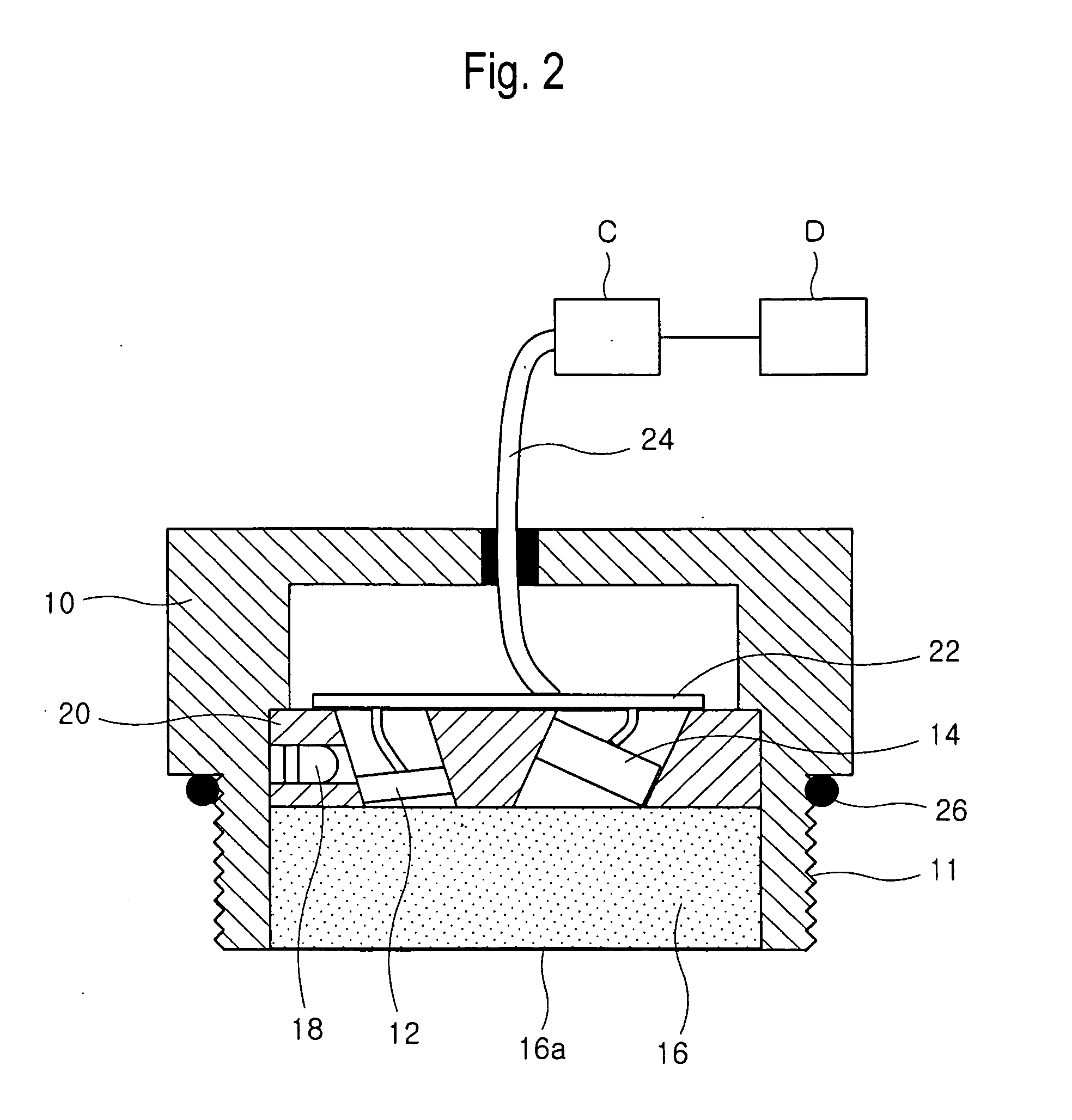
There is provided a method and a device of monitoring oil oxidation in real time. The method of the present invention comprises the steps of: irradiating ultraviolet light into oil to be monitored; measuring fluorescence emission intensity of the oil in red, green and blue wavelength bands; determining one value measured in a relatively long wavelength band and the other value measured in a relatively short wavelength band among the fluorescence emission intensity measured in the red, green and blue wavelength bands; calculating a fluorescence emission ratio which is defined as a ratio of the value measured in the relatively long wavelength band to the value measured in the relatively short wavelength band; and monitoring a change in the fluorescence emission ratio. It is then determined whether the fluorescence emission ratio reaches a predetermined critical magnitude. When the fluorescence emission ratio reaches the critical magnitude, the necessity of replacing the oil with new one is indicated.
Owner:KOREA INST OF SCI & TECH
Phosphor and manufacturing method of the same, and light emitting device using the phosphor
InactiveUS20060220520A1Improve efficiencyIncrease intensityDischarge tube luminescnet screensLamp detailsLuminous intensityUltraviolet
To provide a phosphor having an emission spectrum with a broad peak in a range of blue color (peak wavelength range from 400 nm to 500 nm) and a broad and flat excitation band in a range of near ultraviolet / ultraviolet, and having an excellent emission efficiency and emission intensity / luminance, a manufacturing method of the same, and a light emitting device using the phosphor. The phosphor is provided, which is given as a general composition formula expressed by MmAaBbOoNn:Z, (where element M is the element having bivalent valency, element A is the element having tervalent valency, element B is the element having tetravalent valency, O is oxygen, N is nitrogen, and element Z is more than one kind of element acting as an activator.), satisfying 5.0<(a+b) / m<9.0, 0≦a / m≦2.0, 0≦o<n, and n=2 / 3m+a+4 / 3b−2 / 3o, and having an emission spectrum with a maximum peak wavelength from 400 nm to 500 nm under an excitation of the light in a wavelength range from 350 nm to 430 nm.
Owner:MITSUBISHI CHEM CORP
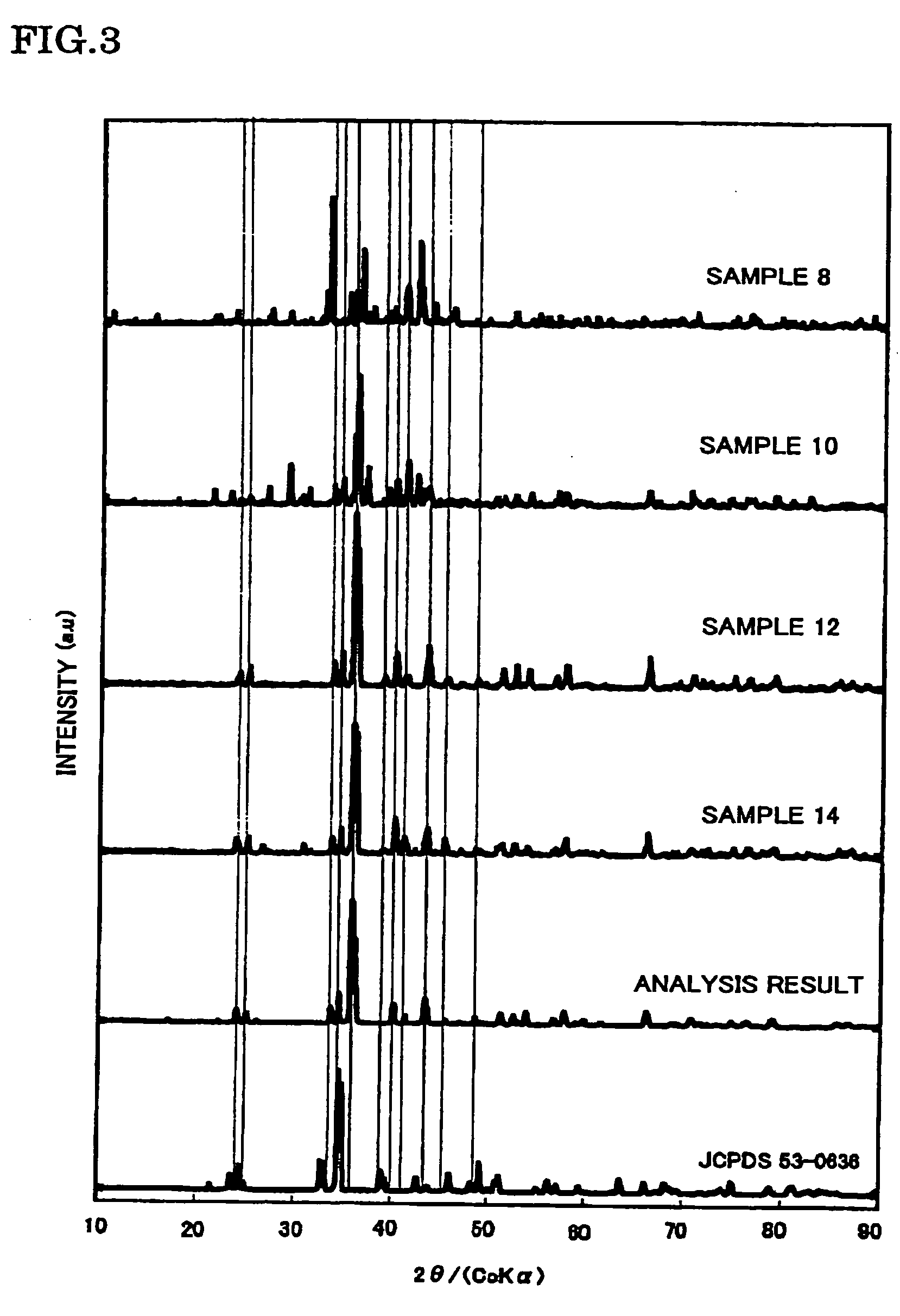
Method, system, and computer software for the presentation and storage of analysis results
A computer program product, and related systems and methods, are described that processes emission intensity data corresponding to probes of a biological probe array. The computer program includes a genotype and statistical analysis manager that determines absolute or relative expression values based, at least in part, on a statistical measure of the emission intensity data and at least one user-selectable statistical parameter. The analysis manager may also determine genotype calls for one or more probes based, at least in part, on the emission intensity data, The analysis manager may further display the absolute or relative expression values based, at least in part, on at least one user-selectable display parameter and / or a measure of normalized change between genotype calls. The measure of normalized change may be based, at least in part, on a comparison of genotype calls and a reference value.
Owner:AFFYMETRIX INC
Light emitting apparatus, display apparatus and method for controlling light emitting apparatus
InactiveUS20070284994A1High color reproductionDischarge tube luminescnet screensElectroluminescent light sourcesLuminous intensityPhosphor
In a light emitting apparatus using LEDs, luminance and chromaticity of the LED changes largely due to a temperature change. Meanwhile, by using phosphors, it is possible to improve the stability of the luminance and chromaticity against the temperature change. However, color reproducibility of the light emitting apparatus decreases. Therefore, the light emitting apparatus includes a first LED, a second LED, a first phosphor which is excited by the second LED and emits light whose color is the same as or similar to a color of light emitted from the first LED, and a control circuit which controls a ratio between an emission intensity of the first LED and an emission intensity of the second LED.
Owner:LEDCOMM LLC
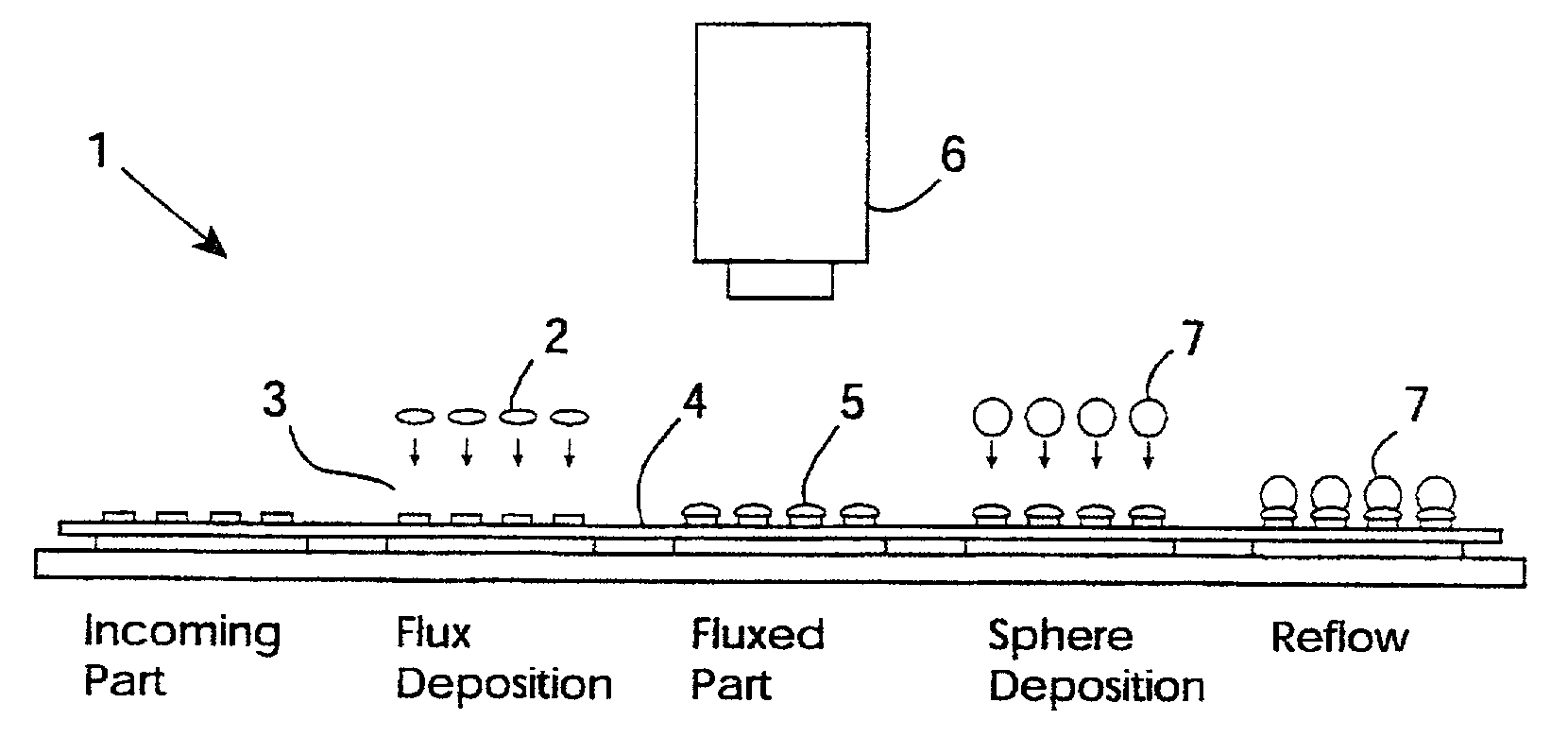
Material inspection
InactiveUS20020053589A1Avoid downstream processing defectMinimize sensationAutomatic control devicesPrecision positioning equipmentDiffuse illuminationFluorescence
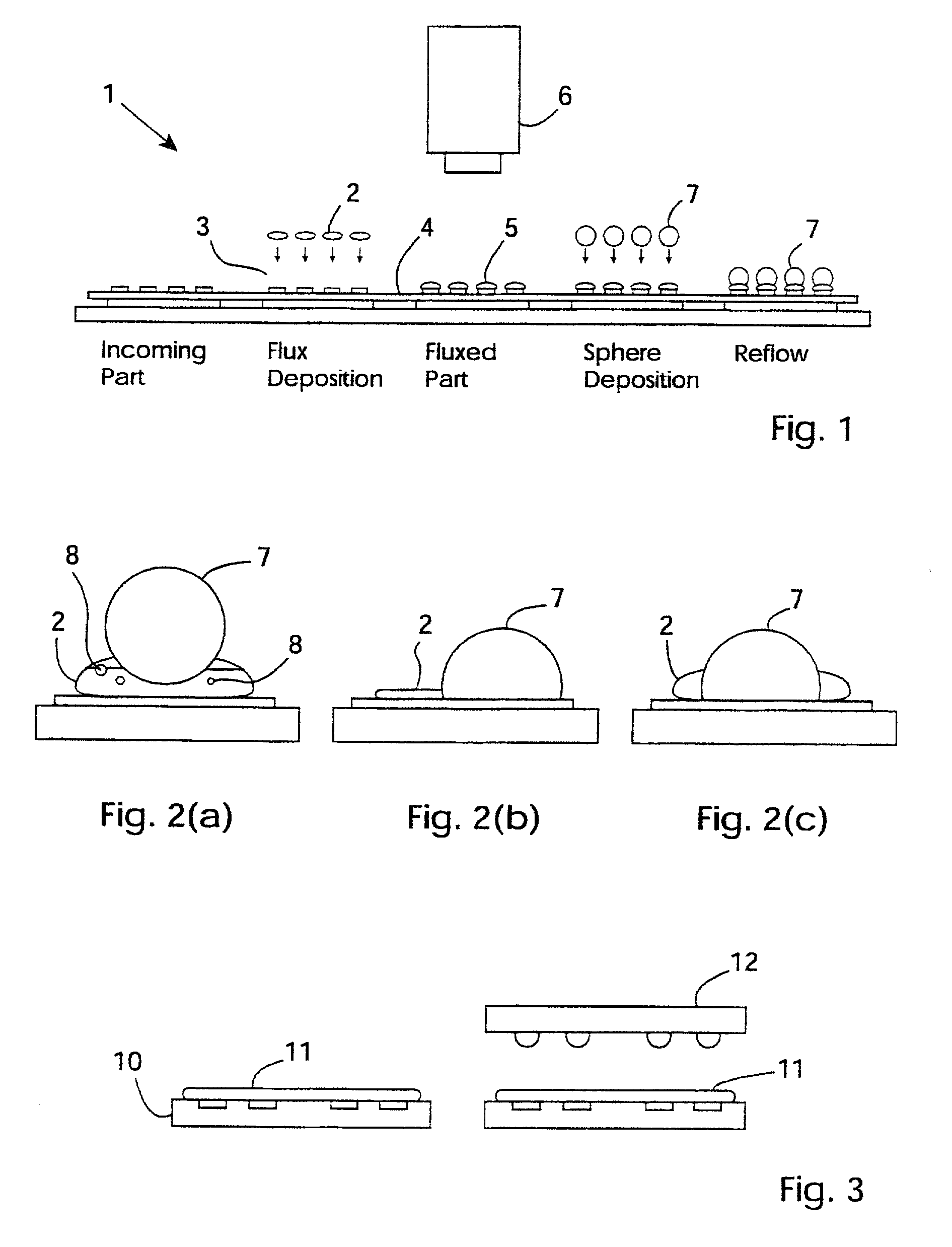
An inspection station (6) has a ring of 370 nm LEDs (24) for low-angle diffuse illumination of flux. This stimulates inherent fluorescent emission of the flux without the need for flux additives or pre-treatment. A CCD sensor (20) detects the emission. An image processor generates output data indicating flux volume according to a relationship between emission intensity and volume over the surface of the flux. Intensity non-uniformity indicates either height non-uniformity or hidden voids, both of which give rise to defects after application of solder paste and reflow. The inspection is particularly effective for pre solder application flux inspection.
Owner:AGILENT TECH INC
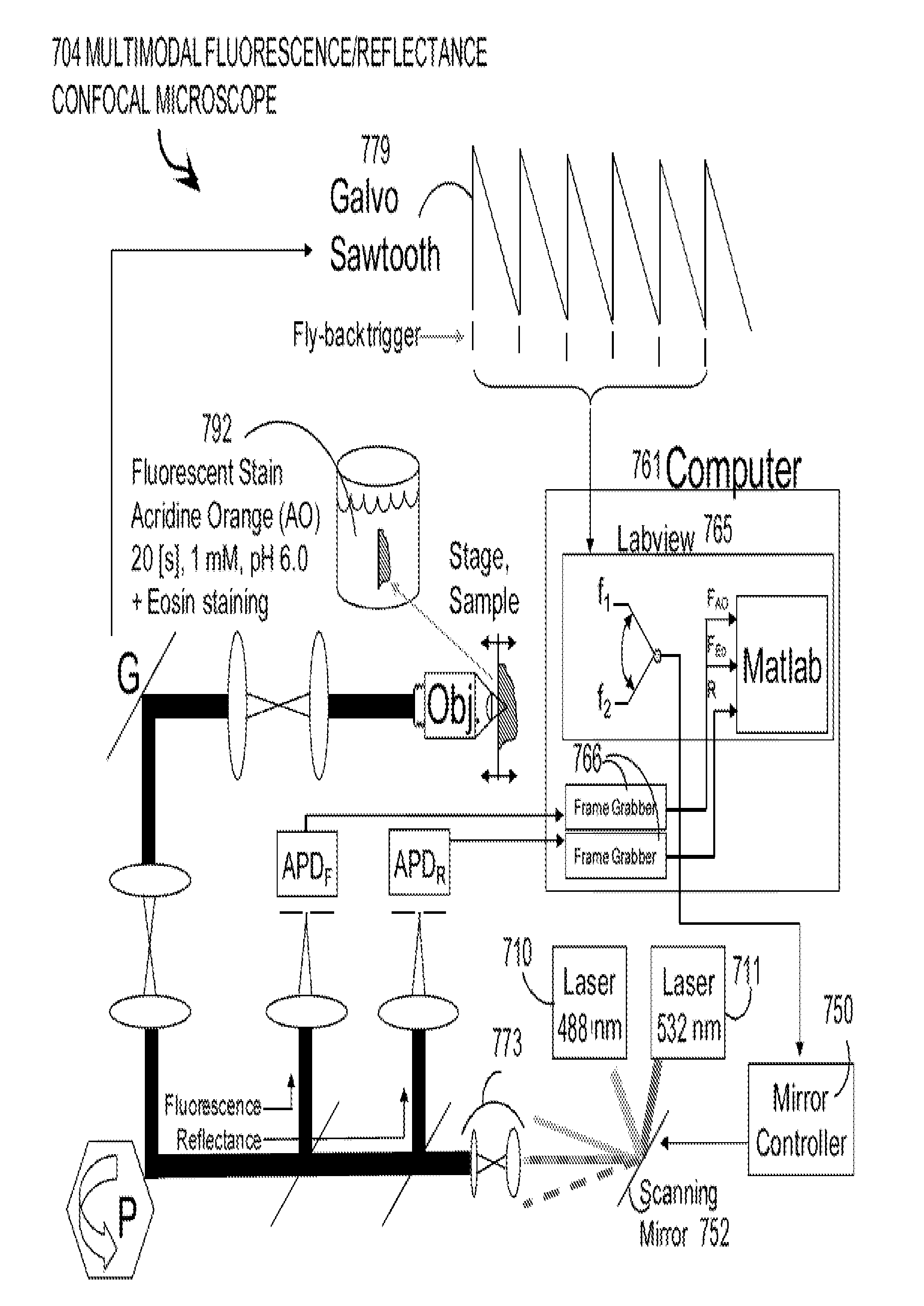
Rapid confocal microscopy to support surgical procedures
ActiveUS20110116694A1Prevent lateral movementMicrobiological testing/measurementPreparing sample for investigationOptical propertyFluorescence
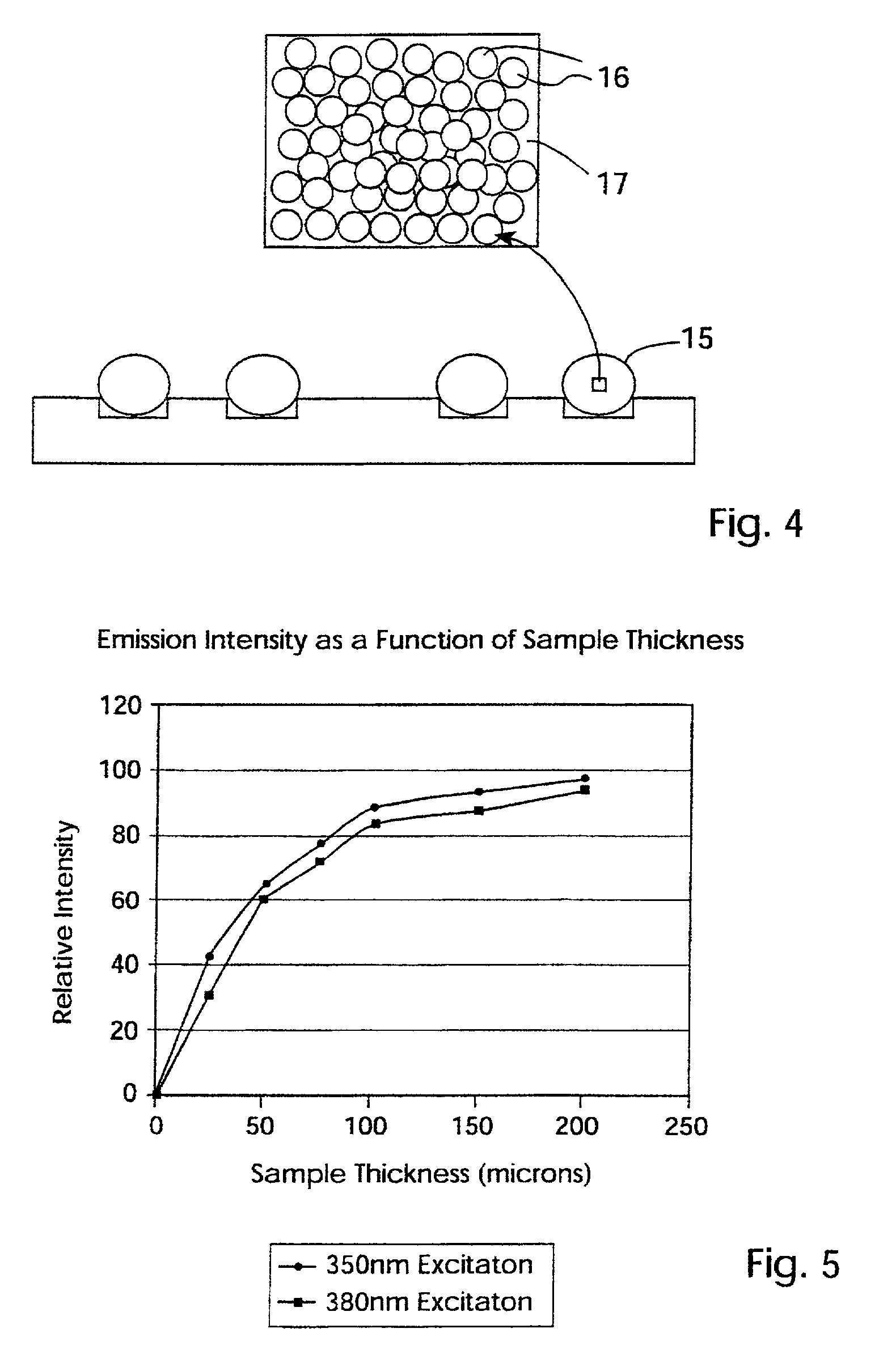
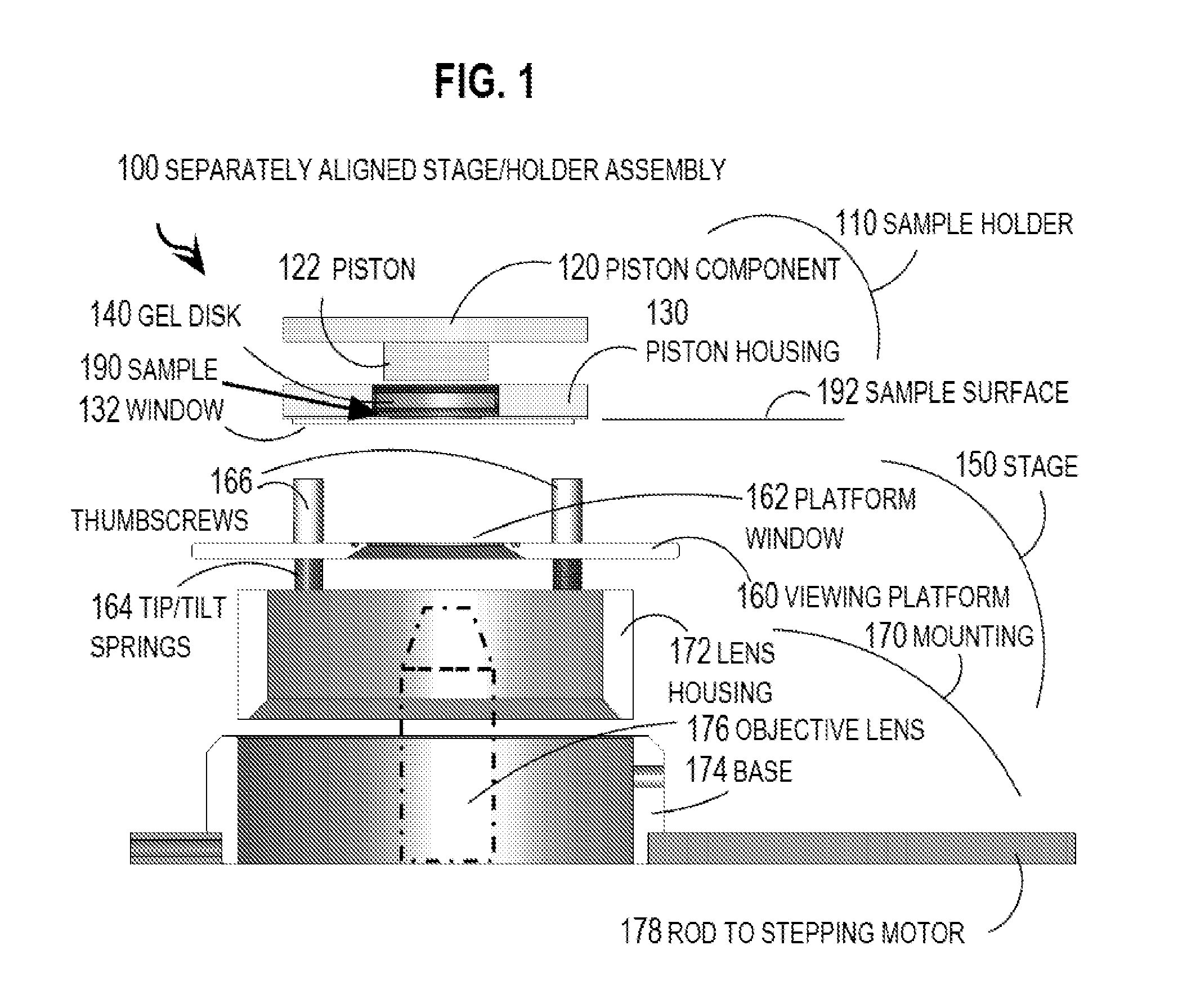
One embodiment of techniques for confocal microscopy includes illuminating a spot on a surface of a biological sample. A first emission intensity from the spot is detected in a first range of optical properties; and a second emission intensity in a second range. A pixel that corresponds to the spot is colored using a linear combination of the first and second emission intensities. Sometimes, the pixel is colored to approximate a color produced by histology. In some embodiments, a surface of a sample is contacted with a solution of acridine orange. Then, a spot is illuminated with a laser beam of wavelength about 488 nanometers (nm). Fluorescence emission intensity is detected above about 500 nm. Sometimes, a certain illumination correction is applied. In some embodiments, a sample holder that compresses a sample is removable from a stage that is fixed with respect to a focal plane of the microscope.
Owner:SLOAN KETTERING INST FOR CANCER RES +1
Phosphor, Phosphor Sheet, and Manufacturing Method Therefore, and Light Emission Device Using the Phosphor
InactiveUS20090026915A1High efficient excitation bandImprove emission efficiencyDischarge tube luminescnet screensLamp detailsUltravioletEmission efficiency
To provide a phosphor having a broad emission spectrum in a range of blue color (in a peak wavelength range from 400 nm to 500 nm), having a broad flat excitation band in a near ultraviolet / ultraviolet range, and having excellent emission efficiency and emission intensity / luminance. The phosphor is given as a general composition formula expressed by MmAaBbOoNn:Z, (where element M is the element having bivalent valency, element A is the element having tervalent valency, element B is the element having tetravalent valency, O is oxygen, N is nitrogen, and element Z is more than one kind of element acting as an activator), satisfying 5.0<(a+b) / m<9.0, 0≦a / m≦2.0, 0≦o≦n, n=2 / 3m+a+4 / 3b−2 / 3o, and has an emission spectrum with a maximum peak in the wavelength range from 400 nm to 500 nm under an excitation of the light in a wavelength range from 250 nm to 430 nm.
Owner:MITSUBISHI CHEM CORP
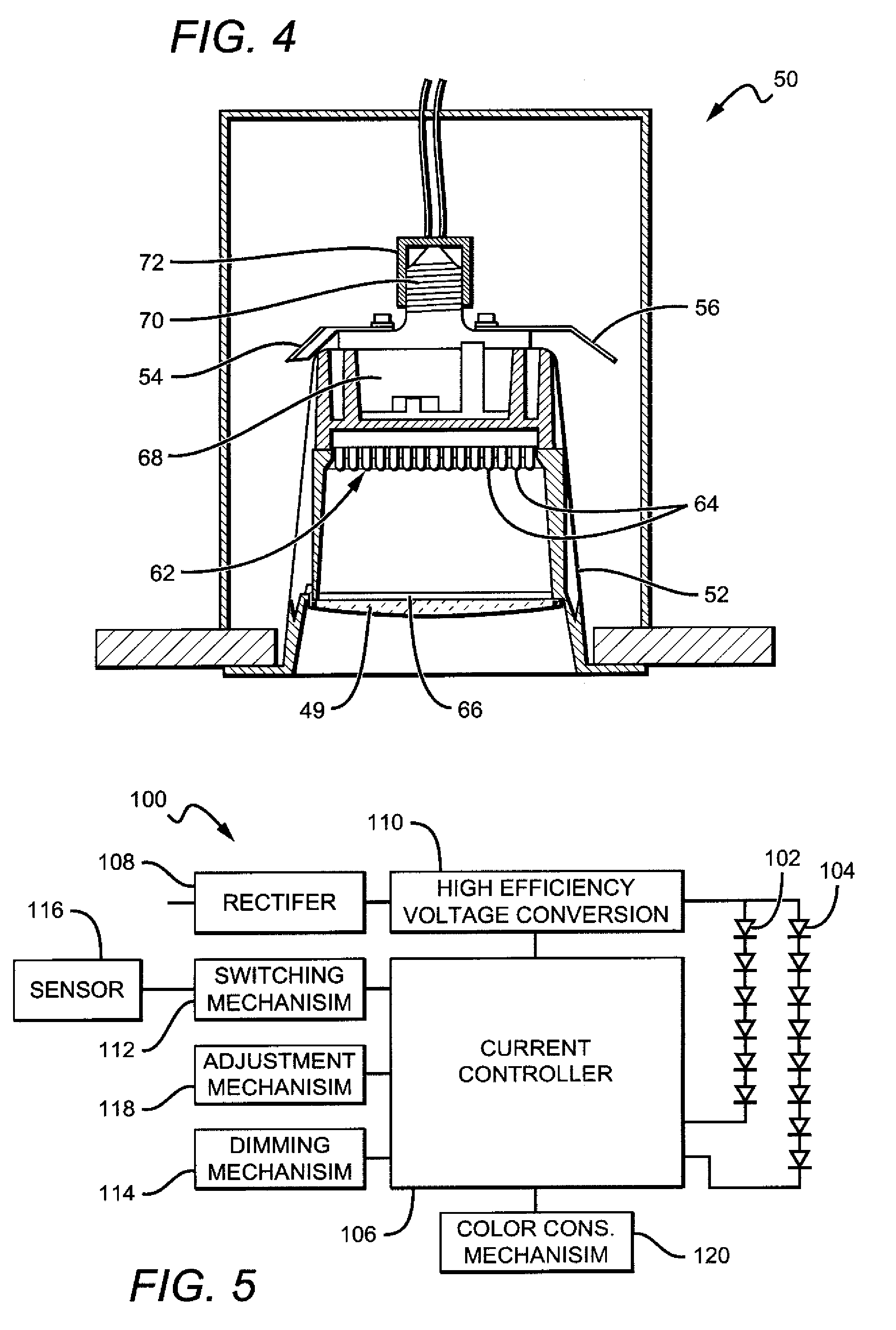
Light emitting apparatus
ActiveUS20080030976A1High emission intensityImprove reliabilityDischarge tube luminescnet screensLamp detailsWeather resistancePhosphor
A light emitting apparatus with high emission intensity and that is superior in weather resistance and reliability is obtained. The light emitting apparatus includes a light source and a wavelength-converting member for converting the wavelength of light emitted from the light source, wherein the wavelength-converting member contains a phosphor subjected to a cleaning treatment and / or a coating treatment, in a glass material having a composition of SiO2: 30 to 50%, Li2O: 0 to 15%, Na2O: 0 to 10%, K2O: 0 to 10%, Li2O+Na2O+K2O: 20 to 30%, B2O3: 5 to 15%, MgO: 0 to 10%, BaO: 0 to 10%, CaO: 0 to 10%, SrO: 0 to 10%, Al2O3: 0 to 10%, ZnO: 0 to 15%, TiO2: 10 to 20%, Nb2O5: 1 to 5%, La2O3: 0 to 5%, and TiO2+Nb2O5+La2O3: 11 to 20% by mole percentage.
Owner:NICHIA CORP
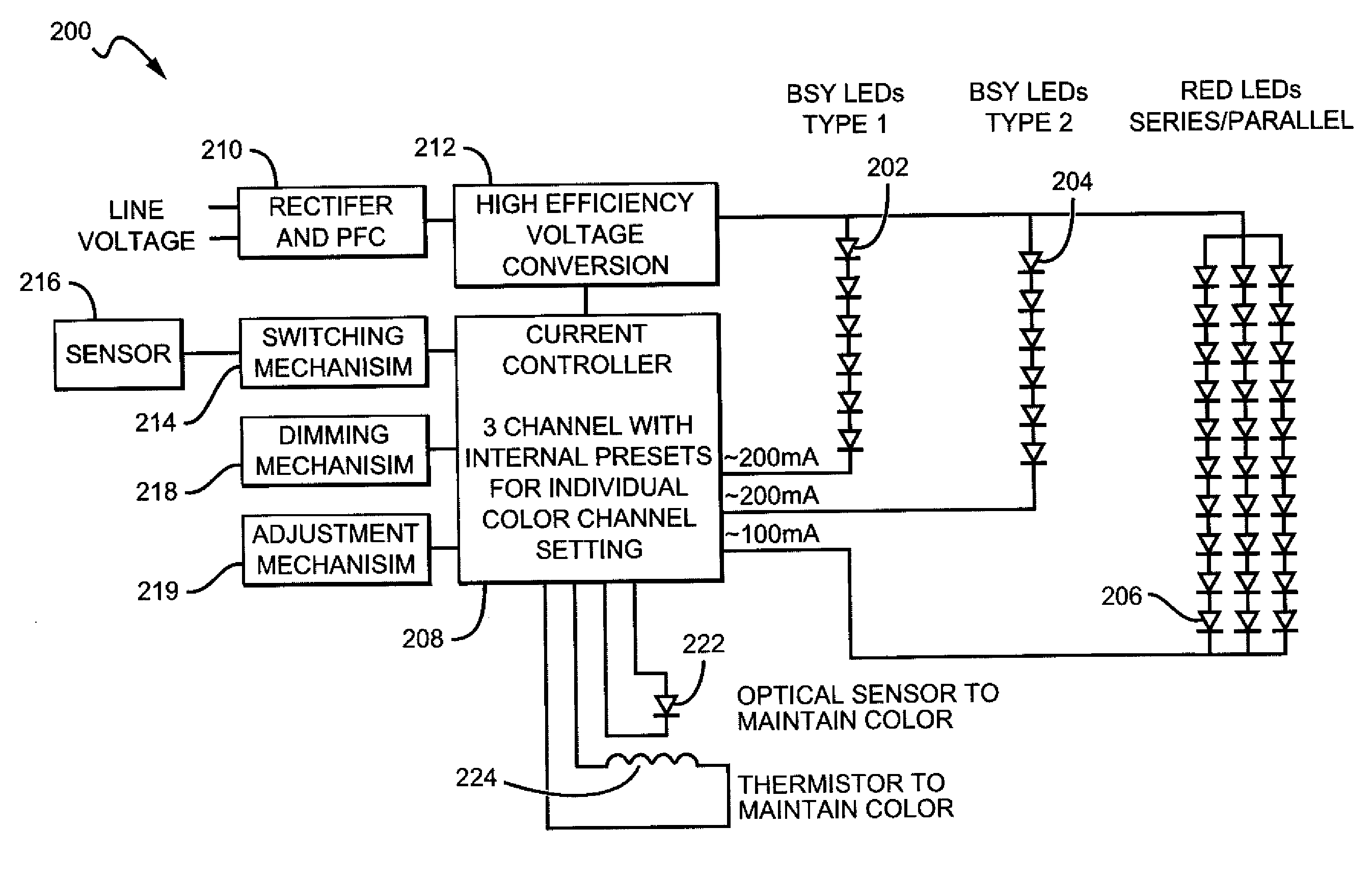
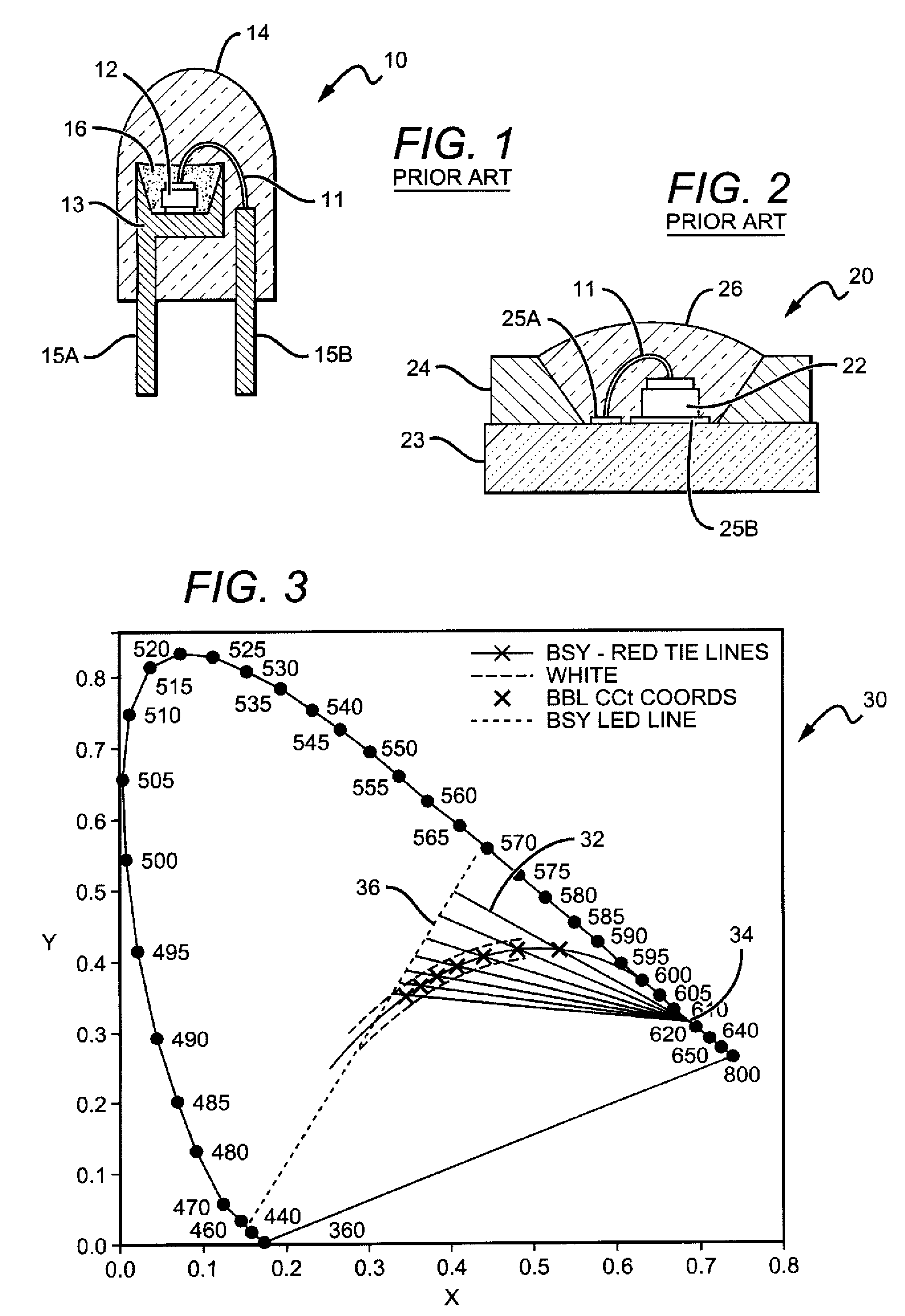
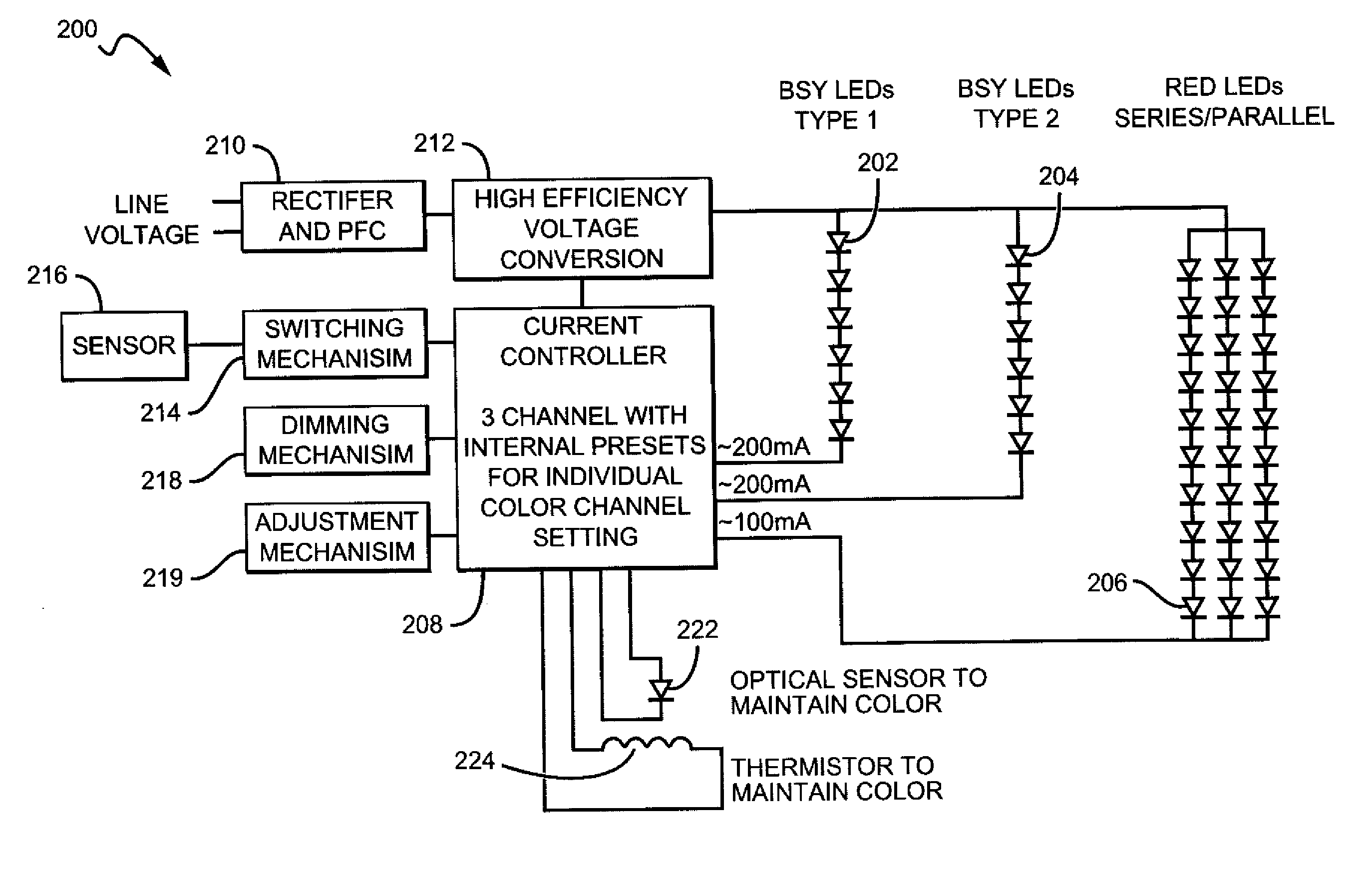
White light color changing solid state lighting and methods
Solid state lighting (SSL) luminaries are disclosed wherein the emission intensity of discrete light sources within the SSL luminaire can be varied to produce luminaire light having different characteristics. The present invention can utilize the unique circuit topology of SSL luminaires to vary the emission intensity of different types of LEDs in the luminaire. In some embodiments, the different types of LEDs are connected in respective serial strings, and the intensity of emission of the LEDs in each of the strings can be varied by changing the electrical signal driving the strings. In some of these embodiments, white light is emitted from the SSL luminaire by combining emission from BSY and red LEDs. For these embodiments the color changing solutions according to the present invention can include, as an example, changing color while dimming the luminaire, changing color between daytime and nighttime modes, and changing between most efficient and points in between.
Owner:IDEAL IND LIGHTING LLC
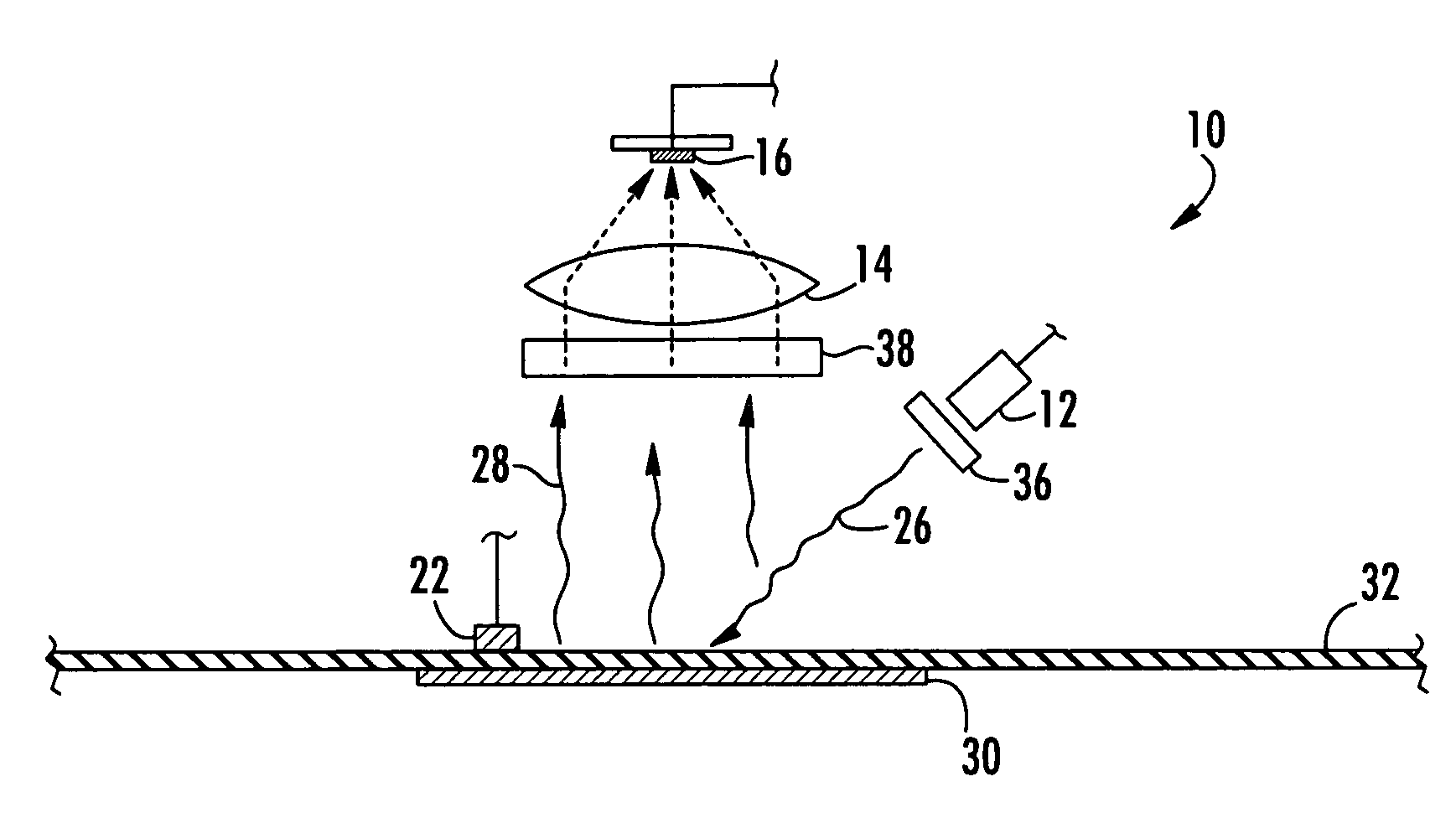
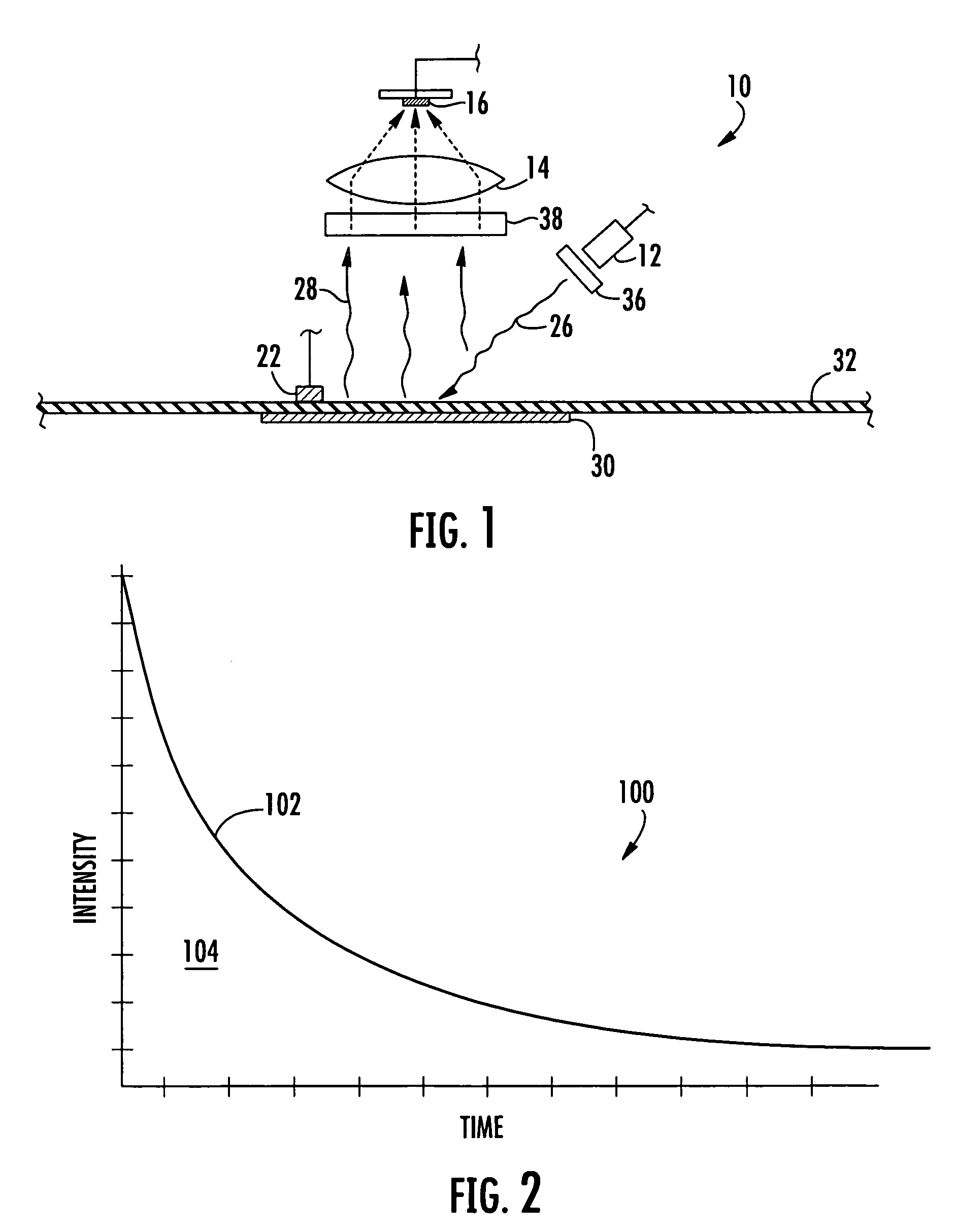
Method and apparatus for measuring oxygen concentration
InactiveUS7569395B2Lower Level RequirementsHigh sensitivityChemiluminescene/bioluminescenceWithdrawing sample devicesOxygenNon invasive
An apparatus and non-invasive method of measuring oxygen by exciting a luminescent compound disposed in a container and then measuring the intensity of the light emitted by the excited luminescent compound as it relaxes to the ground state. A plot of emission intensity as a function of time results in an exponential decay curve the area of which is inversely proportional to the oxygen concentration. The oxygen concentration can be determined over a wide temperature range by measuring the temperature of the container and the emission intensity and then applying the following equation:[O2]=(ATa(T)2+BTa(T)+CTa)(tau)2+(ATb(T)2+BTb(T)+CTb)(tau)+(ATc(T)2+BTc(T)+CTc)T is the measured temperature;tau is the area of the exponential decay curve; andATa, BTa, CTa, ATb, BTb, CTb, ATc, BTc, and CTc are coefficients that are specific to the luminescent compound being examined.
Owner:CRYOVAC ILLC
Method, device and security system, all for authenticating a marking
InactiveUS7067824B2Guaranteed high speed operationImprove signal-to-noise ratioPaper-money testing devicesPhotometrySecurity systemAtomic physics
A luminescent probe marking is excited with at least one excitation pulse of at least one excitation source. The probe intensity values of emission intensity from emission radiation of the luminescent probe marking are measured in response to the excitation pulse(s) at time intervals. A probe intensity-versus-time emission function is formed of the probe intensity values, and the probe intensity-versus-time emission function is compared with at least one reference intensity-versus-time emission function after the probe intensity-versus-time emission function and the reference intensity-versus-time emission function have been normalized.
Owner:SICPA HLDG SA
Phosphor and manufacturing method for the same, and light source
ActiveUS7476337B2Easy to manufactureReduce manufacturing costElectrical apparatusElectroluminescent light sourcesPhosphorX-ray
To provide a phosphor having an emission characteristic such that a peak wavelength of light emission is in a range from 580 to 680 nm, and having a high emission intensity, and having a flat excitation band with high efficiency for excitation light in a broad wavelength range from ultraviolet to visible light (wavelength range from 250 nm to 550 nm). For example, Ca3N2(2N), AlN(3N), Si3N4(3N), Eu2O3(3N) are prepared, and after weighing and mixing a predetermined amount of each raw material, raw materials are fired at 1500° C. for 6 hours, thus obtaining the phosphor including a product phase expressed by a composition formula CaAlSiN3:Eu and having an X-ray diffraction pattern satisfying a predetermined pattern.
Owner:CITIZEN ELECTRONICS CO LTD +1
White light color changing solid state lighting and methods
Solid state lighting (SSL) luminaries are disclosed wherein the emission intensity of discrete light sources within the SSL luminaire can be varied to produce luminaire light having different characteristics. The present invention can utilize the unique circuit topology of SSL luminaires to vary the emission intensity of different types of LEDs in the luminaire. In some embodiments, the different types of LEDs are connected in respective serial strings, and the intensity of emission of the LEDs in each of the strings can be varied by changing the electrical signal driving the strings. In some of these embodiments, white light is emitted from the SSL luminaire by combining emission from BSY and red LEDs. For these embodiments the color changing solutions according to the present invention can include, as an example, changing color while dimming the luminaire, changing color between daytime and nighttime modes, and changing between most efficient and points in between.
Owner:IDEAL IND LIGHTING LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com