Doped-state spherical FeF<3>.0.33H<2>O positive electrode material and preparation method therefor
A positive electrode material, spherical technology, applied in the field of new energy material preparation, can solve the problems of poor electrochemical performance, low electronic conductivity of ferric fluoride, low tap density, etc., to achieve high tap density, excellent electrochemical performance, rough surface effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
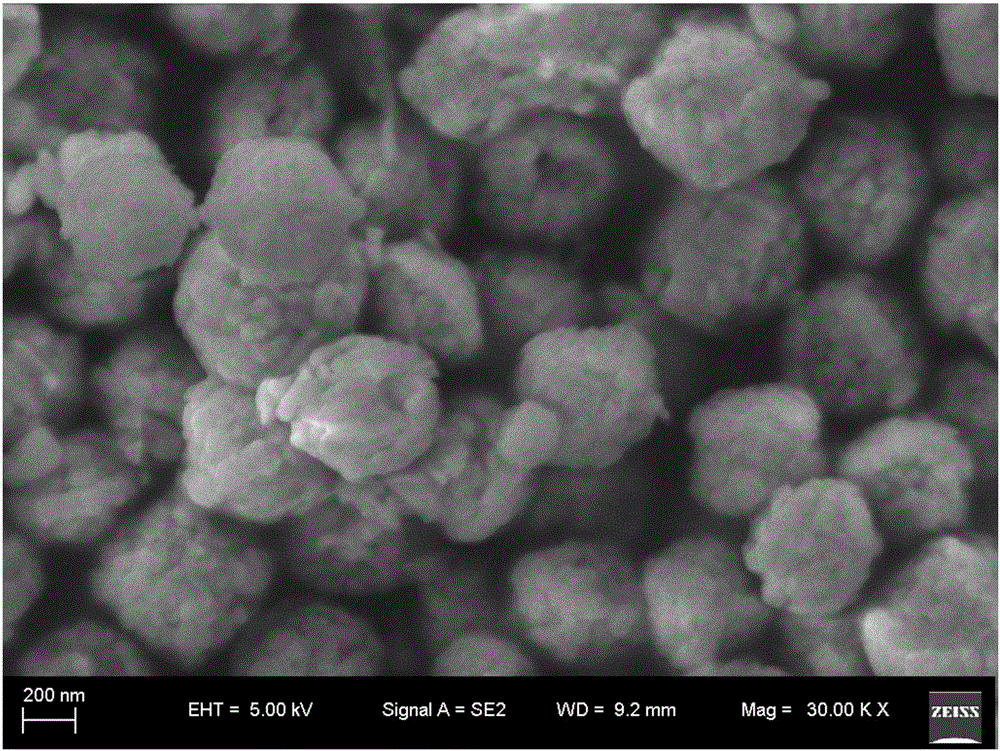
[0034] At room temperature, weigh 4.04g Fe(NO 3 ) 3 9H 2 O, 0.384g Mg(NO 3 ) 2 ·6H 2 O was placed in a polytetrafluoroethylene reactor, and 50 mL of anhydrous methanol was added, magnetically stirred to dissolve, and 2 mL of HF aqueous solution with a mass fraction of 40% was added dropwise under constant stirring, and stirred for 15 minutes. Put the reactor in a hydrothermal reaction kettle and seal it, and heat it at 180°C for 6 hours to obtain a mixed solution with a layer of sediment on the bottom and a clarified upper part. Pour off the supernatant, wash the precipitate into a centrifuge tube with absolute ethanol and centrifuge to obtain a white Precipitate, wash twice with ethanol, centrifuge, and vacuum dry at 80°C for 12 hours to obtain white Fe 0.87 Mg 0.13 f 2.87 0.33H 2 O powder, by testing it was found that the Fe 0.87 Mg 0.13 f 2.87 0.33H 2 O is a spherical particle with a particle size of about 500 nm.
Embodiment 2
[0036] At room temperature, weigh 4.04g Fe(NO 3 ) 3 9H 2 O, 0.256g Mg(NO 3 ) 2 ·6H 2 O was placed in a polytetrafluoroethylene reactor, and 50 mL of anhydrous methanol was added, stirred magnetically to dissolve, and 2 mL of HF aqueous solution with a mass fraction of 40% was added dropwise under continuous stirring, and stirred for 10 minutes. Put the reactor in a hydrothermal reaction kettle and seal it, and heat it at 180°C for 6 hours to obtain a mixed solution with a layer of sediment on the bottom and a clarified upper part. Pour off the supernatant, wash the precipitate into a centrifuge tube with absolute ethanol and centrifuge to obtain a white Precipitate, wash twice with ethanol, centrifuge, and vacuum dry at 60°C for 16 hours to obtain white Fe 0.91 Mg 0.09 f 2.91 0.33H 2 O powder, by testing it was found that the Fe 0.91 Mg 0.09 f 2.91 0.33H 2 O is a spherical particle with a particle size of about 1 micron.
Embodiment 3
[0038] At room temperature, weigh 4.04g Fe(NO 3 ) 3 9H 2 O, 0.512g Mg(NO 3 ) 2 ·6H 2 O was placed in a polytetrafluoroethylene reactor, and 50 mL of anhydrous methanol was added, stirred magnetically to dissolve, and 2.1 mL of HF aqueous solution with a mass fraction of 45% was added dropwise under constant stirring, and stirred for 15 minutes. Put the reactor in a hydrothermal reaction kettle and seal it, and heat it at 190°C for 6 hours to obtain a mixed liquid covered with a layer of precipitate on the bottom and clear on the upper part. Pour off the supernatant, wash the precipitate into a centrifuge tube with absolute ethanol and centrifuge to obtain a white Precipitated, washed with ethanol for 3 times, centrifuged, and vacuum dried at 80°C for 12 hours to obtain white Fe 0.83 Mg 0.17 f 2.83 0.33H 2 O powder, by testing it was found that the Fe 0.83 Mg 0.17 f 2.83 0.33H 2 O is a spherical particle with a particle size of about 600 nanometers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Quality score | aaaaa | aaaaa |
| Quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com