Technology for producing sodium fluosilicate
A sodium fluorosilicate and production process technology, applied in the field of sodium fluorosilicate production process, can solve the problems of difficult sewage treatment in phosphoric acid production enterprises, large consumption of sodium sulfate, etc., to reduce sodium ion content, save treatment costs, reduce The effect of consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
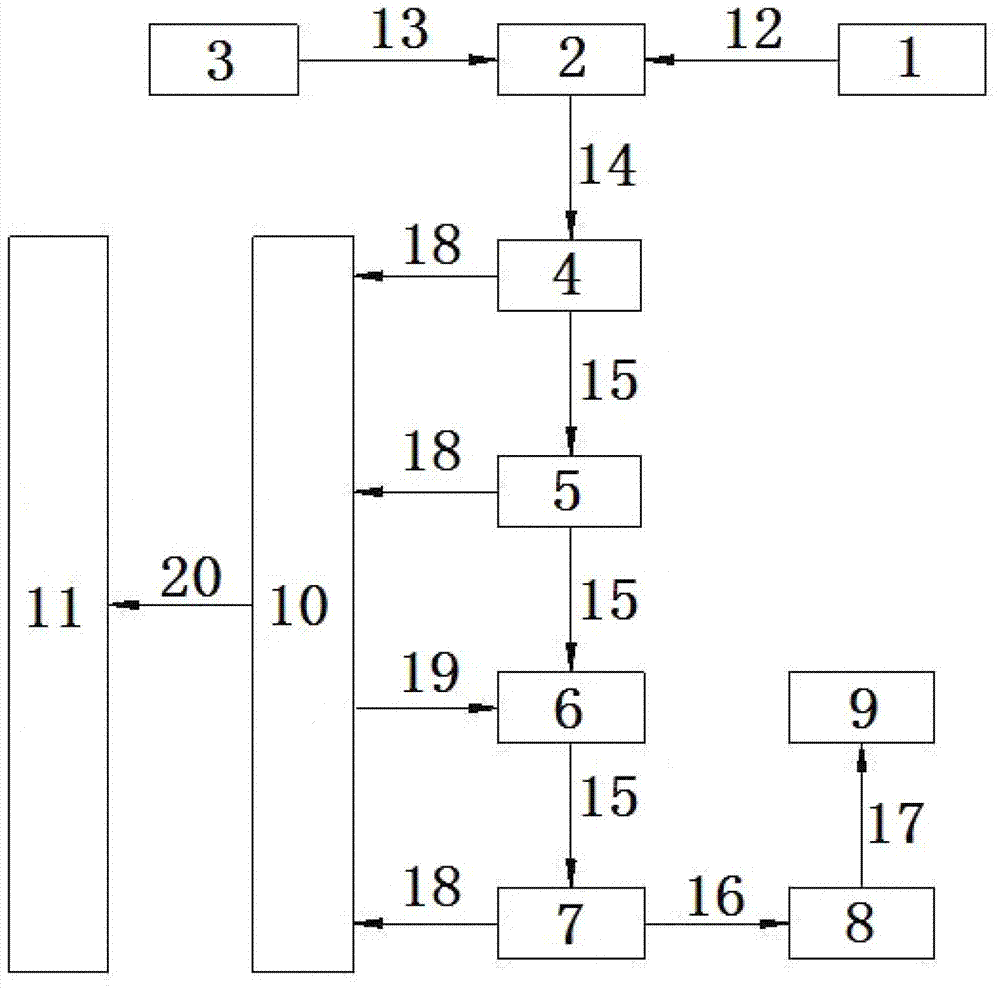
[0040] Sodium sulfate is prepared into sodium sulfate solution 12 with a mass fraction of 26% in the salt tank 1; the sodium sulfate solution 12 is sent to the synthesis tank 2 with a pump, and it is added from the fluosilicic acid storage tank 3 while stirring. The mass fraction is 8% fluosilicic acid 13; control the consumption of sodium sulfate solution 12, adjust the consumption of fluosilicic acid 13, make the consumption of fluosilicic acid 13 more than 4% of the theoretical dosage calculated according to the chemical equation; sulfuric acid The sodium fluorosilicate magma 14 obtained after the reaction of sodium 12 and fluorosilicate 13 in the synthesis tank 2 is fed into the first crystallizer 4 to grow crystals to obtain sodium fluorosilicate crystals 15, and then enters the second crystallizer 5 to grow crystal; after the crystal growth is finished, wash off excess free acid with appropriate amount of industrial clear water 19 in the washing tank 6 to ensure the cryst...
Embodiment 2
[0042] Sodium sulfate is prepared into sodium sulfate solution 12 with a mass fraction of 29% in the chemical salt tank 1; the sodium sulfate solution 12 is sent to the synthesis tank 2 with a pump, and it is added from the fluosilicic acid storage tank 3 while stirring. The mass fraction is 11% fluosilicic acid 13; control the consumption of sodium sulfate solution 12, regulate the consumption of fluosilicic acid 13, make the consumption of fluosilicic acid 13 more than 4% of the theoretical consumption calculated according to chemical equation; Sulfuric acid The sodium fluorosilicate magma 14 obtained after the reaction of sodium 12 and fluorosilicate 13 in the synthesis tank 2 is fed into the first crystallizer 4 to grow crystals to obtain sodium fluorosilicate crystals 15, and then enters the second crystallizer 5 to grow crystal; after the crystal growth is finished, wash off excess free acid with appropriate amount of industrial clear water 19 in the washing tank 6 to ens...
Embodiment 3
[0044] Sodium sulfate is prepared into sodium sulfate solution 12 with a mass fraction of 32% in the salt tank 1; the sodium sulfate solution 12 is sent to the synthesis tank 2 with a pump, and is added from the fluosilicic acid storage tank 3 while stirring. The mass fraction is 14% fluosilicic acid 13; control the consumption of sodium sulfate solution 12, regulate the consumption of fluosilicic acid 13, make the consumption of fluosilicic acid 13 more than 6% of the theoretical consumption calculated according to chemical equation; Sulfuric acid The sodium fluorosilicate magma 14 obtained after the reaction of sodium 12 and fluorosilicate 13 in the synthesis tank 2 is fed into the first crystallizer 4 to grow crystals to obtain sodium fluorosilicate crystals 15, and then enters the second crystallizer 5 to grow crystal; after the crystal growth is finished, wash off excess free acid with appropriate amount of industrial clear water 19 in the washing tank 6 to ensure the crys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com