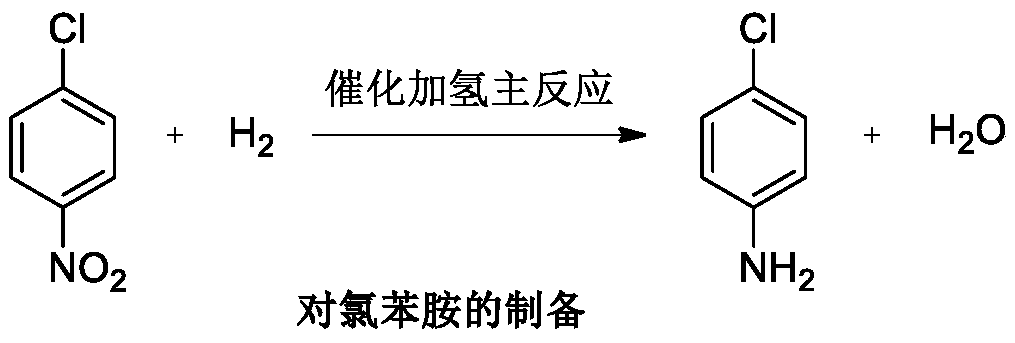
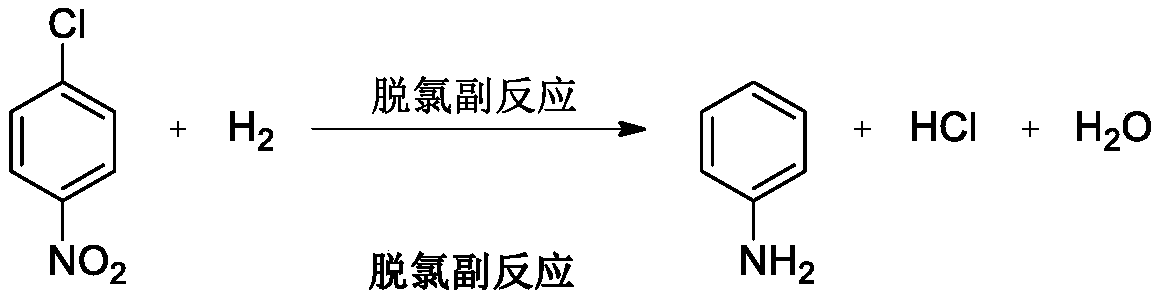
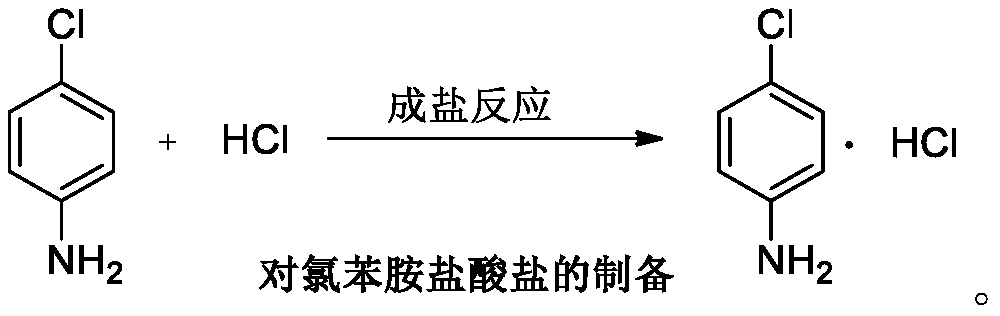
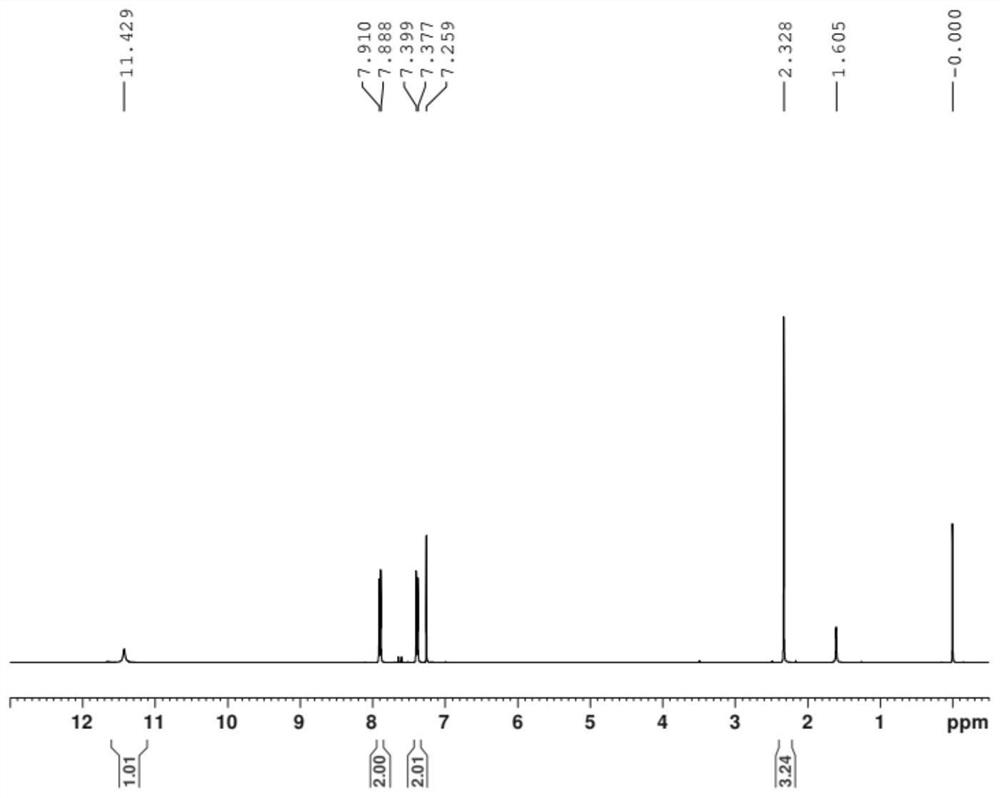
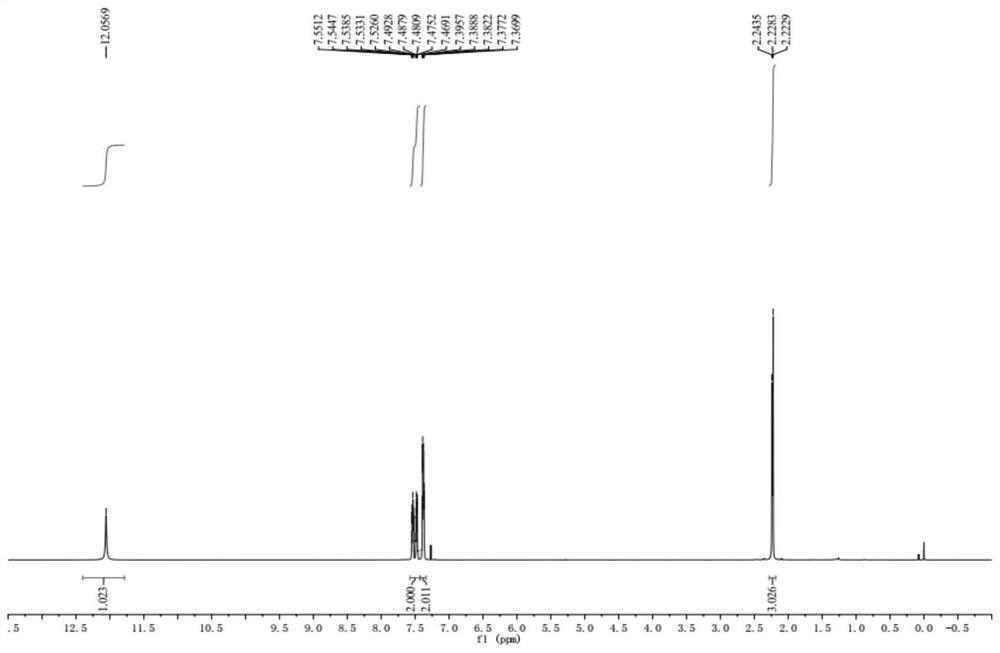
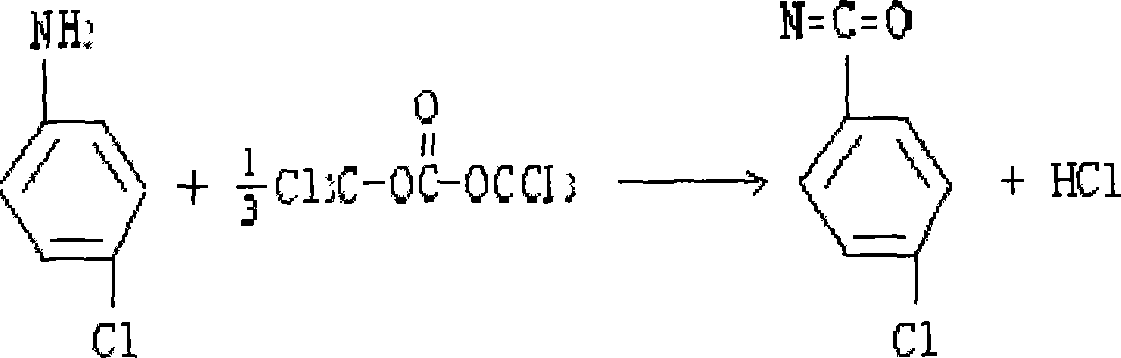Patents
Literature
99 results about "P-chloroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Water-soluble, chlorhexidine-containing compositions and use thereof
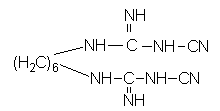
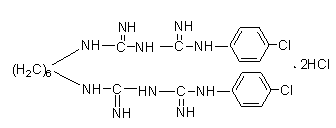
The invention relates to water-soluble, chlorhexidine-containing compositions having a long shelf life. The compositions are in the form of powder, granules or tablets and consist substantially of (i) a salt of chlorhexidine with 2 moles of a sugar acid and a microbicidally active quaternary ammonium bromide, or of (ii) chlorhexidine, a sugar acid or sugar lactone, with at least 2 moles of sugar acid or sugar lactone being present per mole of chlorhexidine, and a microbicidally active quaternary ammonium bromide. Peferred compositions consist substantially of chlorhexidine digluconate and N-cetyl-N,N,N-trimethylammonium bromide (cetrimide) in a weight ratio of 1:1 to 1:10. The solid compositions are stable, with little or no p-chloroaniline splitting off during storage.
Owner:EVONIK DEGUSSA GMBH
Treatment method for acyl-chlorination reaction tail gas
InactiveCN103908870ASafe and easy production operationDoes not affect product qualityHydrazine preparationDispersed particle separationP-chloroanilineAcyl group
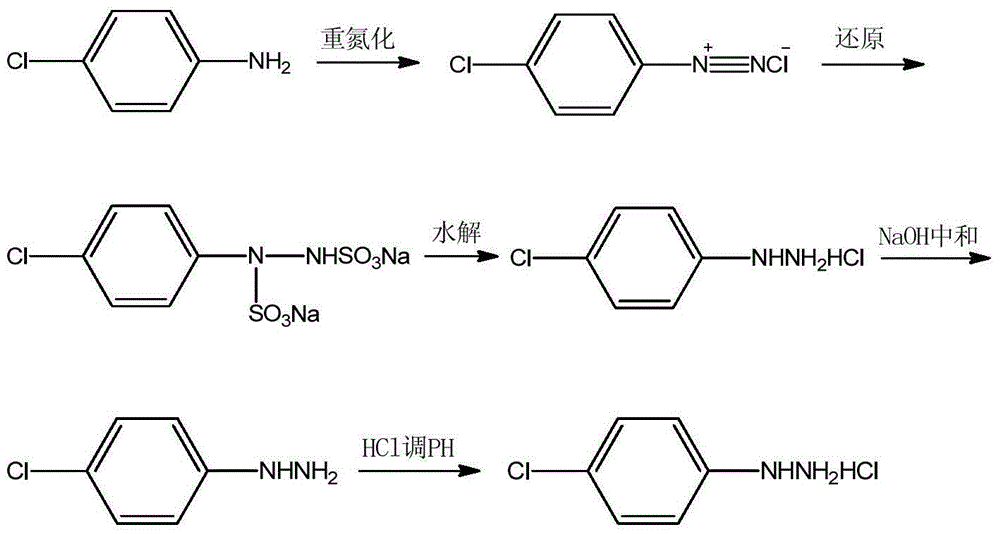
The invention relates to a treatment method for acyl-chlorination reaction tail gas. The reaction tail gas successively enters a three-step series hydrogen chloride falling-film absorption tower and a two-step series ammonium hydroxide absorption tower; p-chloroaniline and recovered hydrochloric acid are taken as raw materials and subjected to diazotization reaction for generating a diazo salt; and an aqueous solution of ammonium sulfite is taken as a reducing agent, after the diazo salt is dropwise added at room temperature, the recovered hydrochloric acid is dropwise added for acidification hydrolysis and further for preparing p-chlorophenylhydrazine hydrochloride. By employing the method to treat the acyl-chlorination reaction tail gas, tedious technologies for discharging and drying solid materials and selecting low-temperature equipment for liquefying sulfur dioxide are avoided, and the production operation is safe and convenient; and the produced tail gas absorption solution can be used as a raw material for preparing organic chemical products, is environment-friendly and is capable of creating economic benefit.
Owner:TIANJIN QIDONG CHEM PLANT
Novel synthesis process of P-chlorophenylhydrazine hydrochloride
InactiveCN103848752ASimplified processing stepsGood processing effectHydrazine preparationP-chloroanilineReaction temperature
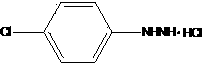
The invention discloses a preparation method of P-chlorophenylhydrazine hydrochloride. The method comprises the following steps: diazotizing chloroaniline taken as a raw material so as to prepare a corresponding diazonium salt; mixing anhydrous sodium sulfite with sodium hydrogen sulfite in a stirring manner, maintaining a pH (Power Of Hydrogen) value within 6 to 7, controlling a temperature below 40 DEG C, slowly adding the diazonium salt below a liquid surface and reacting at the temperature of 60 DEG C to 90 DEG C according to the molar ratio of the p-chloroaniline to the sodium hydrogen sulfite being 1:(1-1):3; maintaining the unchanged pH value in the step 2, slowly adding hydrochloric acid so as to maintain the pH value within 1 to 3, reacting for 1 to 3 hours at the reaction temperature of 40 DEG C to 90 DEG C, cooling to a room temperature, filtering and drying so as to obtain a reddish P-chlorophenylhydrazine hydrochloride solid. The pH value of the reaction system is maintained within 6 to 7 by taking a mixture of the sodium sulfite and the sodium hydrogen sulfite as a reducing agent, so that the phenomenon that the pH value is always regulated by NAOH (sodium hydroxide) or HCL (hydrogen chloride) in a reaction process is avoided; and the repeated heating during the reaction process is not required, so that the temperature is convenient to control; and the adding amount of water is reduced, so that the discharge of wastewater is greatly reduced, namely, the wastewater treatment at a later period is avoided. As a result, the cost is lowered.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of chlorhexidine compound
InactiveCN102993056ASimple processMild reaction conditionsOrganic chemistryOrganic compound preparationP-chloroanilineChemical synthesis
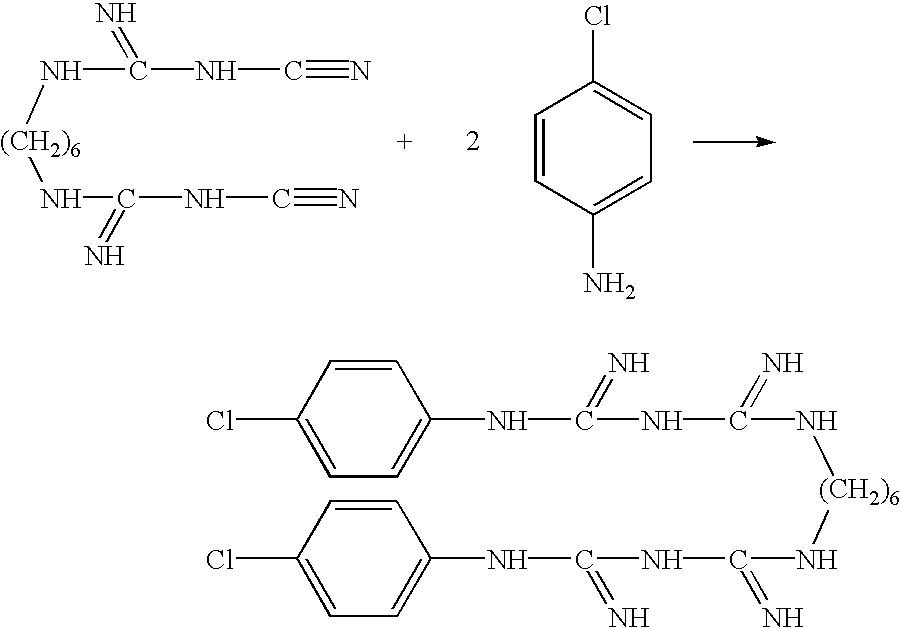
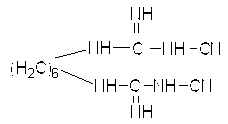
The invention discloses a preparation method of chlorhexidine compound and belongs to the field of organic chemical synthesis. The preparation method comprises the following steps: carrying out a heating reflux reaction on hexamethylene-dicyanoguanidine and chloroaniline hydrochloride as raw materials and glycol ether or normal butanol as a solvent to directly obtain a target compound in a single step. The preparation method is simple in process, mild in reaction condition, short in reaction time, rapid and efficient; as the glycol ether or normal butanol is used as the solvent, the influence of the toxic or side effect of the solvent on a human body is reduced, meanwhile the environmental pollution is reduced; and as a catalyst is not used in the synthesis process, an aftertreatment process is simplified, the synthetic comprehensive cost is effectively reduced, the yield of a final product can reach as high as 84.7%, the purity of the final product can reach as high as 97%, thus the preparation method has a better industrial application prospect.
Owner:GANSU RES INSTION OF CHEM IND GRICI
Detecting method of aromatic amine compound in water sample
InactiveCN102323342ARapid enrichmentHigh sensitivityComponent separationP-chloroaniline4-Aminobiphenyl
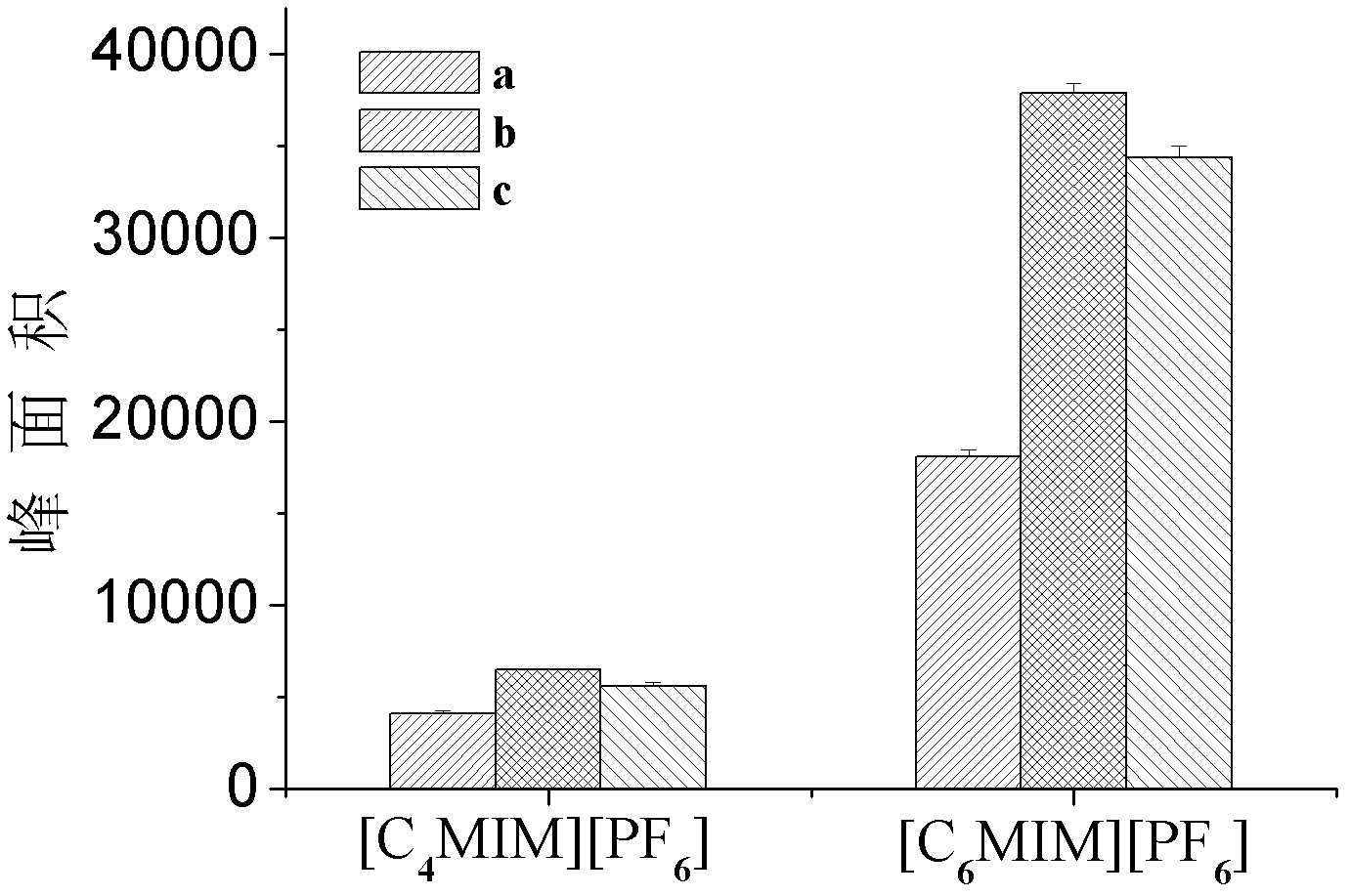
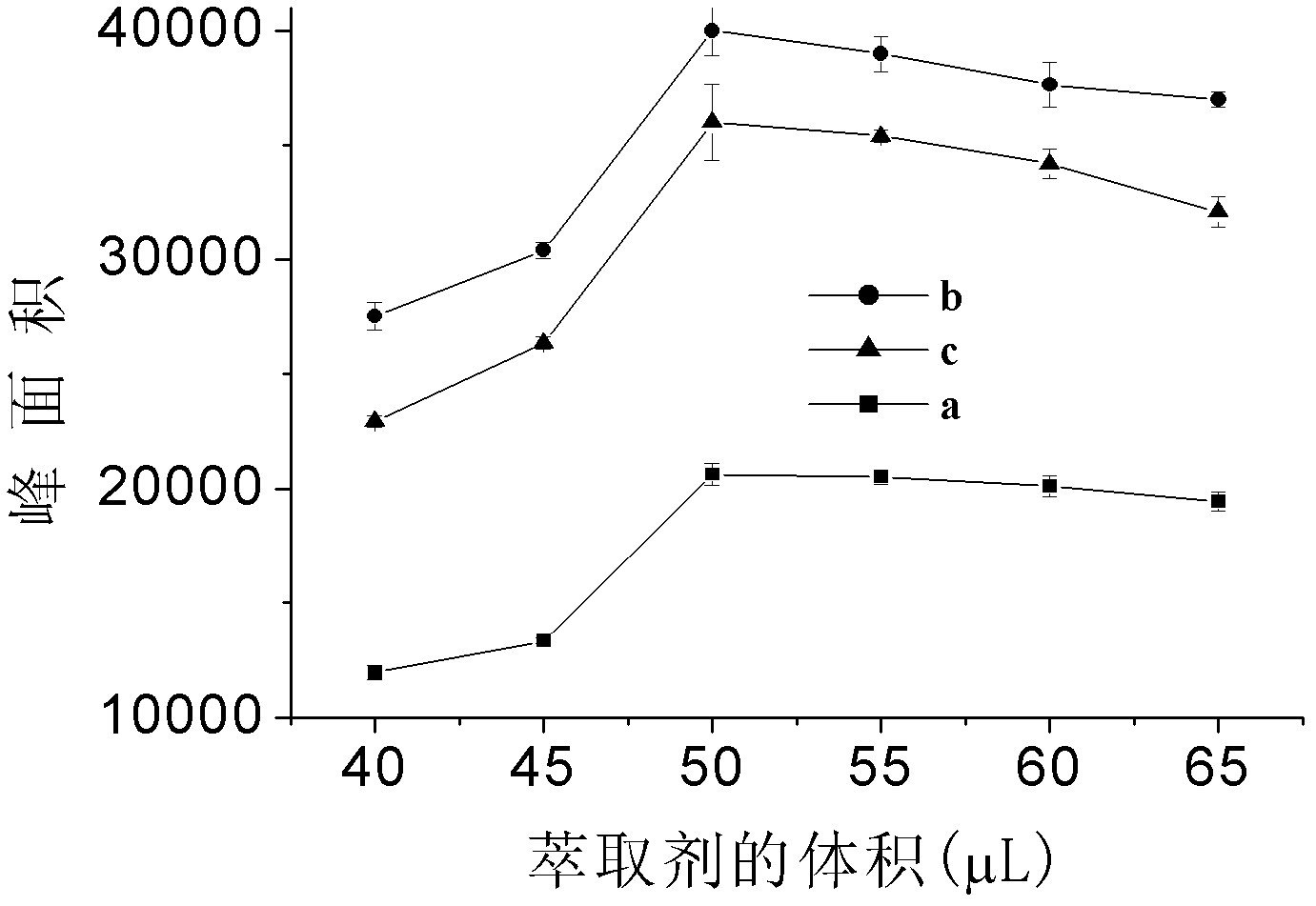
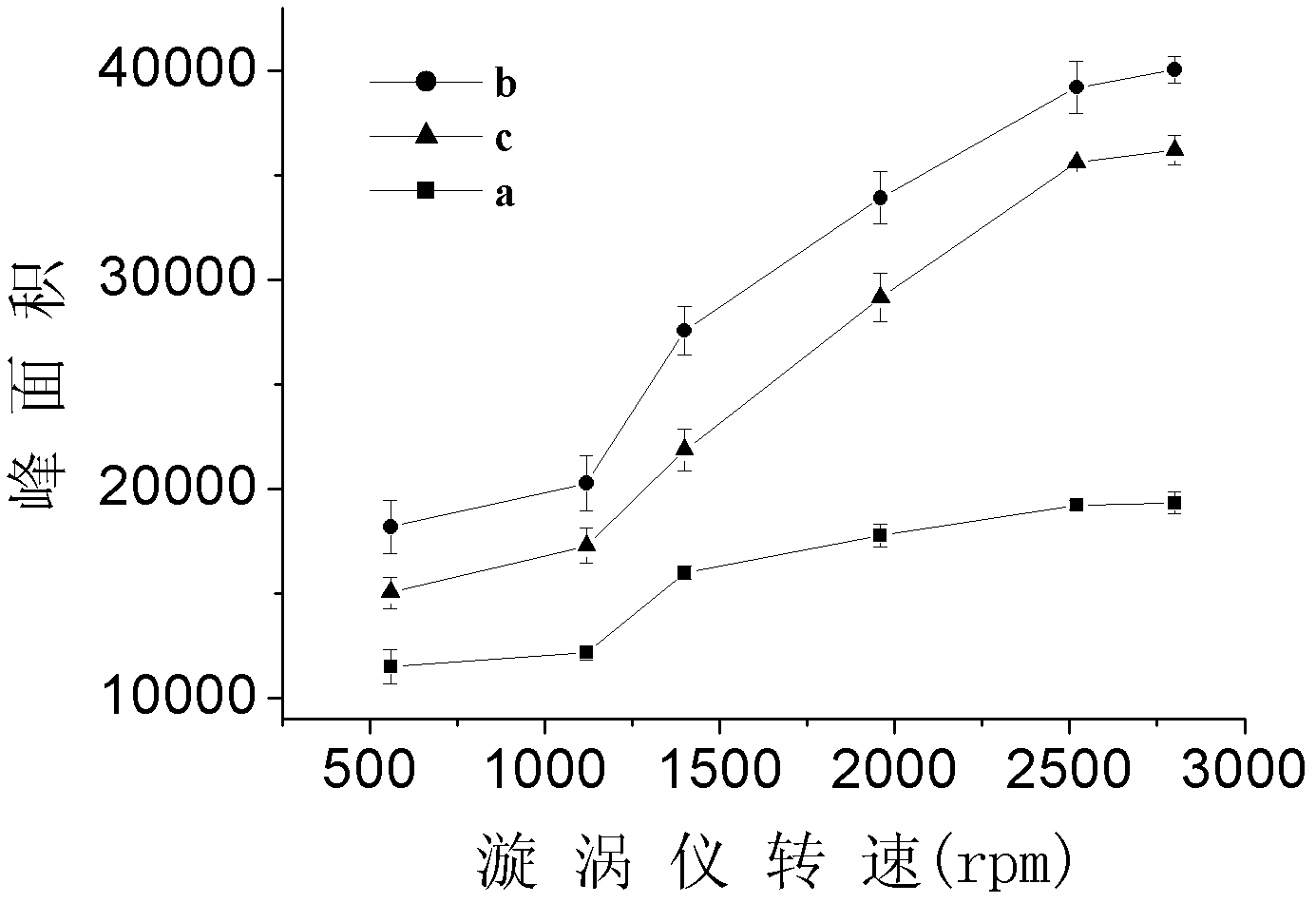
The invention discloses a detecting method of an aromatic amine compound in a water sample, belonging to the technical field of analytical chemistry. The aromatic amine compound is p-chloroaniline, 1-naphthylamine and 4-aminobiphenyl. The detecting method comprises extraction and detection processes, ionic liquid [C6MIN][PF6]or[C4MIM][PF6] is used as an extracting agent, vortex oscillation is adopted to assist the ionic liquid in carrying out microextraction, an ultra fast liquid chromatograph is used for detecting, and a regressive curve equation is used for obtaining a detection result. The detecting method provided by the invention has high sensitivity, low detection limit and good degree of precision; a green reagent is used as the extracting agent, thus, using an organic reagent isavoided, and pollution to the environment is reduced; the detecting method has the characteristics of rapidness, stability and the like, and the time for detecting samples is greatly shortened; and the detecting method can be applied to the detection of the aromatic amine compounds in various water samples and has a certain social significance on protecting the environment and the health of humanbeings.
Owner:JILIN UNIV
Cyclodextrin-modified hypercrosslinked resin and method for adsorbing and recovering aniline compounds in industrial wastewater by using same
ActiveCN107434852AReduce contentImprove stabilityOther chemical processesWater contaminantsP-chloroanilineMaterials preparation
The invention discloses cyclodextrin-modified hypercrosslinked resin and a method for adsorbing and recovering aniline compounds in industrial wastewater by using the same. The method is used for adsorbing aniline compounds such as aniline, p-toluidine, p-chloroaniline and p-aminobenzoic acid in wastewater by taking the synthesized cyclodextrin-modified polystyrene-chloromethylstyrene hypercrosslinked resin as an adsorption material, and the maximum adsorption capacities can respectively reach 149mg / g, 198mg / g, 294mg / g and 623mg / g. Experimental results show that the adsorption capacity is obviously higher than the maximum adsorption capacities of adsorption materials such as activated carbon, coal ash and modified zeolite to the aniline compounds. The method disclosed by the invention has the advantages such as simple material preparation process, repeated utilization, mild adsorption condition, energy saving, environment friendliness and low cost.
Owner:HUIZHOU RES INST OF SUN YAT SEN UNIV
Synthesis method of parachlorophenylhydrazine hydrochloride
InactiveCN105837466AHigh yieldThe synthesis process is simpleHydrazine preparationAfter treatmentSynthesis methods
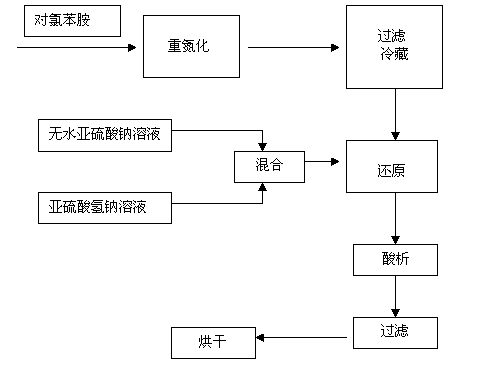
The invention provides a synthesis method of parachlorophenylhydrazine hydrochloride. The synthesis method comprises the following steps: 1. diazotization of parachloroaniline; 2. reduction; 3. acidification; and 4. after-treatment. The synthesis method has the advantages of environment-friendly raw materials, simple synthesis technique, environment friendliness, low cost, high product yield, energy saving and consumption reduction, effectively lowers the production cost, and can enhance the economic benefit of the enterprise.
Owner:JIANGSU TUOQIU AGRI CHEM CO LTD
P-chlorophenylu hydrazine hydrochloride preparation method
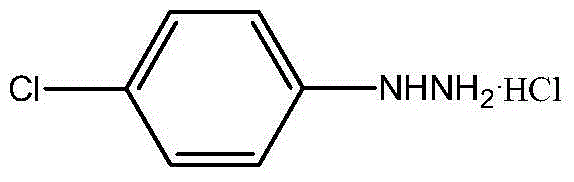
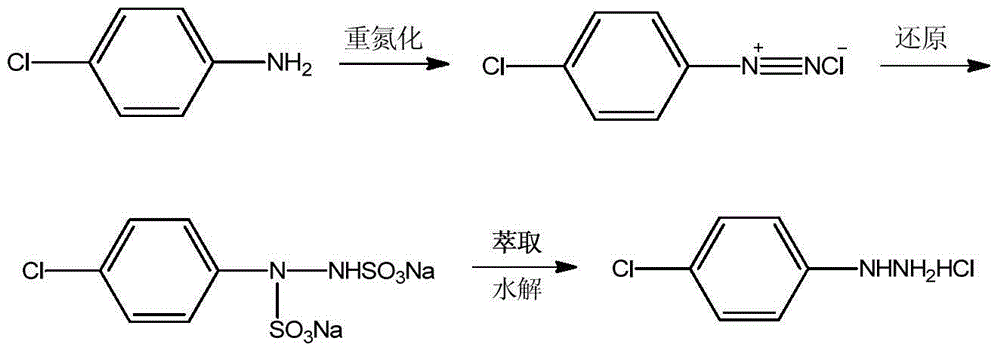
The invention discloses a p-chlorophenylu hydrazine hydrochloride preparation method. P-chloroaniline serves as a raw material, after diazotization and reduction, non-polar solvent toluene or benzene, dichloromethane, trichloromethane and dichloroethane are used for extracting an aqueous phase to remove impurities, and then hydrochloric acid is added into the aqueous phase for hydrolysis to obtain p-chlorophenylu hydrazine hydrochloride through cooling, filtering and during. The chemical equation is shown as the following (please see the formula in the specification). Compared with the prior art, the non-polar solvents are used for extraction and impurity removal, the yield and the purity of an obtained product are high, the purity of the p-chlorophenylu hydrazine hydrochloride is larger than 99%, the yield is larger than 86%, filtering only needs to be carried out once, and operation is easy. Waste water amount is reduced by half compared with the prior art, and environmental protection pressure is reduced.
Owner:HUNAN HAILI CHEM IND
Method for preparing p-chloroaniline through catalytic hydrogenation of p-chloronitrobenzene
InactiveCN102351714AEasy to solveReduce post-processingOrganic compound preparationAmino compound preparationP-chloroanilineOrganic synthesis
The invention belongs to the technical field of organic synthesis and provides a method for performing catalytic hydrogenation on p-chloronitrobenzene with a modified Raney nickel catalyst and preparing p-chloroaniline. The method comprises the following steps: adding p-chloronitrobenzene, the modified Raney nickel catalyst with an additional metal material and a solvent in a high-pressure reaction kettle, wherein the weight ratio of p-chloronitrobenzene to the solvent is 1:2-1:5 and the weight ratio of p-chloronitrobenzene to the catalyst is 1:(0.02-0.06); performing nitrogen replacement, then performing hydrogen replacement, wherein the pressure of hydrogen is 1.0-3.5MPa and the reaction temperature is 60-100 DEG C; and stirring to react for 1-4 hours to obtain the product p-chloroaniline. The method has simple technology, low demand on equipment and low environmental pollution and is easy to realize industrialization; and the reaction conversion rate is 99.9%, the selectivity is not less than 98% and the yield is not less than 95%.
Owner:CHINA PETROCHEMICAL CORP +1
Method of preparing 1-(4-chloro-phenyl)-3-(3,4-dichloro-phenyl)-urea
ActiveCN101033203ALow environmental impactReduce energy consumptionUrea derivatives preparationOrganic compound preparationSolventChemistry
This invention relates to a method for preparing 3, 4, 4'-trichloro-Triclosan benzene and two urea as a antiseptic including two-step reaction method, the first one is to take chloraniline as the raw material and toluene as the solvent to be reacted with solid phosgene to remove un-reacted solid phosgene and generated HCl gas to be decolored and filter solid impurities to get chlorophenyl phenyl isocyanate-toluene mother liquid, the second step is to synthesize said mother liquid with the 3, 4-dichloroaniline directly then to be filtered, cleaned and dried to get 3, 4, 4'-trichloro-triclosan benzene and two urea and the solvent is distilled and washed by acid, alkali and water to be reused.
Owner:宁波翔神生化有限公司
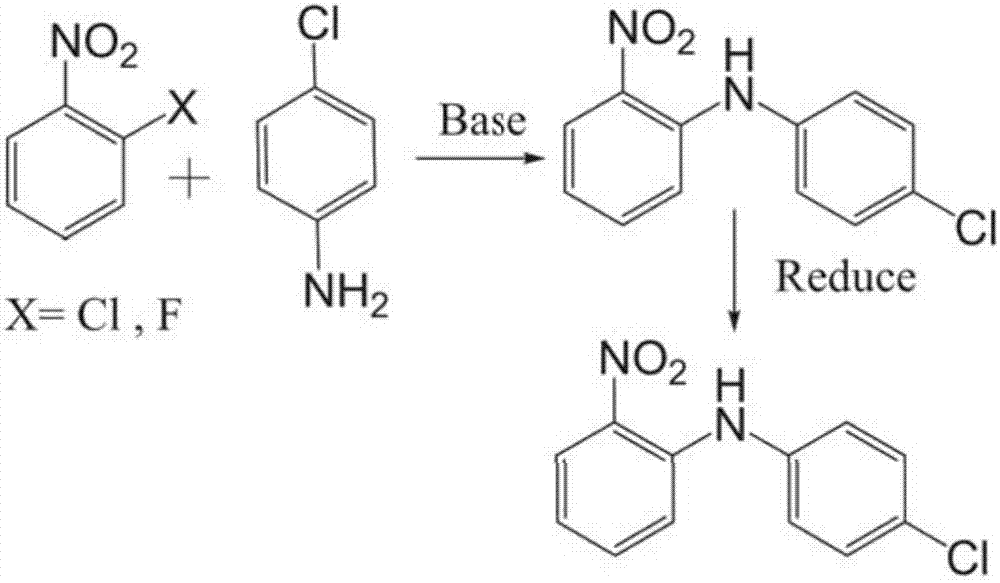
Method for synthesizing N-(4-chlorphenyl)-1,2-phenylenediamine as key clofazimine intermediate
InactiveCN107445845AOrganic compound preparationAmino compound preparation by condensation/addition reactionsP-chloroanilineOrganic solvent
The invention provides a method for synthesizing N-(4-chlorphenyl)-1,2-phenylenediamine as a key clofazimine intermediate. The method specifically comprises the following steps: 1) by taking o-fluoronitrobenzene and p-chloroaniline as raw materials and utilizing organic base as a catalyst for condensation reaction, reacting in an organic solvent and performing conventional treatment after ending the reaction, thereby acquiring a condensation intermediate N-(4-chlorphenyl)-2-nitryl-1-aniline; 2) by utilizing metallic nickel as the catalyst, performing catalytic hydrogenation reaction on the acquired condensation intermediate in the organic solvent and performing conventional treatment after reducing, thereby acquiring a rough product of N-(4-chlorphenyl)-1,2-phenylenediamine; and 3) re-crystallizing the acquired rough product with the organic solvent, thereby acquiring the end product.
Owner:CHONGQING WERLCHEM FINE CHEM
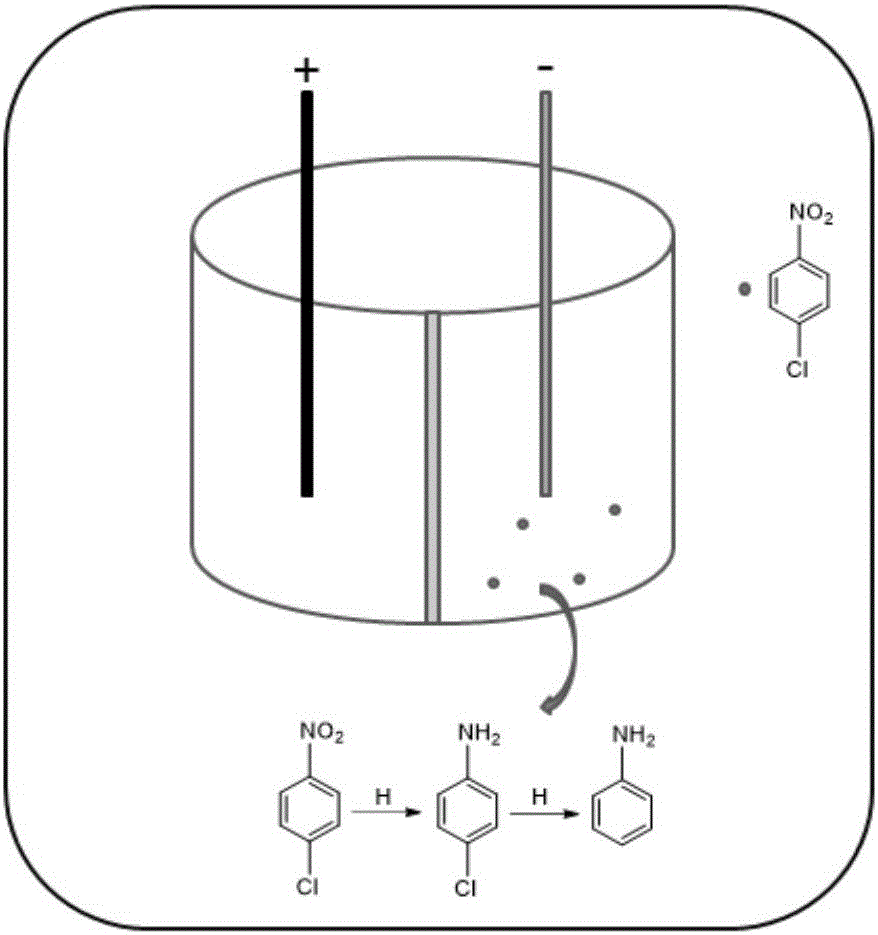
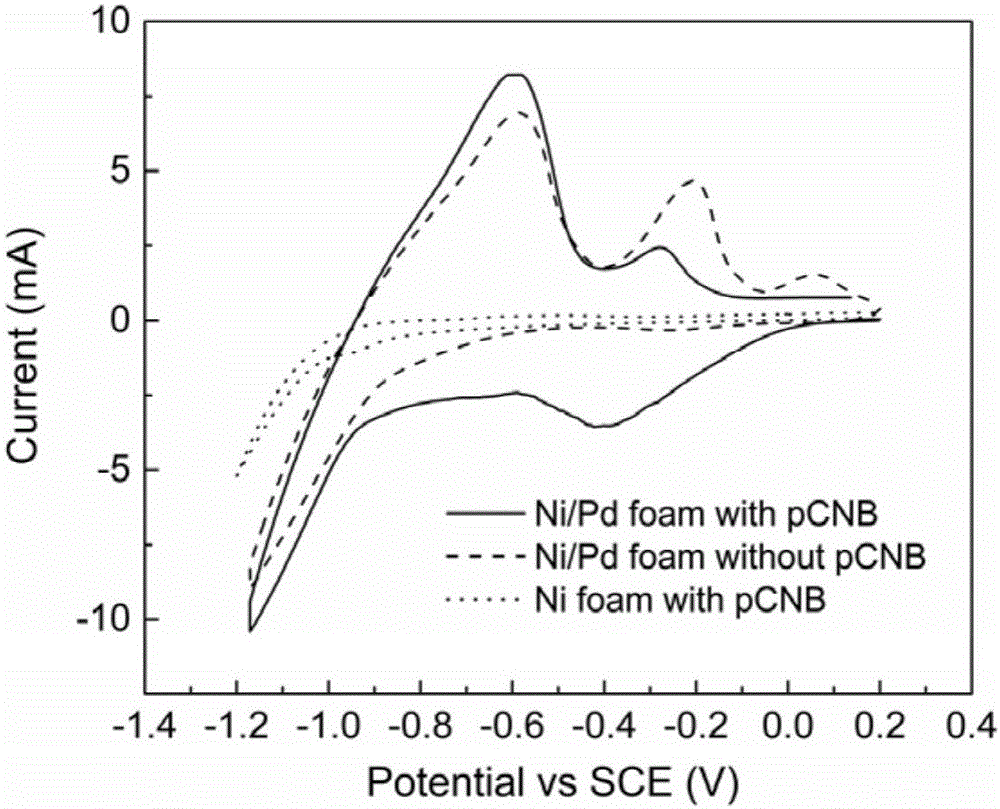
Electrocatalytic dechlorination method for parachloronitrobenzene
InactiveCN105712447AAchieve one-time removalGood dechlorination effectWater contaminantsWater/sewage treatmentChemical treatmentNitrobenzene
The invention discloses an electrocatalytic dechlorination method for parachloronitrobenzene. The method comprises the following steps: loading precious metal palladium on a foam nickel material with a three-dimensional structure by an impregnation method to obtain a cathode of an electrocatalytic reactor; and introducing parachloronitrobenzene-containing wastewater into the electrocatalytic reactor to fill the whole reactor, wherein electrochemical treatment is started under constant current, the parachloronitrobenzene experiences a reduction reaction in a cathode chamber and is reduced into parachloroaniline under the effect of hydrogen radical, and then a dechlorination effect can be realized to form aniline due to the excellent performance of the palladium-containing foam nickel electrode. In the invention, metal reduction is replaced by electrocatalytic reduction, the problems of easy passivation and low mass transfer efficiency of metals are solved, the difficulty in dechlorination of parachloronitrobenzene in the traditional electrode is overcome, and the chlorine group of chloronitrobenzene is effectively removed.
Owner:NANJING UNIV
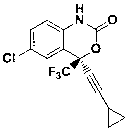
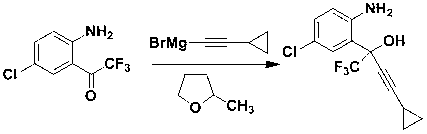
Preparation method of efavirenz intermediate
InactiveCN103254087AImprove stabilityReduce dosageOrganic compound preparationAmino-hyroxy compound preparationP-chloroanilineAnti virus
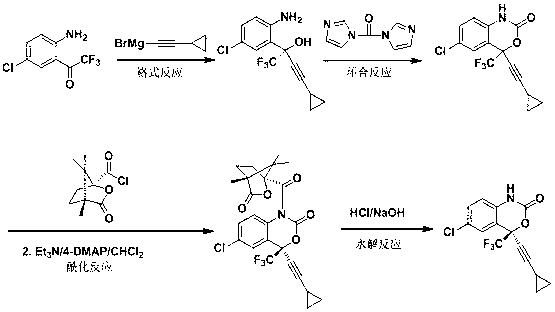
The invention discloses a preparation method of an efavirenz intermediate, relating to synthesis of an anti-virus medicine, namely an efavirenz key intermediate by adopting a green solvent, namely 2-methyltetrahydrofuran as a Grignard reaction solvent, and belonging to the technical field of organic synthesis. The preparation method comprises the following steps of: taking the 2-methyltetrahydrofuran as a solvent, enabling metal magnesium to react with ethyl bromide to obtain ethyl magnesium bromide, then dripping cyclopropylacetylene to generate cyclopropyne ethyl magnesium bromide, and finally performing addition reaction with 2-trifluoroacetyl p-chloroaniline to obtain 2-(2-amino-5-chlorophenyl)-4-cyclopropyl-1, 1, 1-trifluoro-3-butyn-2-ol. According to the method, the 2-(2-amino-5-chlorophenyl)-4-cyclopropyl-1, 1, 1-trifluoro-3-butyn-2-ol can be prepared with high selectivity and high yield, the product purity is more than 99.8%, and the yield can achieve 95.2-97.1%. Compared with traditional technologies, the preparation method disclosed by the invention has the following advantages: as the green solvent, namely the 2-methyltetrahydrofuran is adopted in Grignard reaction, the yield is high, the selectivity is good, the product is easy to separate, the reaction conditions are easy to control, the using quantity of the solvent is small, and the solvent is easy to recover, so that the preparation method is in line with a green chemical idea and is suitable for industrial production.
Owner:ZHENGZHOU UNIV +1
Process for photo catalytic reduction preparation of p-chloroaniline
InactiveCN1634862AChemically stableLow priceOrganic compound preparationAmino compound preparationP-chloroanilineScavenger
The invention provides a method for reduction preparation of p-chloroaniline by photocatalysis. The process comprises the following steps: solving p-chloronitrobenzene with organic solvent in photocatalysis reactor allowing over 300nm ultraviolet light to pass with the mass ratio of p-chloronitrobenzene to solvent between 1í†40 and 1í†225, adding hole scavenger and photocatalyst with the volume ratio of hole scavenger to organic solvent between 1í†3 and 1í†18 with the concentration of photocatalyst in organic solvent between 0.5g / L and 10g / L, adding nitrogen for removing oxygen, and irradiating for 3 hours to 6 hours by ultraviolet light at normal pressure. The reduction reaction can be carried out at normal temperature without high temperature energy consumption device, initiation light can be ultraviolet light or sunlight, and nano photocatalyst can be titanium dioxide or zinc oxide, etc. The invention has higher reduction conversion and yield.
Owner:TIANJIN UNIV
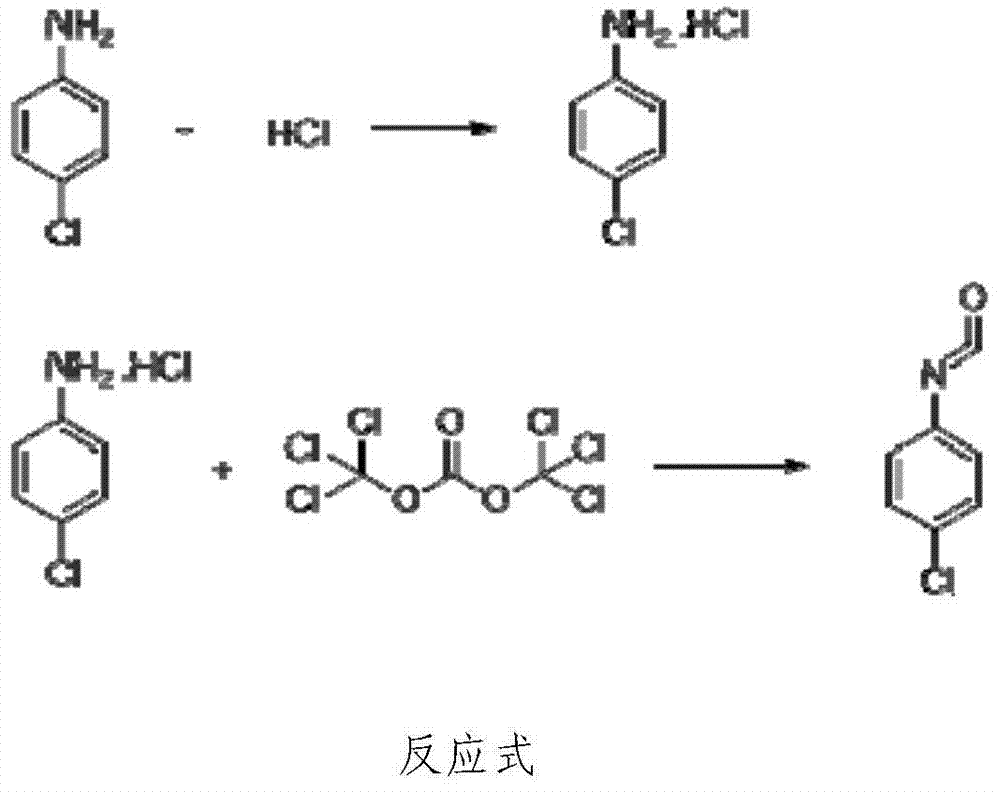
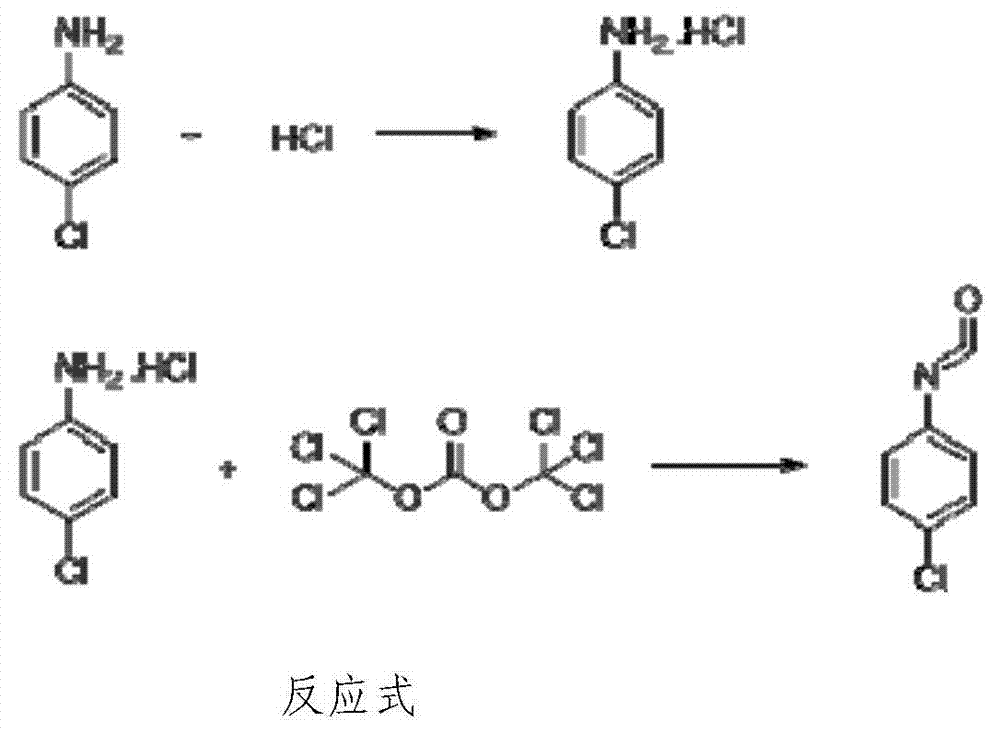
P-chloroaniline isocyanate preparation method
InactiveCN104744306AAdvanced process routeReasonable process conditionsIsocyanic acid derivatives preparationOrganic compound preparationP-chloroanilineSocial benefits
The invention relates to a p-chloroaniline isocyanate preparation method comprising the following steps: (1) p-chloroaniline hydrochloride is synthesized, wherein p-chloroaniline is adopted as a raw material and is subjected to a reaction with hydrochloric acid in an organic solvent, such that p-chloroaniline hydrochloride is synthesized; (2) p-chloroaniline isocyanate is synthesized, wherein p-chloroaniline hydrochloride and triphosgene are adopted as raw material, and p-chloroaniline isocyanate is synthesized in an organic solvent. The method provided by the invention has the advantages of advanced process route, reasonable process conditions, and high reaction yield. The materials used in production are not highly excessive; solvent dose in each step is low; and the solvent can be directly used without treatment. An intermediate is not needed to be dried, and can be directly used in the next step of reaction. Three-waste amount is low, and the waste is easy to treat. Therefore, the method has good implementation value and social benefit.
Owner:HUNAN LIJIE BIOCHEM
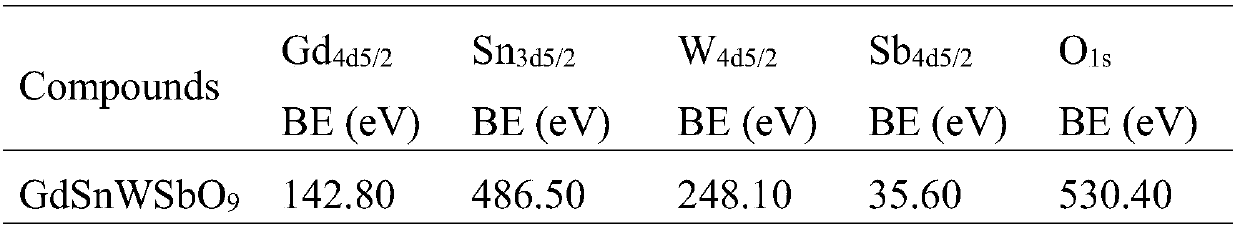
Preparation and application of powder catalytic material, nanoribbon catalytic material and composite porous catalytic material
ActiveCN107803197AImprove catalytic performanceLarge specific surface areaWater/sewage treatment by irradiationWater treatment compoundsP-chloroanilineDecabromodiphenyl ether
The invention discloses a powder catalyst, composed of GdSnWSbO9 and having a particle size of 0.15-0.30 mu m. The powder catalyst is capable of optically catalytically degrading organic pollutants inwater, such as p-chloroaniline, methyl orange and decabromodiphenyl ether. Also disclosed is a preparation method of the powder catalyst, comprising high-temperature solid sintering process, direct deposition process and chemical vapor condensation deposition process. The invention also discloses a preparation method of a composite nano catalytic material; GdSnWSbO9-Na-montmorillonite composite nano catalytic material prepared by the using the preparation method is applicable to removing the organic pollutants in water, such as p-chloroaniline, methyl orange and decabromodiphenyl ether, by photocatalysis.
Owner:NANJING UNIVERSTIY SUZHOU HIGH TECH INST
Parachloroaniline imprinted polymer membrane electrode, and preparation method and application method thereof
The invention discloses a preparation method of a parachloroaniline imprinted polymer membrane electrode. The preparation method comprises the specific steps: 1, preparing a graphite electrode; 2, preparing a parachloroaniline imprinted polymer membrane on the graphite electrode; 3, eluting to prepare the parachloroaniline imprinted polymer membrane electrode. The invention further discloses a method for detecting parachloroaniline by using the parachloroaniline imprinted polymer membrane electrode. The method comprises the specific steps: connecting the prepared graphite electrode with the parachloroaniline imprinted polymer membrane on the surface according to a zero-current potential system; testing a to-be-tested sample solution by using a formula of relation between the zero-current potential EZCP and parachloroaniline concentration. According to the invention, the defects of low sensitivity, complicated operation and long detection period of the prior art when parachloroaniline is detected by using a chromatographic analysis technology are solved.
Owner:XI'AN POLYTECHNIC UNIVERSITY
Preparation method for high-purity pyraclostrobin
InactiveCN109438354AIncrease profitReduce manufacturing costOrganic chemistryP-chloroanilineCombinatorial chemistry
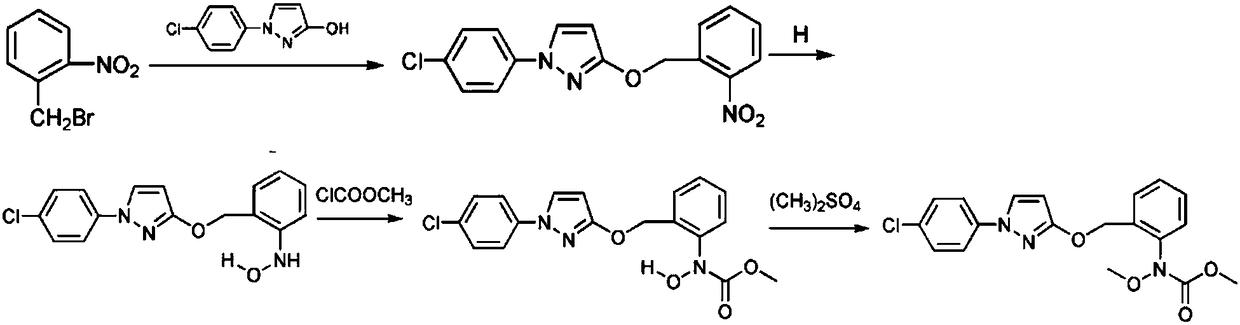
The invention relates to the technical field of compound synthesis, in particular to a preparation method for high-purity pyraclostrobin. The pyraclostrobin is synthesized from p-chloroaniline and o-nitrosotoluene as initial raw materials through the steps of diazotizing, cyclizing, oxidizing, bromizing, condensating, reducing, acylating, methylating and the like. According to the preparation method, the problem that the purity of the pyraclostrobin in the prior art is low is solved; and the preparation method for the pyraclostrobin has the advantages of high yield, high purity and high material utilization rate.
Owner:新沂市永诚化工有限公司
Synthetic process of 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole
The invention relates to a synthetic process of 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole, which is characterized by comprising the following steps: firstly, preparing heavy nitrogen liquid by using p-chloroaniline as a raw material; then reducing the heavy nitrogen liquid by sodium sulfite solution; next, reacting with dimethyl ester, carrying out acidification by hydrochloric acid so as to obtain a mixture of 1-(4-chlorophenyl) pyrazolidine-3-ketone; finally, reacting with sodium hydroxide solution, carrying out acid modulation, and filtration and rectification, so as to obtain a final product. According to the synthetic process disclosed by the invention, after the heavy nitrogen liquid mixture is obtained in the step 1, the heavy nitrogen liquid mixture is directly used for the next step of reaction without being treated, the process of acidification of the hydrochloric acid is omitted temporarily through the reaction in the step 2, and the hydrochloric acid is directly adopted to carry out acidification and acid modulation in the step 3, so that one acidification process is omitted, and outputs of waste acid and waste water are reduced; synthesis of each ton of the 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole can reduce generation of 10 tons of waste water and reduce consumption of 5 tons of hydrochloric acid.
Owner:ANHUI GUANGXIN AGROCHEM
Modification method for raney nickel catalyst for p-chloronitrobenzene hydrogenation
InactiveCN102527393AEasy to solveReduce post-processingOrganic compound preparationAmino compound preparationP-chloroanilineGeneration rate
The invention relates to a modification method for a raney nickel catalyst for p-chloronitrobenzene hydrogenation, and belongs to the technical field of organic synthesis. According to the method, a catalyst is added to deionized water containing 1-5% of a modifier, a stirring reaction is performed for 20-40 minutes at a temperature of 30-70 DEG C, and a standing treatment is performed for 30-90 minutes; a reduction agent with the concentration of 5-15% is added, a stirring reaction is performed for 30-60 minutes at the temperature of 40-50 DEG C, and NaHCO3 is adopted to adjust the pH value to 8-10, wherein a mass ratio of the reduction agent to the raney nickel catalyst is 4:1-8:1; a standing treatment is performed for 30-60 minutes, and deionized water is adopted to wash until the solution is neutral, a filtering treatment is performed, water is added, and sealed preservation is performed to prepare the modified raney nickel catalyst. According to the present invention, under the condition of no addition of the dechlorination inhibitor, the p-chloronitrobenzene conversion rate is more than or equal to 99.9%, the dechlorination side reaction generation rate in the p-chloronitrobenzene hydrogenation process is less than or equal to 2%, and the yield of p-chloroaniline is more than or equal to 95%; compared to the existing methods for preparing the hydrogenation catalyst, the method of the present invention has the following advantages that: the hydrogenation process is simplified, the three waste is less after the hydrogenation, the cost is low, the energy consumption is low, and the industrial requirements of quality increasing, consumption reducing, and environmental protection are achieved.
Owner:CHINA PETROLEUM & CHEM CORP +1
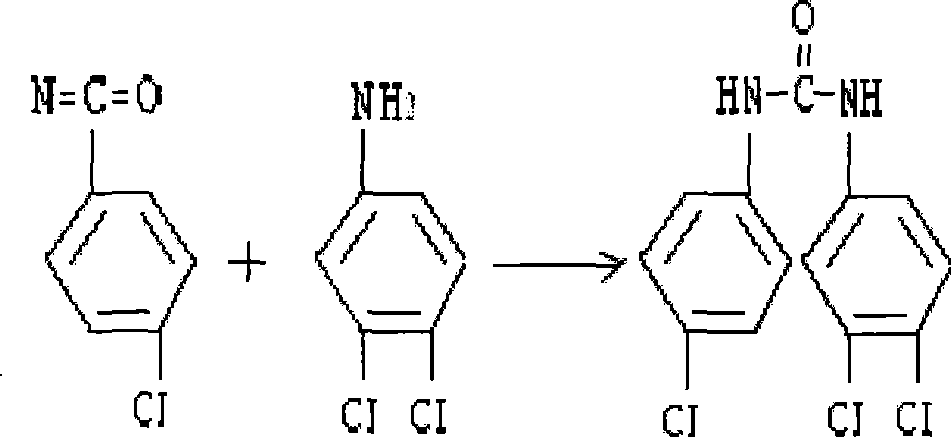
Synthesis method for co-producing p-chloroaniline and p-chlorophenol isocyanate
InactiveCN102617401AAvoid pollutionAvoid poisoningIsocyanic acid derivatives preparationOrganic compound preparationP-chloroanilineReflux
The invention relates to a synthesis method for co-producing p-chloroaniline and p-chlorophenol isocyanate. The method comprises the following steps of: reducing parachloronitrobenzene by using iron powder, separating water, dehydrating, adding a solvent, reacting with a bis(trichloromethyl) carbonate solution, distilling to recover the solvent, and distilling to obtain a p-chlorophenol isocyanate finished product. The method has the advantages that a process is advanced; process conditions are reasonable; the production of p-chloroaniline is organically combined with that of p-chlorophenol isocyanate; the environment pollution and an intoxicating phenomenon which are brought by p-chloroaniline used as an industrial product in the processes of aftertreatment, conveying, carrying and feeding can be avoided; virulent phosgene and diphosgene are avoided during production of p-chlorophenol isocyanate, and production is safe and reliable; the residual bis(trichloromethyl) carbonate is removed by normal-pressure reflux; the improvement on the quality of a product is facilitated; and the method has great implementation value and social and economic benefits.
Owner:象山志华新材料有限公司
P-chloroaniline hydrochloride preparation method
InactiveCN110467533AModerate reaction conditionsSuppress generationAmino compound purification/separationOrganic compound preparationP-chloroanilineThermal insulation
The invention provides a p-chloroaniline hydrochloride preparation method, which comprises: in the presence of an organic solvent azeotropic with water, using p-chloronitrobenzene as a starting raw material, adding anti-dehalogenation agent and an acid binding agent, sealing, introducing hydrogen gas, carrying out a hydrogenation reaction under the catalysis of a platinum-carbon catalyst at a hightemperature under a high pressure, cooling, filtering to remove the catalyst, collecting the filtrate, adding concentrated hydrochloric acid to the filtrate, carrying out thermal insulation for 0.5-2h at a temperature of 80-95 DEG C, continuously heating to achieve a boiling state, refluxing by a water separator until no water is separated, filtering, and drying to obtain p-chloroaniline hydrochloride. According to the present invention, the preparation method has characteristics of mild and controllable reaction conditions, simple operation and low cost, performs the two steps through the one-pot method, and provides the advanced preparation method for industrial production.
Owner:辽宁顺通化工股份有限公司
Preparation method of tolvaptan key intermediate
InactiveCN104829533ASimple and fast operationShorten the production cycleOrganic chemistryEthyl ChlorideOxygen
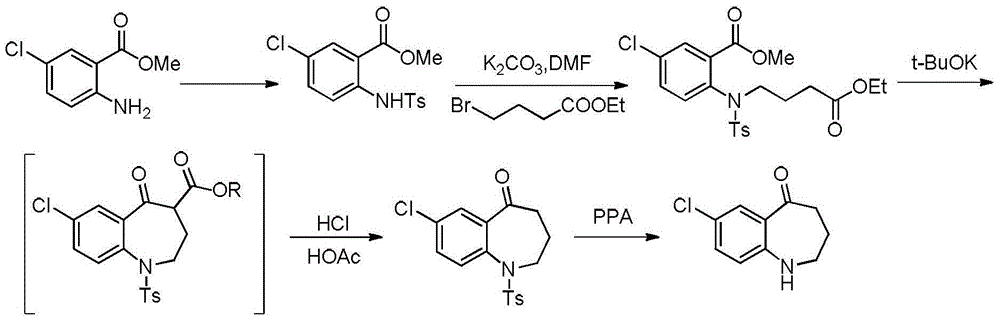
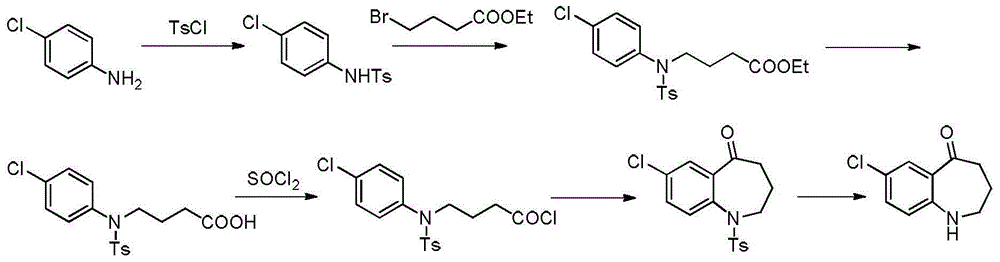
The invention discloses a preparation method of tolvaptan key intermediate 7-chloro-5-oxo-1,2,3,4-tetrahydro-benzazepine. The preparation method comprises the steps of condensing parachloroaniline, which is taken as a starting raw material, with 4-chlorobutyric acid methyl ester, carrying out hydrolysis de-esterification and acylating chlorination, and carrying out ring closure under an acidic condition, so as to obtain a target product, wherein the total yield of the target product can reach above 80%, and the HPLC (High Performance Liquid Chromatography) purity reaches above 99.5%.
Owner:广安凯特制药有限公司
Method for producing o-nitro-p-chloroaniline by using chloridized aromatic hydrocarbon waste as raw material
InactiveCN102358718AReduce pollutionSimple processOrganic compound preparationAmino compound preparationP-chloroanilineAromatic hydrocarbon
The invention relates to a method for producing o-nitro-p-chloroaniline by using chloridized aromatic hydrocarbon waste as a raw material. The method comprises the following three parts of: processing the chloridized aromatic hydrocarbon waste by utilizing a physical synthesis technology; separating high-content p-dichlorobenzene from the chloridized aromatic hydrocarbon waste by using a separated integration technology; and obtaining the o-nitro-p-chloroaniline as a target product by utilizing nitrification and ammonification technologies. The method provided by the invention has the advantages of effectively utilizing the waste and reducing the environmental pollution. In addition, by adopting the process provided by the invention, the problems described in the specification are effectively solved, and the process is simple and convenient for control and large-scale production.
Owner:JIANGSU LONGCHANG CHEM
Synthetic method of Oxazolam drug intermediate 2-amino-5-chlorobenzophenone
InactiveCN105461576AReduce intermediate linksLow reaction temperatureOrganic chemistryOrganic compound preparationP-chloroanilineSulfite salt
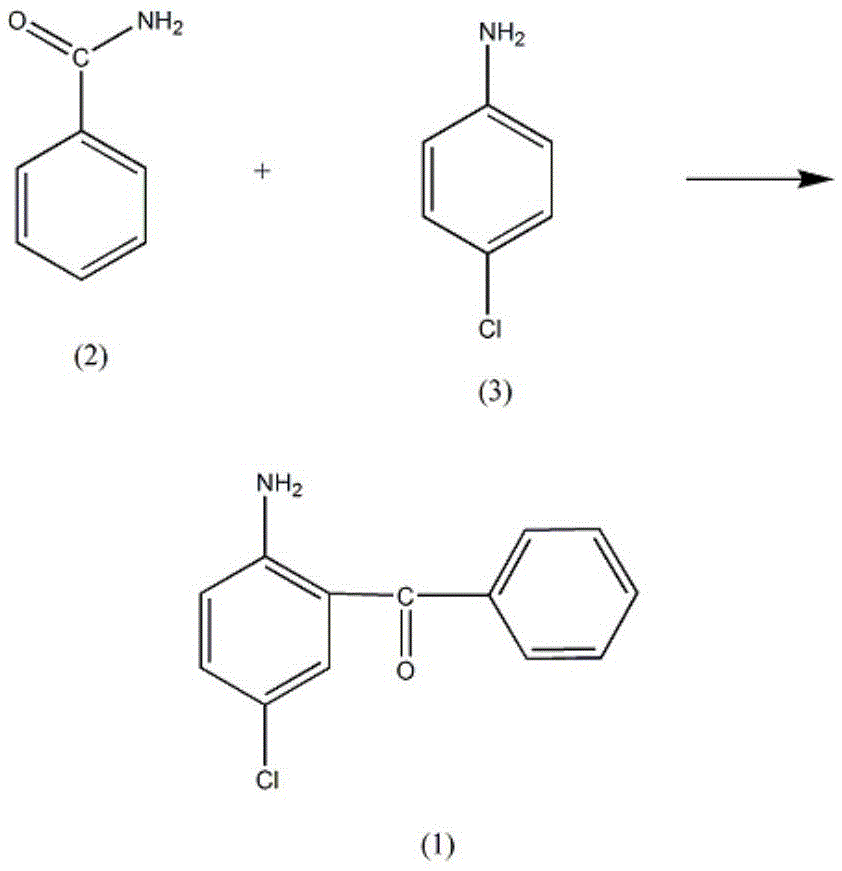
A synthetic method of an Oxazolam drug intermediate 2-amino-5-chlorobenzophenone comprises the following steps: adding 3.51-3.55mol of benzamide into a reaction container provided with a stirrer, a thermometer and a reflux condenser, rising the temperature of a solution to 120-123 DEG C, dropwise adding 1.41mol of p-chloroaniline, after adding, slowly rising the temperature to 190-195 DEG C, adding 5.5-5.9 mol of stannous chloride, rising the temperature of a solution to 240-250 DEG C, reacting for 3-4h at the temperature until no gas is generated, reducing the temperature of a solution to 90-95 DEG C, adding 500ml of a potassium chloride solution, rising the temperature of a solution to 130 DEG C, performing backflow, pouring out a water layer after 3-4h, cooling a solution, then separating out a dark suspended matter, adding the suspended matter into 2.5L of a phosphoric acid solution, performing backflow for 20-23h, cooling, then pouring a solution into 1200ml of a sodium nitrate solution, reducing the temperature of a solution to 5-8 DEG C, adding cyclohexane for extraction, adding 300ml of a sodium sulfite solution in a cyclohexane layer, performing reduced pressure distillation, adding acetonitrile and extracting for 5-7 times, separating out a solid, filtering, and dehydrating by a dehydrating agent, thus obtaining the yellow crystal 2-amino-5-chlorobenzophenone.
Owner:CHENGDU KA DI FU TECH
Synthesis method of sulfentrazone intermediate
ActiveCN113402472AReduce manufacturing costAvoid supply constraintsOrganic chemistryP-chloroanilineChlorobenzene
The present invention provides a synthesis method of asulfentrazone intermediate. The methodcomprises: S1) carrying out a nitration reaction on chlorobenzene in a nitration reagent to obtain a mixture of o-chloronitrobenzene and p-chloronitrobenzene, wherein the product does not need to be separated; s2) performing catalytic hydrogenation reaction on the mixture of o-chloronitrobenzene and p-chloronitrobenzene to obtain a mixture of o-chloroaniline and p-chloroaniline, wherein the product does not need to be separated; s3) making the mixture of o-chloroaniline and p-chloroaniline subjected to a diazotization reaction to obtain a mixture of o-chlorophenylhydrazine and p-chlorophenylhydrazine, wherein the product does not need to be separated; s4) performing condensation reaction on the mixture of the o-chlorophenylhydrazine and the p-chlorophenylhydrazine and aldehyde to obtain triazole ring mixtures as shown in a formula I-a and a formula I-b; and S5) carrying out chlorination reaction on the triazole ring mixtures to obtain the sulfentrazone intermediate shown in the formula I. According to the method, 2, 4-dichloroaniline is not used as a raw material, so that the production cost of sulfentrazone is reduced, and the limitation of raw material supply is avoided.
Owner:SHANDONG WEIFANG RAINBOW CHEM
Application of antioxidant in distillation reaction of 2, 5-dichloroaniline
ActiveCN103467307AImprove stabilityImprove heat resistanceAmino compound purification/separationP-chloroanilineHydrogen
The invention relates to an application of an antioxidant in distillation reaction of 2, 5-dichloroaniline. The process comprises the following steps: simultaneously adding a generated crude product, namely the 2, 5-dichloroaniline and the antioxidant into a distillation kettle after the reaction of 2, 5-dichloronitrobenzene and hydrogen is completed and reacting during the distillation process to get a final product, namely p-chloroaniline. The antioxidant comprises the component of sodium sulfide, and the concentration is 0.1%. The temperature of the distillation reaction is 130 DEG C, and the pH value during the reaction process is 10-11. The application disclosed by the invention has the advantages that compared with other antioxidants, the application of the antioxidant has better stability, good heat resistance and good oxidation resistance, and can keep the color and the appearance of the material for a long time. The application disclosed by the invention further has the advantages of simple process, low production cost, low toxicity and good oxidation resistance of the product, and is suitable for green industrial production.
Owner:HULUDAO TIANQI SHENGYE CHEM
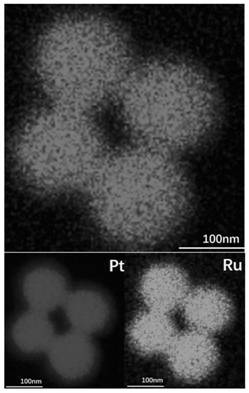
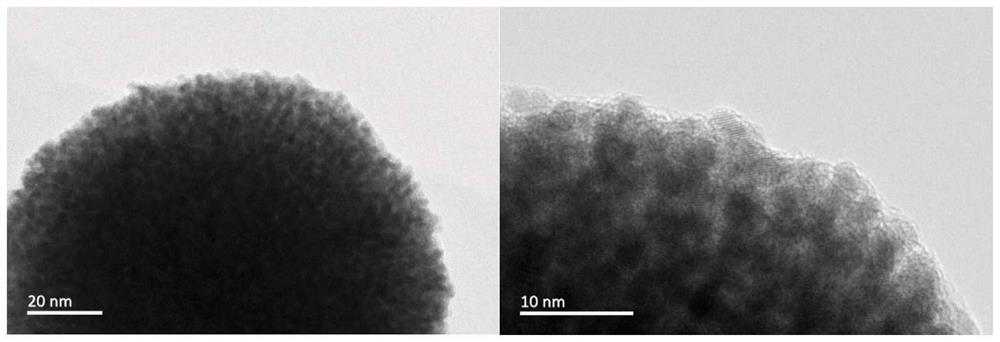
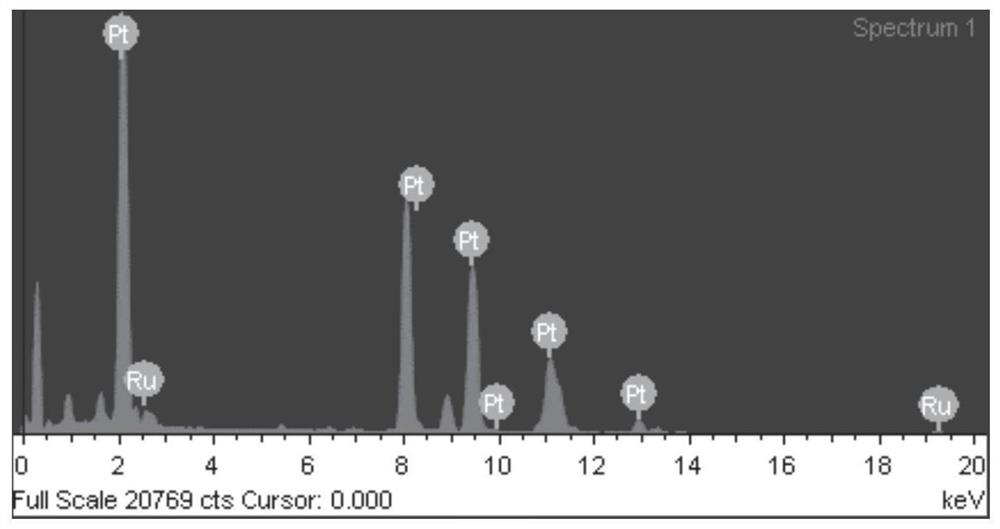
Preparation of supported porous nano platinum-ruthenium alloy catalyst and application of supported porous nano platinum-ruthenium alloy catalyst in preparation of chloroaniline through chloronitrobenzene hydrogenation
PendingCN113578316AHigh activityFacilitate desorptionOrganic compound preparationAmino compound preparationP-chloroanilinePtru catalyst
The invention provides an application of a supported porous nano platinum-ruthenium alloy catalyst in preparation of o-(m-, p-) chloroaniline by hydrogenation of o-(m-, p-) chloronitrobenzene. The catalyst takes a metal oxide or a carbon material as a carrier and a porous nano platinum-ruthenium alloy as an active component, and the porous nano platinum-ruthenium alloy is formed by mutually connecting platinum-ruthenium alloy particles; in the porous nano platinum-ruthenium alloy, ruthenium is enriched on the outer layer of the porous nano platinum-ruthenium alloy. The preparation process of the catalyst comprises the following steps: (1) preparing the porous nano platinum-ruthenium alloy; (2) loading the porous nano platinum ruthenium alloy; and (3) post-treating the catalyst. The optimized catalyst is obtained by optimizing the preparation conditions of the porous nano platinum-ruthenium alloy, screening the carrier and optimizing the operation method of post-treatment. The catalyst prepared by the invention can catalyze the hydrogenation of ortho (m, p) chloronitrobenzene to prepare ortho (m, p) chloroaniline with high conversion rate and high selectivity under the reaction conditions of no use of a dechlorination inhibitor and wide range.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesis method of intermediate 4'-chloro-2-aminobiphenyl of boscalid
ActiveCN106366002AReduce transfer lossRaw materials are easy to getAmino preparation from aminesP-chloroanilineSynthesis methods
The invention belongs to the technical field of pesticides, and relates to a preparation technology of an intermediate of an agricultural bactericide, in particular to a preparation technology of 4'-chloro-2-aminobiphenyl. The preparation technology uses p-chloroaniline and aniline as raw materials, at least comprises a diazotization reaction, alkalization, a Gomberg-Buckman reaction and a post-treatment process, and is characterized by comprising the following specific steps: under the action of sodium nitrite, performing the diazotization reaction on the p-chloroaniline, water and hydrochloric acid to generate diazonium salt; with water, tetrahydrofuran or DMF and a mixed solvent thereof as a solvent, performing the Gomberg-Buckman reaction on the diazonium salt and the aniline under an alkaline condition so as to achieve synthesis of a key intermediate 4'-chloro-2-aminobiphenyl of boscalid and a structural analogue thereof. Through inflation of hydrochloric acid gas, 4'-chloro-2-aminobiphenyl hydrochloride is obtained; by virtue of recrystallization, purification of the intermediate is achieved; mother liquor is continuously used, and the total yield reaches 80% or higher.
Owner:JINGBO AGROCHEM TECH CO LTD
Salicylic acid modified styrene polymer and method for adsorbing and recovering aniline components in industrial waste water by using salicylic acid modified styrene polymer
InactiveCN108479727AImprove stabilitySimple methodOther chemical processesSpecific water treatment objectivesP-chloroanilineMaterials preparation
The invention discloses a salicylic acid modified styrene polymer and a method for adsorbing and recovering aniline components in industrial waste water by using the salicylic acid modified styrene polymer. According to the method, the synthesized salicylic acid modified styrene polymer serves as an adsorbing material to adsorb the aniline components such as aniline, p-aminotoluene, p-chloroaniline and para aminobenzoic acid in the waste water, and the maximal adsorption capacity can reach to 132, 155, 229 and 113 mg / g respectively. Experimental results show that the adsorption capacity is obviously higher than the maximal adsorption capacity, on the aniline compounds, of the adsorbing materials such as active carbon, coal ash and modified zeolite. The method provided by the invention hasthe advantages of simple material preparation process, reusability, mild adsorption condition, energy conservation, environmental friendliness, low cost and the like.
Owner:HUIZHOU RES INST OF SUN YAT SEN UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com