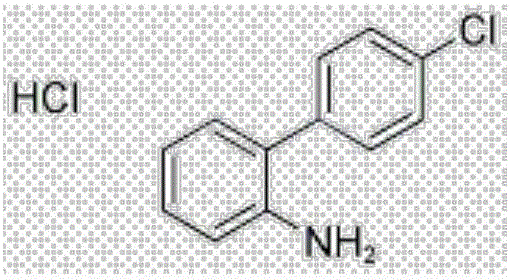
Synthesis method of intermediate 4'-chloro-2-aminobiphenyl of boscalid
A technology of boscalid and aminobiphenyl, applied in the field of pesticides, can solve the problems of high cost of raw materials, complicated process, poor operability, etc., and achieve the effect of easy-to-obtain raw materials, simple process flow, and reduce material transfer loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add p-chloroaniline (3.86g, 0.03mol), 5.8g water, 12mL hydrochloric acid in a 250mL four-neck round bottom flask, adjust the system temperature to 50-70°C, then cool down to 0-5°C, and add sodium nitrite ( 2.26g, 0.033mol) in aqueous solution. The reaction is about 2h to produce the diazonium salt. The yield measured by acid titration (calculated as p-chloroaniline) was 97.5%. Under nitrogen protection, the above diazonium salt was cooled to -13°C, and sodium hydroxide solution (23.5ml, 8mol / L) was added dropwise to prepare diazonium salt lye. Aniline (28g, 0.3mol), 5ml of water, heated to 70°C, added the lye solution of the above-mentioned diazonium salt into the system dropwise, and added dropwise for 2 hours, and detected 0.2% of the remaining diazonium salt, and the vacuum was above 0.095Mpa, and the 20g of aniline and 9g of water, add 20ml of toluene to the bottom liquid, pass through hydrochloric acid gas, continue to stir, after fully separated, cool to -5-0°C ...
Embodiment 2
[0034] Add p-chloroaniline (3.86g, 0.03mol), 6g water, 10mL hydrochloric acid in a 250mL four-neck round bottom flask, adjust the system temperature to 50-70°C, then cool to 0-5°C, add sodium nitrite (2.2 g, 0.032mol) in aqueous solution. The reaction is about 2h to produce the diazonium salt. The yield measured by acid titration (calculated as p-chloroaniline) was 97.2%. Under nitrogen protection, the above diazonium salt was cooled to -15°C, and sodium hydroxide solution (25ml, 8mol / L) was added dropwise to prepare diazonium salt lye. Aniline (25.11g, 0.27mol), 10ml THF, heated to 60°C, added the lye solution of the above-mentioned diazonium salt into the system dropwise, and added dropwise for 2 hours, and detected that 0.2% of the diazonium salt was left, the vacuum was above 0.095Mpa, and the 19.5g of aniline and 11g of water, add 25ml of toluene to the bottom liquid, pass through hydrochloric acid gas, continue to stir, after fully separated, cool down to -5-0°C to coo...
Embodiment 3
[0036] Add p-chloroaniline (3.86g, 0.03mol), 6g water, 13mL hydrochloric acid in a 250mL four-neck round bottom flask, adjust the system temperature to 50-70°C, then cool to 0-5°C, add sodium nitrite (2.24 g, 0.0324mol) in water solution. The reaction is about 2h to produce the diazonium salt. The yield measured by acid titration (calculated as p-chloroaniline) was 97.6%. Under the protection of argon, the above-mentioned diazonium salt was cooled to -18°C, and sodium hydroxide solution (24ml, 8mol / L) was added dropwise to prepare a lye solution of the diazonium salt. Aniline (23.25g, 0.25mol), 10ml of DMF, heated to 85°C, dropwise added the lye solution of the above-mentioned diazonium salt into the system, and added dropwise for 2 hours, detected 0.2% of the remaining diazonium salt, and the vacuum was above 0.095Mpa, and the 18g of aniline and 7.6g of water, add 20ml of ethanol to the bottom solution, pass through hydrochloric acid gas, continue to stir, after full separa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com