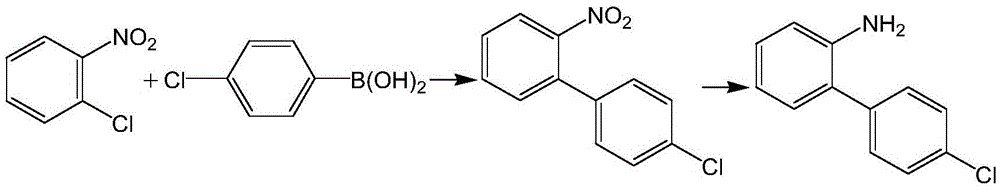
The preparation method of boscalid intermediate 2-(4-chlorophenyl)aniline
A technology for boscalid and intermediates, applied in the field of preparation of boscalid intermediate 2-aniline, can solve the problems of harsh reaction conditions, complex post-processing, high price, etc., and achieve simple reaction, good product purity and low cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Room temperature, under nitrogen protection, add 45ml ethanol, 5.3g potassium hydroxide and 0.15mg KI, 15g (98%, 0.093mol) o-chloronitrobenzene, 15.3g (99%, 0.097mol) p-chlorobenzene to four-necked flask Boric acid, after stirring and dissolving evenly, add 0.75mg of Pd(PPh 3 ) 4 , and stirred to heat up to 75 ° C for 3 hours, the system was cooled to 40 ° C, filtered, and the filtrate was used;
[0031] Under nitrogen protection, add 0.45mg of Pd (PPh 3 ) 4 , adjust the temperature to 25°C and maintain a positive pressure of 0.01MPa to feed hydrogen for 4 hours;
[0032] Concentrate the reacted feed solution to 85°C, cool down, discharge, and suction filter to obtain a solid product while recovering the solvent to obtain 18.11g of the product 2-(4-chlorophenyl)aniline with a content of 93.5% and a yield of 95.6%.
Embodiment 2
[0034] At room temperature, under nitrogen protection, add 60ml isopropanol, 5.58g sodium hydroxide and 0.45mg KI to the four-necked flask, add 15g (98%, 0.093mol) o-chloronitrobenzene, 14.69g (99%, 0.093mol) For p-chlorophenylboronic acid, stir and dissolve evenly, then add 1.2 mg of Pd / C, stir and heat up to 83°C for 3 hours, cool the system to 50°C and filter, and the filtrate is ready for use;
[0035] Under the protection of nitrogen, add 0.75mg of Pd / C to the above filtrate, adjust the temperature to 30°C, maintain a positive pressure of 0.03MPa, and pass in hydrogen for 4h;
[0036] After the reaction, the feed liquid was filtered and concentrated to 90 ° C, cooled, discharged, and suction filtered to obtain a solid product and recover the solvent at the same time to obtain 18.07 g of the product 2-(4-chlorophenyl) aniline with a content of 93.8% and a yield of 95.4 %.
Embodiment 3
[0038] At room temperature, under nitrogen protection, add 105ml tert-butanol, 12.52g potassium tert-butoxide and 0.30mg KI to the four-necked flask, add 15g (98%, 0.093mol) o-chloronitrobenzene, 15.3g (99%, 0.097mol ) p-chlorophenylboronic acid, stir and dissolve evenly, then add 15mg of Pd / molecular sieve, and stir to raise the temperature to 78°C for 5h, then cool the system to 45°C and filter, and the filtrate is ready for use;
[0039] Under the protection of nitrogen, add 0.45 mg of Pd / molecular sieve to the above filtrate, adjust the temperature to 27 ° C, maintain a positive pressure of 0.02 MPa, and pass in hydrogen for 4 hours;
[0040] Concentrate the reacted feed solution to 95°C, cool down, discharge, and suction filter to obtain a solid product while recovering the solvent to obtain 18.20 g of the product 2-(4-chlorophenyl)aniline with a content of 94.1% and a yield of 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com