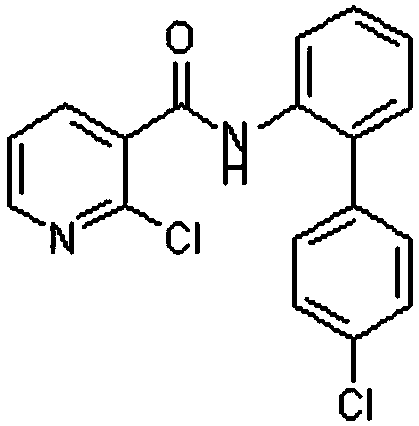
A kind of preparation method of boscalid intermediate 2-(4-chlorophenyl)nitrobenzene
A boscalid and chlorophenyl technology, which is applied in the field of preparation of boscalid intermediate: 2-nitrobenzene, can solve the problems of poor operability, complicated process and high cost of raw materials, achieves low environmental protection pressure, The effect of simple process flow and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Under nitrogen protection, in a 250mL four-necked round-bottomed flask, magnesium chips (2.88g, 0.12mol), 10mL of anhydrous tetrahydrofuran, 15mL of toluene, 1.8% p-chlorobromobenzene (0.35 g, 0.0018mol) and 0.01g of iodine, stir slowly at 20-25°C and wait for initiation. After the system is initiated, the temperature rise is completed. Adjust the system temperature to 40-50°C, and slowly add p-chlorobromobenzene (18.9g, 0.0982 mol) and a mixture of 20mL tetrahydrofuran and 20mL toluene. The Grignard reagent was prepared after about 4 hours of reaction. The yield measured by acid titration (calculated as p-chlorobromobenzene) was 97.5%.
[0032] Under nitrogen protection, the Grignard reagent solution prepared above was slowly added dropwise to a solution of 30 mL of tetrahydrofuran, o-chloronitrobenzene (17.59 g, 0.11 mol), 0.539 g of anhydrous NiCl2 and 3.66 g of P(Ph)3. The reaction temperature is kept at 40-45°C. After the Grignard reagent is slowly added, the rea...
Embodiment 2
[0034] Under nitrogen protection, add zinc powder (7.816g, 0.12mol), 20mL of anhydrous tetrahydrofuran, p-chlorobromobenzene (0.35g, 0.0018 mol) and 0.02g of iodine, stirred slowly at 25-30°C and waited for initiation. After the system was initiated and the temperature rise was completed, adjust the temperature of the system to 50-60°C, and slowly add p-chlorobromobenzene (18.9g, 0.0982mol) and 40mL of tetrahydrofuran dropwise of the mixture. The reaction is about 4h to obtain the zinc reagent. The yield measured by acid titration (calculated as p-chlorobromobenzene) was 97.5%. Under nitrogen protection, the zinc reagent solution prepared above was slowly added dropwise to a solution of 30 mL of tetrahydrofuran, o-chloronitrobenzene (17.59 g, 0.11 mol), 0.539 g of anhydrous NiCl2 and 3.66 g of P(Ph)3. The reaction temperature is maintained at 45-50°C. After the Grignard reagent is slowly added, the gas-phase detection format reagent remains less than 0.5%, and the zinc reage...
Embodiment 3
[0036]Under nitrogen protection, add zinc powder (9.77g, 0.15mol), 10mL anhydrous tetrahydrofuran, 15mL toluene, p-chlorobromobenzene (0.318 g, 0.00165mol) and 0.015g iodine, stirred slowly at 20-25°C and waited for initiation. After the system was initiated and the temperature rise was completed, adjust the system temperature to 40-50°C, and slowly add p-chlorobromobenzene (18.93g, 0.0983mol) dropwise and 40mL tetrahydrofuran mixture. The Grignard reagent was prepared after about 3 hours of reaction. The yield measured by acid titration (calculated as p-chlorobromobenzene) was 98.1%. Under nitrogen protection, the Grignard reagent solution prepared above was slowly added dropwise to a solution of 35 mL THF, o-chloronitrobenzene (18.39 g, 0.115 mol), 0.577 g anhydrous NiCl2 and 3.46 g P(Ph)3. The reaction temperature is maintained at 35-45°C. After the Grignard reagent is slowly added, the remaining gas phase detection format reagent is less than 0.5%, and the zinc reagent i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com