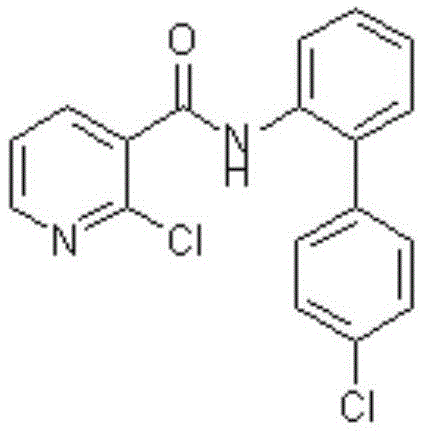
Preparation method of boscalid intermediate 2-(4-chlorophenyl)nitrobenzene
A technology of boscalid and o-chloronitrobenzene, applied in the field of pesticides, can solve the problems of poor operability, complex process, high cost of raw materials, and achieve the effects of low environmental protection pressure, simple process flow, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Under nitrogen protection, in a 250mL four-necked round-bottomed flask, magnesium chips (2.88g, 0.12mol), 10mL of anhydrous tetrahydrofuran, 15mL of toluene, 1.8% p-chlorobromobenzene (0.35 g, 0.0018mol) and 0.01g of iodine, stir slowly at 20-25°C and wait for initiation. After the system is initiated, the temperature rise is completed. Adjust the system temperature to 40-50°C, and slowly add p-chlorobromobenzene (18.9g, 0.0982 mol) and a mixture of 20mL tetrahydrofuran and 20mL toluene. The Grignard reagent was prepared after about 4 hours of reaction. The yield measured by acid titration (calculated as p-chlorobromobenzene) was 97.5%.
[0036] Under nitrogen protection, the Grignard reagent solution prepared above was slowly added dropwise to a solution of 30 mL of tetrahydrofuran, o-chloronitrobenzene (17.59 g, 0.11 mol), 0.539 g of anhydrous NiCl2 and 3.66 g of P(Ph)3. The reaction temperature is kept at 40-45°C. After the Grignard reagent is slowly added, the rea...
Embodiment 2
[0038] Under nitrogen protection, add zinc powder (7.816g, 0.12mol), 20mL of anhydrous tetrahydrofuran, p-chlorobromobenzene (0.35g, 0.0018 mol) and 0.02g of iodine, stirred slowly at 25-30°C and waited for initiation. After the system was initiated and the temperature rise was completed, adjust the temperature of the system to 50-60°C, and slowly add p-chlorobromobenzene (18.9g, 0.0982mol) and 40mL of tetrahydrofuran dropwise of the mixture. The reaction is about 4h to obtain the zinc reagent. The yield measured by acid titration (calculated as p-chlorobromobenzene) was 97.5%. Under nitrogen protection, the zinc reagent solution prepared above was slowly added dropwise to a solution of 30 mL of tetrahydrofuran, o-chloronitrobenzene (17.59 g, 0.11 mol), 0.539 g of anhydrous NiCl2 and 3.66 g of P(Ph)3. The reaction temperature is maintained at 45-50°C. After the Grignard reagent is slowly added, the gas-phase detection format reagent remains less than 0.5%, and the zinc reage...
Embodiment 3
[0040]Under nitrogen protection, add zinc powder (9.77g, 0.15mol), 10mL anhydrous tetrahydrofuran, 15mL toluene, p-chlorobromobenzene (0.318 g, 0.00165mol) and 0.015g iodine, stirred slowly at 20-25°C and waited for initiation. After the system was initiated and the temperature rise was completed, adjust the system temperature to 40-50°C, and slowly add p-chlorobromobenzene (18.93g, 0.0983mol) dropwise and 40mL tetrahydrofuran mixture. The Grignard reagent was prepared after about 3 hours of reaction. The yield measured by acid titration (calculated as p-chlorobromobenzene) was 98.1%. Under nitrogen protection, the Grignard reagent solution prepared above was slowly added dropwise to a solution of 35 mL THF, o-chloronitrobenzene (18.39 g, 0.115 mol), 0.577 g anhydrous NiCl2 and 3.46 g P(Ph)3. The reaction temperature is maintained at 35-45°C. After the Grignard reagent is slowly added, the remaining gas phase detection format reagent is less than 0.5%, and the zinc reagent i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com