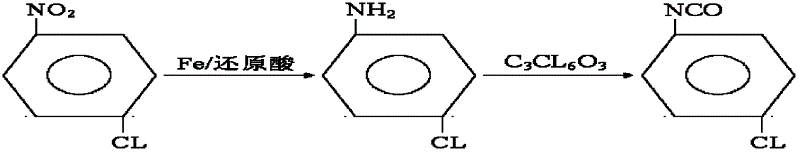
Synthesis method for co-producing p-chloroaniline and p-chlorophenol isocyanate
A technology of chloroisocyanate and p-chloroaniline, which is applied in the field of pesticides, dyes, and synthetic medicines, can solve the problems of easy poisoning in transportation and handling, large safety hazards, equipment corrosion, etc., achieve great implementation value and social and economic benefits, and safe and reliable production , the effect of reasonable process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: In a 1000mL reactor, equipped with a stirring paddle, a thermometer, and a condenser, add 800mL of water, 4.6g of formic acid, add 216.2g of iron powder under stirring, raise the temperature to 98°C, and slowly add p-chloronitro Benzene 218.8g, (the dripping device needs to be kept warm at more than 90°C), the time is 2 to 2.5 hours, the reaction temperature is 98 to 102°C, and after 2 hours of heat preservation reaction time, the reaction is detected by gas chromatography without raw material peaks, and the reaction Finish. Separate the water layer, and dehydrate under a vacuum of 0.08mp to 0.09mp until there is no water drop at the receiving part of the condenser, and the temperature is 80 to 120°C. Add 1175 g of solvent ethyl acetate to the above material, stir and mix thoroughly for half an hour, the material is filtered and washed into the next reaction kettle, and 438 g of bis(trichloromethyl)carbonate solution is added dropwise, which contains 294 g of...
Embodiment 2
[0013] Embodiment 2: change iron powder into 194kg, other conditions are unchanged as embodiment 1, finally get p-chloroisocyanate 185g, its content 99.5%, total yield 86.8%.
Embodiment 3
[0014] Embodiment 3: change iron powder into 221.5kg, other conditions are unchanged as embodiment 1, finally get p-chloroisocyanate 185g, its content 99.7%, total yield 93.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com