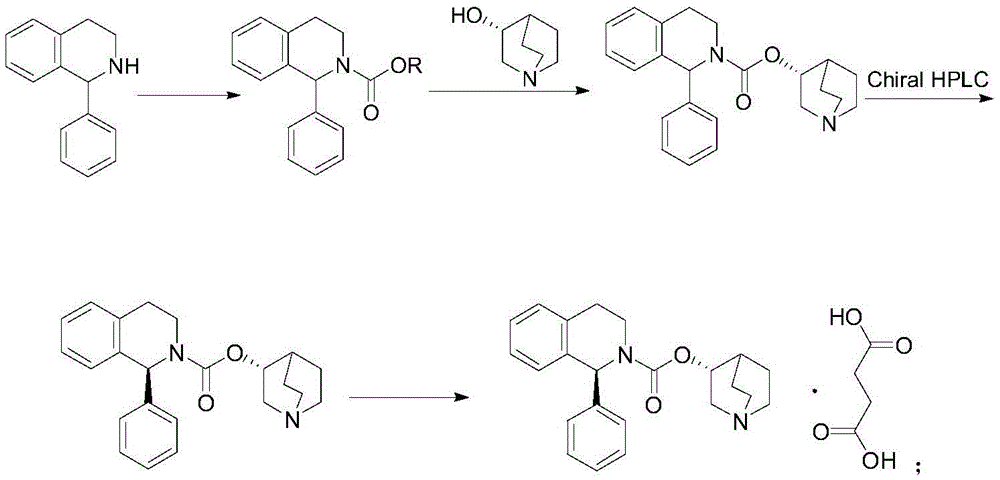
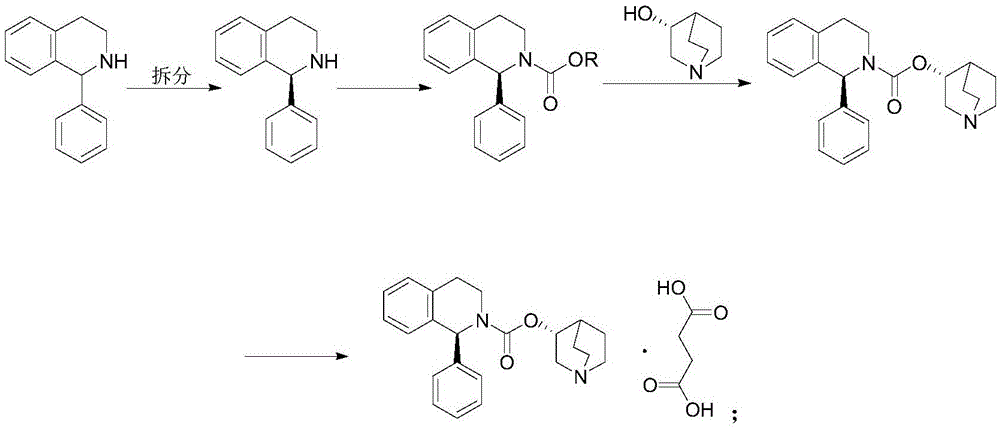
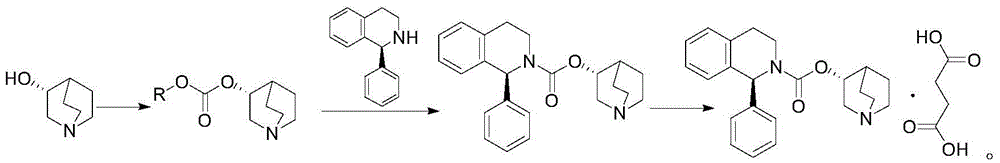
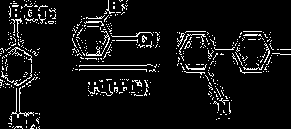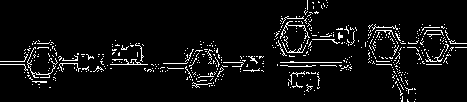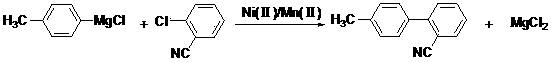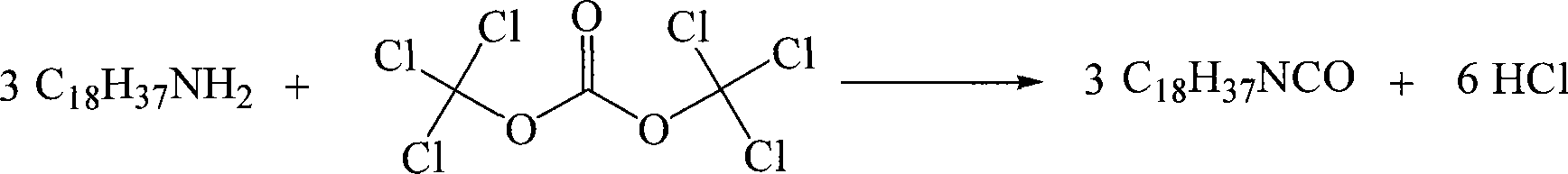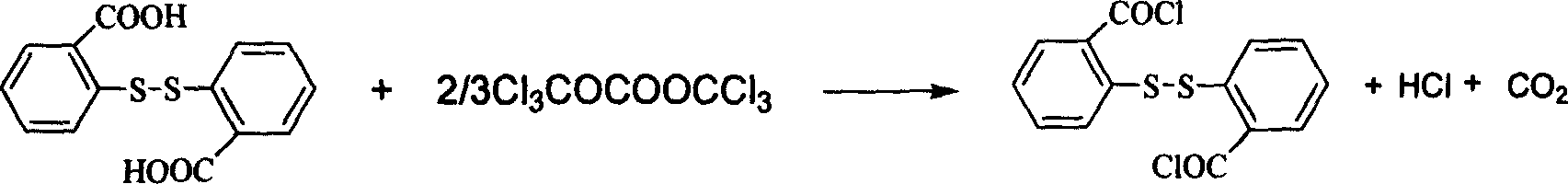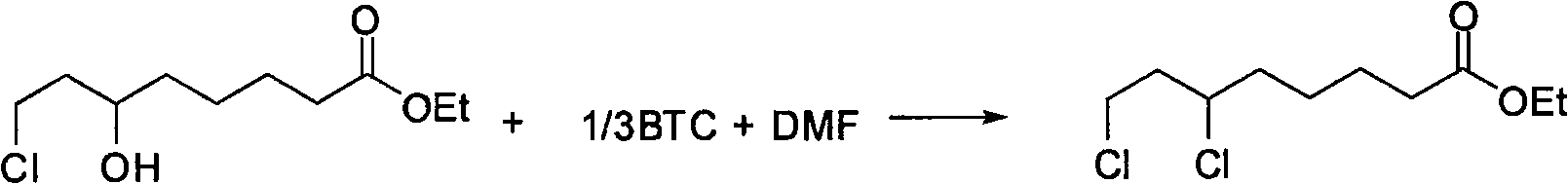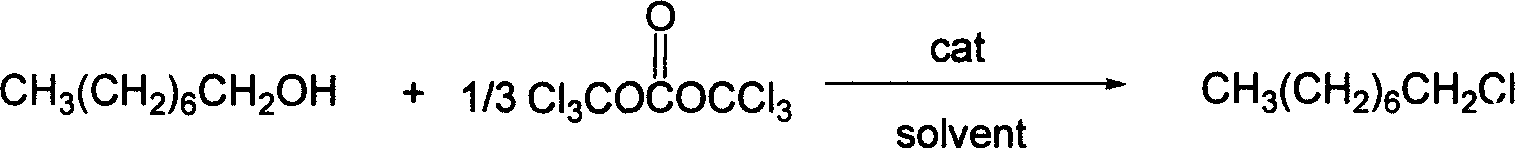Patents
Literature
95results about How to "Advanced process route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
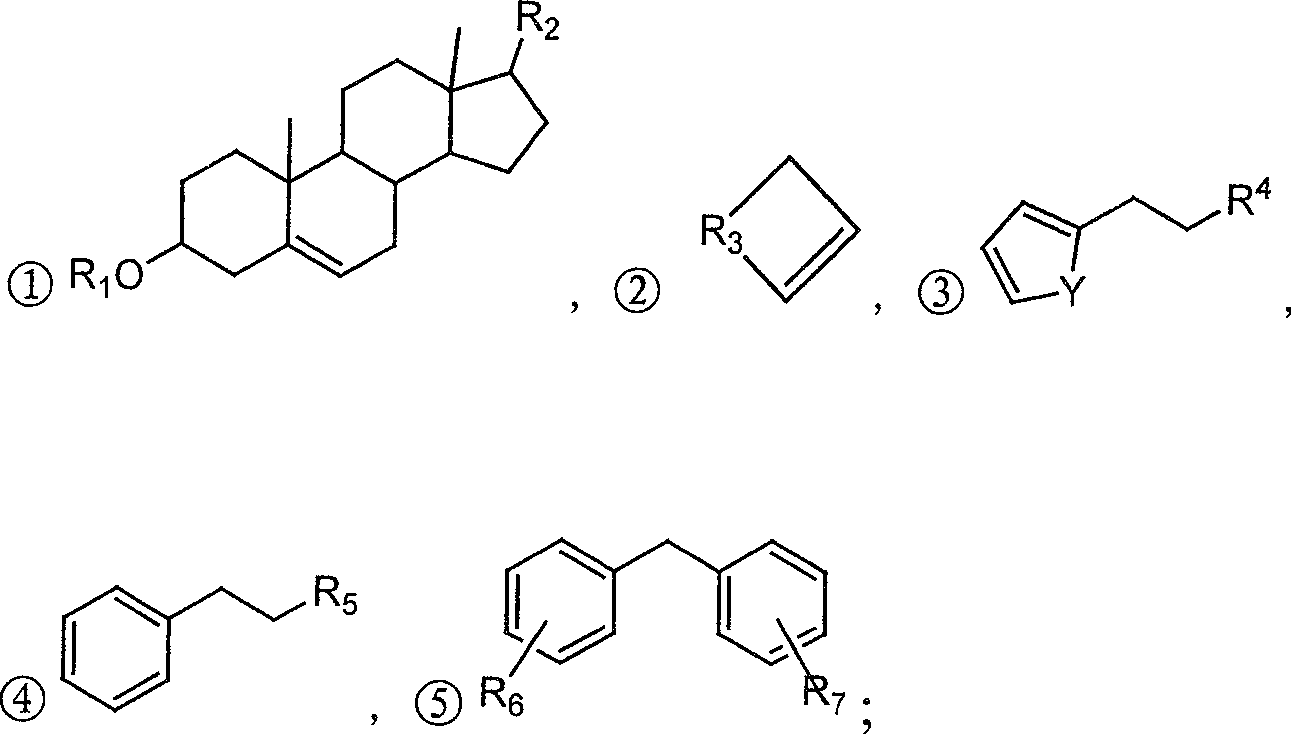
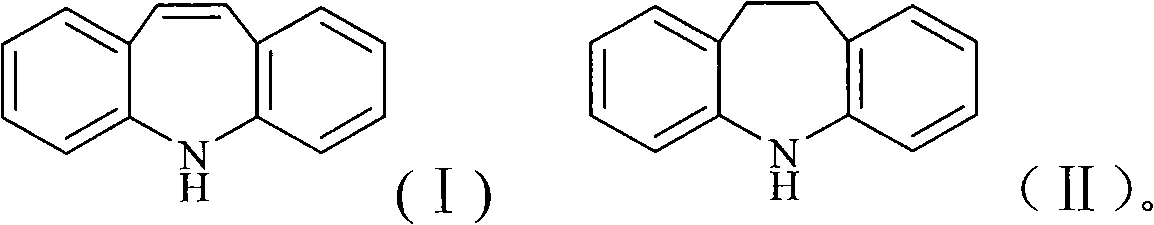
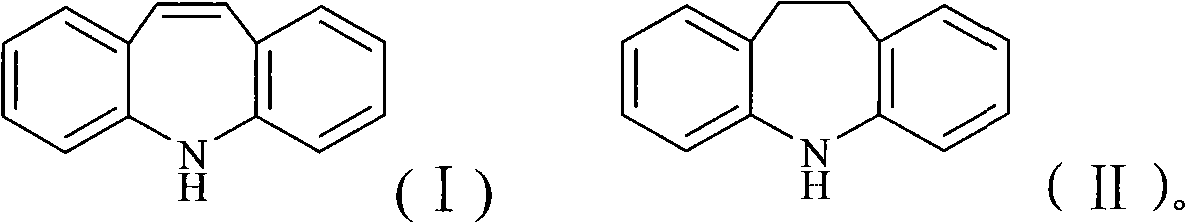
Chemical synthesis process for iminostilbene
ActiveCN101307021AHigh reaction yieldReduce manufacturing costOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsChemical synthesisElectricity
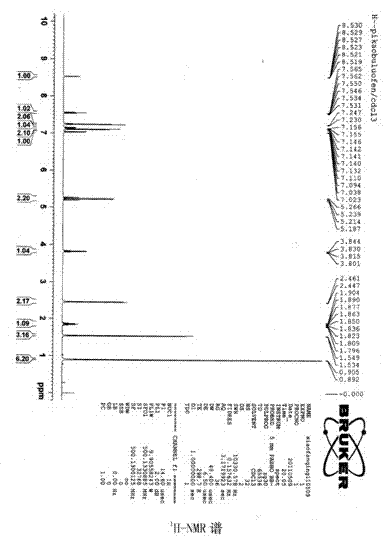
The invention provides a method for chemically synthesizing iminostilbene which is shown as a formula (I), comprising the following steps that: catalyst is filled on a fixed catalyst bed, the temperature of the catalyst bed is increased to between 400 and 600 DEG C by heating the catalyst bed electrically, iminodibenzyl which is shown as a formula (II) and is pre-heated to be liquated is sent to the fixed catalyst bed through high-pressure water vapour, the flow rate of the water vapour is controlled to between 0.2 and 20l / h by the iminodibenzyl fluid volume, and the material which passes through the fixed catalyst bed is put into a receiving device holding cooling water to produce the mixed fluid which is subject to moisture evaporation to dryness is crystallized again so that the iminostilbene is obtained. The method mainly has the advantages of high reaction yield coefficient up to 93.8 percent, low production, advanced process flow and convenient operation.
Owner:ZHEJIANG UNIV OF TECH +1
Synthesis method of palmitoyl amino acid sodium
ActiveCN102863348AAdvanced process routeHigh reaction yieldOrganic compound preparationCarboxylic acid amides preparationPalmitoyl chlorideActive agent
The invention relates to a synthesis method of palmitoyl amino acid sodium in the surfactant field, in particular relates to a method for preparing palmitoyl amino acid sodium by using palmitoyl chloride synthesized by a phosgene method. The synthesis method comprises the following steps: by taking palmitic acid and phosgene as raw materials, preparing palmitoyl chloride through reaction in the presence of an organic amide catalyst; dropwise adding palmitoyl chloride in an amino acid alkaline solution to obtain palmitoyl amino acid through reaction; and dissolving obtained palmitoyl amino acid with ethanol and adding the ethanol solution of sodium hydroxide to finally obtain palmitoyl amino acid sodium. In the synthesis method, a lot of phosphorus-containing wastewater is not produced even though phosphorus trichloride is used as a chlorination agent; sulfur dioxide generated by a thionyl chloride method can heavily pollute the environment; and the synthesis method reasonable in process, high in reaction yield and good in product quality, and the product does not contain residual phosphorus and sulfur and can be widely used in high-end anti-aging cosmetics.
Owner:CHANGSHA PUJI BIOTECH
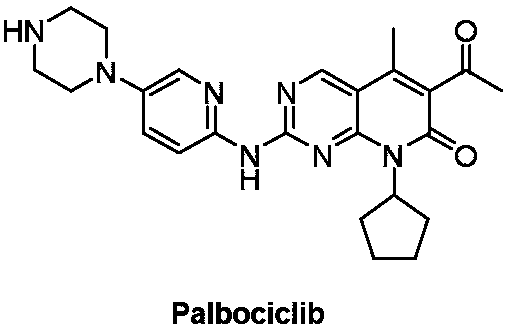
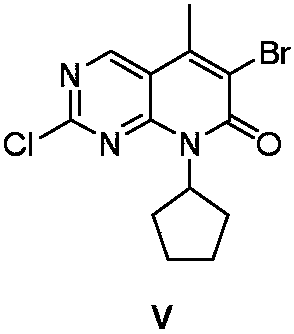
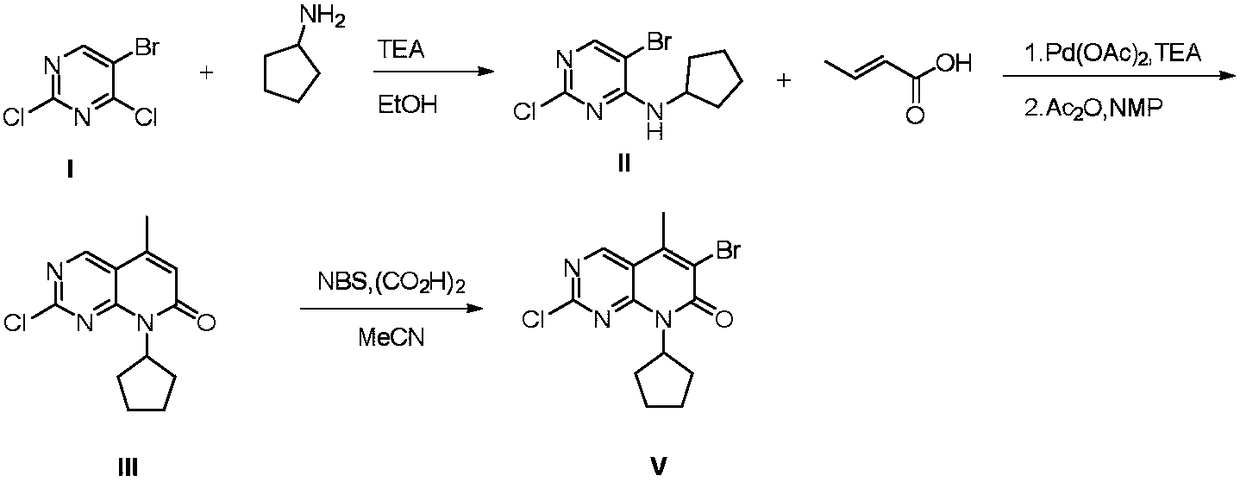
Preparation method of Palbociclib intermediate
The invention relates to the field of medicine synthesis, and discloses a preparation method of a Palbociclib intermediate. In the preparation process, 5-bromine-2,4-dichloropyrimidine is used as a starting raw material; through ammoniation substitution reaction, green solvent PEG (polyethylene glycol) promotion palladium catalysis coupled reaction, BTC (triphosgene) promotion cyclization reactionand NBS (N-bromosuccinimide) bromination reaction, a target compound V is finally obtained; through aftertreatment improvement, the HPLC purity of the final product can reach 99 percent or higher. Compared with a traditional process, the preparation method has the main beneficial effects that the reaction conditions are mild; the operation is simple and convenient; the palladium catalyst consumption is low; the yield is high; the cost is low; the three-waste quantity is small; the industrialization is easy; high implementation values and socioeconomic benefits are realized.
Owner:HANGZHOU FST PHARMA +1
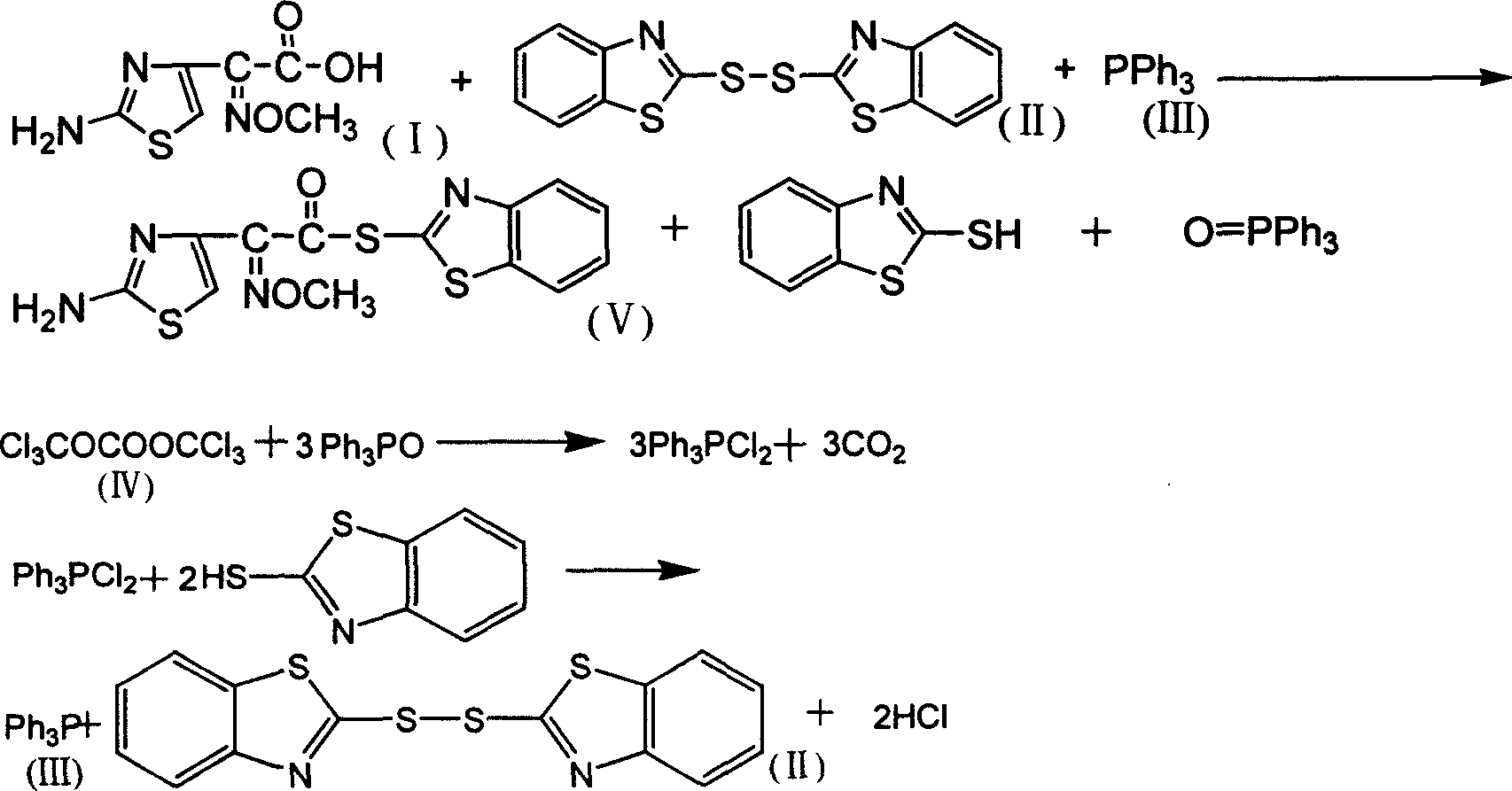
AE-active ester chemical synthesizing method
InactiveCN1709880AAdvanced process routeReasonable process conditionsOrganic chemistryChemical synthesisOrganic solvent
This invention has offered a chemosynthesis method to AE-activity ester, using ATMAA, dibenzthiazole sulfide, triphenylphosphine as raw materials, in existing of catalyst, after sufficient response of the organic solvent, filter the reaction solution, filter cake is washed and dried to get stated AE-activity ester, join di(trichloromethyl)carbonic acid ester in filtrate, the reaction gets dibenzthiazole and triphenylphosphine, stated benzthiazole sulfide and triphenylphosphine can be recycled after being retrieved. This invention has advanced craft route, rational process conditions, cheap and easy getting raw materials, simple and safe operation.
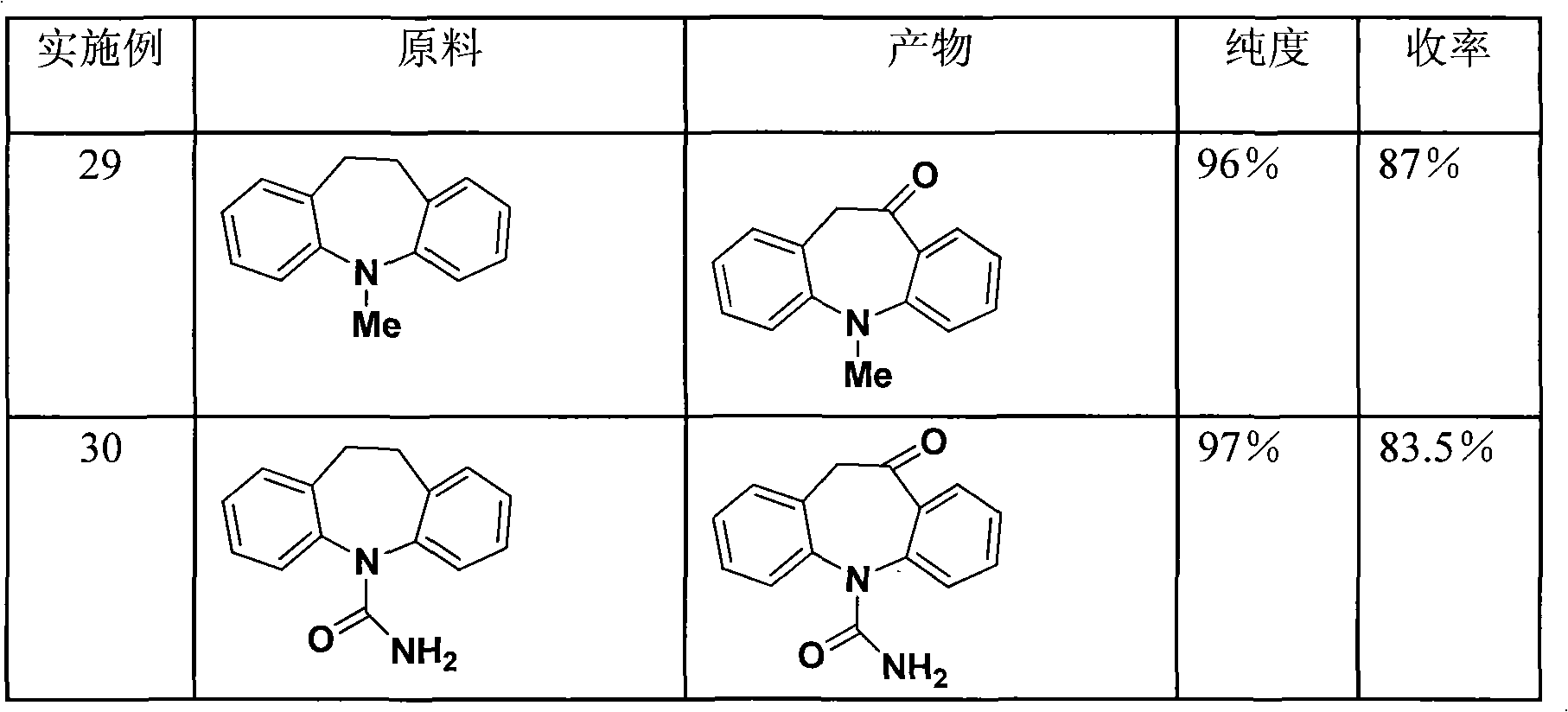
Owner:ZHEJIANG UNIV OF TECH
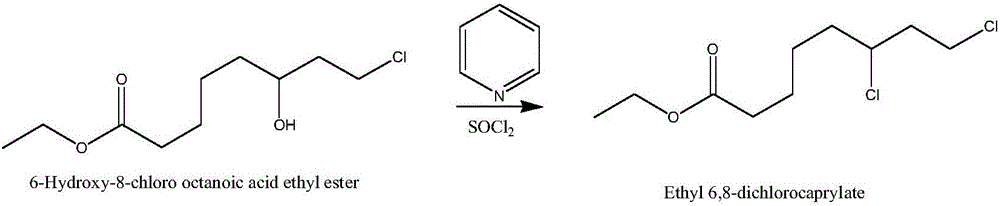
Chemical method for synthesizing 6,8-dichloro ethyl cacodylic acid caprylate
ActiveCN101157614AAdvanced process routeReasonable process conditionsOrganic compound preparationCarboxylic acid esters preparationChemical synthesisWater baths
The invention discloses a chemical synthetic method for 6, 8-ethyl octylate chloride. The method comprises the steps as follows that: 6-hydroxyl-8-ethyl octylate chloride is dissolved in N, N-dimethylformamide; solution which is dissolved with organic solution of double (trichloride trichloromethyl)-carbonic acid ester is dropped in when in mixing under cold water bath condition; the solution is gradually warmed to 50 to 90 DEG C for reacting for 2 to 8 hours after dropping; and the reaction solution is disposed into 6, 8-ethyl octylate chloride after finishing the reaction. As the invention replaces sulphoxides chloride with double (trichloride trichloromethyl)-carbonic acid ester, the invention produces environment friendly Vilsmeier agent which needs not to be separated in the reaction, and the Vilsmeier agent directly reacts with 6-hydroxyl-8-ethyl octylate chloride to produce 6, 8-ethyl octylate chloride. Besides, the invention has the advantages of advanced technique, rational technological condition, simple and safe operation, high reaction yield, low production cost, low three-waste emission and great implementation value and social and economic efficiency.
Owner:ZHEJIANG UNIV OF TECH +1
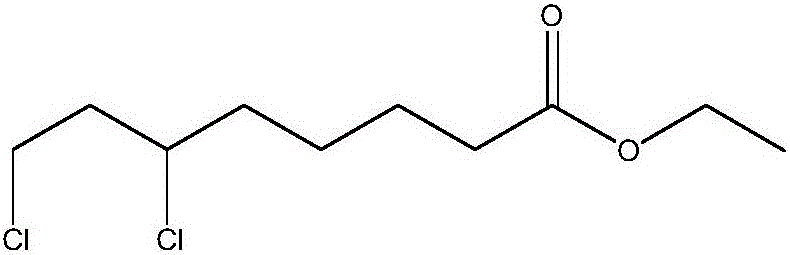
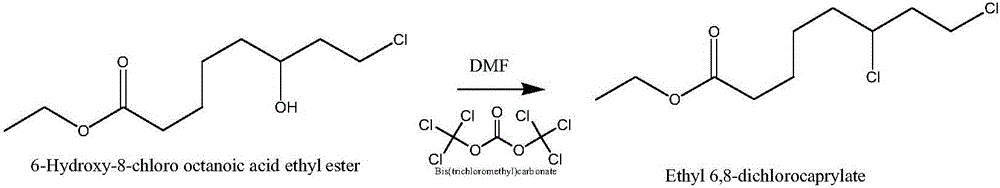
Preparation method of ethyl 6,8-dichlorocaprylate
InactiveCN105693510AEmission reductionImprove chlorination abilityOrganic compound preparationCarboxylic acid esters preparationOrganic solventReaction temperature
The invention relates to a preparation method of ethyl 6,8-dichlorocaprylate, belonging to the technical field of preparation of organic compound intermediates. The preparation method comprises the steps of reacting ethyl 6-hydroxy-8-chlorocaprylate with a chlorination agent in an organic solvent by taking N,N-dimethylbenzylamine as an acid-binding agent, controlling the reaction temperature, maintaining the temperature after the reaction is finished, and controlling the temperature maintaining temperature and the temperature maintaining time to obtain reaction liquid, adding water for layering to obtain an organic phase at an upper layer, carrying out reduced pressure rectification to obtain ethyl 6,8-dichlorocaprylate, adding alkali to a lower layer to regulate pH for layering, and carrying out dehydration on the upper layer by virtue of anhydrous sodium sulphate so as to obtain N,N-dimethylbenzylamine, and recycling. By virtue of the preparation method, the chlorination capacity is improved, the yield reaches about 95%, the cycle use is realized, the discharging of wastewater is reduced, the process route is advanced, the process conditions are reasonable, the operation is simple and safe, the reaction yield is high, the production cost is relatively low, and three wastes are little.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Green synthesis method for ibuprofen piconol and medicinal preparation thereof
InactiveCN102304081AAdvanced process routeReasonable process conditionsOrganic active ingredientsOrganic chemistryChemical synthesisIbuprofen Piconol
The invention discloses a green synthesis method for ibuprofen piconol and a medicinal preparation method thereof and provides a formula process of and a green chemical synthesis method of a medicinal compound, in particular the method for preparing ibuprofen piconol, a medicinal composition prepared by using ibuprofen piconol as an active ingredient, and a new formula of an externally-applied preparation for diminishing inflammation, relieving pain and treating infectious diseases. Compared with the prior art, the method has the advantages that: the technical route is advanced, the technical conditions are reasonable, the use of halogenating agents such as thionyl chloride which produce lots of toxic and harmful gases is avoided, a reactions are replaced by one reaction, the operation is simple, convenient and safe, the reaction yield is high, the reaction conditions are easy to control, the solvent and a dehydration condensation agent can be recycled and reused, the problems of trouble of treatment after a reaction process, serious waste gas, waste water and waste residue pollution and the like in the conventional process are solved from the source, environment pollution is avoided, little waste gas, waste water and water residue are produced, and high implementation value and great social and economic benefit are realized.
Owner:湖南医药工业研究所有限公司
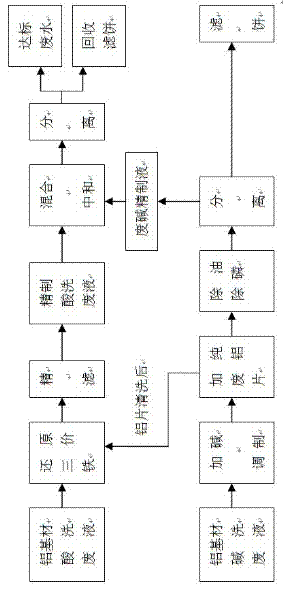
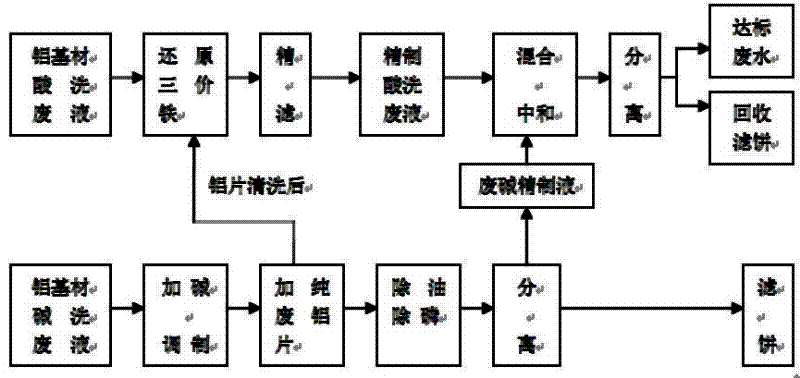
Technique for recycling aluminum hydroxide from waste liquid for acid washing and alkaline washing aluminum-based material
InactiveCN102642945ALower iron levelsIncrease added valueAluminates/aluminium-oxide/aluminium-hydroxide purificationMultistage water/sewage treatmentSocial benefitsAluminium hydroxide
The invention relates to a technique for recycling aluminum hydroxide from acid washing waste liquor and alkaline washing waste liquor of an aluminum-based material. The technique is characterized by comprising the following steps of: (1) respectively pre-treating the acid washing waste liquor and the alkaline washing waste liquor of the aluminum-based material; (2) mixing and separating the pretreated acid washing and the alkaline washing liquor after being neutralized, discharging the waste water after reaching the standard and directly utilizing a filter cake - aluminum hydroxide as an industrial raw material. According to the technique, not only is a great amount of aluminum-based alkaline washing waste liquid and acid washing waste liquid successfully treated, but also byproducts can be recycled, no secondary pollution occurs, and the technical difficulty both at home and abroad for a long time can be solved. A pilot scale test shows that the technique is reasonable in design and easy to implement and has great social benefit, environmental-protection benefit and economic benefit.
Owner:LUCKY HUAGUANG GRAPHICS +1
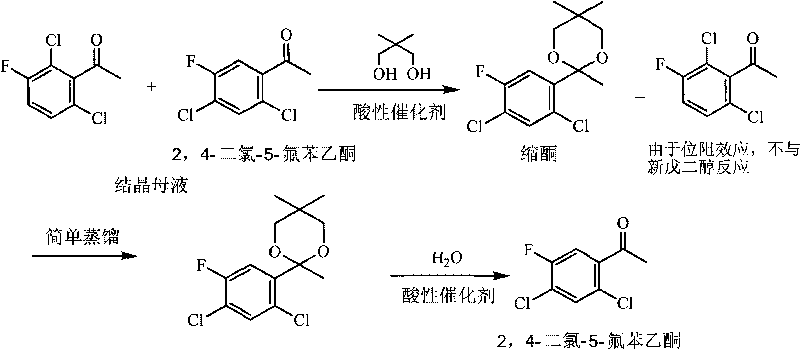
Method for effectively recycling 2,4-dichloro-5-fluoro acetophenone from crystallization mother liquor
ActiveCN101747167AAdvanced process routeEasy to operateMultistage water/sewage treatmentNature of treatment waterChemistryMother liquor
The invention discloses a method for effectively recycling 2,4-dichloro-5-fluoro acetophenone from crystallization mother liquor, which comprises the following steps of: (a) reacting 2,4-dichloro-5-fluoro acetophenone crystallized mother liquor and neopentyl glycol as raw materials in an inert organic solution A under the action of an acidic catalyst A, then removing water continuously produced in the reaction process, and after sufficient reaction, separating and purifying to respectively obtain 2,6-dichloro-3-fluoro acetophenone and 2,4-dichloro-5-fluoro acetophenone ketal; and (b) carrying out the hydrolysis on the 2,4-dichloro-5-fluoro acetophenone ketal and water in an inert organic solution B under the action of an acidic catalyst B, and after sufficient reaction, carrying out aftertreatment to obtain the 2,4-dichloro-5-fluoro acetophenone. The invention has advanced process route, simple operation, mild reaction condition, high recycle rate of the 2,4-dichloro-5-fluoro acetophenone, environment protection and low production cost; and the neopentyl glycol of the auxiliary reagent can be recycled repeatedly, and the solidoid acidic catalyst can be recycled, thereby the invention is suitable for industrialized large-scale production.
Owner:ZHEJIANG UNIV OF TECH +1
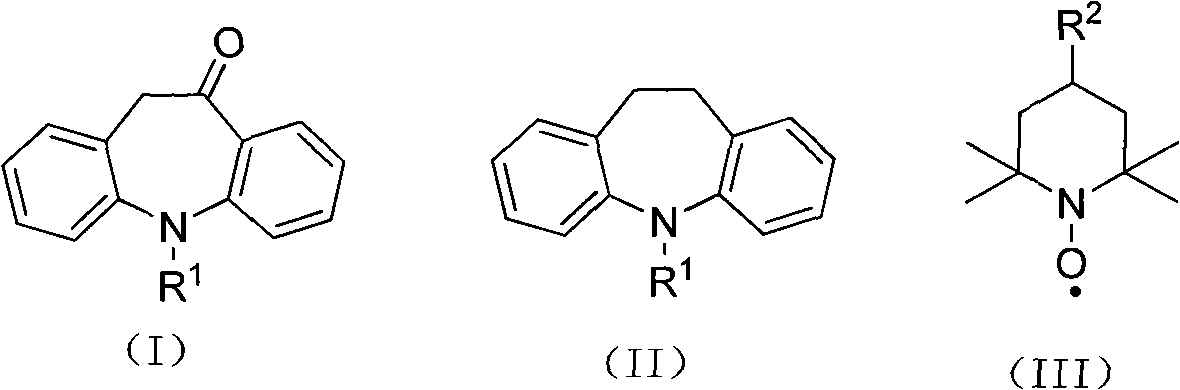
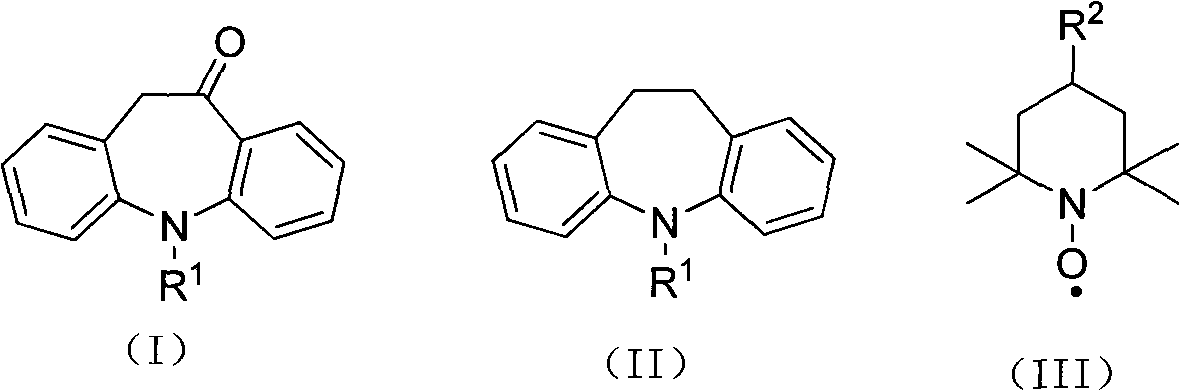
Synthesis method of 10-oxa-10,11-dihydro-5H-dibenzo(b,f) azepine
The invention relates to a synthesis method of 10-oxa-10,11-dihydro-5H-dibenzo(b,f) azepine shown in the formula (I). The synthesis method comprises the following steps of: carrying out oxidizing reaction at 0-200 DEG C by taking 10,11-dihydro-5H-dibenzo(b,f) azepine shown in the formula (II) as a raw material, nitroxides shown in the formula (III) as a catalyst, acetate as a catalyst promoter, calcium hypochlorite as an oxidant and inorganic salt as a carrier, and after reaction, processing reaction liquid to obtain the 10-oxa-10,11-dihydro-5H-dibenzo(b,f) azepine shown in the formula (I). The invention has the advantages of short reaction step, high conversion rate and yield, advanced process path, mild reaction conditions, small catalyst consumption, no use of brom-containing reagent like NBS (N-bromosuccinimide), liquid bromine and the like, simple after-treatment and less pollution to the environment.
Owner:ZHEJIANG UNIV OF TECH +1
Chemical synthesis method of 3,4-dichlorobenzene isocyanate
InactiveCN1478769AAdvanced process routeReasonable process conditionsIsocyanic acid derivatives preparationOrganic compound preparationChemical synthesisOrganic solvent
A process for chemically synthesizing 3,4-dichlorobenzene isocyanate includes such steps as proportionally mixing 3,4-dichlorophenylamine, catalyst and organic solvent to reactor, stirring, dripping the solution of bicarbonate in organic solvent into the solution, reacting at 20-180 deg.C for 3-10 hr, vacuum distilling to recover organic solvent, collecting fraction, cooling and solidifying. Its advantages are high output rate, low cost and less corrosion to equipment.
Owner:蔡戈冬
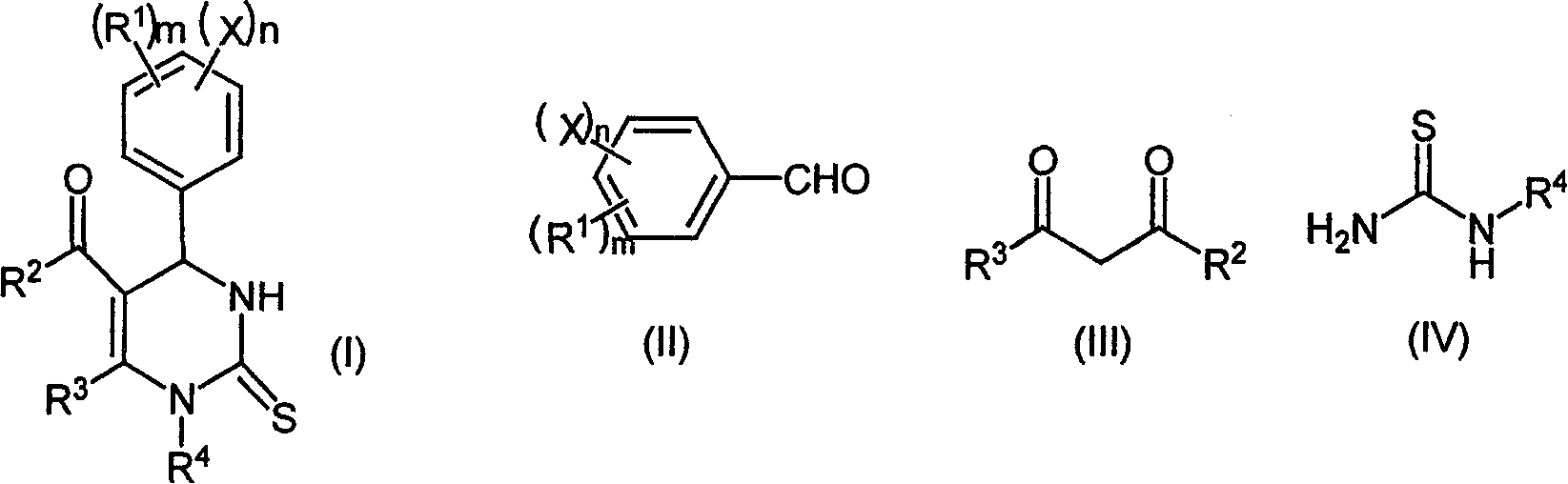
Chemical synthesis method of O-acylcalix[4]arene
ActiveCN101781199AReduce dosageHigh reaction yieldPreparation from carboxylic acid halidesOrganic compound preparationChemical synthesisOrganic solvent
The invention relates to a chemical synthesis method of O-acylcalix[4]arene shown in the formula (I). The synthesis method comprises the following steps: dissolving the calixarene shown in the formula (II) in organic solvent, reacting with the acylation reagent shown in the formula (IV) at 0-150 DEG C for 0.1-10 hours under the catalysis of lithiumtrifluoromethanesulfonate shown in the formula (III), and reprocessing reaction liquid to obtain O-acylcalix[4]arene shown in the formula (I), wherein in the formula (I) and (II), R1 represents C1-C6 alkyl or hydrogen; in the formula (I) and (IV), R2 represents C1-C6 alkyl, phenyl or benzyl; and in the formula (IV), X is Cl or R2COO. The beneficial effects of the invention are as follows: the catalyst used in the reaction can be reused; the yield is high (generally over 80%); the process route is advanced, the reaction conditions are mild; and the amount of catalyst is small and the method is environmentally friendly.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing solifenacin intermediate
ActiveCN105541712AReduce manufacturing costAdvanced process routeOrganic chemistryDistillationNitromethane
The invention belongs to the field of medicine and particularly relates to a method for preparing a solifenacin intermediate. After 2-halogenated diphenyl ketone is used for carbonyl protection, n-butyllithium is used for removing bromine, then the formyl group is added; a condensation reaction with nitromethane and catalytic hydrogenation for reduction are carried out, and then acidification is conducted; later, cyclization is carried out, and a solifenacin intermediate compound I is obtained through alkaline hydrolysis after reduction and chiral resolution. The structural formula of the solifenacin intermediate is shown in the description. According to the method for preparing the solifenacin intermediate, the initial raw materials easy to obtain are utilized, and the production cost is reduced. The process route is advanced, the reaction conditions are mild, the reaction yield is high, less three wastes are caused, expensive and toxic reagents do not exist, reaction solvents can be used repeatedly after distillation, industrial production is facilitated, and implementation value and social, economic and environmental protection benefits are high.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
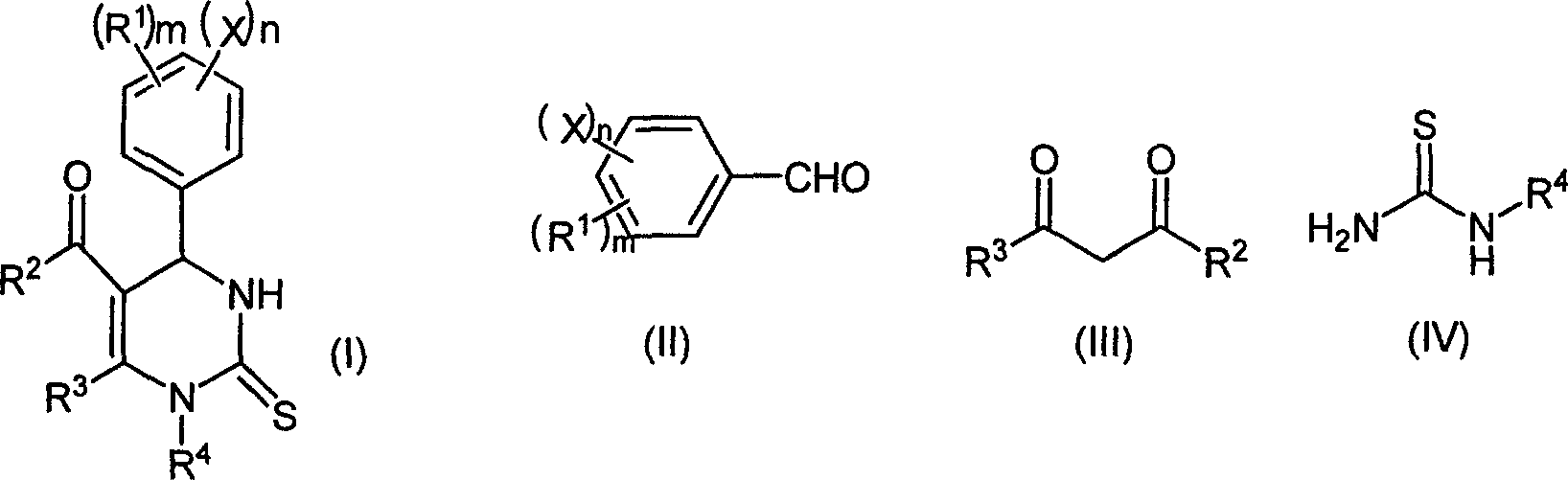
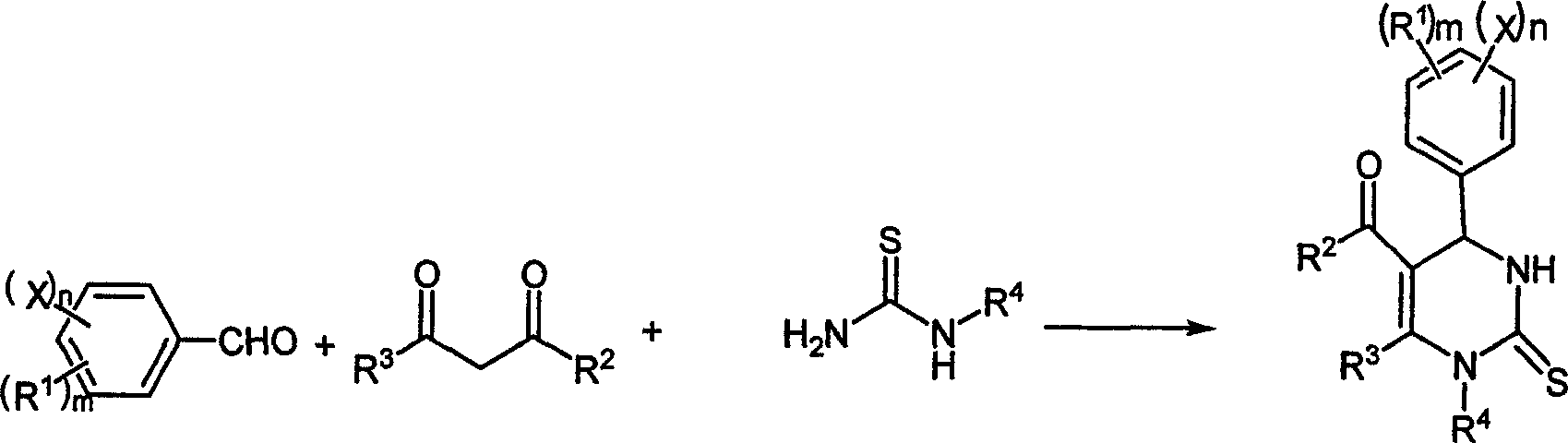
Chemical synthesis method of pyrimidine thioketone
InactiveCN1803777AAdvanced process routeMild reaction conditionsOrganic chemistryChemical synthesisDiketone
The related synthesis method for pyrimidinethione compound comprises: using trifluoro magnesium methanesulfonate to replace traditional Lewis acid catalyst of strong HCl and ZnCl2, selecting aldehyde and beta-diketone instead of thiourea as material to reaction at 20-150Deg. This invention has yield and purity more than 85% and 98.5% respectively with low cost and mild condition, and produces almost no three wastes.
Owner:ZHEJIANG UNIV OF TECH
Alpha,beta-unsaturated ketone or arone environment-friendly synthesis method
ActiveCN100494149CReduce dosageStop pollutionOrganic compound preparationCarbonyl group formation/introductionImideBenzoyl peroxide
The invention discloses a green synthesizing method of alpha, beta-unsaturated ketone or aromatic ketone, which is characterized by the following: adopting relative unsaturated hydrocarbons or aromatic hydrocarbons as raw material; setting imide as catalyst; making azobisisobutyronitrile (AIBN) or benzoyl peroxide (BPO) as initiator; oxidizing in the organic solvent; dehydrating through dehydrant; separating and purifying to obtain the alpha, beta-unsaturated ketone; improving reacting receiving rate; reducing manufacturing cost; possessing mild reacting condition; using little catalyst; avoiding pollution.
Owner:ZHEJIANG UNIV OF TECH +1
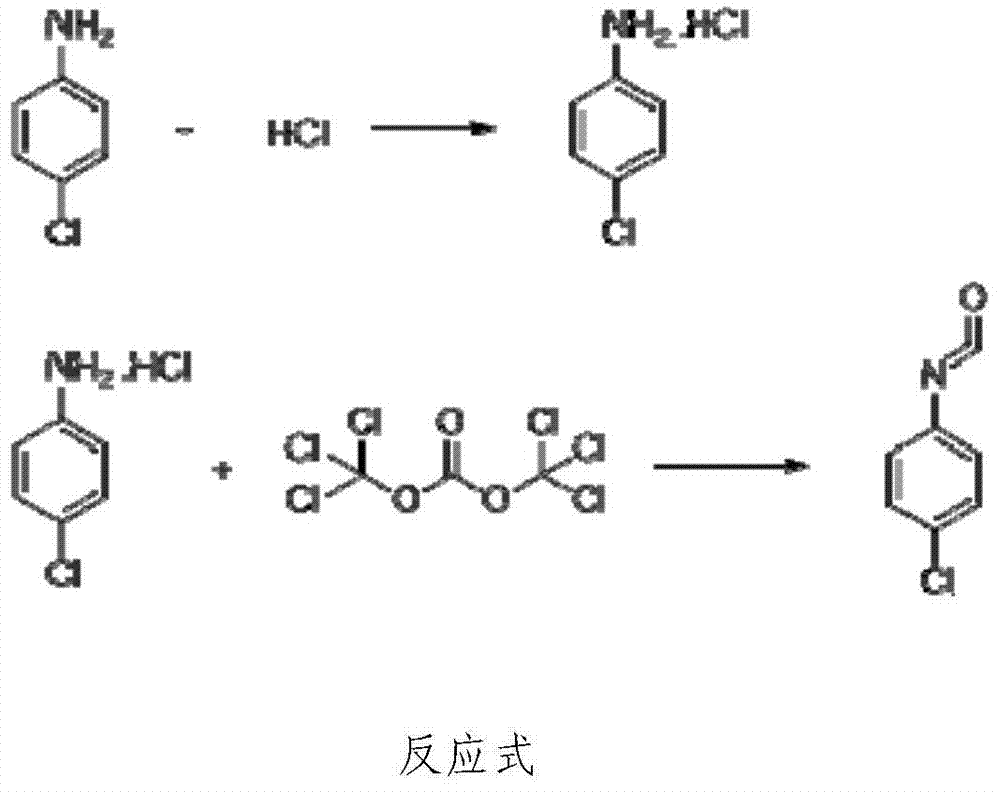
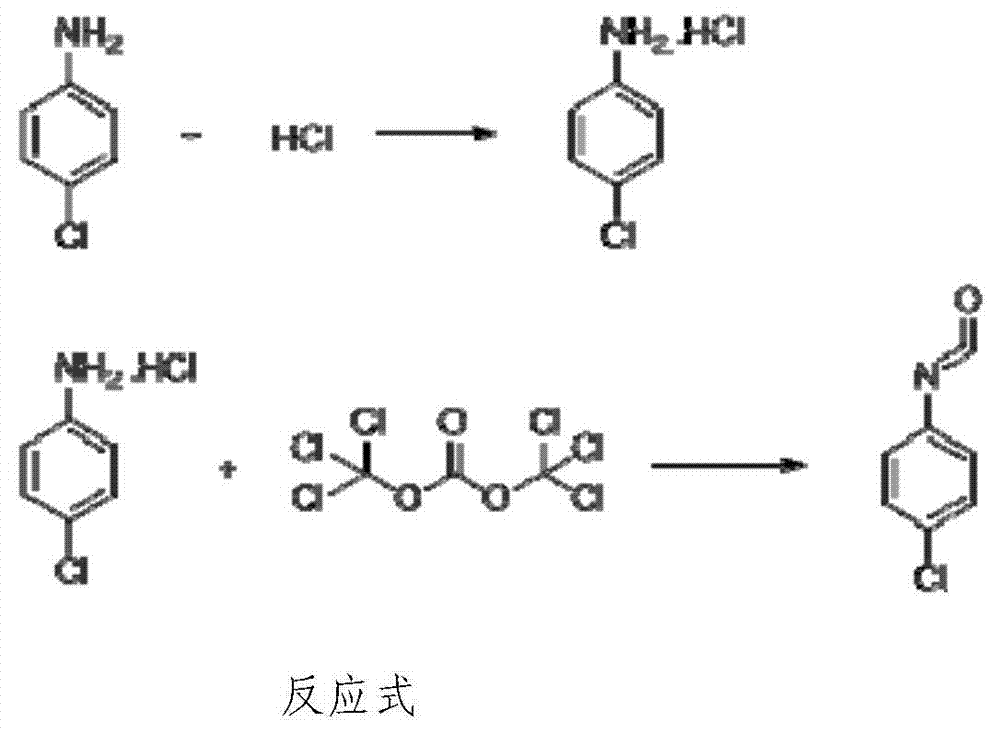
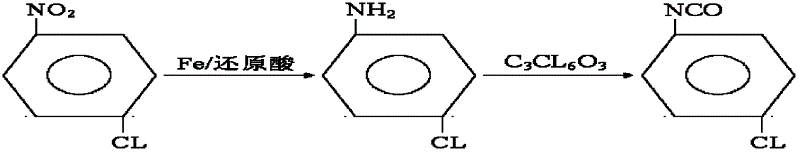
P-chloroaniline isocyanate preparation method
InactiveCN104744306AAdvanced process routeReasonable process conditionsIsocyanic acid derivatives preparationOrganic compound preparationP-chloroanilineSocial benefits
The invention relates to a p-chloroaniline isocyanate preparation method comprising the following steps: (1) p-chloroaniline hydrochloride is synthesized, wherein p-chloroaniline is adopted as a raw material and is subjected to a reaction with hydrochloric acid in an organic solvent, such that p-chloroaniline hydrochloride is synthesized; (2) p-chloroaniline isocyanate is synthesized, wherein p-chloroaniline hydrochloride and triphosgene are adopted as raw material, and p-chloroaniline isocyanate is synthesized in an organic solvent. The method provided by the invention has the advantages of advanced process route, reasonable process conditions, and high reaction yield. The materials used in production are not highly excessive; solvent dose in each step is low; and the solvent can be directly used without treatment. An intermediate is not needed to be dried, and can be directly used in the next step of reaction. Three-waste amount is low, and the waste is easy to treat. Therefore, the method has good implementation value and social benefit.
Owner:HUNAN LIJIE BIOCHEM
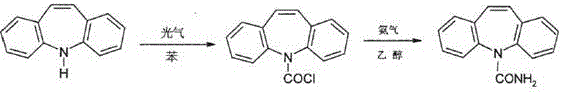
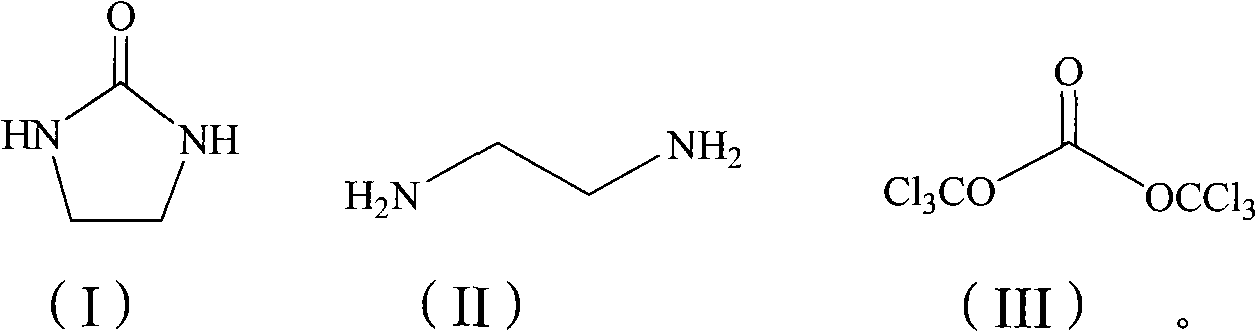
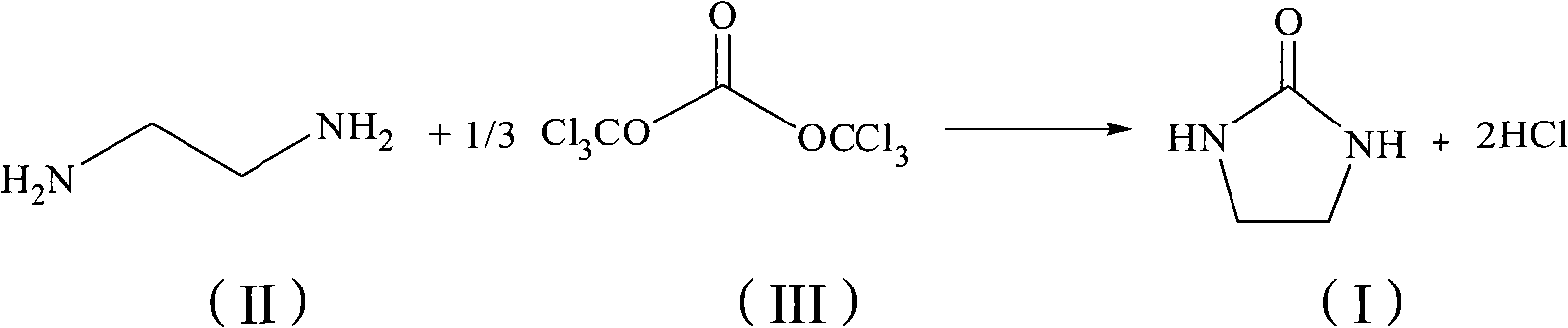
Method for synthesizing carbamazepine
Provided is a method for synthesizing carbamazepine. The method comprises the steps that 1 part by weight of iminostilbene and 10 parts by weight of benzene are put into a glass lining reaction vessel of 1,000 L to be dissolved, and the materials react with 0.7 part of triphosgene for 5 h to 6 h at 70 DEG C to 80 DEG C after being dissolved to enable all the materials to react completely; 2 / 3 of benzene is distilled off through reduced pressure distillation, suction filtration is conducted after cooling is conducted, the materials are put into a drying oven or a drying room to be dried for 3 h to 4 h at 60 DEG C after being filtered, iminostilbene carbonyl chloride is obtained and put into a glass lining reaction vessel of 1,000 L, 15 parts of 95% ethyl alcohol is added, the materials are dissolved in the glass lining reaction vessel, 0.8 part of liquid ammonia is dropwise added, reacting is conducted for 8 h at 50 DEG C to 60 DEG C, heat preservation is conducted for 30 min, all the materials are cooled to room temperature, 1%-3% by weight of activated carbon is added, the temperature is increased to 78 DEG C, decoloration is conducted for 2 h, a mother solution is subjected to hot filtration, residues are discarded, and the mother solution is sent back to the glass lining reaction vessel; 1 / 2 of ethyl alcohol is distilled off, after cooling is conducted, natural crystallization and suction filtration are conducted, a crystal substance obtained after filtration is conducted is put into the drying oven or drying room to be dried for 3 h to 4 h at 60 DEG C, and the finished carbamazepine product is obtained. The yield ranges from 82% to 90%, and the content ranges from 91.5% to 99.1%.
Owner:陈建国
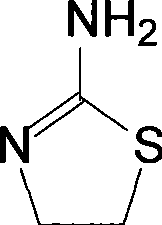
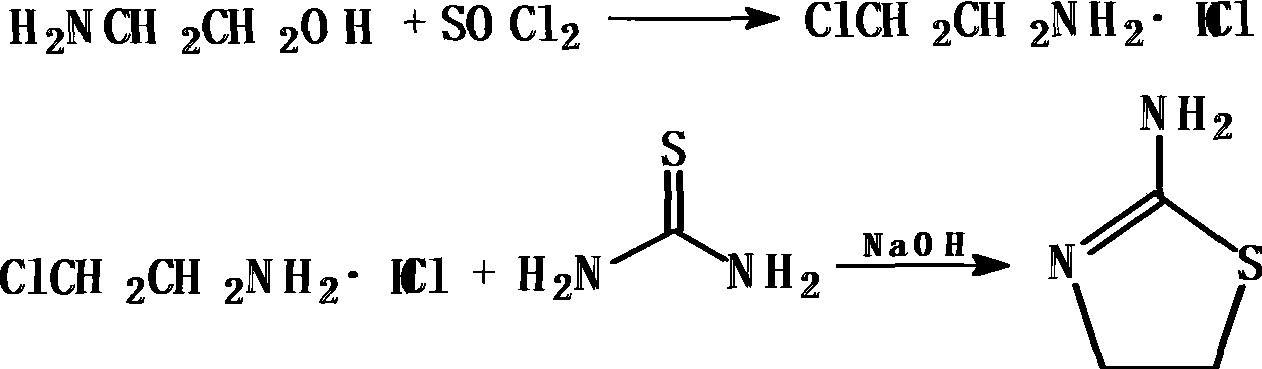
Method for synthesizing 2-amino thizaoline
ActiveCN101417985AFew reaction stepsAdvanced process routeOrganic chemistry2-aminothiazolineOrganic solvent
The invention relates to a method for synthesizing 2-aminothiazoline, which comprises the following steps: firstly, performing chlorinated reaction between ethanolamine and thionyl chloride in an organic solvent to generate 2-chloro-ethylamine hydrochloride; and secondly, performing cyclization reaction between the 2-chloro-ethylamine hydrochloride and thiourea to generate the 2-aminothiazoline. Compared with the prior art, the method does not need introduce hydrogen chloride gas and concentrated hydrochloric acid to synthesize ethanolamine hydrochloride, thereby reducing reaction reagents and the reaction steps; besides, the process route is more advanced, environment-friendly and safer, and the cyclization yield reaches more than 70 percent.
Owner:山西新天源药业有限公司
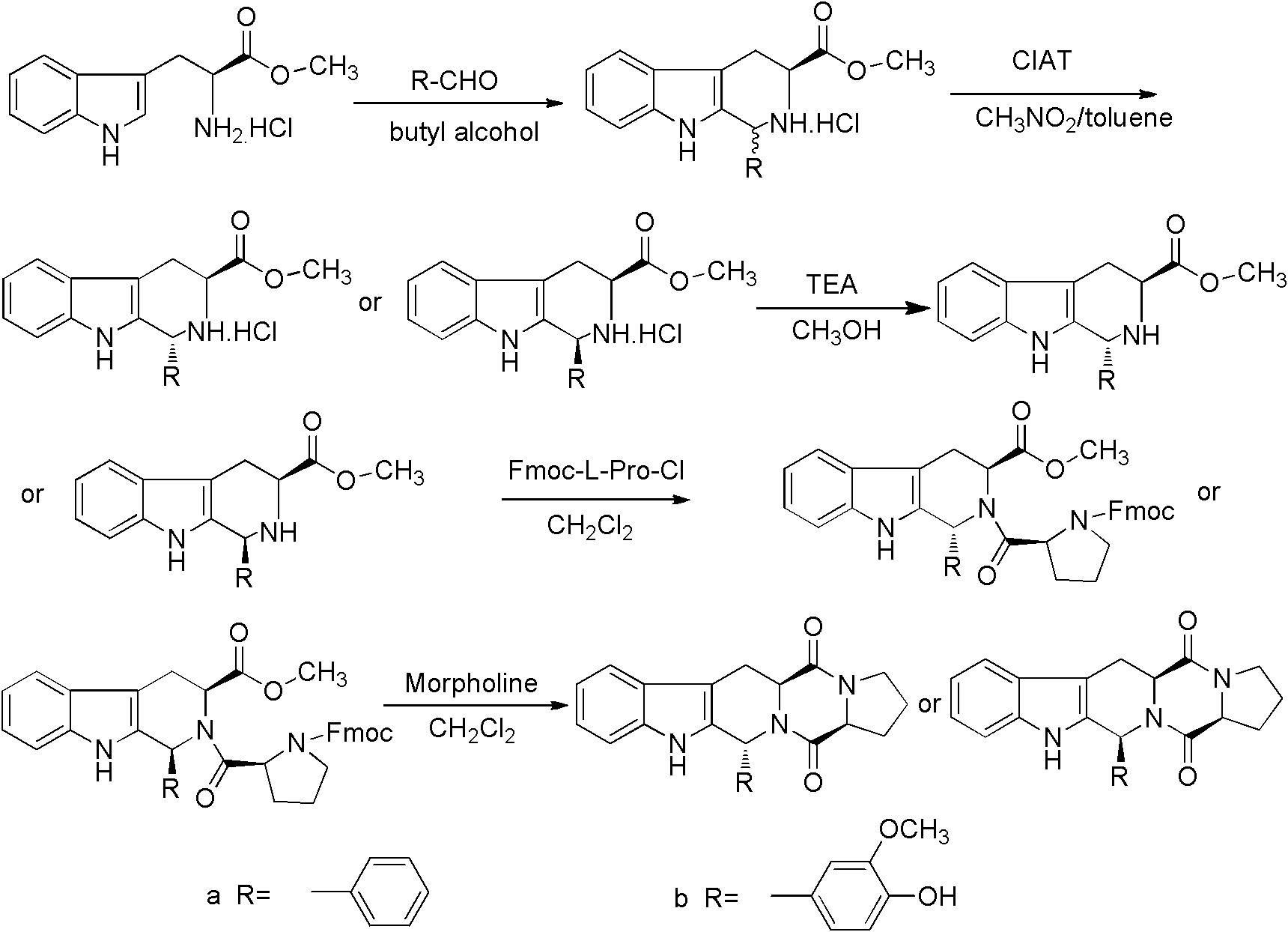
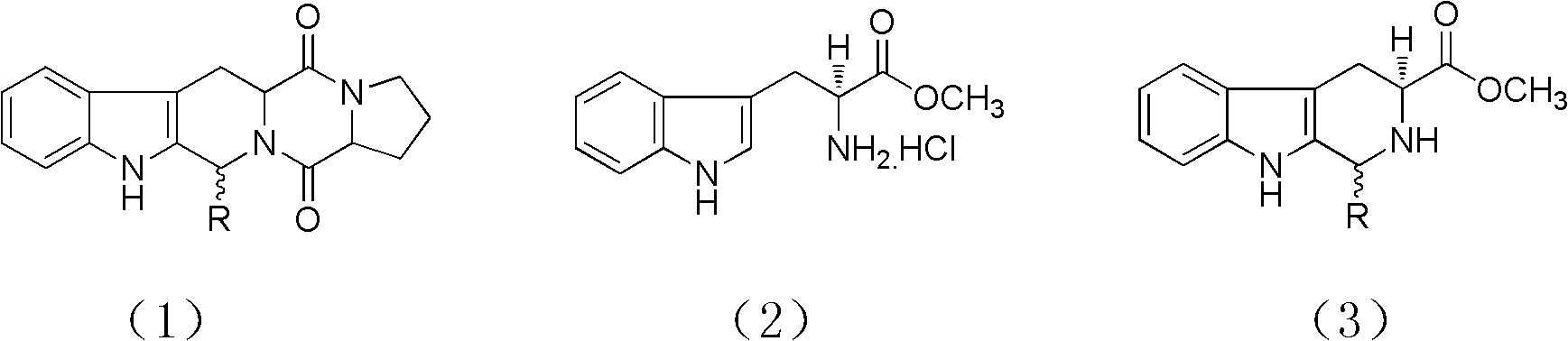
Method for synthesizing tetrahydro-beta-carboline diketopiperazine compound
InactiveCN102276608AAdvanced process routeSimple reaction conditionsOrganic chemistrySchotten–Baumann reactionDiketopiperazines
The invention provides a method for synthesizing a tetrahydro-beta-carboline diketopiperazine compound. The method comprises the following steps of: reacting Pictet-Spengler reaction on L-tryptophane methyl ester hydrochloride serving as a starting raw material with aldehyde; performing a crystallization induced asymmetric transformation (CIAT) process to obtain a tetrahydro-beta-carboline ring; performing Schotten-Baumann reaction in a two-phase solvent comprising saturated sodium carbonate and dichloromethane; and finally forming an amido bond and performing a deprotection process to obtaina target product. The method has the beneficial effects of fewer reaction steps, high transformation rate and yield, advanced technological line, simple post-treatment, easiness in purification and the like.
Owner:SHAANXI UNIV OF SCI & TECH
Preparation method for 2-cyano-4'-methylbiphenyl
InactiveCN103467341ALow costHigh activityCarboxylic acid nitrile preparationOrganic compound preparationBenzeneNitrogen
The invention discloses a preparation method for 2-cyano-4'-methylbiphenyl. The invention adopts the following technical scheme: the preparation method comprises the following steps: taking p-chlorotoluene as a raw material for Grignard reaction with magnesium powder in a solvent in the absence of water under nitrogen protection, and coupling a reaction product with chlorobenzonitrile under the catalytic effect of a nickel-manganese composite catalyst to prepare 2-cyano-4'-methylbiphenyl. A Ni (II) / Mn (II) composite catalyst is added into a 2-cyano-4'-methylbiphenyl synthesis reaction system, so that the reaction activity is enhanced and the reaction selectivity is improved; the catalyst is lower in price and easy to recover, so that the postprocessing difficulty is lowered and the production cost is reduced; a recrystallization method is adopted in postprocessing and refining processes, so that the product purity is further improved, a white product is obtained, and the industrialization operation is more convenient.
Owner:HENAN NORMAL UNIV
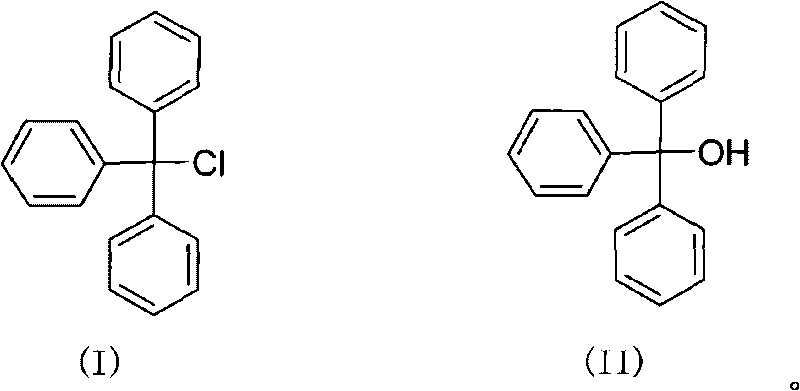
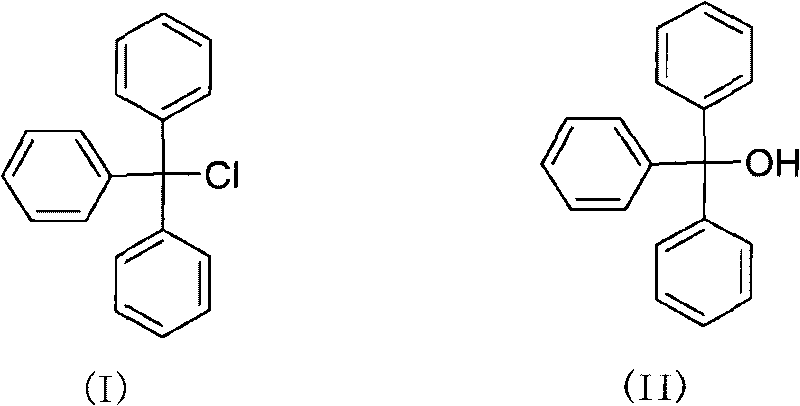
Green synthesizing method of triphenylchloromethane
ActiveCN101717324AHigh reaction yieldReduce manufacturing costHalogenated hydrocarbon preparationOrganic solventTriphosgene
The invention discloses a green synthesizing method of triphenylchloromethane. The synthesizing method comprises the following steps of: using triphenylmethanol as raw material; reacting the triphenylmethanol with triphosgene in an organic solvent for 0.1-20 hours at a temperature of -20-150 DEG C; after reacting, separating to obtain a crude product; and recrystallizing the crude product to obtain the triphenylchloromethane, wherein the mass ratio of the triphenylchloromethane to the triphosgene is 1:(0.33-2). The synthesizing method has the advantages of reasonable process, high reaction yield, low production cost, less three waste and simple aftertreatment.
Owner:ZHEJIANG UNIV OF TECH
Chemical synthesis method for octadecyl isocyanic ester
InactiveCN101245036AAdvanced process routeReasonable process conditionsIsocyanic acid derivatives preparationOrganic compound preparationIsocyanateChemical synthesis
The invention discloses a chemical synthesis method of octadecyl isocyanate; wherein, the method adopts octadecyl amine and bi(trichloromethyl) carbonate as raw materials, organic solvents are used for dissolving the octadecyl amine solution to be slowly dropped into the solution of the bi(trichloromethyl) carbonate dissolved by the same organic solvent, the temperature of a reaction system is kept from 0 to 5 DEG C, and after the dropping is finished, the solution is stirred for 0.5 hour to 5 hours at room temperature, then the temperature is increased to 35 to 150 DEG C and the solution is reacted at the temperature of 35 to 150 DEG C for 0.5 hour to 10 hours and then the octadecyl isocyanate is obtained after the reaction solution is post treated. The chemical synthesis method is advanced in technical route, reasonable in technical condition and eliminates potential safety hazard from headstream; the used raw materials are cheap and easy to be obtained; the use of highly toxic gas phosgene controlled internationally is abolished and the problems of big potential safety hazard and severe three wastes, etc. of traditional technologies are eliminated; the chemical synthesis method is simple and safe in operation, high in reaction yield, low in production cost and basically has no three wastes and has greater implement value and social economic benefits.
Owner:WENZHOU UNIVERSITY
Chemical synthesis method for 2-imidazole alkyl ketone
ActiveCN101270088AHigh reaction yieldReduce manufacturing costOrganic chemistryEthylenediamineChemical synthesis
The present invention discloses a chemical synthetic method of 2-imidazole flavanone. The synthetic method comprises the following steps: ethylenediamine and 2 to 20 percent of sodium hydroxide solution are added in a reactor; at the temperature from minus 10 DEG C to 50 DEG C, bi(trichloromethyl) carbonate is slowly dropped into the reactor for 1 to 15 hours; then the mixture reacts for 1 to 20 hours at the temperature from 0 DEG C to 80 DEG C; after the reaction, the reaction solution can be treated to prepare the 2-imidazole flavanone. The chemical synthetic method has high yield (generally above 80 percent) and high purity (generally above 98.0 percent). Thus the chemical synthetic method is an effective synthetic method with reasonable process, low production cost, high yield and less three-waste.
Owner:浙江遂昌利民科技有限公司
2,2'-di-thio-bibenzoyl cholride chemical synthesis method
InactiveCN1762998AAdvanced process routeReasonable process conditionsSulfide preparationChemical synthesisOrganic solvent
The present invention provides the reaction process of di(trichloromethyl) carbonate and 2, 2'-dithio diphenyl formic acid at 20-150 deg.c inside organic solvent under the action of organic amine catalyst to prepare 2, 2'-dithio diphenyl formyl chloride. The chemical synthesis process eliminates unsafe hidden trouble and environmental pollution radically, and has the advantages of cheap material, high safety and reliability, high reaction yield, low production cost and no waste generated.
Owner:ZHEJIANG UNIV OF TECH
Synthesis method for co-producing p-chloroaniline and p-chlorophenol isocyanate
InactiveCN102617401AAvoid pollutionAvoid poisoningIsocyanic acid derivatives preparationOrganic compound preparationP-chloroanilineReflux
The invention relates to a synthesis method for co-producing p-chloroaniline and p-chlorophenol isocyanate. The method comprises the following steps of: reducing parachloronitrobenzene by using iron powder, separating water, dehydrating, adding a solvent, reacting with a bis(trichloromethyl) carbonate solution, distilling to recover the solvent, and distilling to obtain a p-chlorophenol isocyanate finished product. The method has the advantages that a process is advanced; process conditions are reasonable; the production of p-chloroaniline is organically combined with that of p-chlorophenol isocyanate; the environment pollution and an intoxicating phenomenon which are brought by p-chloroaniline used as an industrial product in the processes of aftertreatment, conveying, carrying and feeding can be avoided; virulent phosgene and diphosgene are avoided during production of p-chlorophenol isocyanate, and production is safe and reliable; the residual bis(trichloromethyl) carbonate is removed by normal-pressure reflux; the improvement on the quality of a product is facilitated; and the method has great implementation value and social and economic benefits.
Owner:象山志华新材料有限公司
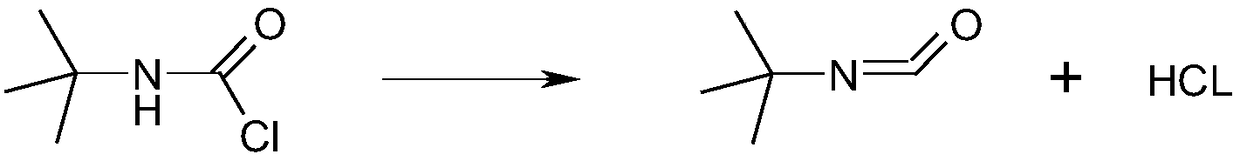
Synthesis method of tert-butyl isocyanate
InactiveCN108689882AEliminate potential safety hazardsPromote safe productionPreparation from carbamatesRotary evaporatorSynthesis methods
The invention relates to a synthesis method of tert-butyl isocyanate. The synthesis method comprises following steps: (1) evenly mixing a proper amount of catalyst and a solvent (90% of the theoretical amount) at a temperature of 90-180 DEG C to obtain a premixed solution, gradually dropwise adding a fed-batch solution into the premixed solution, after addition, maintaining the temperature of reaction solution in a range of 90-180 DEG C, keeping a reflux state, and carrying out reactions for 15 to 25 hours under stirring; and (2) after the reactions (1), delivering the mixed solution to a rotary evaporator, carrying out rotary evaporation, washing, and drying to obtain a tert-butyl isocyanate product. According to the synthesis method, tert-butyl amino formyl chloride directly carries outreactions in an inert solvent to prepare tert-butyl isocyanate; the safety hazard is radically inhibited from the source of the technology, and the preparation method has the advantages of reasonabletechnology, safety, high yield, and low production cost, and does not generate any wastewater, waste gas, or waste solid.
Owner:XINYI AGRI CHEM PLANT JIANGSU PROV
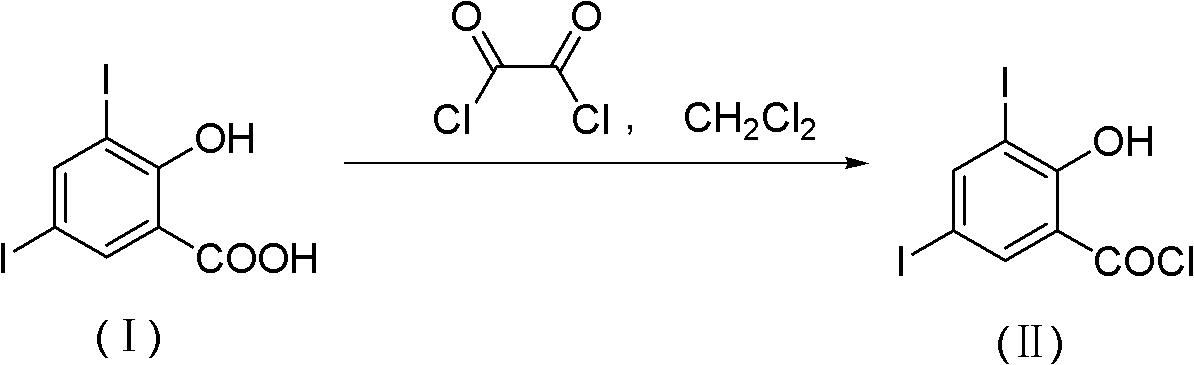
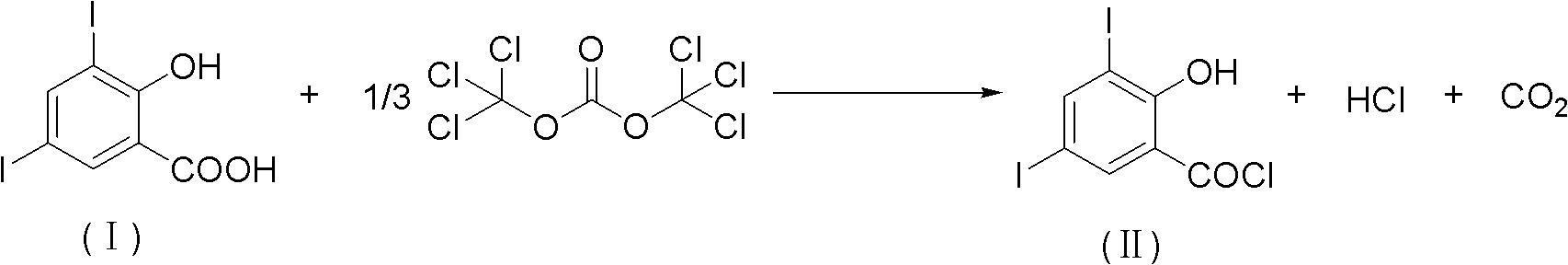
Method for chemically synthesizing 3,5-diio-2-hydroxybenzene formyl chloride
InactiveCN102206152AHigh yieldHigh purityOrganic compound preparationCarboxylic compound preparationChemical synthesisOrganic solvent
The invention discloses a method for chemically synthesizing 3,5-diio-2-hydroxybenzene formyl chloride. The method comprises the step of: with 3,5-diio-2-hydroxybenzoic acid and di(trichloromethyl) carbonic ester as raw materials, reacting for 1-10 hours at the temperature of 40-150 DEG C in an organic solvent under the action of an organic amine catalyst, and processing reaction liquid to obtain 3,5-diio-2-hydroxybenzene formyl chloride, wherein the charging mol ratio of the 3,5-diio-2-hydroxybenzoic acid to the di(trichloromethyl) carbonic ester is 1: (0.34-2.0), the charging mol ratio of the 3,5-diio-2-hydroxybenzoic acid to the organic amine is 1: (0.01-1.0), and the mass ratio of the organic solvent to the 3,5-diio-2-hydroxybenzene formyl chloride is (5-20): 1. The method disclosed by the invention has the advantages of available raw materials, low cost, simplicity and safety in operation, environmental friendliness and higher product yield and purity and has greater application value and good social and economical benefits.
Owner:ZHEJIANG RONGYAO CHEM +2
Crude iminostilbene product recrystallization method
The invention discloses a crude iminostilbene product recrystallization method. The crude iminostilbene product recrystallization method comprises the steps that a crude iminostilbene product is added into a reactor with a heating, stirring and reflux device, then a certain proportion of a mixed solvent is added, electric heating is performed to reach a reflux state, stirring is maintained for a certain period of time, then cooling is performed, a certain mass ratio of decolorized activated carbon is added for decolorization, temperature rise is performed to reach the reflux state, the reflux state is kept for 20 minutes, the decolorized activated carbon is filtered and removed when being hot, and a crude iminostilbene producti is obtained through filtration after standing and crystallization. The crude iminostilbene product recrystallization method has the main advantages that compared with an existing single solvent crystallization process, a mixed solvent crystallization process is high in reaction efficiency (can be up to 94.2%), the product purity is high (can be up to 99.96%), recycle and reuse can be achieved after solvent rectification, a process route is advanced, the maneuverability is good, and the production costs are saved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Chemical method for synthesizing 6,8-dichloro ethyl cacodylic acid caprylate
ActiveCN100593534CAdvanced process routeReasonable process conditionsOrganic compound preparationCarboxylic acid esters preparationWater bathsChemical synthesis
The invention discloses a chemical synthetic method for 6, 8-ethyl octylate chloride. The method comprises the steps as follows: 6-hydroxyl-8-ethyl octylate chloride is dissolved in N, N-dimethylformamide; solution which is dissolved with organic solution of double (trichloride trichloromethyl)-carbonic acid ester is dropped in when in mixing under the cold water bath condition; the solution is gradually warmed to 50 to 90 DEG C for reacting for 2 to 8 hours after dropping; and the reaction solution is disposed into 6, 8-ethyl octylate chloride after finishing the reaction. As the invention replaces sulphoxides chloride with double (trichloride trichloromethyl)-carbonic acid ester, the invention produces environment friendly Vilsmeier agent which needs not to be separated in the reaction,and the Vilsmeier agent directly reacts with 6-hydroxyl-8-ethyl octylate chloride to produce 6, 8-ethyl octylate chloride. Besides, the invention has the advantages of advanced art, rational technological condition, simple and safe operation, high reaction yield, low production cost, low three-waste emission as well as great implementation value and social and economic efficiency.
Owner:ZHEJIANG UNIV OF TECH +1
Synthesis process of 1-chloro octane
InactiveCN1931805AAdvanced process routeReasonable process conditionsHalogenated hydrocarbon preparationOrganic solventOctanol
The present invention is synthesis process of 1-chloro octane with bis(trichloromethyl) carbonate as one of the materials. Under the action of organic amine catalysis, n-octanol and bis(trichloromethyl) carbonate react in organic solvent at 60-150 deg.c for 4-9 hr to obtain 1-chloro octane. The present invention has advanced technological path, reasonable technological conditions, high safety, high yield, low cost, no environmental pollution and simple post-treatment.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
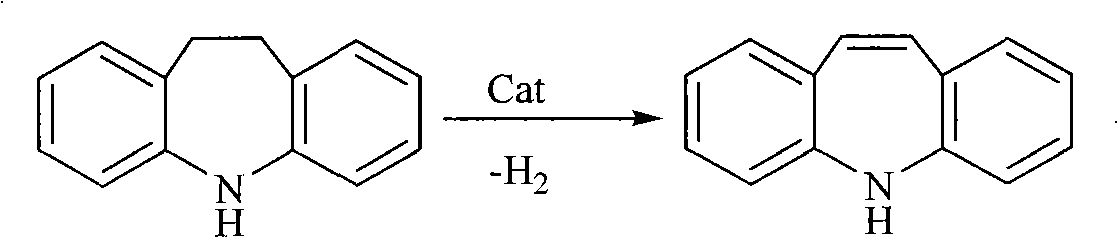
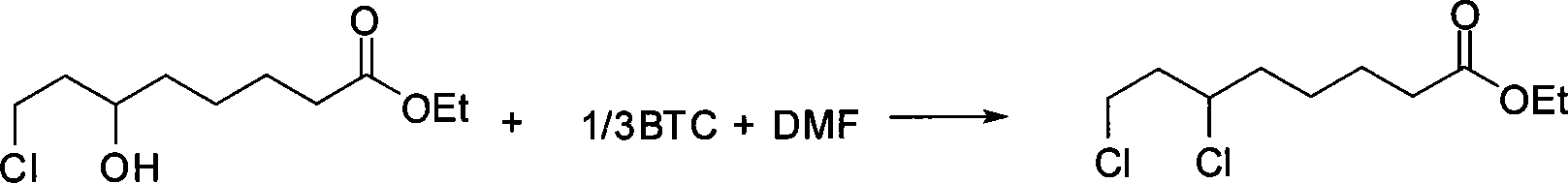
![Chemical synthesis method of O-acylcalix[4]arene Chemical synthesis method of O-acylcalix[4]arene](https://images-eureka.patsnap.com/patent_img/4897fd20-c18d-4c8c-94ed-ea8e6d2cf7da/FSA00000012082600011.PNG)
![Chemical synthesis method of O-acylcalix[4]arene Chemical synthesis method of O-acylcalix[4]arene](https://images-eureka.patsnap.com/patent_img/4897fd20-c18d-4c8c-94ed-ea8e6d2cf7da/GSA00000012082700021.PNG)