Green synthesis method for ibuprofen piconol and medicinal preparation thereof
A green synthesis technology of ibuprofen pyridoxine, which is applied in the field of western medicine, can solve the problems of non-recycling, high production cost, and unfriendly environment, and achieve the effects of less three wastes, eliminating troublesome disposal, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Add 206.2g (1.0mol) ibuprofen and 2000ml dichloromethane into a 5000ml dry single-necked bottle, stir mechanically, dissolve, add 109g (1.0mol) 2-hydroxymethylpyridine, then add 206.3g (1.0mol) N , N'-dicyclohexylcarbodiimide and 3.6g (0.03mol) 4-dimethylaminopyridine, stirred overnight at room temperature in the dark, and a large amount of white crystals were precipitated. After the reaction was completed, filter and wash with an appropriate amount of dichloromethane. Obtain filter residue dicyclohexyl urea, combine and wash filtrate, use 200ml 5%Na 2 CO 3 , 300ml water washing twice to neutral, anhydrous Na 2 SO 4 Drying, vacuum distillation to recover the solvent, and high vacuum rectification to obtain 280.1 g of ibuprofen pyridoxine (170-172 / 0.133kPa) as an oily liquid, with a yield of 94.5%.
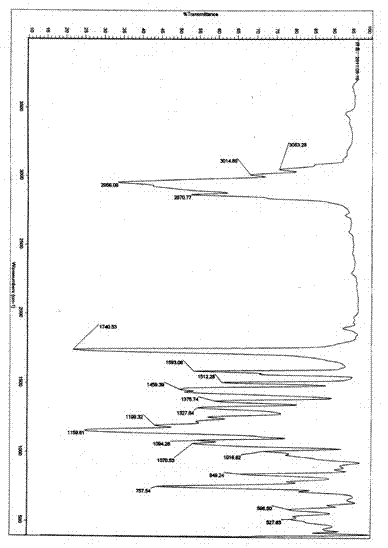
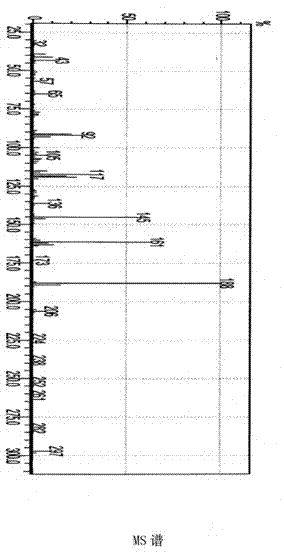
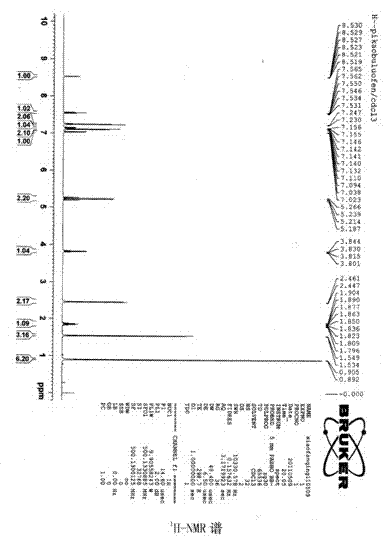
[0052] Analyze the product as figure 1 , 2 , 3, 4, 5, 6, 7, the following conclusions are obtained:
[0053] Light yellow clear liquid, boiling point 178℃ / 0.133kPa. E...
Embodiment 2
[0055] Add 224.3g (1.0mol) of dicyclohexylurea and 800ml of dichloromethane into a 2000ml dry single-necked bottle, stir and dissolve, keep the reaction temperature at about 40°C, and slowly add 212g (1.2mol) of benzene Sulfonyl chloride, stirred and reacted for 2 hours, cooled to room temperature, slowly added dropwise 15% sodium hydroxide solution to neutrality, continued to stir for 30 minutes, separated the organic layer, washed with water, anhydrous Na 2 SO 4 Dry, distill to recover the solvent, vacuum distill and collect the 156-159°C / 15mmHg fraction to obtain 171g of N,N'-dicyclohexylcarbodiimide with a yield of 83%. After solidification, the melting point is 33-35°C.
Embodiment 3
[0057] Add 206.2g (1.0mol) ibuprofen and 2000ml dichloromethane into a 5000ml dry single-necked bottle, stir mechanically, dissolve, add 109g (1.0mol) 2-hydroxymethylpyridine, then add 126.2g (1.0mol) N , N'-diisopropylcarbodiimide and 3.6g (0.03mol) 4-dimethylaminopyridine, stirred overnight at room temperature in the dark, after the reaction was completed, washed with 300ml of water, 200ml of 5% Na 2 CO 3 , 300ml water washing twice to neutral, anhydrous Na 2 SO 4 Drying, vacuum distillation to recover the solvent, and high vacuum rectification to obtain 275.1 g of ibuprofen pyridoxine (170-172 / 0.133kPa) as an oily liquid, with a yield of 92.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com