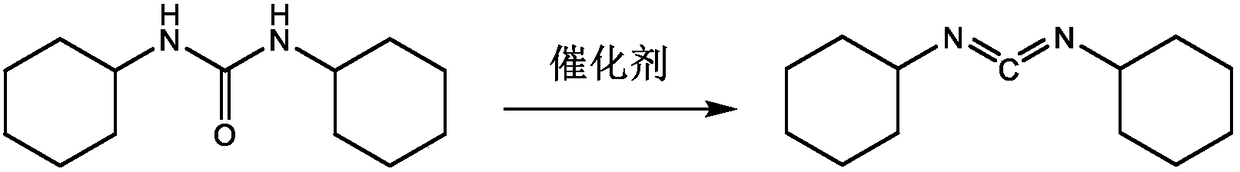
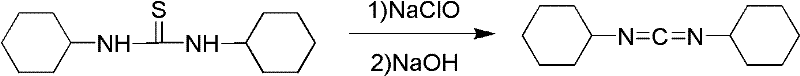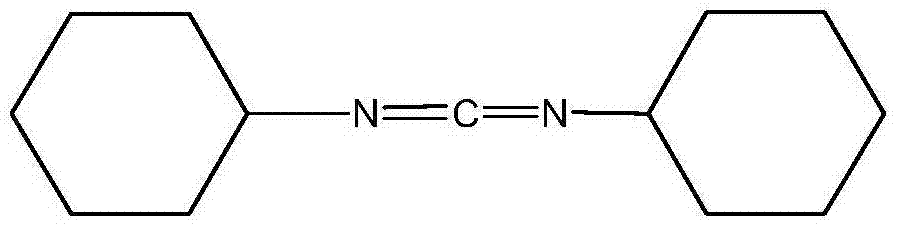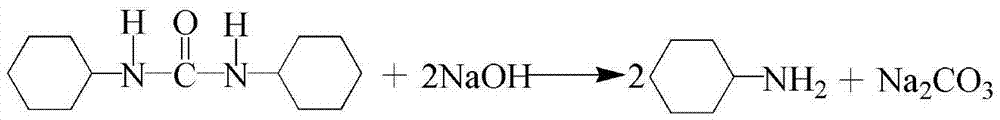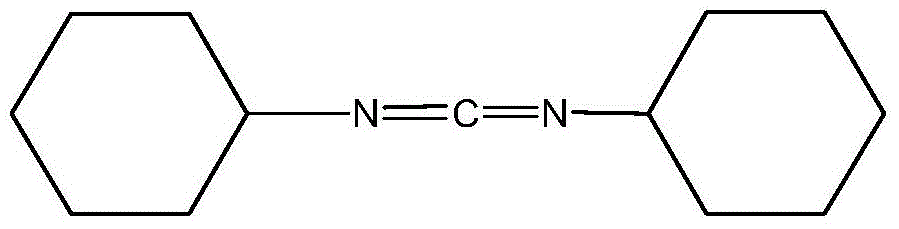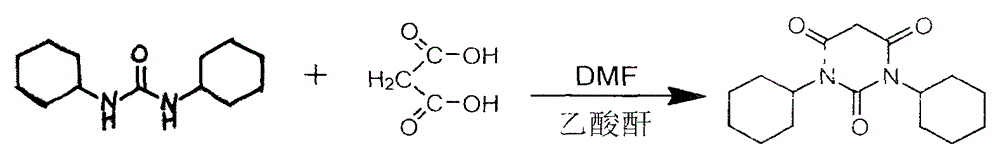Patents
Literature
43 results about "Dicyclohexylurea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
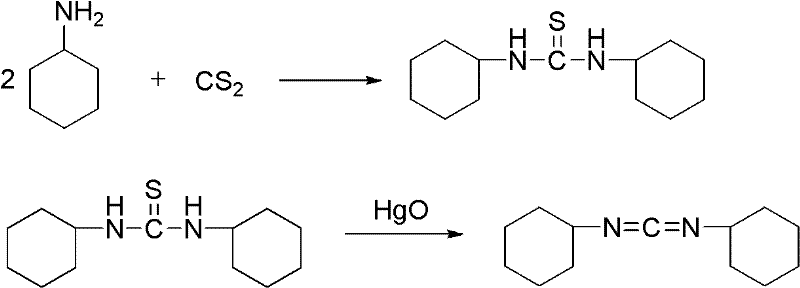
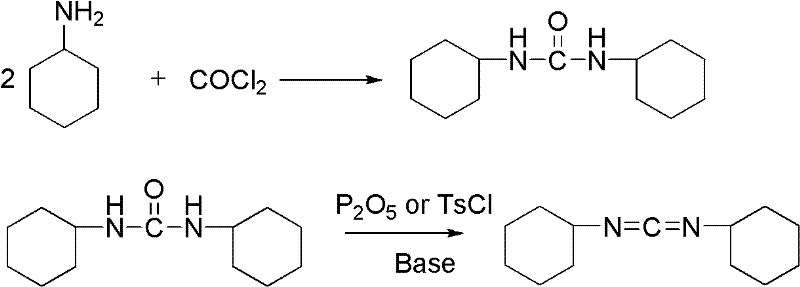
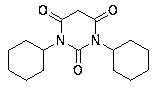
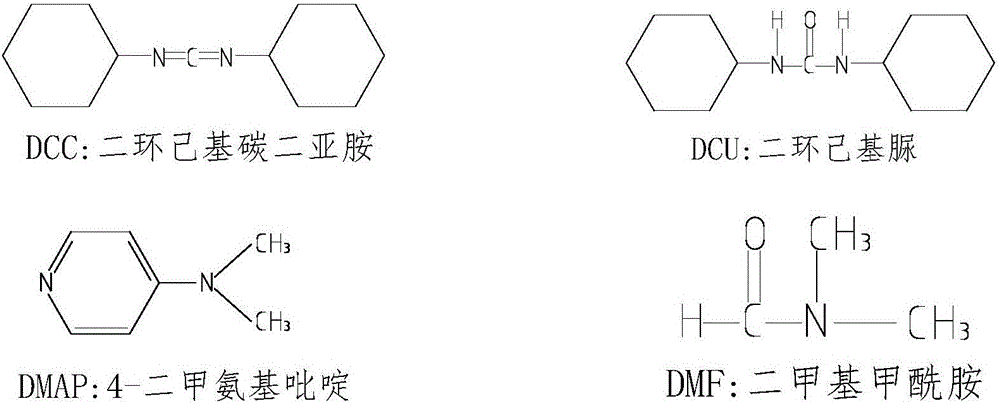
Dicyclohexylurea is an organic compound, specifically, a urea. It is the byproduct of the reaction of dicyclohexylcarbodiimide with amines or alcohols. It may be prepared by the reaction of cyclohexylamine and S,S-dimethyl dithiocarbonate. 1,3-Dicyclohexyl urea (DCU) is a potent soluble epoxide hydrolase (sEH) inhibitor.
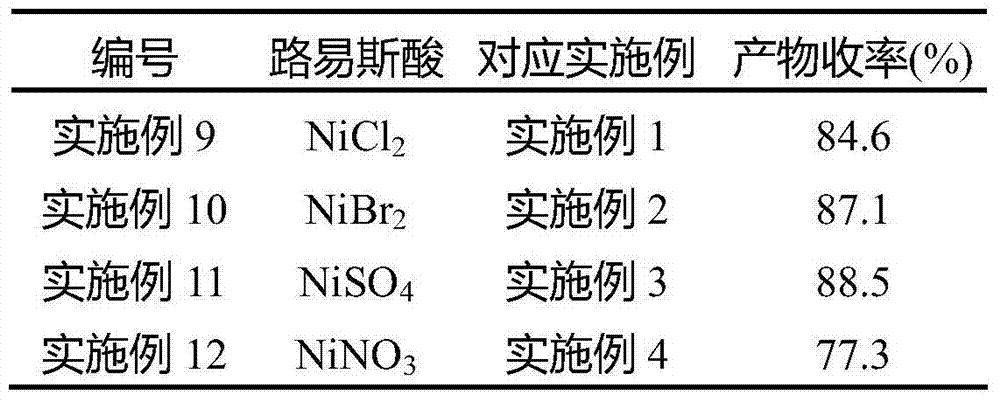
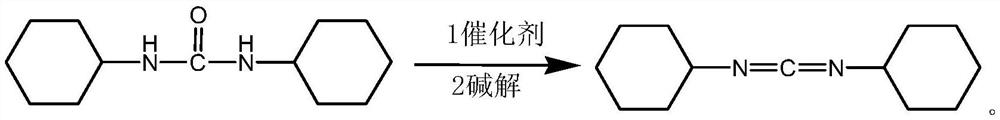
Process for preparing N,N'-dicyclohexyl carbodiimide by regeneration method
The invention discloses a process for preparing N,N'-dicyclohexyl carbodiimide by a regeneration method, which comprises the following steps of: removing impurities from N,N'-dicyclohexyl urea (DCU) serving as a raw material through alkaline wash, and performing regeneration by using an oxidizing agent; and reacting under stirring of an organic solvent at the temperature of between 40 and 42 DEG C for 0.15 to 2 hours to regenerate the N,N'-dicyclohexyl carbodiimide (DCC), wherein the regeneration rate is up to between 60 and 78 percent, and the DCC content of the product reaches over 99.5 percent. The process has the advantages of readily available raw materials, convenient operation and low cost, and provides the beneficial condition for the circular production and use of the DCC and particular the application to industry of medicine, the synthesis of amide and polypeptide and the like.
Owner:JINAN JUYE FINE CHEM
Method for synthesizing dicyclohexylcarbodiimide compound
InactiveCN102408355AEliminate security concernsEliminate pollutionOrganic chemistryChlorobenzeneNitrobenzene
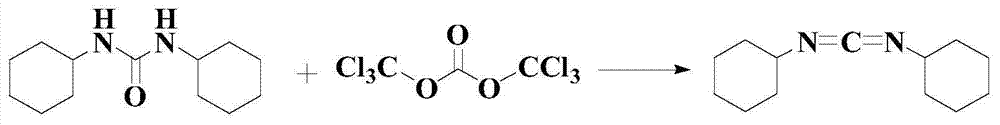
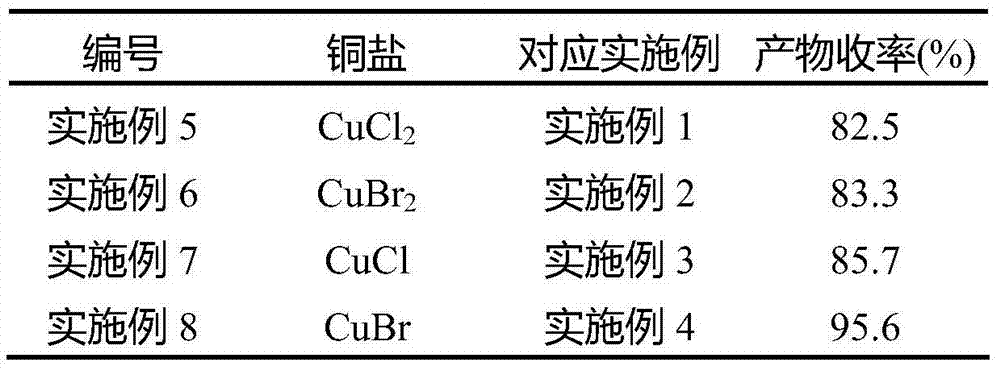
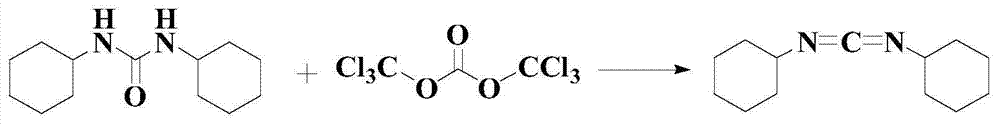
The invention discloses a synthetic method of a dicyclohexylcarbodiimide compound. The method comprises the following steps: taking dicyclohexylurea and di(trichloromethyl) carbonate as a raw material, fully reacting in an organic solvent A at the temperature of 20-110 DEG C, cooling to the room temperature after finishing the reaction, adding triethylamine and neutralizing to pH value of 7-8, post-treating a reaction solution to prepare the dicyclohexylcarbodiimide compound; wherein the organic solvent A is C1-C5 haloalkanes, C3-C8 fatty ester, ketones, ethers, benzene, toluene, xylene, chlorobenzene, nitrobenzene, cyclohexane or nitromethane. The method basically eliminates the problems of big hidden trouble of traditional technical safety and serious three wastes pollution. The method for synthesizing dicyclohexylcarbodiimide compound has the advantages of safety, reliability, simple process, less three wastes, small energy consumption, high overall yield, low cost and excellent product quality, and is suitable for industrialization production and has good economic benefit.
Owner:ZHEJIANG UNIV OF TECH +1
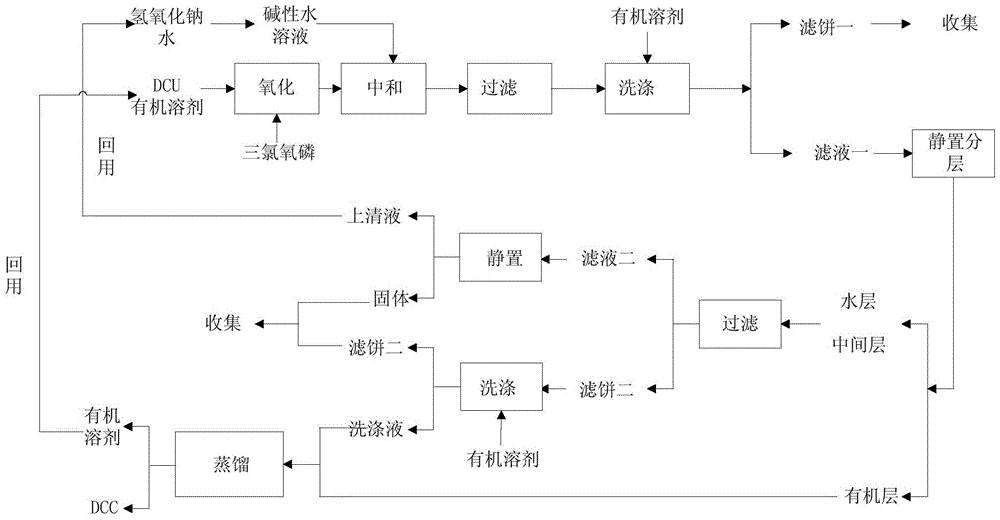
Production method for preparing N,N'-dicyclohexylcarbodiimide (DCC) by recycling wastewater
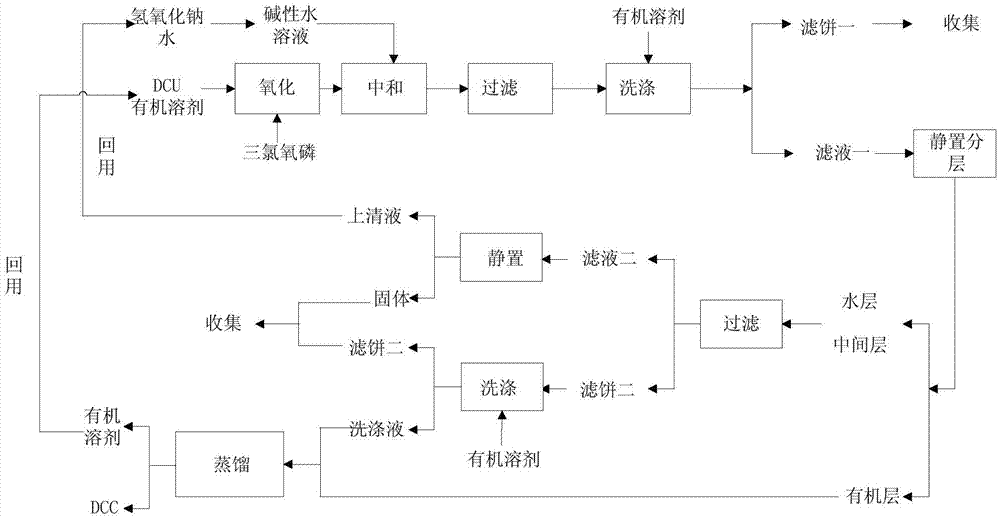
The invention discloses a production method for preparing N,N'-dicyclohexylcarbodiimide (DCC) by recycling wastewater, belonging to the technical field of organic compound synthesis. The method solves the problem of unmanageable brine sewage at present. The method comprises the following steps: washing N,N'-dicyclohexylurea (DCU) generated after using DCC with water to remove impurities, reacting with an oxidizer in an organic solvent, dropwisely adding the reaction solution into an alkali water solution to carry out neutralization reaction, filtering the neutralization reaction product, standing to stratify, and carrying out distillation separation on the organic layer to obtain the DCC product, wherein the distilled solvent is directly recovered; and filtering out inorganic salts from the water layer to obtain recovered water for preparing the alkali solution. The DCU generated after using the DCC is recovered to obtain the DCC; and the generated brine wastewater is recycled, thereby reducing the discharge of the brine sewage and greatly lowering the brine sewage treatment.
Owner:山东巨业精细化工有限公司
Synthesis method of dicyclohexyl carbodiimide
ActiveCN103922970AHigh reaction yieldGuaranteed stabilityOrganic chemistrySynthesis methodsFiltration
The invention relates to a synthesis method of dicyclohexyl carbodiimide. The method comprises the following steps of sequentially adding a solvent and dicyclohexylurea into a reaction kettle at room temperature, then uniformly stirring, adding solid phosgene, then adding a catalyst and a reaction aid, increasing the temperature, and performing heat preservation and stirring reaction; introducing ammonia gas to regulate the pH value to 7.5-9 after the end of reaction, then performing suction filtration while hot, firstly performing evaporation at normal pressure on obtained filtrate to remove the solvent, then performing reduced pressure distillation at the pressure of 1.35-1.54kPa, collecting a fraction at the temperature of 153-156 DEG C, and performing vacuum drying in an oven to obtain dicyclohexyl carbodiimide. The process has the advantages of high yield, fast reaction and strong safety, overcomes the shortcomings that the existing solid phosgene is unstable in reaction and not high enough in yield, and has very broad industrial application prospects and market development potentials.
Owner:SHANDONG FENGYUAN CHEM CO LTD
Preparation of N,Ní»-dicyclohexylurea
InactiveCN101279932AGood miscibilityIncrease temperatureUrea derivatives preparationOrganic compound preparationDicyclohexylureaOrganic solvent
The invention discloses a method to prepare N,N`-dicyclohexylurea. The method takes carbamide and cyclohexane as material and water as solvent; a stirring device, a heating device, a reflux device and a water separating apparatus are arranged inside a reactor; the mixture is stirred and heated to flow back and the phegma flows into the water segregator; the azeotropic liquid in the water segregator is gradually discharged; with the reduction of the water, the temperature of the reaction gradually rises and when the temperature rises to 180-240 DEG C, the reaction will be continued for 10-30 min at this temperature, producing N,N`-dicyclohexylurea. The method greatly shortens preparation period with a yield over 95%, and meanwhile the method requires no organic solvent.
Owner:SHANGHAI INST OF TECH
Method for synthesizing hexamethylene carbamate
InactiveCN101468959AMild reaction conditionsEasy to operateCarbamic acid derivatives preparationOrganic compound preparationDicyclohexylureaCarbamate
The invention discloses a method for synthesizing cyclohexyl carbamate, which is to select dialkyl carbonate and N,N'-dicyclohexylurea as reactants, control the reaction temperature to be between 100 and 250 DEG C and the reaction pressure to be between 0.5 and 2.0 MPa, and synthesize the cyclohexyl carbamate under the action of a catalyst. The method mainly has the characteristics of mild reaction conditions, cheap and easily obtained catalyst, easy reclaiming, good reusability, economic atomic reaction, high purity of products obtained by separation, small number of byproducts, and high industrial application value.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of dicyclohexylcarbodiimide
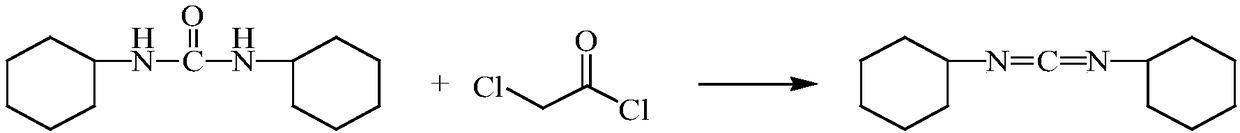
InactiveCN109232319ASimple processSimple and fast operationOrganic chemistryDicyclohexylureaFiltration
The invention belongs to the technical field of fine chemical engineering and in particular relates to a preparation method of dicyclohexylcarbodiimide. The preparation method comprises the followingsteps: dissolving chloroacetyl chloride in a solvent to obtain a solution A; stirring dicyclohexylurea, a catalyst and a solvent uniformly to obtain a solution B; dropwise adding the solution A into the solution B at a certain temperature; then carrying out an insulating reaction; after the reaction, adding a pH regulator to regulate the solution to be neutral; carrying out suction filtration on the reaction solution; distilling the filtrate at constant pressure to remove the solvent; and then carrying out distillation at a reduced pressure to obtain dicyclohexylcarbodiimide. The preparation method of dicyclohexylcarbodiimide is simple in process, the reaction stability and the product yield are improved greatly, dicyclohexylcarbodiimide can be produced industrially on a large scale, and the preparation method has the advantages of being simple and feasible and simple to operate.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
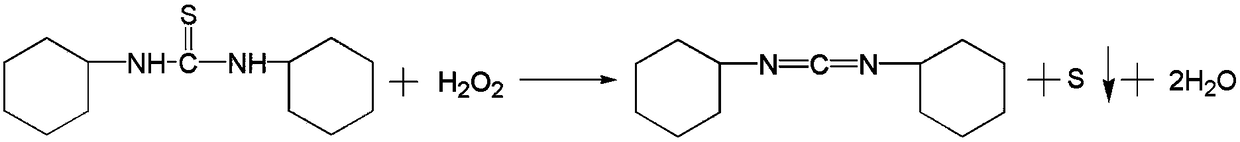
Method for synthesizing N, N'-dicyclohexylcarbodiimide through hydrogen peroxide serving as oxidizing agent
InactiveCN109336786ASolve processing problemsThorough responseOrganic chemistryFiltrationDistillation
The invention relates to a method for synthesizing N, N'-dicyclohexylcarbodiimide through hydrogen peroxide serving as an oxidizing agent. The method comprises the following steps: mixing N, N'-dicyclohexyl thiocarbamide and a solvent, slowly adding a catalyst, dropwise adding hydrogen peroxide at 15 to 25 DEG C to perform oxidation reaction, performing heat preservation for 1.5 h after dropwise adding is accomplished, then performing suction filtration at 15 DEG C, and performing decoloration and reduced pressure distillation, so as to obtain the N, N'-dicyclohexylcarbodiimide. The method hasthe advantages that the oxidizing agent hydrogen peroxide is adopted to ensure that reaction is more complete, the content of by-products N, N'-dicyclohexylurea and cyclohexyl isothiocyanate is controlled to be lower than 100 ppm, the product quality is improved, and the problem that effluent brine is difficult to treat is solved; a reaction system is optimized, a triethylamine catalyst is adopted to ensure that the reaction is performed in a homogeneous reaction system, the reaction yield is improved, the DCC yield is improved to greater than 93.5 percent, and the reaction is easy to control; and the material utilization is improved, the amount of waste is reduced, the investment in environmental protection is reduced, and the production cost is reduced.
Owner:SHANDONG HUIHAI PHARMA & CHEM
Preparation method of N,N'-dicyclohexylurea
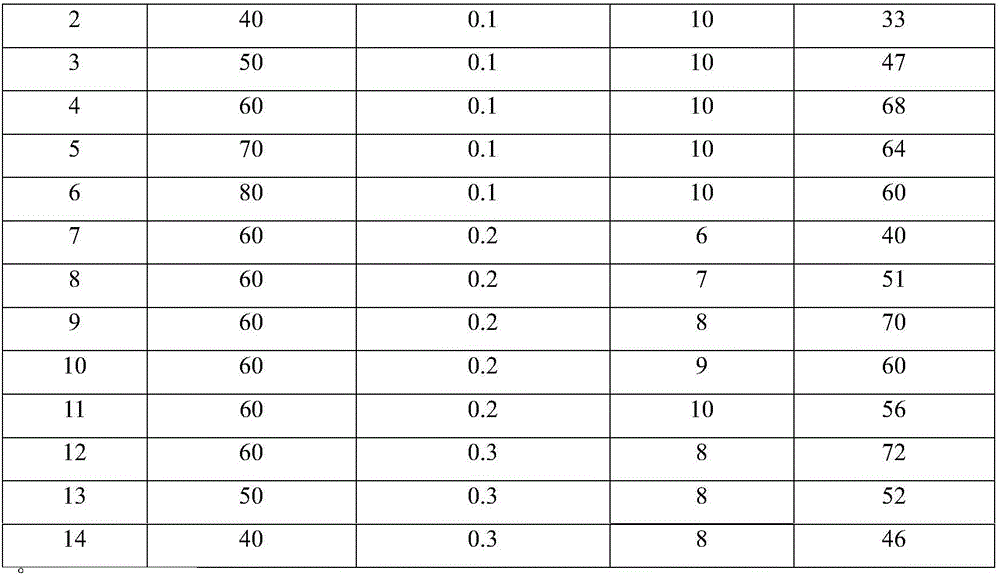
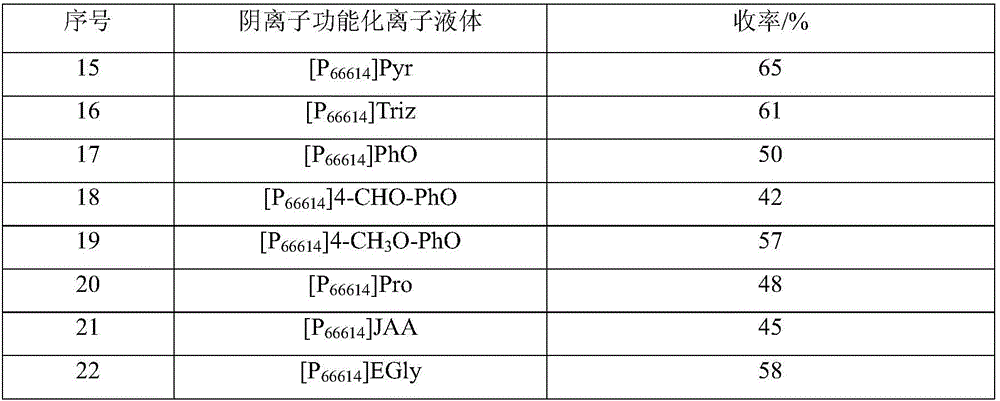
InactiveCN106008274ALarge absorption capacityFast absorption rateUrea derivatives preparationOrganic compound preparationChemical effectsDicyclohexylurea
The invention discloses a preparation method of N,N'-dicyclohexyl urea, which is characterized in that: anionic functionalized ionic liquid absorbs CO through chemical action 2 In situ reaction of carbon source and cyclohexylamine, N,N'-dicyclohexylurea was prepared under mild conditions. with traditional CO 2 Compared with the method of preparing N,N'-dicyclohexyl urea directly with cyclohexylamine reaction, this method has the advantages of low reaction temperature and pressure, CO 2 The utilization rate is high, the anionic functionalized ionic liquid can be recycled, and no additional catalyst, dehydrating agent and solvent are needed. It is a preparation method of N,N'-dicyclohexyl urea with industrial application potential.
Owner:SHAOXING UNIVERSITY
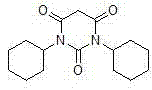
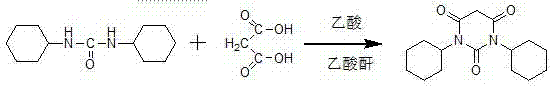
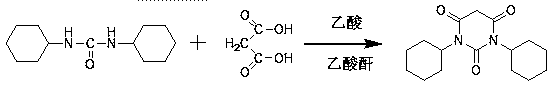
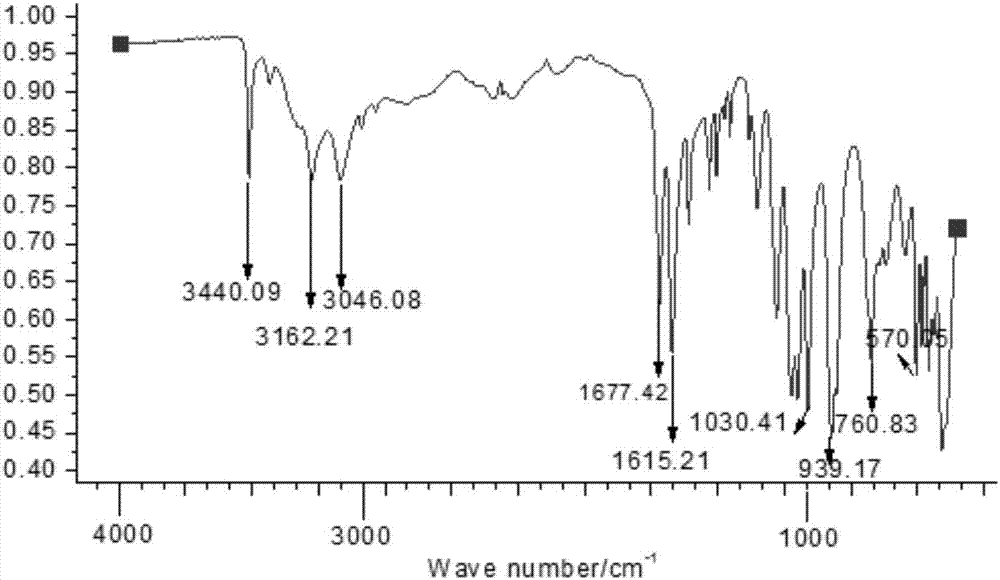
Preparation method of drug intermediate 1,3-dicyclohexyl barbituric acid for inhibiting angiogenesis, tumorigenesis and proliferative diseases
The invention discloses a preparation method of a drug intermediate, namely 1,3-dicyclohexyl barbituric acid, for inhibiting angiogenesis, tumorigenesis and proliferative diseases. The preparation method comprises the step of: under the conditions that acetic acid serves as a solvent and acetic anhydride serves as a dehydrating agent, reacting N,N'-dicyclohexylurea (DCU) with malonic acid to generate the 1,3-dicyclohexyl barbituric acid. The preparation method is simple in process, easy to operate, and high in product yield; and by adoption of the preparation method, the purity of the 1,3-dicyclohexyl barbituric acid is more than 99.5 percent.
Owner:姜树林
Method for recycling recycled N,N'-dicyclohexylurea
ActiveCN105439870ASolve the difficult problems of industrializationAchieve regenerationOrganic compound preparationAmino compound preparationDicyclohexylureaOrganic solvent
The invention relates to a method for recycling recycled N,N'-dicyclohexylurea. The method comprises the steps of adding N,N'-dicyclohexylurea and sodium hydroxide into a reaction still, starting stirring, raising temperature to 80-450 DEG C and reacting for 2-8 hours to steam out yielded fluid of cyclohexane in a reaction process. The method has the advantages that the N,N'-dicyclohexylurea of a bio-polypeptide condensing agent by-product is recycled to achieve reclamation of wastes, the problem that the industrialization of the N,N'-dicyclohexylurea is hard to be processed is solved, and the regeneration from the N,N'-dicyclohexylurea to cyclohexylamine to N,N'-dicyclohexylcarbodiimide is achieved, a solvent is not used, and the shortcomings that an organic solvent is flammable and combustible and high in cost are overcome, the yield of the cyclohexylamine is larger than 99%, the purity is larger than 99.3%, raw materials only have the N,N'-dicyclohexylurea and the sodium hydroxide, the products are only cyclohexylamine and sodium carbonate, and the cyclohexylamine and the sodium carbonate can be separated easily. The method is safe and environmentally friendly, low in cost and suitable for industrial production.
Owner:SHANDONG HUIHAI PHARMA & CHEM
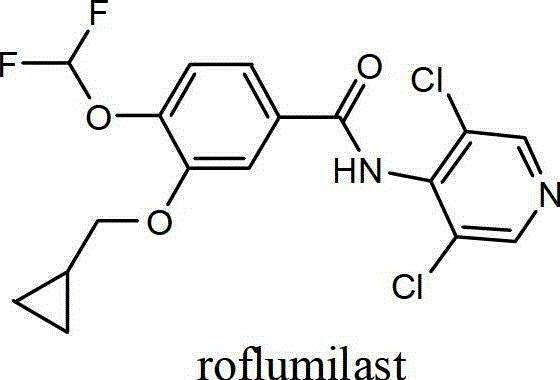
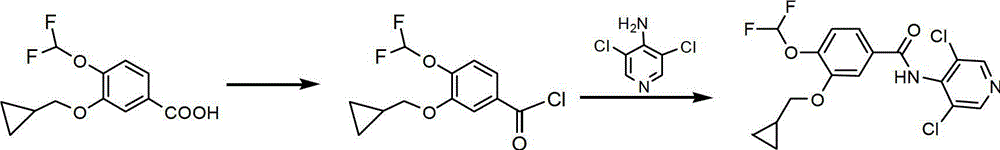
Preparation method of roflumilast
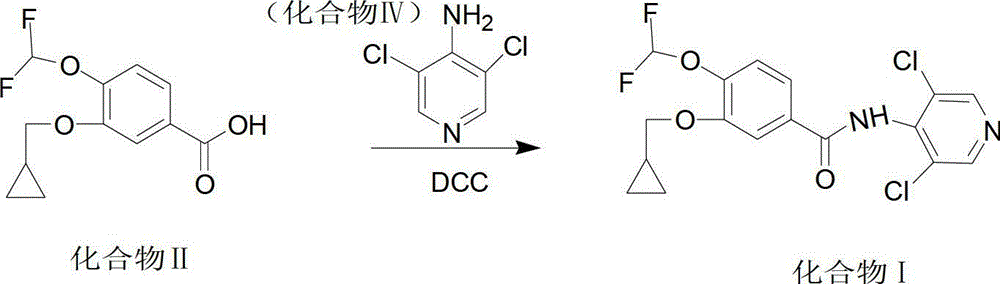
The invention provides a preparation method of roflumilast. The preparation method of roflumilast comprises the following steps of : (1) taking 3-cyclopropyl methoxy group-4-difluoro methoxy group benzoic acid and 4-amino-3, 5-dichloropyridine, and adopting N, N'-dicyclohexylcarbodiimide as the catalytic agent to perform amide reaction, wherein mole dosage ratio of 4-amino-3, 5-dichloropyridine and 3-cyclopropyl methoxy group-4-difluoro methoxy group benzoic acid is equal or greater than 1; mole dosage ratio of N'-dicyclohexylcarbodiimide and 4-amino-3, 5-dichloropyridine is equal or greater than 1, and solvent used by the amide reaction is aprotic polar solvent; and (2) after the reaction is finished, adding poor solvent of N, N'-dicyclohexylurea to perform purification, and accordingly roflumilast refined products are obtained. The preparation method of roflumilast adopts DCC (N, N'-dicyclohexylcarbodiimide) as the catalytic agent, guarantees good product yield and purity, can directly perform amidate condensation reaction without performing acylating chlorination reaction on intermediates, simplifies reaction steps, simultaneously avoids corrosivity from acylating chlorination solvent thionyl chloride to equipment, and is more suitable for industrialized mass production.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Preparation method of dicyclohexylcarbodiimide
InactiveCN110903218AAchieve regenerative conversionSimple processOrganic chemistryDicyclohexylureaDistillation
The invention relates to a method for preparing dicyclohexylcarbodiimide by using recycled dicyclohexylurea, and the method comprises the following steps: adding the recycled dicyclohexylurea into a mixed system composed of water and an organic solvent, stirring, washing, filtering, and drying; uniformly stirring the dried dicyclohexylurea and an organic solvent, adding oxalyl chloride at a certain temperature for reaction, keeping the temperature for 0.5 h after dripping is finished, dripping reaction liquid into an aqueous solution of sodium hydroxide, filtering, standing filtrate for layering to obtain an organic phase, concentrating the organic phase, and performing reduced pressure distillation to obtain the dicyclohexylcarbodiimide. According to the method, DCU generated after DCC isused is regenerated into DCC, cyclic application is achieved, the technological method has the advantages of being safe, reliable and high in recovery rate, and industrial production can be achieved.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
Method for preparing dicyclohexylcarbodiimide by using vilsmeier reagent
InactiveCN112079748AMild process conditionsSimple and fast operationOrganic chemistryDicyclohexylureaBiochemical engineering
The invention discloses a method for preparing dicyclohexylcarbodiimide by using a vilsmeier reagent. The method comprises the following steps: preparing a vilsmeier reagent by using N, N-dimethylformamide (DMF) and thionyl chloride (SOCl2), reacting the vilsmeier reagent with dicyclohexylurea (DCU), and carrying out aftertreatment to obtain the dicyclohexylcarbodiimide. According to the method, the vilsmeier reagent is adopted, the reaction can be carried out under mild conditions, the process is safe and stable, and the method is suitable for industrial large-scale production.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
Synthesis method of dicyclohexyl carbodiimide
ActiveCN103922970BHigh reaction yieldGuaranteed stabilityOrganic chemistryDistillationSynthesis methods
The invention relates to a synthesis method of dicyclohexyl carbodiimide. The method comprises the following steps of sequentially adding a solvent and dicyclohexylurea into a reaction kettle at room temperature, then uniformly stirring, adding solid phosgene, then adding a catalyst and a reaction aid, increasing the temperature, and performing heat preservation and stirring reaction; introducing ammonia gas to regulate the pH value to 7.5-9 after the end of reaction, then performing suction filtration while hot, firstly performing evaporation at normal pressure on obtained filtrate to remove the solvent, then performing reduced pressure distillation at the pressure of 1.35-1.54kPa, collecting a fraction at the temperature of 153-156 DEG C, and performing vacuum drying in an oven to obtain dicyclohexyl carbodiimide. The process has the advantages of high yield, fast reaction and strong safety, overcomes the shortcomings that the existing solid phosgene is unstable in reaction and not high enough in yield, and has very broad industrial application prospects and market development potentials.
Owner:SHANDONG FENGYUAN CHEM CO LTD
Method for synthesizing cyclohexyl carbamate
ActiveCN101759602AMild reaction conditionsEasy to operateCarbamic acid derivatives preparationOrganic compound preparationDicyclohexylureaCarbamate
The invention discloses a method for synthesizing cyclohexyl carbamate. The invention chooses a supported metal oxide solid catalyst to catalyze dialkyl carbonate and N,N'-dicyclohexylurea to obtain the product. The method is mainly characterized in that reaction condition is relatively mild; the catalyst is easy to recycle and good in repeated use; the reaction is atom economy; and the separatedproduct has high purity.
Owner:青岛奥立科新材料科技有限公司
A kind of production method of recycling waste water to prepare n,n'-dicyclohexylcarbodiimide
The invention discloses a production method for preparing N,N'-dicyclohexylcarbodiimide by recycling waste water, and belongs to the technical field of organic compound synthesis. It solves the problem that the existing salty sewage is difficult to treat. The N,N'-dicyclohexylurea produced after the use of N,N'-dicyclohexylcarbodiimide is washed with water to remove impurities, and then reacted with an oxidant in an organic solvent, and the reaction solution is added dropwise to an aqueous alkali solution for Neutralization reaction, the neutralization reaction product is filtered and then stratified, the organic layer is distilled and separated to obtain the DCC product, and the evaporated solvent is directly reused; the water layer is filtered to remove the inorganic salt and reused as the water required for the preparation of the lye. The production method reuses the DCU produced after the use of DCC to obtain DCC, and at the same time, recycles the generated saline wastewater, which reduces the discharge of saline wastewater and greatly reduces the treatment of saline wastewater.
Owner:山东巨业精细化工有限公司
Preparation method of drug intermediate 1,3-dicyclohexyl barbituric acid for inhibiting angiogenesis, tumorigenesis and proliferative diseases
The invention discloses a preparation method of a drug intermediate, namely 1,3-dicyclohexyl barbituric acid, for inhibiting angiogenesis, tumorigenesis and proliferative diseases. The preparation method comprises the step of: under the conditions that acetic acid serves as a solvent and acetic anhydride serves as a dehydrating agent, reacting N,N'-dicyclohexylurea (DCU) with malonic acid to generate the 1,3-dicyclohexyl barbituric acid. The preparation method is simple in process, easy to operate, and high in product yield; and by adoption of the preparation method, the purity of the 1,3-dicyclohexyl barbituric acid is more than 99.5 percent.
Owner:姜树林
Microwave-assisted method for synthesizing N,N'-dicyclohexyl carbodiimide
PendingCN112661669ALarge specific surface areaHigh catalytic efficiencyOrganic chemistryPtru catalystOrganic synthesis
The invention relates to the technical field of organic synthesis, in particular to a method for synthesizing N,N'-dicyclohexyl carbodiimide through a microwave-assisted method. The method comprises the following steps: dissolving N,N'-dicyclohexyl urea in a solvent under microwave radiation, adding an oxidant and a catalyst loaded on a molecular sieve, and carrying out heat preservation reflux; adding a phase transfer catalyst, adjusting the pH value, and carrying out alkaline hydrolysis reaction; filtering, separating liquid, evaporating out the solvent, and carrying out reduced pressure distillation to obtain N,N'-dicyclohexyl carbodiimide. The catalyst loaded on the molecular sieve is large in specific surface area and high in catalytic efficiency; a microwave-assisted method is adopted, and a phase transfer catalyst is added, so that the problems of difficult two-phase reaction and incomplete reaction in the traditional process are solved, the reaction conversion rate is greatly improved, the reaction time is shortened, and the product has the characteristics of high purity and yield.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
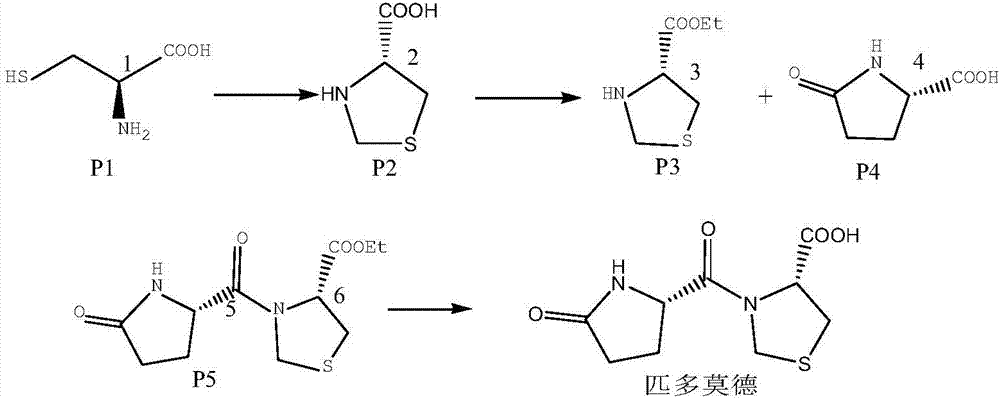
Process for removing pidotimod condensation impurities
ActiveCN108003221AQuality assuranceShorten cycle timePeptide preparation methodsDicyclohexylureaHydrolysis
The invention relates to pidotimod, in particular to a process for removing pidotimod condensation impurities. The process for removing the pidotimod condensation impurities includes the step of preparing dichloromethane mother liquor containing dicyclohexylurea in a process for preparing pidotimod. According to the process for preparing pidotimod, dicyclohexyl carbodiimide serves as a condensingagent, the dichloromethane mother liquor containing dicyclohexylurea is subjected to hydrolysis and skimming, a water phase is obtained, the water phase is treated through a macroporous resin filler column, salification is achieved after the water phase is treated, and pidotimod is obtained after refining is carried out. The dichloromethane mother liquor containing dicyclohexylurea is subjected tohydrolysis and skimming, the water phase is obtained, the water phase is treated through the macroporous resin filler column, and the impurities, namely dicyclohexylurea, in pidotimod products are completely removed. The defect that the impurities, namely dicyclohexylurea cannot be completely removed through an existing process is overcome, and the method which is simple, effective, safe, environmentally friendly and suitable for industralization is found.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
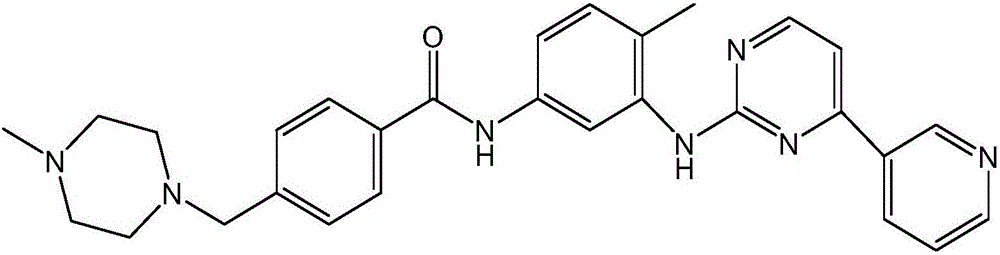
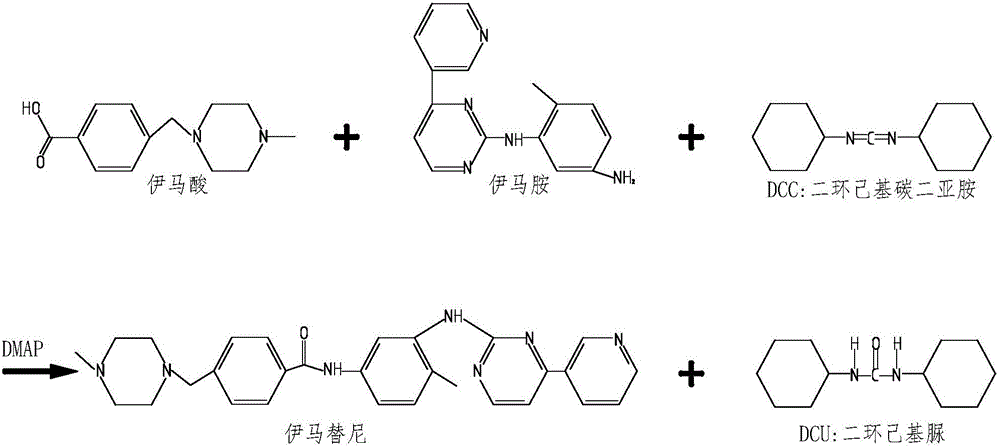
Synthesizing method of imatinib
InactiveCN105732582AIncrease productivityReduce manufacturing costOrganic chemistrySimple Organic CompoundsBenzoic acid
The invention relates to the field of organic compound synthesis, in particular to a synthesizing method of imatinib.The materials of 4-[(4-methylpiperazin-1-yl)methyl]benzoic acid dihydrochloride, N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine, dicyclohexylcarbodiimide and 4-dimethylaminopyridine are added.According to the method, 4-[(4-methylpiperazin-1-yl)methyl]benzoic acid dihydrochloride and N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine are added to an organic solution of dicyclohexylcarbodiimide, the catalyst 4-dimethylaminopyridine is added, imatinib is synthesized in one step, the production efficiency is improved, and meanwhile the production cost is reduced.Meanwhile, the dehydrating agent dicyclohexylcarbodiimide is used, the reaction between 4-[(4-methylpiperazin-1-yl)methyl]benzoic acid dihydrochloride and N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine is thorough, dicyclohexylurea (DCU) is generated from dicyclohexylcarbodiimide (DCC) after the reaction is completed, recycling can be achieved, and the degree of contamination is reduced.
Owner:ANHUI HAIKANG PHARMA
Preparation method to recycle pyribenzoxim byproduct dicyclohexylurea
InactiveCN109081792ASafe and efficient productionSuitable for industrial productionOrganic chemistryChemical recyclingDicyclohexylureaPollutant emissions
The invention discloses a preparation method to recycle pyribenzoxim byproduct dicyclohexylurea and belongs to the field of agricultural chemistry. The byproduct N,N'-dicyclohexylurea is used as a rawmaterial; N,N'-dicyclohexylurea and organic phosphorus catalyst are heated to 140-160 for reacting; the temperature is then lowered to 60-80 DEG C for reacting; after reacting, the catalyst is recycled by filtering, and filtrate is distilled under reduced pressure; distillate at 125-140 DEG C (6-8 mmHg) is collected and cooled to obtain white solid, N,N'-dicyclohexylcarbodiimide; the highest yield reaches 88.2%, and purity reaches 98.4%. The preparation method is efficient, environmentally friendly, sustainable, and suitable for industrial production; the preparation method has the advantagesof good energy efficiency, low consumption, lower pollutant emission and the like, allows a preparation technique of recycling byproducts of pyribenzoxim production process to be implemented, and meets the green manufacturing principle.
Owner:CHANGZHOU UNIV
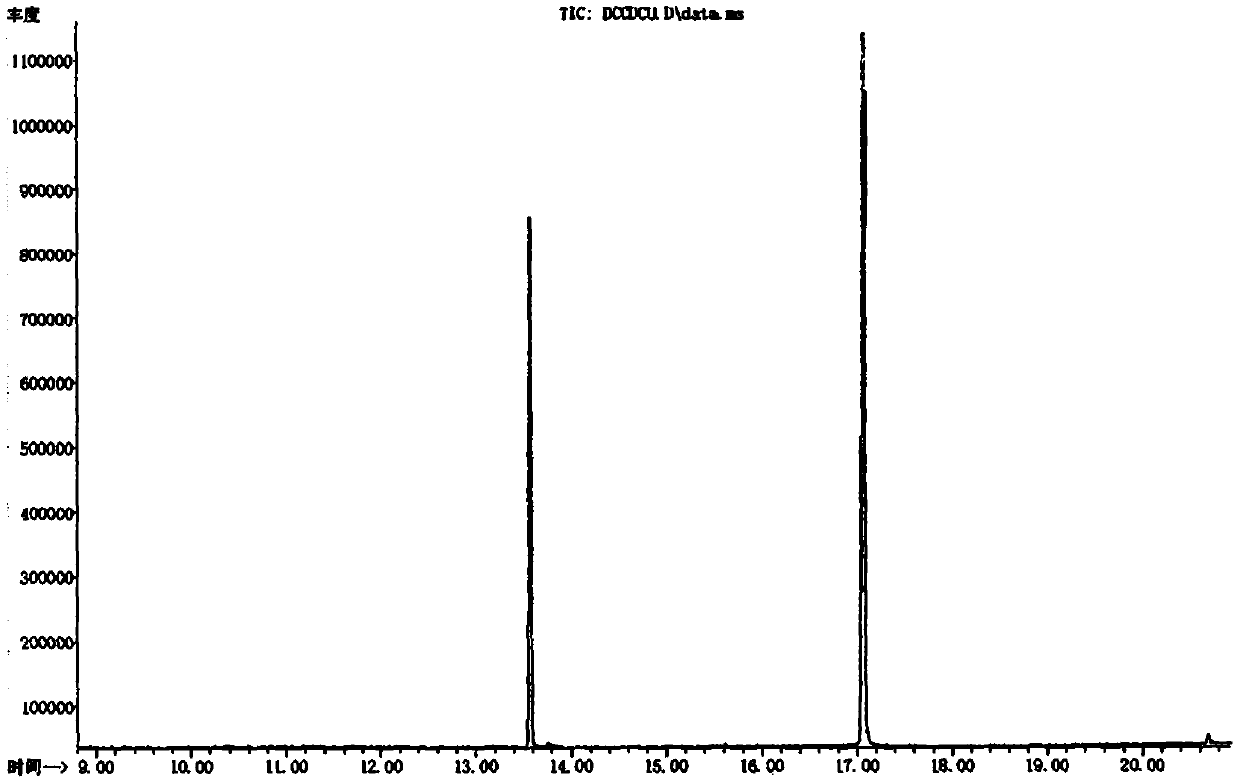
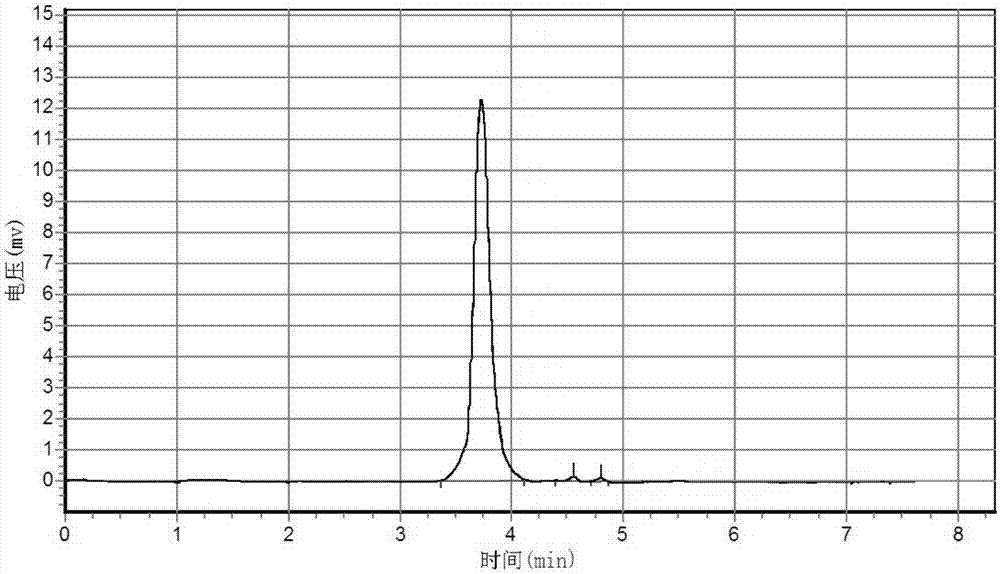
Detection method of N,N'-dicyclohexylurea and N,N'-dicyclohexylcarbodiimide
InactiveCN110749677AKeep timeRetain qualifier ion qualifierComponent separationDicyclohexylureaGas liquid chromatographic
The invention belongs to the field of chemical detection, and discloses a detection method of N,N'-dicyclohexylurea and N,N'-dicyclohexylcarbodiimide. The detection method comprises the following steps: dissolving a sample with methanol; diluting with acetone; filtering with an organic filter membrane; and detecting with a gas chromatograph-mass spectrometer. The method has the advantages of highseparation degree, symmetrical peak pattern and relatively higher accuracy and precision, and fills up the technical blank of the simultaneous qualitative and quantitative detection of the N,N'-dicyclohexylurea and the N,N'-dicyclohexylcarbodiimide.
Owner:谱尼测试集团股份有限公司
Preparation method for benzyloxycarbonyl alanyl alanine
The invention relates to a preparation method for two peptides, in particular to the preparation method for benzyloxycarbonyl alanyl alanine, and mainly solves the technical problem that the conventional synthetic method is not suitable for industrial production. The invention adopts the following technical scheme: the preparation method comprises the steps as follows: 1, adding L-alanine methyl ester hydrochloride into dichloromethane, adding organic alkali and benzyloxycarbonyl alanine, carrying out ice-bath treatment, dropwise adding a dichloromethane solution of N,N'-dicyclohexyl carbodiimide, reacting overnight, filtering out dicyclohexylurea, rotationally steaming to remove dichloromethane, carrying out conventional treatment to obtain a white solid, and re-crystallizing to obtain benzyloxycarbonyl alanyl alanine methyl ester; and 2, dissolving benzyloxycarbonyl alanyl alanine methyl ester in alcohol, adding a sodium hydroxide solution for saponification reaction, and carrying out conventional treatment to obtain benzyloxycarbonyl alanyl alanine as a final product.
Owner:GL BIOCHEM SHANGHAI +2
Method for synthesizing cyclohexyl carbamate
ActiveCN101759602BMild reaction conditionsEasy to operateCarbamic acid derivatives preparationOrganic compound preparationDicyclohexylureaCarbamate
The invention discloses a method for synthesizing cyclohexyl carbamate. The invention chooses a supported metal oxide solid catalyst to catalyze dialkyl carbonate and N,N'-dicyclohexylurea to obtain the product. The method is mainly characterized in that reaction condition is relatively mild; the catalyst is easy to recycle and good in repeated use; the reaction is atom economy; and the separatedproduct has high purity.
Owner:青岛奥立科新材料科技有限公司
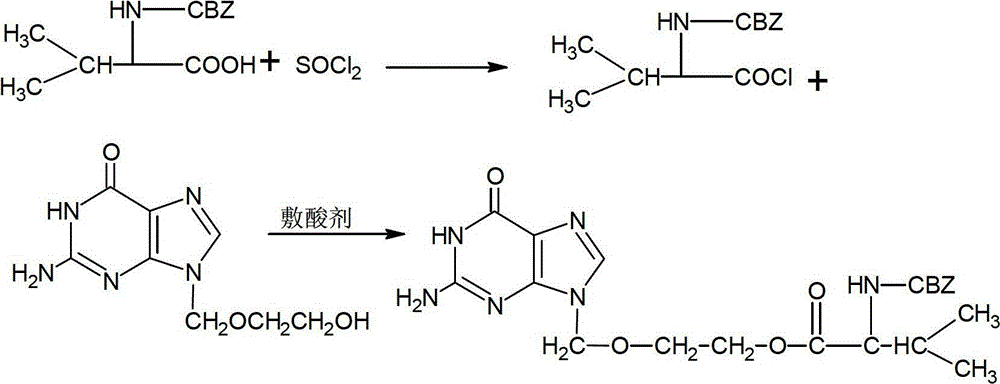
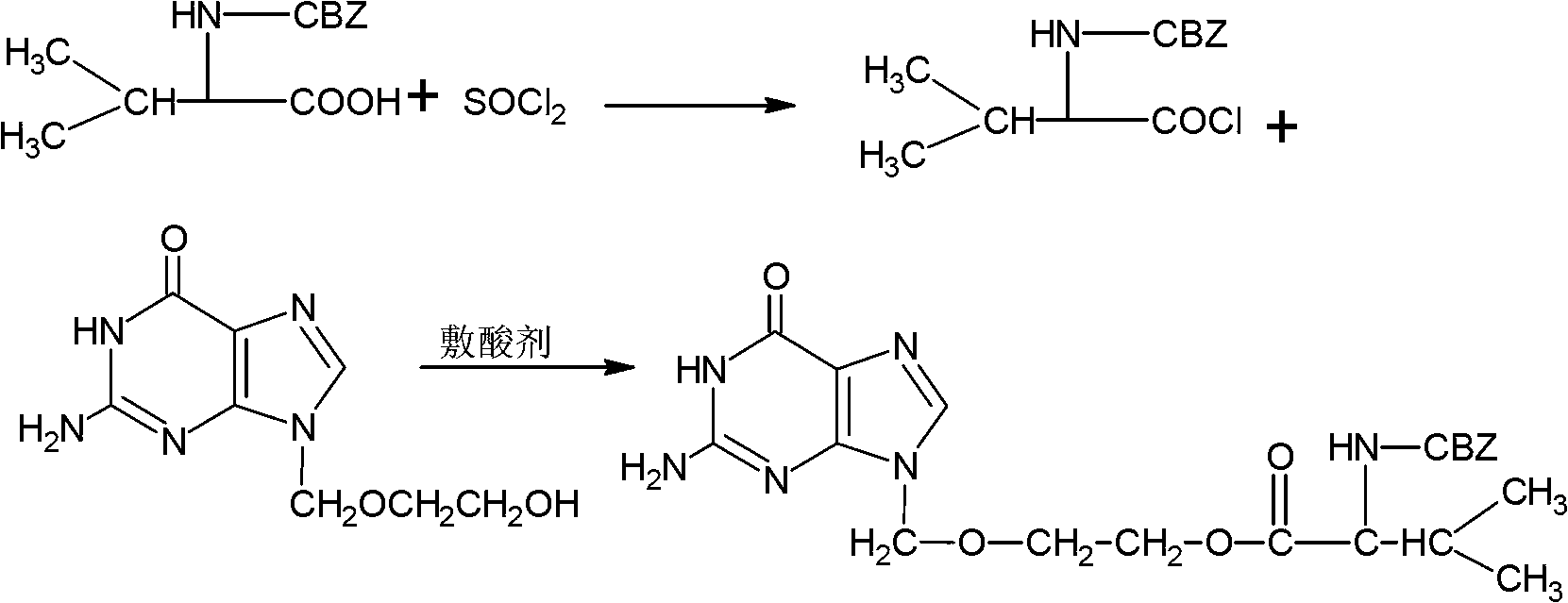
Preparation method of carbamazepine (CBZ)-valaciclovir
ActiveCN102850353BImprove overall utilizationHigh purityOrganic chemistryDicyclohexylureaCarbamazepine
The invention relates to the field of a medicine synthesis technology, and in particular relates to a preparation method of carbamazepine (CBZ)-valaciclovir. The preparation method comprises the steps of: reacting CBZ-L-valine with an acyl chlorination reagent to obtain acyl chloride; then, reacting acyl chloride with a mixed system of acyclovir and an acid-binding agent; and finally, filtering and refining to obtain the CBZ-valaciclovir. According to the method, acyl chloride is utilized to prepare ester, dicyclohexylcarbodiimide (DCC) is not used in the reaction process and does not generate dicyclohexylurea (DCU) is not remianed in the product, thus being beneficial to improvement of the palladium-carbon utilization rate; and the preparation method disclosed by the invention is an effective method for preparing the high-purity CBZ-valaciclovir.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Preparation method of carbamazepine (CBZ)-valaciclovir
ActiveCN102850353AImprove overall utilizationHigh purityOrganic chemistryDicyclohexylureaCarbamazepine
The invention relates to the field of a medicine synthesis technology, and in particular relates to a preparation method of carbamazepine (CBZ)-valaciclovir. The preparation method comprises the steps of: reacting CBZ-L-valine with an acyl chlorination reagent to obtain acyl chloride; then, reacting acyl chloride with a mixed system of acyclovir and an acid-binding agent; and finally, filtering and refining to obtain the CBZ-valaciclovir. According to the method, acyl chloride is utilized to prepare ester, dicyclohexylcarbodiimide (DCC) is not used in the reaction process and does not generate dicyclohexylurea (DCU) is not remianed in the product, thus being beneficial to improvement of the palladium-carbon utilization rate; and the preparation method disclosed by the invention is an effective method for preparing the high-purity CBZ-valaciclovir.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
A kind of preparation method of inositol phosphate
ActiveCN107445984BHigh average yieldEasy to separate and purifyPhosphorus organic compoundsPhosphatePhosphoric acid
The invention discloses a method for preparing inositol phosphate, which comprises the following steps: after mixing ethyl acetate, inositol phosphate, p-toluenesulfonic acid and dicyclohexylcarbodiimide evenly, heating and refluxing reaction to obtain inositol phosphate alcohol crystals. P-toluenesulfonic acid acts as a catalyst to catalyze the esterification of inositol and phosphoric acid in inositol phosphate. Dicyclohexylcarbodiimide (DCC) is used as a dehydrating agent to remove the water generated by the esterification reaction, so that the whole reaction is carried out thoroughly and the reaction yield is improved. At the same time, the reaction solvent ethyl acetate can be recycled, and the by-products and impurities generated during the reaction remain in the ethyl acetate mother liquor, and the remaining dicyclohexylcarbodiimide (DCC) absorbs water to produce 1,3-bicyclic Hexylurea (DCU), which is insoluble in water, is easy to separate and purify during the recrystallization process, effectively ensuring product purity.
Owner:TAISHAN MEDICAL UNIV
Preparation method of creatinol phosphate
ActiveCN107445984AEasy to controlEmission reductionPhosphorus organic compoundsSolventDicyclohexylurea
The invention discloses a preparation method of creatinol phosphate. The method comprises the following steps: uniformly mixing ethyl acetate, creatinol phosphate, p-toluenesulfonic acid and dicyclohexylcarbodiimide; heating and carrying out reflux reaction to obtain a creatinol phosphate crystal. As a catalyst, p-toluenesulfonic acid plays an effect of catalyzing creatinol and phosphoric acid in the creatinol phosphate to form ester. By using dicyclohexylcarbodiimide (DCC) as a dehydrating agent, water generated by performing esterification reaction is removed, so that the whole reaction is carried out completely, and the reaction yield is improved. Meanwhile, the ethyl acetate reaction solvent can be recycled, by-products and impurity parts produced in the reaction process are remained in ethyl acetate mother liquid, and the remaining DCC absorbs the water to produce 1,3-dicyclohexylurea (DCU); moreover, the DCU substance is insoluble in water, so that separation and purification are easily carried out in a recrystallization process, and the purity of the product is effectively ensured.
Owner:TAISHAN MEDICAL UNIV
Method for preparing 1,3-bicyclo hexyl barbituric acid
InactiveCN104151254AReasonable process structureEasy to operateOrganic chemistryAcetic acidMalonic acid
The invention discloses a method for preparing 1,3-bicyclo hexyl barbituric acid. The method comprises the following steps: reacting N,N'-dicyclohexyl urea and malonic acid in the presence of glacial acetic acid and acetic anhydride to generate 1,3-bicyclo hexyl barbituric acid, and further crystallizing, filtering, and drying, thereby obtaining refined 1,3-bicyclo hexyl barbituric acid. The method is reasonable in process structure, easy to operate and high in product yield, the purity of a product is high, the production cost is lowered, and the production benefits are increased.
Owner:NANTONG HUAFENG CHEM
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com