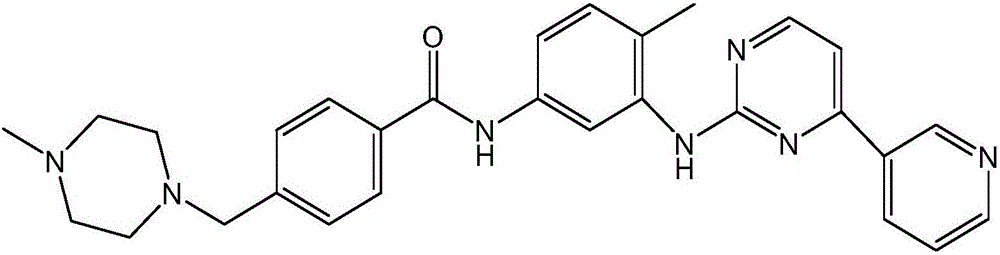
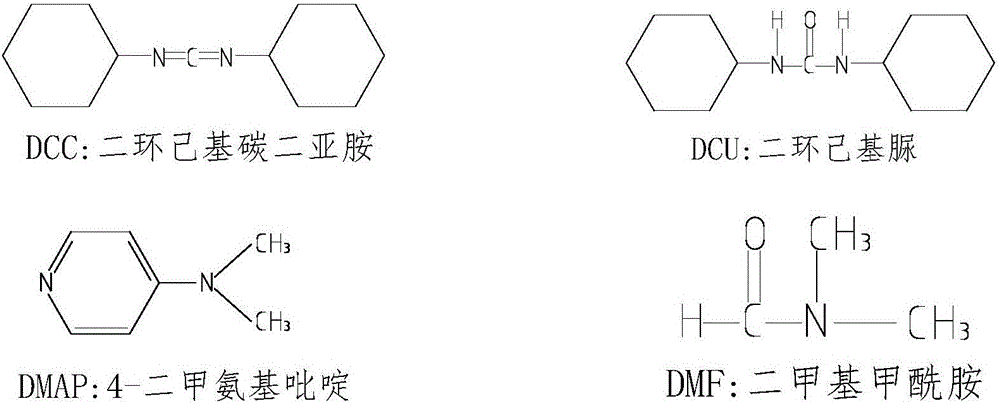
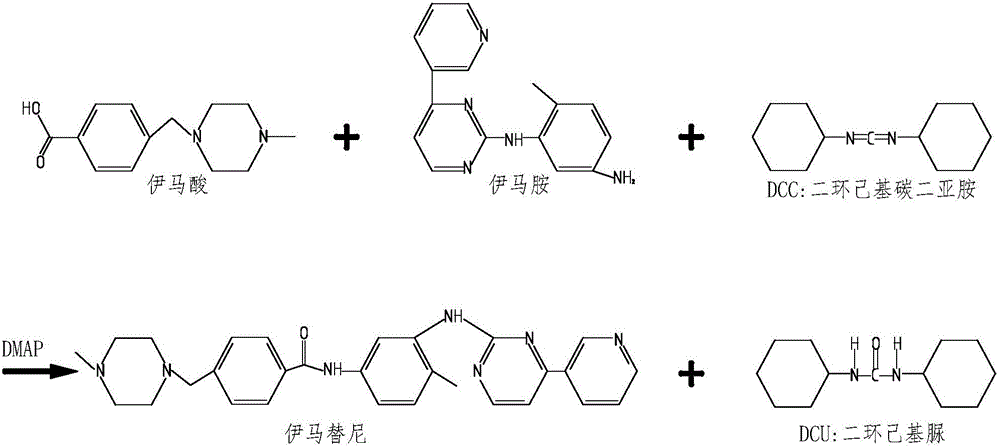
Synthesizing method of imatinib
A synthetic method, imatinib technology, applied in the field of organic compound synthesis, can solve the problems of large pollution, low cost, and prolonged cycle, and achieve the effects of reducing pollution, reducing production costs, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 200ml of dimethylformamide into a 1000ml reaction bottle, then add 28g of imamic acid under stirring, stir until the reaction system is dissolved and transparent, continue stirring, and then add 41g of dicyclohexylcarbodiimide and 35g of imaic acid , 1g of 4-dimethylaminopyridine, then, the temperature in the reaction system will rise, cool with cold water on the outside of the reaction bottle, keep the reaction temperature between 20-30 degrees Celsius, the reaction time needs to be overnight; Observe that the raw material point imamamine basically disappears, and stop the reaction; then add 200ml of water to the reaction bottle, a white solid will precipitate in the system, exotherm, continue to stir for 2 hours and then filter, the filter cake is washed with a small amount of dimethyl Add 200ml of water to the filtrate and continue to stir, and the stirring time needs to be overnight; the next day, filter with suction, rinse the filter cake with a small amount of ...
Embodiment 2
[0027] Add 200ml of tetrahydrofuran into a 1000ml reaction bottle, and then add 28g of imaamine under stirring, stir until the reaction system is dissolved and transparent, continue stirring, and then add 41g of dicyclohexylcarbodiimide, 35g of imaic acid, 4-di 1g of methylaminopyridine, then, the reaction system will heat up, cool with cold water on the outside of the reaction bottle, keep the reaction temperature between 20-30 degrees Celsius, the reaction time needs to be overnight; on the second day, by spotting the plate, observe The raw material point imamamine basically disappeared, and the reaction was stopped; then 200ml of water was added to the reaction flask, and a white solid would precipitate out of the system, exothermic, continue to stir for 2 hours and then filter, the filter cake was rinsed with a small amount of tetrahydrofuran, and the filtrate was added to Continue to stir with 200ml of water, and the stirring time needs to be overnight; the next day, sucti...
Embodiment 3
[0029] Add 200ml of dichloromethane into a 1000ml reaction bottle, then add 28g of imaamine under stirring, stir until the reaction system is dissolved and transparent, continue stirring, then add 41g of dicyclohexylcarbodiimide, 35g of imaic acid, 4 -Dimethylaminopyridine 1g, then, the reaction system will heat up, cool with cold water on the outside of the reaction bottle, keep the reaction temperature between 20-30 degrees Celsius, the reaction time needs to be overnight; the second day by spotting the plate , observe that the raw material point imamamine basically disappears, and stop the reaction; then add 200ml of water to the reaction bottle, a white solid will be precipitated in the system, exothermic, continue to stir for 2 hours and then filter, and the filter cake is drenched with a small amount of dichloromethane Wash, add 200ml water to the filtrate and continue to stir, and the stirring time needs to be overnight; the next day, filter the filter cake with a small ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com