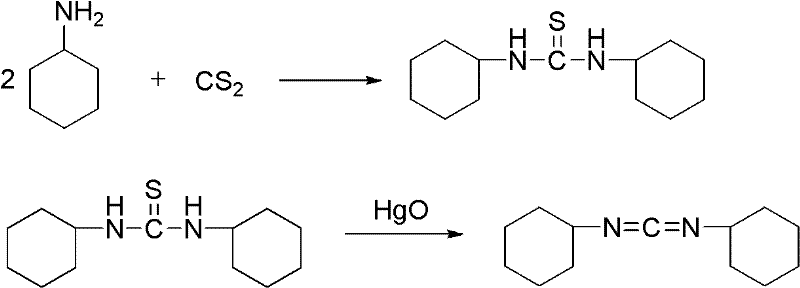
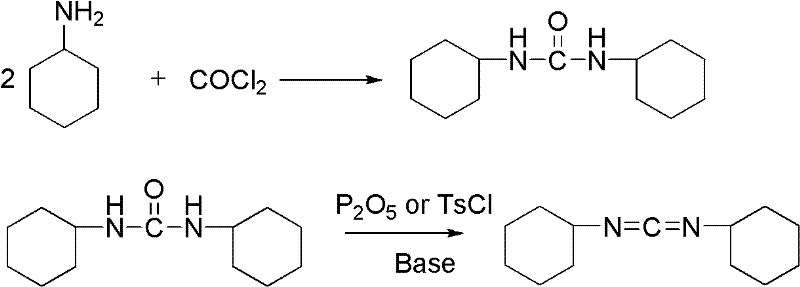
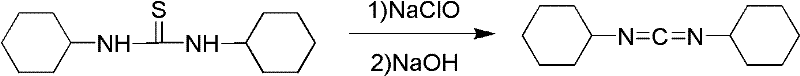Method for synthesizing dicyclohexylcarbodiimide compound
A technology of dicyclohexylcarbodiimide and its synthesis method, which is applied in the direction of organic chemistry, can solve the problems of large safety hazards, high equipment requirements, inconvenient operation, etc., and achieve the goal of less three wastes, excellent product quality, and high total yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] According to dicyclohexyl urea: the amount ratio of bis(trichloromethyl) carbonate feed material is 1: 1 feed intake, dicyclohexyl urea 112g (0.5mol), organic solvent is methyl tert-butyl ether 750mL, bis(trichloromethyl) Chloromethyl)carbonate 149g (0.5mol).
[0034] Dissolve bis(trichloromethyl)carbonate in 500mL of methyl tert-butyl ether, and slowly add it dropwise to a solution of dicyclohexylurea dissolved in 250mL of methyl tert-butyl ether at room temperature (20°C). After the addition, the temperature was raised to reflux temperature, and the reaction was completed after 5 hours of heat preservation reaction, and then the temperature was lowered to room temperature, and 100 mL of triethylamine was added dropwise to adjust the pH value of the reaction solution to 8. Tert-butyl ether was washed 3 times, filtered, and all filtrates (comprising the filtrate obtained by suction filtration and the filtrate obtained by filtering after washing) were combined and distil...
Embodiment 2
[0036] According to dicyclohexyl urea: bis (trichloromethyl) carbonate feed material ratio is 1: 0.5 feeding, dicyclohexyl urea 112g, bis (trichloromethyl) carbonate 74.5g, organic solvent is toluene 750mL.
[0037] The reaction temperature was 110° C., and other operations were the same as in Example 1 to obtain 95 grams of dicyclohexylcarbodiimide with a yield of 92% and a purity of 98%.
Embodiment 3
[0039] According to dicyclohexyl urea: bis (trichloromethyl) carbonate feed material ratio is 1: 0.33 feeding, dicyclohexyl urea 112g, organic solvent dichloromethane 500mL;
[0040] The reaction temperature was reflux temperature, and other operations were the same as in Example 1 to obtain 83.4 g of dicyclohexylcarbodiimide with a yield of 81% and a purity of 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com