Preparation method of N,N'-dicyclohexylurea
A technology of dicyclohexyl urea and cyclohexylamine, which is applied to the preparation of urea derivatives, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of high pressure reaction temperature, reduce reaction temperature and pressure, promote activation, The effect of large absorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] When [P 66614 ]Im anion functionalized ionic liquid for CO absorption 2 After reaching the absorption equilibrium, according to the CO2 Saturation absorption capacity, when absorbing CO 2 Add 2 times the molar amount of cyclohexylamine to the final ionic liquid system, mix it evenly, transfer it to a stainless steel autoclave at 60°C and carry out the in-situ conversion reaction under normal pressure, stop the reaction after reacting for 8 hours, and add a certain amount of distilled water, separated the crude product from the ionic liquid, and recrystallized to obtain N,N'-dicyclohexylurea with a yield of 65%.
Embodiment 2-14
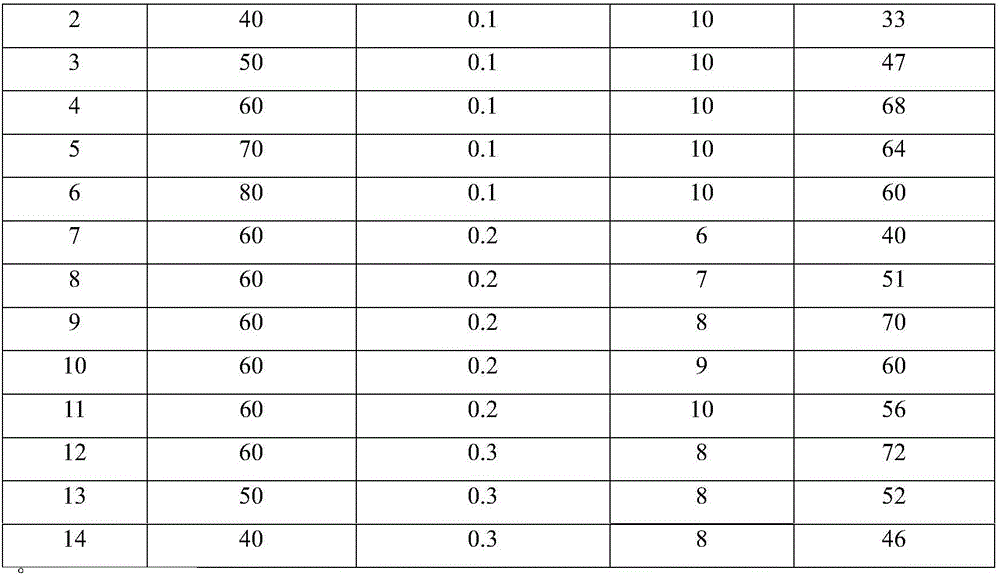
[0031] Embodiment 2-14: the influence of different reaction temperature, pressure and time on product yield.
[0032] According to the preparation method of embodiment 1, adopt [P 66614 ] Im anion-functionalized ionic liquid absorbed CO 2 In situ reaction of carbon source and cyclohexylamine with 2 times the molar amount, the temperature, pressure and time of the reaction were changed, and the yield of N,N'-dicyclohexylurea was calculated as shown in Table 1:
[0033] Table 1, the influence of temperature of reaction, pressure and time on product yield
[0034]
[0035]
[0036] As shown in Table 1, due to the anion functionalization of ionic liquids with CO 2 chemical interaction to activate CO 2 molecules, thereby effectively reducing the CO 2 Reaction temperature and pressure with cyclohexylamine, under mild conditions (reaction temperature is 40-80°C, pressure is 0.1-0.3Mpa, reaction time is 6-10 hours) to achieve 2 In situ transformation and preparation of N,N'...
Embodiment 15-34
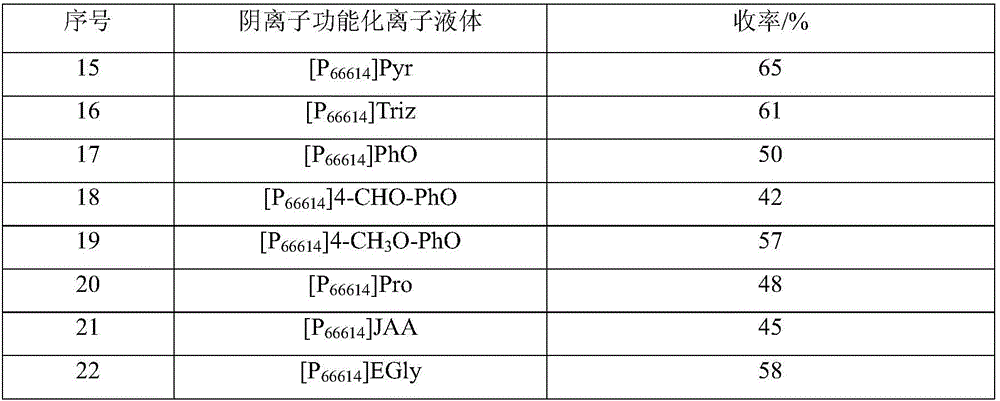
[0038] Examples 15-34: Effects of different types of anion-functionalized ionic liquids on product yield.
[0039] According to the preparation method of Example 1, the difference is: change the type of anion-functionalized ionic liquid, and the CO absorbed by the anion-functionalized ionic liquid 2 In situ reaction of carbon source with 2 times the molar amount of cyclohexylamine, reacted at 60°C and normal pressure for 8 hours, and investigated the effect of the type of anion functionalized ionic liquid on CO 2 The effect of in situ conversion to N,N'-dicyclohexylurea, the results are shown in Table 2.
[0040] Table 2. The effect of the type of anionic functionalized ionic liquid on the product yield
[0041]
[0042]
[0043] As shown in Table 2, different anion functionalized ionic liquids, for CO 2的 Absorption capacity and absorption rate are different, for CO 2 The activation of the molecule is also different, therefore, its impact on the yield is more obvious,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com