Preparation method of carbamazepine (CBZ)-valaciclovir
A CBZ-L-, solvent technology, applied in the direction of organic chemistry, can solve the problem of DCU affecting the product content, and achieve the effect of benefiting the utilization rate of palladium carbon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
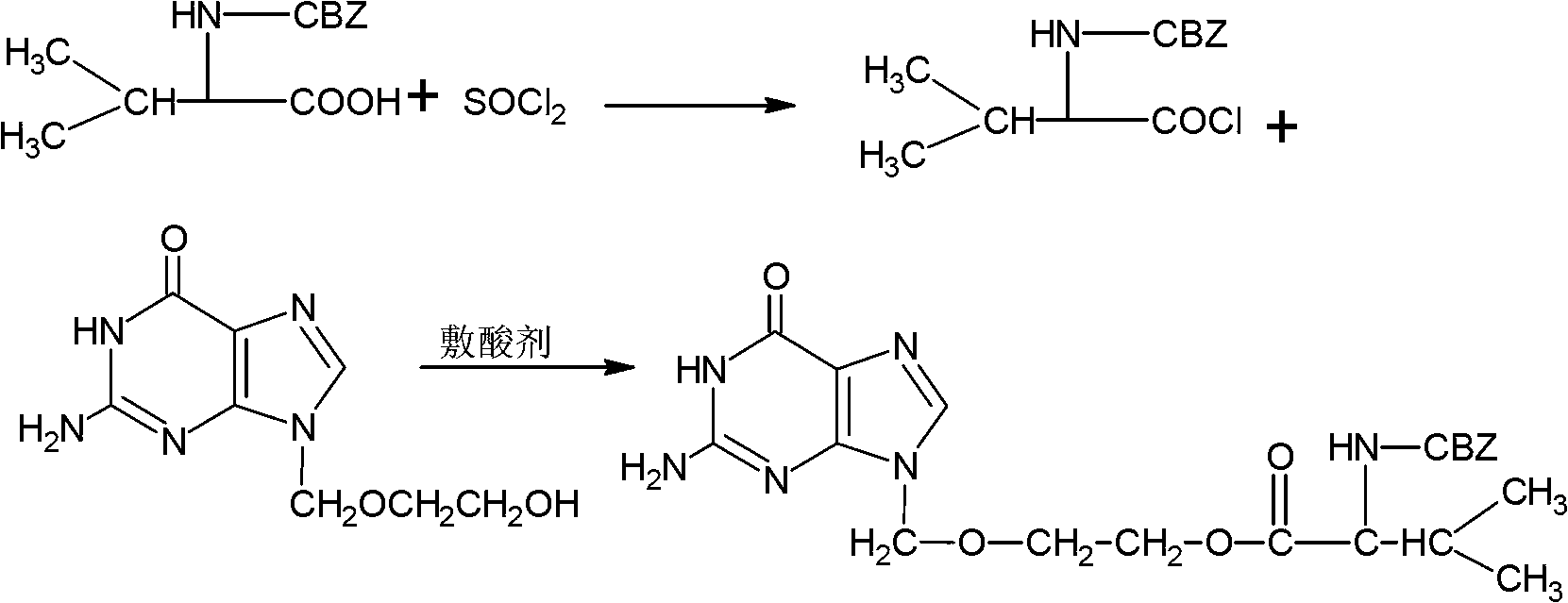
[0023] Dissolve 25.1g of CBZ-L-valine in 150ml of dichloromethane, control the reaction temperature at 5°C, slowly add 12.8g of oxalyl chloride dropwise, control the dropping time for 1h, after the dropping is completed, keep warm for 2h, stop the reaction, and depressurize Concentrate, distill off dichloromethane and excess oxalyl chloride, and prepare the acid chloride for use in the next step;
[0024] Add 20g of acyclovir to 300ml of N,N-dimethylformamide, control the reaction temperature at 20°C, add 13.8g of potassium carbonate, then slowly add the acid chloride prepared in the previous step dropwise, control the dropping time for 1h, and then raise the temperature To 40°C, continue to react for 4h, through liquid-phase central control reaction, after aciclovir remaining less than 1%, carry out vacuum concentration, add 600ml methanol to recrystallize the crude product after concentration, obtain 34g CBZ-valaciclovir, product yield 85% %, its purity measured by liquid ch...
Embodiment 2
[0026] Dissolve 25.1g of CBZ-L-valine in 150ml of benzene, control the temperature at 10°C, slowly add 13g of thionyl chloride dropwise, control the dropping time for 5h, after the dropwise addition is completed, keep warm for 2h, stop the reaction, concentrate under reduced pressure, Evaporate excess thionyl chloride, and prepare the acid chloride for use in the next step;
[0027] Add 20g of acyclovir to 300ml of benzene, control the temperature at 20°C, add 13.8g of potassium carbonate, then slowly drop in the acid chloride prepared in the previous step, control the drop time for 1h, then raise the temperature to 40°C, and continue the reaction for 4h, Through the liquid-phase control reaction, the remaining acyclovir was less than 1%, and then concentrated under reduced pressure. After concentration, the crude product was recrystallized by adding 600ml of methanol to obtain 31.6g of CBZ-valaciclovir. The product yield was 79%. The purity is 99.0%.
Embodiment 3
[0029] Dissolve 25.1g of CBZ-L-valine in 150ml of benzene, control the reaction temperature at 40°C, slowly add 13g of thionyl chloride dropwise, control the dropping time for 1h, after the dropping is completed, keep warm for 2h, stop the reaction, and concentrate under reduced pressure , distill off benzene and excess thionyl chloride, and prepare the acid chloride for use in the next step;
[0030] Add 20g of acyclovir to 300ml of N,N-dimethylformamide, control the reaction temperature at 20°C, add 12g of triethylamine, then slowly add the acid chloride prepared in the previous step, control the dropping time for 1h, and then raise the temperature To 60°C, continue to react for 4 hours, through the liquid phase control reaction, the remaining acyclovir is less than 1%, then concentrate under reduced pressure, after concentration, the crude product is recrystallized by adding 600ml of ethanol to obtain 35.6g of CBZ-valaciclovir, the product yield 89%, its purity measured by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com