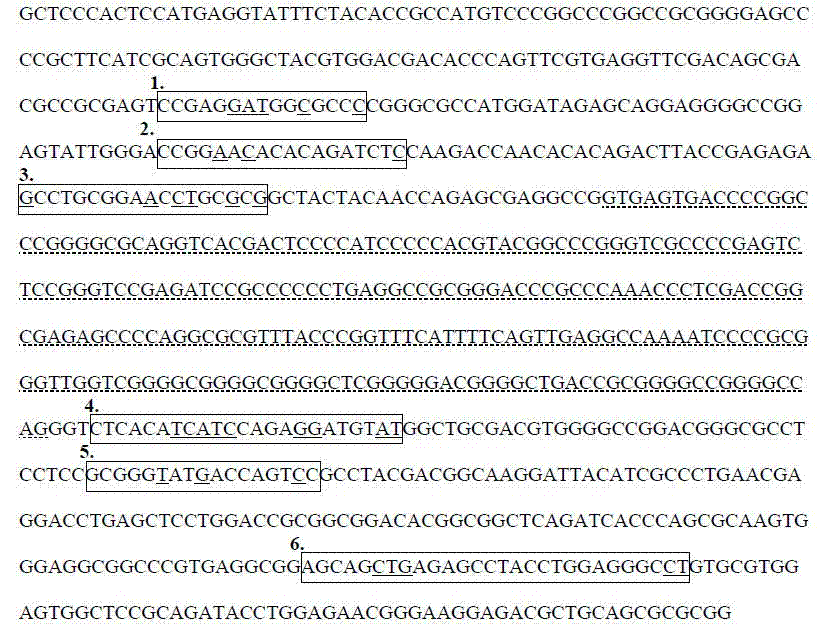Patents
Literature
162 results about "Carbamazepine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
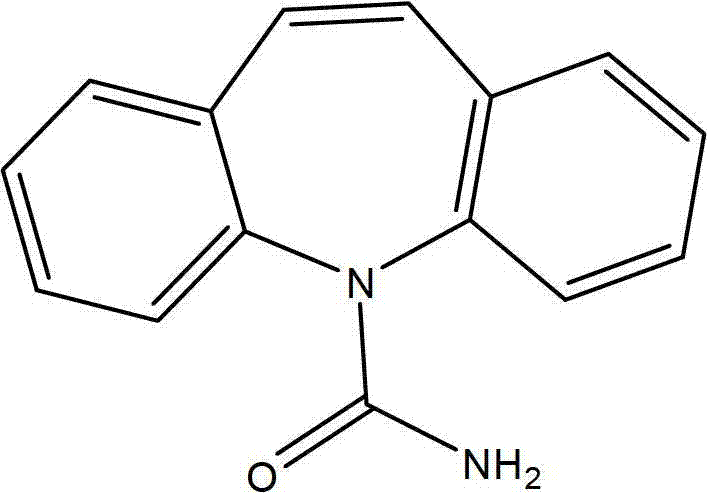
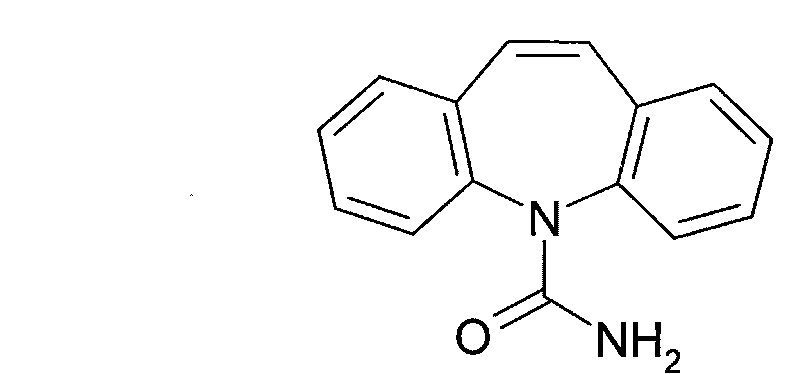
Carbamazepine is used to prevent and control seizures.
Zero-order sustained release delivery system for carbamazephine derivatives
A zero-order sustained-release delivery system for delivery of carbamazepine or a derivative thereof. A polymeric matrix formulation of carbamazepine comprises hydrophilic polymer or hydrophilic / hydropholic polymer mixture which permits carbamazepine or carbamezepine derivative to be released from the polymer matrix in zero-order release kinetics.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Preparation method of ticagrelor
ActiveCN102675321AHigh yieldReduce manufacturing costOrganic chemistryBulk chemical productionNitriteTicagrelor
The invention provides a preparation method of ticagrelor, belonging to the technical field of medicine manufacturing. According to the method, a compound VII is taken as a raw material, and the method comprises the steps of: carrying out a nucleophilic substitution reaction on the raw material to obtain a compound VI; hydrogenating the VI, removing carbamazepine (Cbz) protection to obtain a compound V; carrying out a reaction on the V and 4, 6-dichloro-2-(allyl sulfide)-5-amio-pyrimidine to obtain a compound IV; carrying out a reaction on the IV and nitrite of alkali metal to obtain a compound III; carrying out a reaction on the III and (1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine to obtain a compound II; and finally, removing protecting group of the II to obtain a compound I.
Owner:SHANGHAI HAOYUAN CHEMEXPRESS
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
Risk assessment for adverse drug reactions
The present invention provides a method of predicting the risk of a patient for developing adverse drug reactions, particularly SJS or TEN. It was discovered that an HLA-B allele, HLA-B* 1502, is associated with SJS / TEN that is induced by a variety of drugs. The correlation with HLA-B* 1502 is most significant for carbamazepine-induced SJS / TEN, wherein all the patients tested have the HLA-B* 1502 allele. In addition, another HLA-B allele, HLA-B*5801, is particularly associated with SJS / TEN induced by allopurinol. Milder cutaneous reactions, such as maculopapular rash, erythema multiforme (EM), urticaria, and fixed drug eruption, are particularly associated with a third allele, HLA-B *4601. For any of the alleles, genetic markers (e.g., HLA markers, microsatellite, or single nucleotide polymorphism markers) located between DRB1 and HLA-A region of the specific HLA-B haplotype can also be used for the test.
Owner:ACAD SINIC
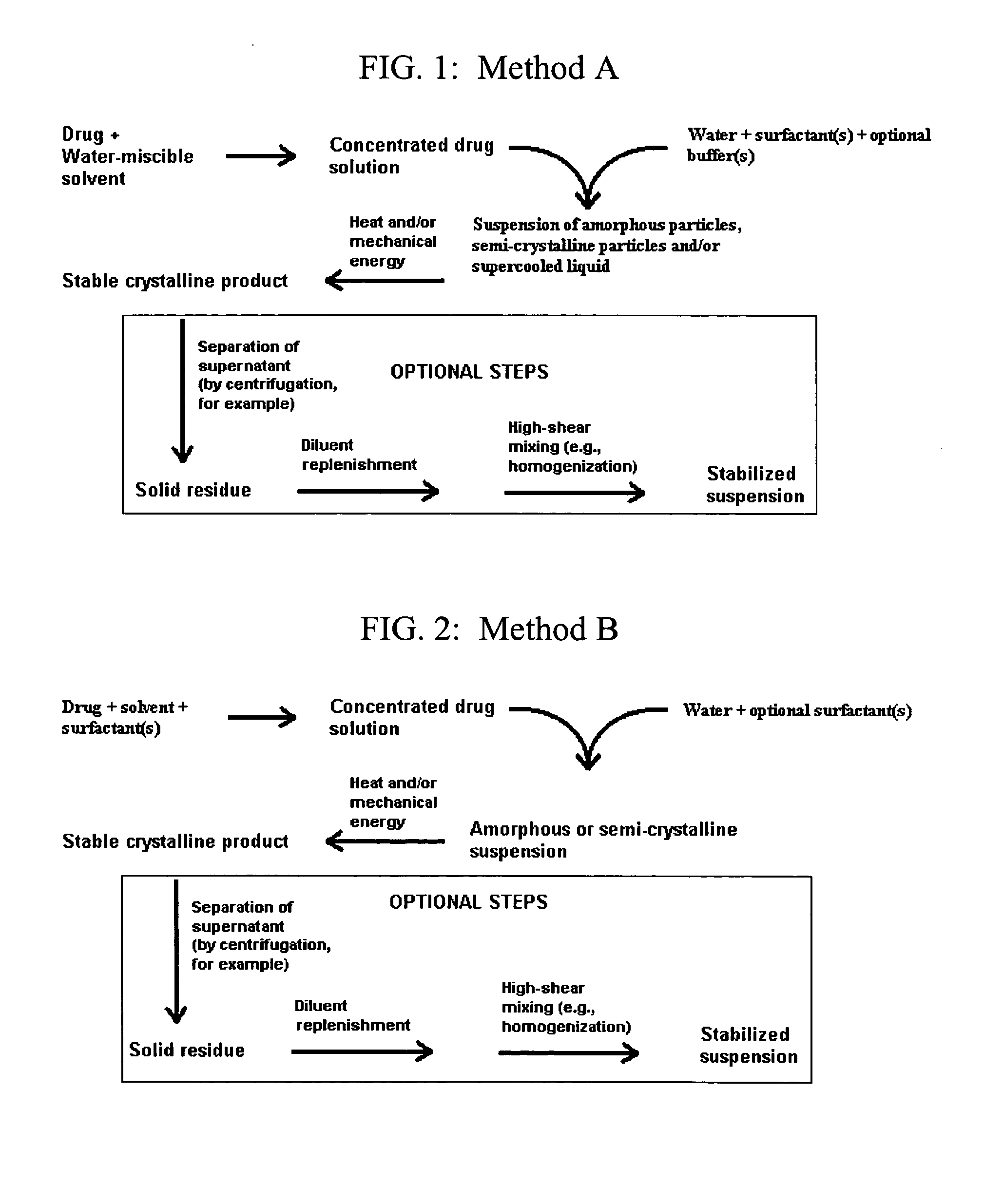
Small-particle pharmaceutical formulations of antiseizure and antidementia agents and immunosuppressive agents
InactiveUS20050244503A1High drug loadingMinimize side effectsPowder deliveryCyclic peptide ingredientsMedicineCyclosporins
This invention pertains to the formulation of small-particle suspensions of anticonvulsants and antidementia, particularly carbamazepine, for pharmaceutical use. This invention also pertains to the formulation of small-particle suspensions of immunosuppressive agents, particularly cyclosporin, for pharmaceutical use.
Owner:BAXTER INT INC +1
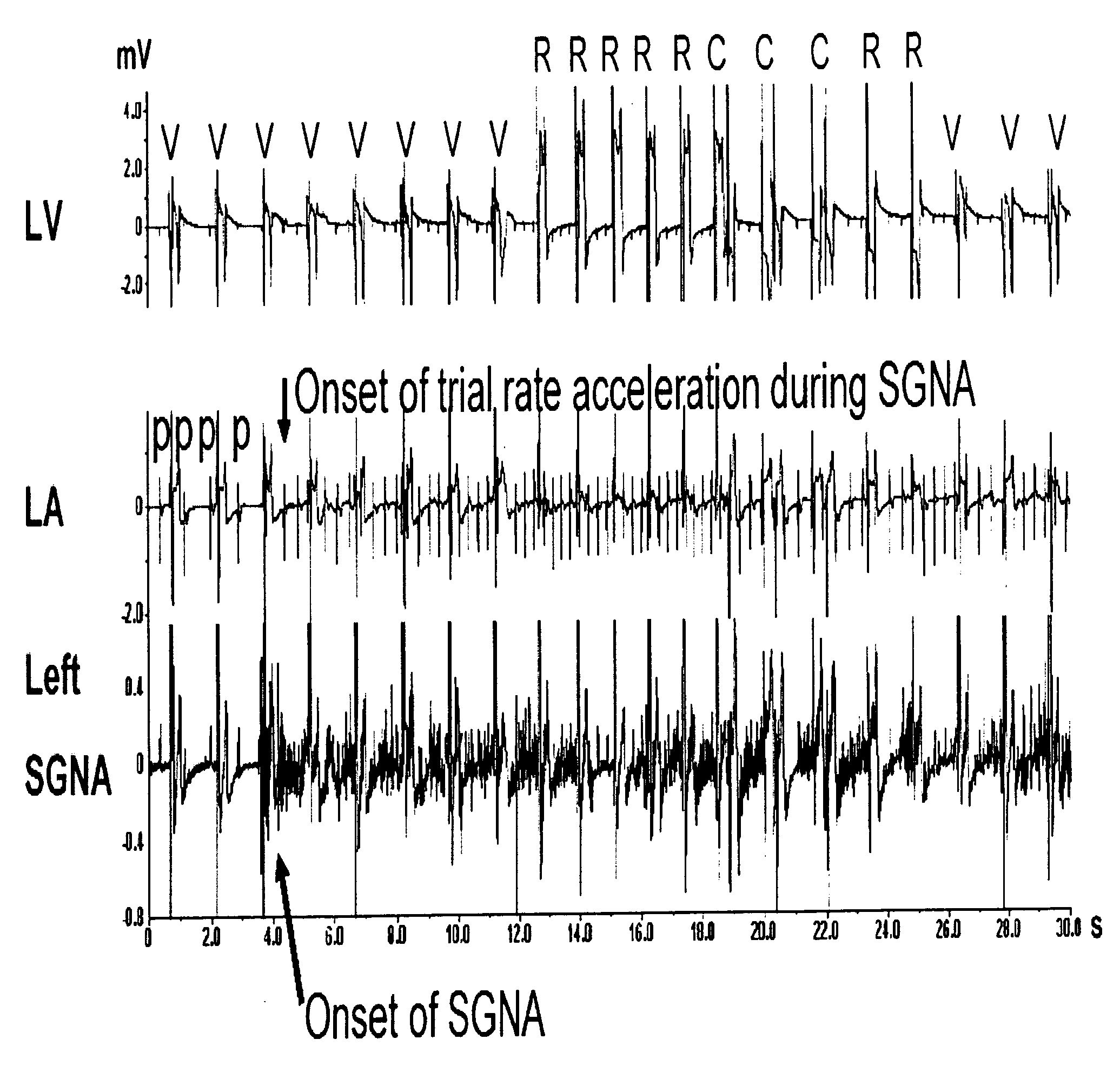
Method and system for the prediction of cardiac arrhythmias, myocardial ischemia, and other diseased condition of the heart associated with elevated sympathetic neural discharges
InactiveUS20060074451A1Raise the possibilityIncrease heart rateSpinal electrodesHeart stimulatorsDiseaseAntiarrhythmic effect
Methods and systems are provided for determining an increased likelihood of the occurrence of a cardiac arrhythmia, myocardial ischemia, congestive heart failure and other diseased conditions of the heart associated with elevated sympathetic neural discharges in a patient. The methods and systems comprise monitoring the sympathetic neural discharges of a patient from the stellate ganglia, the thoracic ganglia, or both, and detecting increases in the sympathetic neural discharges. The methods and systems may further comprise delivering therapy to the patient in response to a detected increase in the sympathetic neural discharge, such as delivering one or more pharmacological agents; stimulating myocardial hyperinnervation in the sinus node and right ventricle of the heart of the patient; and applying cardiac pacing, cardioversion or defibrillation shocks. Pharmacologic agents which may be used in connection with the delivery of include those which are known to exert anti-arrhythmic effect and anti-convulsant agents, such as phenytoin, carbamazepine, valproate, and phenobarbitone. Other pharmacologic agents may be used to treat impending myocardial ischemia and other diseased conditions of the heart associated with elevated sympathetic neural discharges.
Owner:CEDARS SINAI MEDICAL CENT
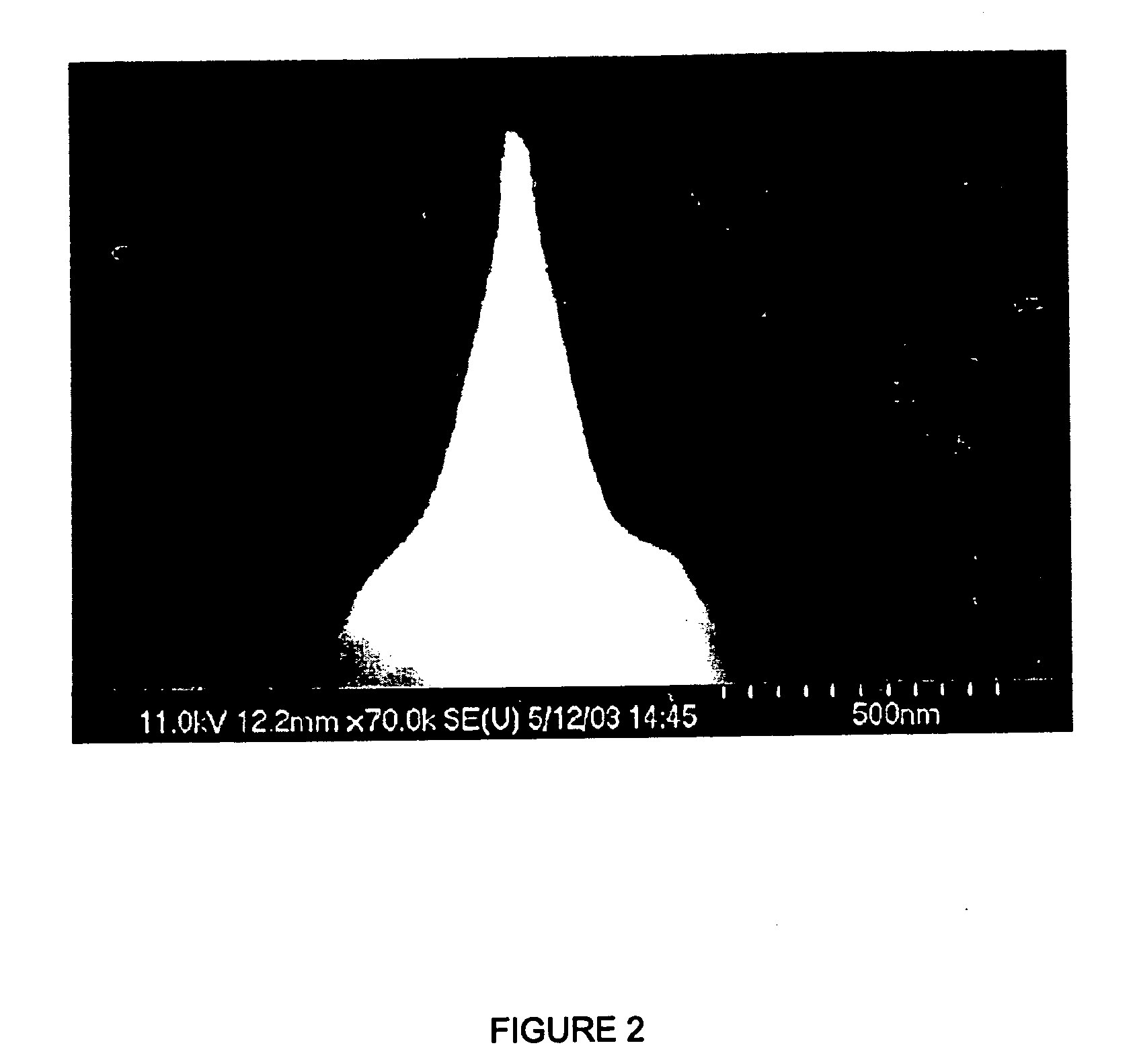
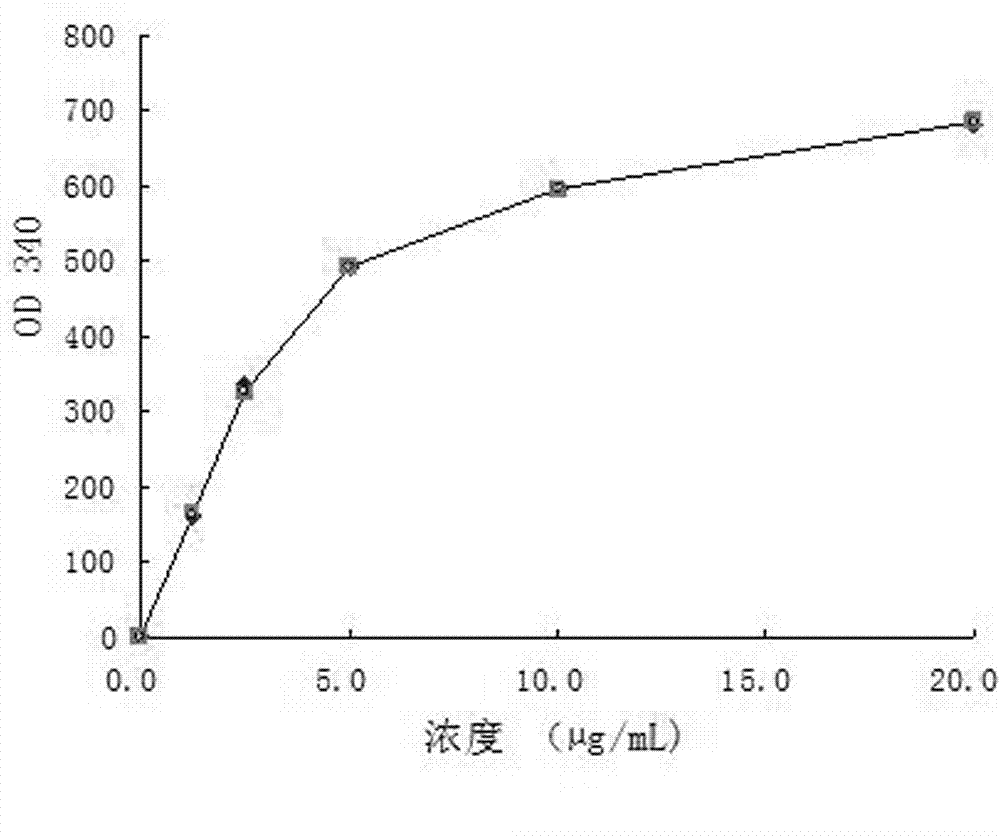
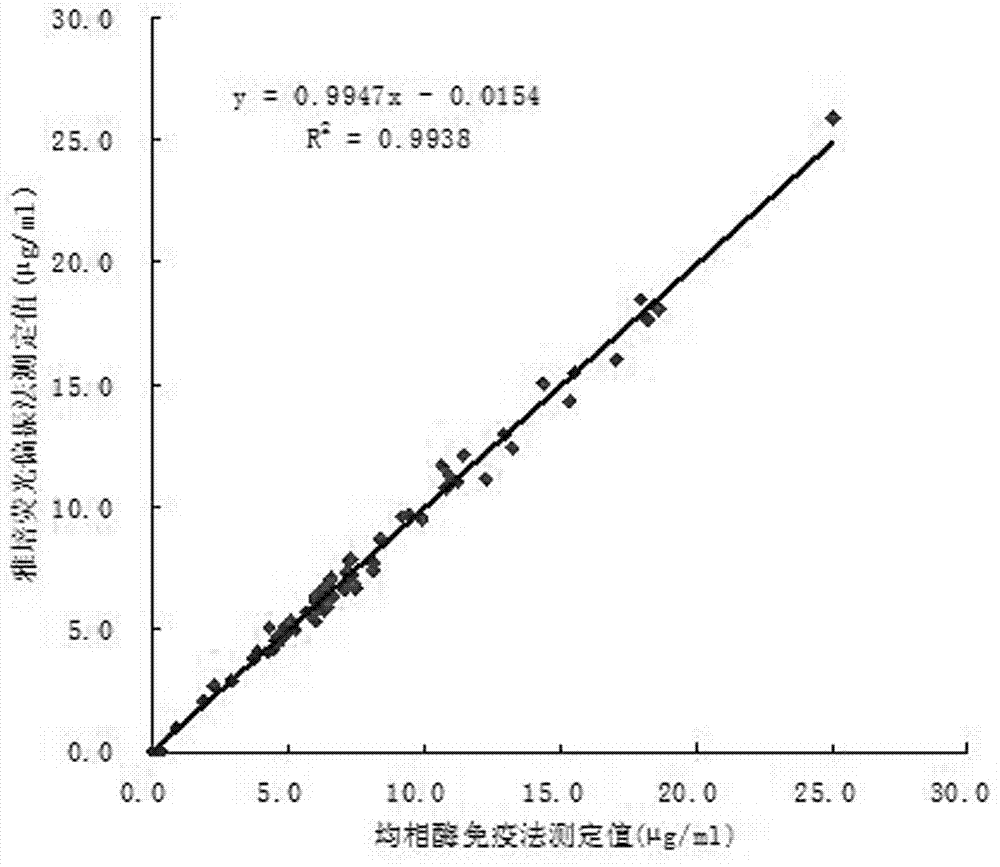
Immunodetection reagent of carbamazepine homogeneous enzyme and detection method thereof
The invention discloses a carbamazepine immunogen, a carbamazepine specific antibody directly obtained through the immunogen, a detection reagent containing the specific antibody and a method for detecting the content of the carbamazepine in samples to be tested. The immunodetection reagent and the detection method have the advantages that the carbamazepine immunogen is strong in specificity and high in immunogenicity; the prepared carbamazepine immunogen is strong in specificity and high in titer and has no cross reaction with 45 common drugs; the homogeneous enzyme immunodetection reagent containing the carbamazepine specific antibody can conveniently, fast and accurately determine the content of the carbamazepine in the samples and can measure a plurality of samples simultaneously on a full-automatic biochemical analyzer, so that high-throughput and fast measuring of the carbamazepine are achieved, the accuracy is high, the specificity is strong, the accuracy and the detection efficiency are greatly improved compared with the prior art, full automation in the detection process is achieved simultaneously, the requirements on detection staff is not high, and the immunodetection reagent and the detection method are easy to achieve, popularize and use.
Owner:苏州博源医疗科技有限公司
Multiple unit modified release compositions of carbamazepine and process for their preparation
The present invention relates to multiple-unit modified release carbamazepine compositions for oral administration which include: (i) at least one extended release unit, and (ii) at least one enteric release unit. Also provided are processes for the preparation of multiple-unit modified release compositions of carbamazepine.
Owner:RANBAXY LAB LTD
Method and system for the prediction of cardiac arrhythmias, myocardial ischemia, and other diseased condition of the heart associated with elevated sympathetic neural discharges
Owner:CEDARS SINAI MEDICAL CENT
Oral controlled drug delivery system
InactiveUS20030175353A1Easy to manufactureAvoid conversionBiocidePowder deliveryHydrophobic polymerCarbamazepine
The oral controlled drug delivery system of the present invention comprises carbamazepine and one or more hydrophobic polymers in homogenous admixture, wherein the system does not comprise any means capable of preventing the conversion of carbamazepine to its dihydrate form. The present invention provides an oral controlled drug delivery system for carbamazepine having a desirable controlled rate of delivery of carbamazepine, which system is simple, uncomplicated and easy to manufacture.
Owner:SUN PHARMA INDS
Preparation method and application for magnetic molecularly imprinted polymers for selectively separating carbamazepine
ActiveCN102827321AUniquely identifiableSuperparamagneticOther chemical processesPreparing sample for investigationMagnetic mediaSuperparamagnetism
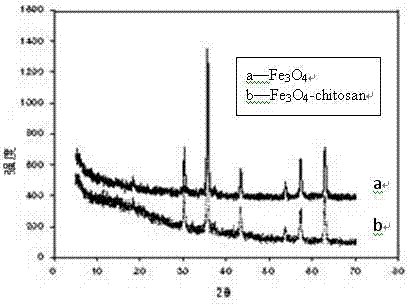
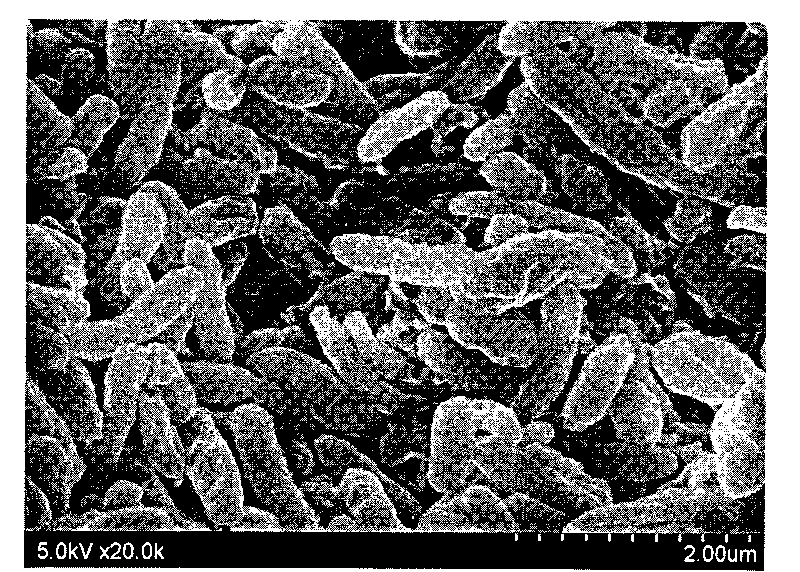
The invention relates to a preparation method and an application for magnetic molecular imprinting polymers for selectively separating carbamazepine and belongs to the technical fields of environmental material preparation and pollution regulation. Nanometer ferroferric oxide modified on a surface of chitosan serves as a magnetic medium, molecular imprinting material polymerization is carried out on the surface of the nanometer ferroferric oxide by using a sediment polymerization method, and a magnetic molecular imprinting adsorption agent is obtained after template molecular carbamazepine is eluted. Obtained molecular imprinting composite materials are stable in physicochemical property, have high adsorption capacity and specific identification characteristics on the carbamazepine, have superparamagnetism, can be rapidly separated under an outside magnetic field effect, therefore, interference of a complex substrate in an actual sample is avoided, and sample treatment processes are greatly simplified. The preparation method is simple, reliable and low in cost and has a wide application prospect in analysis, detection and pollution treatment of a complex environment sample.
Owner:TONGJI UNIV
Carbamazepine immunogen, anti-carbamazepine specific antibody, detection reagent and detection kit
ActiveCN102180965AImproving immunogenicityStrong specificitySerum albuminDepsipeptidesCarbamazepineSpecific antibody
The invention discloses a carbamazepine immunogen, an anti-carbamazepine specific antibody, a detection reagent and a detection kit.
Owner:HAINAN JINYU MEDICAL TESTING CENT CO LTD
Method and system for the prediction of cardiac arrhythmias, myocardial ischemia, and other diseased condition of the heart associated with elevated sympathetic neural discharges
InactiveUS20060004414A1Raise the possibilityIncrease heart rateCatheterHeart stimulatorsDiseaseAntiarrhythmic effect
Methods and systems are provided for determining an increased likelihood of the occurrence of a cardiac arrhythmia, myocardial ischemia, congestive heart failure and other diseased conditions of the heart associated with elevated sympathetic neural discharges in a patient. The methods and systems comprise monitoring the sympathetic neural discharges of a patient from the stellate ganglia, the thoracic ganglia, or both, and detecting increases in the sympathetic neural discharges. The methods and systems may further comprise delivering therapy to the patient in response to a detected increase in the sympathetic neural discharge, such as delivering one or more pharmacological agents; stimulating myocardial hyperinnervation in the sinus node and right ventricle of the heart of the patient; and applying cardiac pacing, cardioversion or defibrillation shocks. Pharmacologic agents which may be used in connection with the delivery of include those which are known to exert anti-arrhythmic effect and anti-convulsant agents, such as phenytoin, carbamazepine, valproate, and phenobarbitone. Other pharmacologic agents may be used to treat impending myocardial ischemia and other diseased conditions of the heart associated with elevated sympathetic neural discharges.
Owner:CEDARS SINAI MEDICAL CENT
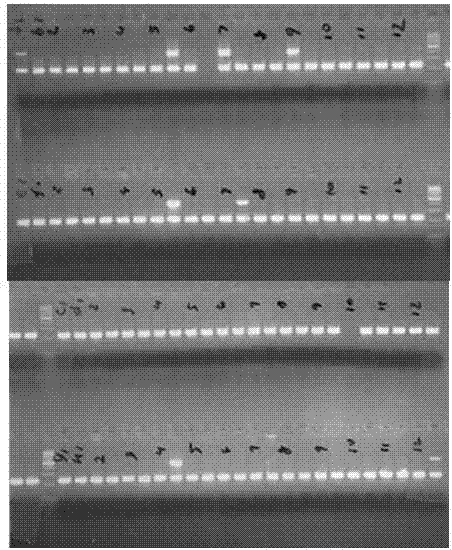
Reagent for detecting antiepileptic drug allergic reaction associated antigen genetype and clinical application method
InactiveCN101353698AReduce riskImprove accuracyMicrobiological testing/measurementAntigenAntiepileptic drug
The invention relates to a reagent used for detecting antigen genotypes relevant to the anaphylactic reaction of antiepileptic drugs, and a clinical application method thereof. The reagent is characterized in that: 1) the reagent comprises Taq enzyme, a chain of seven tubes and a PCR primer; 2) the PCR primer is shown in the sequence list SEQ ID No.1. The clinical application method is characterized in that: 1) four pairs of the sequence specific primers and two pairs of internal reference primers are designed by utilizing a sequence specific primer PCR-SSP according to an HLA-B sequence, and PCR amplification is carried out by taking gDNA extracted from human peripheral blood or other issues as a template; 2) gel electrophoresis is adopted for the detection so as to confirm that the genotype of the sample is HLA-B multiplied by 1502. The reagent and the method of the invention have the advantages of simple operation, high accuracy and low cost, and are especially suitable for determining that whether the antiepileptic drugs such as carbamazepine, etc. can be taken by the HLA-B multiplied by 1502 genotype detection before patients in China or Asia take antiepileptic drugs such as the carbamazepine, etc.
Owner:THE SECOND AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
Method for preparing carbamazepine PLGA (poly lactic-co-glycolic acid) copolymer micro capsule
ActiveCN103610664AHigh dissolution rateIncrease dissolution ratePharmaceutical non-active ingredientsMicrocapsulesSolubilityAcetic acid
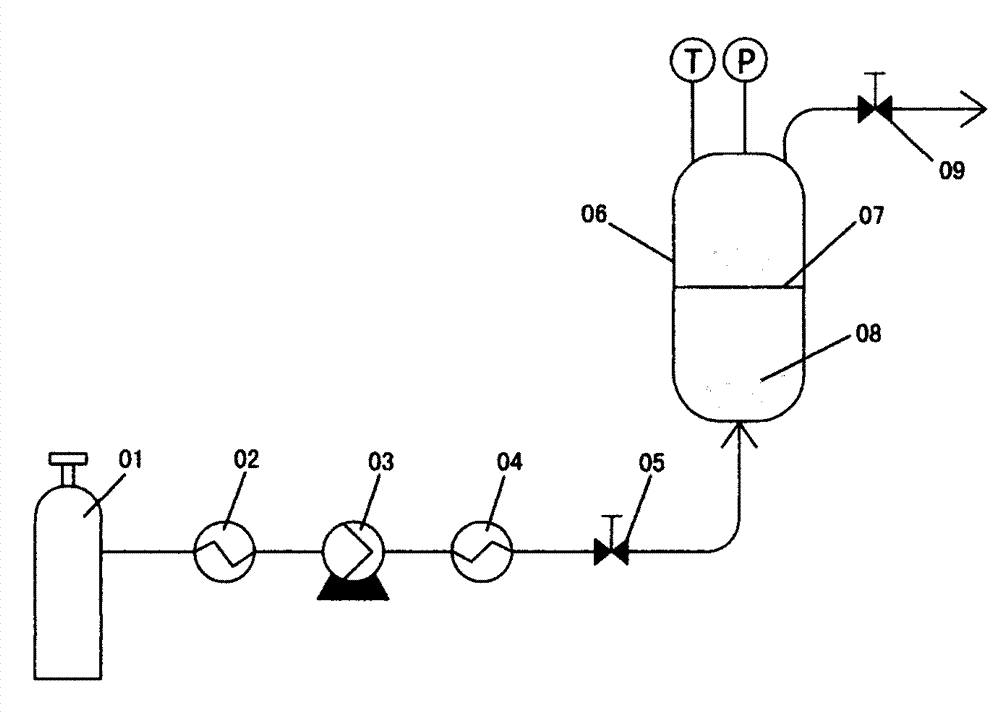
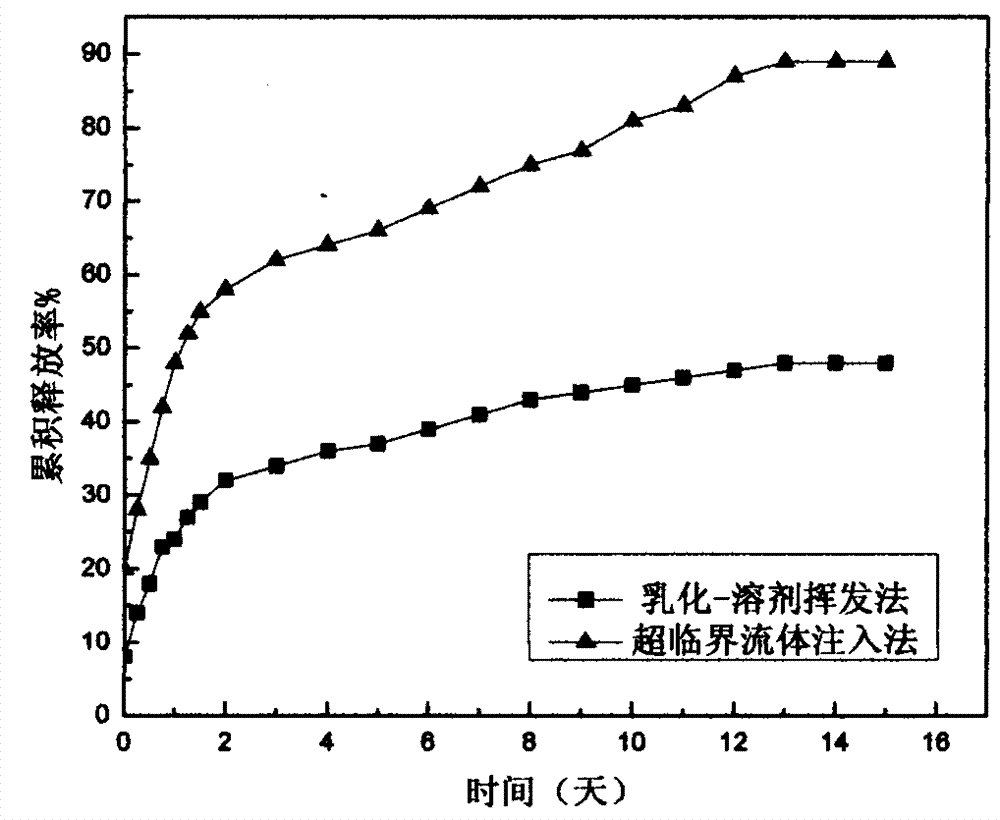
The invention provides a method for preparing a carbamazepine PLGA (poly lactic-co-glycolic acid) copolymer micro capsule through a supercritical fluid injection method. The method comprises the following steps: firstly dissolving carbamazepine into supercritical carbon dioxide; secondly, feeding the supercritical carbon dioxide which is dissolved with the carbamazepine into a substrate to be maintained for 1 to 5 hours through the plasticization and swelling of PLGA copolymer, and enabling the carbamazepine to be balanced in distribution between the supercritical carbon dioxide and the PLGA copolymer; finally escaping the carbon dioxide, reserving the carbamazepine in the PLGA copolymer substrate, and preparing the carbamazepine PLGA copolymer micro capsule. The carbamazepine PLGA copolymer micro capsule which is prepared through the method is high in solubility and dissolution rate.
Owner:CHINA PHARM UNIV
Method for treating antiepileptic drug in water by utilizing MnxCo3-xO4 nanocages for activating monoperoxysulfate
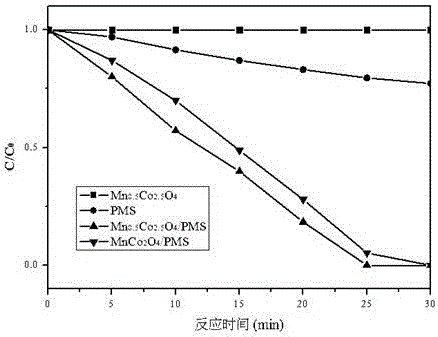
ActiveCN106430699AEasy to recycleEfficient recyclingWater treatment parameter controlWater treatment compoundsCentrifugationMedicine
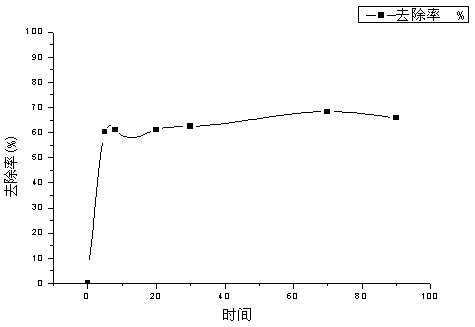
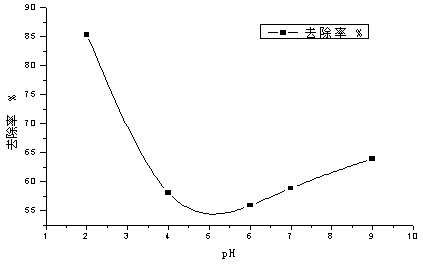
The invention relates to the field of water treatment, and discloses a method for treating an antiepileptic drug in water by utilizing MnxCo3-xO4 nanocages for activating monoperoxysulfate; the method particularly includes the following steps: one, mixing monoperoxysulfate with an antiepileptic drug-containing aqueous solution; two, adjusting a reaction pH; three, preparing the MnxCo3-xO4 nanocages; four, adding the MnxCo3-xO4 nanocages; five, recycling the MnxCo3-xO4 nanocages, and thus completing the method for treating the antiepileptic drug in the water by utilizing the MnxCo3-xO4 nanocages for activating monoperoxysulfate. The removal effect of the typical antiepileptic drug carbamazepine in water by using the method is obvious, the removal rate reaches 95%-99%, a catalytic material can be recovered and reused through centrifugation and separation, and the cost is saved.
Owner:ZHEJIANG UNIV OF TECH
Method and system for the prediction of cardiac arrhythmias, myocardial ischemia, and other diseased condition of the heart associated with elevated sympathetic neural discharges
Methods and systems are provided for determining an increased likelihood of the occurrence of a cardiac arrhythmia, myocardial ischemia, congestive heart failure and other diseased conditions of the heart associated with elevated sympathetic neural discharges in a patient. The methods and systems comprise monitoring the sympathetic neural discharges of a patient from the stellate ganglia, the thoracic ganglia, or both, and detecting increases in the sympathetic neural discharges. The methods and systems may further comprise delivering therapy to the patient in response to a detected increase in the sympathetic neural discharge, such as delivering one or more pharmacological agents; stimulating myocardial hyperinnervation in the sinus node and right ventricle of the heart of the patient; and applying cardiac pacing, cardioversion or defibrillation shocks. Pharmacologic agents which may be used in connection with the delivery of include those which are known to exert anti-arrhythmic effect and anti-convulsant agents, such as phenytoin, carbamazepine, valproate, and phenobarbitone. Other pharmacologic agents may be used to treat impending myocardial ischemia and other diseased conditions of the heart associated with elevated sympathetic neural discharges.
Owner:CEDARS SINAI MEDICAL CENT
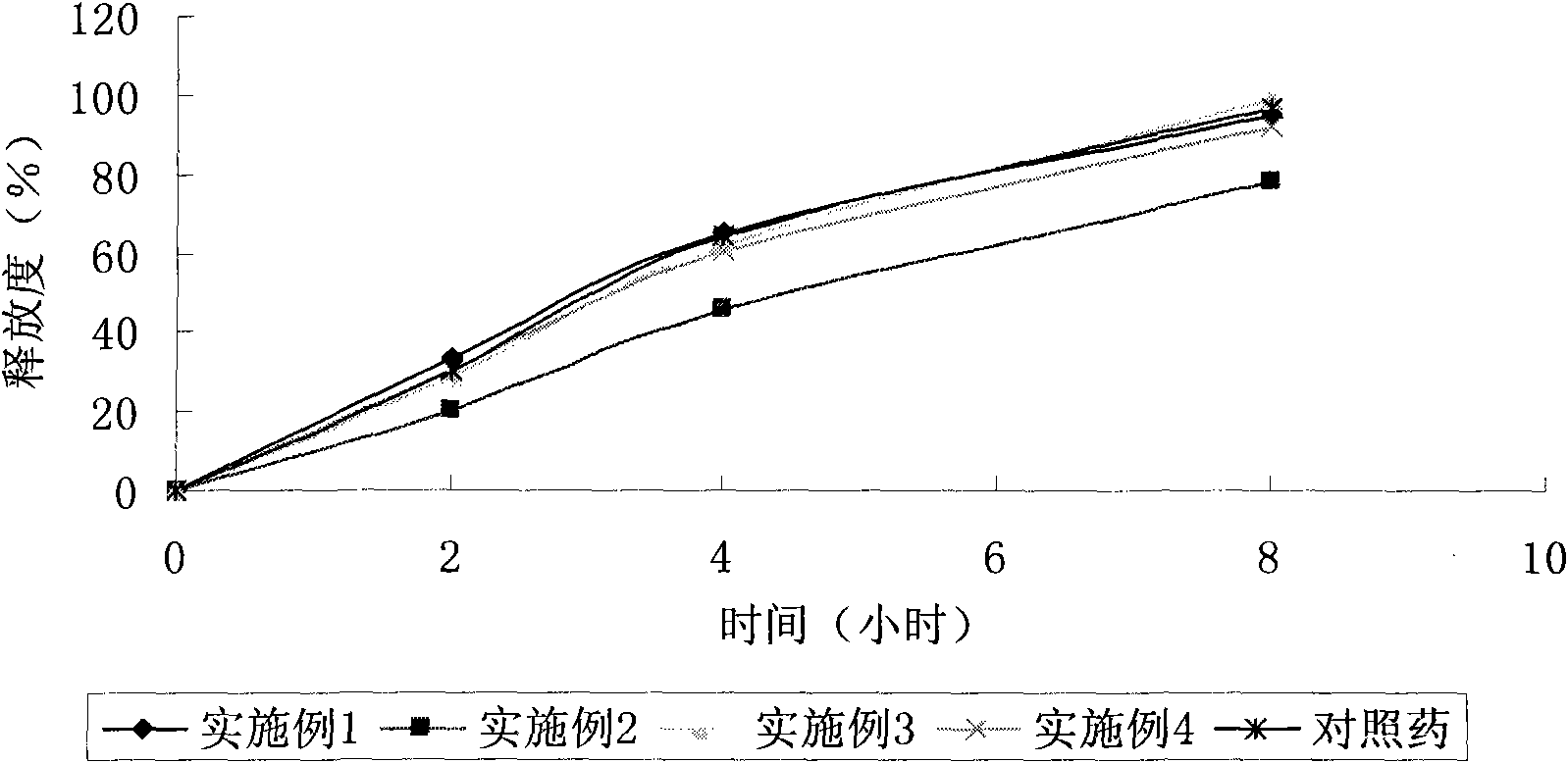
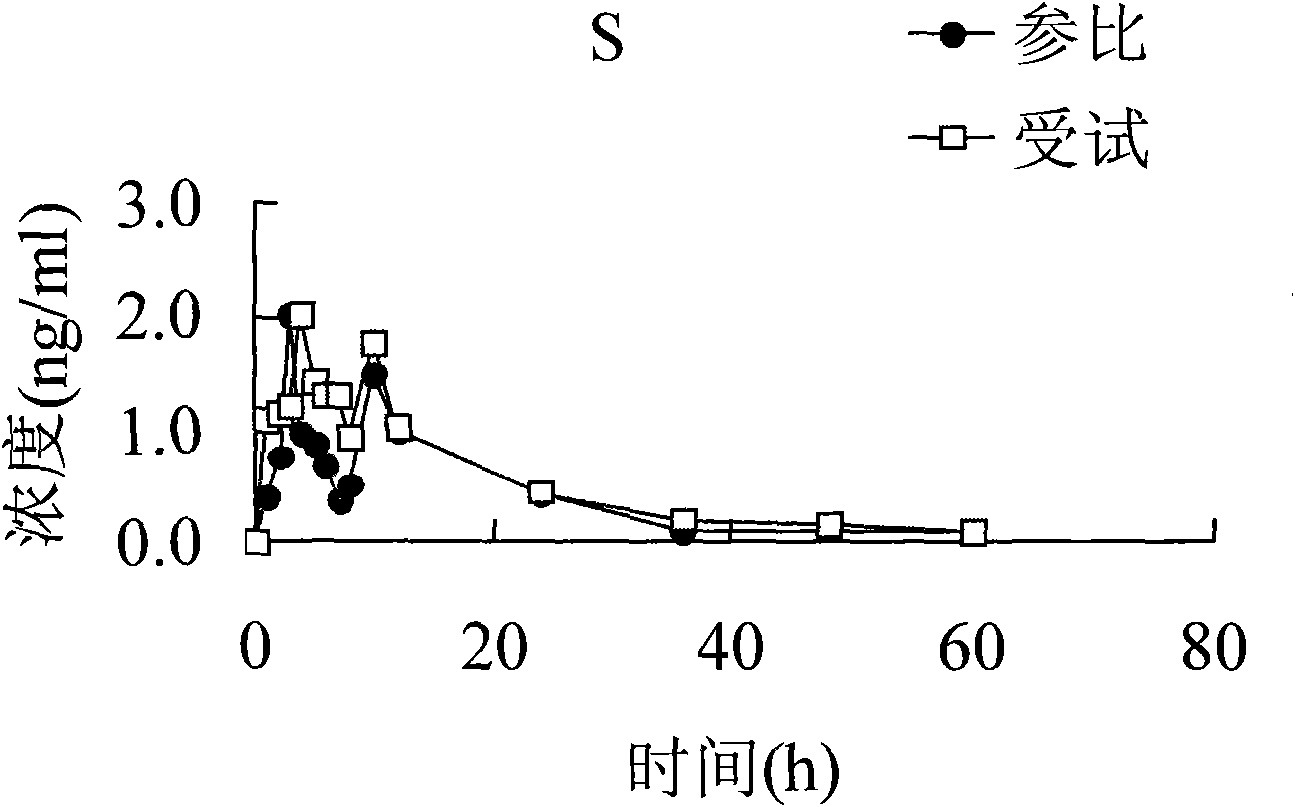
Carbamazepine sustained-release tablet and preparation method thereof
ActiveCN101647784AGood curative effectImprove securityNervous disorderPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention aims to provide a carbamazepine sustained-release tablet having higher medicament release stability and higher medication security. The carbamazepine sustained-release tablet is characterized by consisting of an effectively therapeutic dose of carbamazepine and physiologically acceptable pharmaceutic adjuvant, and has the characteristics of convenient administration, lasting effect,stable curative effect, small toxic and side effect and the like.
Owner:COSCI MED TECH CO LTD
Method and system for the prediction of cardiac arrhythmias
InactiveUS20060004413A1Raise the possibilityHeart stimulatorsSensorsAntiarrhythmic effectValproic Acid
Methods and systems are provided for determining an increased likelihood of the occurrence of a cardiac arrhythmia in a patient. The methods and systems comprise monitoring the sympathetic neural discharges of a patient from the left stellate ganglion, the thoracic ganglia, or both, and detecting increases in the sympathetic neural discharges. The methods and systems may further comprise delivering anti-arrhythmic therapy to the patient in response to a detected increase in the sympathetic neural discharge, such as delivering one or more pharmacological agents; stimulating myocardial hyperinnervation in the sinus node and right ventricle of the heart of the patient; and applying cardiac pacing, cardioversion or defibrillation shocks. Pharmacologic agents which may be used in connection with the delivery of anti-arrhythmic therapy include those which are known to exert anti-arrhythmic effect and anti-convulsant agents, such as phenyloin, carbamazepine, valproate, and phenobarbitone.
Owner:CEDARS SINAI MEDICAL CENT
Genetic testing for risk of causing serious adverse reactions of skin of carbamazepine
InactiveCN102108382AMicrobiological testing/measurementFluorescence/phosphorescenceAdverse drug reactionAntiepileptic drug
The invention discloses a genetic testing method for risk of causing serious adverse reactions of carbamazepine. The genetic testing method comprises the following steps: collecting oral mucosa cells of a subject, extracting genome DNA (deoxyribonucleic acid) of the oral mucosa cells, testing HLA (human leukocyte antigen)-B*1502 allelotype of the genome DNA and evaluating the risk of causing the serious adverse reactions of the skin of an anti-epileptic medicament-carbamazepine, thereby providing reference basis for clinical individual medication.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Absorbent comprising hydrophobic mesoporous material for removal of harmful pharmaceuticals from aqueous environment
ActiveUS20120172213A1High activityEliminate damageSilicon organic compoundsOther chemical processesTrimethoprimCarbamazepine
This invention relates to an absorbent including trimethylsilylated mesoporous silica SBA-15, and more particularly to an absorbent including trimethylsilylated mesoporous silica SBA-15, which can effectively remove 90% or more of the seven pharmaceuticals of carbamazepine, diclofenac, estrone, gemfibrozil, ibuprofen, ketoprofen, and trimethoprim which are present in high concentration.
Owner:GWANGJU INST OF SCI & TECH
Risk assessment for adverse drug reactions
ActiveUS20080227109A1Microbiological testing/measurementBiological testingFixed drug eruptionsMicrosatellite
The present invention provides a method of predicting the risk of a patient for developing adverse drug reactions, particularly SJS or TEN. It was discovered that an HLA-B allele, HLA-B* 1502, is associated with SJS / TEN that is induced by a variety of drugs. The correlation with HLA-B* 1502 is most significant for carbamazepine-induced SJS / TEN, wherein all the patients tested have the HLA-B* 1502 allele. In addition, another HLA-B allele, HLA-B*5801, is particularly associated with SJS / TEN induced by allopurinol. Milder cutaneous reactions, such as maculopapular rash, erythema multiforme (EM), urticaria, and fixed drug eruption, are particularly associated with a third allele, HLA-B *4601. For any of the alleles, genetic markers (e.g., HLA markers, microsatellite, or single nucleotide polymorphism markers) located between DRB 1 and HLA-A region of the specific HLA-B haplotype can also be used for the test.
Owner:ACAD SINIC
Method for removing drugs in soil by utilizing magnetism molecular imprinting-electromagnetism grating combination
InactiveCN103170496AAchieve recyclingSimple structureContaminated soil reclamationDrug ContaminationClofibric acid
The invention relates to a method for removing drugs in soil by utilizing magnetism molecular imprinting-electromagnetism grating combination. The drugs are one or more of carbamazepine, clofibric acid or diclofenac. The method comprises the following specific steps of: fully mixing preprocessed soil and a magnetism molecularly imprinted polymer, absorbing the drugs in the soil on the surface of the magnetism molecular imprinting, and separating the water soil and the magnetism molecularly imprinted polymer after reaction through an electromagnetism grid; and absorbing the magnetism molecular imprinting on the grid through a magnetic field generated by the electromagnetism grid after electrification, and effectively separating the magnetism molecular imprinting and the grid. The method provided by the invention has the advantages that the operation is simple, the effect is obvious, the arbamazepine, the clofibric acid and the diclofenac and the like in the soil are removed in one time, the drugs absorbed on the magnetism molecular imprinting are recovered and separated through the electromagnetism grid; a magnetism molecular imprinting technology is utilized to restore the soil polluted by the drugs and has the advantages that the cost is low, the restoring efficiency is high and the like; and an electromagnetism grid device is utilized to recycle the resources, so that secondary pollution is not caused.
Owner:TONGJI UNIV
Preparation method of high-purity carbamazepine (CBZ)-valaciclovir
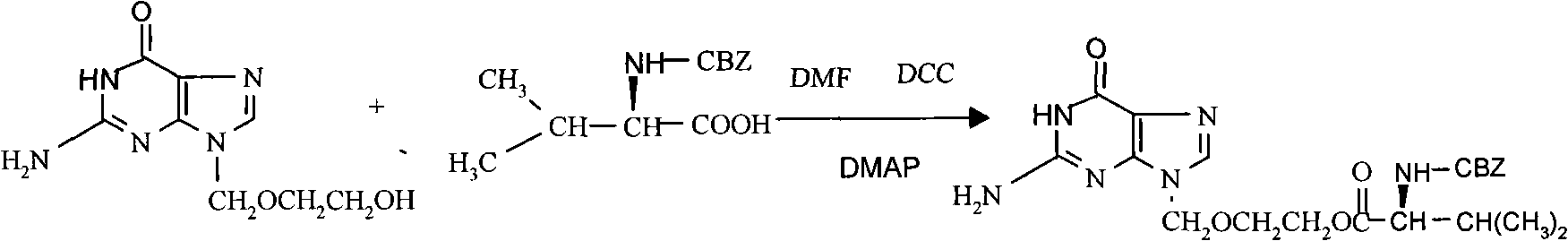
ActiveCN101787027AAvoid generating too muchShort reaction timeOrganic chemistryN dimethylformamideCarbamazepine
The invention relates to the technical field of pharmaceutical chemistry, in particular to a preparation method of high-purity carbamazepine (CBZ)-valaciclovir, which comprises the steps of: suspending acyclovir into N,N-dimethylformamide, adding CBZ-L-valine and 4-dimethylamino pyridine to obtain a turbid liquid, cooling the turbid liquid to 0-10 DEG C, adding dicyclohexylcarbodiimide, preserving the temperature for 1-5h, raising the temperature to 18-30 DEG C, controlling the temperature raising time to be 1-2h, filtering solid in a reaction system after carrying out heat preservation reaction for 10-20h at a temperature of 18-30 DEG C, decompressing and concentrating filtrate to obtain an oily matter, dissolving concentrate by heating through using alcohol, cooling crystal after being dissolved and filtering, recrystallizing and filtering the obtained solid by using alcohol, dissolving the obtained solid by heating through using the N,N-dimethylformamide with matched quantity to obtain a crude solution of the CBZ-valaciclovir, adding the solution into water with a temperature of 50-80 DEG C to make the solid to separate out, filtering, washing with water with a temperature of 50-80 DEG C to obtain a filter cake, and drying to obtain the high-purity CBZ-valaciclovir.
Owner:LIVZON GROUP CHANGZHOU KONY PHARMA
HLA-B*1502 detection kit
InactiveCN105296615AStrong specificityReduce the possibility of non-specific amplificationMicrobiological testing/measurementDNA/RNA fragmentationHLA-BCarbamazepine
The invention provides an HLA-B*1502 detection kit and belongs to the technical field of carbamazepine use detection. The HLA-B*1502 detection kit is characterized in that the Allele-Specific LAMP technology is used for designing specific primers aiming at the C allele of rs10484555, the relevance between the C allele of rs10484555 and HLA-B*1502 is used for judging whether a patient carries HLA-B*1502 allele or not, so that personalized carbamazepine use can be guided, and problems caused by direct testing for HLA-B*1502 are avoided. The HLA-B*1502 detection kit is low in cost, short in detecting time, high in accuracy and easy to popularize.
Owner:BEIJING JINQI BIOLOGICAL TECH CO LTD
Method for extracting efficient degrading bacteria of carbamazepine
InactiveCN101691558AHigh removal rateDoes not affect degradationBacteriaMicroorganism based processesInorganic saltsPurification methods
The invention relates to a method for extracting efficient degrading bacteria of carbamazepine, which comprises the following steps: (1) taking active sludge of a secondary sedimentation tank of an urban sewage treatment plant, inoculating the active sludge into an inorganic salt culture medium according to an inoculating volume of 5 weight percent, and acclimating the mixture by a gradient pressure type acclimation method for 2 months; and (2) standing a sample after the 2 months of the acclimation for 30 minutes, taking 1 milliliter of an upper layer water sample to perform gradient dilution, taking 1 milliliter of a bacterial solution of each diluted concentration respectively, inoculating the bacterial solution into a solid culture medium to perform enrichment culture so as to ensure that a pure single bacterial strain grows out, then inoculating the single bacterial strain into a slant culture medium, and preserving the mixture at a temperature of 4 DEG C. The method extracts the degrading bacteria of the carbamazepine from the active sludge of the secondary sedimentation tank of the urban sewage treatment plant, the strain source is easy to obtain and has a simple, convenient and quick separation and purification method and a low cost, and is environment-friendly; and the extracted strain has a degradation rate of over 75 percent to the carbamazepine, and has a good application prospect.
Owner:DONGHUA UNIV
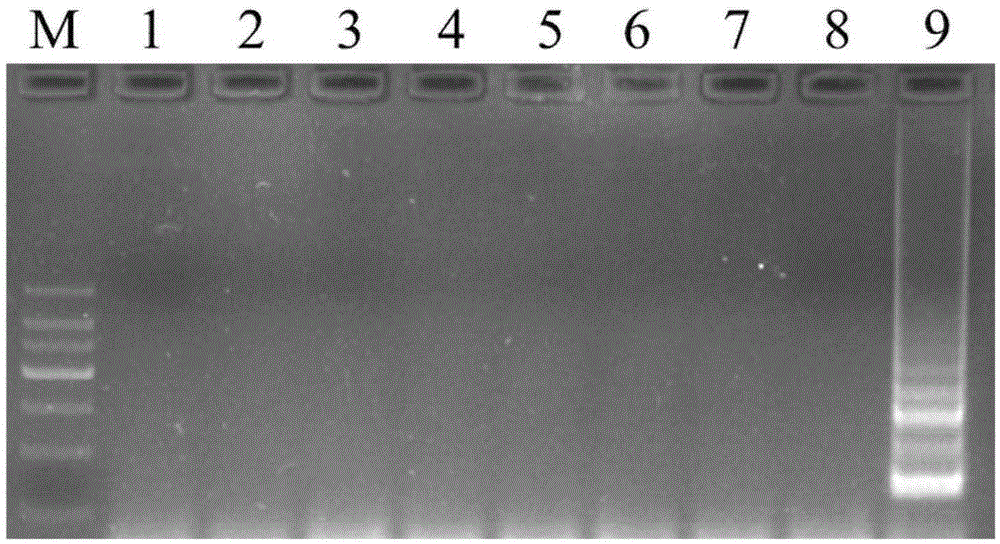
Method for qualitatively detecting HLA-B*1502 gene with PCR-SSP method and clinical kit
ActiveCN103114138ALow costLow instrument requirementsMicrobiological testing/measurementCarbamazepineMedication use
The invention belongs to the field of biotechnology, and particularly relates to a method for qualitatively detecting HLA-B*1502 gene with a PCR-SSP method and a clinical detection kit. The method comprises the following steps of: finding 6 specific areas capable of effectively identifying HLA-B*1502 allele type; designing 6 specific primers covering the 6 specific areas, and screening out a high-specificity primer pair applicable to PCR-SSP; and adding dUTP and UDG enzyme (uracil-DNA glycosylase) into a reaction system to solve the problem of PCR cross pollution and further improve the detection reliability. An HLA-B*1502 quick and convenient qualitative detection kit is researched and developed accordingly. The method and the clinical detection kit provided by the invention have the advantages of convenience in operation, short time, strong specificity, high accuracy, low cost and the like, and is suitable for realizing individual safe and reasonable drug use through HLA-B*1502 genotype detection before the Chinese or Asian epileptics take carbamazepine and phenytoin sodium.
Owner:GRACELL BIOTECH SHANGHAI CO LTD
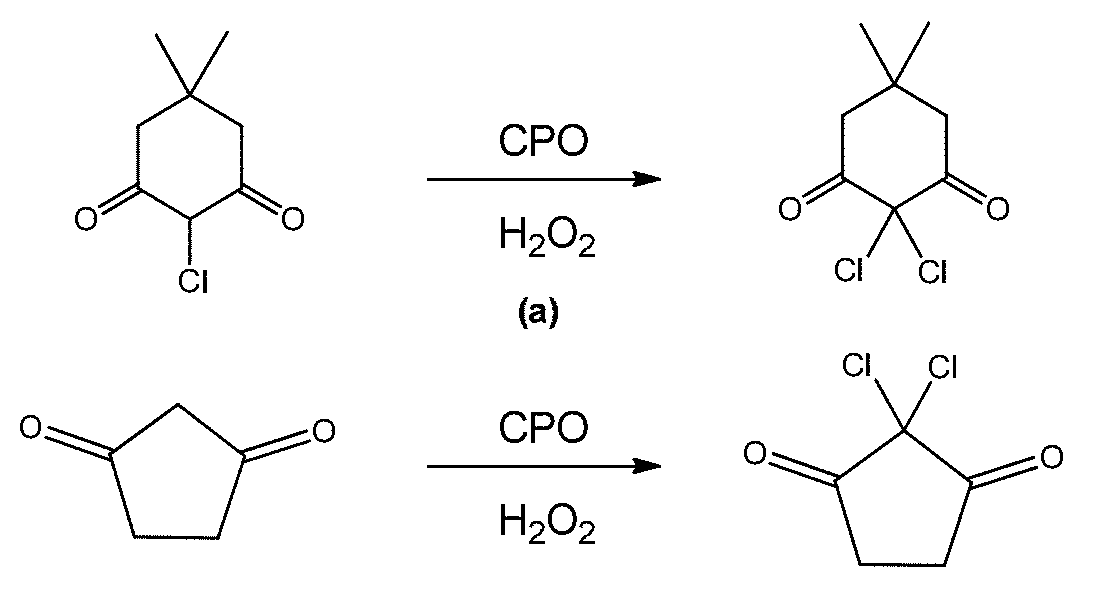
Chloroperoxidase-catalyzed degradation of pharmaceutical pollutants in wastewater
ActiveUS9938176B1Guaranteed economic efficiencyRemoving pollutants from water sourcesWater contaminantsOxidoreductasesWater sourceWastewater
The present invention provides efficient, economical, and environmentally-friendly compositions and methods for removing pollutants from water sources. In particular embodiments, the present invention provides compositions and methods for catalyzing the degradation of pharmaceutical pollutants in wastewater using the enzyme chloroperoxidase (CPO). Another embodiment provides a method of degrading pollutants in wastewater and other water sources. In specific embodiments, the claimed composition and method can be used to degrade pharmaceutical pollutants selected from the group consisting of: acetaminophen, carbamazepine, sulfamethazine, diclofenac, and naproxen.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Transdermal delivery of dicolfenac, carbamazepine and benzydamine
The invention discloses solutions of diclofenac, carbamazepine and benzydamine, at therapeutically desirable concentrations and the solutions stable for extended periods of time at room temperature.
Owner:KYDES PHARMA
Kit and detection method for accurately determining blood concentration of multiple antiepileptic drugs in human serum
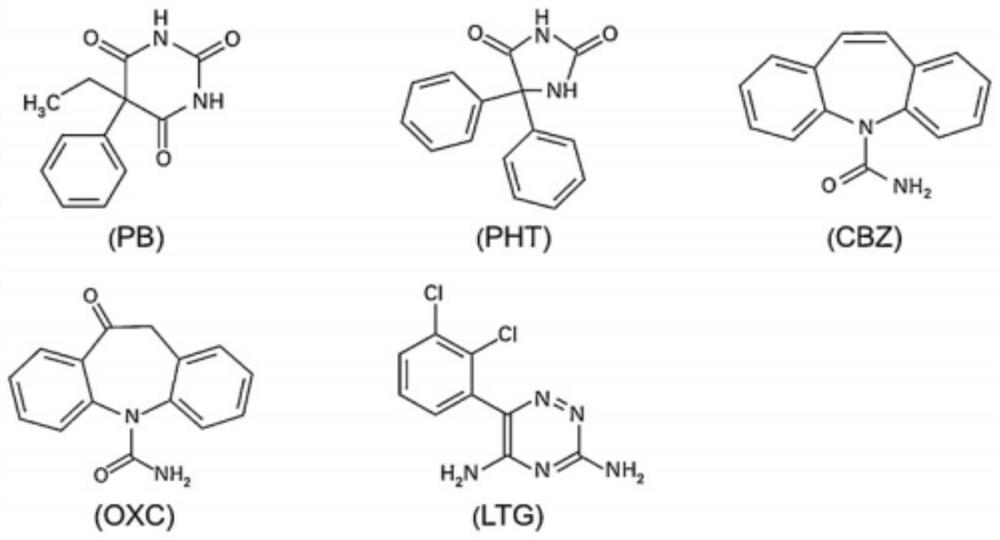
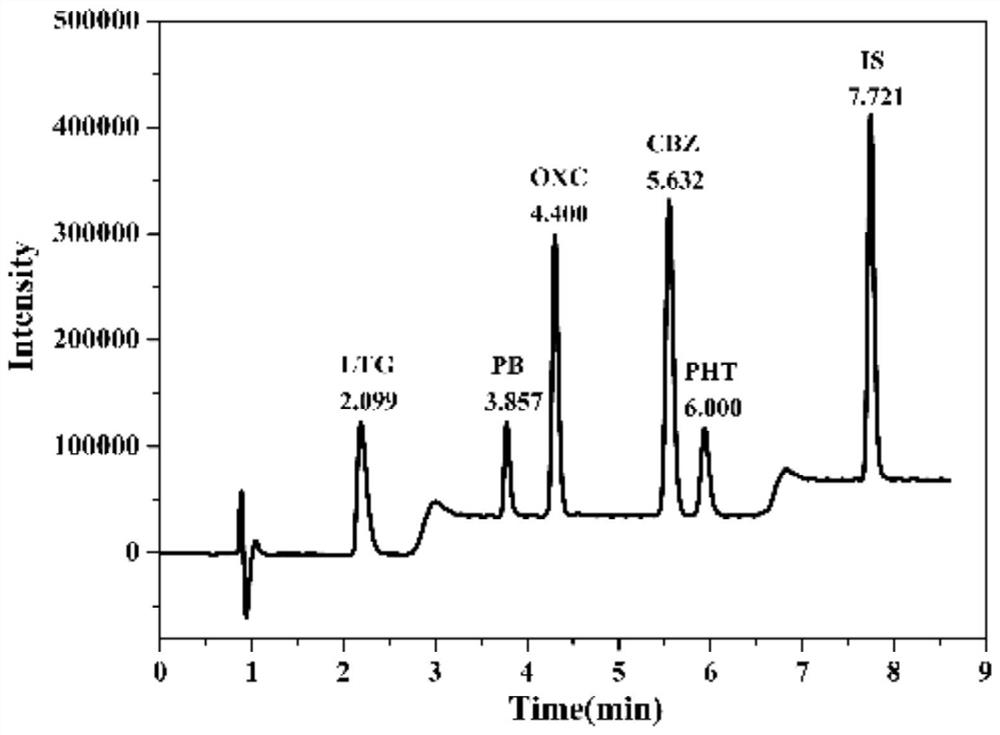
The invention discloses a kit and a detection method for accurately determining the blood concentration of multiple antiepileptic drugs in human serum. The kit mainly comprises a calibration product mother liquor, methanol or acetonitrile is used as a diluent to prepare the calibration product mother liquor comprising six concentration points, the calibration product mother liquor includes lamotrigine, phenobarbital, oxcarbazepine, carbamazepine and phenytoin in order; the kit also comprises an internal standard, a quality control material, a matrix correction solution and an extract, whereinthe extract is a tert-butyl methyl ether solution, a reconstitution solution and a mobile phase. Complex purification steps are not needed, and the required analysis time is short; and the detection speed is increased.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com