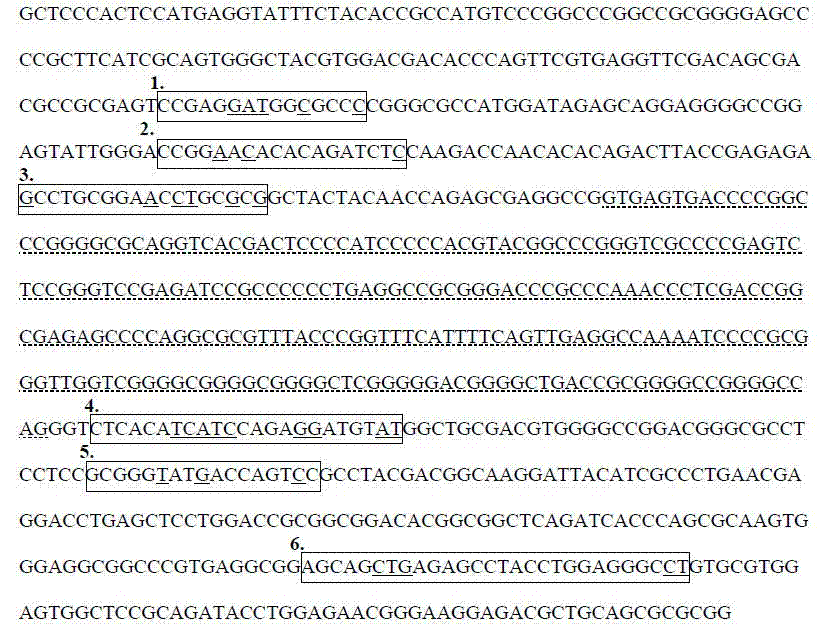Method for qualitatively detecting HLA-B*1502 gene with PCR-SSP method and clinical kit
A technology of B1502-SSP-F2 and B1502-SSP-R5, which is applied in the field of qualitative detection of HLA-B*1502 gene and clinical detection kits, can solve problems such as damage, pollution, and mental decline of children, and achieve control of false positives , increase the pollution system, and reduce the effect of false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0036] 1. Extract the genomic DNA of the subject's whole blood, saliva or other tissues according to the manufacturer's instructions.
[0037] 2. Main reagents: HLA-B*1502 clinical kit reagents. The main components include 2×PCR Mix (Roche), dUTP, UDG enzyme (Thermo Scientific).
[0038] 3. Reaction system and PCR program.
[0039] Configure 12 μl PCR reaction system: including 11 μl kit reagents, 1 μl (1-10ng) of subject sample DNA. PCR reaction program: first incubate at 37°C for 3min, then hot start at 95°C for 10min, 1 cycle; then denature at 95°C for 20sec, extend at 68°C for 50sec, a total of 40 cycles; finally, 72°C for 5min.
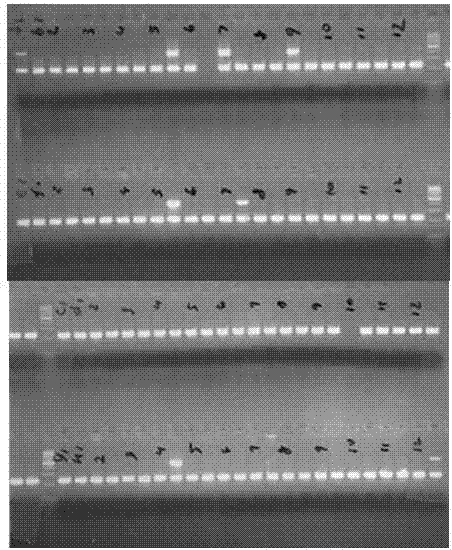
[0040] 4. Separation of PCR fragments: 2% agarose gel electrophoresis detection, electric field strength not higher than 5V / cm, time 20-30min, gel imaging system imaging. If there is an HLA-B*1502-specific band with a length of 430 bp bases and an internal reference control band with a length of 219 bp bases, the tested sample is positive for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com