Patents
Literature
4925 results about "Saliva" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Saliva is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is 99.5% water plus electrolytes, mucus, white blood cells, epithelial cells (from which DNA can be extracted), enzymes (such as amylase and lipase), antimicrobial agents such as secretory IgA, and lysozymes.
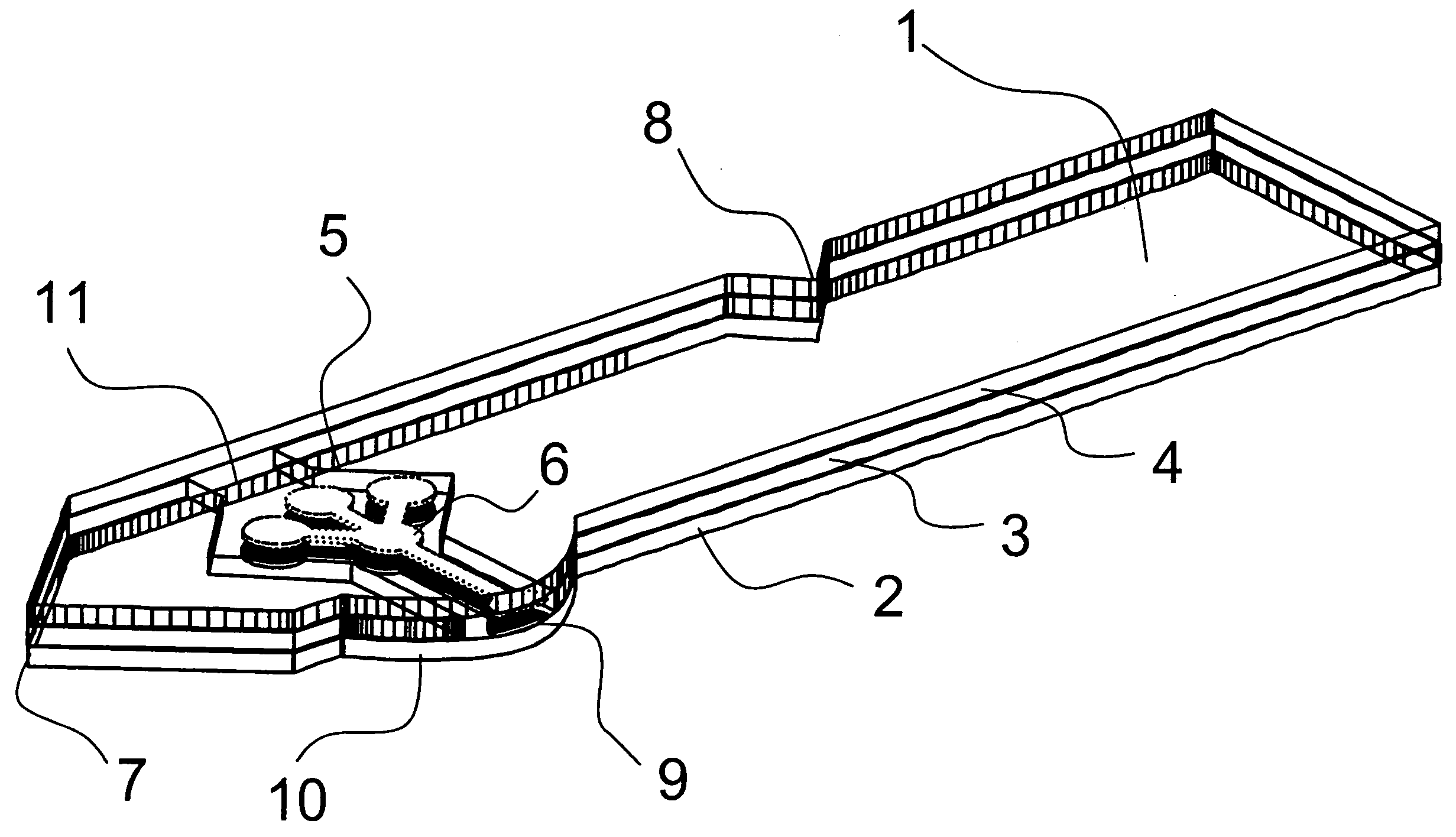
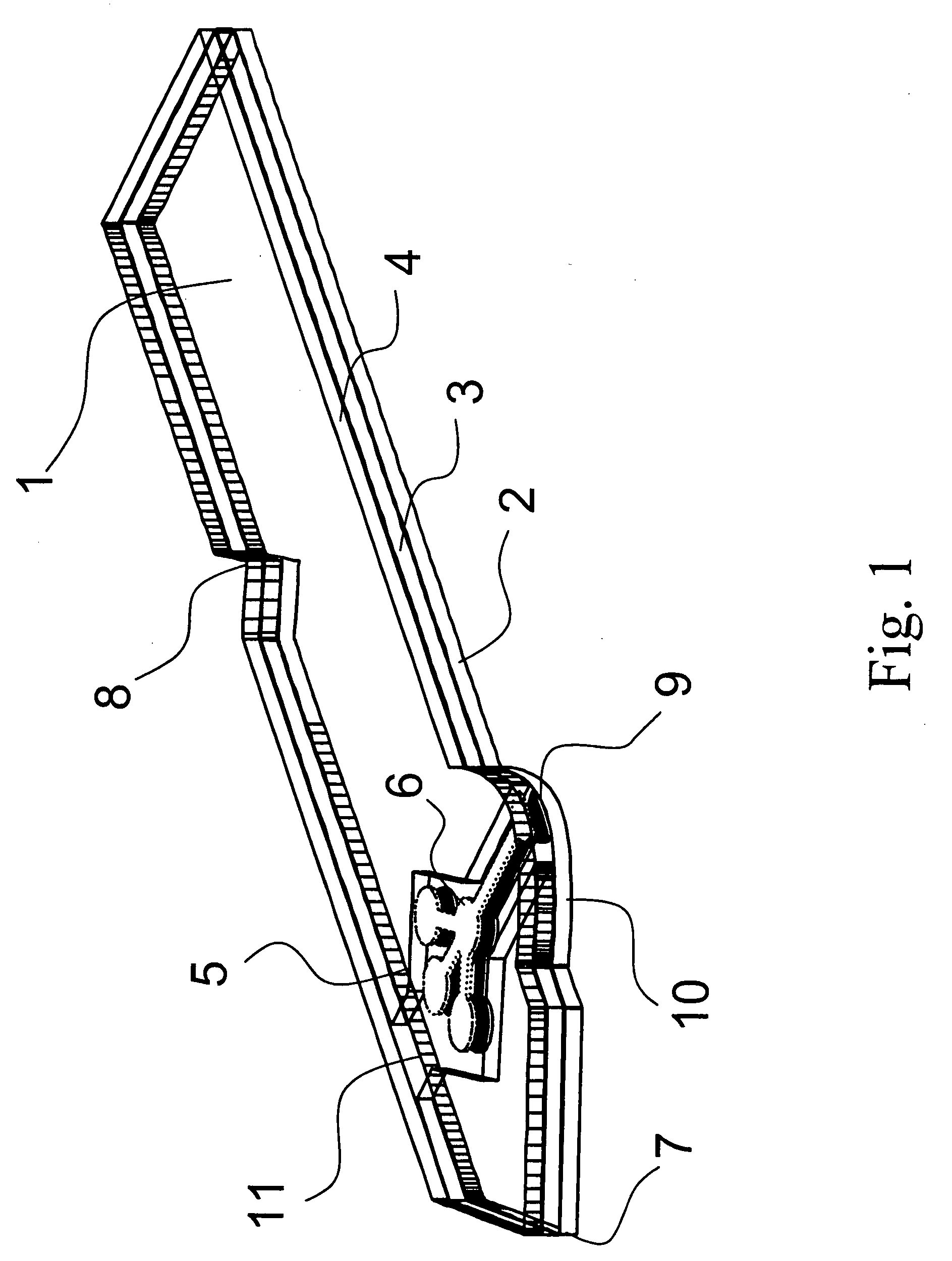
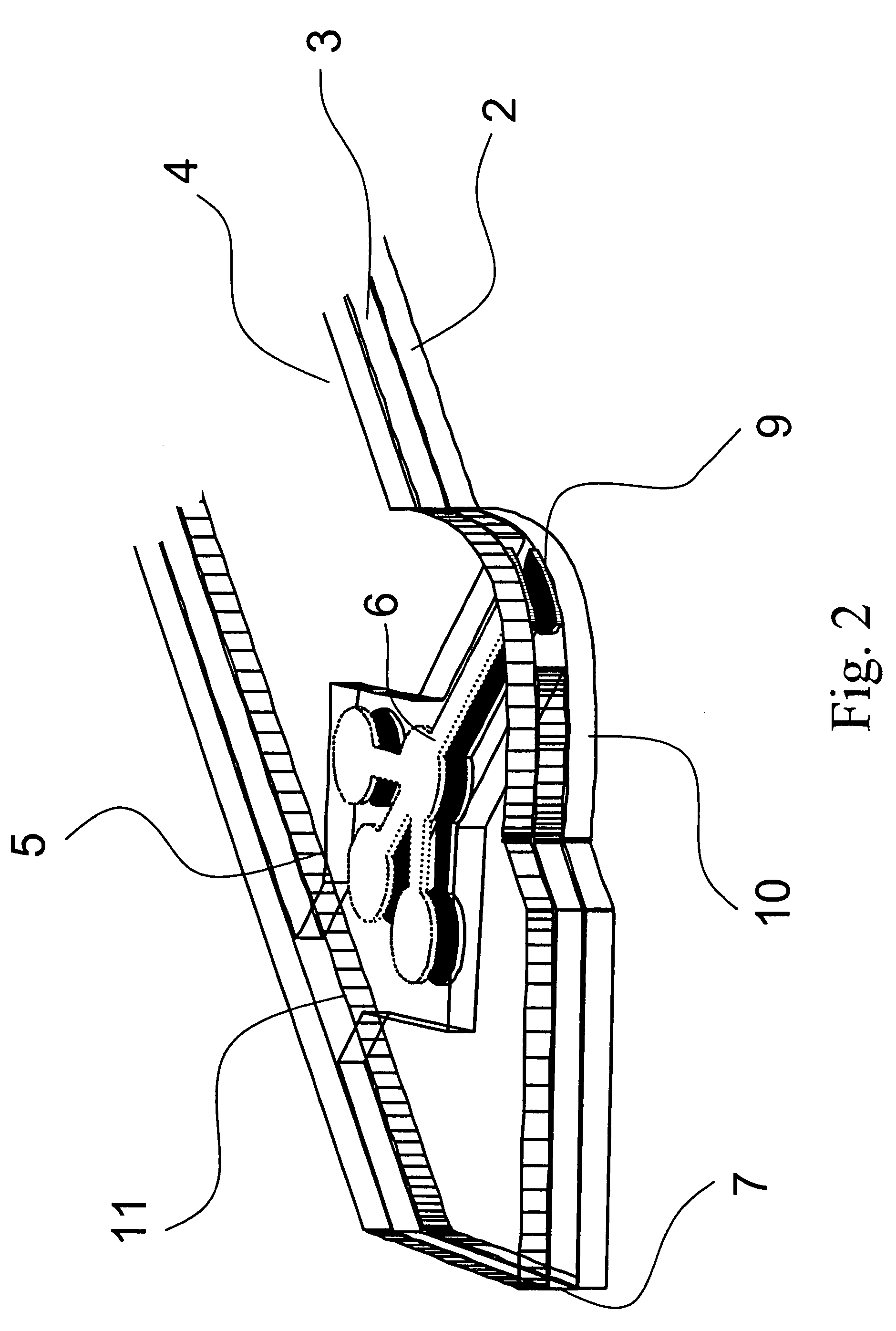
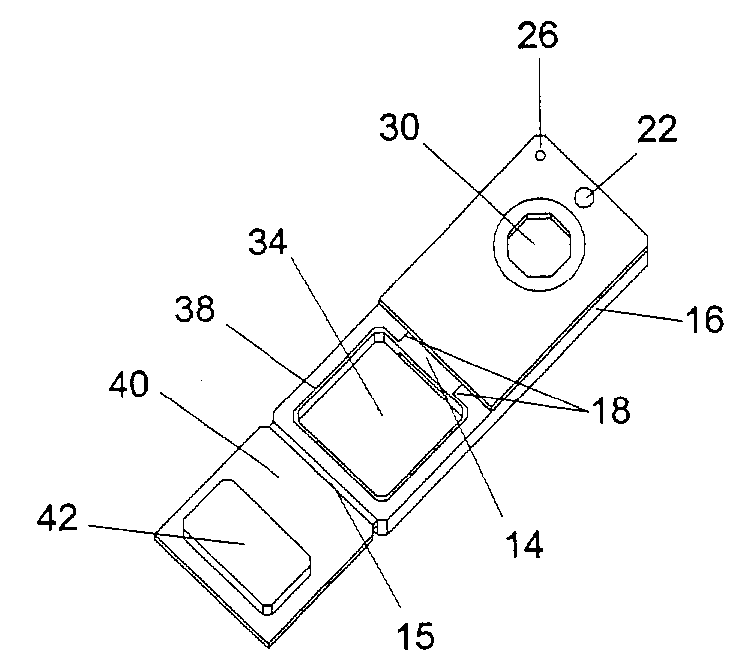
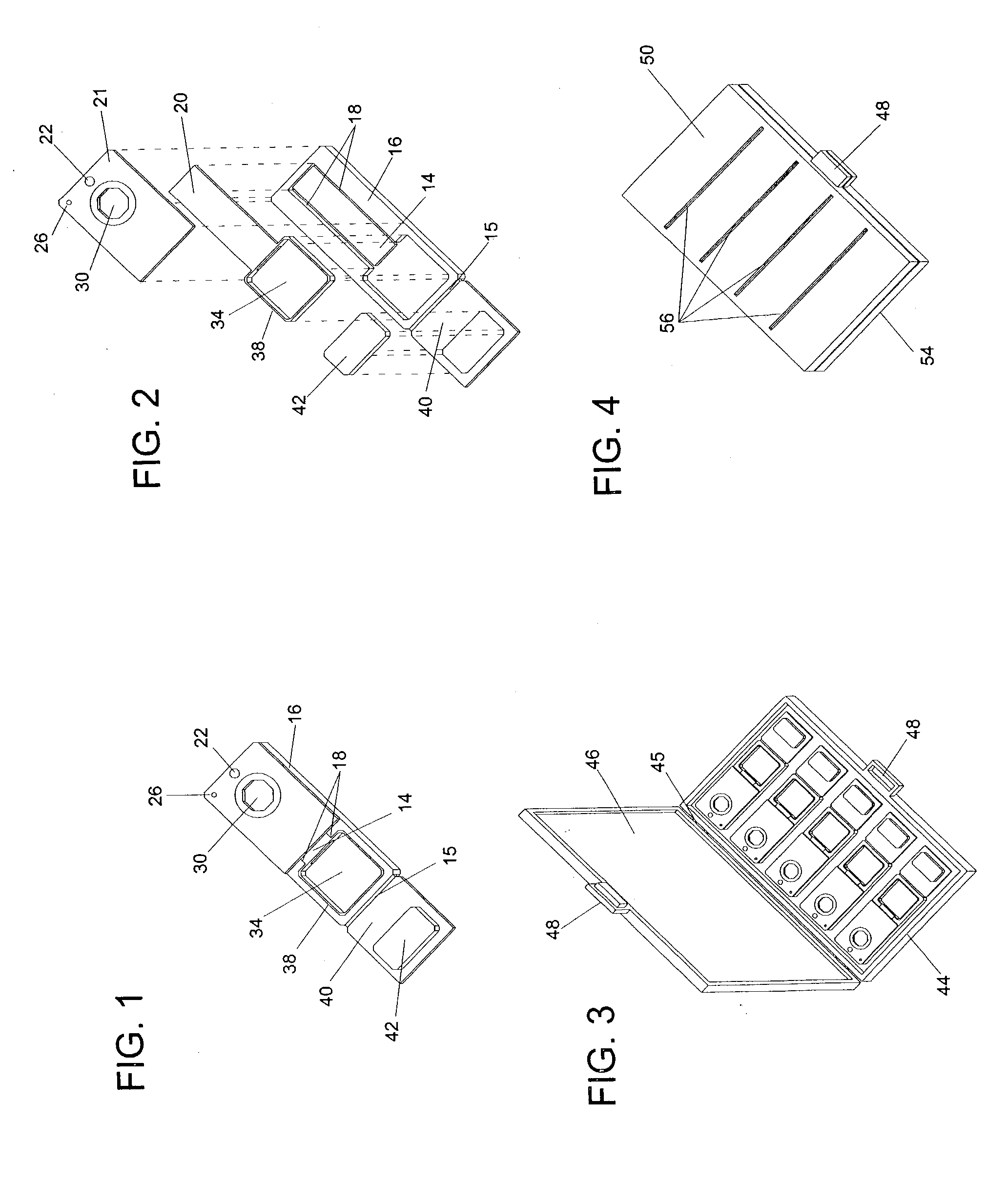
Devices, systems and methods for minimally invasive surgery of the throat and other portions of mammalian body
ActiveUS20050059960A1Simple structureLittle precisionMechanical apparatusCannulasThroatLess invasive surgery
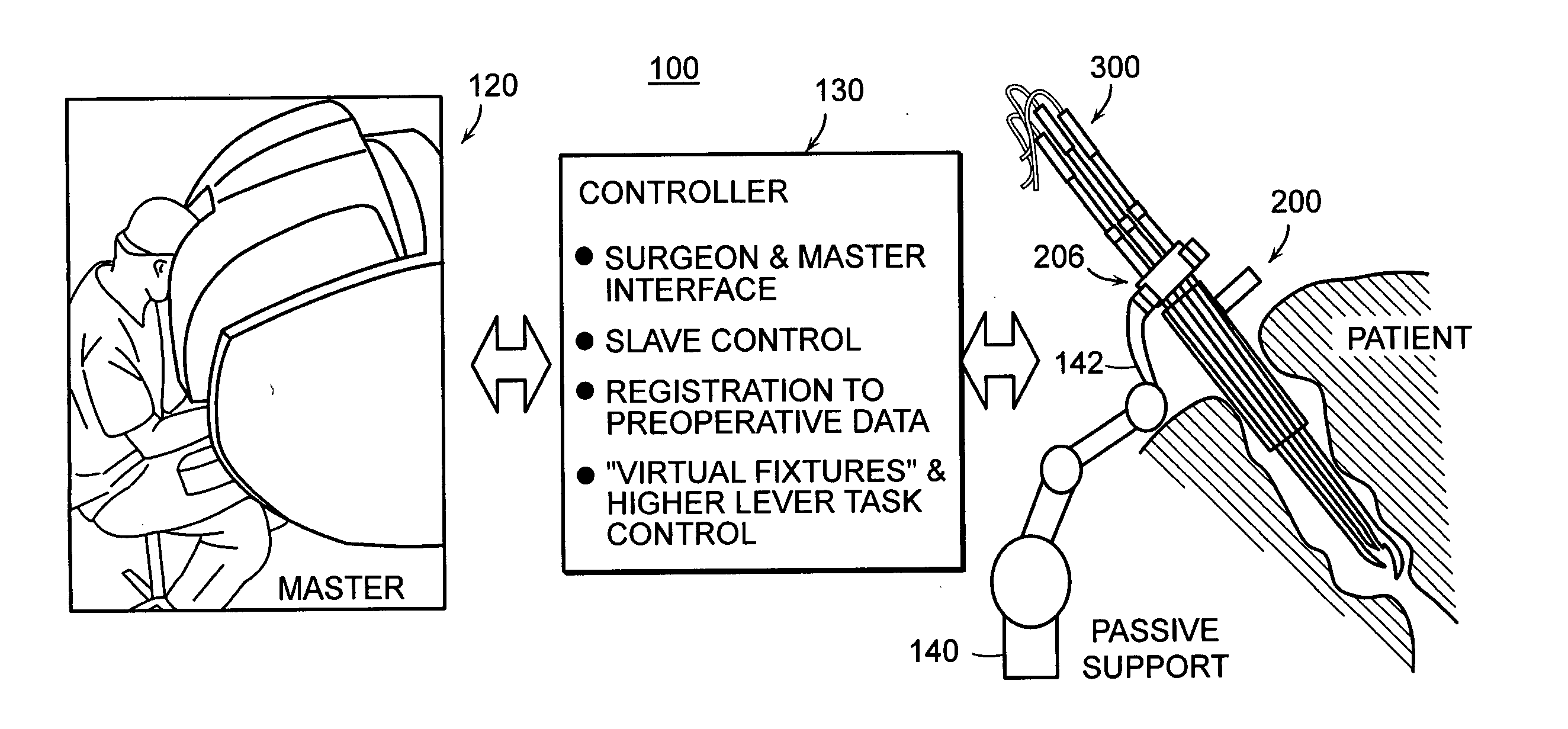
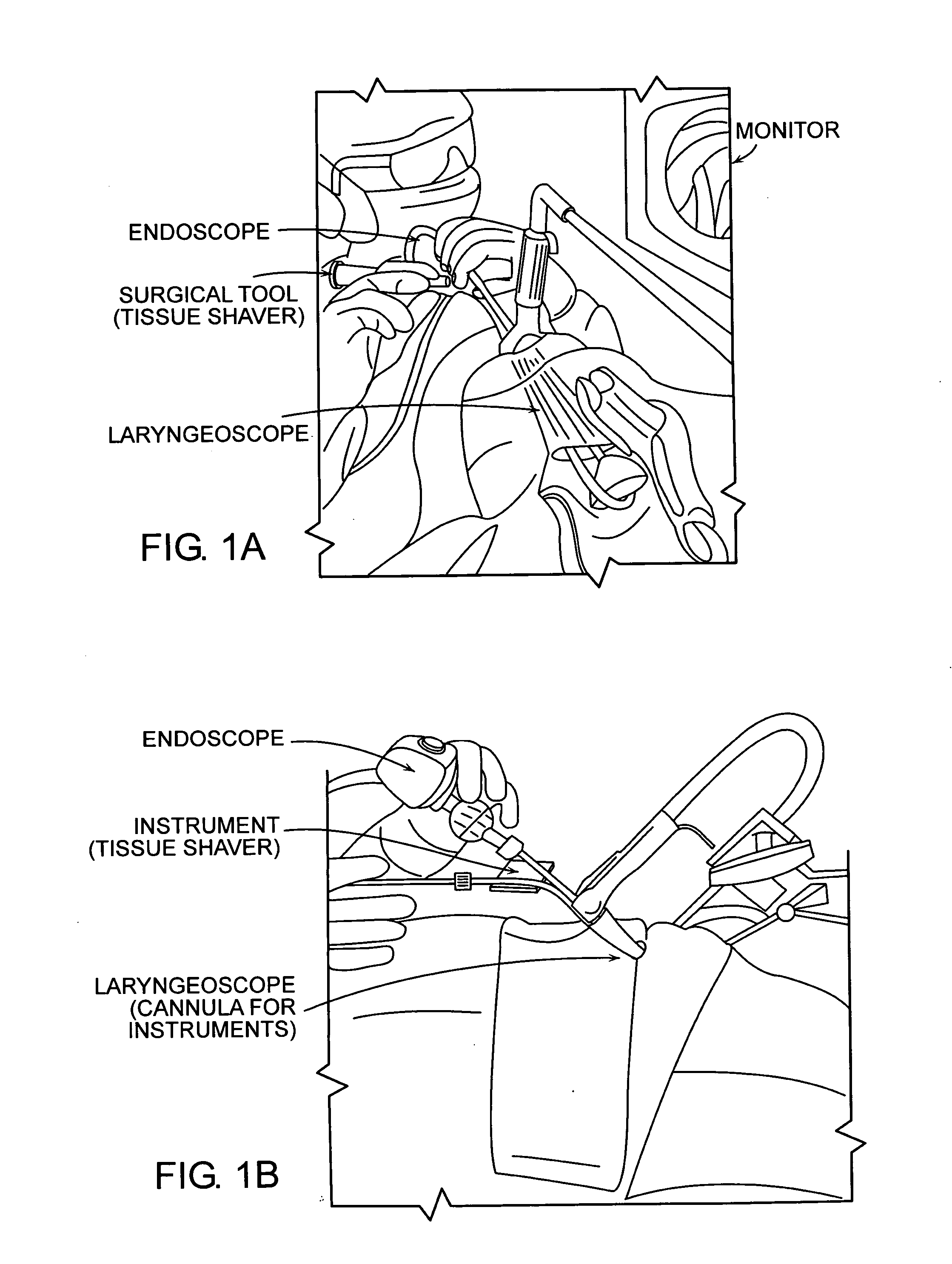
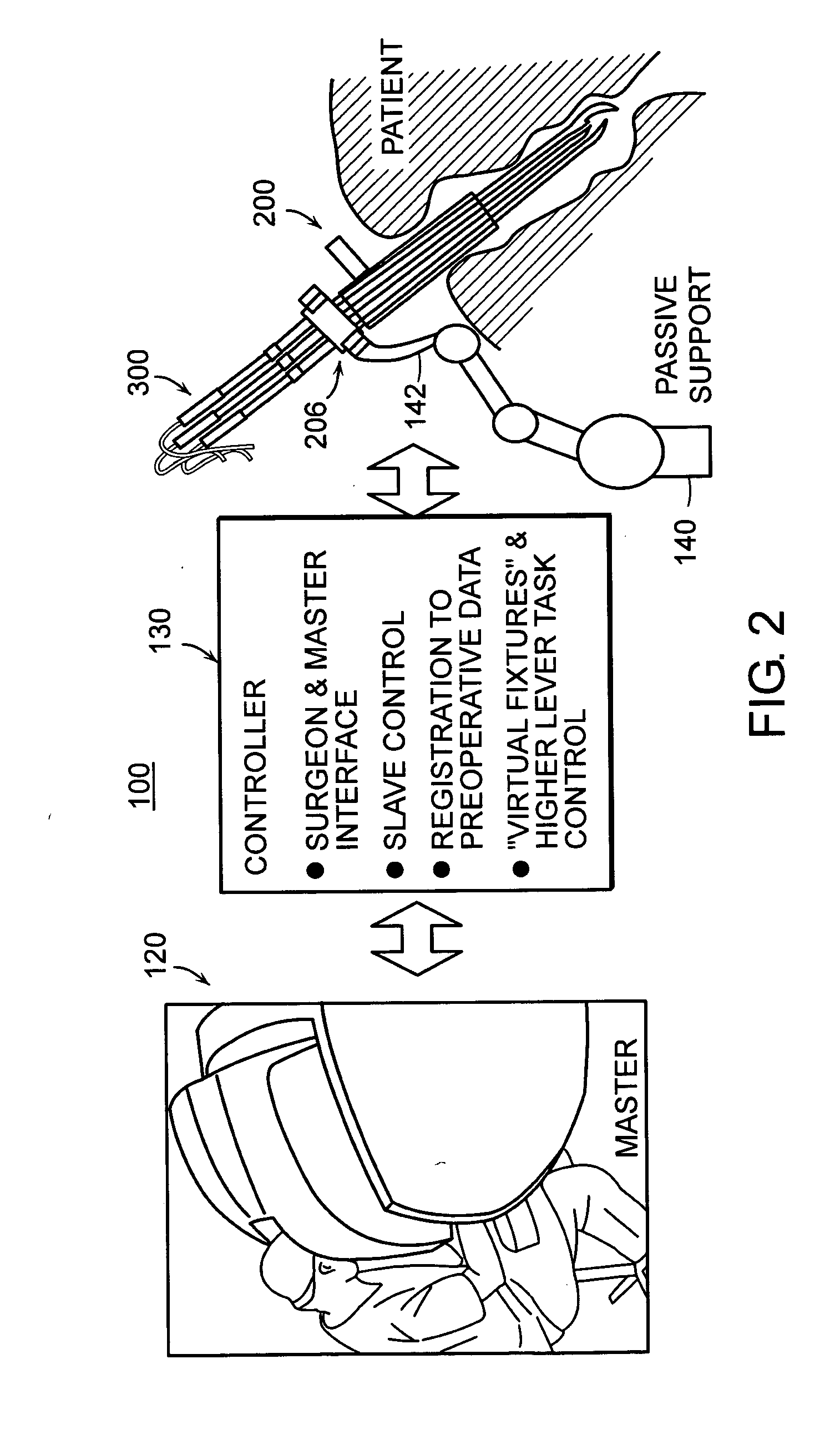
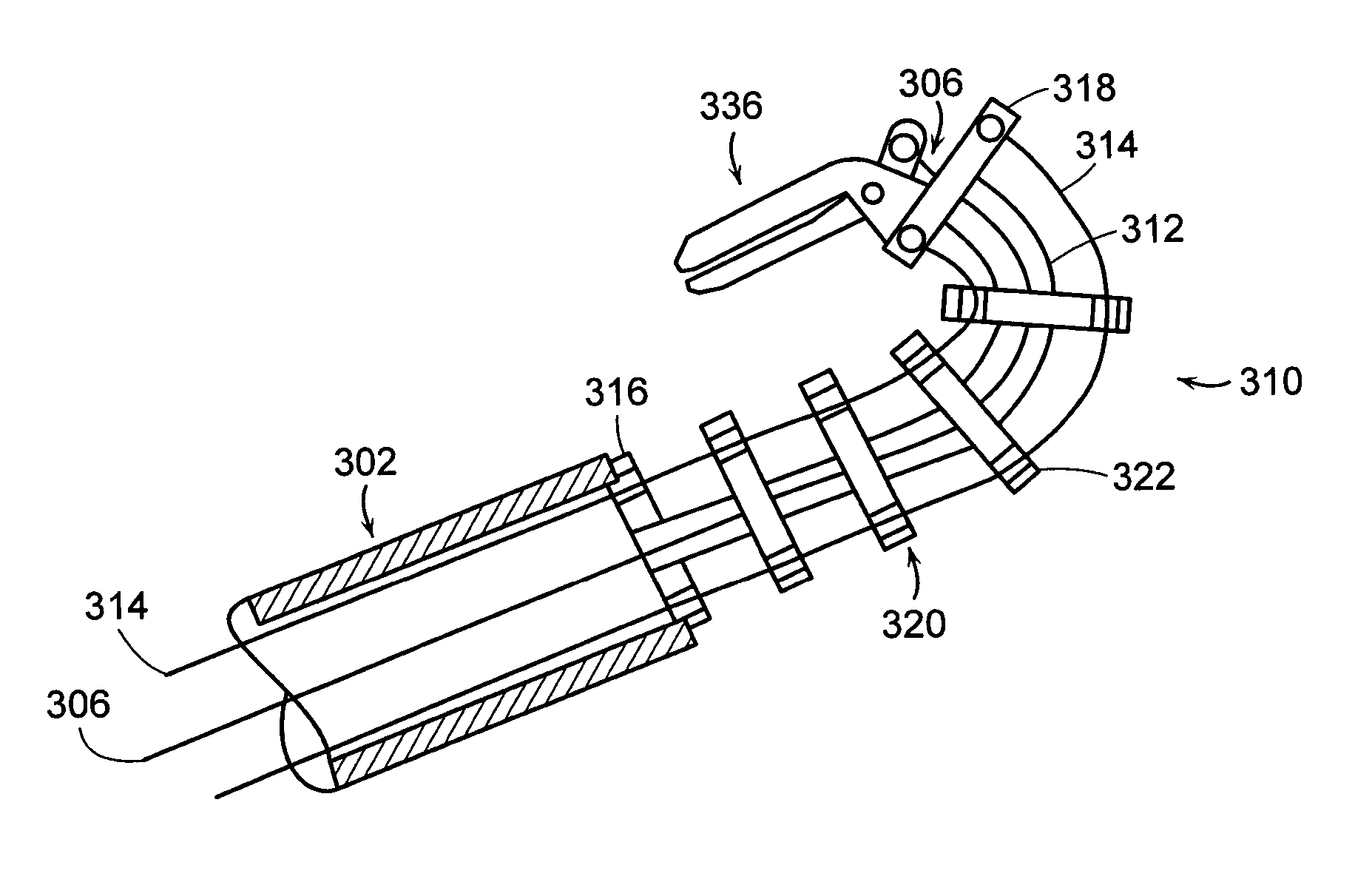
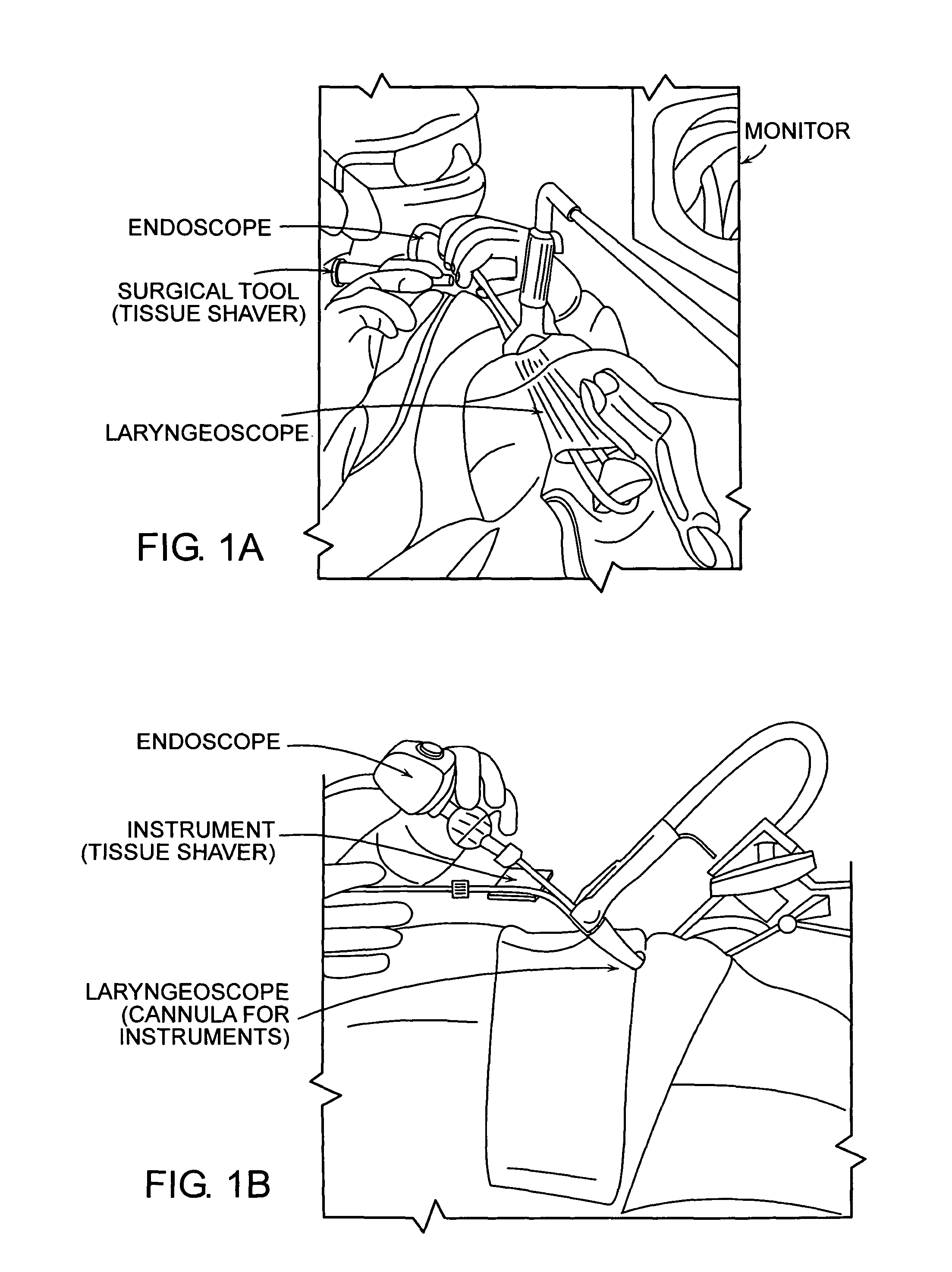
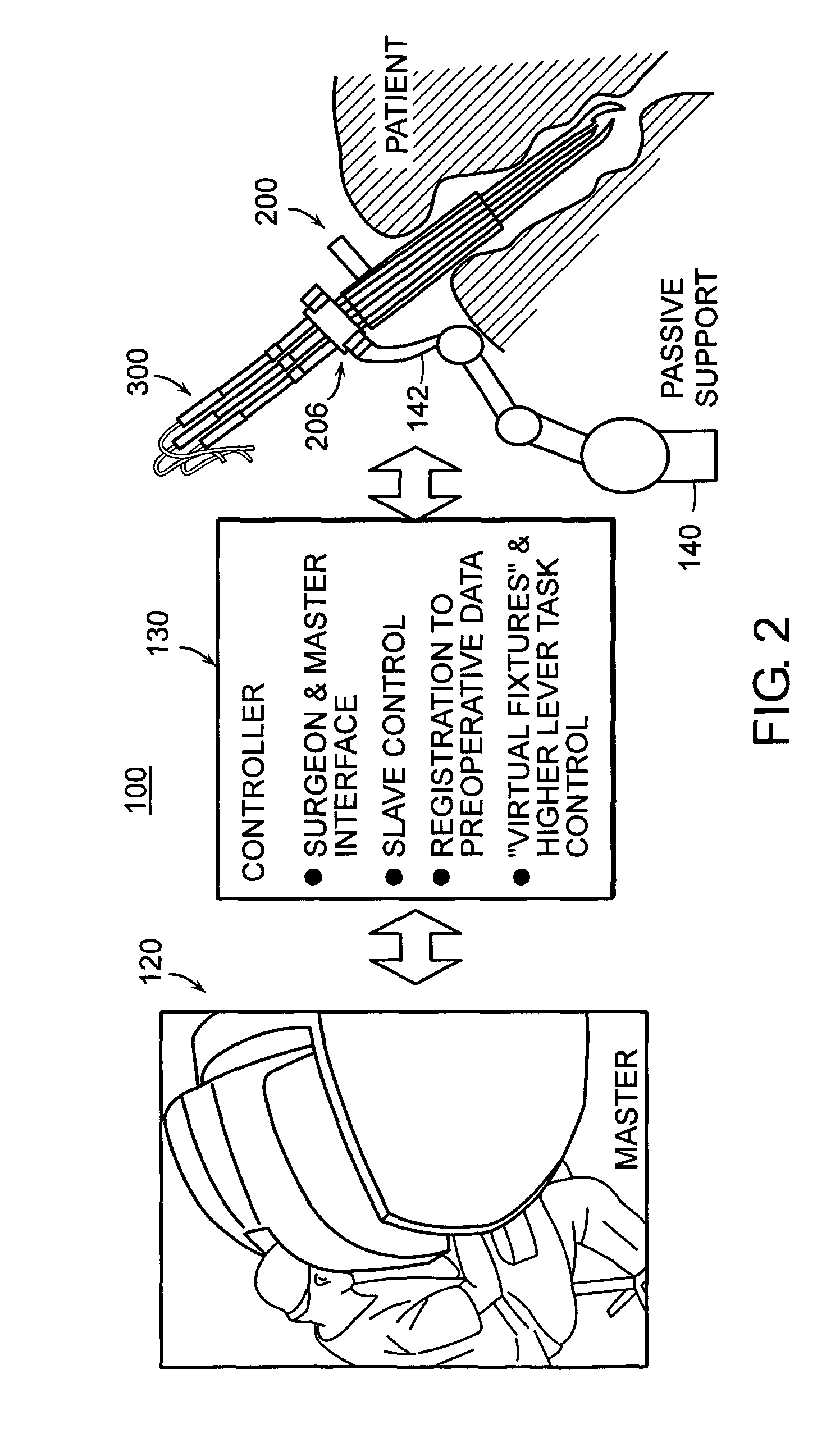
Featured are systems, devices and apparatuses for use in minimally invasive surgical, diagnostic or therapeutic methods and / or techniques, in particular methods and / or techniques for a mammalian throat. In particular embodiments, a dexterity apparatus including one or more dexterity devices is featured, where each of the dexterity devices comprises surgical tools and each is configured and arranged with end-tip dexterity for enhanced manipulation. A portion of the dexterity devices is snake like, which is re-configurable (i.e., can be bent) so as to in effect maneuver the surgical tool and put the tool in a desired position with respect to the surgical site. Another portion of the dexterity device includes the surgical tool thereby providing the capability of performing surgical actions such as sewing, gripping, soft tissue manipulation, cutting and suction of saliva, blood and other materials from the surgical site.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Devices, systems and methods for minimally invasive surgery of the throat and other portions of mammalian body
ActiveUS8365633B2Simple structureLittle precisionMechanical apparatusCannulasLess invasive surgeryThroat
Featured are systems, devices and apparatuses for use in minimally invasive surgical, diagnostic or therapeutic methods and / or techniques, in particular methods and / or techniques for a mammalian throat. In particular embodiments, a dexterity apparatus including one or more dexterity devices is featured, where each of the dexterity devices comprises surgical tools and each is configured and arranged with end-tip dexterity for enhanced manipulation. A portion of the dexterity devices is snake like, which is re-configurable (i.e., can be bent) so as to in effect maneuver the surgical tool and put the tool in a desired position with respect to the surgical site. Another portion of the dexterity device includes the surgical tool thereby providing the capability of performing surgical actions such as sewing, gripping, soft tissue manipulation, cutting and suction of saliva, blood and other materials from the surgical site.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
High titer production of highly linear poly (alpha 1,3 glucan)
ActiveUS20130244287A1Improve mechanical propertiesMore amenable to preparing fibersTransferasesFermentationSucroseSaccharophagus degradans
Owner:NUTRITION & BIOSCIENCES USA 4 INC
Process for discriminating between biological states based on hidden patterns from biological data
The invention describes a process for determining a biological state through the discovery and analysis of hidden or non-obvious, discriminatory biological data patterns. The biological data can be from health data, clinical data, or from a biological sample, (e.g., a biological sample from a human, e.g., serum, blood, saliva, plasma, nipple aspirants, synovial fluids, cerebrospinal fluids, sweat, urine, fecal matter, tears, bronchial lavage, swabbings, needle aspirantas, semen, vaginal fluids, pre-ejaculate.), etc. which is analyzed to determine the biological state of the donor. The biological state can be a pathologic diagnosis, toxicity state, efficacy of a drug, prognosis of a disease, etc. Specifically, the invention concerns processes that discover hidden discriminatory biological data patterns (e.g., patterns of protein expression in a serum sample that classify the biological state of an organ) that describe biological states.
Owner:ASPIRA WOMENS HEALTH INC +1
Handheld diagnostic device with renewable biosensor
A handheld diagnostic device having a test head and a handle is equipped with an open test channel having sensors and liquid reagent dispensing opening for the diagnostic testing of body fluids. The test channel can draw in fluid sample by capillary force and be closed by a channel cover for mixing the fluid sample with liquid reagent for electrochemical reactions for providing measurement signals for diagnostic analysis by a microprocessor included in the handle. A vibration means is added for stimulating the production of the body fluid sample and for assisting mixing of the sample solution. A renewable biosensor having a reusable electrode system and a dispensing means for providing a new dose of liquid reagent is included in the test head for repeated uses of the test channel and the biosensor. A dual-dispensers system having two reagent cartridges and two dispensing lines is included for simultaneous or selective dispensing of reagents for multiple diagnostic testing. The handheld device can be used for the self-diagnostic testing of saliva, body fluid, blood and vagina fluid for home healthcare and for monitoring predetermined components in a pourable fluid. For vagina fluid applications, a handheld diagnostic device may include cream or foam dispenser for dispensing vagina medication material, lubricant, or spermicide.
Owner:KUO YOUTI
Intraoral apparatus for non-invasive blood and saliva monitoring & sensing
Controlled-specimen-sampling oral devices are described, implanted or inserted into an oral cavity, built onto a prosthetic tooth crown, a denture plate, braces, a dental implant, or the like. The devices are replaced as needed. The controlled specimen sampling may be passive, based on a dosage form, or electro-mechanically controlled, for a high-precision, intelligent, specimen sampling. Additionally, the controlled sampling may be any one of the following: sampling in accordance with a preprogrammed regimen, sampling at a controlled rate, delayed sampling, pulsatile sampling, chronotherapeutic sampling, closed-loop sampling, responsive to a sensor's input, sampling on demand from a personal extracorporeal system, sampling regimen specified by a personal extracorporeal system, sampling on demand from a monitoring center, via a personal extracorporeal system, and sampling regimen specified by a monitoring center, via a personal extracorporeal system. Specimen collection in the oral cavity may be assisted or induced by a transport mechanism, such as any one of, or a combination of iontophoresis, electroosmosis, electrophoresis, electroporation, sonophoresis, and ablation. The oral devices require replacement at relatively long intervals of weeks or months. The oral devices and methods for controlled specimen sampling apply to humans and animals.
Owner:BEISKI BEN ZION +1
Probe set and kit for detecting whole exons of extended genetic diseases and application of probe set
InactiveCN110499364AComprehensive diagnostic extended whole exome testingIncrease positive rateMicrobiological testing/measurementLibrary creationFresh TissueExon
The invention discloses a probe set for detecting whole exons of extended genetic diseases. The probe set for detecting the whole exons of the extended genetic diseases comprises a standard whole exonprobe set, a whole genome copy number variation probe, and a mitochondrial loop full-length probe, and the genetic diseases comprise 6161 genetic diseases; the standard whole exon probe set can detect the genetic diseases caused by whole exon mutation, the genetic diseases comprise nervous system diseases, metabolic system diseases, endocrine system diseases, digestive system diseases, skeletal system diseases, urinary system diseases, immune system diseases, cardiovascular system diseases, blood system diseases, integument system diseases, ophthalmic system diseases, ear system diseases, respiratory system diseases, and genital system diseases; and the density of the mitochondrial probe is 6X; test samples comprise blood, fresh tissue, FFPE samples, and saliva. The invention discloses using method and kit and application of the probe set for detecting the whole exons of the extended genetic diseases.
Owner:北京凯昂医学诊断技术有限公司
Analyte test system for determining the concentration of an analyte in a physiological or aqueous fluid
InactiveUS20050196747A1Cheap productionReliable resultsBioreactor/fermenter combinationsBiological substance pretreatmentsSmall sampleQuality control system
This invention provides a device for determining the concentration of an analyte like glucose, cholesterol, free fatty acids, triglycerides, proteins, ketones, phenylalanine or enzymes, in a physiological or aqueous fluid like blood, serum, plasma, saliva, urine, interstitial and / or intra-cellular fluid, the device having an integrated calibration and quality control system suitable for dry reagent test strips with a very small sample volume of about 0.5 μL based on to a new sample distribution system. The production of the inventive analyte test element involves only a small number of uncomplicated production steps enabling an inexpensive production of the strips.
Owner:EGOMEDICAL SWISS
Strip for whitening tooth surfaces
InactiveUS6514483B2Inhibition releaseCosmetic preparationsImpression capsWhitening AgentsEthylene oxide
A thin, flexible film which when applied to stained teeth is hydrated by saliva and is effective in such form to whiten teeth, the film comprising an anhydrous water hydratable ethylene oxide polymer matrix containing a solid peroxide whitening agent whereby upon application to stained tooth surfaces, the peroxide whitening agent is solublilized by saliva present in the oral cavity into active whitening activity when the film is positioned and placed on the teeth.
Owner:COLGATE PALMOLIVE CO
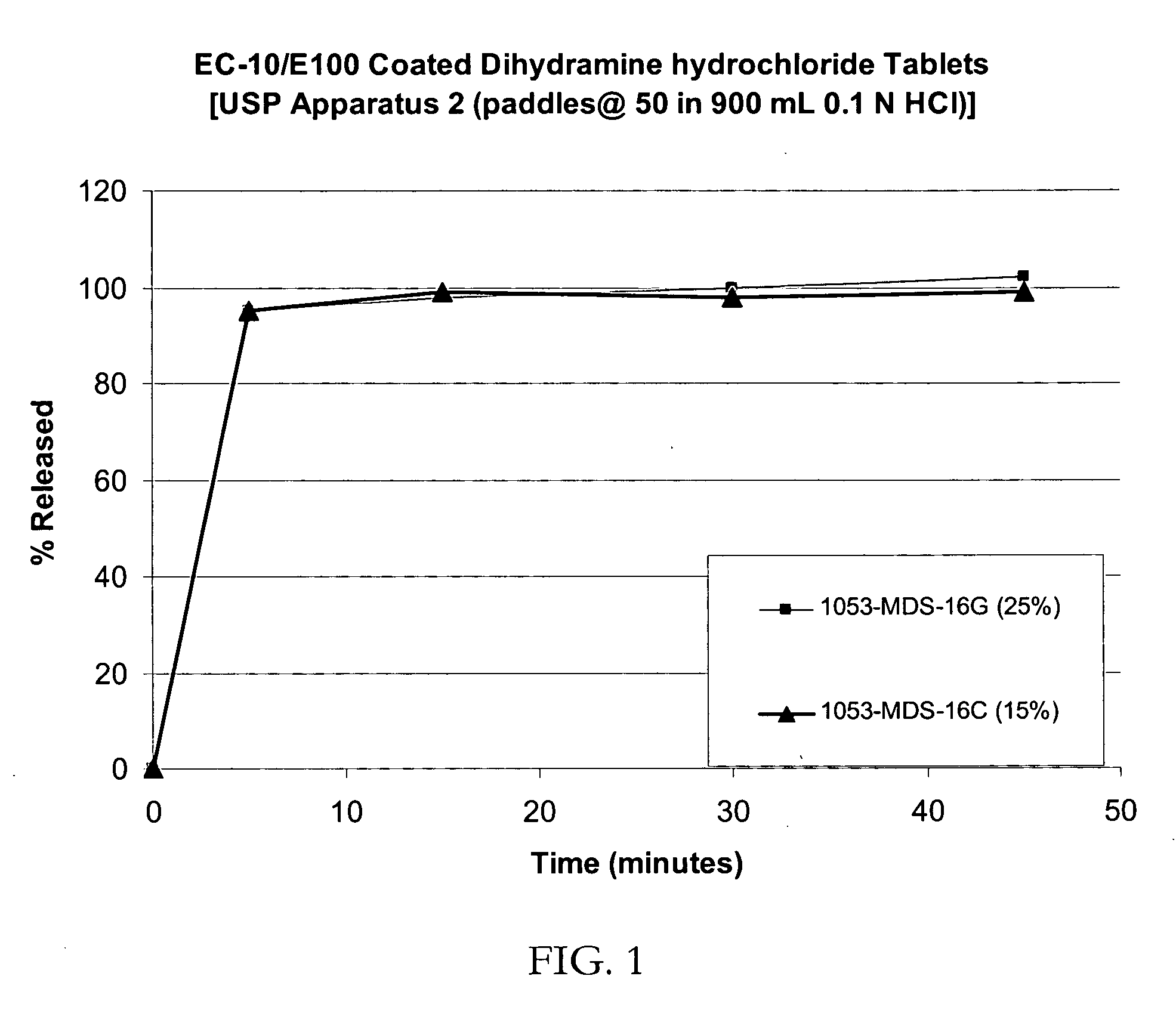
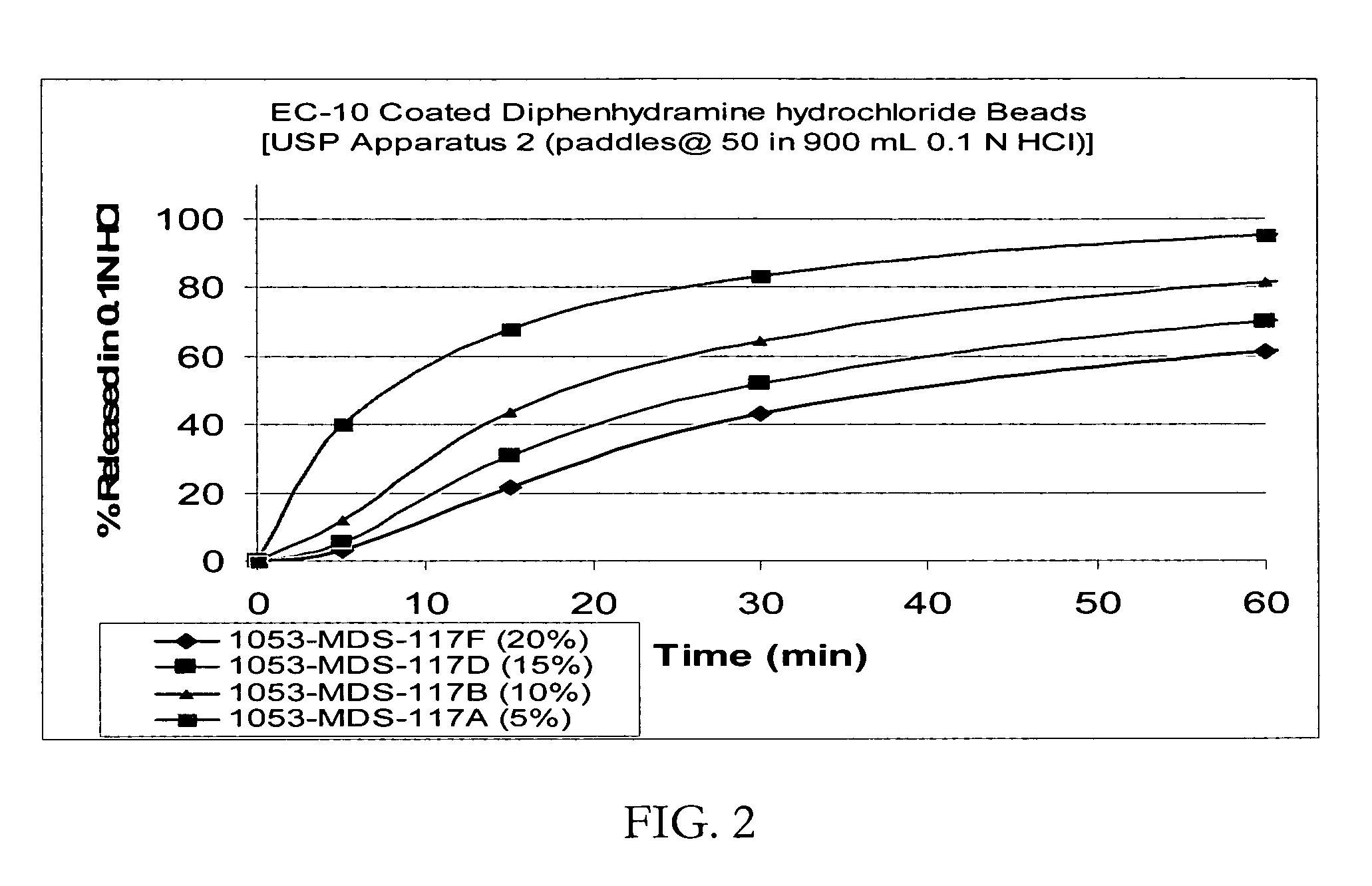
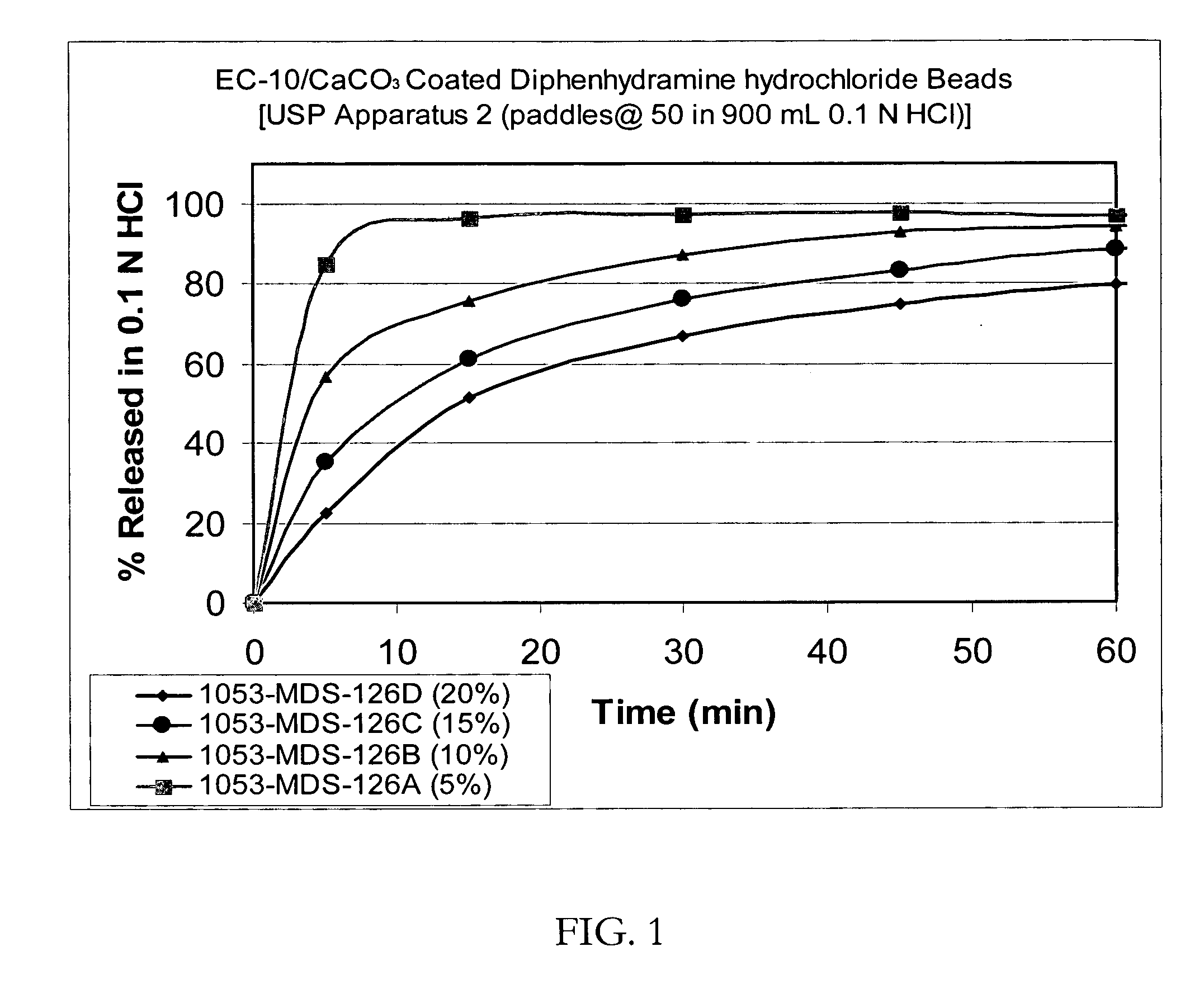
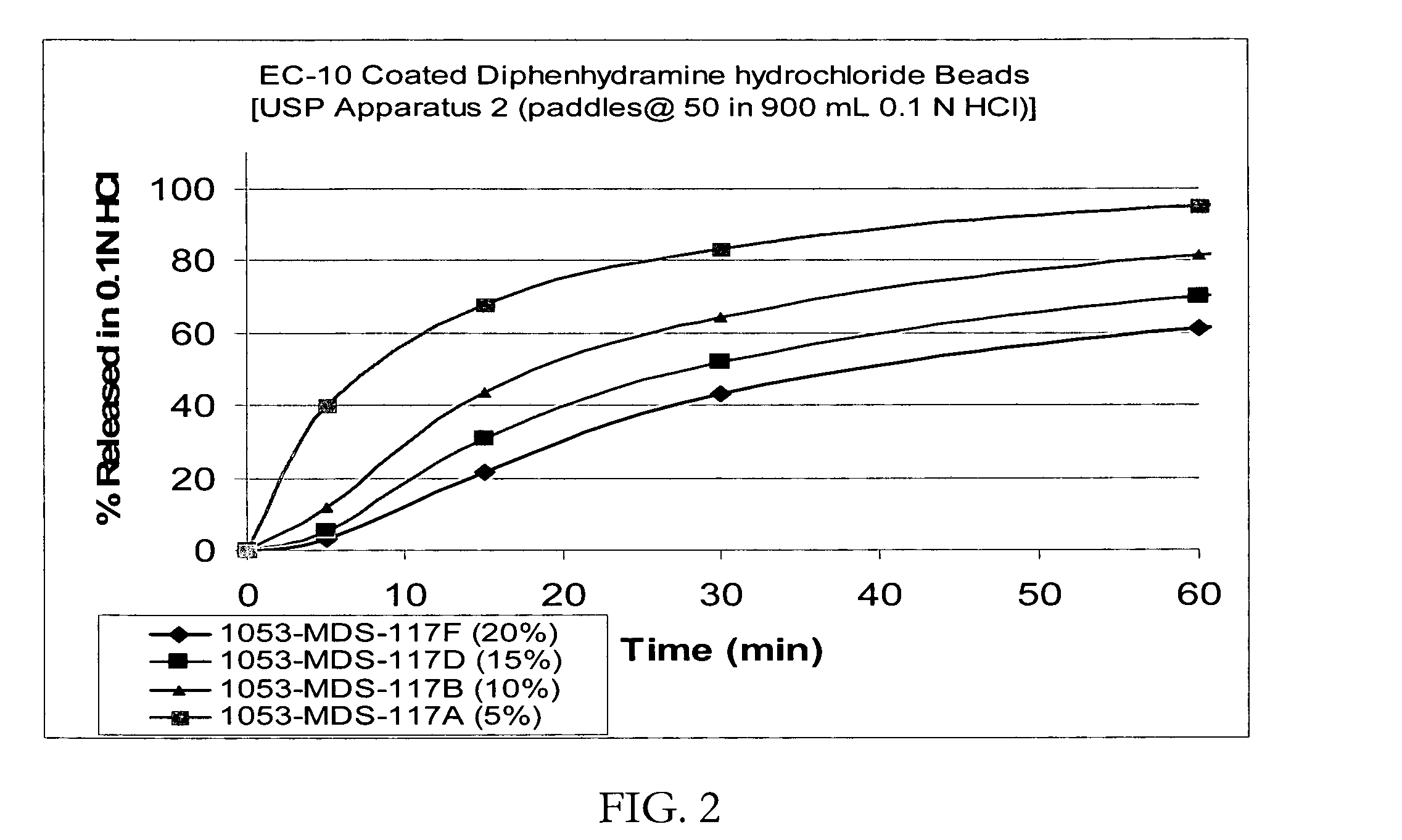
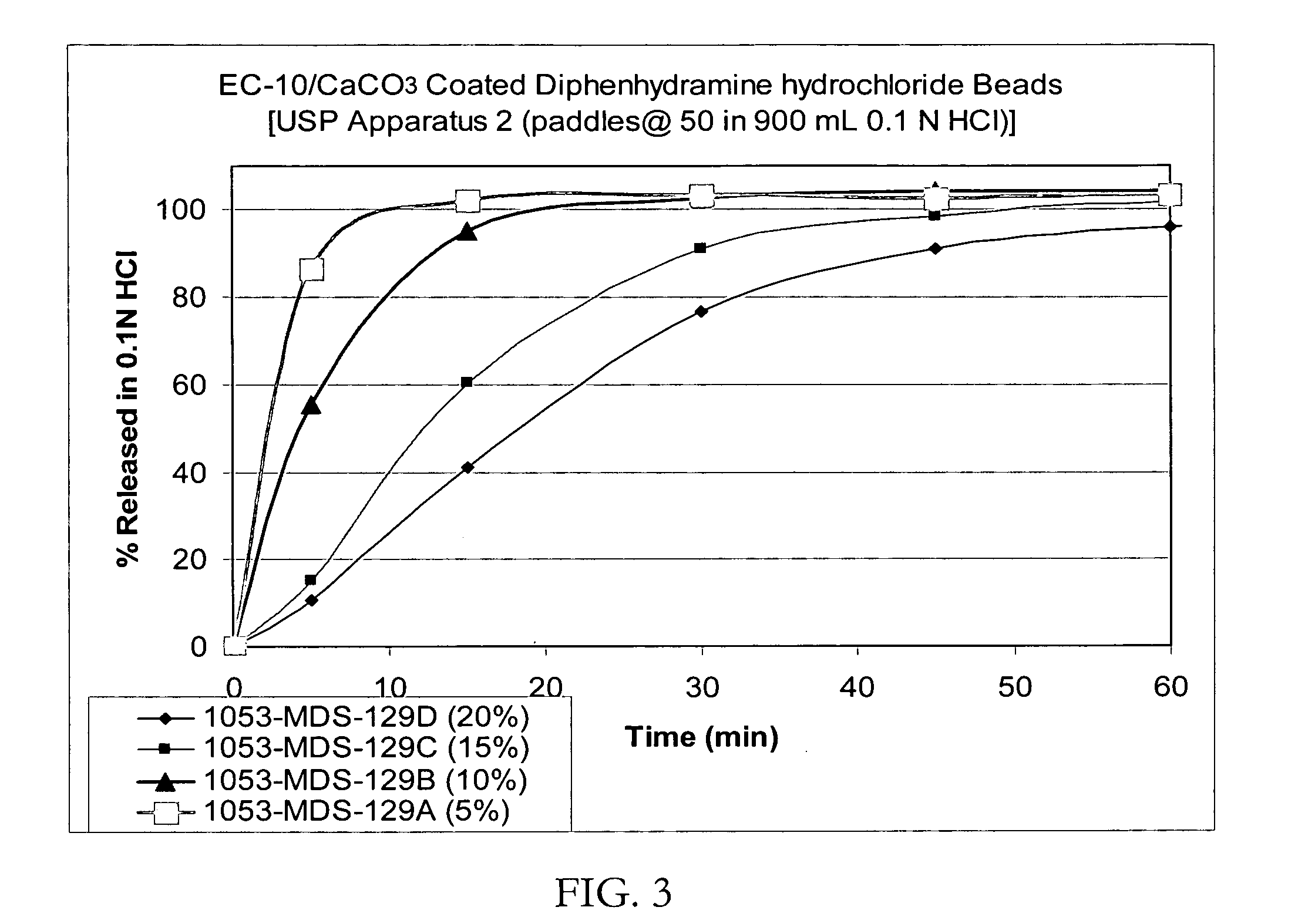
Taste-masked pharmaceutical compositions prepared by coacervation
InactiveUS20060105038A1Effective taste-maskingSmooth tasteOrganic active ingredientsPill deliveryAdditive ingredientWater insoluble
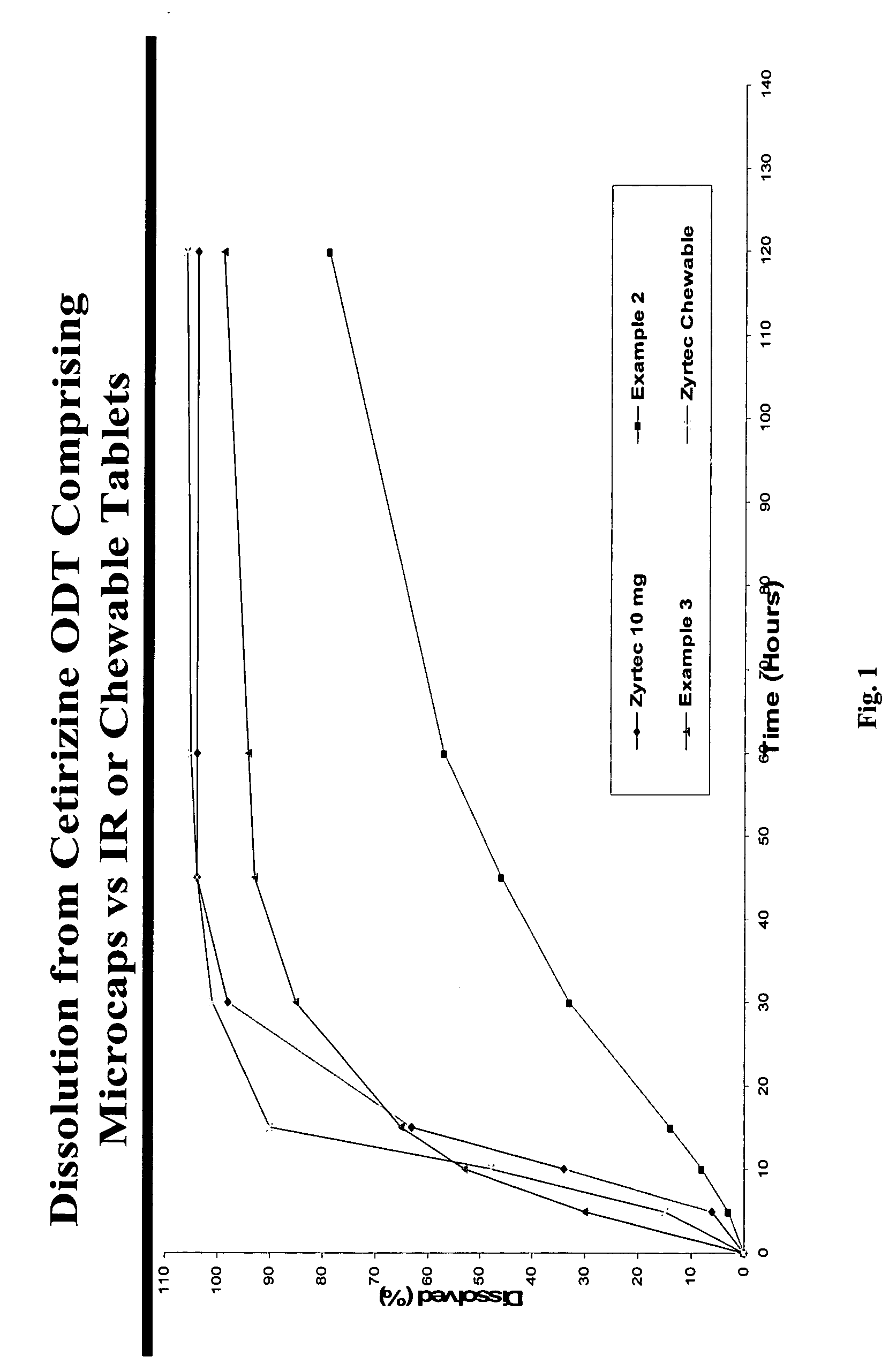
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredients, rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates rapidly with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing one or more actives) with a taste-masking membrane applied by a modified solvent coacervation process comprising a water-insoluble polymer and at least one gastrosoluble inorganic or organic pore-former, exhibit a pleasant taste when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:EURAND PHAMACEUTICALS LTD
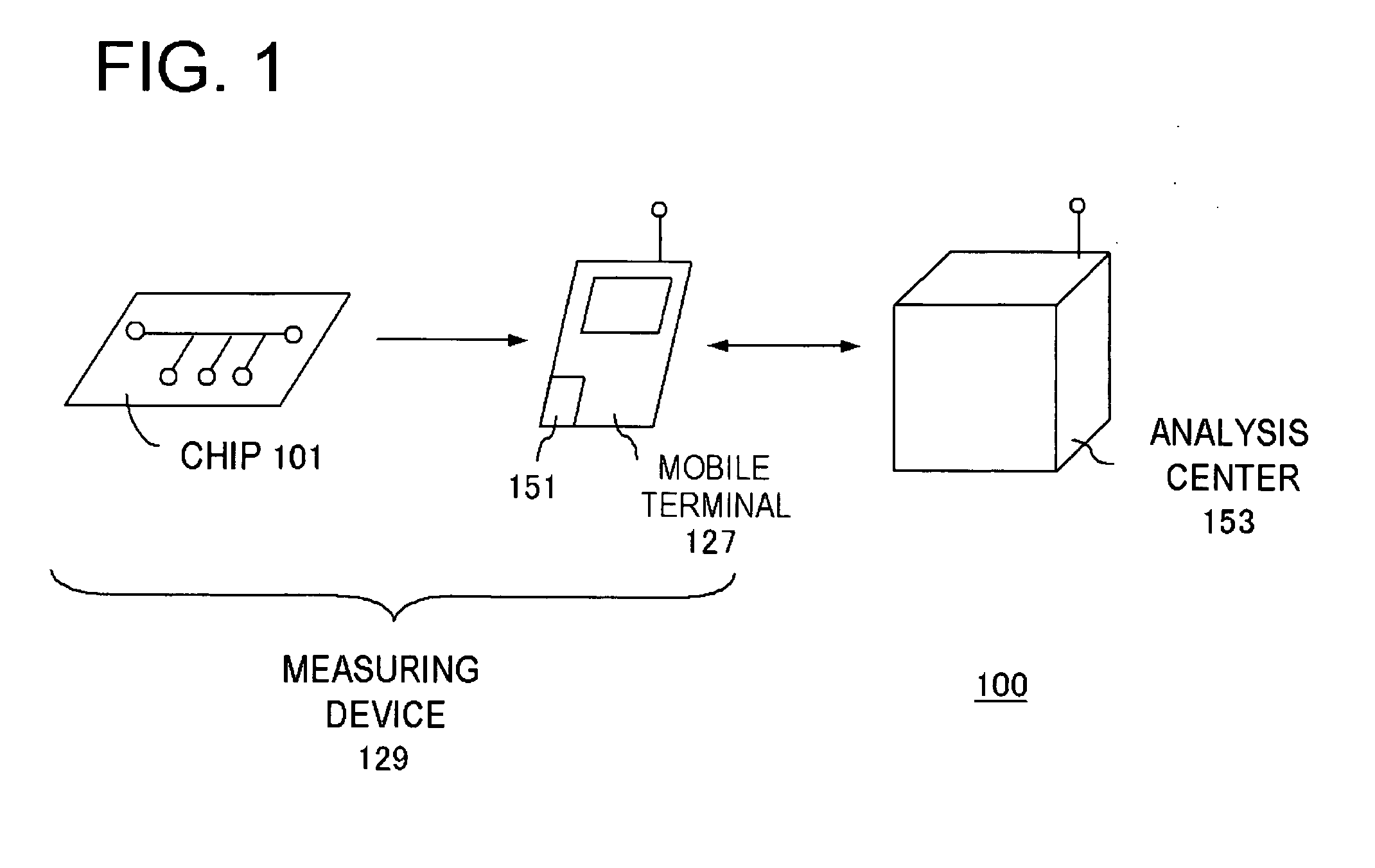
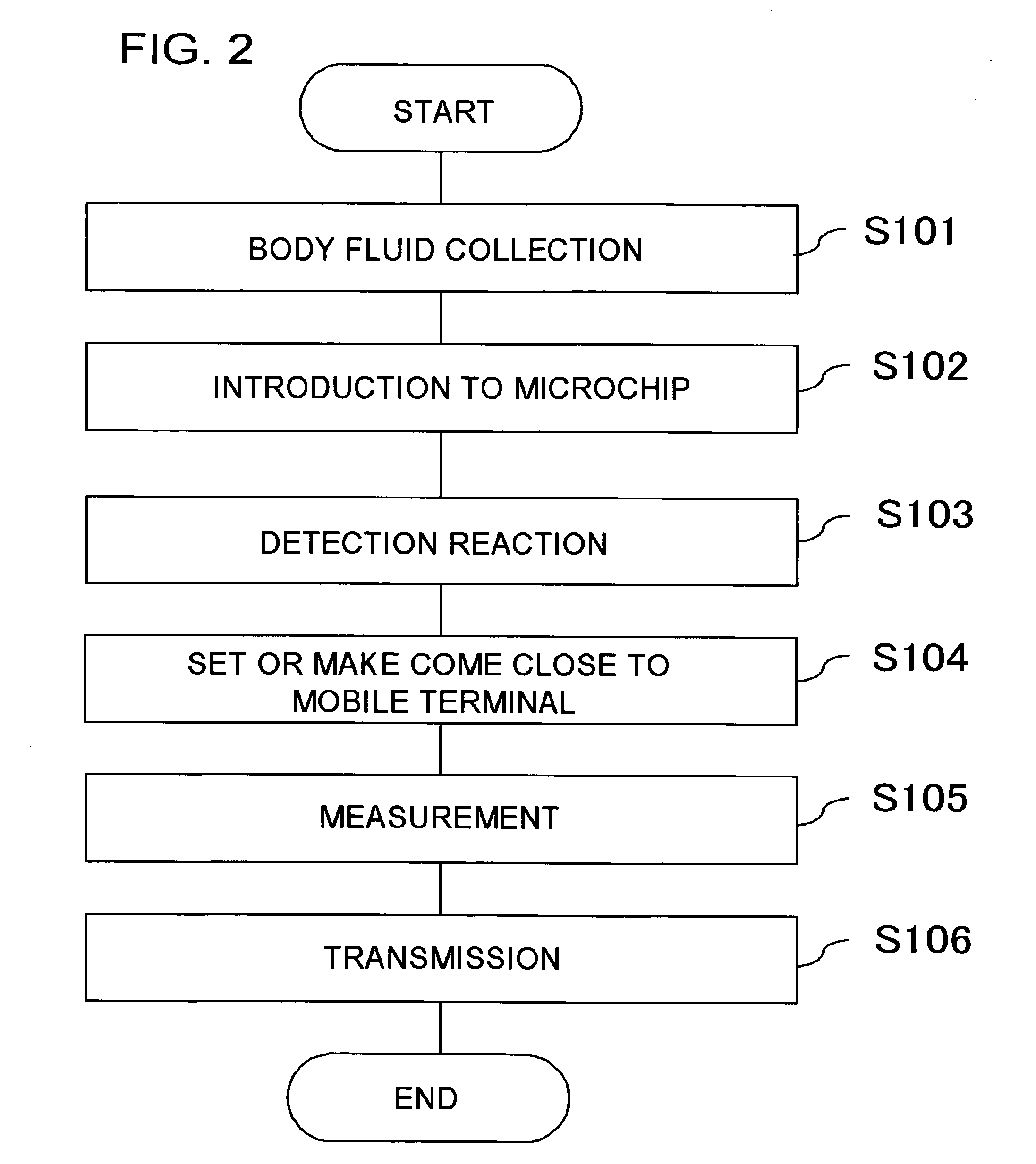
Measuring system
InactiveUS20060292039A1Easy to checkWithdrawing sample devicesMaterial analysis by optical meansAnalysis centerEngineering
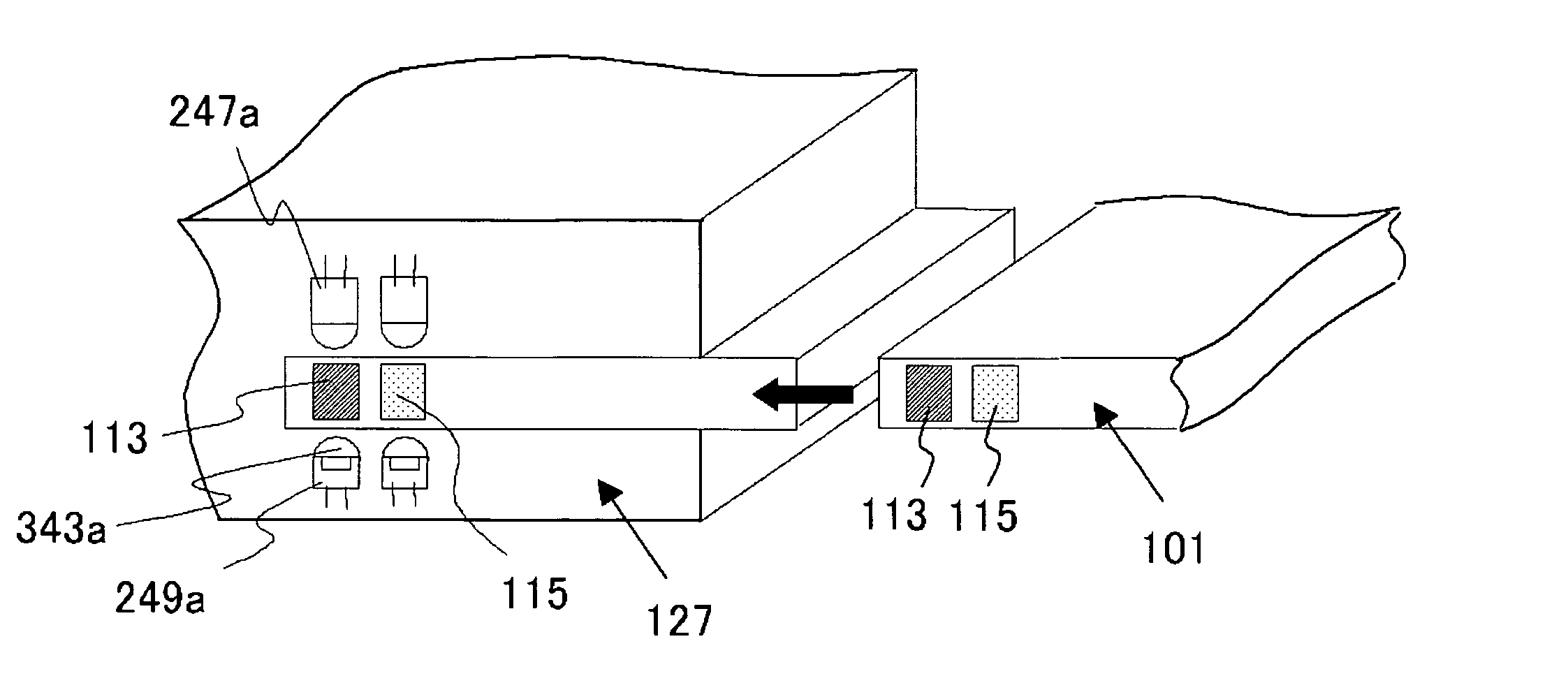
It becomes possible for a user to check his / her own health state at a desired place without visiting inspection agencies. Furthermore, it becomes possible for a user to conveniently check his / her own health state. First, a user collects body fluid to be a measurement object, such as blood, saliva, and urine. Then, the collected body fluid is introduced to the chip (101) as a sample. Then, the sample is made to act on a detection reagent which acts on a specific component in the sample to generate a predetermined detection reaction. This chip (101) is set to the mobile terminal (127). A measurement unit (151) of the mobile terminal (127) measures an amount of a specific component in the sample by means that product of reaction is quantitated by an optical method or the like. The mobile terminal (127) transmits the measurement value to an analysis center (153).
Owner:NEC CORP
Taste-masked pharmaceutical compositions
ActiveUS20060078614A1Effective taste-maskingRapid/complete releasePill deliveryAdditive ingredientOrally disintegrating tablet
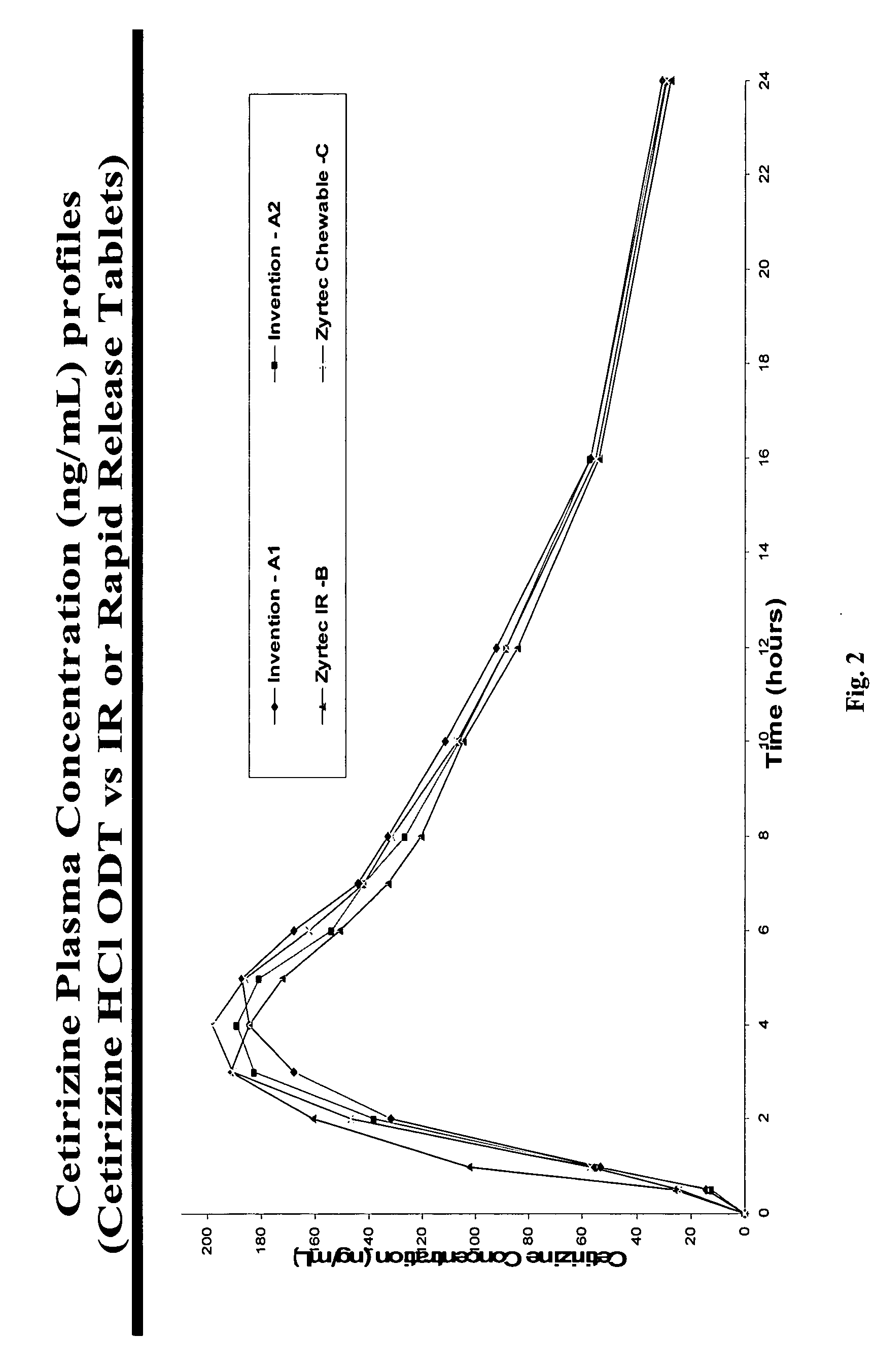
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity in about 60 seconds forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active) applied with a taste-masking membrane comprising a combination of water-insoluble and gastrosoluble polymers release not less than about 60% of the dose is in the stomach in about 30 minutes, thus maximizing the probability of achieving bioequivalence to the reference IR product having rapid onset of action (short Tmax). A process for preparing such compositions for oral administration using conventional fluid-bed equipment and rotary tablet press is also disclosed.
Owner:ADARE PHARM INC
Product quality enhancement in mammalian cell culture processes for protein production
ActiveUS7332303B2Low production costQuality improvementAnimal cellsCell receptors/surface-antigens/surface-determinantsHigh cellBiotechnology
Owner:BRISTOL MYERS SQUIBB CO
Ketone assay
InactiveUS20030175993A1Easy to useLow costMaterial analysis by observing effect on chemical indicatorVaccination/ovulation diagnosticsAnalyteKetone
The present invention relates to analyte detection test systems, including test systems for the oral detection of analytes in saliva. The present invention also provides compositions and methods for storing multiple assay tests and compositions and methods for measuring the concentration of analytes in a sample.
Owner:TORANTO ANTHONY +2
Novel nutritional food products for improved digestion and intestinal absorption
InactiveUS20100239559A1Sustained stabilityAvoiding unstable breakdown of the enzyme and large overdosingHydrolasesPeptide/protein ingredientsDigestionNovel food
The present invention is directed to novel food products, e.g., nutritional food products and infant formula, which contain one ore more enzymes selected from lipase, protease, and amylase that have been formulated / stabilized to have sustained stability in an aqueous medium. Such formulations are intended to provide a greater degree of compliance based on their ability to be incorporated into aqueous media while avoiding unstable breakdown of the enzyme and large overdosing due to expected breakdown when exposed to an aqueous environment, including saliva. Further described in the invention are additives packaged with instructions for combination with an aqueous medium, and instructions for the administration of the resulting mixture to a subject. In certain embodiments, enzyme insufficient patients, e.g., infants and elderly persons, would find particular benefit from the food products described herein.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
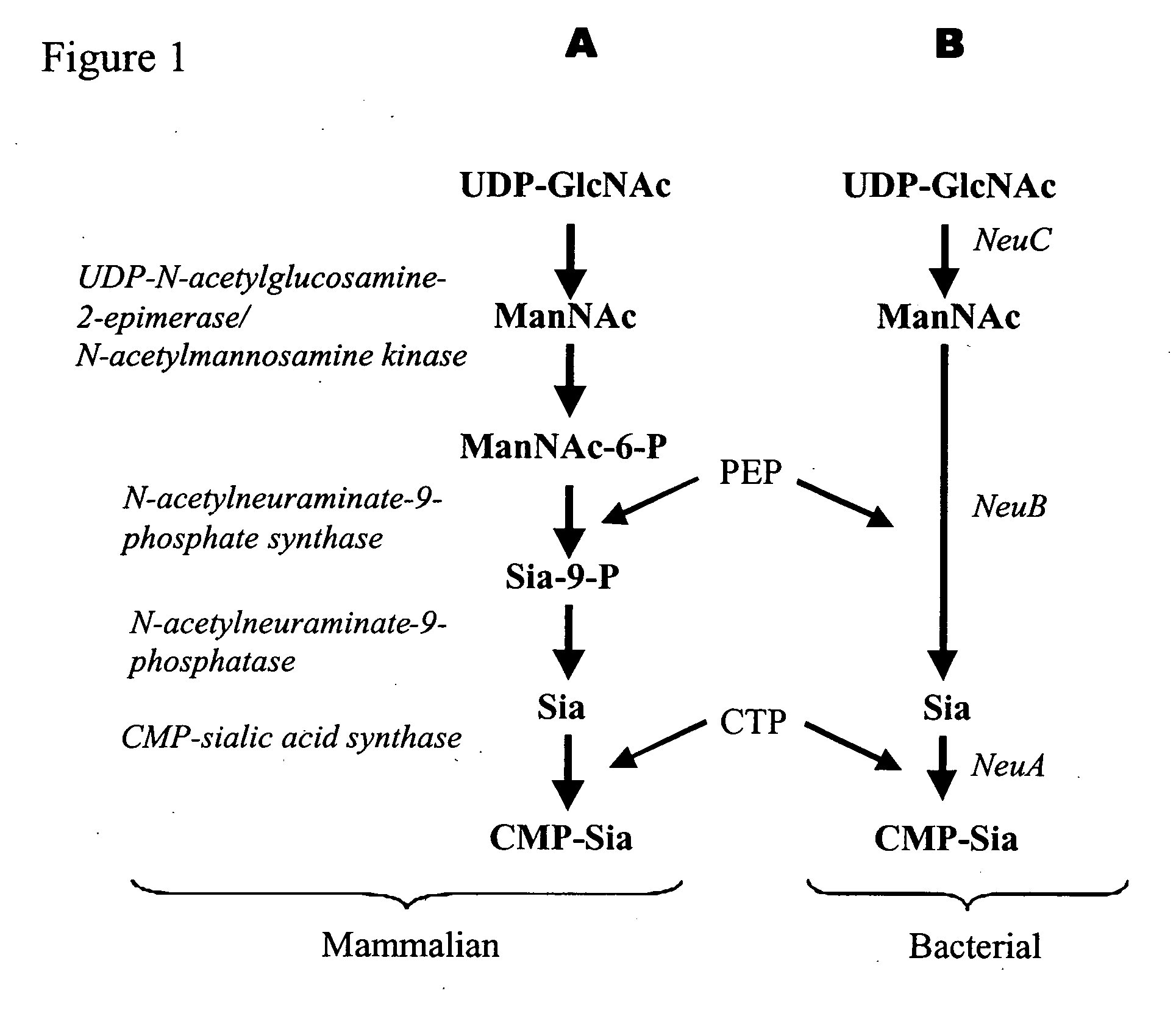
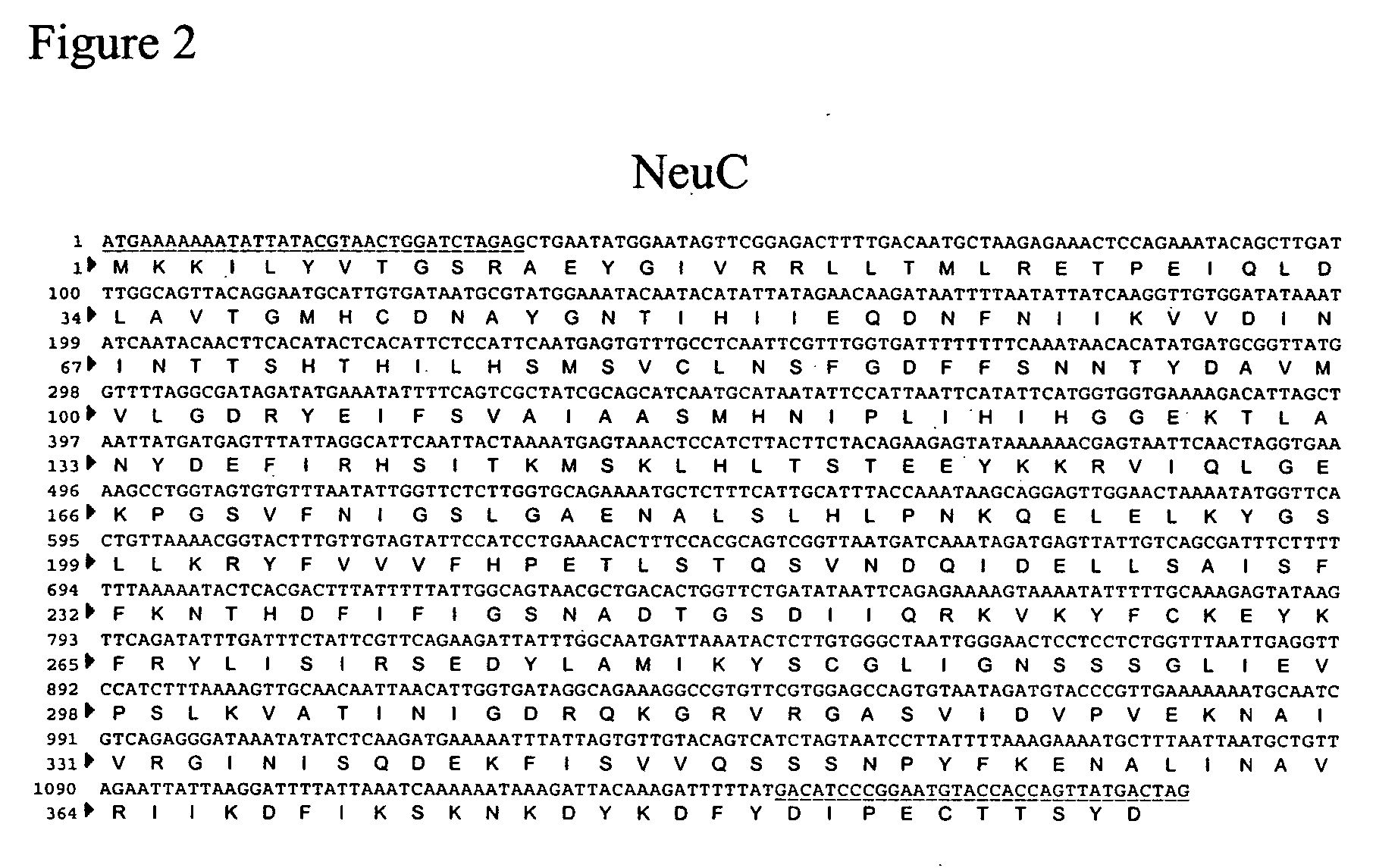
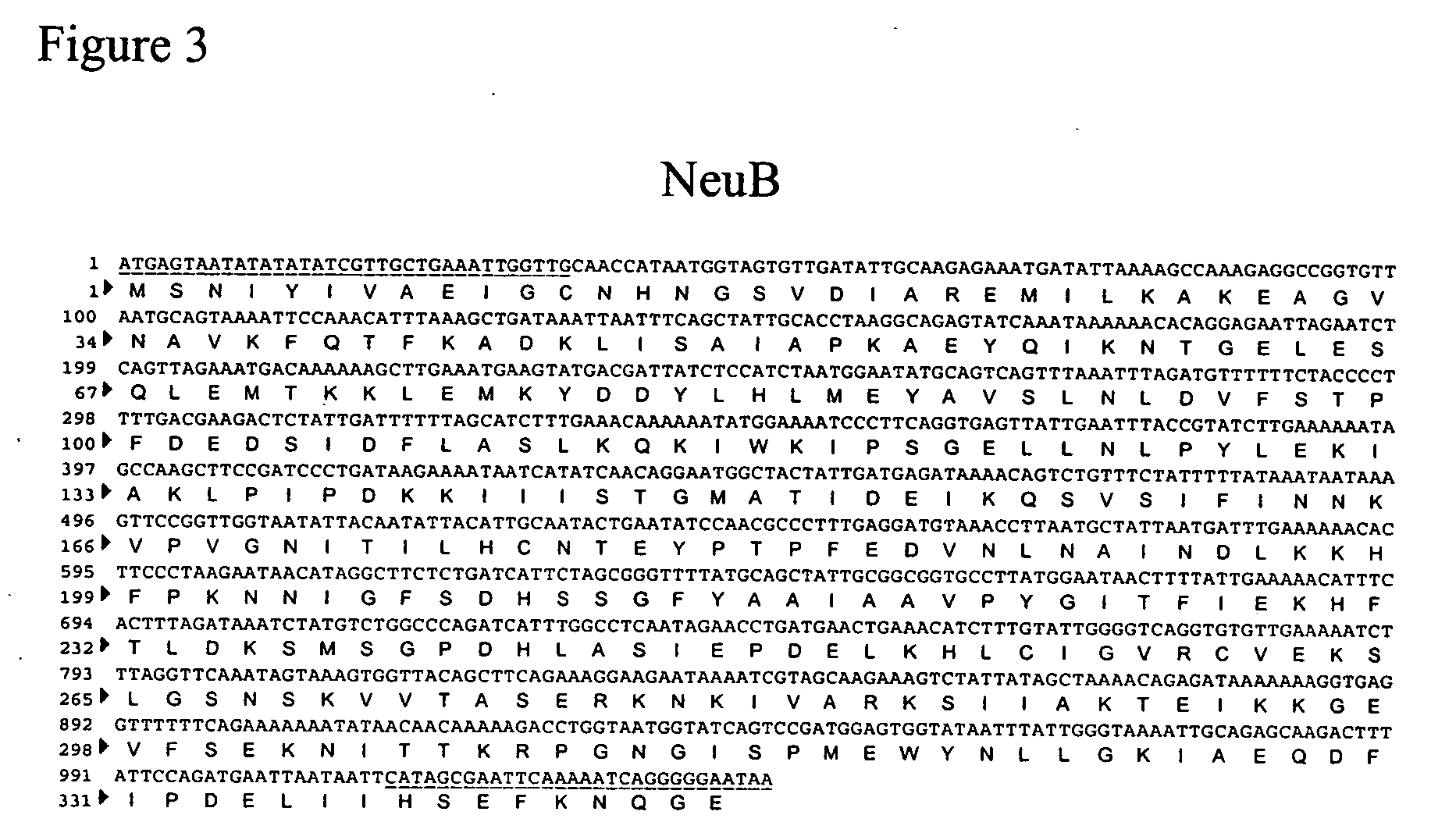
Method of engineering a cytidine monophosphate-sialic acid synthetic pathway in fungi and yeast
The present invention provides methods for generating CMP-sialic acid in a non-human host which lacks endogenous CMP-Sialic by providing the host with enzymes involved in CMP-sialic acid synthesis from a bacterial, mammalian or hybrid CMP-sialic acid biosynthetic pathway. Novel fungal hosts expressing a CMP-sialic acid biosynthetic pathway for the production of sialylated glycoproteins are also provided.
Owner:GLYCOFI
Spicy coarse grain dried bean curd and preparation method thereof
InactiveCN103444883AFull of nutritionHigh nutritional valueCheese manufactureFood scienceReady to eatLiver and kidney
The invention discloses spicy coarse grain dried bean curd. The spicy coarse grain dried bean curd is prepared from the following raw materials in parts by weight: 90 to 100 parts of dried soybeans, 3 to 4 parts of oat, 3 to 4 parts of millet, 4 to 5 parts of sorghum, 4 to 5 parts of corns, 2 to 3 parts of mulberries, 2 to 3 parts of hawthorn, 2 to 3 parts of jujubes, 1 to 2 parts of calamus leaves, 1 to 2 parts of platycladus orientalis leaves, 2 to 3 parts of prepared rehmannia root, 1 to 2 parts of fructus amomi, 1 to 2 parts of rehmannia flower, 1 to 2 parts of loofah sponge, 2 to 3 parts of cassia bark, and 2 to 3 parts of rhodiola rosea. A variety of grains are added into the spicy coarse grain dried bean curd, so that a nutritionally balanced structure system is formed; a plurality of seasonings are added, so that the dried bean curd tastes spicy and is easily preferred by consumers; the healthcare components of a plurality of traditional Chinese medicines are added, so that the nutrition value of the dried bean curd is high; the finished dried bean curd is ready to eat, spicy, unique, rich in nutrients and chewy, has the effects of stimulating appetite, improving digestion, tonifying liver and kidney, tonifying spleen and stomach, cooling blood to stop bleeding, promoting the secretion of saliva or body fluid and lubricating intestines, and is the best healthcare food catering to the market and consumers.
Owner:JINCAIDI FOOD CO LTD
Method for selectively combining multiple membranes for assembly into test strips
ActiveUS7129038B2Improve performanceReduce test strip lot rejectionBioreactor/fermenter combinationsBiological substance pretreatmentsCholesterolKetone
A method for selectively combining multiple membranes for assembly into test strips (such as visual blood glucose test strips with side-by-side membranes). The method includes first measuring a plurality of color parameters (e.g., L*, a* and b*color parameters) associated with membrane samples from at least two membrane lots. Next, response characteristics (e.g., blood glucose response levels) are simulated for a speculative test strip that includes, for purposes of the simulation, combined multiple membranes tentatively selected from the at least two membrane lots. The simulated response characteristics are based on the measured plurality of color parameters of the tentative selection of combined multiple membranes. Optionally, the simulated response characteristics can also be based on simulated color parameters of the tentative selection of combined multiple membranes. Subsequently, assembly of the at least two membrane lots into a test strip with combined membranes is contingent on acceptable simulated response characteristics. Any suitable color parameters can be employed. The method can be used to selectively combine two or more membranes based on any number of color parameters. The assembled test strips can be used to measure glucose, cholesterol, proteins, ketones, phenylalanine or enzymes in blood, urine, saliva or other biological fluid, as well as sample fluid characteristics (e.g., pH and alkalinity).
Owner:LIFESCAN IP HLDG LLC
Systems and methods for analyzing body fluids
ActiveUS20110070606A1Improve accuracyLow costBioreactor/fermenter combinationsBiological substance pretreatmentsBody fluidVaginal tissue
Systems and methods analyzing body fluids contain cells including blood, bone marrow, urine, vaginal tissue, epithelial tissue, tumors, semen, and spittle are disclosed. The systems and methods utilize an improved technique for applying a monolayer of cells to a slide and generating a substantially uniform distribution of cells on the slide. Additionally aspects of the invention also relate to systems and method for utilizing multi-color microscopy for improving the quality of images captured by a light receiving device.
Owner:ROCHE DIAGNOSTICS HEMATOLOGY INC
Nicotine containing stimulant unit
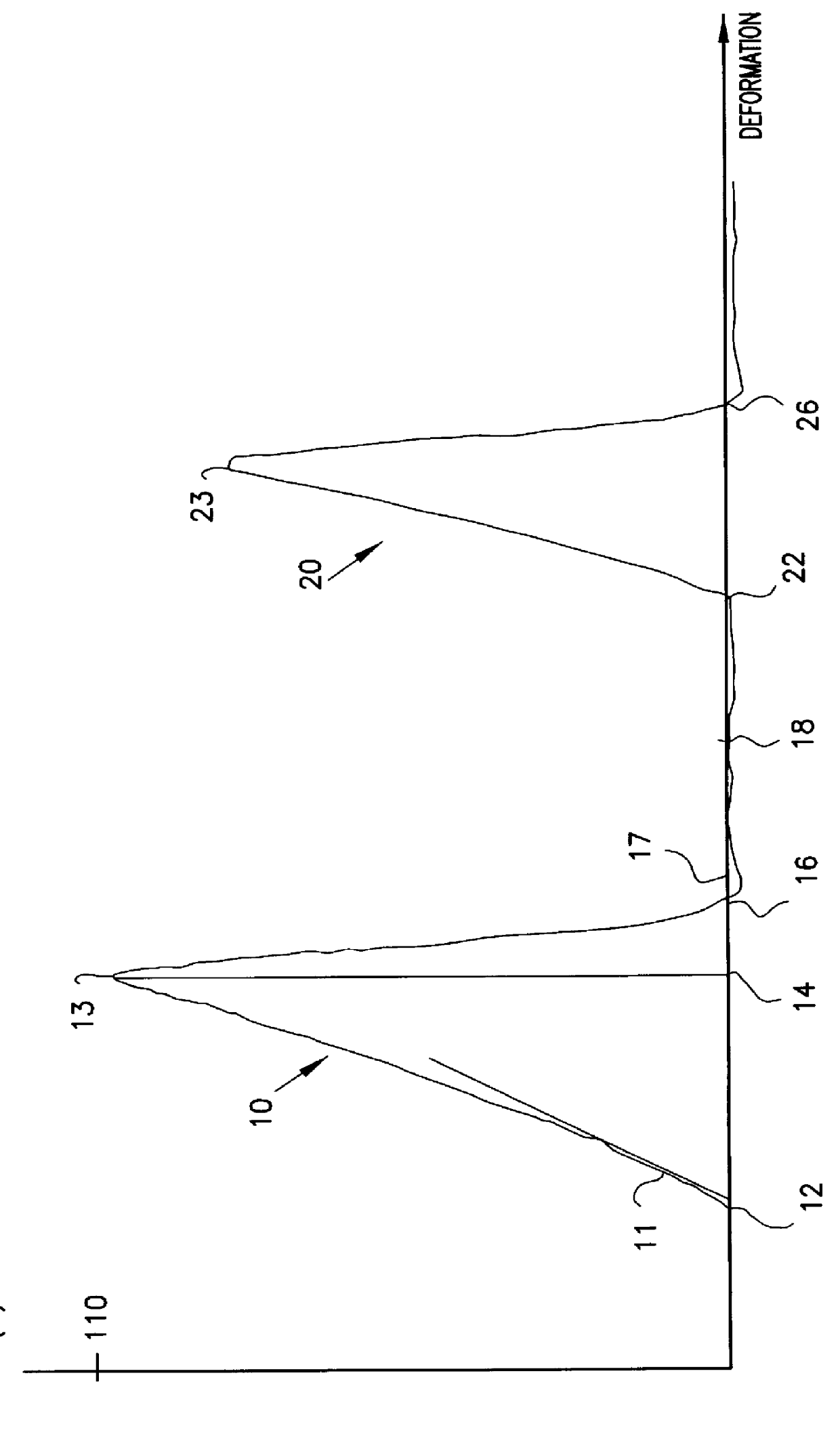
InactiveUS6110495AIncrease stimulationLarge deformationTobacco treatmentConfectioneryStimulantAdditive ingredient
A saliva-soluble stimulant unit comprising an active ingredient and optional ingredients comprising flavor and aroma additives incorporated in a gel prepared by gelling a water-binding gelling agent, in which the active ingredient comprises nicotine or other alkaloids with the same direction of activity, said unit having i) a texture profile, determined by texture profile analysis, with parameter values of firmness, hardness, brittleness, adhesiveness, elasticity, and cohesiveness within given ranges; (ii) a disintegration time within the range 5-60 minutes; and (iii) a nicotine content from 0.5 to 10 mg or a corresponding content of said alkaloids.
Owner:DAM ANDERS
Taste-masked pharmaceutical compositions with gastrosoluble pore-formers
InactiveUS20060105039A1Effective taste-maskingRapid/complete releaseOrganic active ingredientsPill deliveryAdditive ingredientWater insoluble
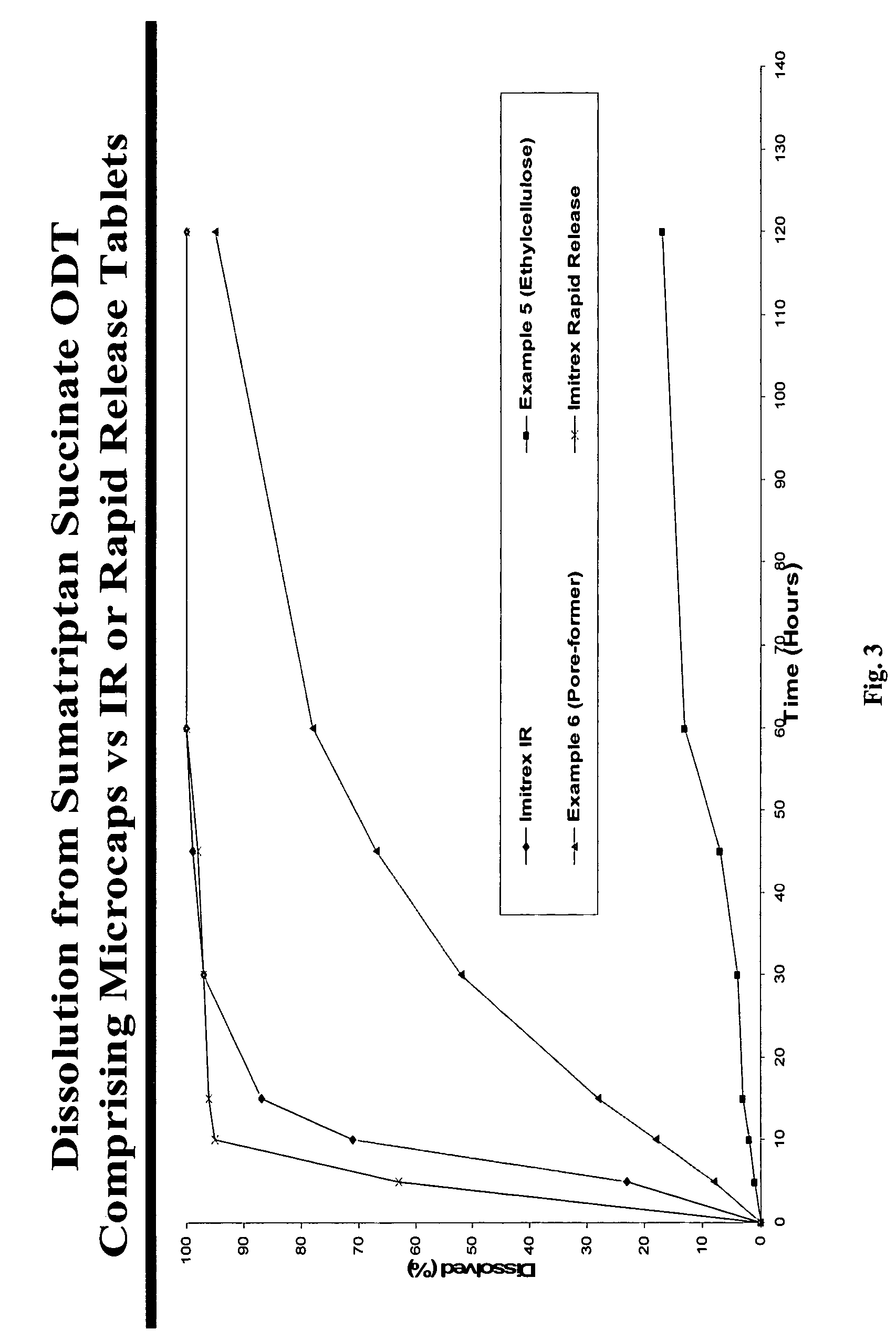
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active), coated with a taste-masking membrane comprising a water-insoluble polymer and one or more gastrosoluble inorganic or organic pore-formers (practically insoluble in water and saliva, but soluble in an acidic buffer), exhibit acceptable taste-masking when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:ADARE PHARM INC
Hand-mouth baby wet tissue and preparation method thereof
InactiveCN103040688APromote secretionLower pHCosmetic preparationsToilet preparationsAdditive ingredientCleansing Agents
The invention relates to a hand-mouth baby wet tissue and a preparation method thereof. The hand-mouth baby wet tissue is composed of compound wet tissue liquid and non-woven fabrics for holding the compound wet tissue liquid, wherein every 100 parts of the compound wet tissue liquid comprises the following components in percent by weight: 0.01%-7.5% of a sweetening agent, 0.01-2.0% of a preservative, 0.01%-2.0% of a cleaning agent, 0.05%-5.0% of a solubilizer, 0-10.0% of a humectant, 0-5.0% of a skin care agent, 0-5.0% of an acidity regulator, 0-5.0% of essential oil, 0-5.0% of a stabilizer, 0-1.0% of natural essence, and the balance of pure water. The hand-mouth baby wet tissue has the advantages that edible sweetening agent is added into the compound wet tissue liquid to promote spittle secretion of babies, relieve the drop of pH value in the oral cavity and prevent the formation of decayed teeth. The product formula is stable and safe, and the material components are non-toxic. The formula is safe, non-irritant and non-corrosive, so that the allergic risks are reduced to be the lowest. The hand-mouth baby wet tissue can be used for cleaning the hands and mouths of an infant, infant tableware and other infant supplies and toys.
Owner:林建广
High titer production of highly linear poly (alpha 1,3 glucan)
ActiveUS8642757B2Improve mechanical propertiesMore amenable to preparing fibersSugar derivativesPeptide/protein ingredientsSucroseSaccharophagus degradans
A process for enzymatic preparation of a highly linear poly(α1,3 glucan) from sucrose is disclosed. The glucosyltransferase enzyme (gtfJ) from Streptococcus salivarius is used to convert sucrose to a highly linear poly(α1,3 glucan) in high titers. Hydrolyzed poly(α1,3 glucan) is used as the primer for the gtfJ enzyme reaction resulting in the formation of highly linear poly(α1,3 glucan).
Owner:NUTRITION & BIOSCIENCES USA 4 INC
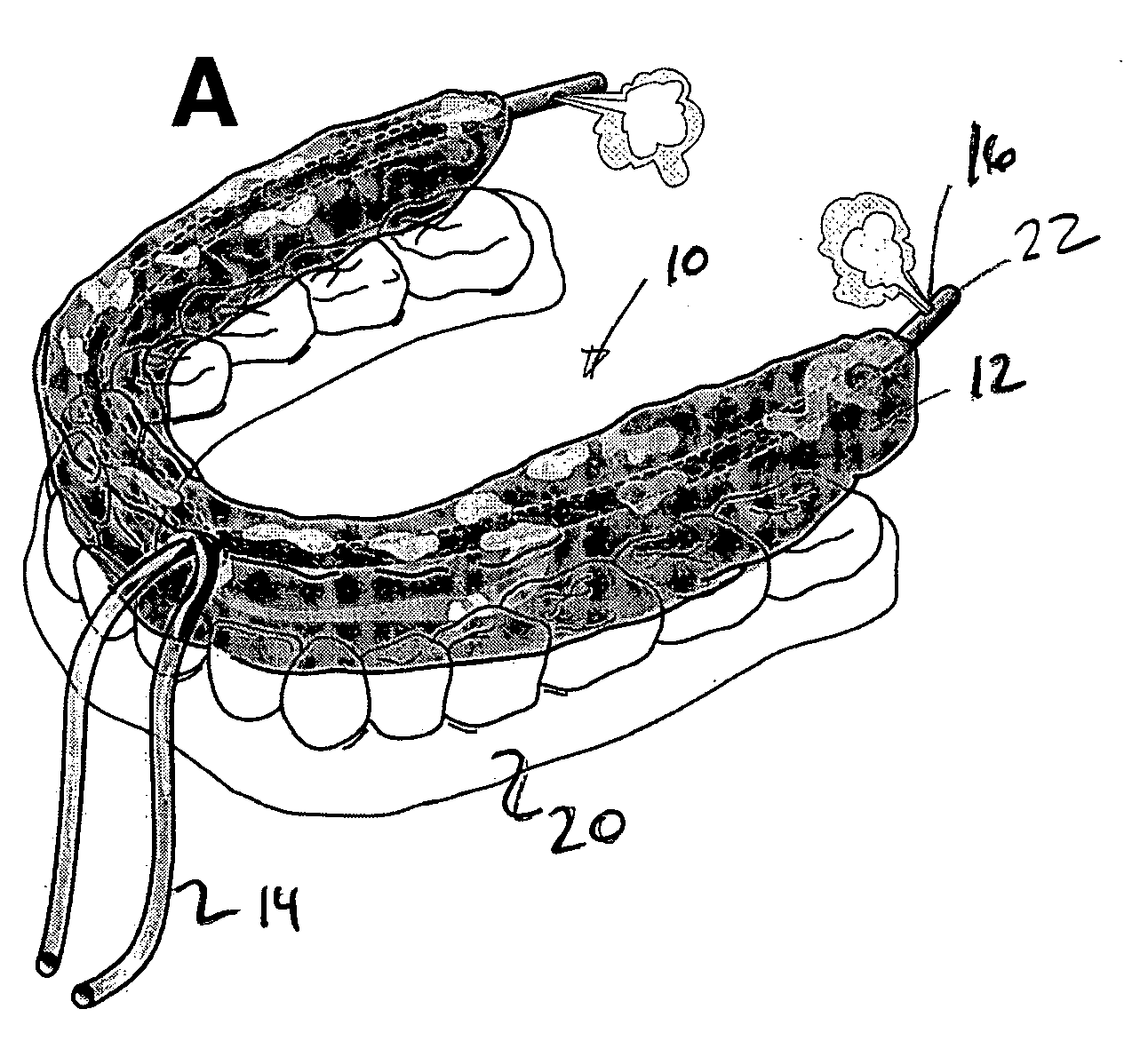
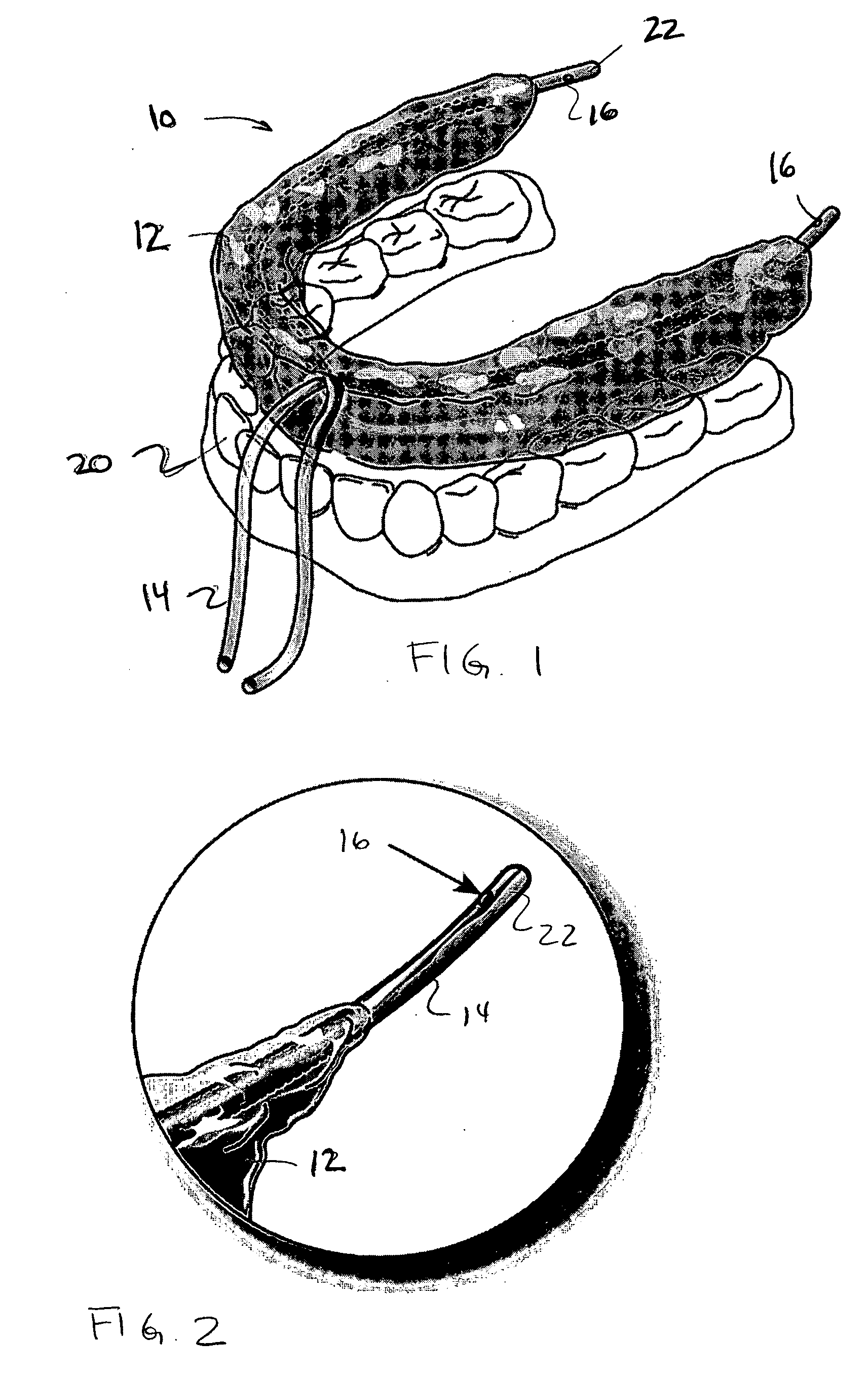
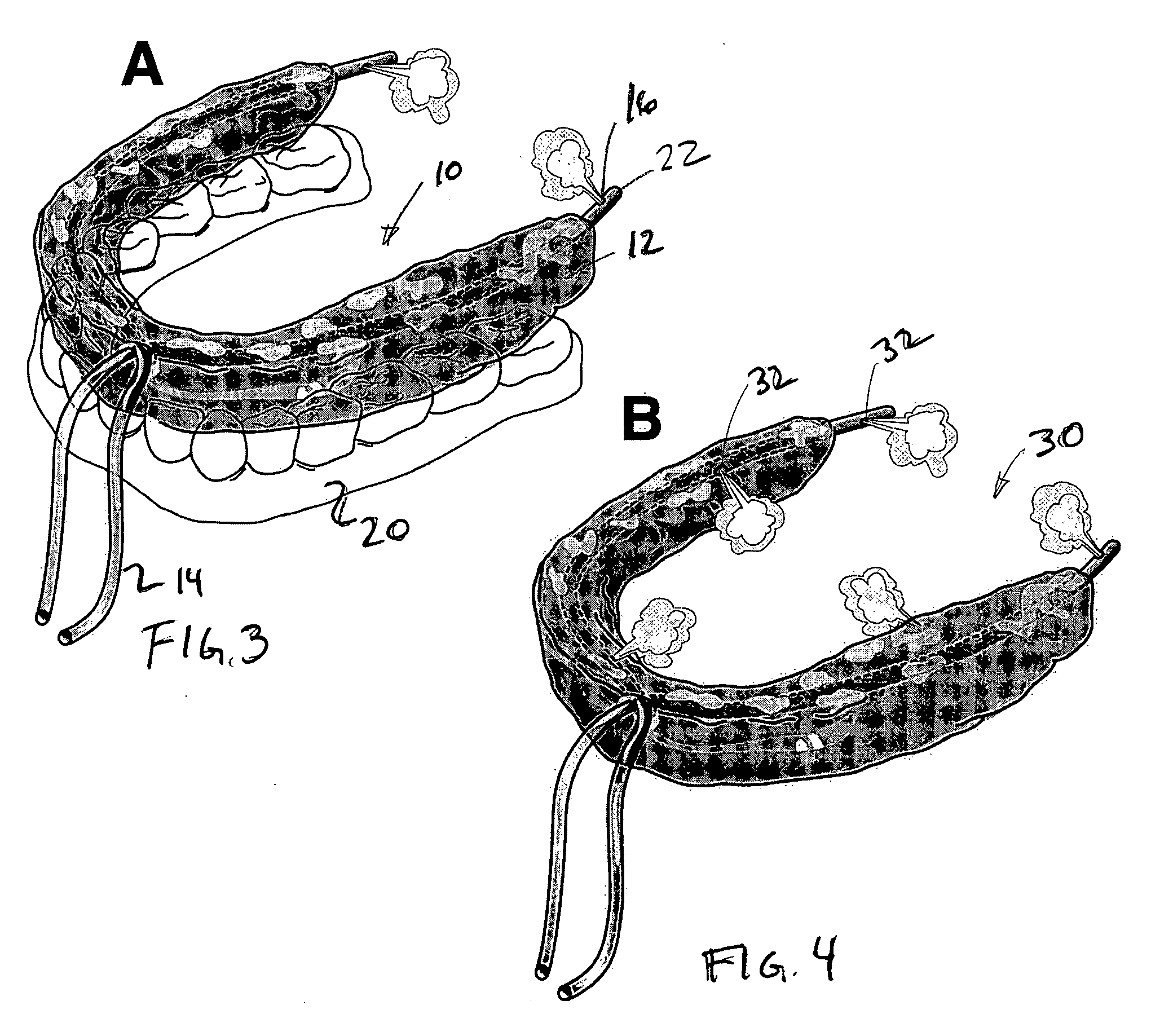
Oral device
An oral device for use with a person in which at least one non-toxic gas pulse is delivered to a predetermined location in the mouth via a device conduit. The oral device may be provided as a kit with at least one device for measuring the subject's responses and representing them as feedback to the subject / clinician. A method of creating a gas bolus pulse train, delivering it to a predetermined mouth area, and monitoring the subject's responses to it, is also shown. The oral device and method may be used as a diagnostic tool, or a therapeutic tool, in swallowing or speech rehabilitation of children and adults who have swallowing, speech, salivary, and / or oral sensorimotor impairments.
Owner:WESTERN ONTARIO THE UNIV OF
Compositions and methods for obtaining nucleic acids from sputum
InactiveUS7482116B2Reduce viscosityEasy extractionSugar derivativesMicrobiological testing/measurementBiologyDNA
The present invention relates to compositions and methods for preserving and extracting nucleic acids from saliva. The compositions include a chelating agent, a denaturing agent, buffers to maintain the pH of the composition within ranges desirable for DNA and / or RNA. The compositions may also include a reducing agent and / or antimicrobial agent. The invention extends to methods of using the compositions of the invention to preserve and isolate nucleic acids from saliva as well as to containers for the compositions of the invention.
Owner:DNA GENOTEK
Medicinal delivery system, and related methods
ActiveUS20080020050A1Rapid and sustained reliefMedication quicklyPowder deliveryBiocideMedicineDelivery system
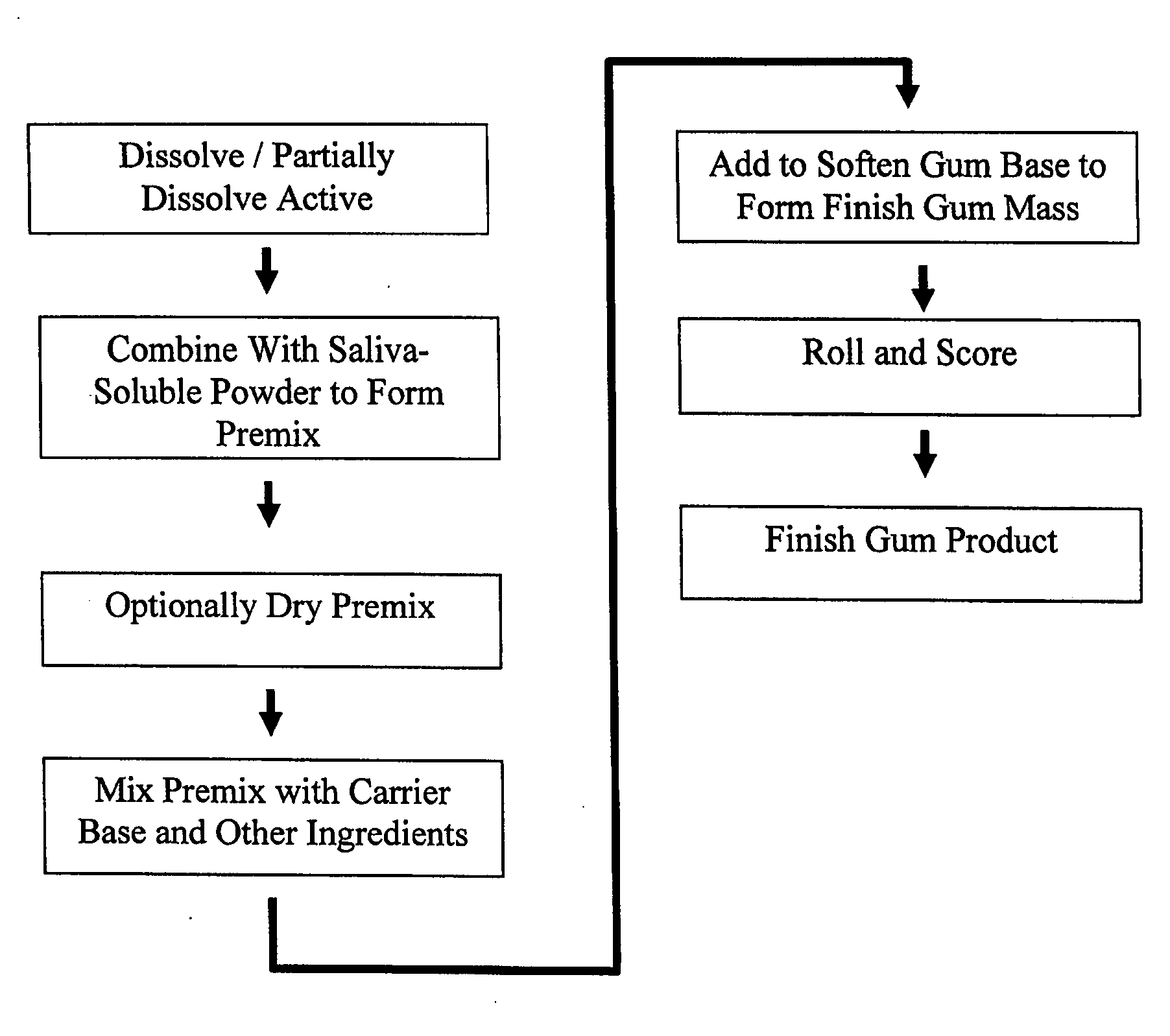
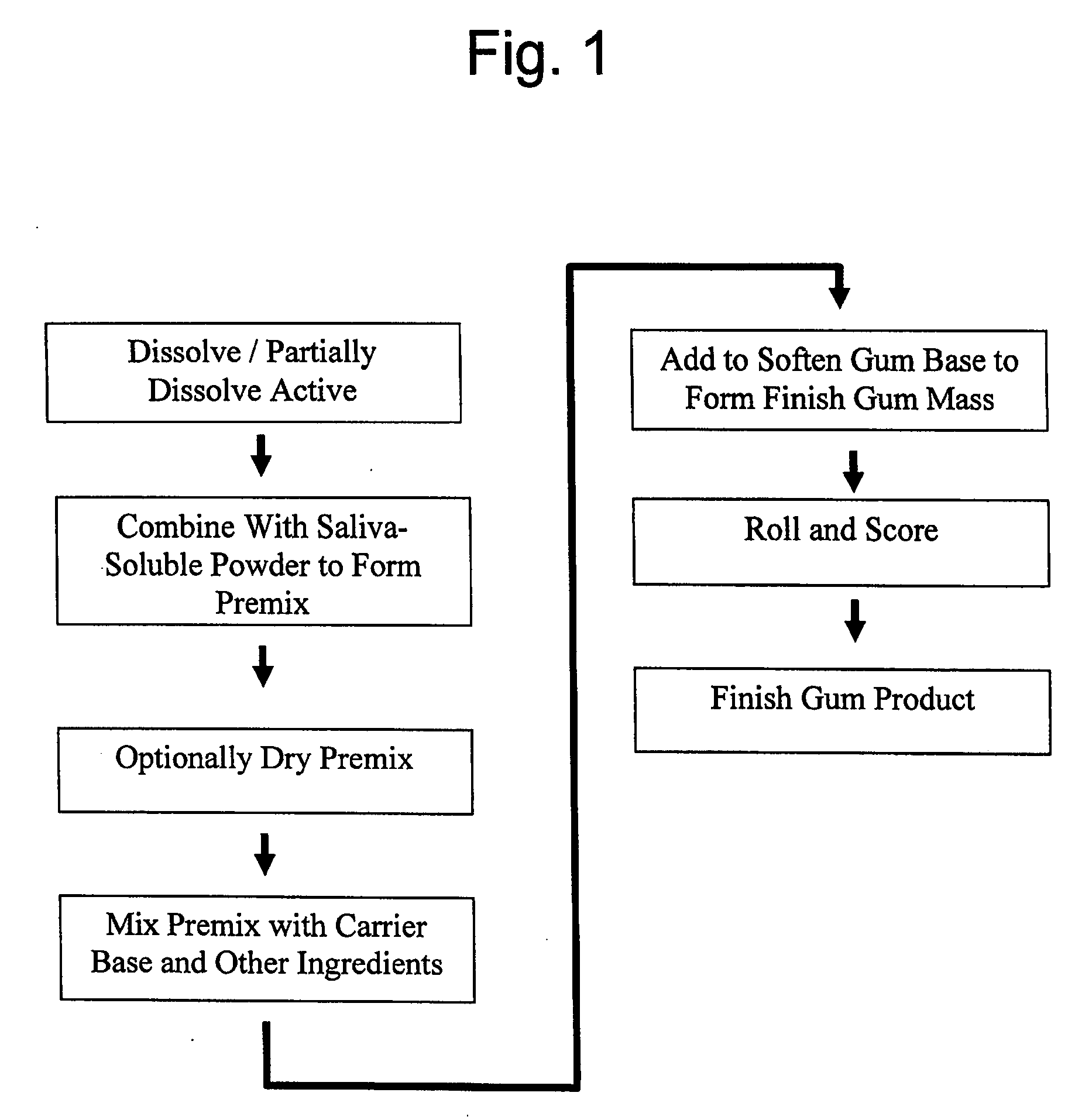
A method is provided of making a medicinal delivery system which satiates a craving in an individual when the medicinal delivery system is administered orally to the individual. A coating composition is applied on a saliva-soluble powder to establish a coated powder, the coating composition featuring an at least partially solubilized craving satiation medicinal compound. The coated powder is combined with an edible carrier base to establish a medicinal delivery system that rapidly releases medicine and buffer preferably followed by slower, sustained release.
Owner:JSR NTI
Chinese medicinal formulation for treating rheumatic and rheumatoid disease
InactiveCN1966018AGood curative effectAnthropod material medical ingredientsAntipyreticBroom RapeGastrodia
The invention discloses a pharmaceutical composition for treating rheumatism and rheumatoid disease, wherein the constituents include black-tail snake, gastrodia tuber, Clematis chinensis, pubescent angelica root, notopterygium root, ledebouriella root, speranskia herb, Loranthus mulberry mistletoe, cortex acanthopanacis, root of herbaceous peony, earthworm, achyranthes and cyathula root, dipsacus root, drynaria, east Asian tree fern rhizome, broomrape, psoralea fruit, eucommia bark, deer horn gum, homalomena rhizoma, hairy birthwort, batryticated silkworm, dragon's blood resin, Moghania philippinensis, futokadsura stem, rhizoma dioscoreae hypoglaueae, herba siehesbeckiae, root of red rooted saliva, buck grass, orienavine, anisetree bark, Japanese yam, pawpaw, large-leaf gentian root, and Tripterygium wilfordii 120-150, prepared Sichuan aconite root, prepared wild aconite root, processed semen strychni each 280-320g, Chinese angelica root, red ginseng, astragalus root each 140-180, pipe-fish, wood louse, buthus martensi karsch, Chinese ephedra, licorice root each 70-80, safflower, Ligusticum wallichii, notoginseng, cassia twig, hornet nest 100-120, centipede 50-60, asaryl, aniseed 80-100 and Aconitum pendulum Busch 15-20.
Owner:雒宏志
Rapidly disintegrating solid preparation
InactiveUS20100233278A1Efficient productionHigh dissolution ratePowder deliveryBiocideCelluloseAlcohol sugars
Provided is a solid preparation which rapidly disintegrates in the presence of saliva or a small amount of water in the oral cavity, particularly, a rapidly disintegrating solid preparation useful as an orally-disintegrating solid preparation.Specifically provided is a rapidly disintegrating solid preparation containing coated granules wherein saccharide or sugar alcohol having an average particle size of not less than 75 μm and a high dissolution rate is coated with cellulose, and a rapidly disintegrating solid preparation containing a) an active ingredient, b) saccharide or sugar alcohol having an average particle size of not less than 400 μm, c) cellulose and d) a disintegrant.
Owner:MITSUBISHI TANABE PHARMA CORP
Oral isolation device with evacuation chambers
An essentially a U-shaped oral isolation device having a tongue arm, a buccal arm, and a hinge section is provided. The device is an essentially hollow member having an upper suction chamber and a lower suction chamber within the hollows of the oral isolation device. Each chamber further comprising suction inlet apertures through which saliva, fluid, aerosol mist, and debris can be evacuated from the operative site, and a suction outlet chamber through which the collected saliva, fluid, and debris are removed from the device. A U-shaped hinge member joins the buccal arm and the tongue arm. It has position memory which permits the user to squeeze the arm members toward one another for placement in the mouth and upon release, the arm members exert opposing positional influence against the tongue and cheek. Also provided is a method of use for the device in which the tongue arm and buccal arms are forced toward one another. The hinged member is then inserted into the patient's oral cavity and placed around the rearmost tooth. Upon proper placement of the device, the user releases the pressure on the arms of the device, the arms thereby exerting opposing positional influence against the cheek and tongue, resulting in retraction of the tongue and cheek, thereby creating a clear operative field. A reduced pressure high volume device is then attached to the upper chamber suction outlet and a low pressure reduced volume device is attached to the lower chamber outlet.
Owner:PREMIER DENTAL PRODS +1
Oral Care Compositions
InactiveUS20090269287A1Prevent significant water lossGood dispersionCosmetic preparationsToilet preparationsCarrageenanRelative humidity
Disclosed are oral care compositions, particularly thickened dentifrices in liquid, paste or gel form comprising a binding / thickening system that also function effectively as humectant agent thereby replacing a significant portion or all of traditional humectant components such as glycerin, sorbitol and other polyols. The binding / thickening system comprise select carrageenans that provide a water viscosity of at least about 20 mPa·s in a 1.5% solution at 25° C. and effective water-binding capacity to prevent significant water loss from the composition when exposed to air to cause unacceptable drying out. For example, the water-binding capacity of the carrageenan must be effective such that there is no more than about 0.75% water loss from a dentifrice composition when exposed to air for 30 minutes at room temperature conditions and 50% relative humidity. The present dentifrice compositions exhibit increased dispersibility in saliva during use, which provides for increased contact time of the composition with the user's teeth and oral cavity tissues such that the active dental agents contained therein are more rapidly available to effect their beneficial activity.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com