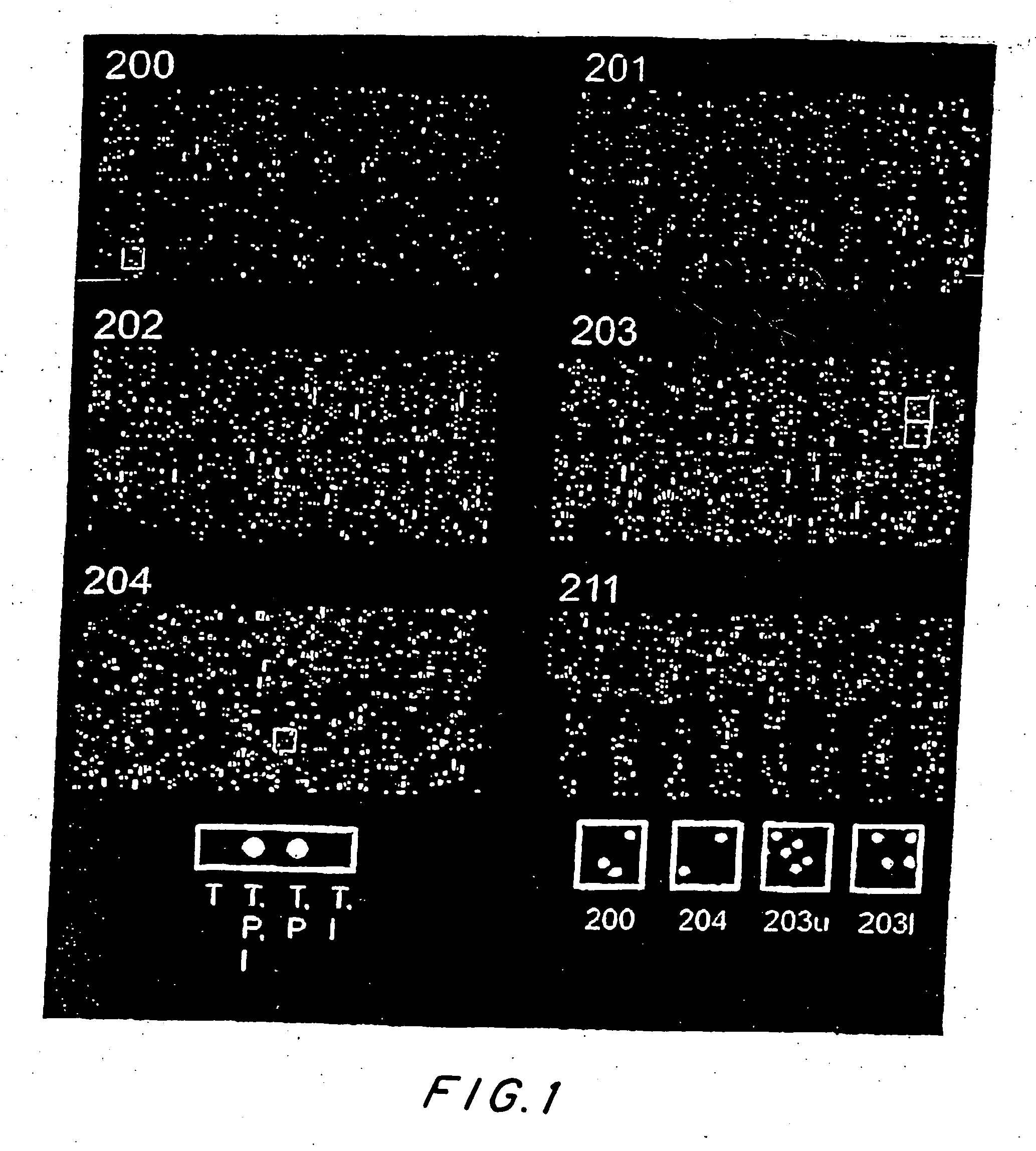
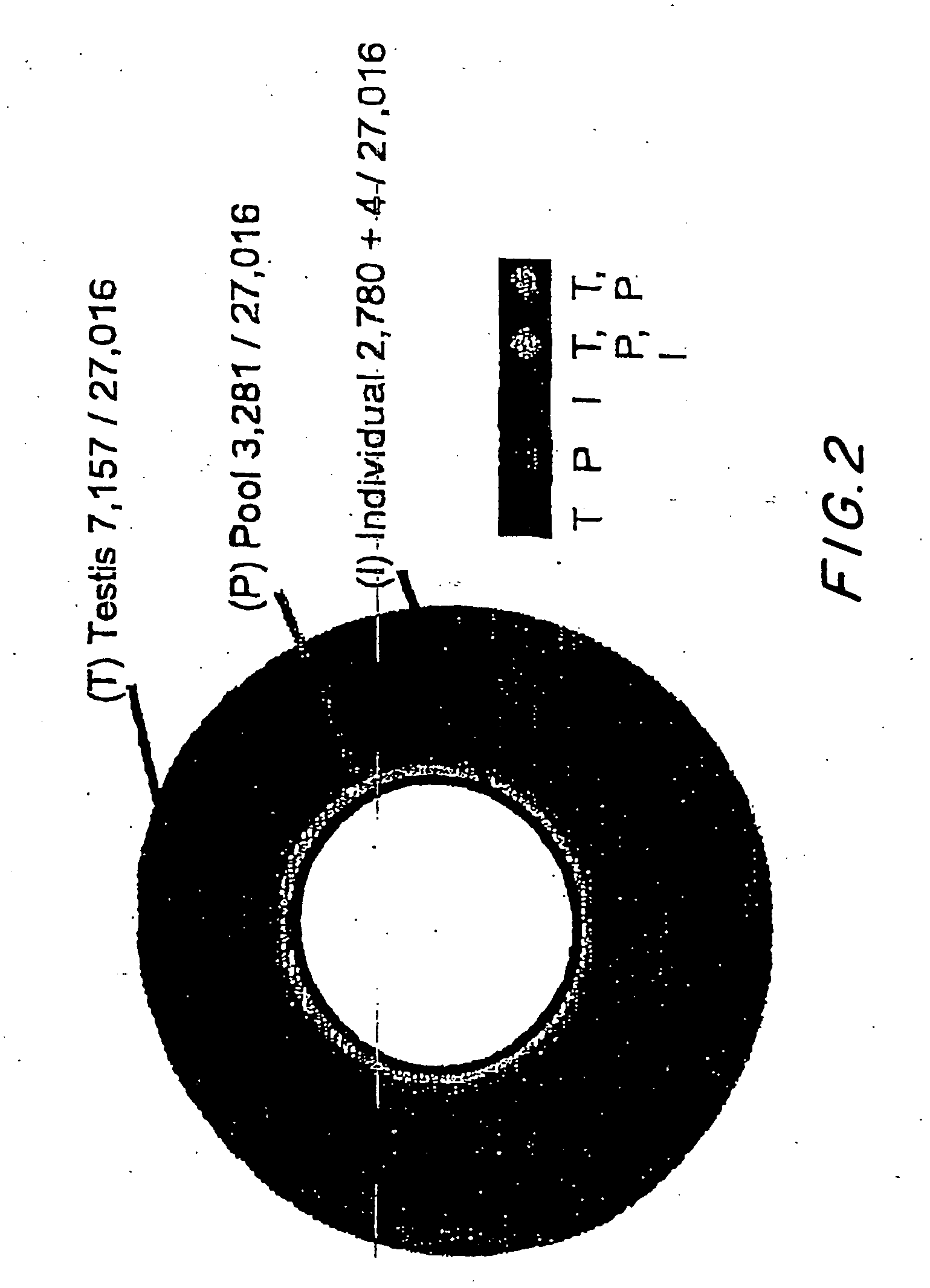
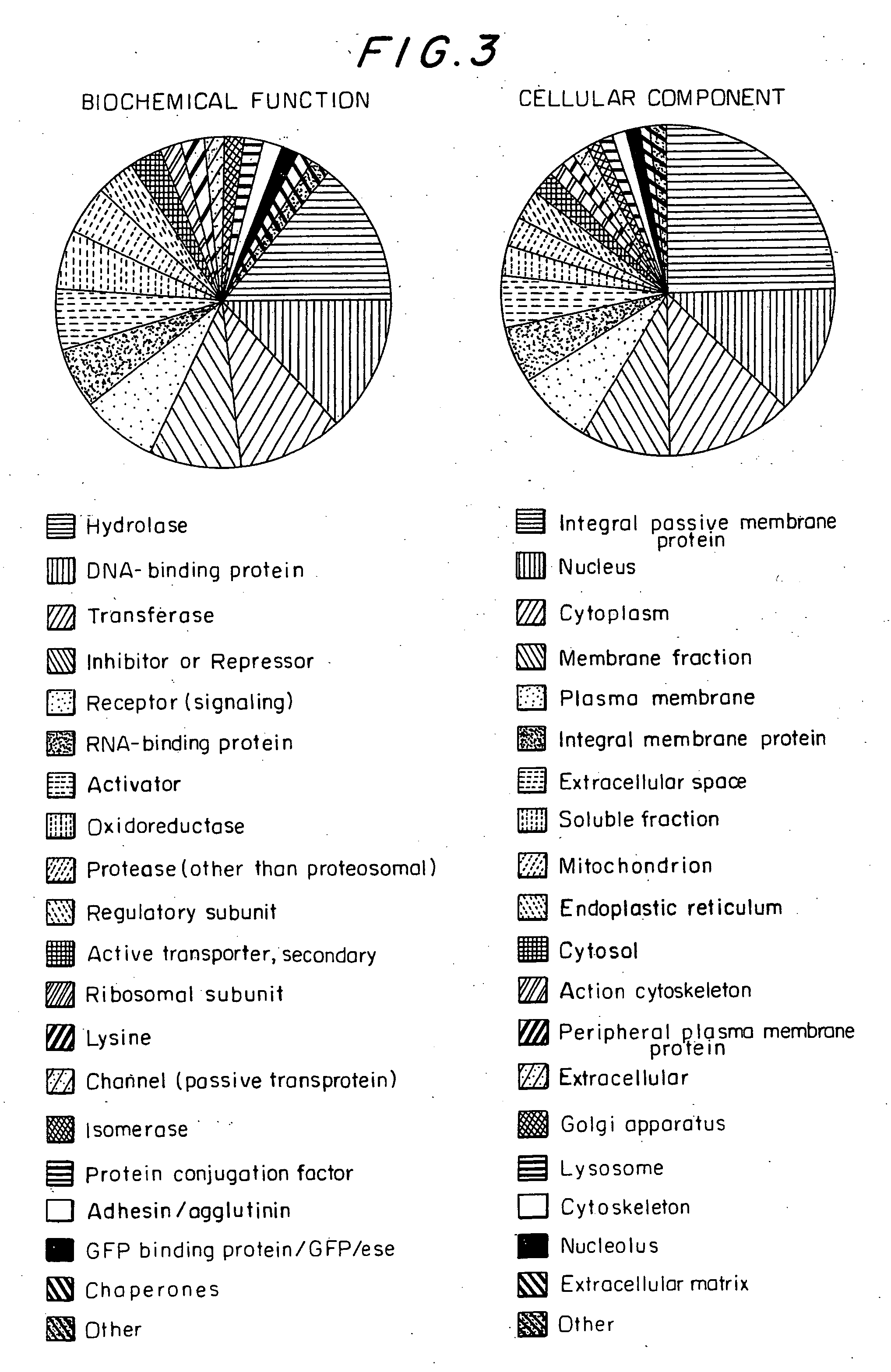
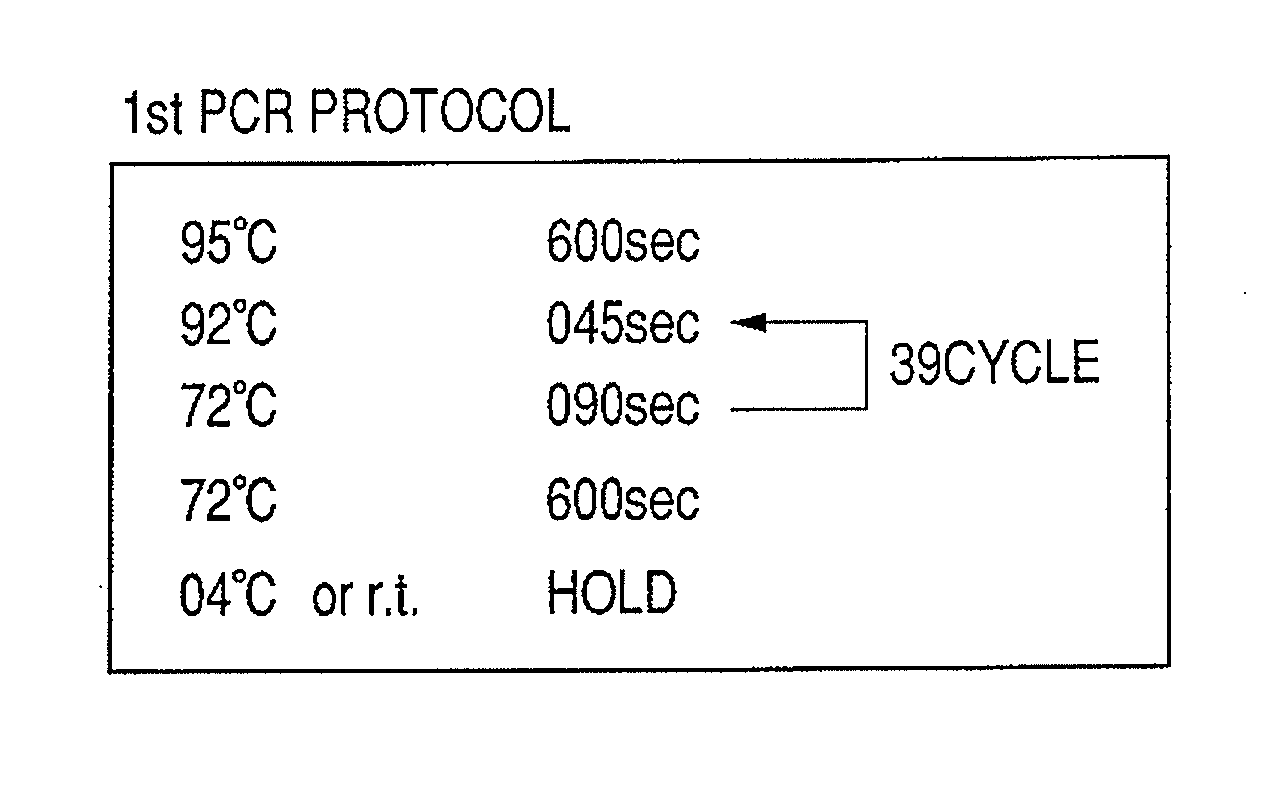
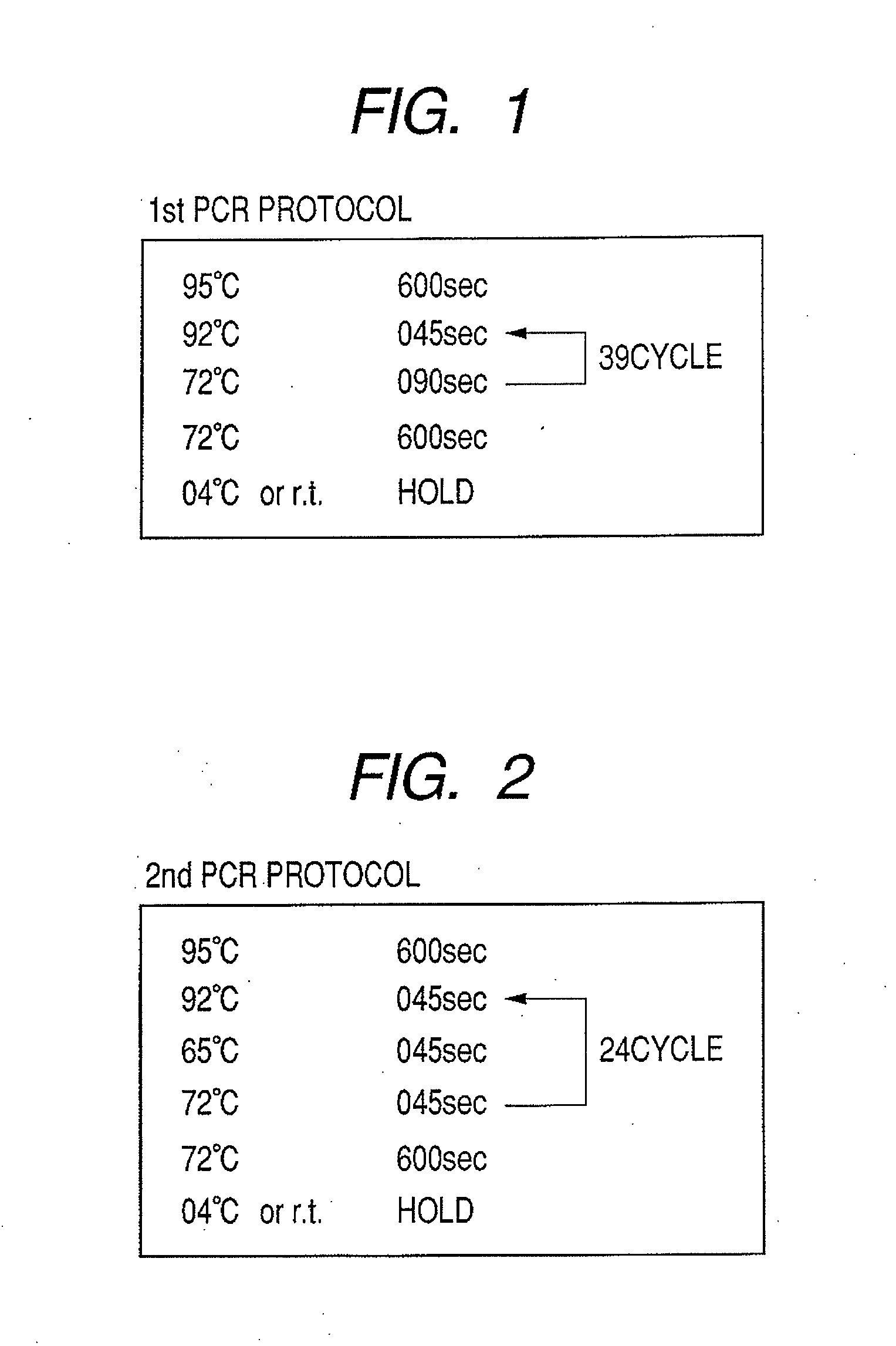
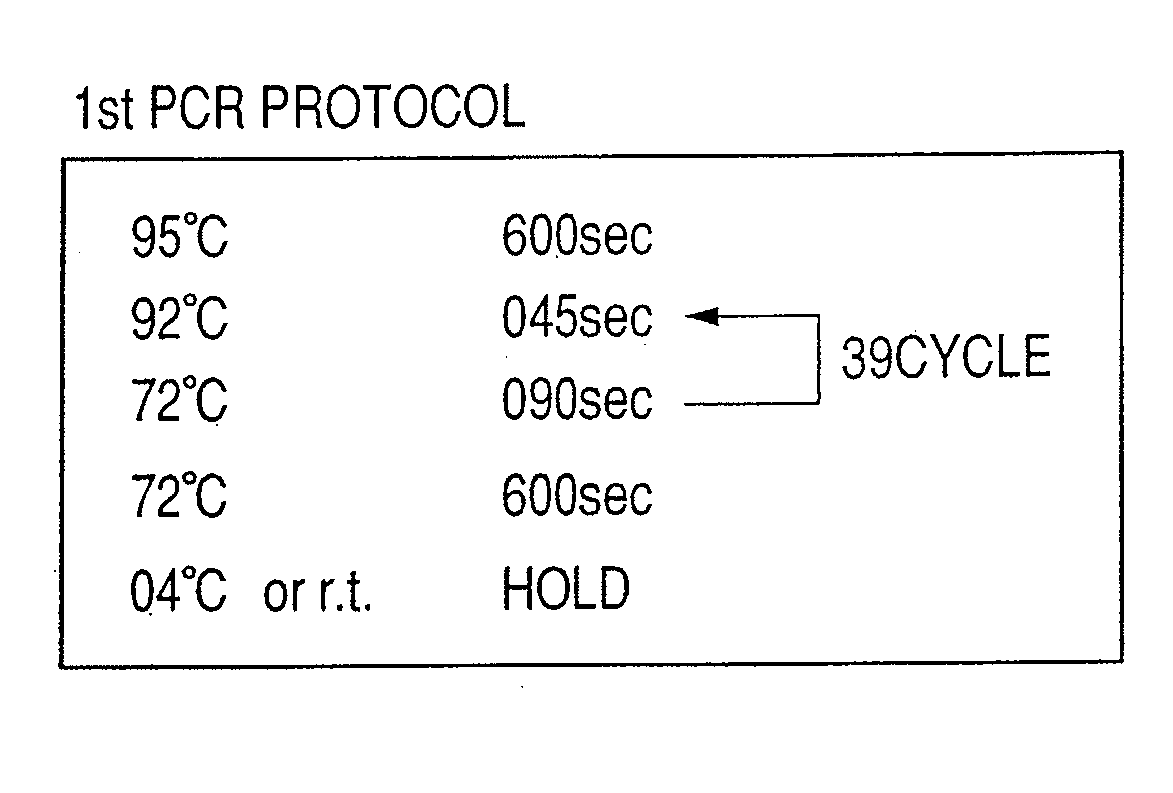
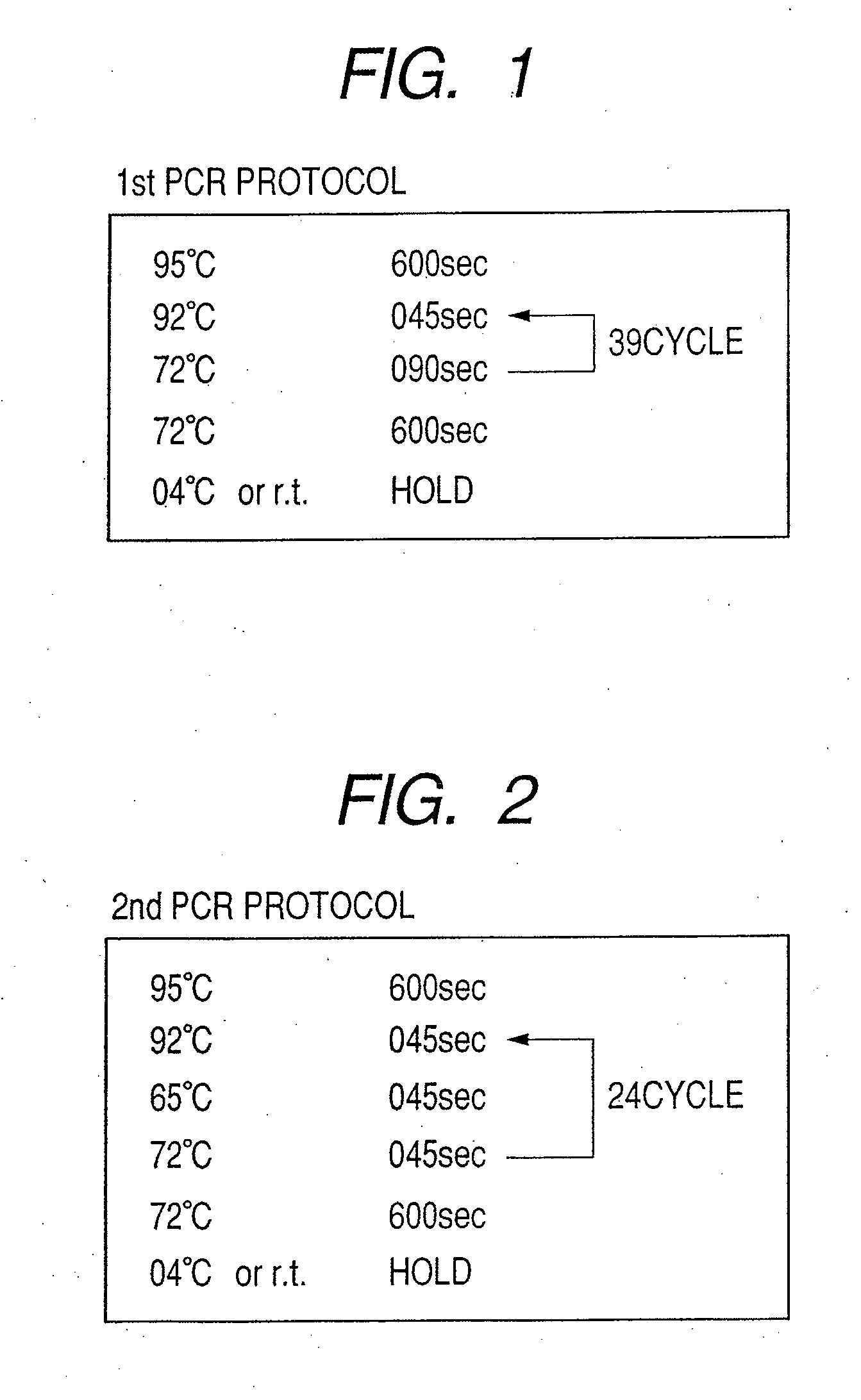
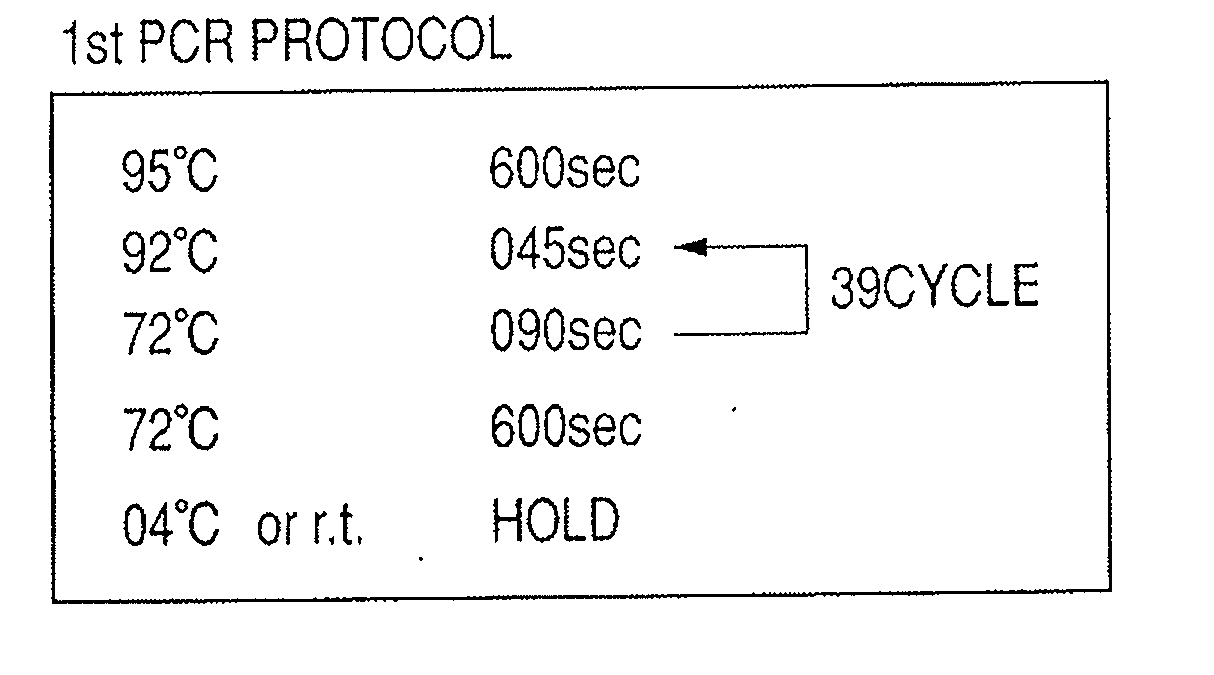
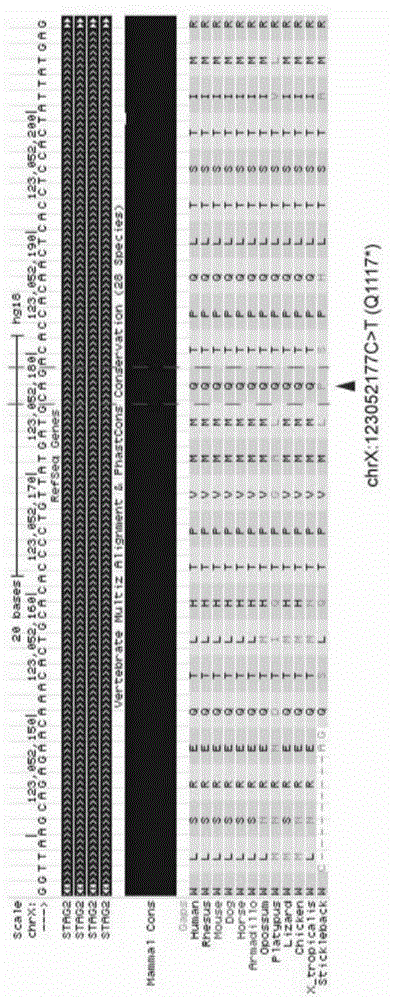
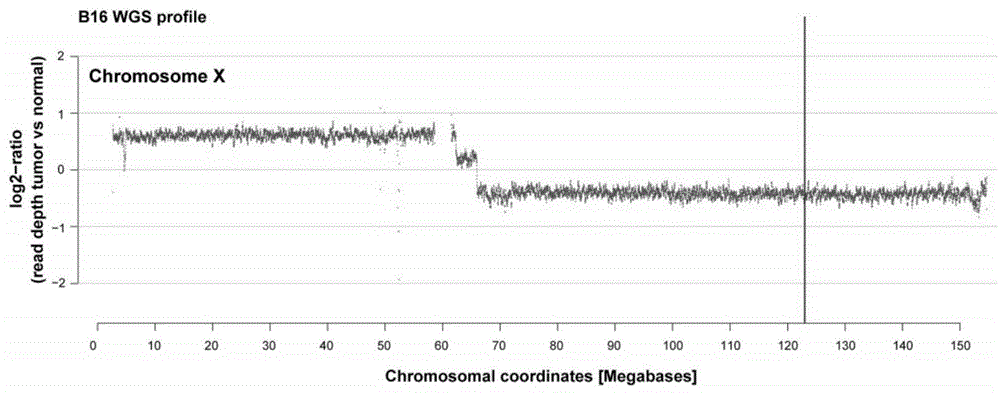
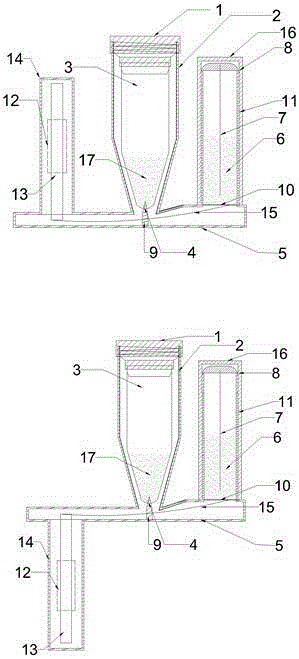
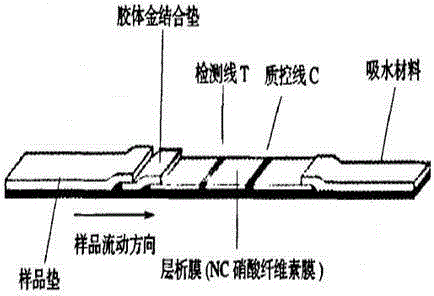
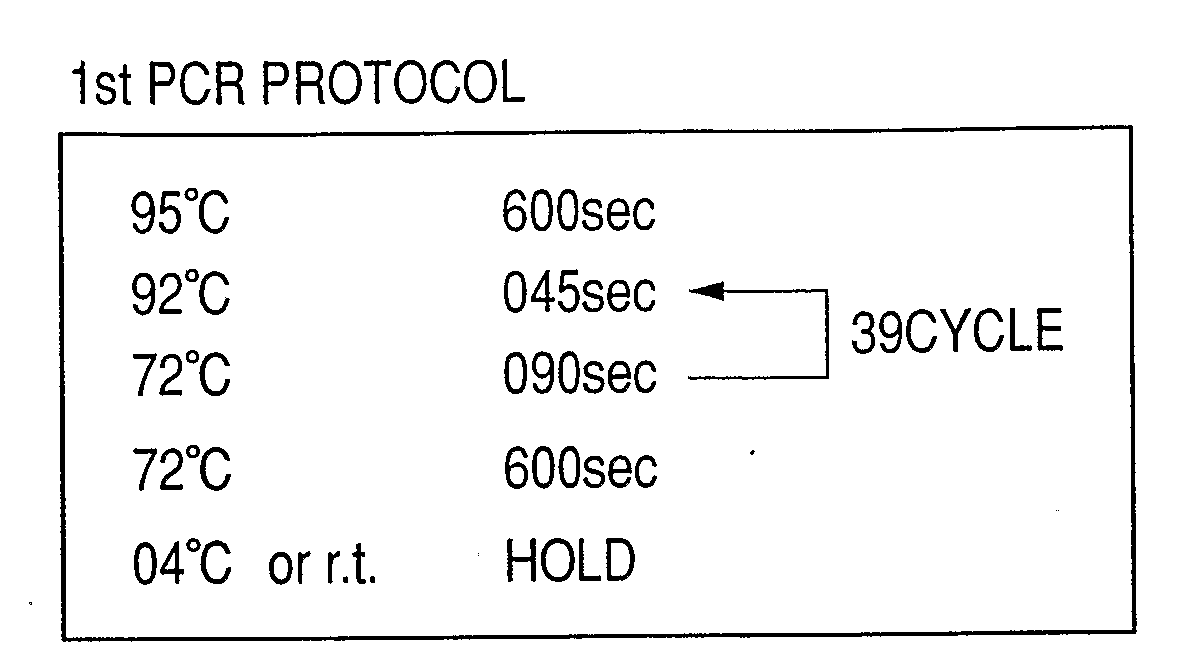
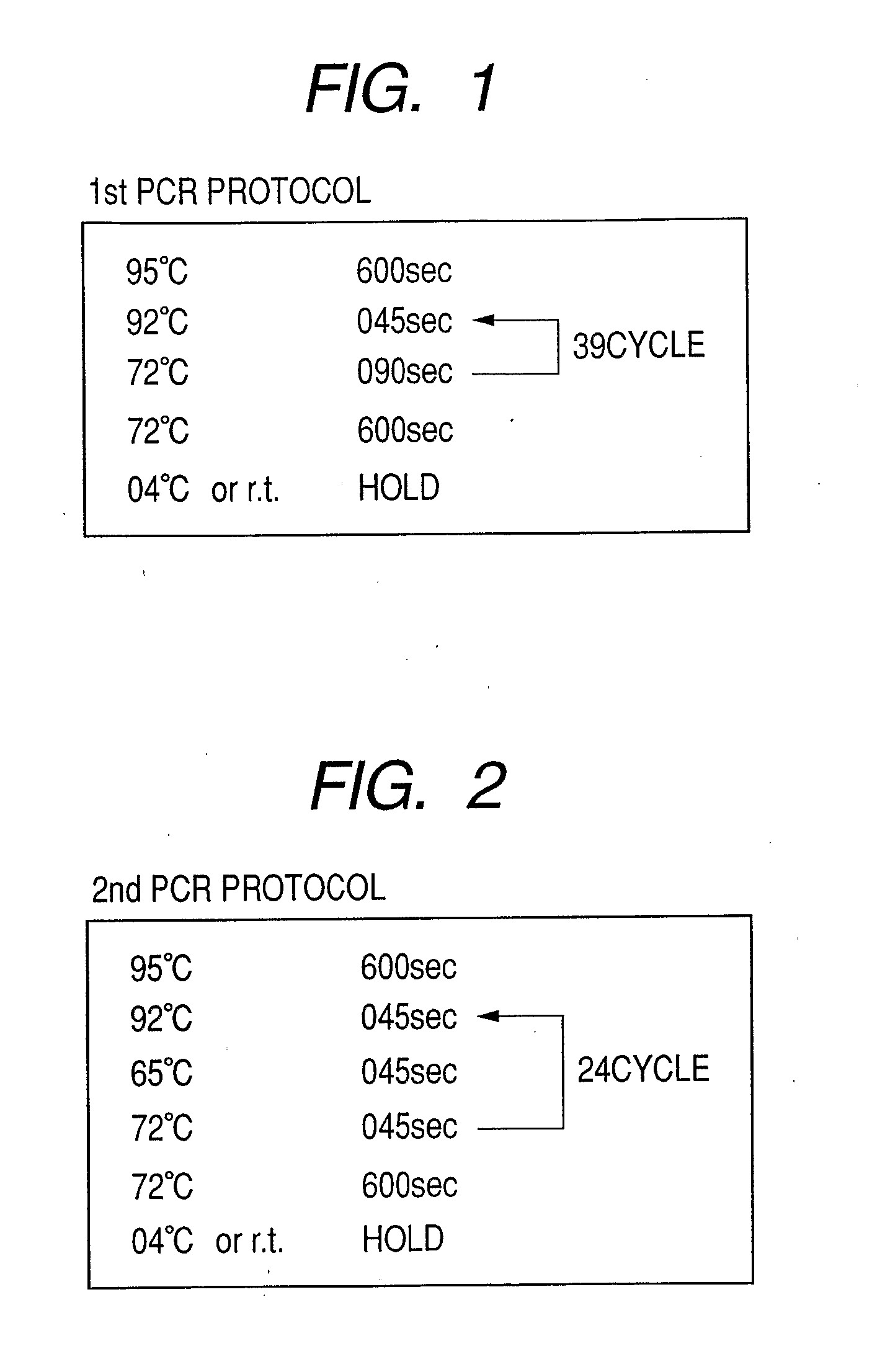
Patents
Literature
2131 results about "Genetic testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
<ul><li>Results of molecular diagnostic tests report mutations that can lead to single gene disorders and chromosomal analyses report abnormalities in chromosomes such as deletions and duplications that could be indicative of specific syndromes.</li><li>Test results can determine the presence of, or risk of developing/passing on certain diseases and sometimes also help to decide the appropriate medical treatment.</li></ul>
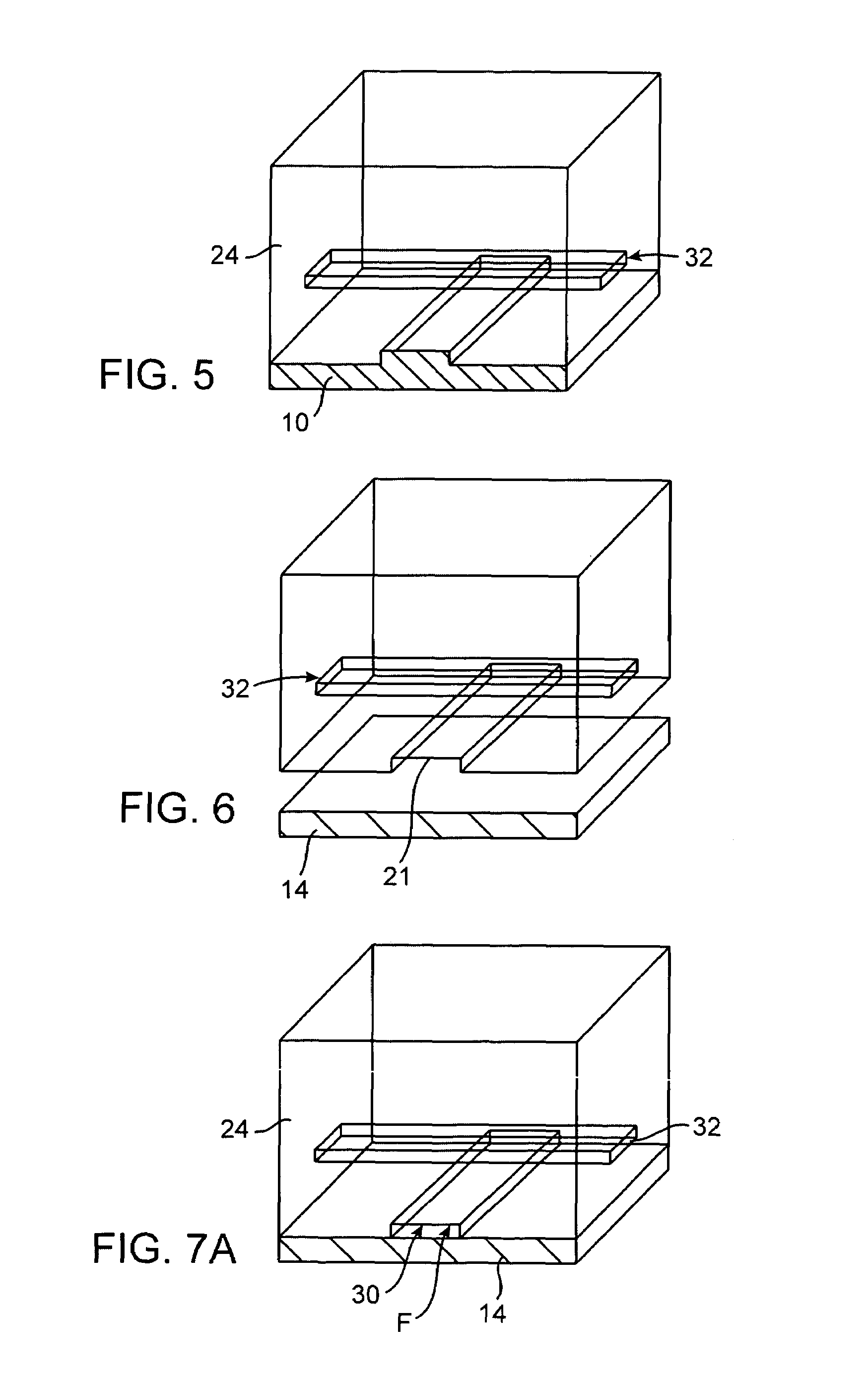
Gene detection field-effect device and method of analyzing gene polymorphism therewith
ActiveUS7695907B2High degreeReduction of size and costBioreactor/fermenter combinationsBiological substance pretreatmentsNucleic Acid ProbesBiology
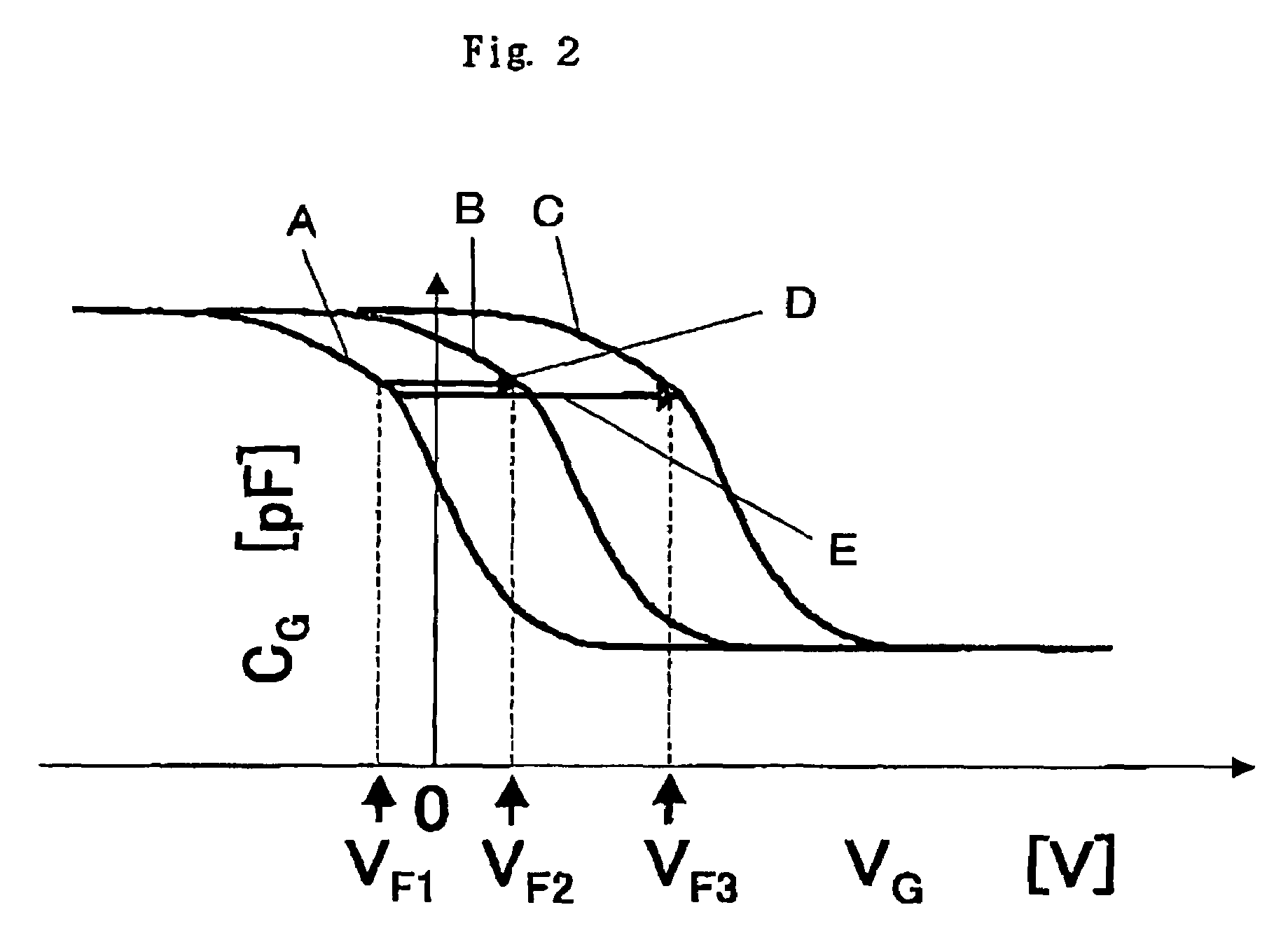
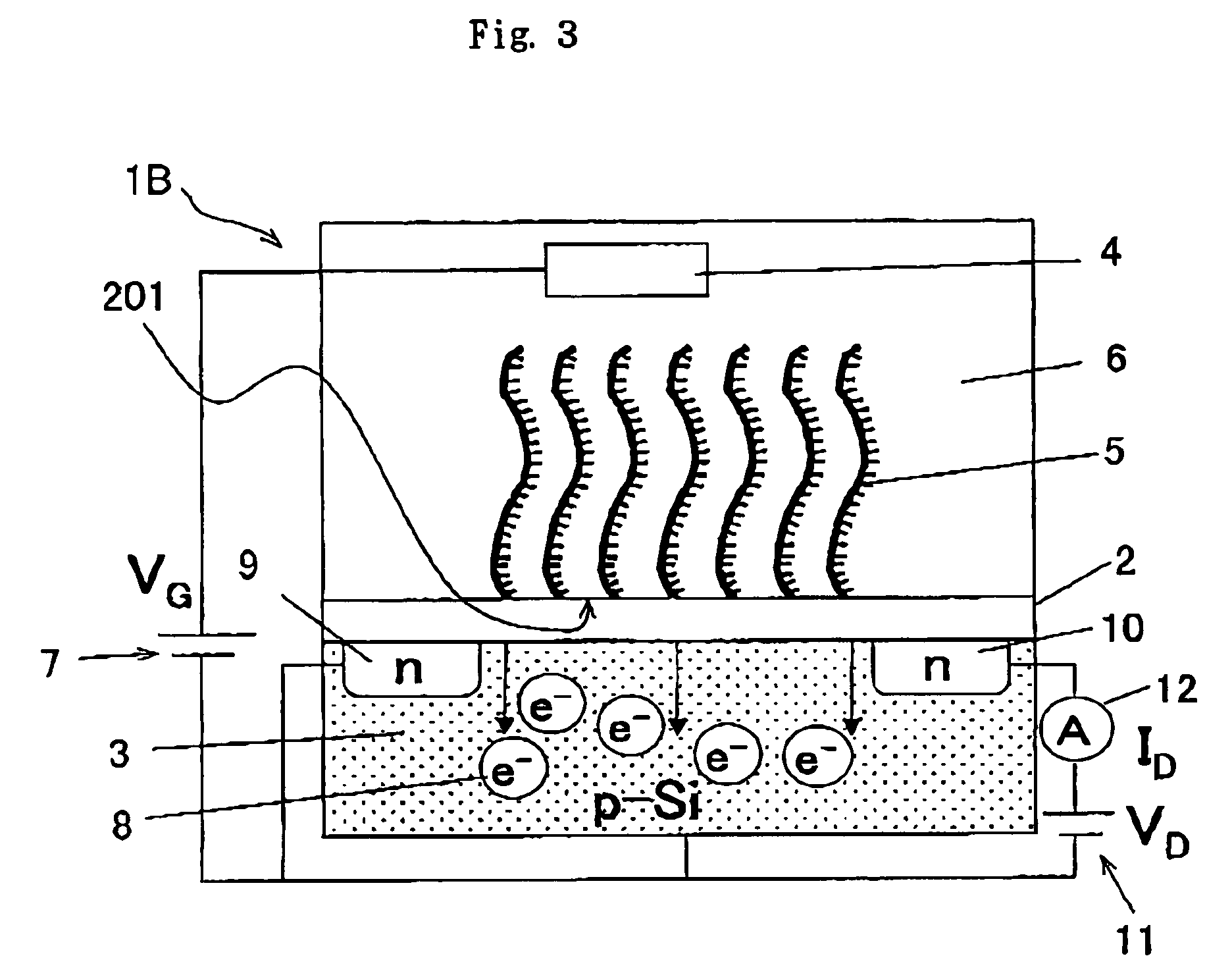
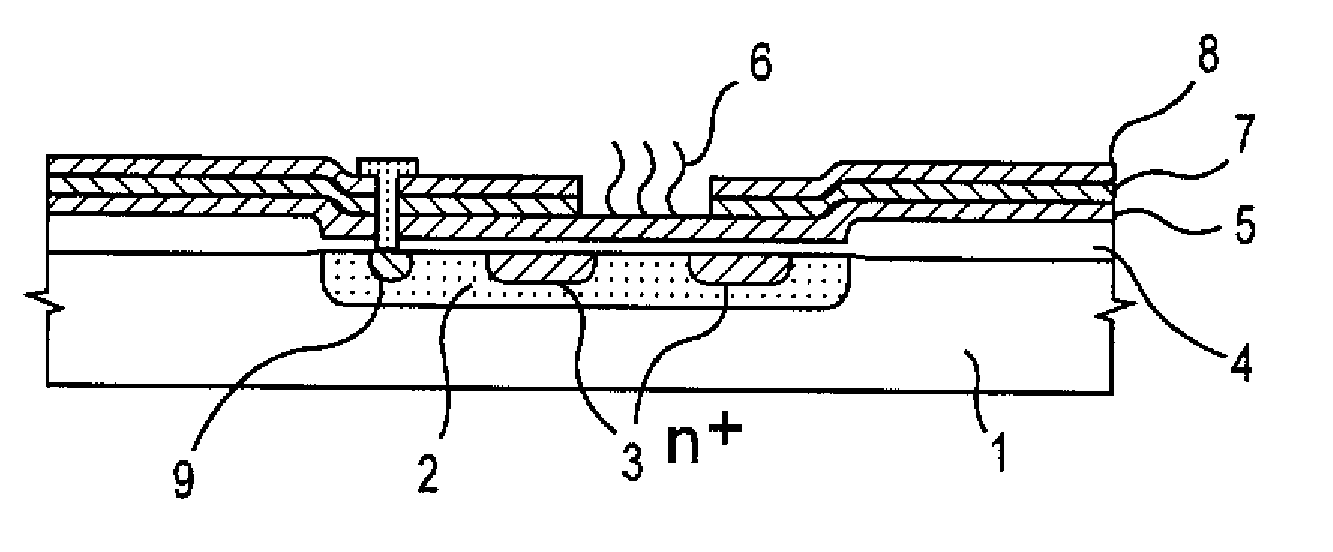
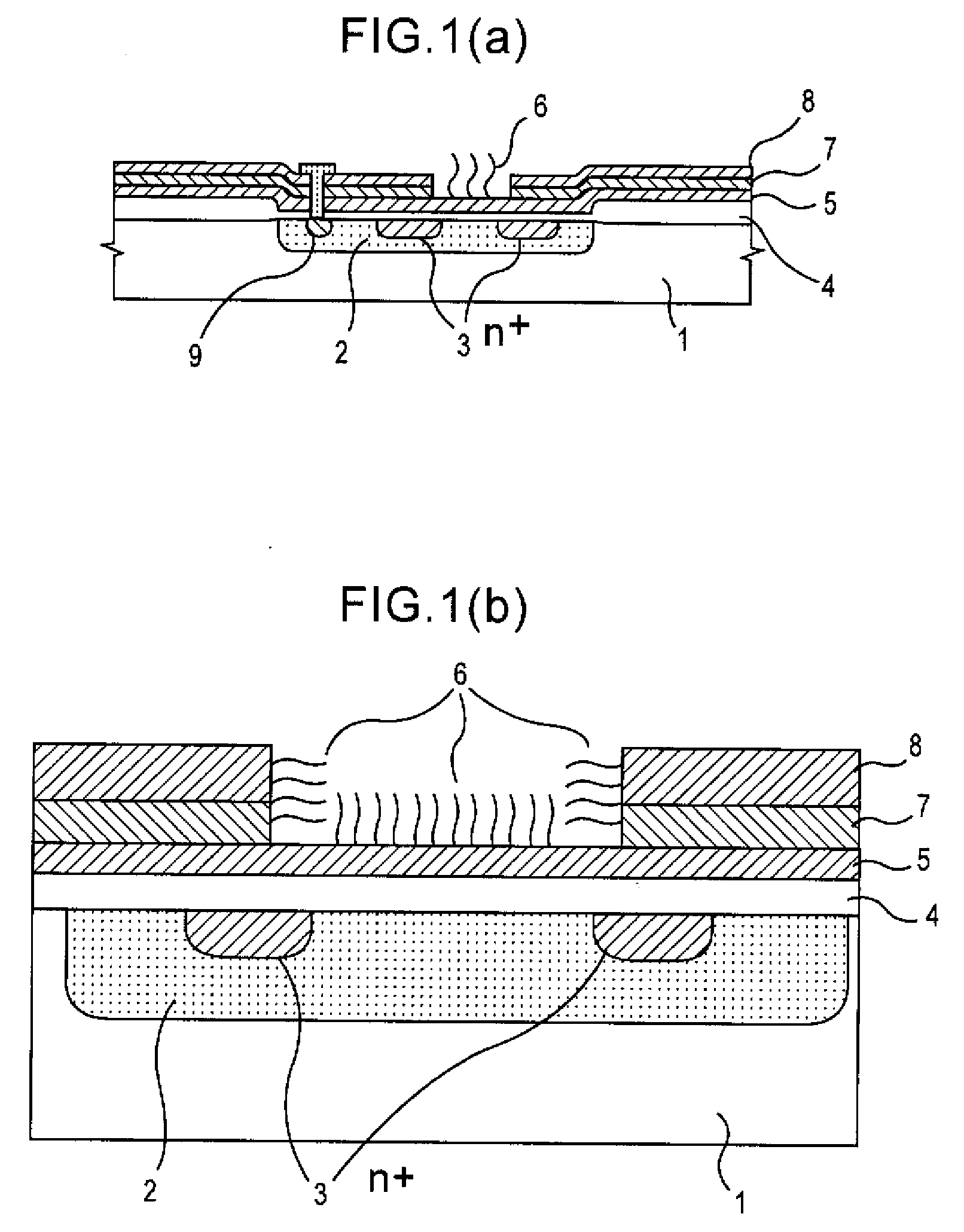
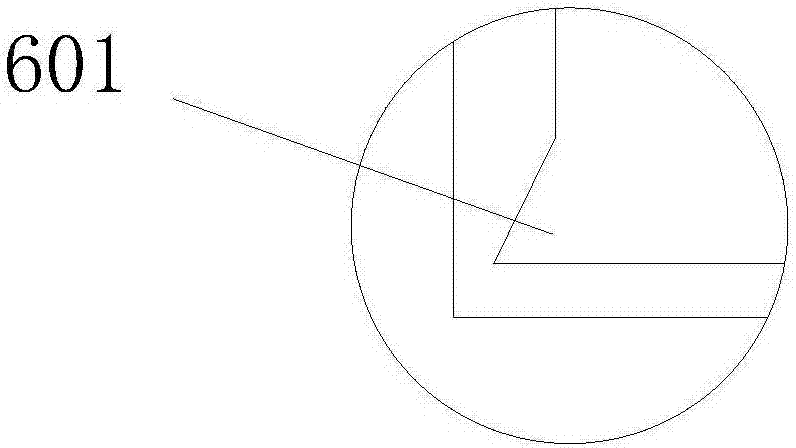
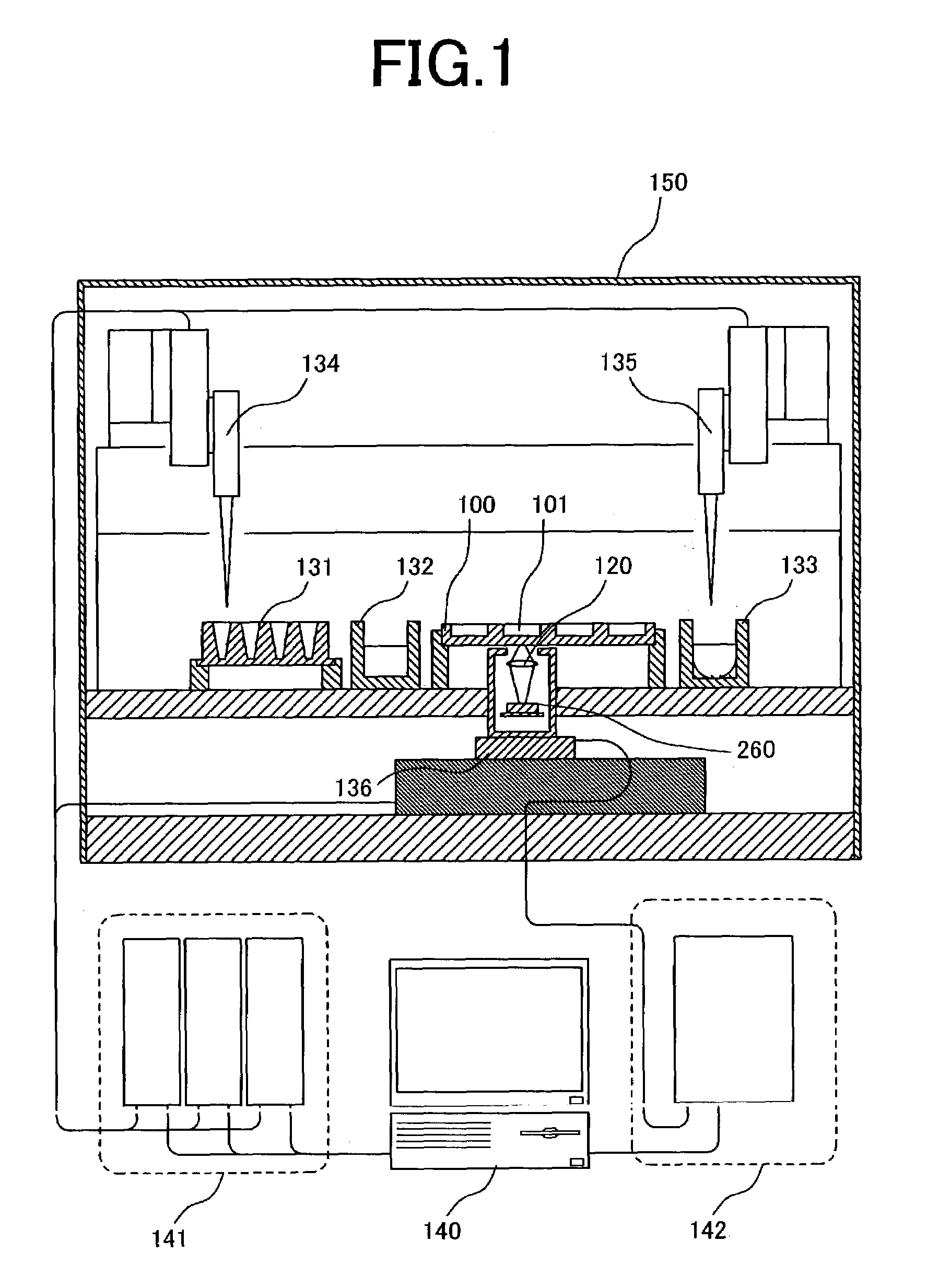
A gene detection field-effect device provided with an insulation film (2), a semiconductor substrate (3), and a reference electrode (4), includes: (a) the insulation film (2) including a nucleic acid probe (5) immobilized on one of the surfaces thereof and is in contact with a sample solution (6) containing at least one type of a target gene (601) for detection and analysis; (b) the semiconductor substrate (3) being installed so as to abut against the other surface of the insulation film (2); and (c) the reference electrode (4) being provided in the sample solution (6).
Owner:NAT INST FOR MATERIALS SCI
Genetic analysis and authentication
InactiveUS7157228B2Reduce morbidityBioreactor/fermenter combinationsBiological substance pretreatmentsBiological bodyGenetic Screening (procedure)
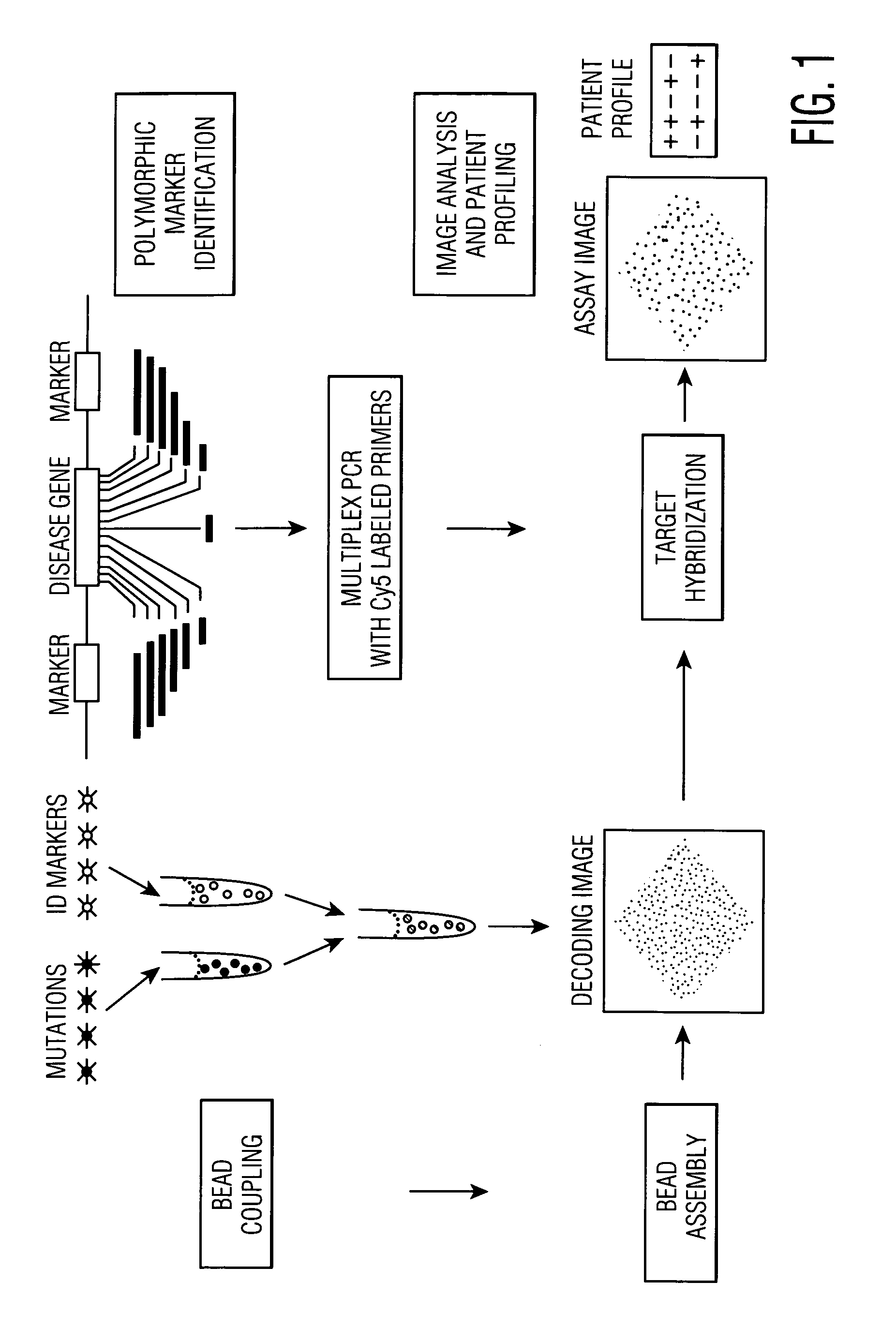
This invention provides compositions and methods for genetic testing of an organism and for correlating the results of the genetic testing with a unique marker that unambiguously identifies the organism. The markers may be internal markers, such as for example single nucleotide polymorphisms (SNPs), short tandem repeats (STRs), or other sites within a genomic locus. Alternatively, the markers may be external, such that they are separately added to the genetic sample before testing.
Owner:BIOARRAY SOLUTIONS
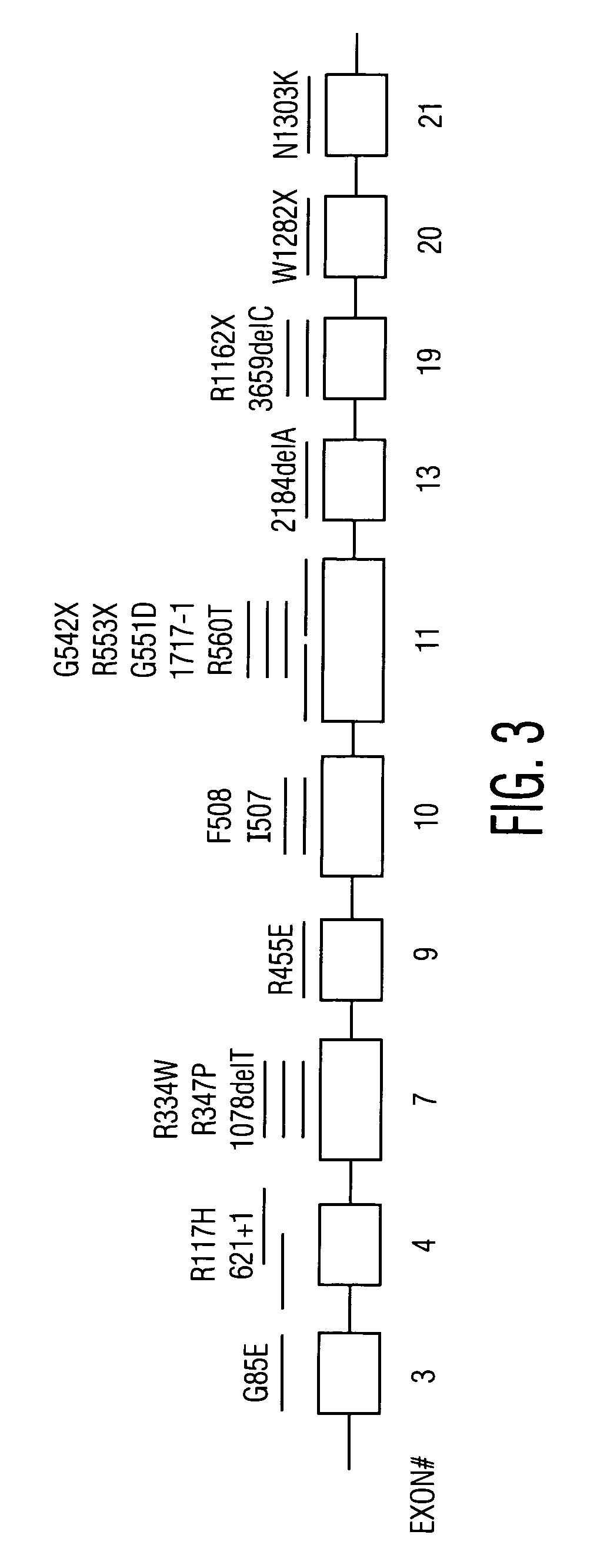
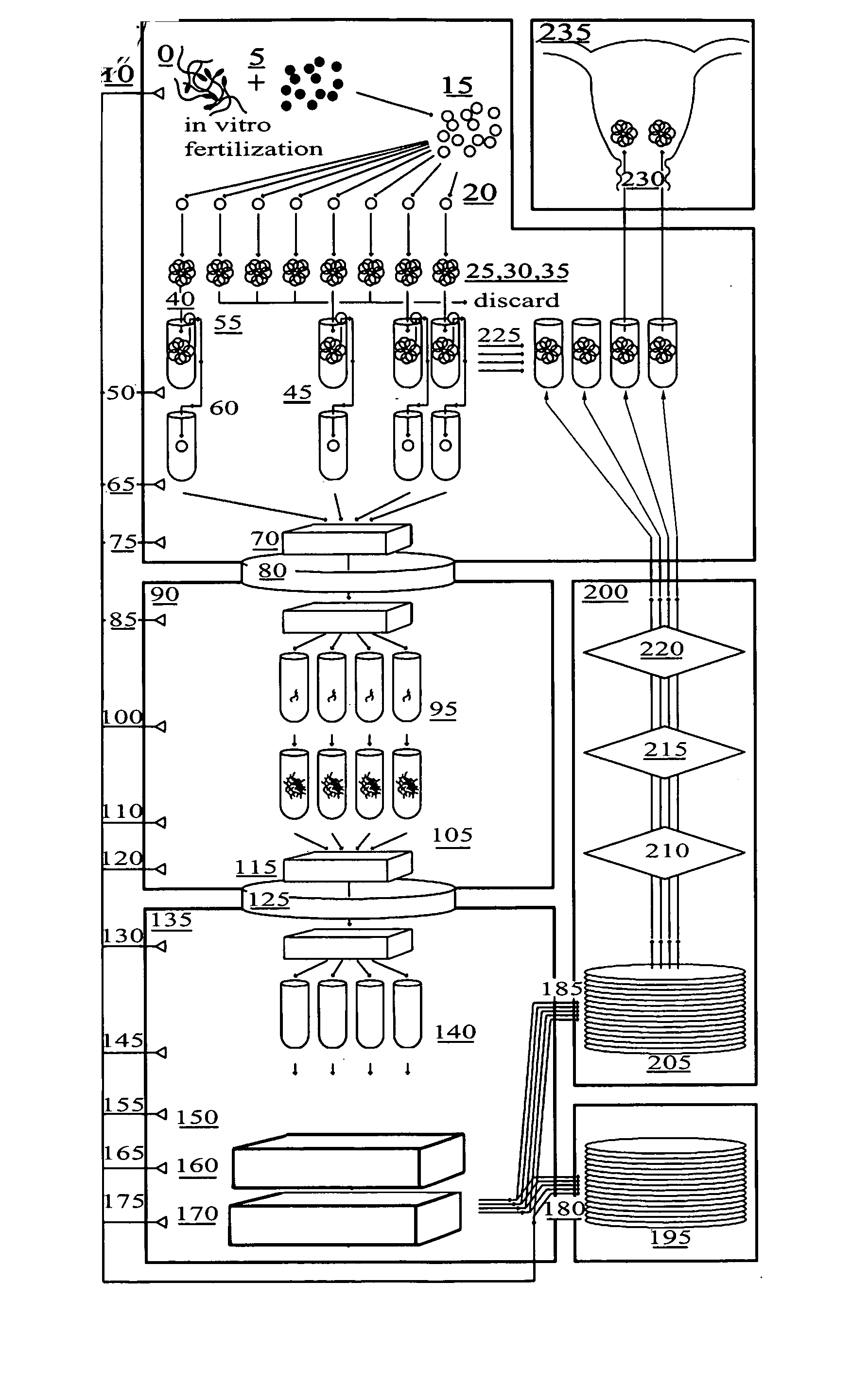
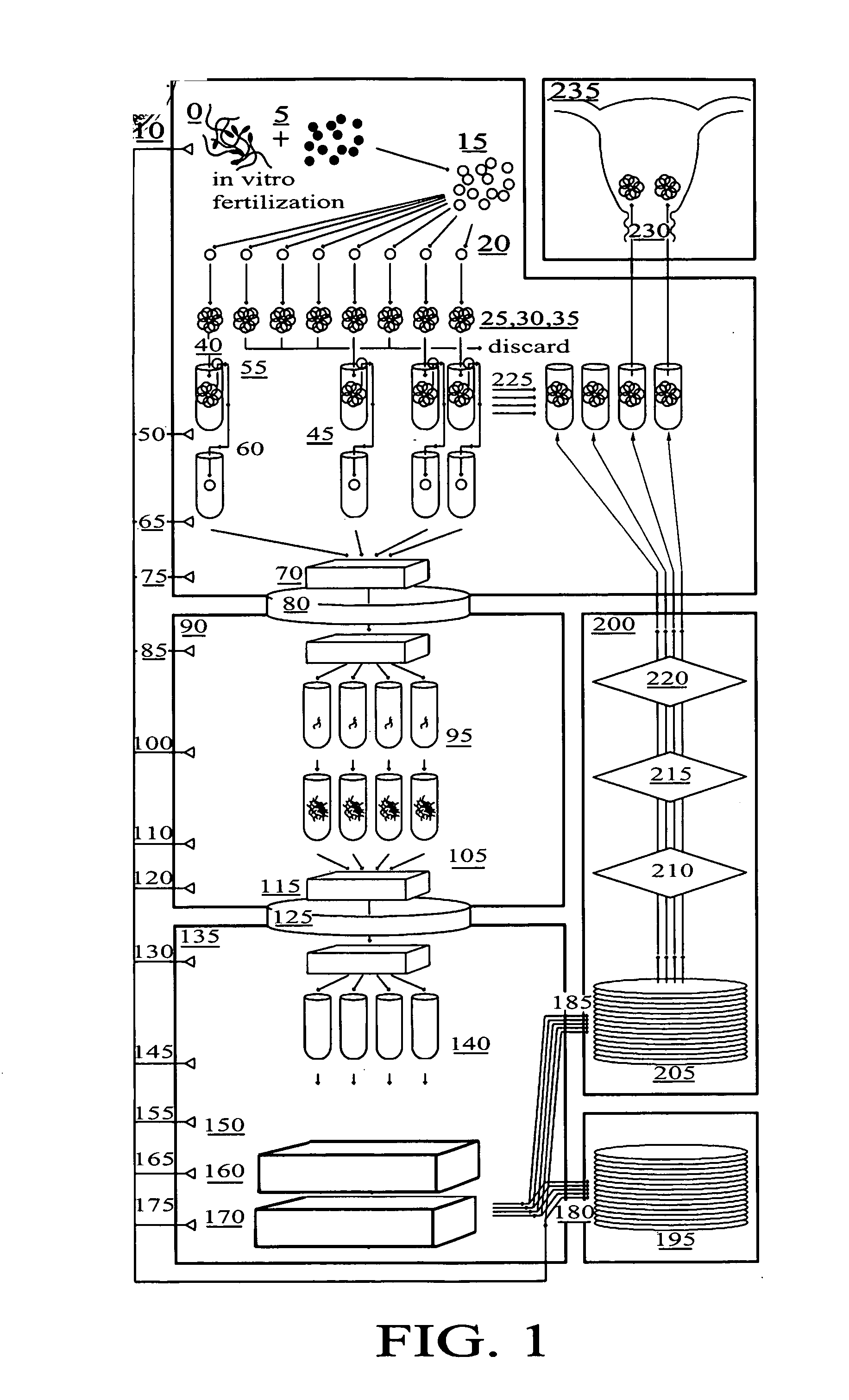
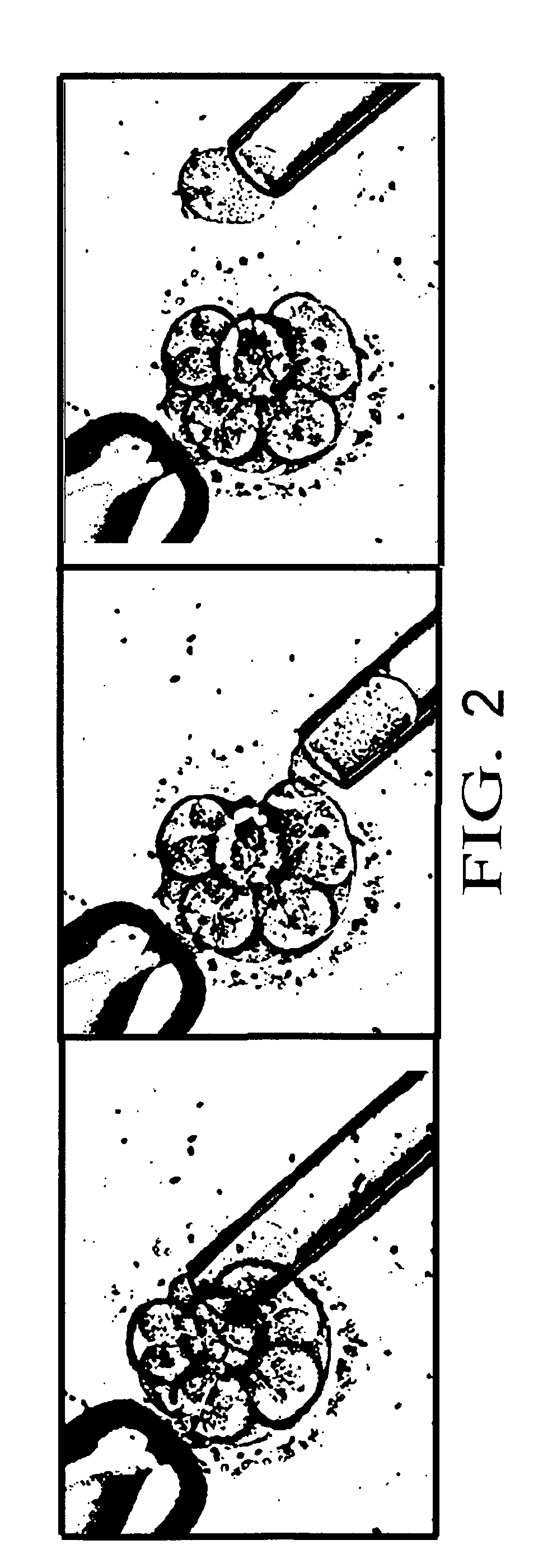
Method for genetic testing of human embryos for chromosome abnormalities, segregating genetic disorders with or without a known mutation and mitochondrial disorders following in vitro fertilization (IVF), embryo culture and embryo biopsy
InactiveUS20080085836A1Reduce significant riskImprove the level ofLibrary screeningLibrary member identificationLess invasiveContamination
We describe a method for interrogating the content and primary structure of DNA by microarray analyses and to provide comprehensive genetic screening and diagnostics prior to embryo transfer within an IVF setting. We will accomplish this by the following claims: 1) an optimized embryo grading system, 2) a less invasive embryo biopsy with reduced cellular contamination, 3) an optimized DNA amplification protocol for single cells, 4) identify aneuploidy and structural chromosome abnormalities using microarrays, 5) identifying sub-telomeric chromosome rearrangements, 6) a modified DNA fingerprinting protocol, 7) determine imprinting and epigenetic changes in developing embryos, 8) performing genome-wide scans to clarify / diagnose multi-factorial genetic disease and to determine genotype / haplotype patterns that may predict future disease, 9) determining single gene disorders with or without a known DNA mutation, 10) determining mtDNA mutations and / or the combination of mtDNA and genomic (nuclear) DNA aberrations that cause genetic disease.
Owner:KEARNS WILLIAM G +1
Method of Analyzing Dna Sequence Using Field-Effect Device, and Base Sequence Analyzer
ActiveUS20080286767A1Bioreactor/fermenter combinationsHeating or cooling apparatusAnalysis dnaFluorescence
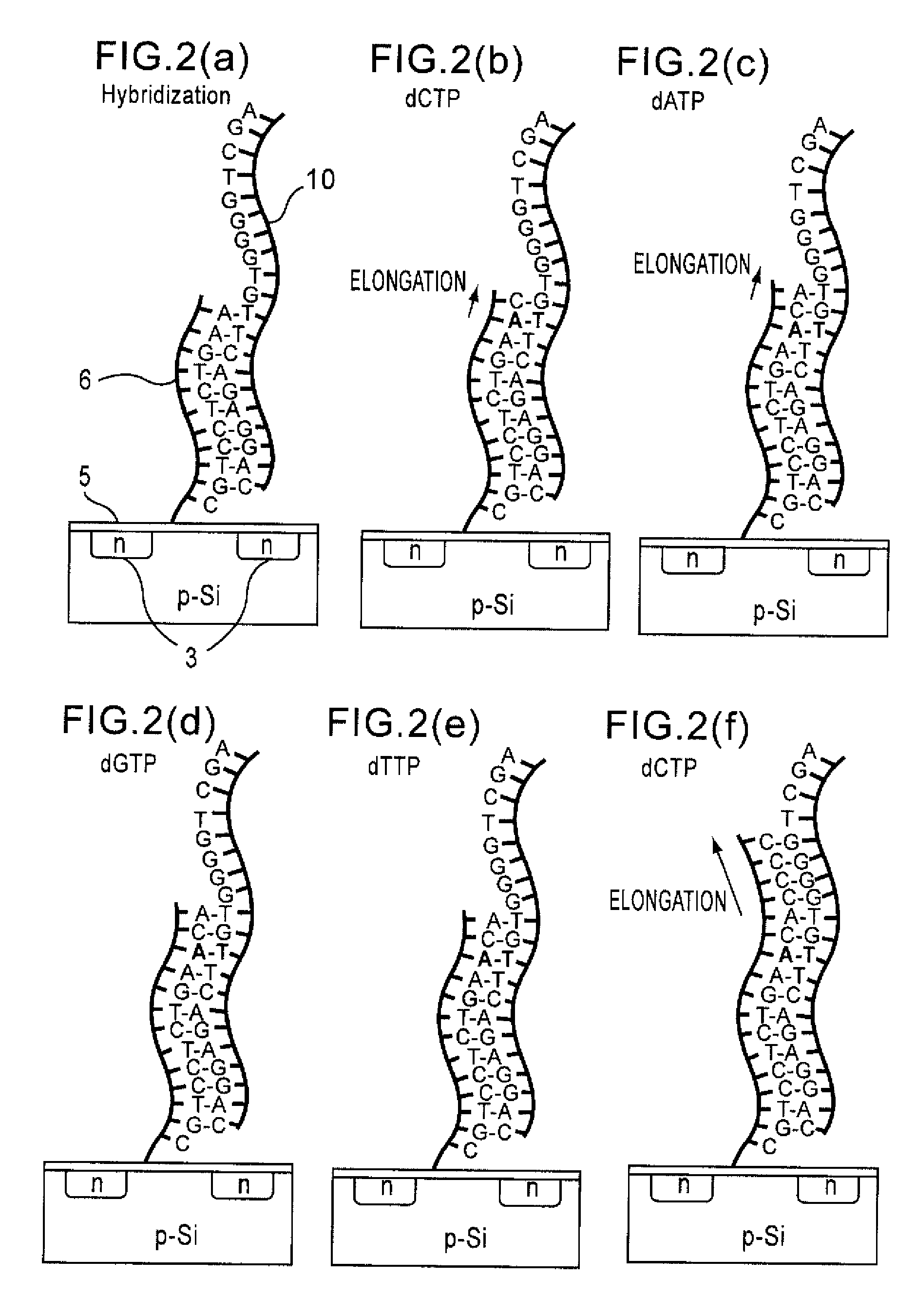
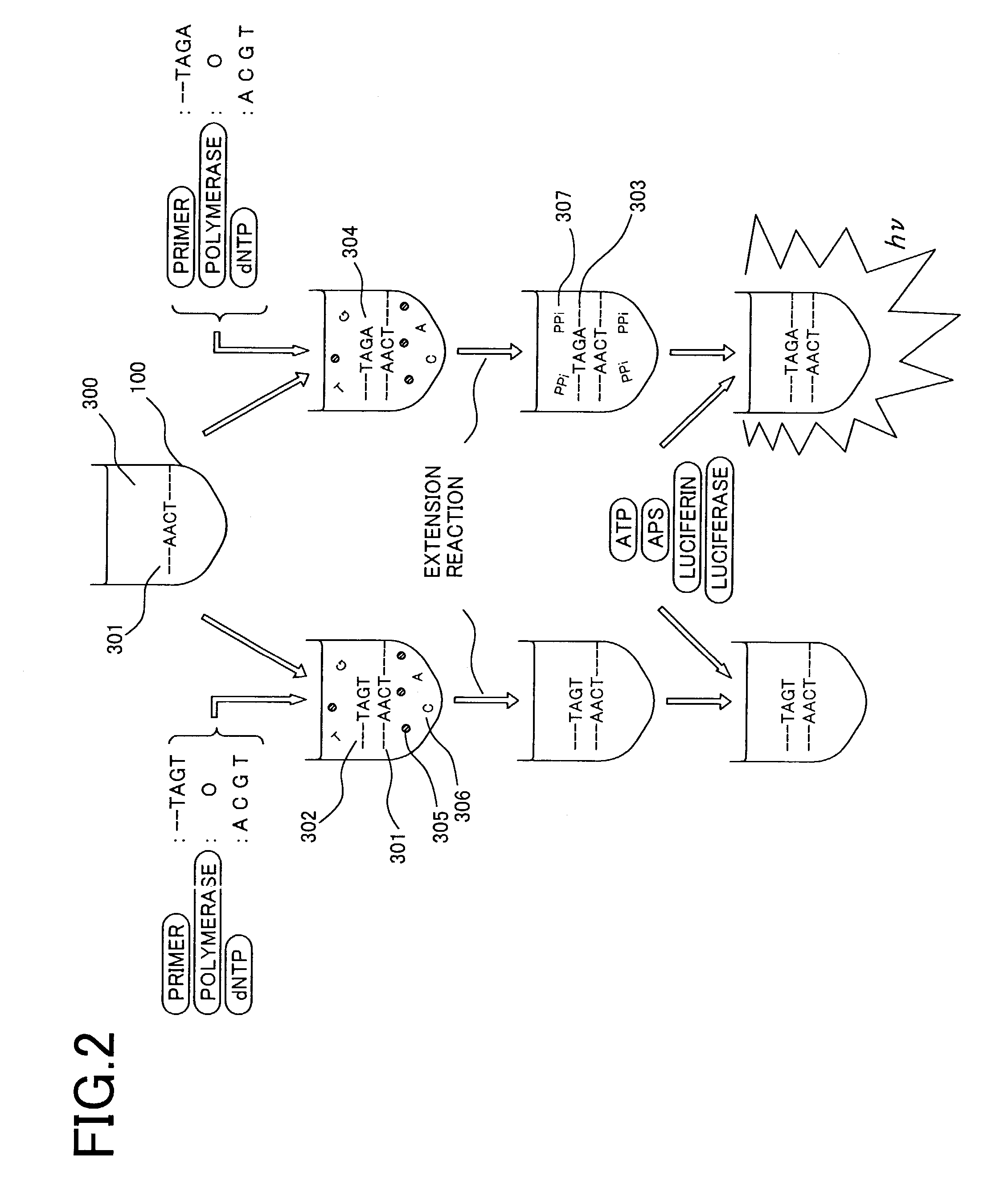
Since conventional DNA sequence analyzing technologies are based on the fundamental principle of fluorescent detection, expensive, complex optical systems and laser sources have been necessary.A field-effect device for gene detection of the present invention analyzes a base sequence by immobilizing a single-strand nucleic acid probe at a gate portion, inducing hybridization at the gate portion to form a double-stranded DNA, inducing elongation reaction by adding a DNA polymerase and one of the substrates, and measuring the electrical characteristic of the field-effect device caused by elongation reaction.Since the elongation reaction of one base induced at the gate portion can be directly converted to an electrical signal, expensive lasers or complex optical systems are not needed. Thus, a small gene polymorphism detection system that can conduct measurement at high precision can be provided.
Owner:NAT INST FOR MATERIALS SCI
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20080124733A1Quickly and precisely identifiedAccurate distinctionSugar derivativesMicrobiological testing/measurementNucleic Acid ProbesMicrobiology
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 90 to 91 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
Probe set, probe immobilized carrier and gene examination method
InactiveUS20070134702A1Improve accuracyThe process is fast and accurateSugar derivativesMicrobiological testing/measurementMicroorganismMicrobiology
A probe and a primer capable of collectively detecting microorganisms of the same species while differentiating microorganisms of other species with an object of classification by species of fungus. An oligonucleotide probe for detecting an infectious etiologic microorganism gene includes at least one base sequence selected from the base sequences belonging to one group of the following first to ninth groups. The base sequence groups of first to ninth groups are: first group (SEQ ID NOS:1 to 5); second group (SEQ ID NOS:6 to 10); third group (SEQ ID NOS:11 to 15); fourth group (SEQ ID NOS:16 to 21); fifth group (SEQ ID NOS:22 to 26); sixth group (SEQ ID NOS:27 to 31); seventh group (SEQ ID NOS:32 to 36); eighth group (SEQ ID NOS:37 to 41); and ninth group (SEQ ID NOS:42 to 46).
Owner:CANON KK
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20080113365A1Quickly and precisely detectAccurate identificationBioreactor/fermenter combinationsBiological substance pretreatmentsInfectious DisorderNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 76 to 77 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
Genetic testing for male factor infertility
RNA in sperm can be used as a diagnostic to distinguish between normal and affected individuals. A list of specific diagnostic transcripts is provided which is compared with transcripts obtained from the sperm of a subject. Correlation between the two transcripts is used to identify normal sperm or affected sperm. Addition, genetic testing for male infertility or damage to spermatozoa is accomplished by providing a microarray of DNA probes with a sample of spermatozoa to determine the mRNA fingerprints of the sample, and comparing the mRNA fingerprints of the sample with the mRNA fingerprints of normal fertile male spermatozoa.
Owner:ENVIRONMENTAL PROTECTION AGENCY US +2
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20080113363A1Quickly and precisely identifiedAccurate distinctionSugar derivativesMicrobiological testing/measurementInfectious DisorderNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 70 to 72 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
Gene detection assay for improving the likelihood of an effective response to a her2 antibody cancer therapy
InactiveUS20070166753A1Great likelihoodChoose accuratelyOrganic active ingredientsBiocideAbnormal tissue growthAssay
The invention provides a method for more effective treatment of patients susceptible to or diagnosed with tumors overexpressing HER2, as determined by a gene amplification assay, with a HER2 antibody. Such method comprises administering a cancer-treating dose of the HER2 antibody, preferably in addition to chemotherapeutic agents, to a subject in whose tumor cells her2 has been found to be amplified e.g., by fluorescent in situ hybridization.
Owner:GENENTECH INC
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20080113366A1Quickly and precisely detectAccurate identificationBioreactor/fermenter combinationsBiological substance pretreatmentsInfectious DisorderNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 68 to 69 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20080161192A1Quickly and precisely identifiedAccurate distinctionSugar derivativesMicrobiological testing/measurementBacteroidesNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 87 to 89 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
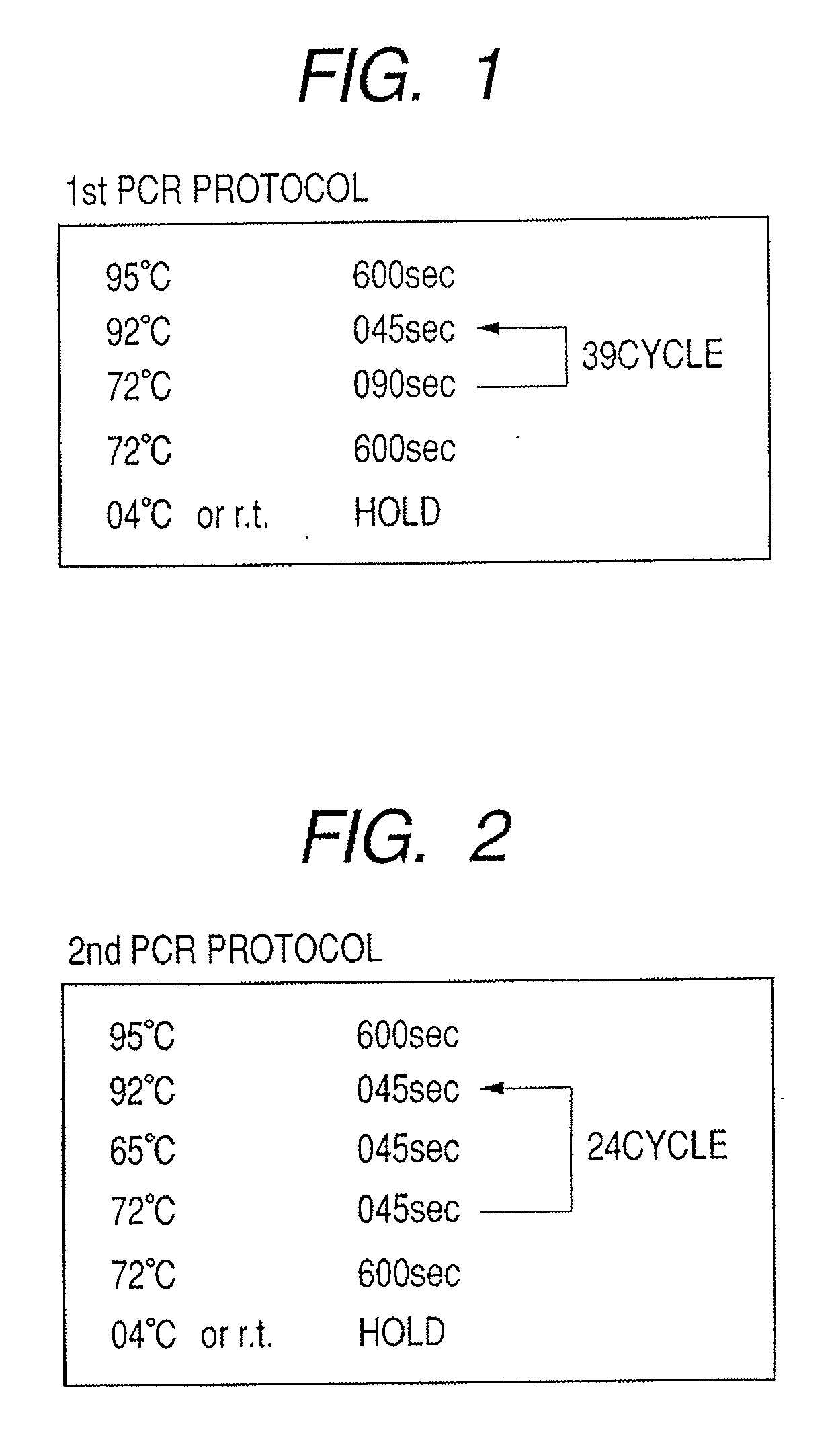
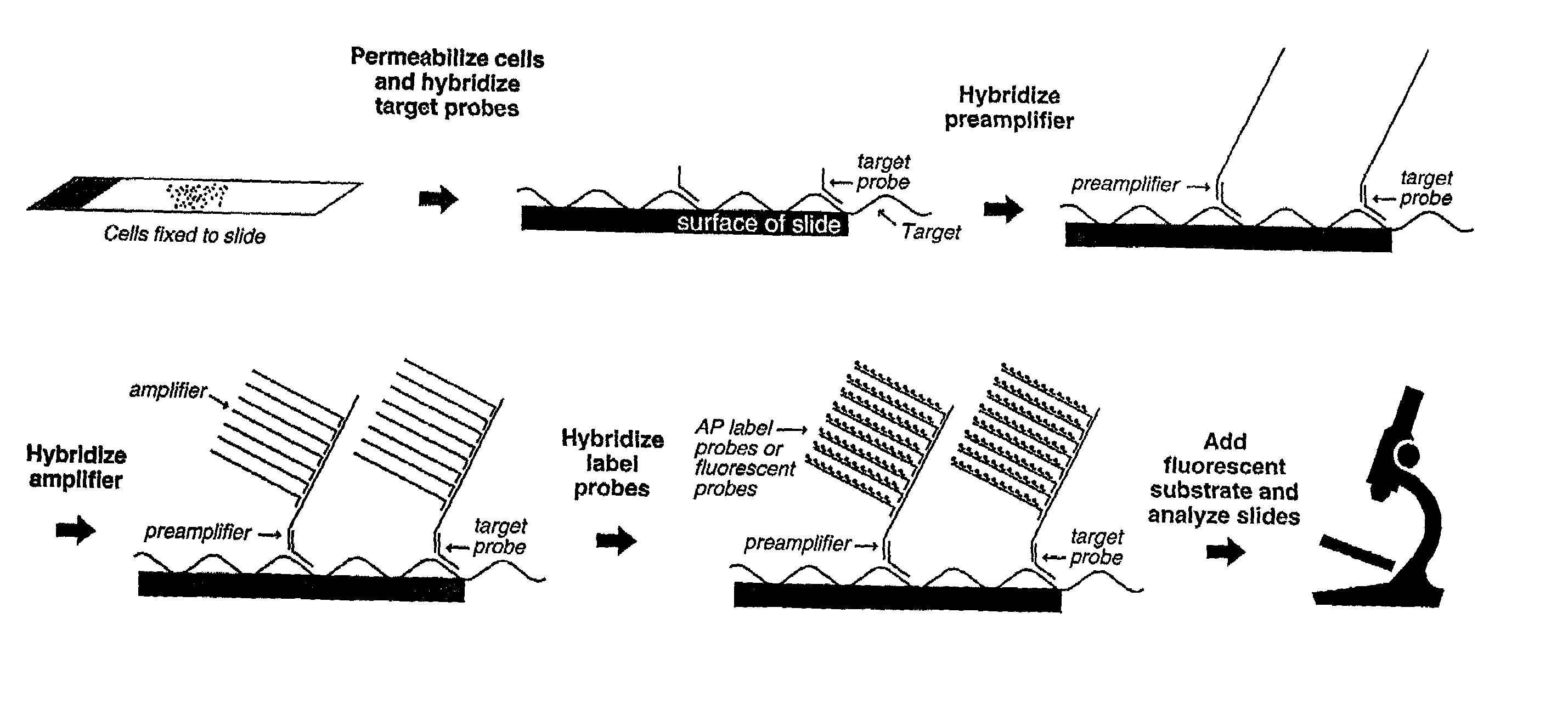
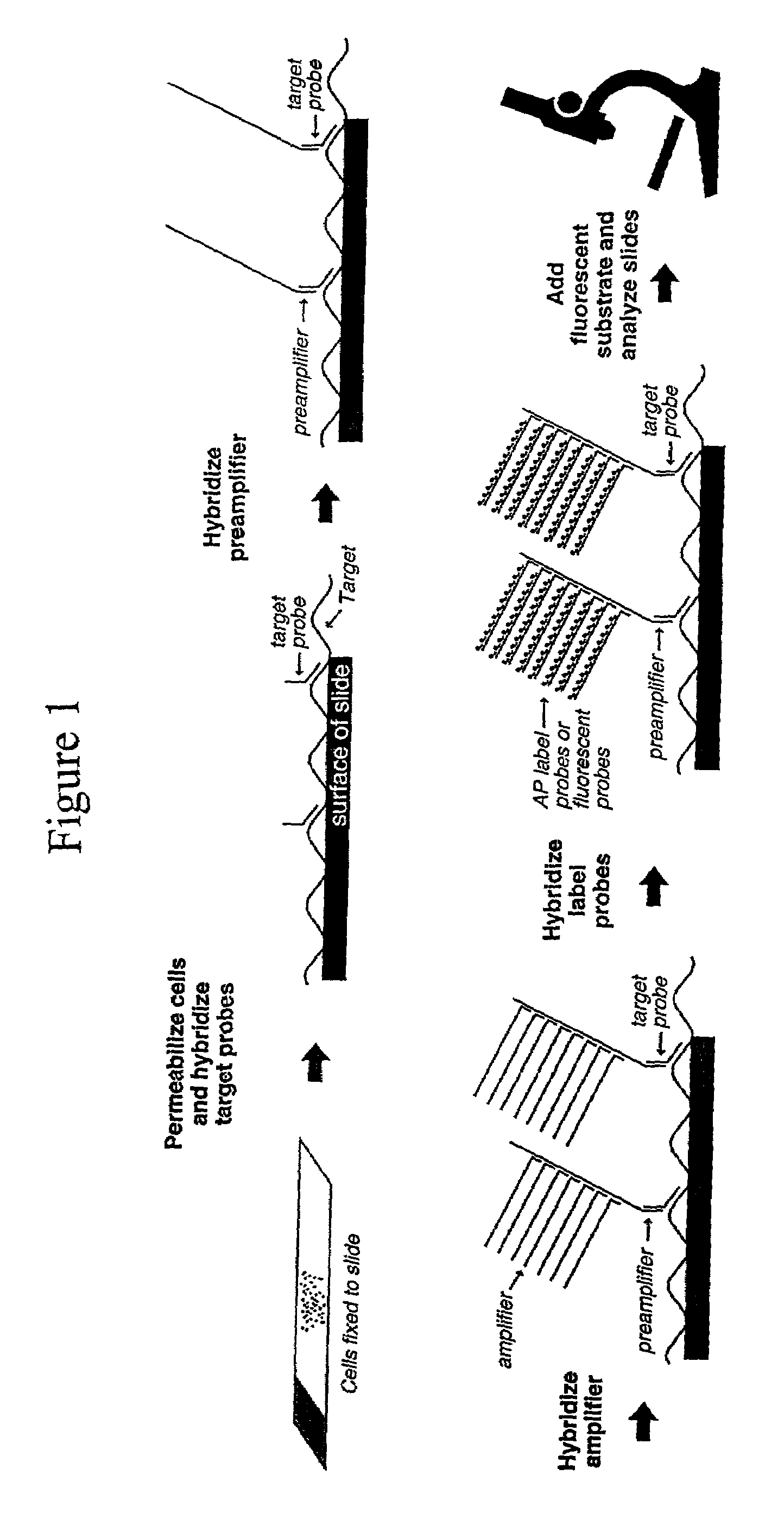
Highly sensitive gene detection and localization using in situ branched-DNA hybridization
InactiveUS7033758B2High sensitivitySugar derivativesMicrobiological testing/measurementAnalyteBiological materials
Methods are provided for highly sensitive and rapid in situ detection of a nucleic acid analyte of a known sequence. The method employs oligonucleotide probes in a series of optimized steps to amplify a signal and decrease background. Sensitivity is enhanced such that the method can detect as few as 1–2 copies of nucleic acid analyte per sample, the sample containing a cell, tissue or similar biological material. Methods of detecting and identifying the position of the nucleic acid analyte in a cell are also provided.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
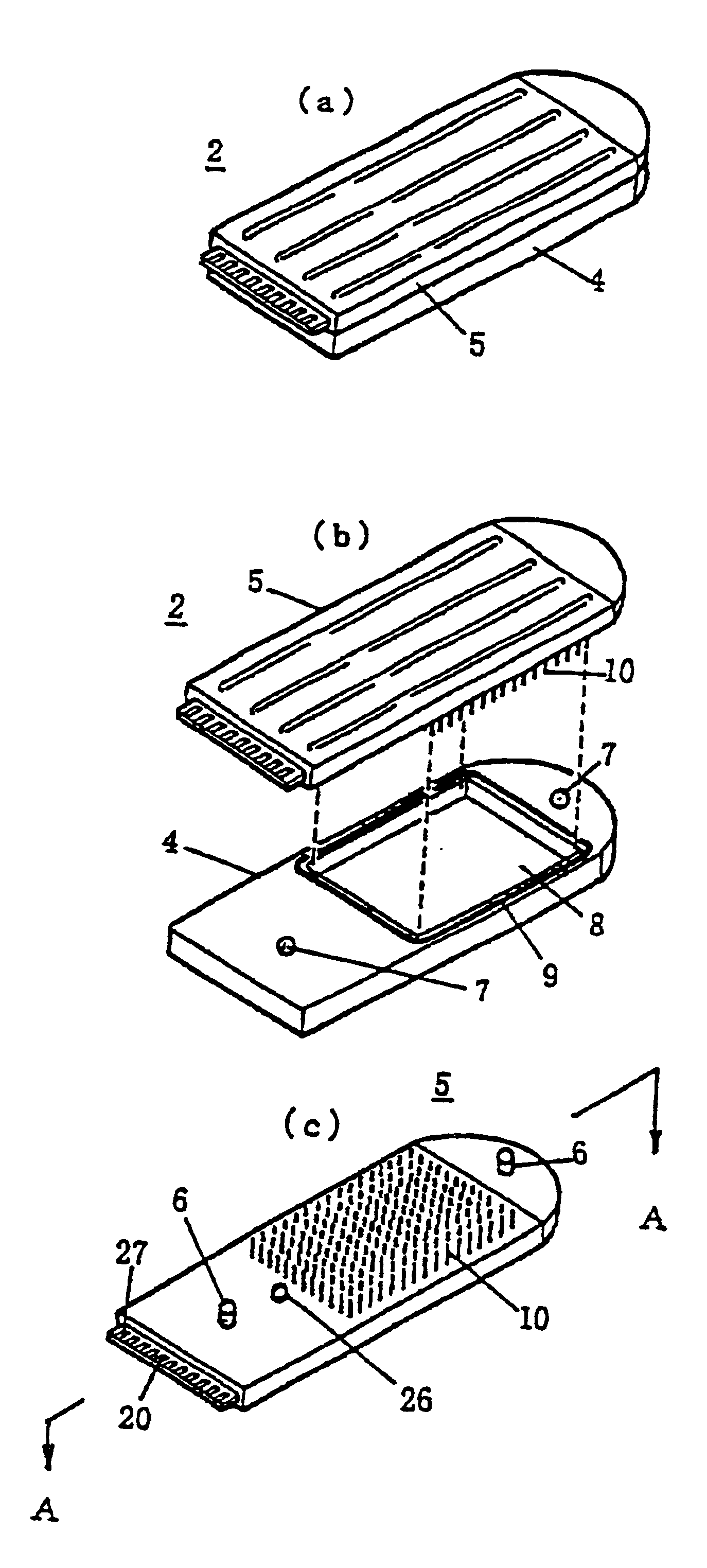
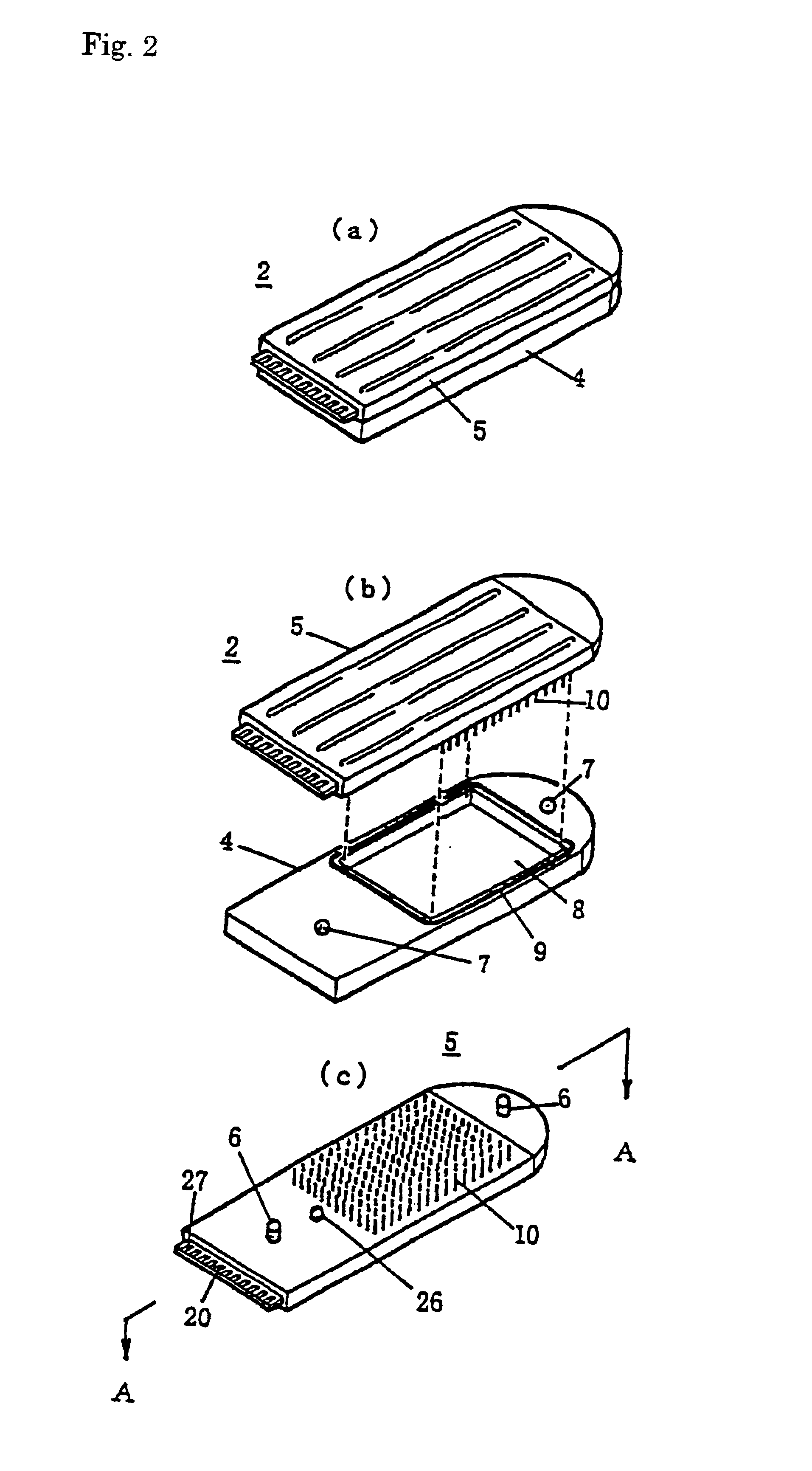
Gene detecting chip, detector, and detecting method
InactiveUS6916614B1Increase the areaImprove precisionImmobilised enzymesBioreactor/fermenter combinationsEngineeringOligonucleotide
A detecting chip (2) comprising a body (5) and a frame (4) both detachable from each other. A large number of projecting pin electrodes (10) are arranged in a matrix inside the body (5). Oligonucleotide consisting of different gene sequences is fixed to the pin electrode (10). A common electrode is so disposed in a recess (8) in a frame (4) as to be out of contact with the pin electrodes (10). A DNA sample is placed in the recess (8). By applying a voltage between the common electrode and the pin electrodes (10), a current is detected to detect a hybridized two-strand DNA, thus analyzing a gene DNA.
Owner:TOPPAN PRINTING CO LTD
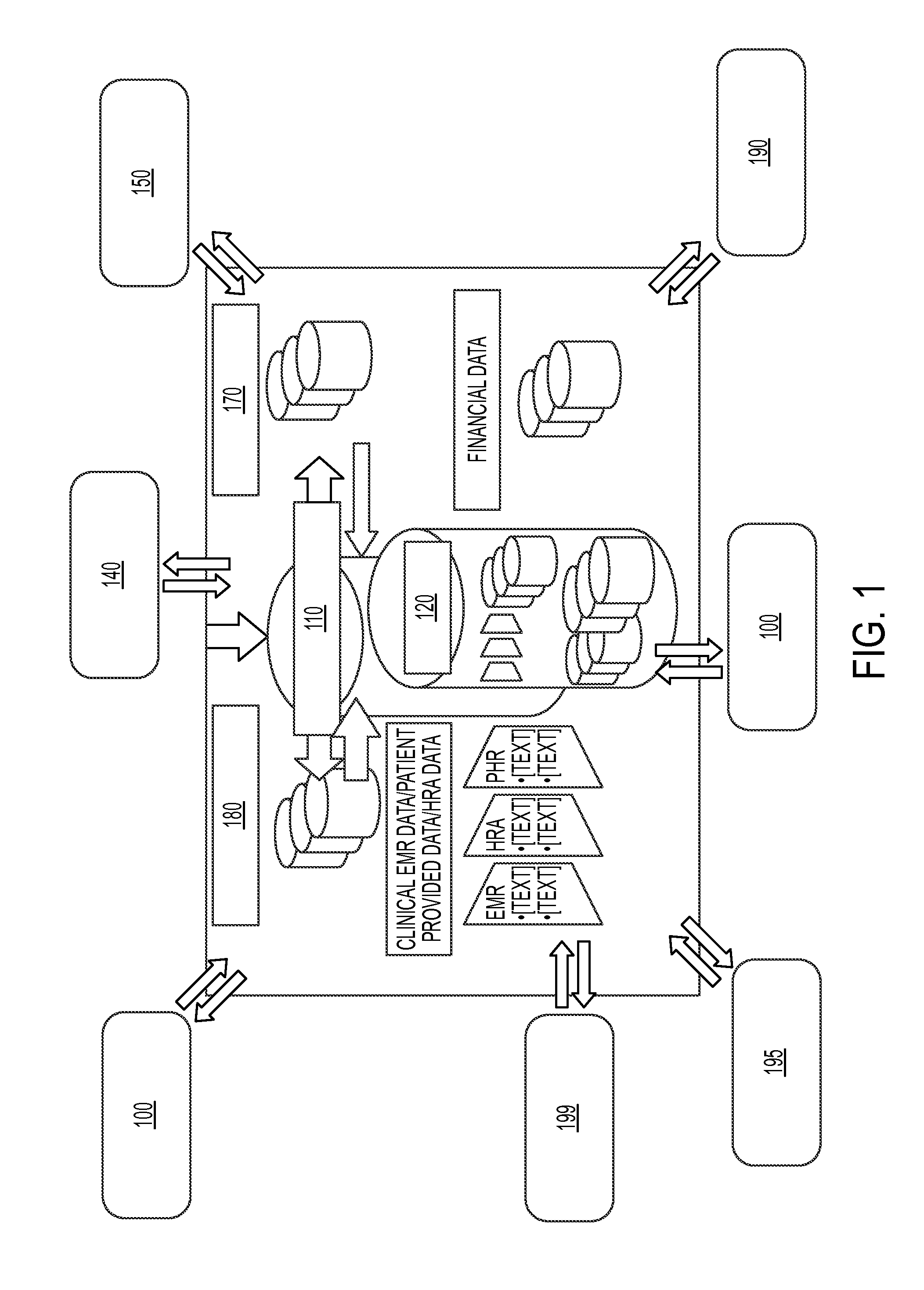
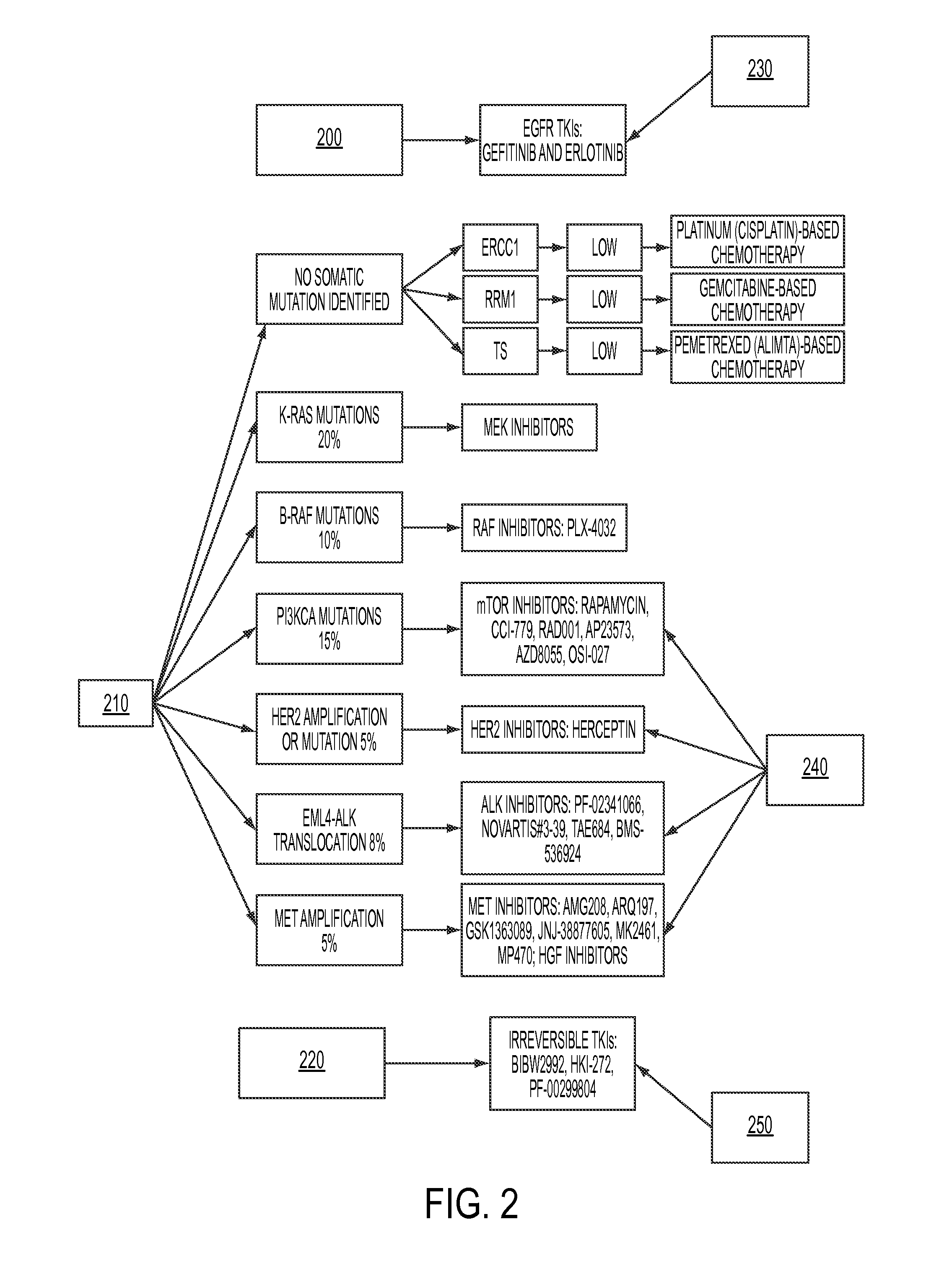
Personalized medical management system, networks, and methods
InactiveUS20120231959A1Low costReduce inefficienciesBioreactor/fermenter combinationsBiological substance pretreatmentsPersonalizationDisease
Disclosed herein are systems and methods for the assignment of therapeutic pathways to members of a network of oncology. The systems and methods allow for storage of disparate information in a database and determine a uniform semantics for all of the stored information. In addition, the systems and methods allow for the calculation of treatment pathways based on patient information as well as publicly-available information relating to particular diseases, and for the refinement of those treatment pathways as new information is added. Robot-assisted genomic labs permit automated genetic testing, which is integrated with the system.
Owner:KEW GROUP
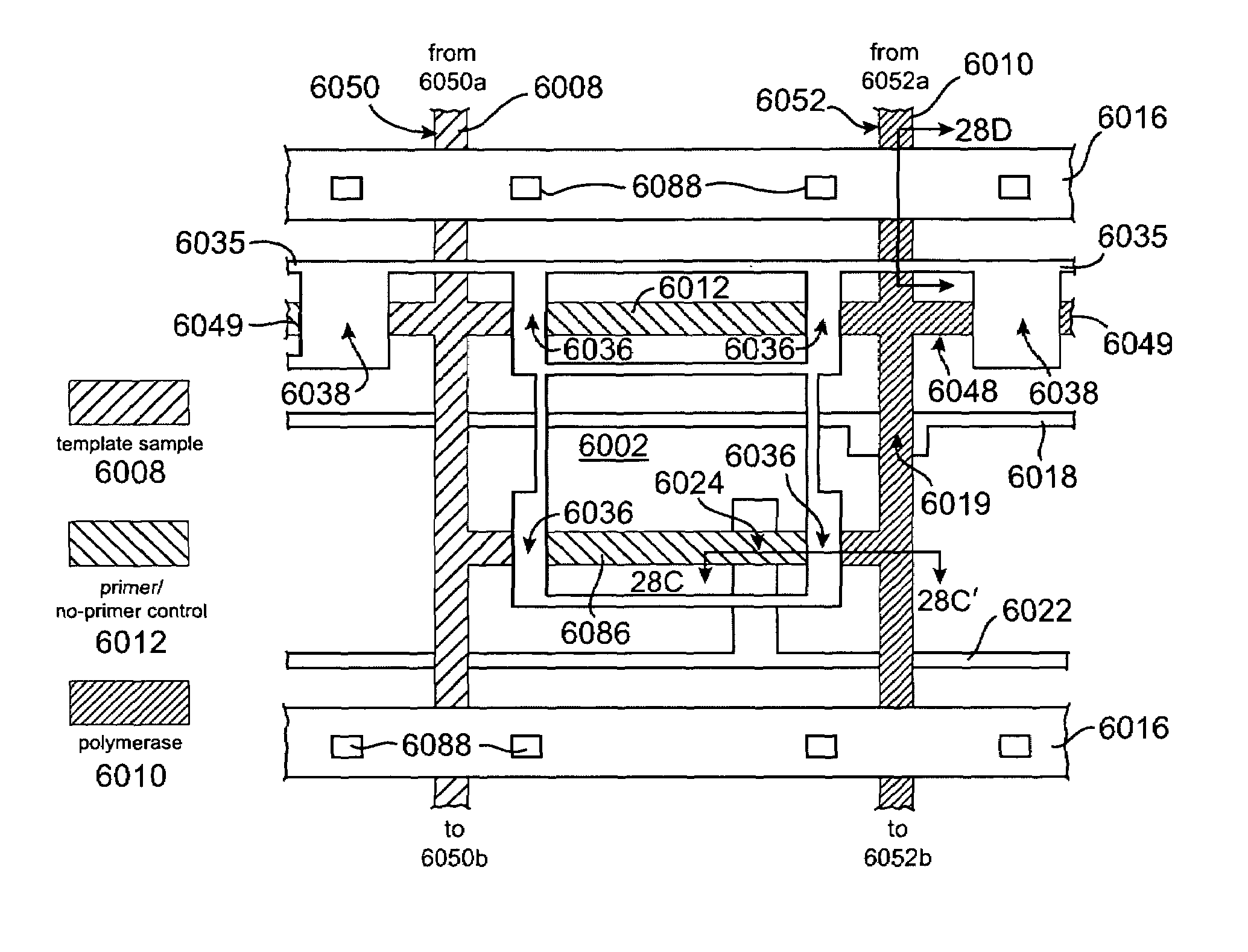
Microfluidic rotary flow reactor matrix
InactiveUS7413712B2Economy of scale in reagent consumptionMinimizes stepBioreactor/fermenter combinationsHeating or cooling apparatusMedical diagnosisEngineering
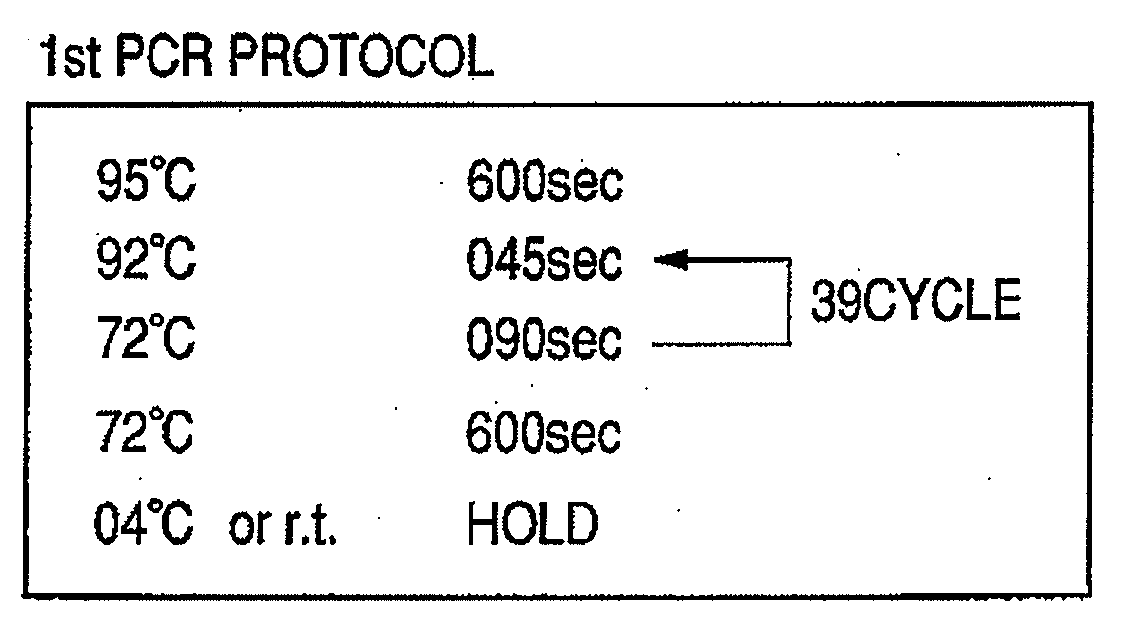
A microfluidic device comprises a matrix of rotary flow reactors. The microfluidic matrix device offers a solution to the “world-to-chip” interface problem by accomplishing two important goals simultaneously: an economy of scale in reagent consumption is achieved, while simultaneously minimizing pipetting steps. N2 independent assays can be performed with only 2N+1 pipetting steps, using a single aliquot of enzyme amortized over all reactors. The chip reduces labor relative to conventional fluid handling techniques by using an order of magnitude less pipetting steps, and reduces cost by consuming two to three orders of magnitude less reagents per reaction. A PCR format has immediate applications in medical diagnosis and gene testing. Beyond PCR, the microfluidic matrix chip provides a universal and flexible platform for biological and chemical assays requiring parsimonious use of precious reagents and highly automated processing.
Owner:CALIFORNIA INST OF TECH
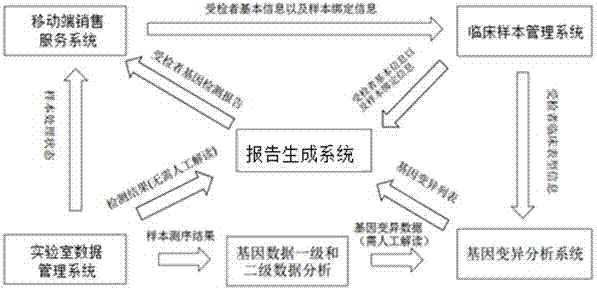
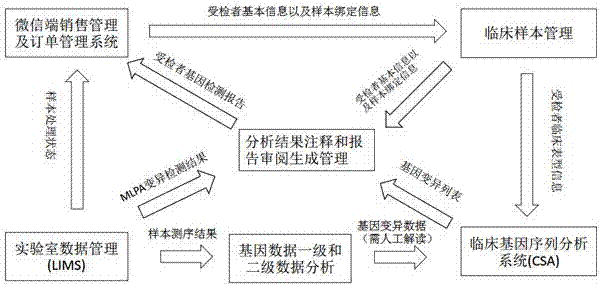
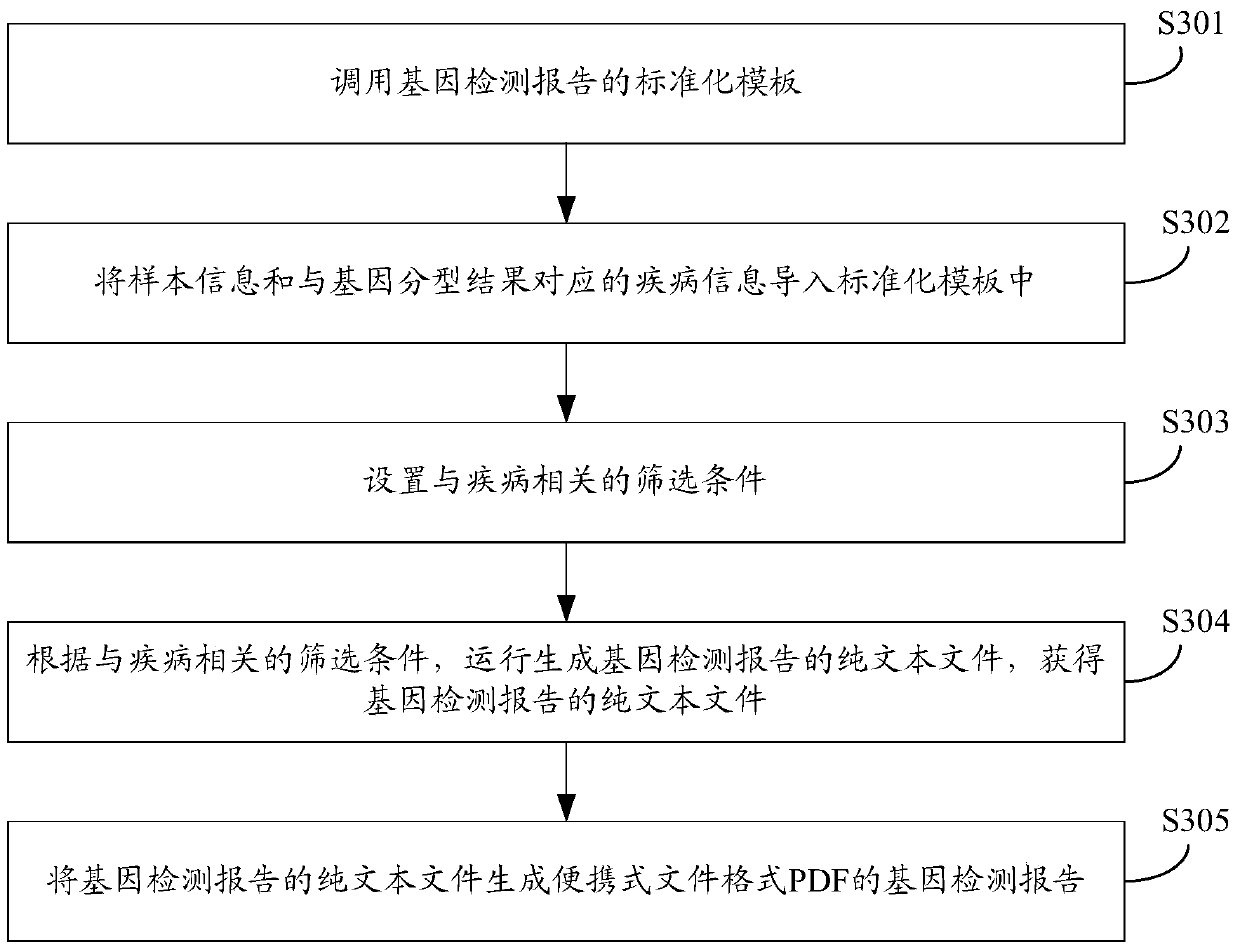
Gene detection product report system and method
InactiveCN107220885AImplement automatic exchangeGuaranteed accuracyProteomicsGenomicsBiologySpecific storage
The invention discloses a gene detection product report system comprising a mobile terminal sales service system which is used for the user to select a gene detection product and place an order to pay and is also used for acquiring basic information of the detected subject and sample binding information; a clinical sample management system which is used for receiving the basic information of the detected subject and the sample binding information from the mobile terminal sales service system; a laboratory data management system which is used for storing samples starting from order placing until the sequencing data are transferred to the specific storage position from a sequencer, wherein a report generation system generates a report to be stored in the laboratory data management system and pushed to the mobile terminal sales service system to be transmitted to the specific report receiver; and the report generation system which is used for receiving the basic information of the detected subject and the sample binding information from the clinical sample management system and the detection result from the laboratory data management system so as to generate the report. Automatic data exchange and centralized report management can be realized, and the report can be automatically generated according to the requirement so as to guarantee the accuracy of the report data.
Owner:MINGMA SHANGHAI BIOLOGICAL TECH CO LTD
Gene detection assay for improving the likelhood of an effective response to an ErbB antagonist cancer therapy
InactiveUS20060228745A1Great likelihoodChoose accuratelyOrganic active ingredientsBiocideFhit geneTumor cells
The invention provides a method for more effective treatment of patients susceptible to or diagnosed with tumors overexpressing ErbB, as determined by a gene amplification assay, with an ErbB antagonist. Such method comprises administering a cancer-treating dose of the ErbB antagonist, preferably in addition to chemotherapeutic agents, to a subject in whose tumor cells ErbB has been found to be amplified e.g., by fluorescent in situ hybridization. ErbB antagonists described include an anti-HER2 antibody. Pharmaceutical packaging for providing the components for such treatment is also provided.
Owner:GENENTECH INC
Gene detection method, gene detection kit and gene detection equipment
PendingCN107151700AEasy to assembleNot easy to produce secondary pollutionBioreactor/fermenter combinationsBiological substance pretreatmentsMagnetic beadTest sample
The invention discloses a gene detection method, a gene detection kit and gene detection equipment. The gene detection method comprises the following steps of arranging a plurality of separation cavities in the kit, blocking the every two adjacent separation cavities by a plunger, and adding a cracking liquid, a cleaning liquid and a reaction liquid into each separation cavity; when a sample is detected, pushing each plunger, aligning a plunger hole of each plunger with the corresponding separation cavity, and further conducting each separation cavity; utilizing an electromagnetic control type to control magnetic beads in the kit to drive the to-be-tested sample to sequentially pass through the separation cavities, and then sequentially cracking, cleaning and reacting; finally, performing optical detection on the gene in the reaction liquid from the exterior.
Owner:HANGZHOU LIFEREAL BIOTECH CO LTD
Apparatus and method for luminometric assay
InactiveUS7163822B2Low cost of reagentsLow costBioreactor/fermenter combinationsTelevision system detailsSensor arrayAssay
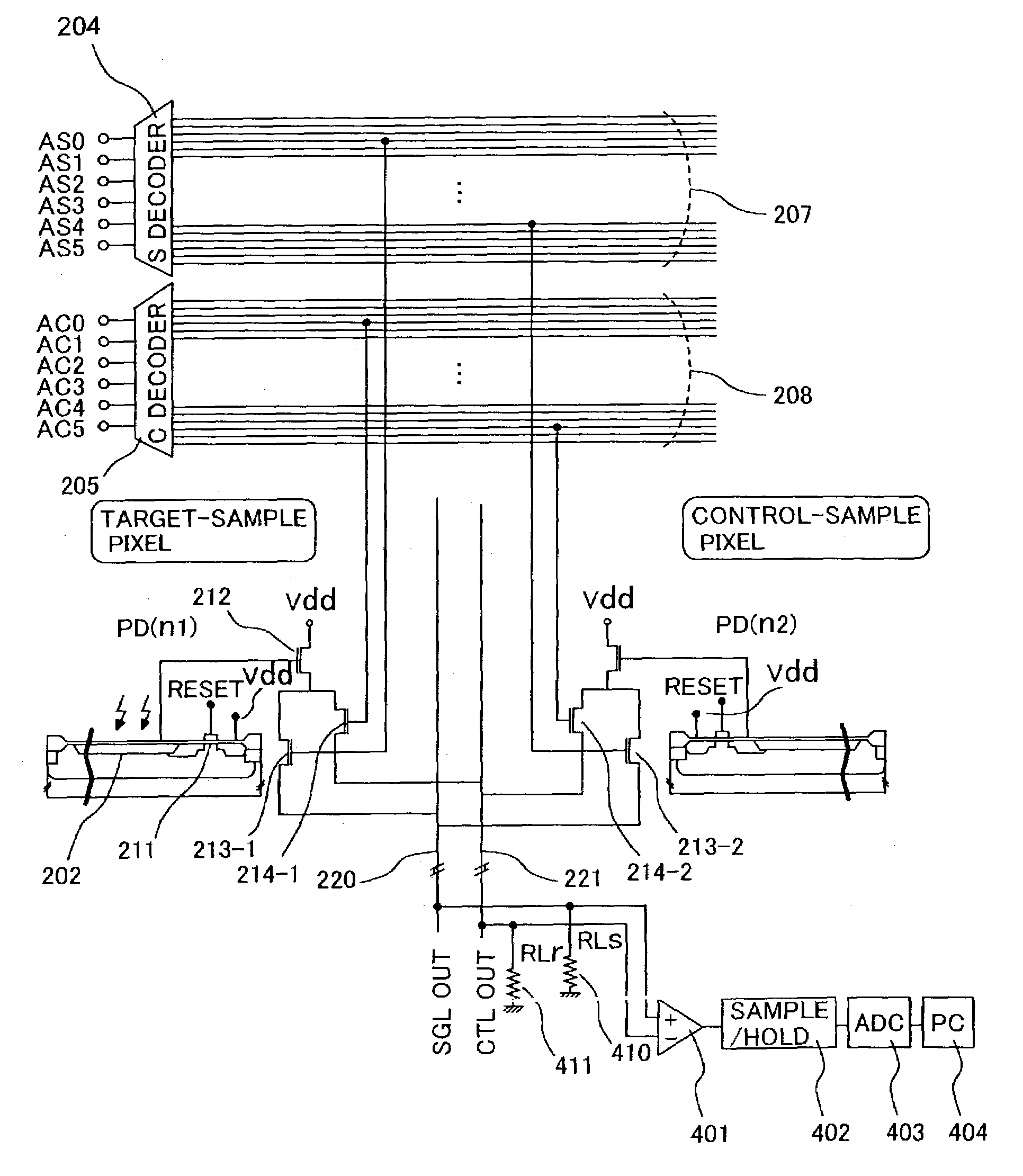
A small sized, cost-effective genetic testing apparatus that provides high sensitivity testing, for performing genetic testing simply and at low cost. An optical sensor array for the apparatus and method for luminometric assay comprises a means that simultaneously selects 2 pixels and detects minute amounts of chemiluminescence by obtaining the differential output of the respective signals.
Owner:HITACHI LTD
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20090130667A1Quickly and precisely identifiedAccurate distinctionSugar derivativesMicrobiological testing/measurementInfectious DisorderNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 81 to 83 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
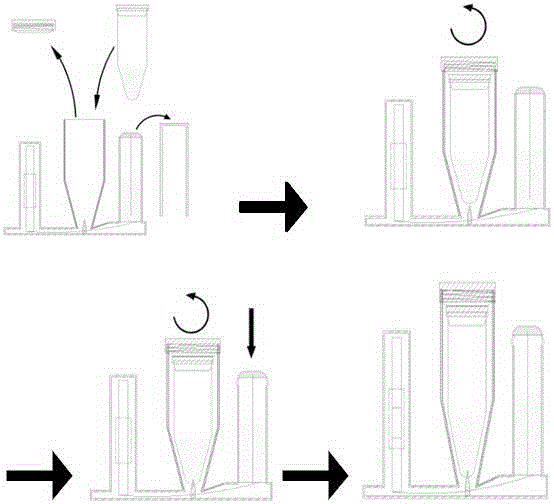
Anti-pollution portable gene detection method and device
InactiveCN105199940AQuickly and reliably determineEasy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsAnimal husbandryGene
The invention relates to an anti-pollution portable gene detection method and device. The operation procedure comprises the steps that a PCR pipe containing amplification products is put into a device to conduct sealing, and by means of a series of simple manual operations, a detection result visible by eyes is obtained; target nucleic acid amplification products can be detected rapidly, nucleic acid amplification products can be prevented from being polluted, and a false positive result is avoided. Application can be achieved in the aspects of species identification on the gene level in the fields such as pathogen detection on clinical infectious diseases, food pathogenic microbe detection, agriculture, industry, customs and animal husbandry.
Owner:GUANGZHOU HEAS BIOTECH CO LTD
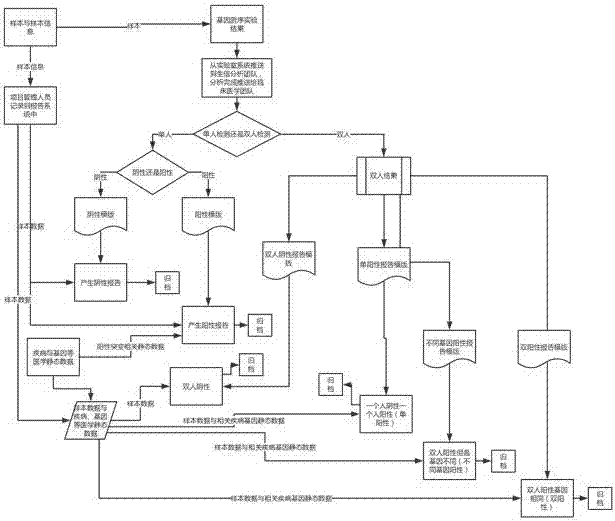
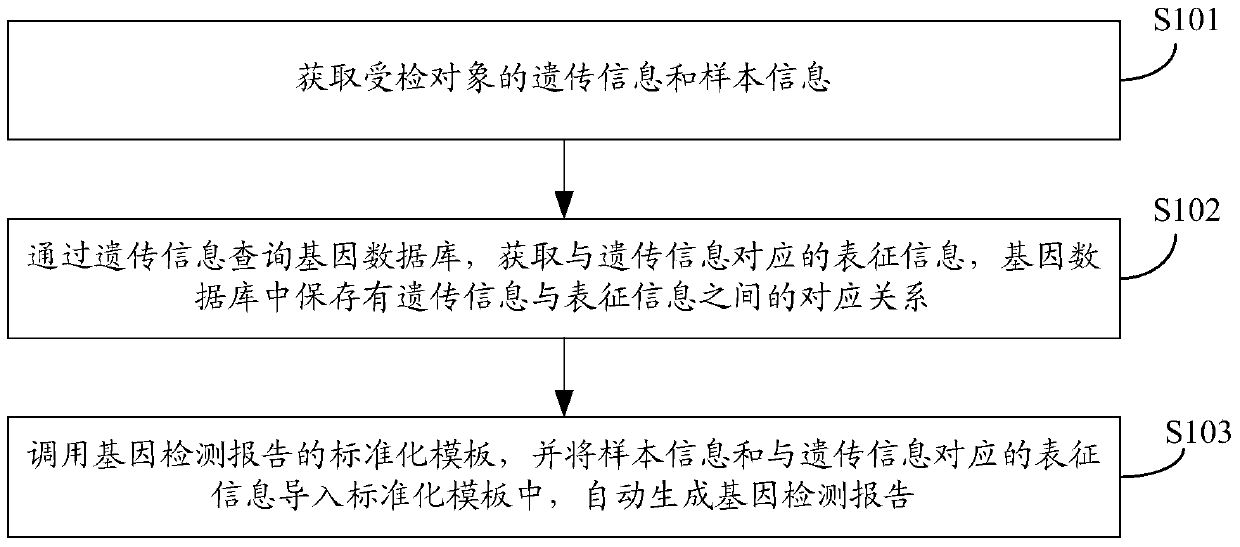
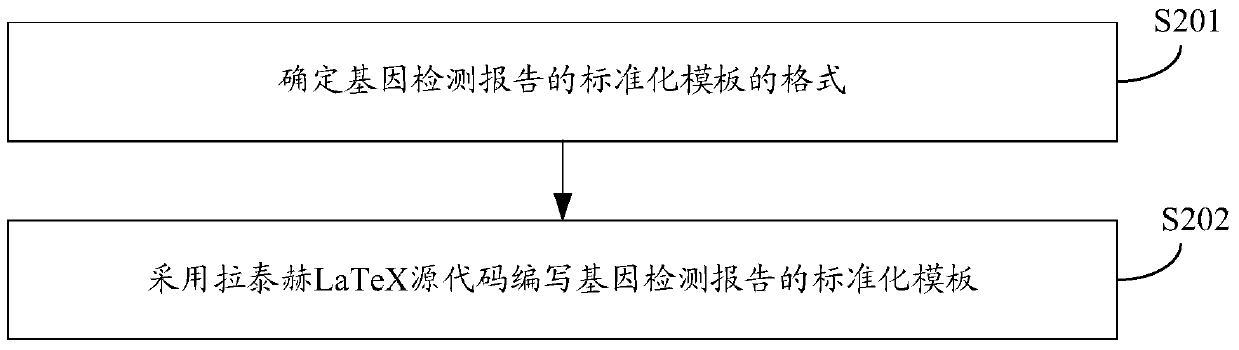
Gene detection report automatic generation method and device
InactiveCN105512508ATimely updateShorten the timeSpecial data processing applicationsComputer scienceStandardization
The invention discloses a gene detection report automatic generation method and device; the method comprises the following steps: obtaining heredity information and sample information of a detected object; querying a gene database by using heredity information, and obtaining representation information corresponding to the heredity information; calling a gene detection report standardization template, and introducing the sample information and the representation information corresponding to the heredity information into the standardization template, thus automatically generating the gene detection report. The novel method and device can save mass time, can ensure accuracy, thus timely updating the gene detection report.
Owner:SHENZHEN HUADA GENE INST
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20090130669A1Quickly and precisely identifiedAccurate distinctionSugar derivativesMicrobiological testing/measurementBacteroidesNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 92 to 93 and complementary or modified sequences thereof or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
Method of genetic testing in heritable arrhythmia syndrome patients
InactiveUS20050142591A1Microbiological testing/measurementBiological testingSyndrome patientGenetic Screening (procedure)
A method of diagnosing heritable arrhythmia syndrome in a patient is disclosed. In one embodiment, the method comprises the steps of (a) isolating a nucleic acid sample from the patient and (b) comparing the nucleic acid sample to the compendium of novel DNA mutations disclosed in Table 1, wherein the comparison is to the mutations described from at least one of the genes selected from the group consisting of KCNQ1, KCNH2, SCN5A, and KCNE2.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Skin Sampling Kit Which Stores Nucleic Acids In Stable Status, Genetic Test Methods By Using The Kit And Their Practical Application
InactiveUS20110033842A1Easily and stably acquiredAccurate conditionBioreactor/fermenter combinationsBiological substance pretreatmentsGenomicsSkin test results
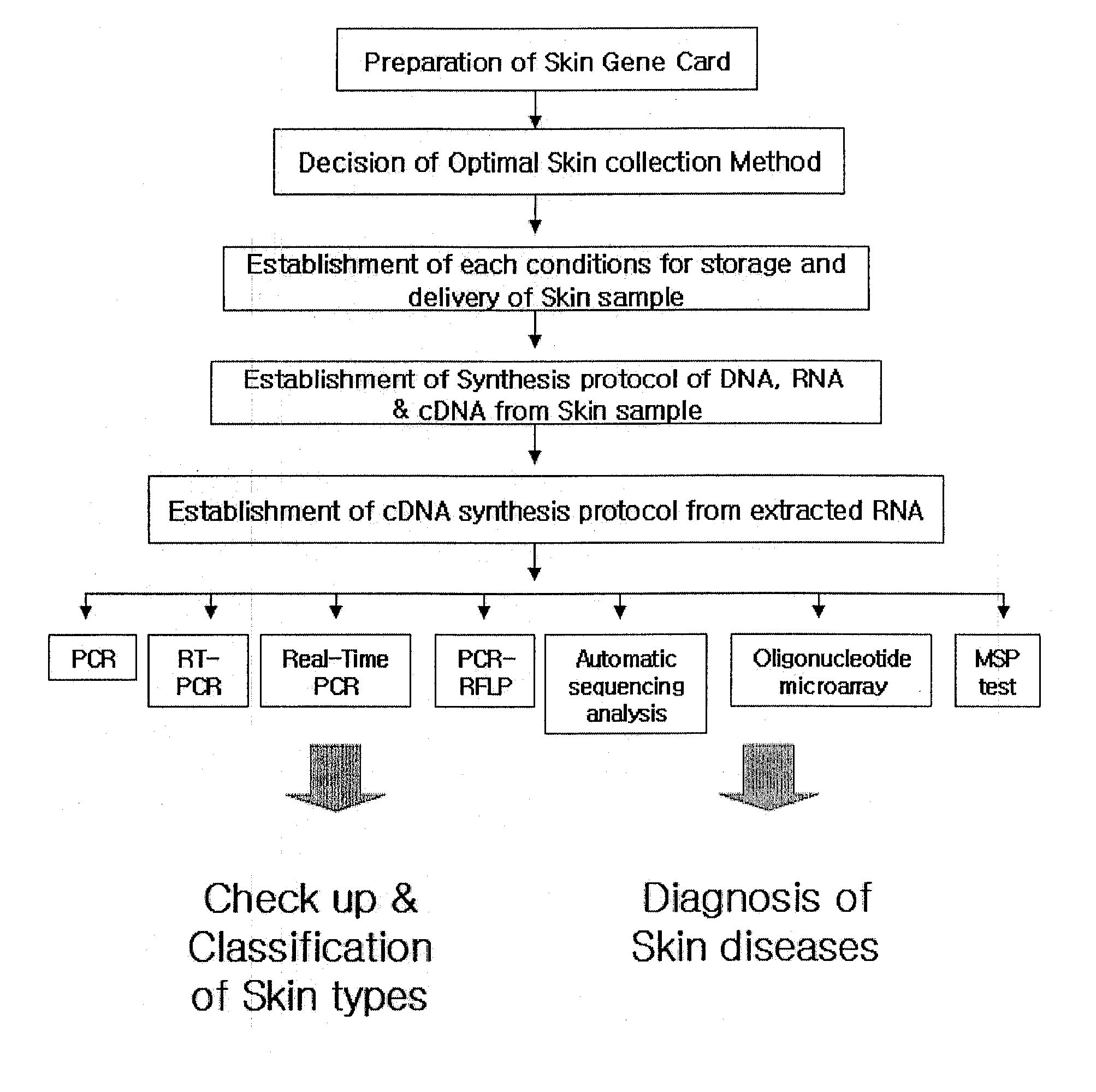
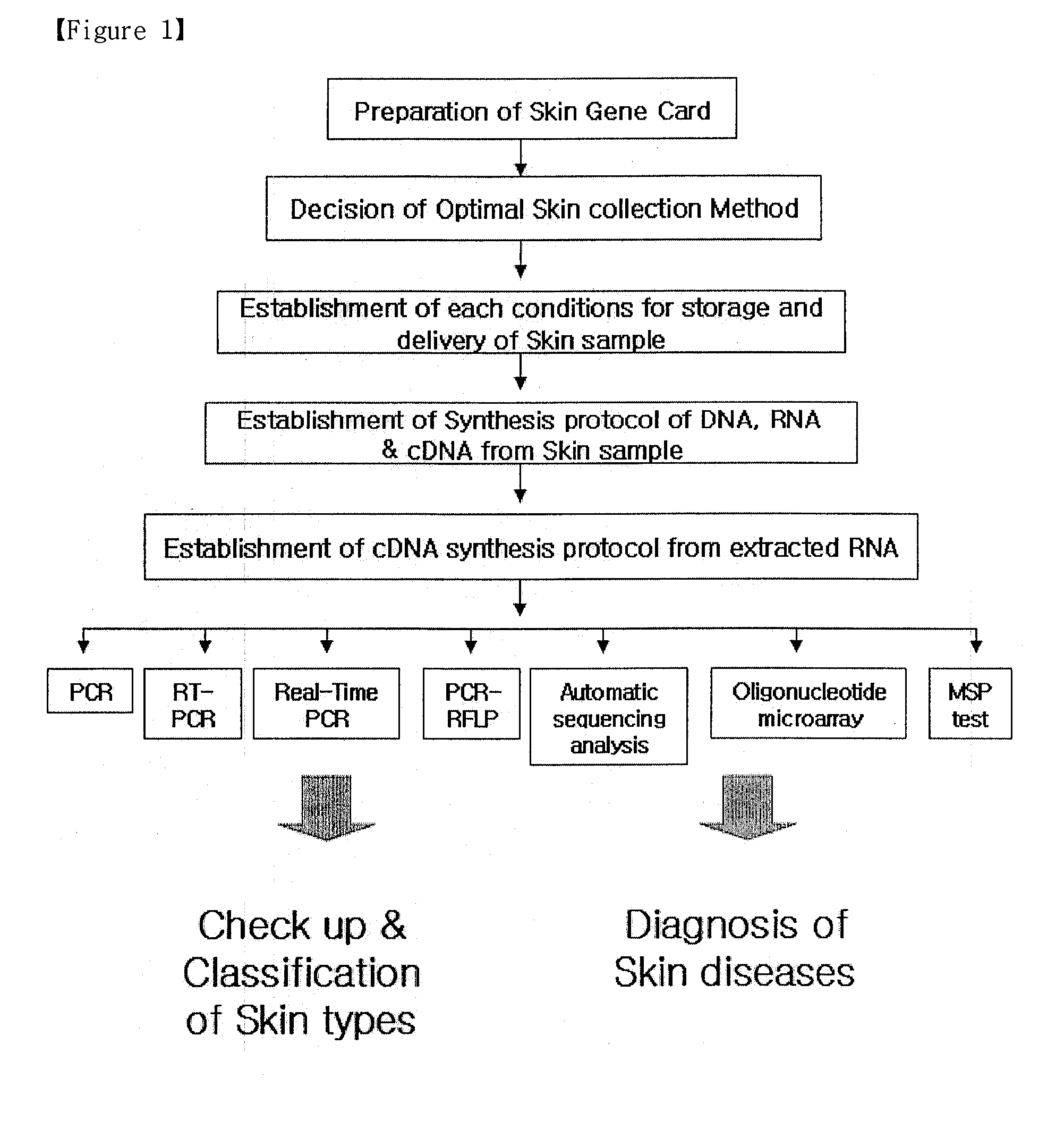
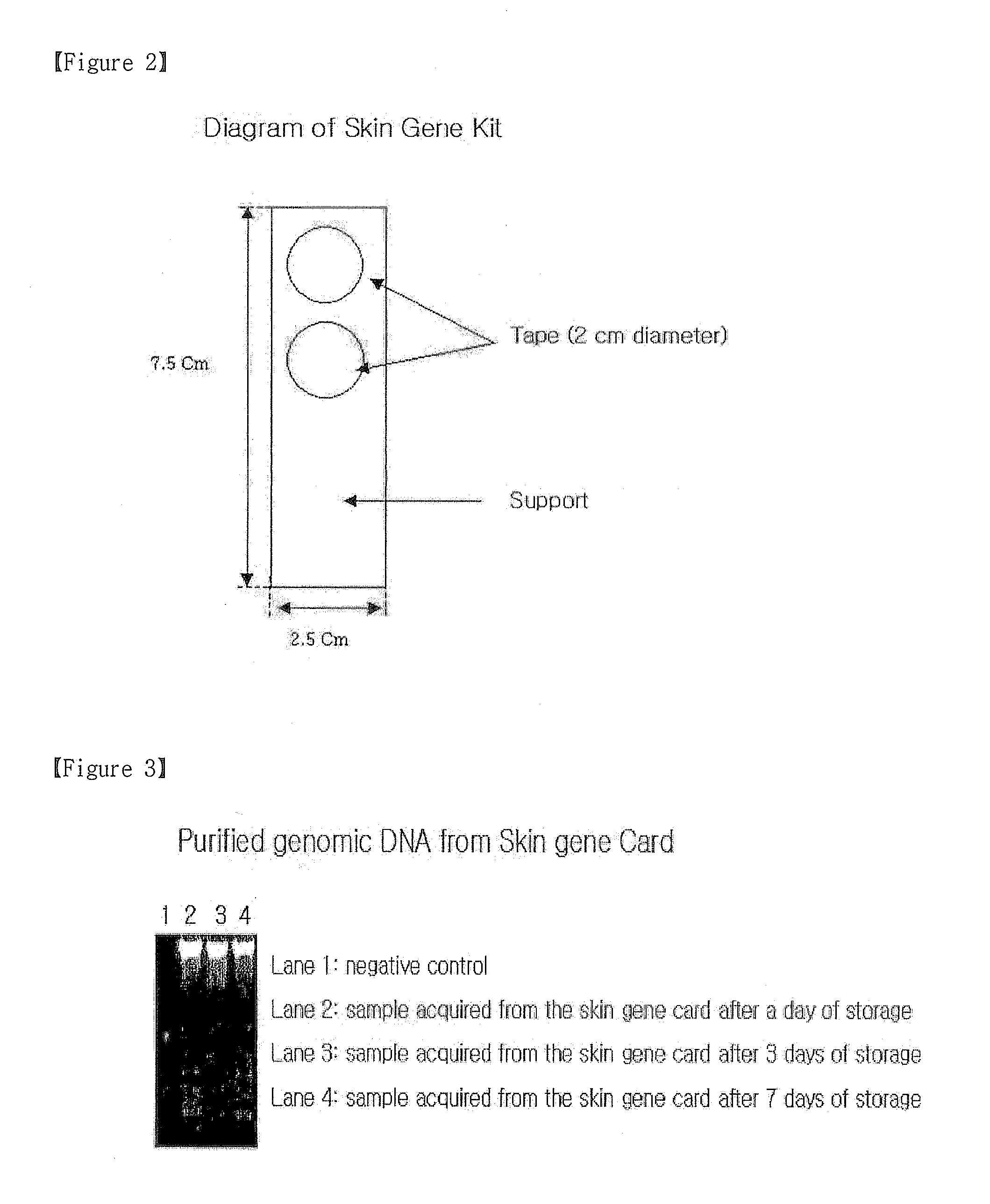
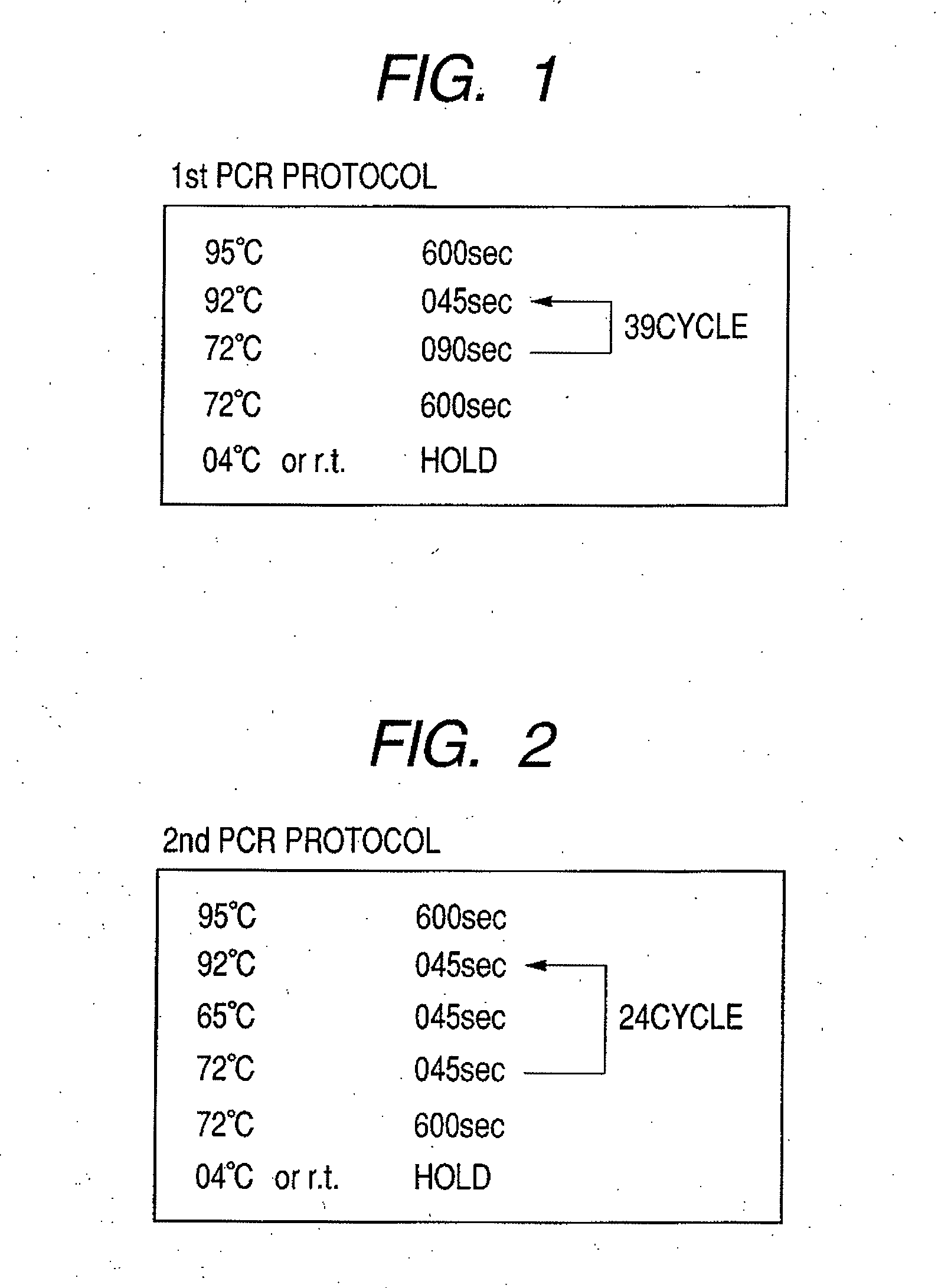
The present invention relates to a new skin gene card for genetic test, a method for acquiring DNA and RNA and performing various genetic tests using the same, and practical applications thereof. More specifically, the inventors of the present invention have developed a skin gene card capable of acquiring samples from human skin, hair or mucosa simply, safely and quickly and enabling stable long-term storage and transport of DNA and RNA included in the acquired sample at room temperature. Various genetic tests may performed using the acquired DNA and RNA, including polymerase chain reaction (PCR), reverse transcription (RT)-PCR, real-time PCR, sequencing, hybridization, DNA chip analysis, single-nucleotide polymorphism (SNP) assay, gene mutation assay, promoter methylation assay, gene expression assay, etc. The genetic skin test result may be utilized for disease prognosis, nutrigenomic test, pharmacogenomic test, forensic test such as personal identification, diagnosis of genetic diseases, diagnosis of skin diseases, or the like. In addition, through an objective evaluation of the skin or hair condition, a personalized cosmetic and skin care system may be established for practical application in beauty care, cosmetology, dermatology, and clinical practice.
Owner:GOODGENE
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20090305262A1Quickly and precisely detectAccurate identificationSugar derivativesNucleotide librariesNucleic Acid ProbesMicrobiology
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 56 to 58 or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
Probe, probe set, probe-immobilized carrier, and genetic testing method
InactiveUS20090317809A1Quickly and precisely detectAccurate identificationSugar derivativesMicrobiological testing/measurementInfectious DisorderNucleic Acid Probes
A nucleic acid probe for classification of pathogenic bacterial species is capable of collectively detecting bacterial strains of the same species and differentially detecting them from other bacterial species. Any one of the base sequences of SEQ ID NOS. 52 to 54 or a combination of at least two of them is used for detecting the gene of an infectious disease pathogenic bacterium.
Owner:CANON KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com