Preparation method of high-purity carbamazepine (CBZ)-valaciclovir
A technology of valacyclovir and CBZ-L-, which is applied in the field of preparation of high-purity CBZ-valacyclovir, can solve the problem of high product impurity content, and achieve the effects of improving product purity, reducing energy consumption and shortening reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
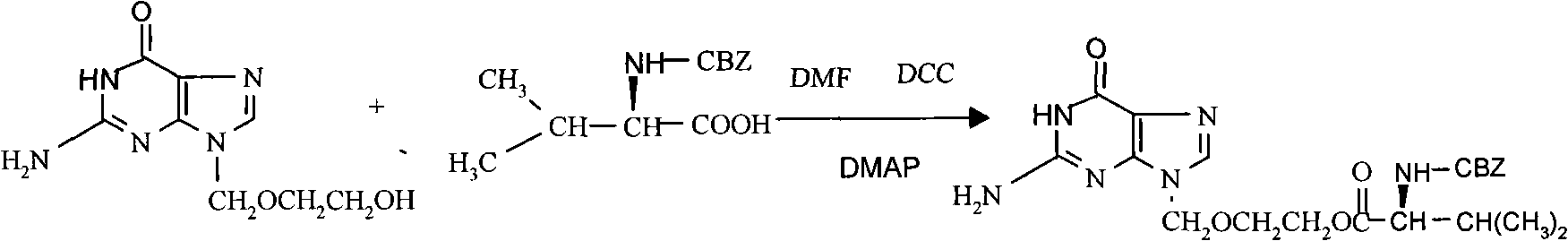
[0031] Put 800ml of N,N-dimethylformamide into the reaction flask, add 25g of acyclovir, 50g of N-benzyloxycarbonyl-L-valine and 0.5g of 4-dimethylaminopyridine under stirring, and cool to 10°C Next, add 40 g of dicyclohexylcarbodiimide, and keep the reaction at 5-10°C for 2 hours, then raise the temperature to 20-25°C within 55-65 minutes, and keep the reaction for 15 hours. The solid in the reaction system (reaction by-product dicyclohexyl urea) was filtered off with suction, the filtrate was concentrated under reduced pressure to obtain an oily substance, and 300ml of ethanol was added to heat to dissolve the concentrate. After the solution was clear, it was cooled to -5~0°C to crystallize for 10 hours. After suction filtration, the resulting solid was heated and dissolved with 250ml of ethanol, and after dissolving, cooled to -5-0°C to crystallize for 10 hours. Suction filtration, the obtained solid was heated and dissolved with 100ml of N,N-dimethylformamide, then the obt...
Embodiment 2
[0033] Put 800ml of N,N-dimethylformamide into the reaction flask, add 25g of acyclovir, 50g of N-benzyloxycarbonyl-L-valine and 0.5g of 4-dimethylaminopyridine under stirring, and cool to 10°C Next, 40 g of dicyclohexylcarbodiimide was added, and the temperature was kept at 5-10° C. for 1 hour, then the temperature was raised to 20-25° C. within 115-125 minutes, and kept for 15 hours. The solid in the reaction system (reaction by-product dicyclohexyl urea) was filtered off with suction, the filtrate was concentrated under reduced pressure to obtain an oily substance, 300ml of ethanol was added to dissolve the concentrate by heating, and after dissolving, cool to -5°C to crystallize for 10 hours. Suction filtration, the obtained solid was heated and dissolved with 100ml of N,N-dimethylformamide, then the obtained N,N-dimethylformamide solution was added to 600ml of hot water at a temperature between 55 and 65°C, and the Stir at 55°C for 30 minutes, filter with suction, and was...
Embodiment 3
[0035] Put 800ml of N,N-dimethylformamide into the reaction flask, add 25g of acyclovir, 50g of N-benzyloxycarbonyl-L-valine and 0.5g of 4-dimethylaminopyridine under stirring, and cool to 5°C Next, add 40 g of dicyclohexylcarbodiimide, and keep the reaction at 0-5°C for 2 hours, then raise the temperature to 20-25°C within 55-65 minutes, and keep the reaction for 15 hours. The solid in the reaction system (reaction by-product dicyclohexyl urea) was filtered off with suction, the filtrate was concentrated under reduced pressure to obtain an oily substance, 300ml of ethanol was added to dissolve the concentrate by heating, and after dissolving, cool to 0°C to crystallize for 10 hours. After suction filtration, the obtained solid was heated and dissolved with 250ml of ethanol, and after dissolving, cooled to 0°C for crystallization for 10 hours. Suction filtration, the obtained solid was heated and dissolved with 100ml of N,N-dimethylformamide, then the obtained N,N-dimethylform...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com