Carbamazepine sustained-release tablet and preparation method thereof
A technology of carbamazepine and carbamazepine, which is applied in the field of carbamazepine sustained-release tablets and their preparation, and can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
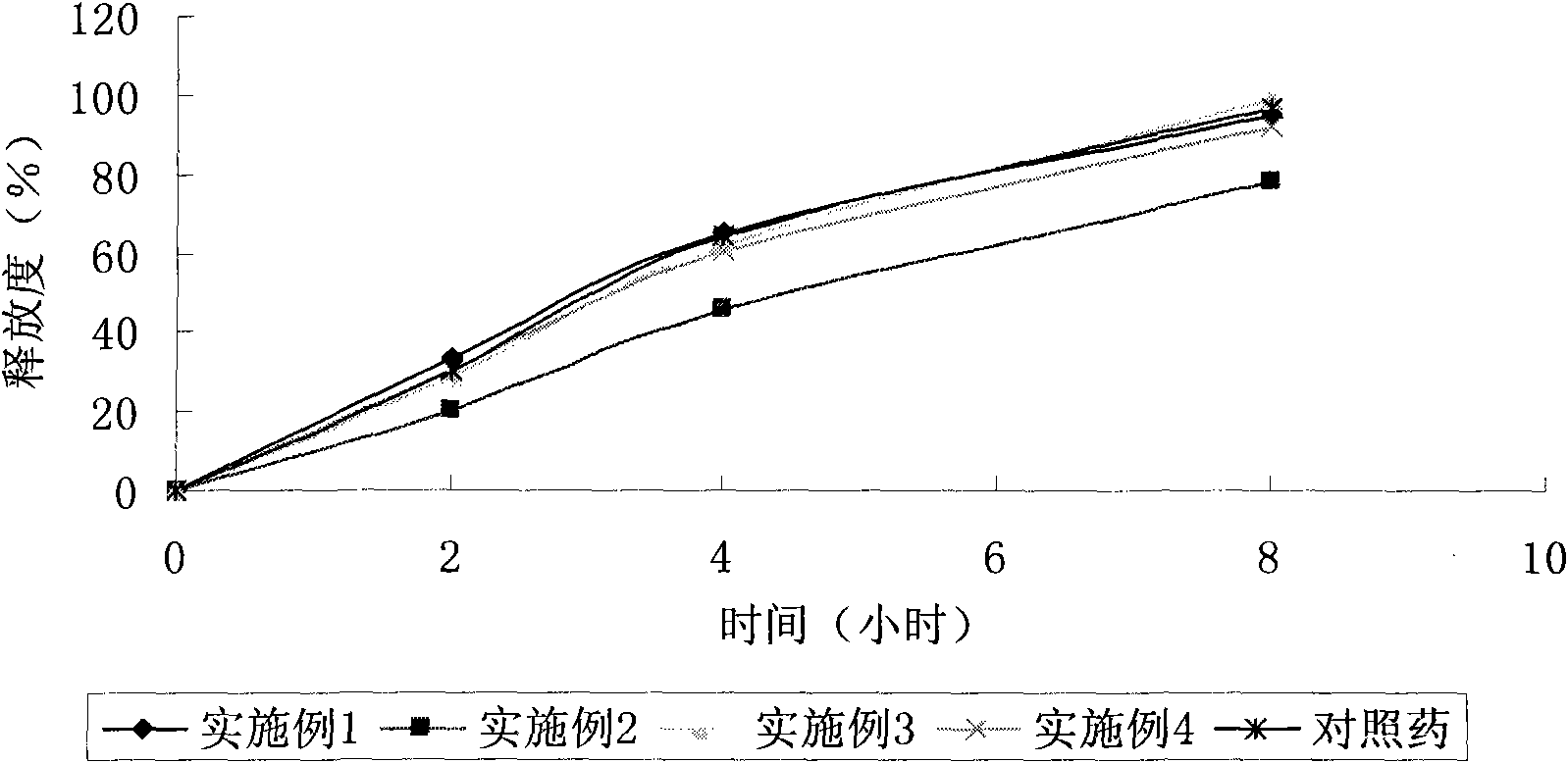
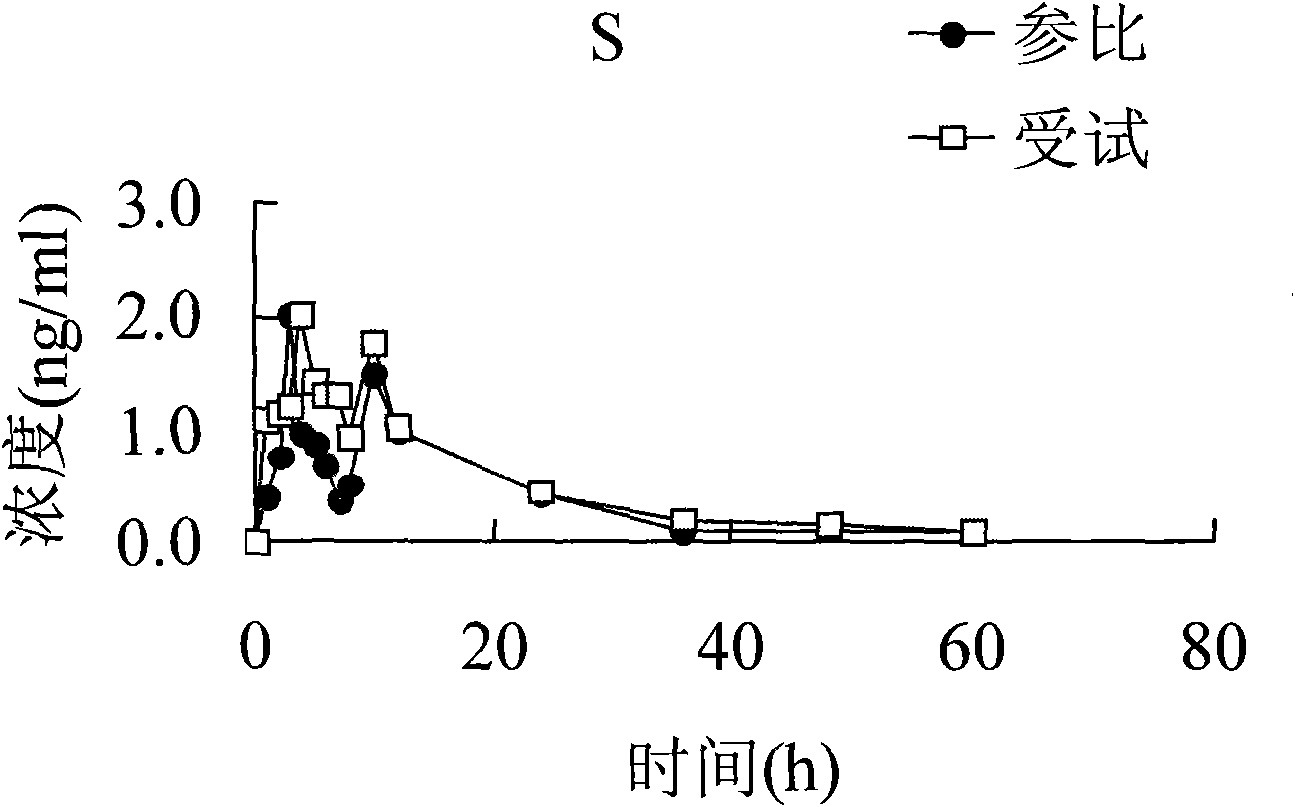
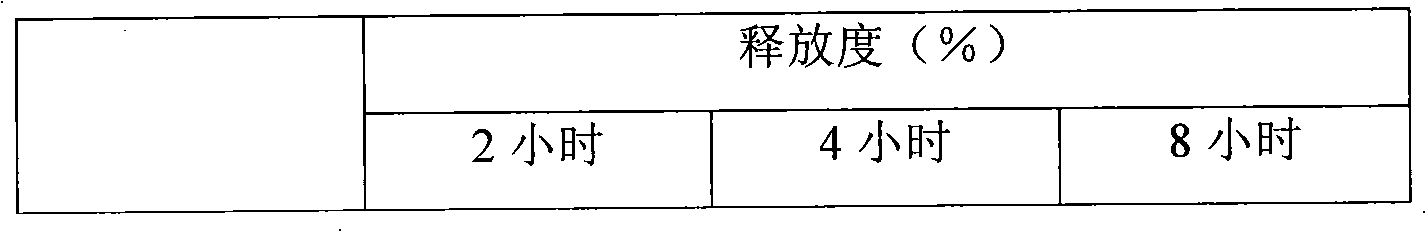
Embodiment 1
[0030] prescription:
[0031] Carbamazepine 200g
[0032] Hypromellose K100 90g
[0033] Microcrystalline Cellulose 101 20g
[0034] Mannitol 200SD 120g
[0035] Povidone K30 15g
[0037] Amount of ethanol
[0038] Make 1000 pieces
[0039] Formulation process
[0040] 1 prepare the povidone ethanol solution to get the povidone of the recipe quantity and make the ethanol solution of 10% (w / w), for subsequent use;
[0041] 2 Granulation After sieving carbamazepine and mannitol with 80 meshes respectively, put them into a mixing granulator together with hypromellose and microcrystalline cellulose for mixing, and then add povidone ethanol solution for wet granulation. granules, take out the granules and carry out wet granulation with a 20-mesh sieve. After drying, pass through a 20-mesh sieve to granulate and set aside.
[0042] 3. Tabletting After mixing the obtained granules and the prescribed amount of magnesium stearate evenly, the con...
Embodiment 2
[0044] prescription:
[0045] Carbamazepine 200g
[0046] Hypromellose K4M 120g
[0047] Microcrystalline Cellulose 101 30g
[0048] Mannitol 200SD 150g
[0049] Copovidone S630 30g
[0051] Amount of ethanol
[0052] Make 1000 pieces
[0053] Formulation process
[0054] 1 prepare the povidone ethanol solution to get the povidone of the recipe quantity and make the ethanol solution of 10% (w / w), for subsequent use;
[0055] 2 Granulation After sieving carbamazepine and mannitol with 80 meshes respectively, put them into a mixing granulator together with hypromellose and microcrystalline cellulose for mixing, and then add povidone ethanol solution for wet granulation. granules, take out the granules and carry out wet granulation with a 20-mesh sieve. After drying, pass through a 20-mesh sieve to granulate and set aside.
[0056] 3. Tabletting After mixing the obtained granules and the prescribed amount of magnesium stearate evenly, the co...
Embodiment 3
[0058] prescription:
[0059] Carbamazepine 200g
[0060] Hypromellose K4M 30g
[0061] Hypromellose E5 90g
[0062] Microcrystalline Cellulose 101 30g
[0063] Mannitol 200SD 150g
[0064] Povidone K30 57g
[0066] Amount of ethanol
[0067] Make 1000 pieces
[0068] Formulation process
[0069] 1 prepare the povidone ethanol solution to get the povidone of the recipe quantity and make the ethanol solution of 10% (w / w), for subsequent use;
[0070] 2 Granulation After sieving carbamazepine and mannitol with 80 meshes respectively, put them into a mixing granulator together with hypromellose and microcrystalline cellulose for mixing, and then add povidone ethanol solution for wet granulation. granules, take out the granules and carry out wet granulation with a 20-mesh sieve. After drying, pass through a 20-mesh sieve to granulate and set aside.
[0071] 3. Tabletting After mixing the obtained granules and the prescribed amount of magne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com