Patents
Literature
6248results about How to "Reduce force" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
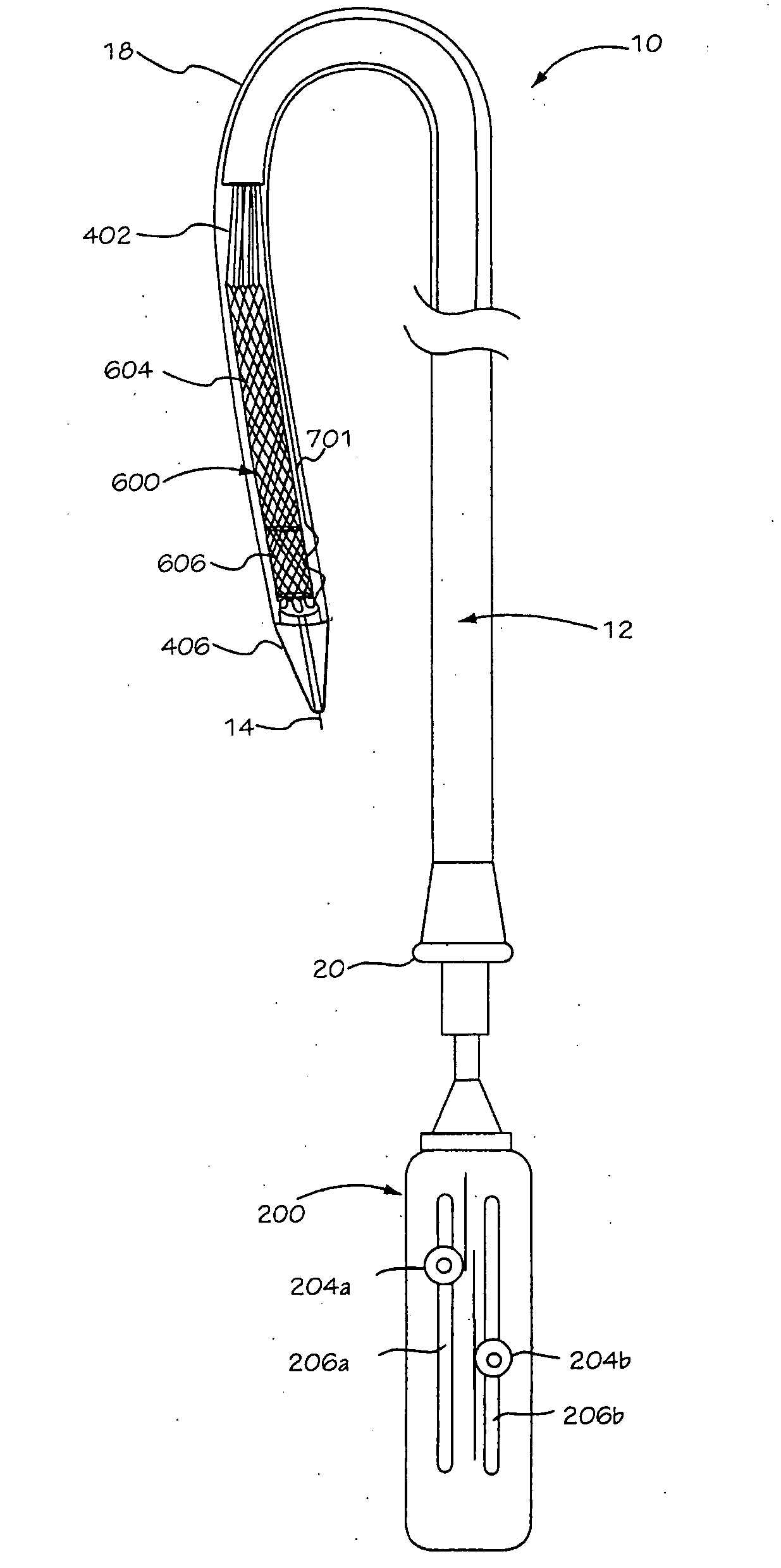
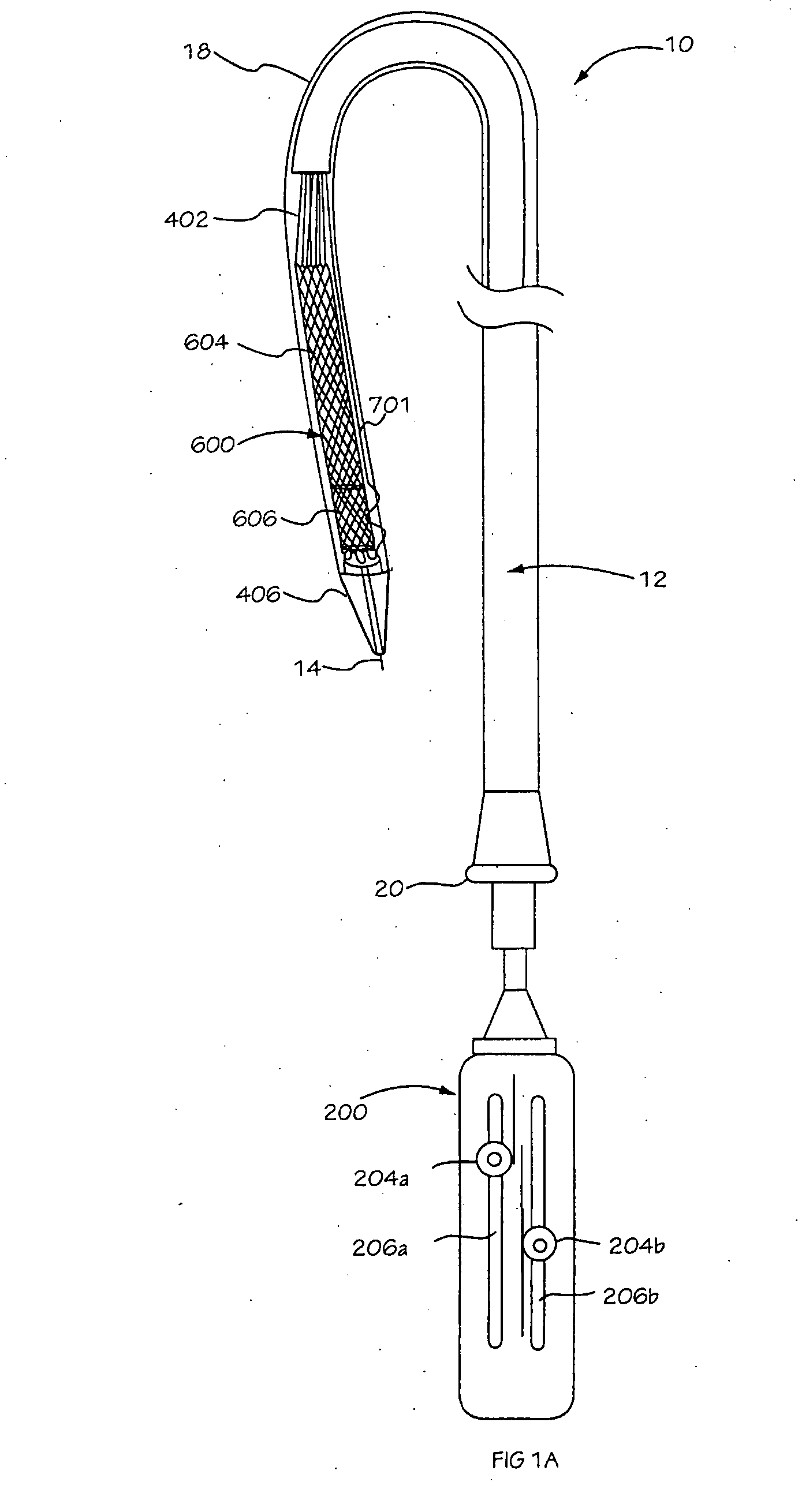
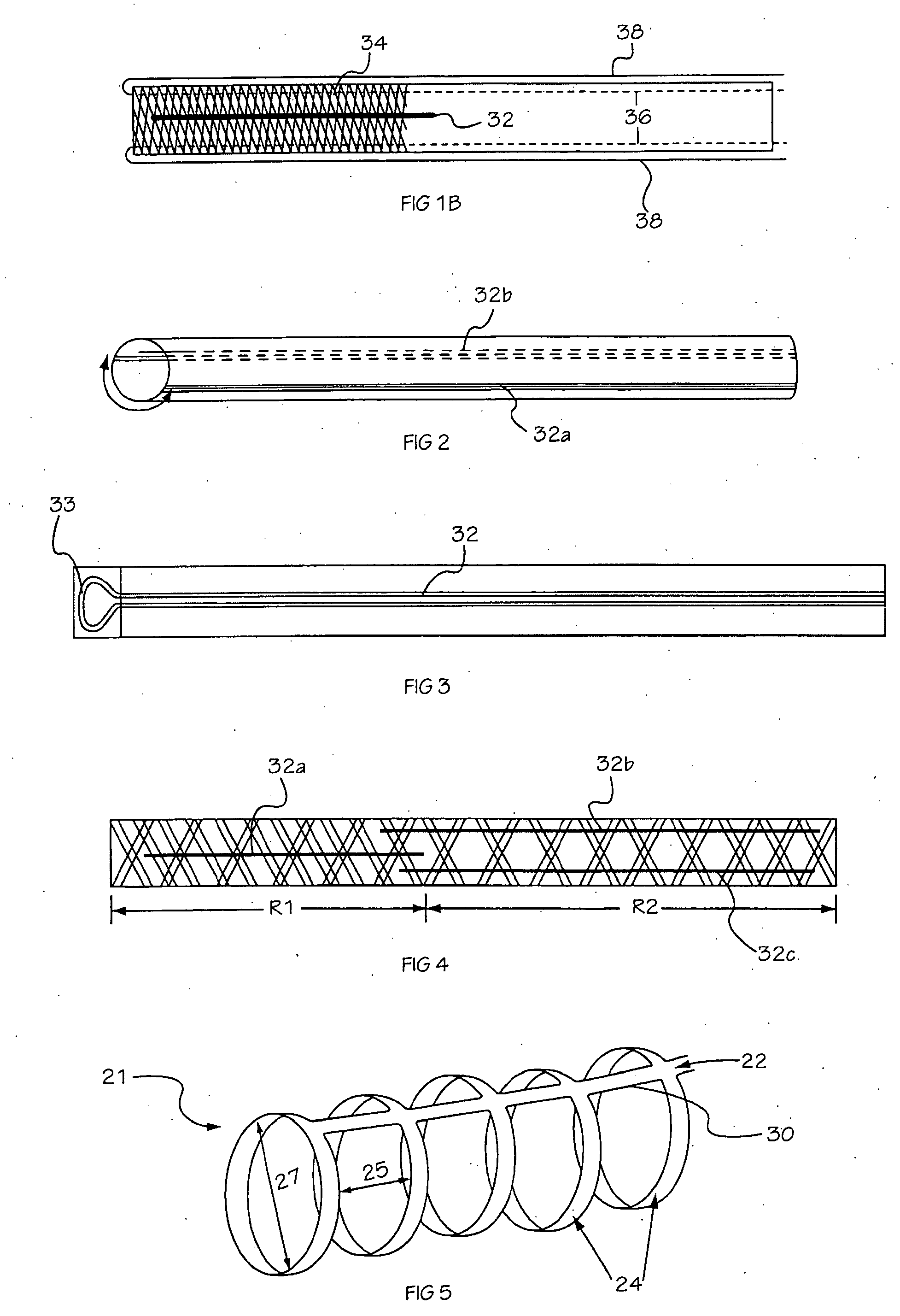
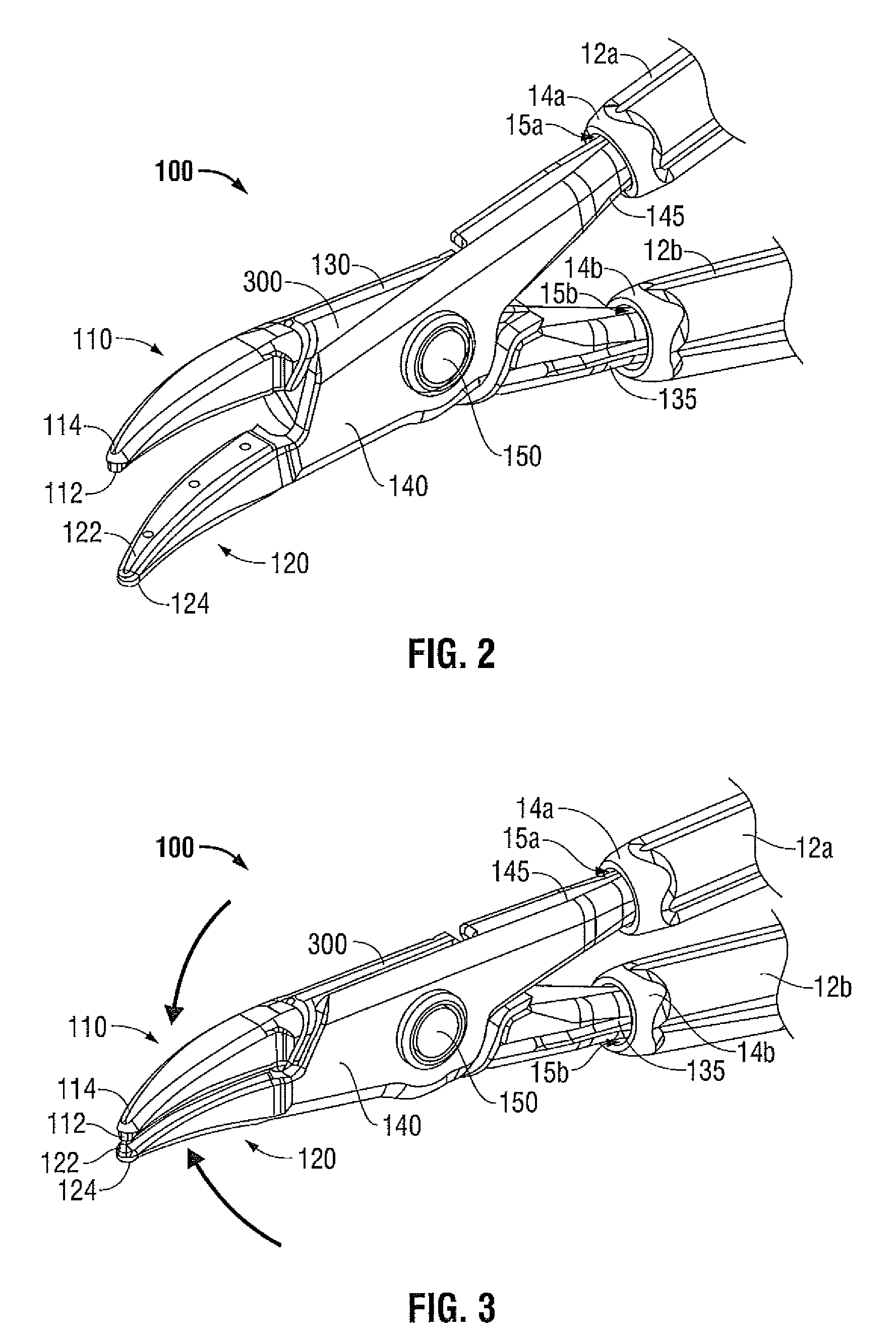
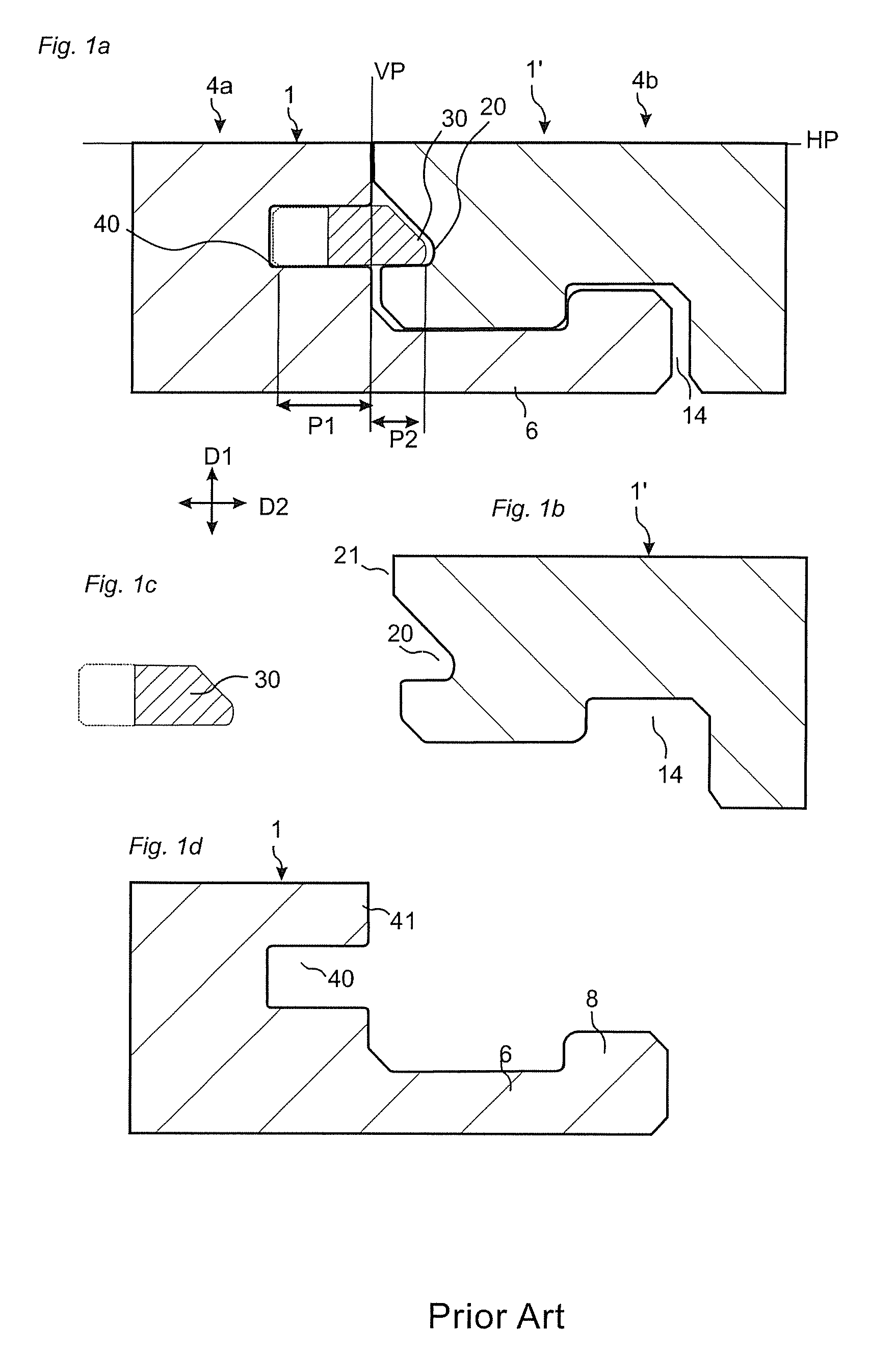
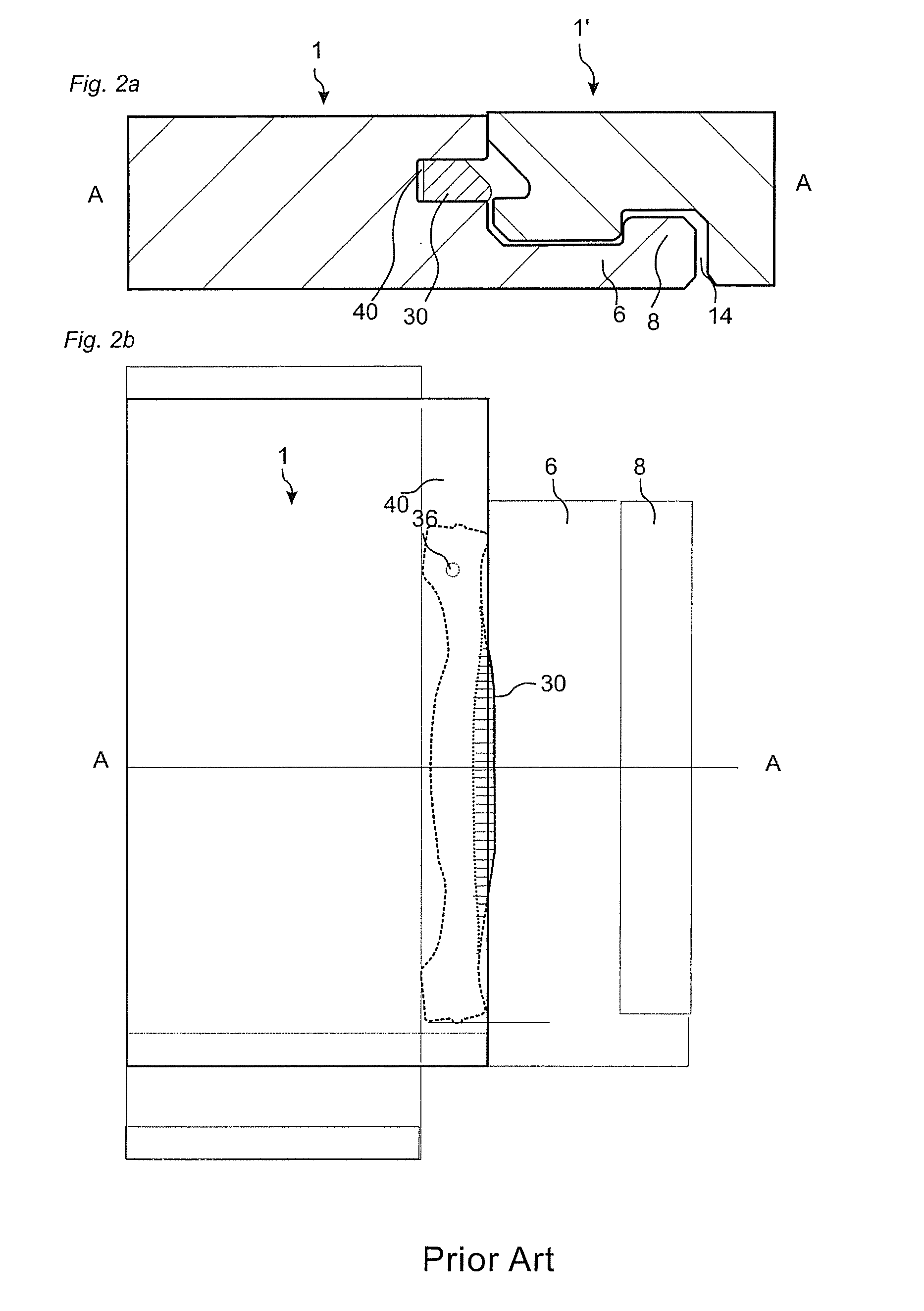
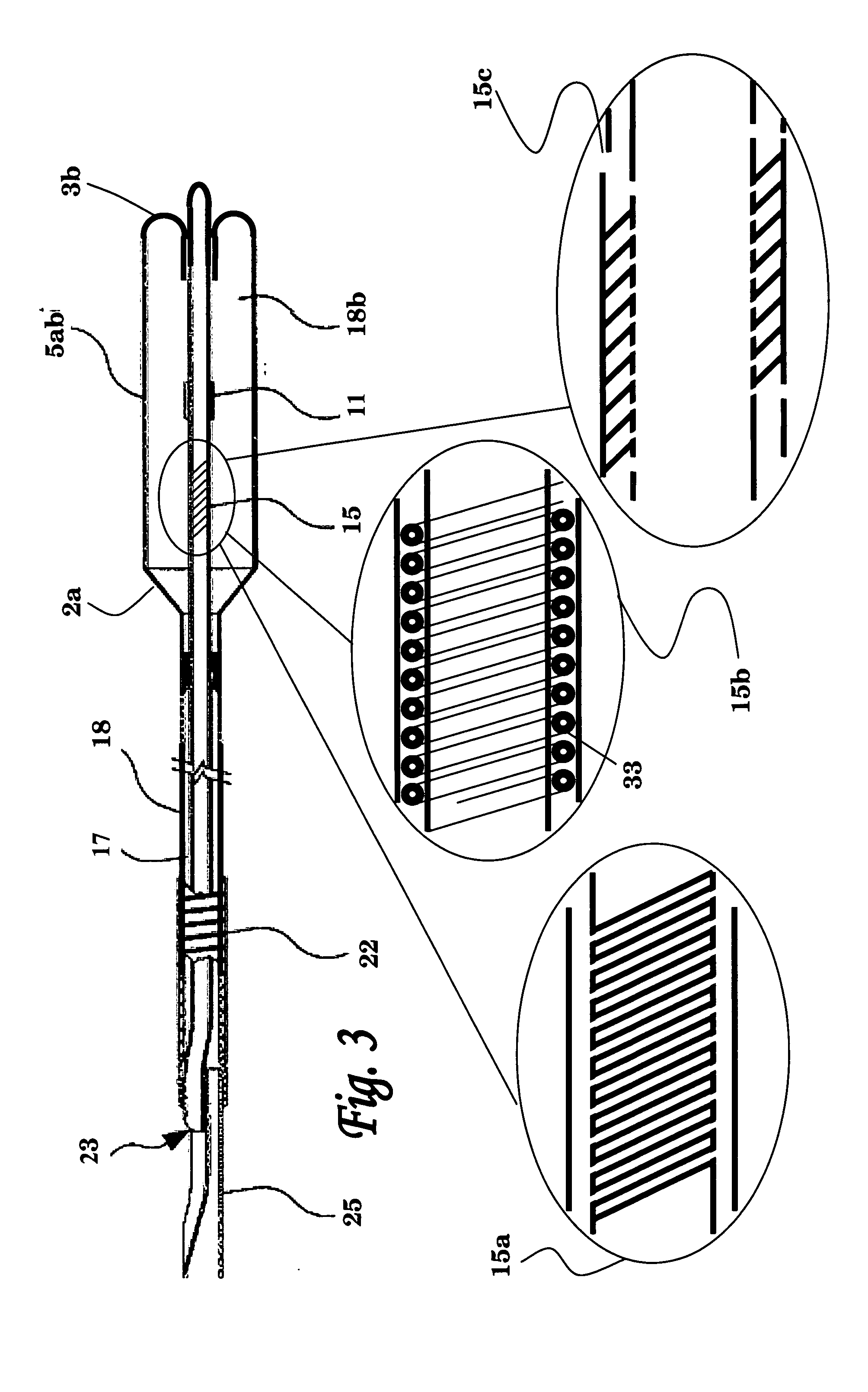
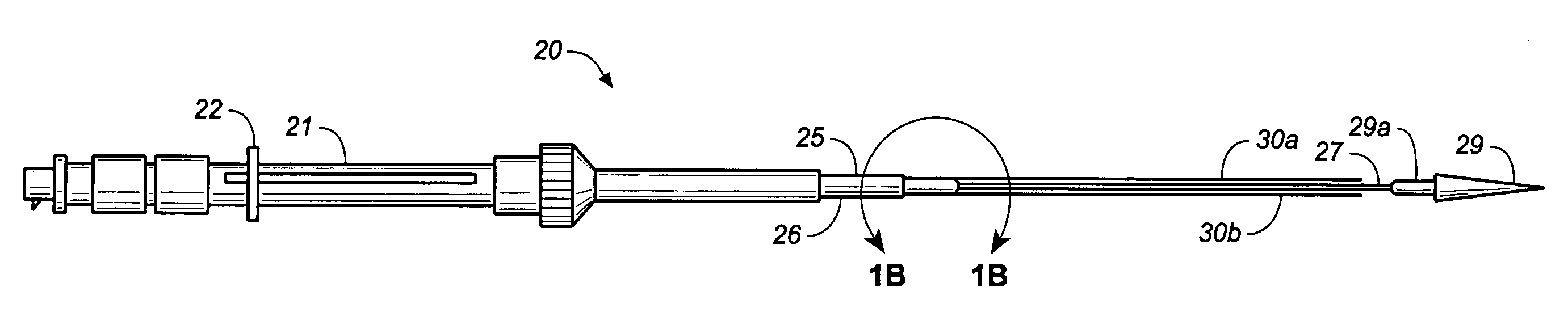
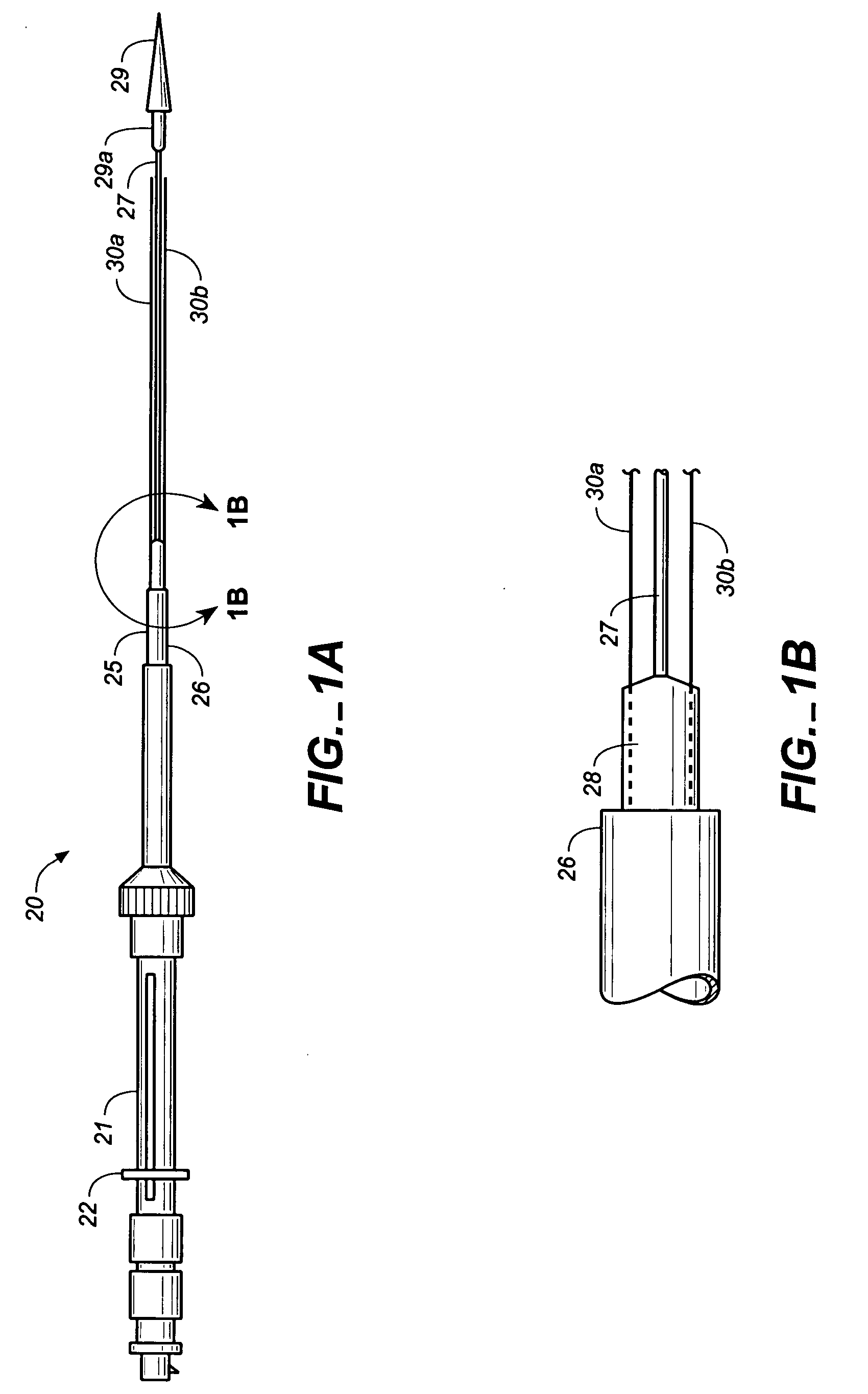
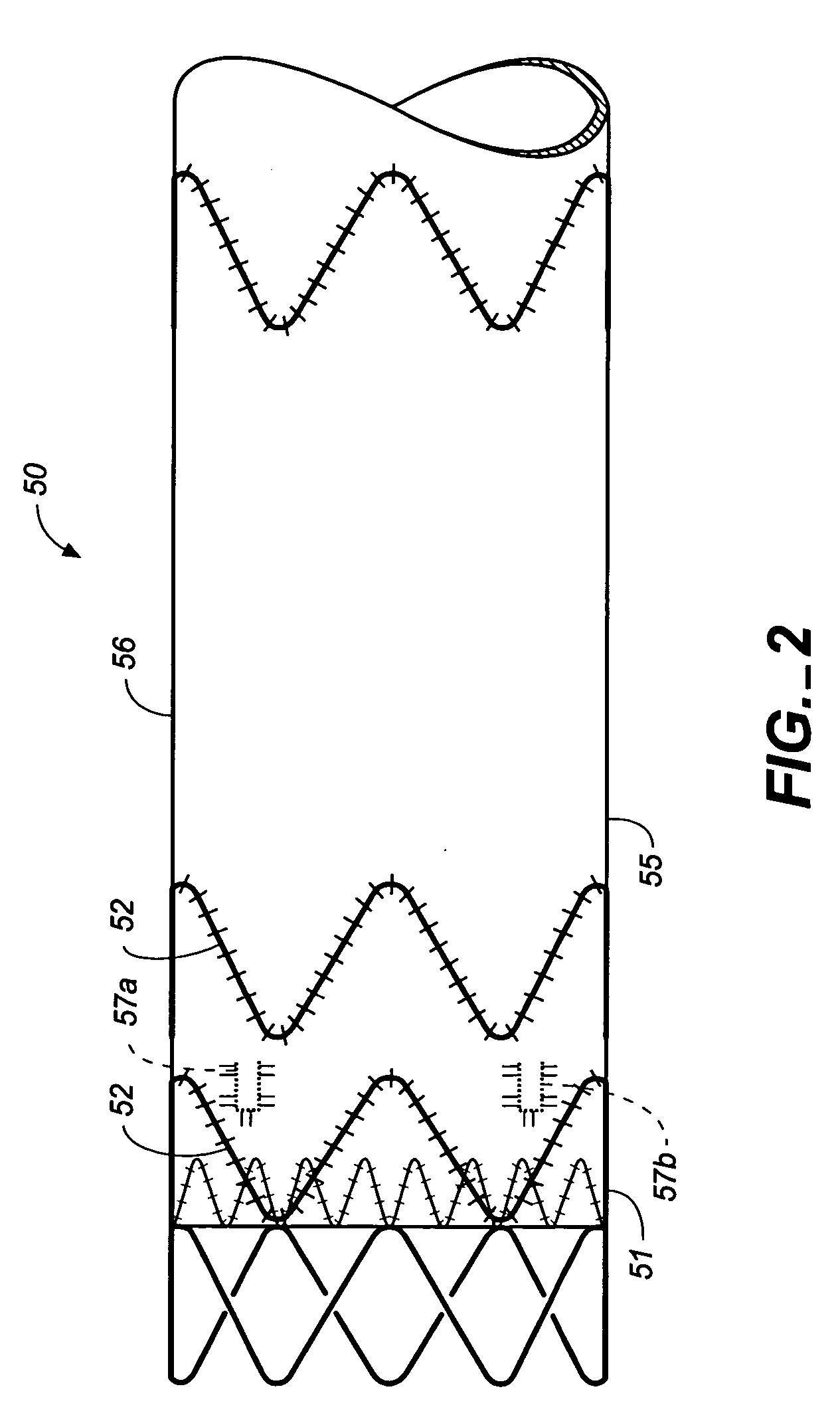
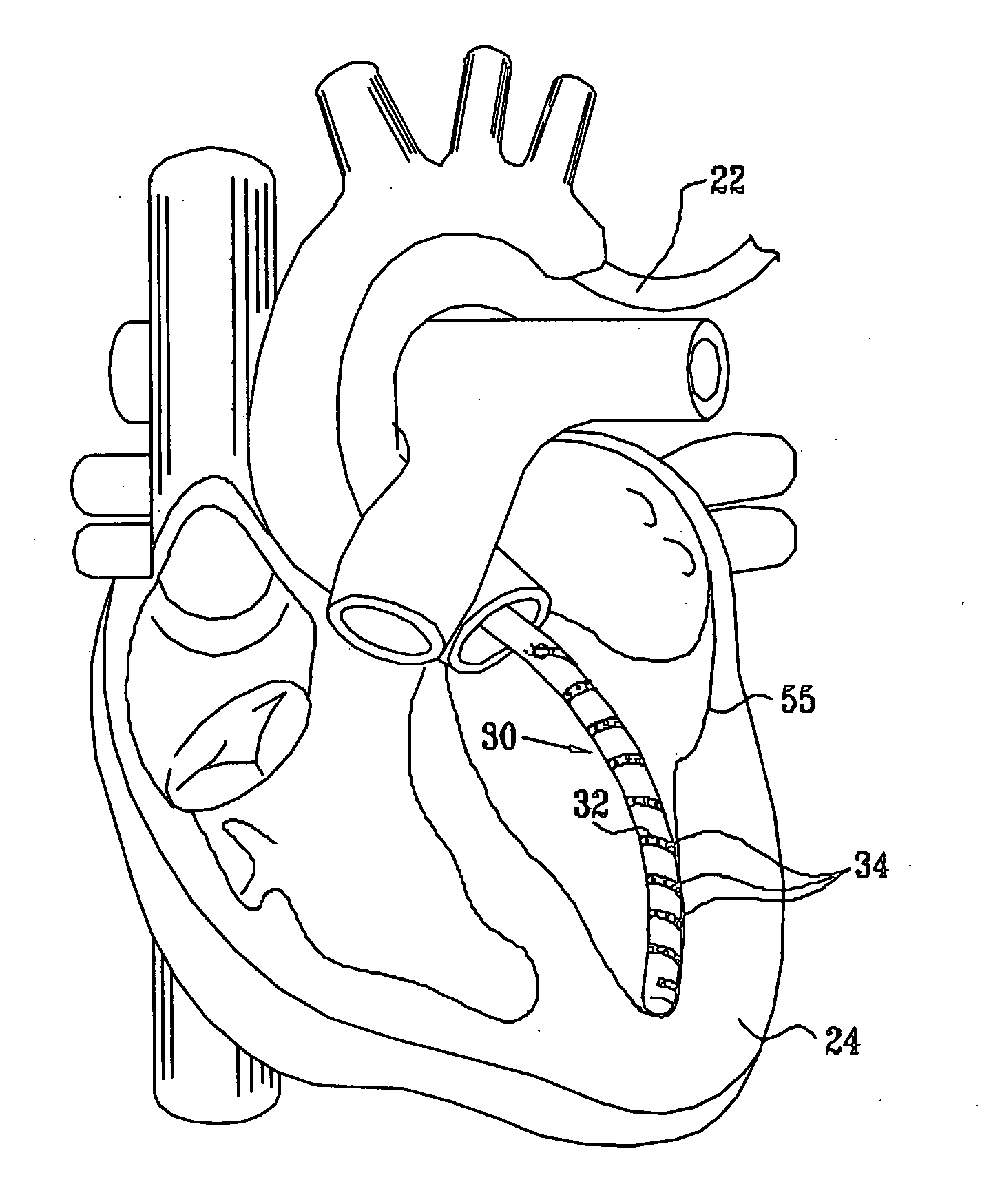
Medical device delivery sheath
InactiveUS20080188928A1Easy to collapseFacilitate resheathingStentsHeart valvesMedical deviceGuide tube
A support member for a catheter sheath is disclosed. The support member has a series of ribs with a distal member having integrated fingers for providing radial compliance. The support member provides sufficient axial stiffness to provide a desired pushability of a minimally invasive device for replacing a heart valve. Various alternative embodiments are also described.
Owner:BOSTON SCI SCIMED INC
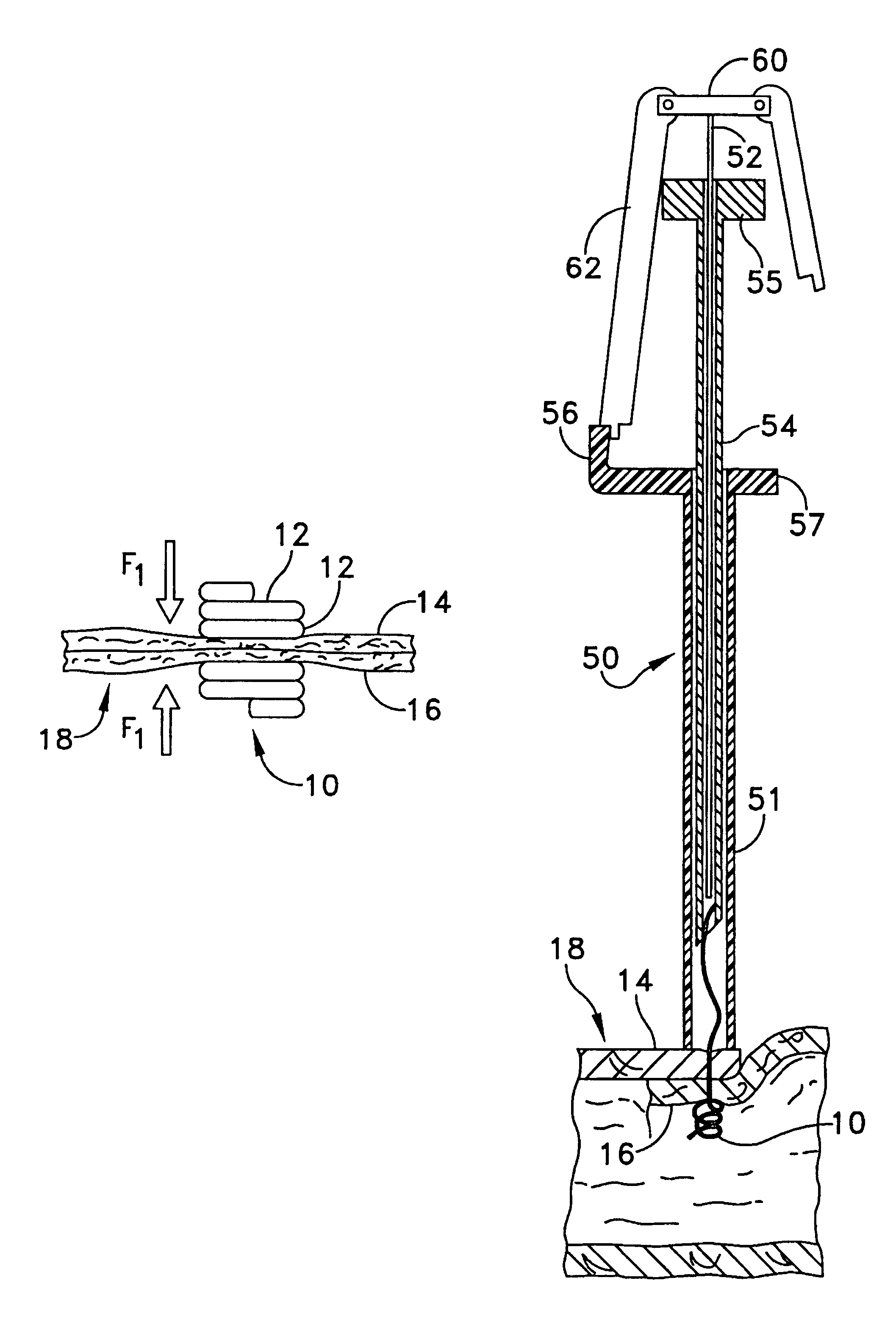
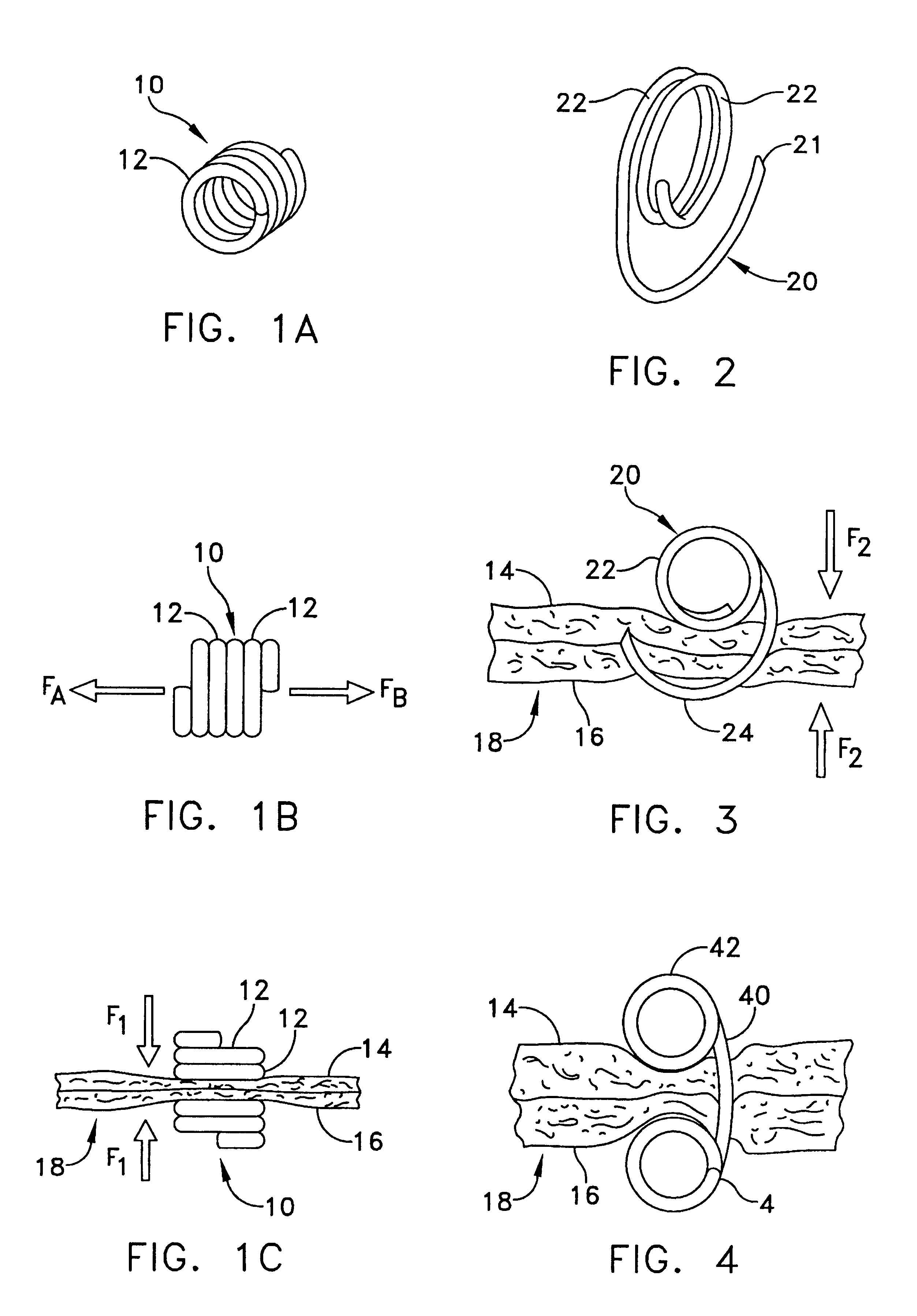
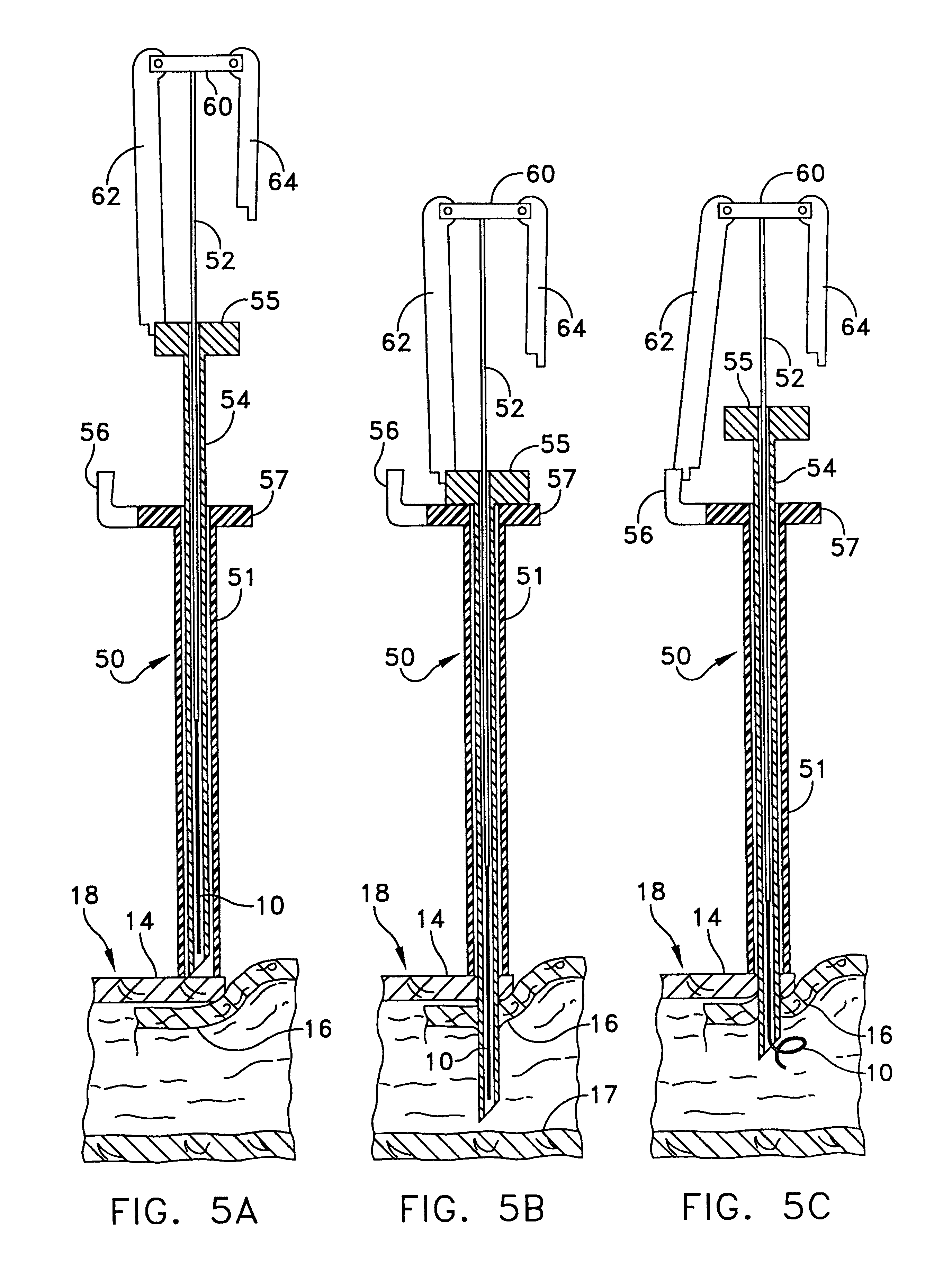
Multi-fastener surgical apparatus and method
A fastener preferably made from a shape memory alloy is provided which can access internal tissue or other synthetic material by catheter delivery through an endovascular pathway. After the fastener is deployed through layers of tissue or other material, it assumes a shape that automatically applies to the layers of tissue or other material an appropriate hemostatic compression which is relatively independent of tissue or material thickness. The fastener is a suitable replacement for conventional non bio-absorbable sutures and staples in certain clinical applications. The shape, method of deployment, and low force requirements make the disclosed apparatus suitable for endosurgical procedures where access to the wound site is limited. A method for deploying the fastener is also provided.
Owner:ONUX MEDICAL
Blunt Tissue Dissection Surgical Instrument Jaw Designs
ActiveUS20110034918A1Reduce forceImproving tissue division and repeatabilityIncision instrumentsBlunt dissectorsSurgical instrumentDissection forceps
A forceps for use in surgery for dissecting tissue includes a pair of jaw members movable from an open position in spaced relation relative to one another to a closed position. The jaw members each have an outer housing extending along the length thereof to a distal end of the jaw members. The outer housing of one of the jaw members includes a textured surface at a distal end configured to interface with and dissect tissue during the movement of the jaw members from the closed to open positions. A dissecting tip may be selectively extendable from a channel defined in one of the jaw members to engage and separate tissue when in the extended position.
Owner:TYCO HEALTHCARE GRP LP
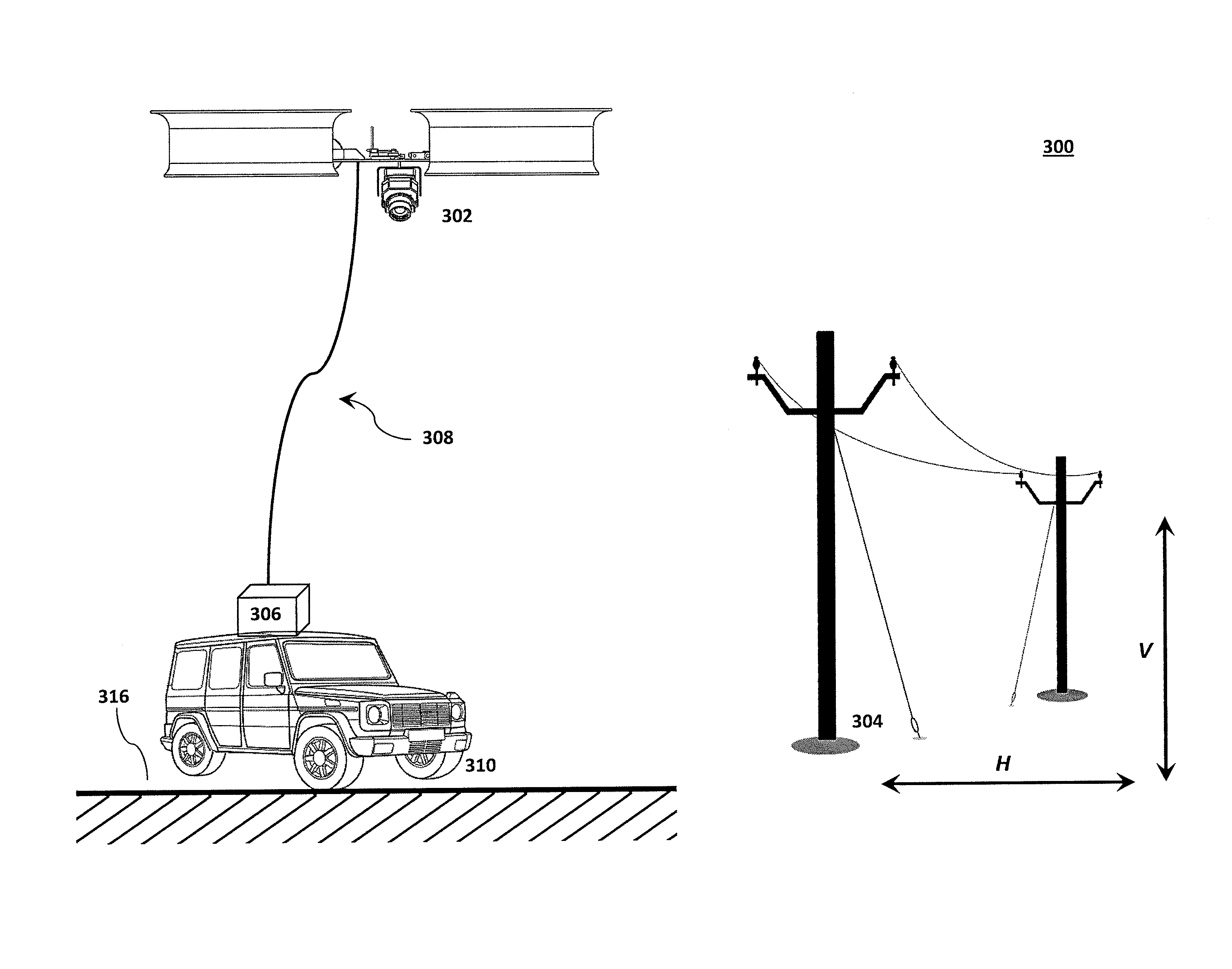
Tethered aerial system for data gathering
InactiveUS20130233964A1Increase horizontal rangeExtending flight spaceTethered aircraftActuated automaticallyLevel flightFlight vehicle
A tethered unmanned aerial vehicle (“UAV”) may be outfitted with a sensor payload for data gathering. The tethered UAV may be tethered to a ground station for constricting the flight space of the UAV while also providing the option for power delivery and / or bidirectional communications. The tethered UAV's flight path may be extended by introducing one or more secondary UAVs that cooperate to extend the horizontal flight path of a primary UAV. The ground station, which may be coupled with the tethered aerial vehicle, may comprise a listening switch configured to determine a condition of the tether such that the supply of power to the tether may be terminated when tether damage or a tether severance is detected.
Owner:AURORA FLIGHT SCI CORP
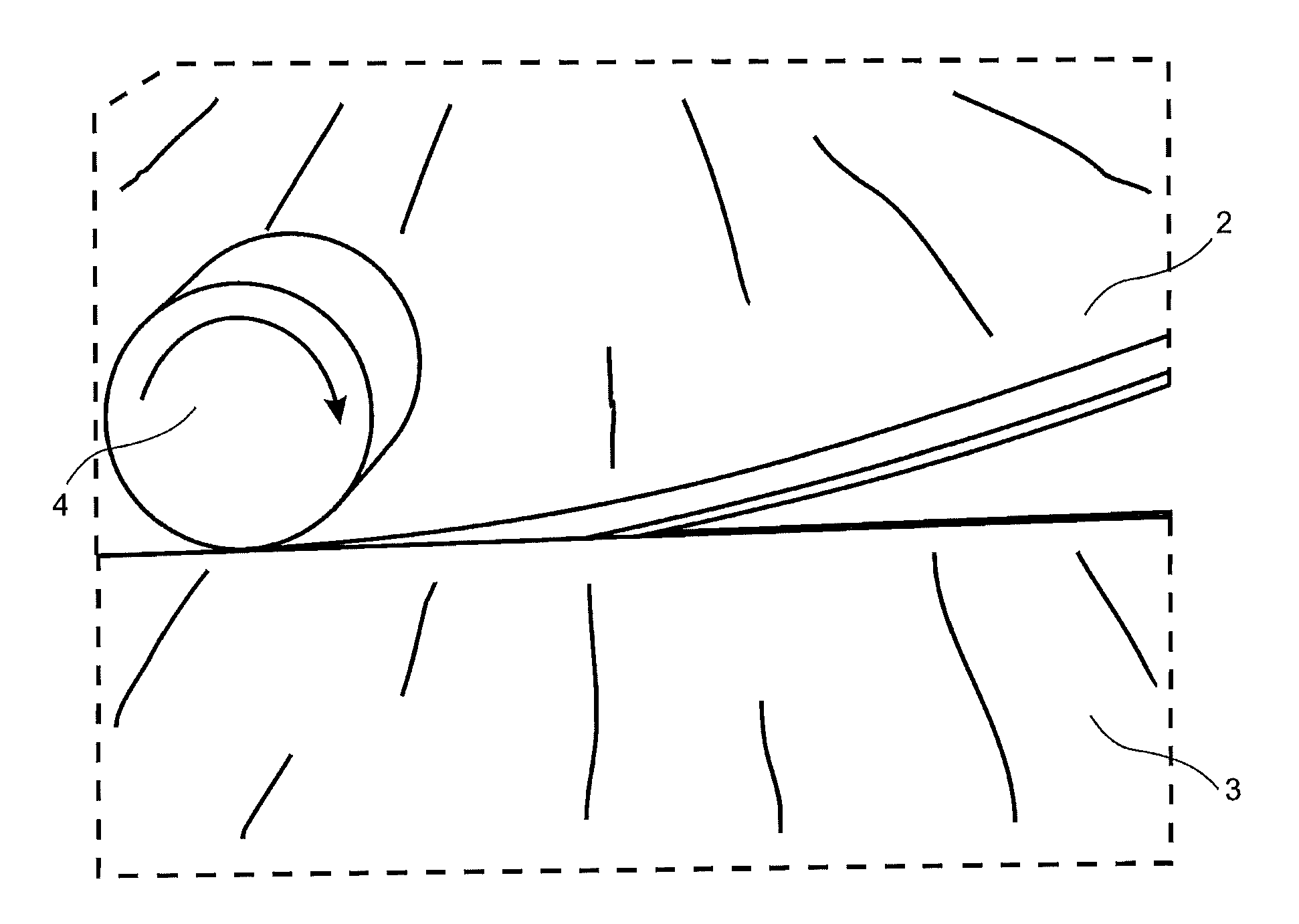
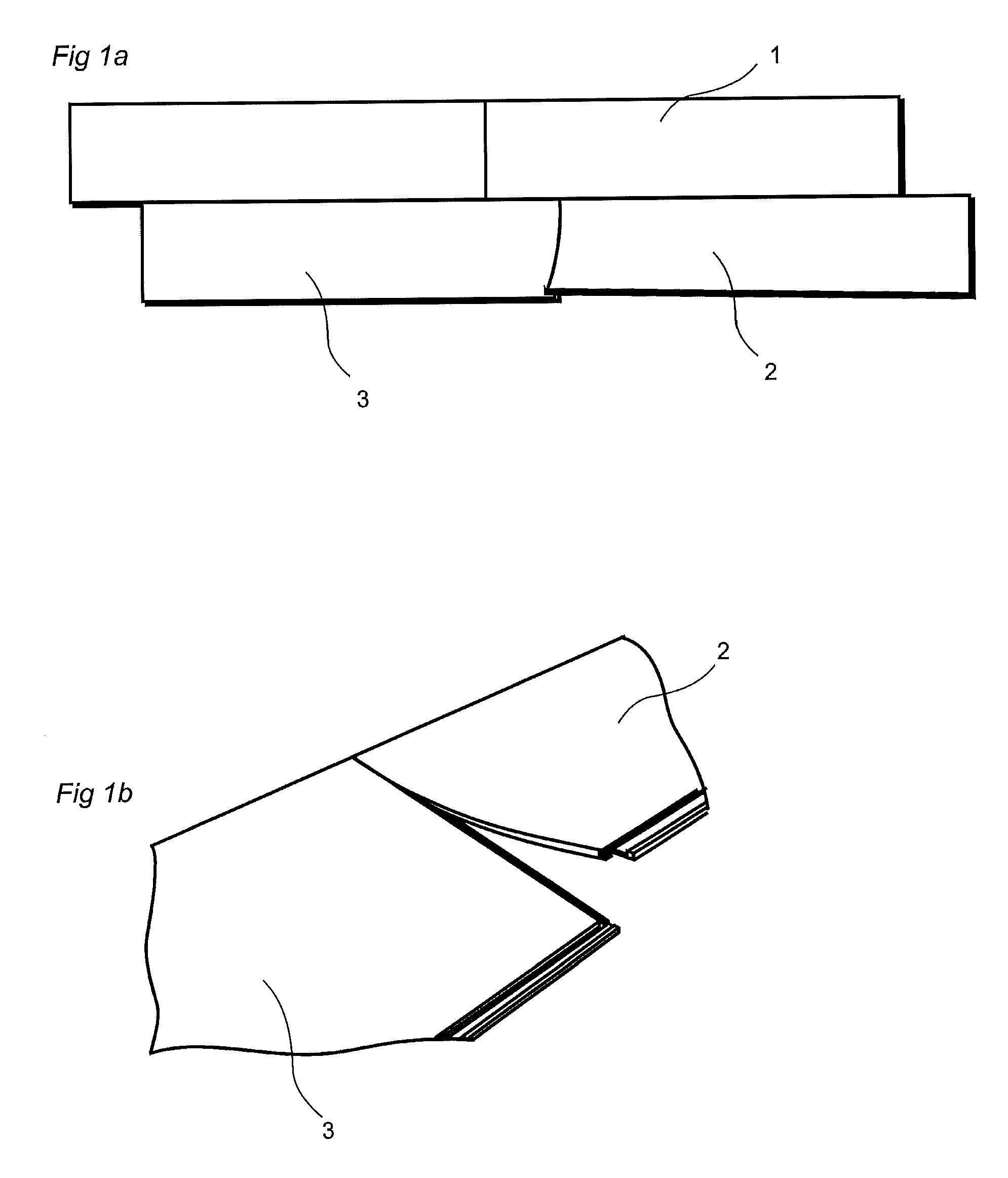
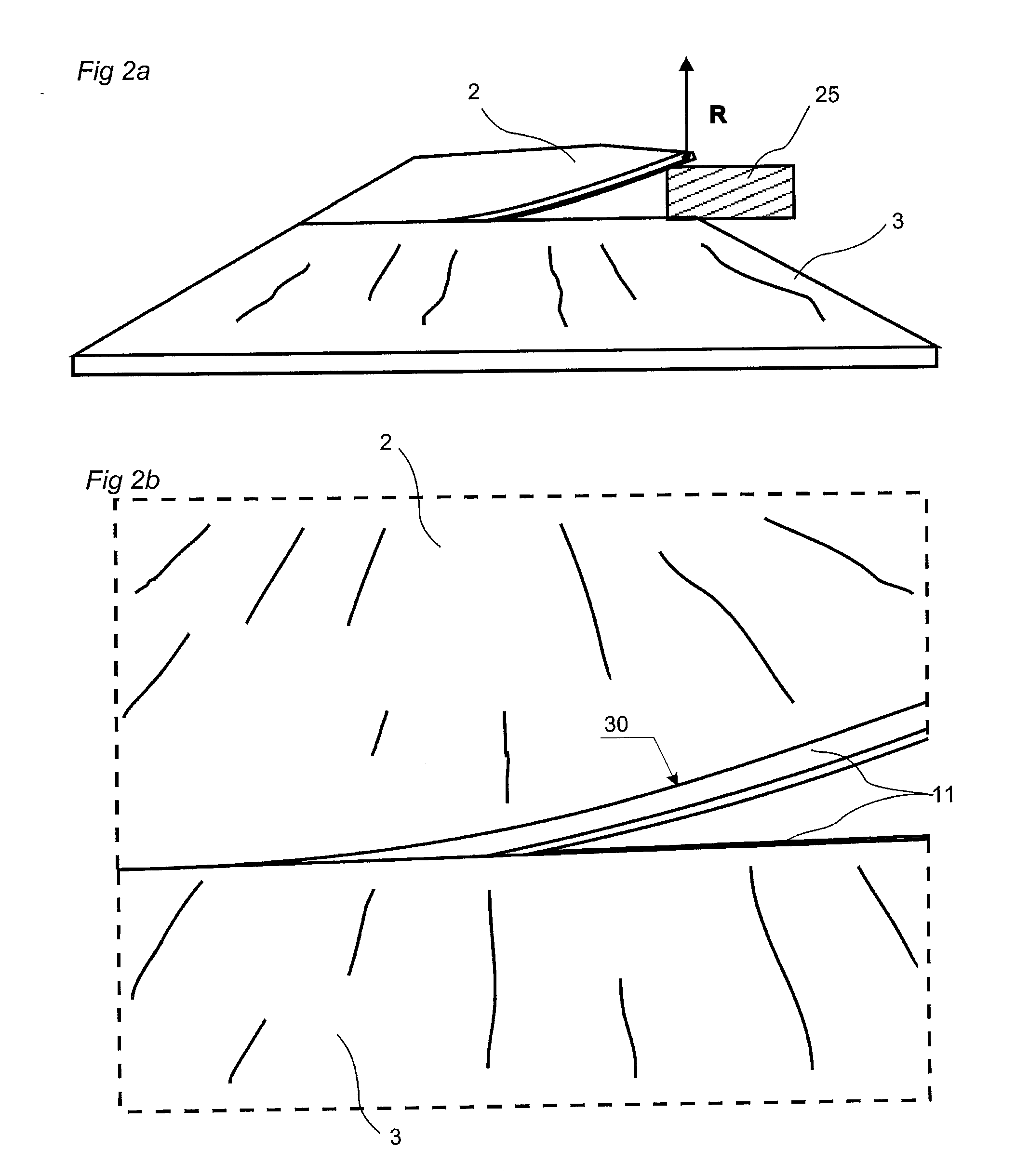
Floorboards, flooring systems and methods for manufacturing and installation thereof
ActiveUS20040139678A1Increase wear resistanceCost reductionStrutsWallsMechanical engineeringFloating floor
Floorboards with a format corresponding to a traditional parquet block for laying of mechanically joined floating flooring. Rectangular floorboards include a surface layer and a core with two long sides and two short sides, for making a floating flooring, which floorboards are mechanically lockable and which along their four sides have pairs of opposing connectors for locking similar, adjoining floorboards to each other both vertically and horizontally wherein the long sides have a length not exceeding 80 cm and the short sides have a width not exceeding 10 cm.
Owner:VÄLINGE INNOVATION AB
Mechanical Locking of Floor Panels
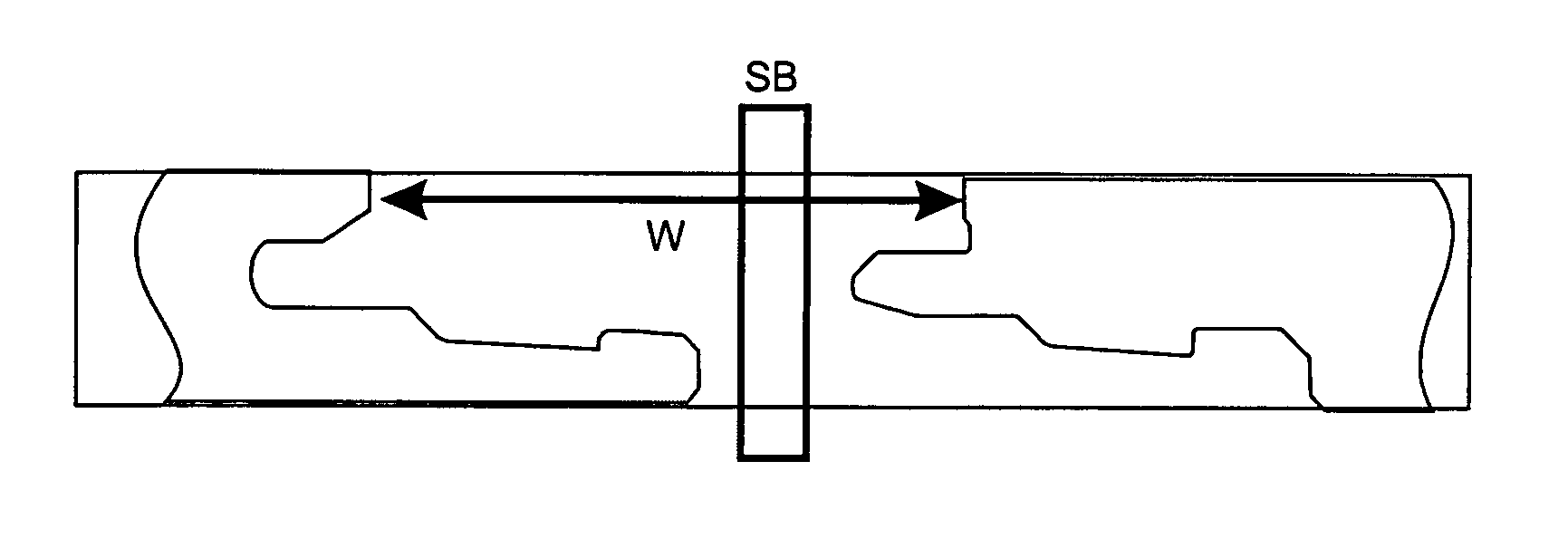
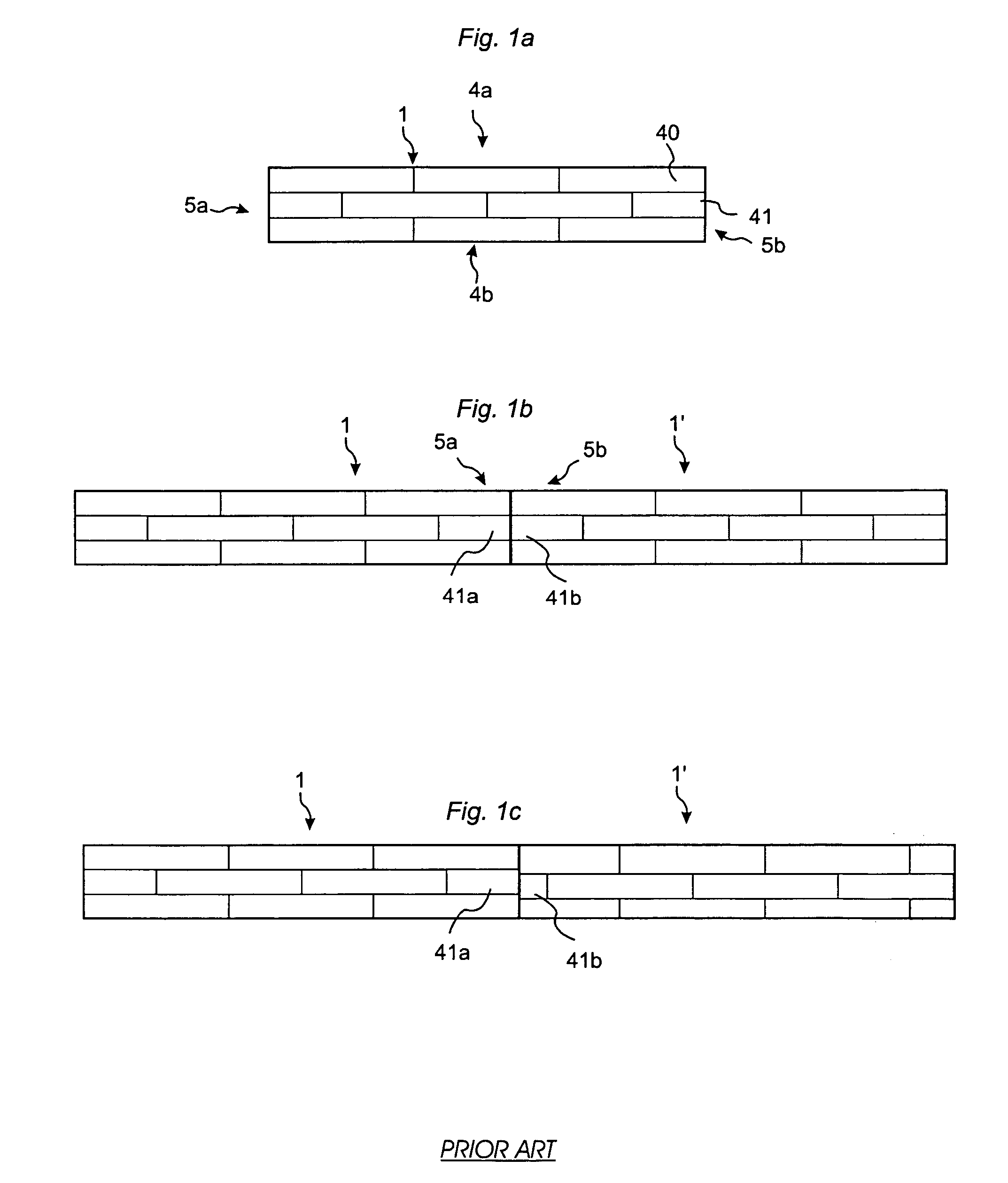
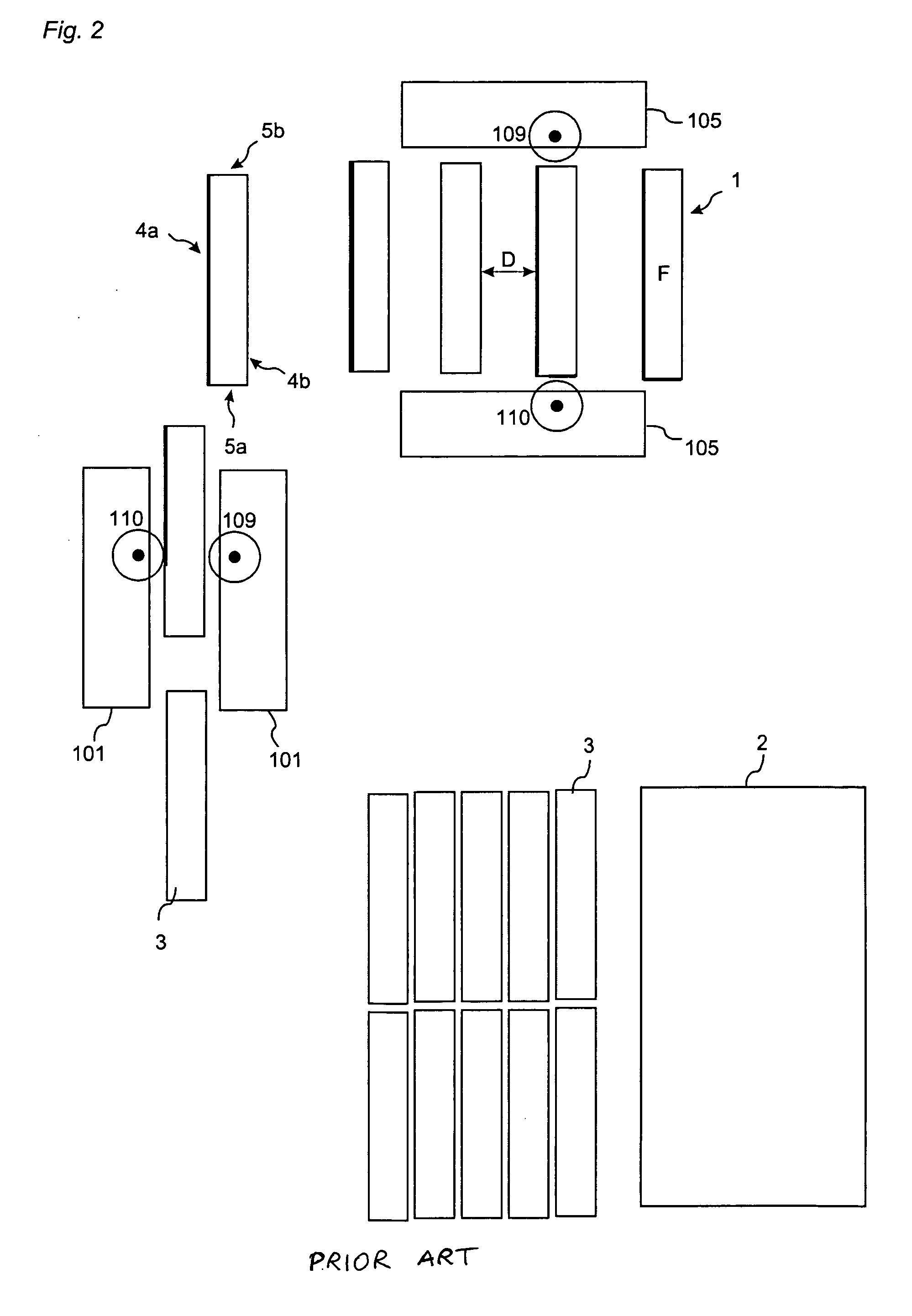
ActiveUS20080134613A1Prevent separationHigh separation forceCovering/liningsFloorsEngineeringPanelling
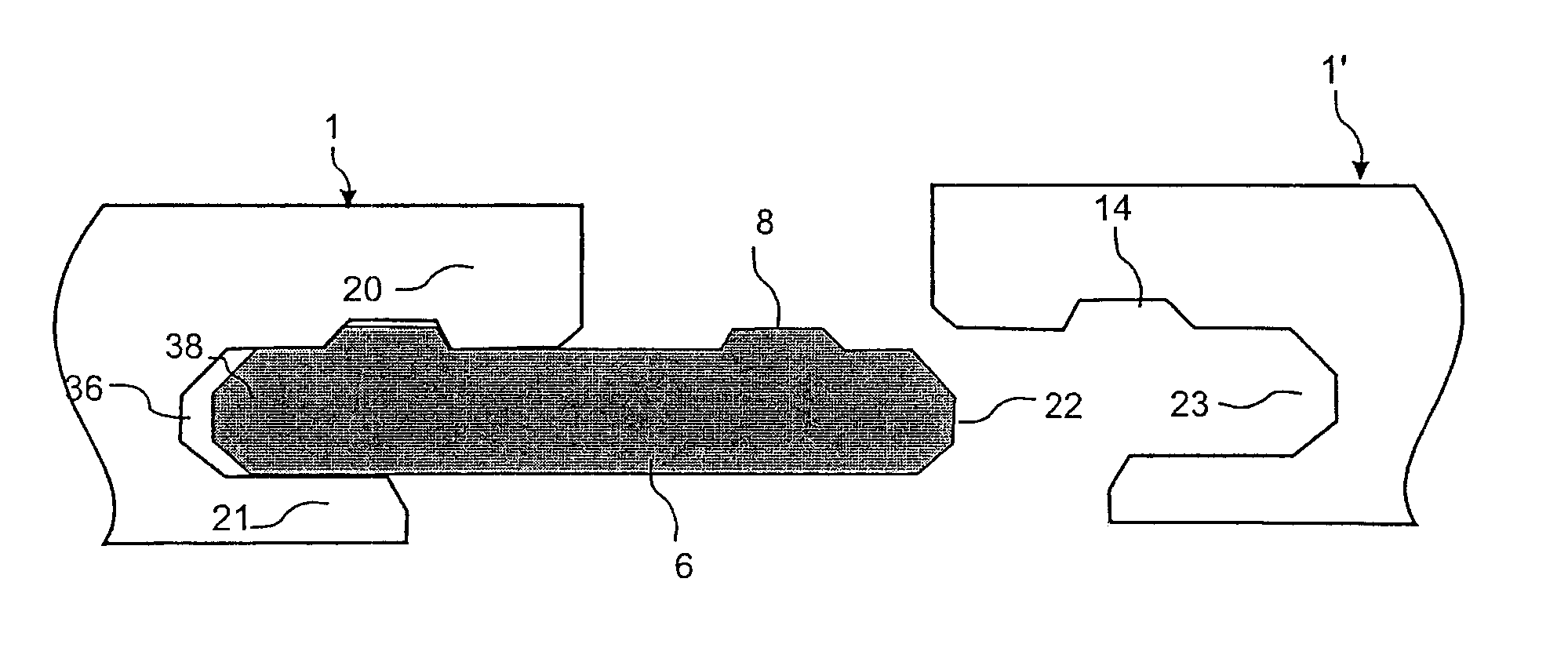
Floor panels (1, 1′) are shown, which are provided with a mechanical locking system on long (5a, 5b) and short edges (4a, 4b) allowing installation with angling of long edges and where the short edge locking system has a displaceable tongue that is displaceable essentially in one direction from an inner unlocked position to an final locked position.
Owner:VÄLINGE INNOVATION AB
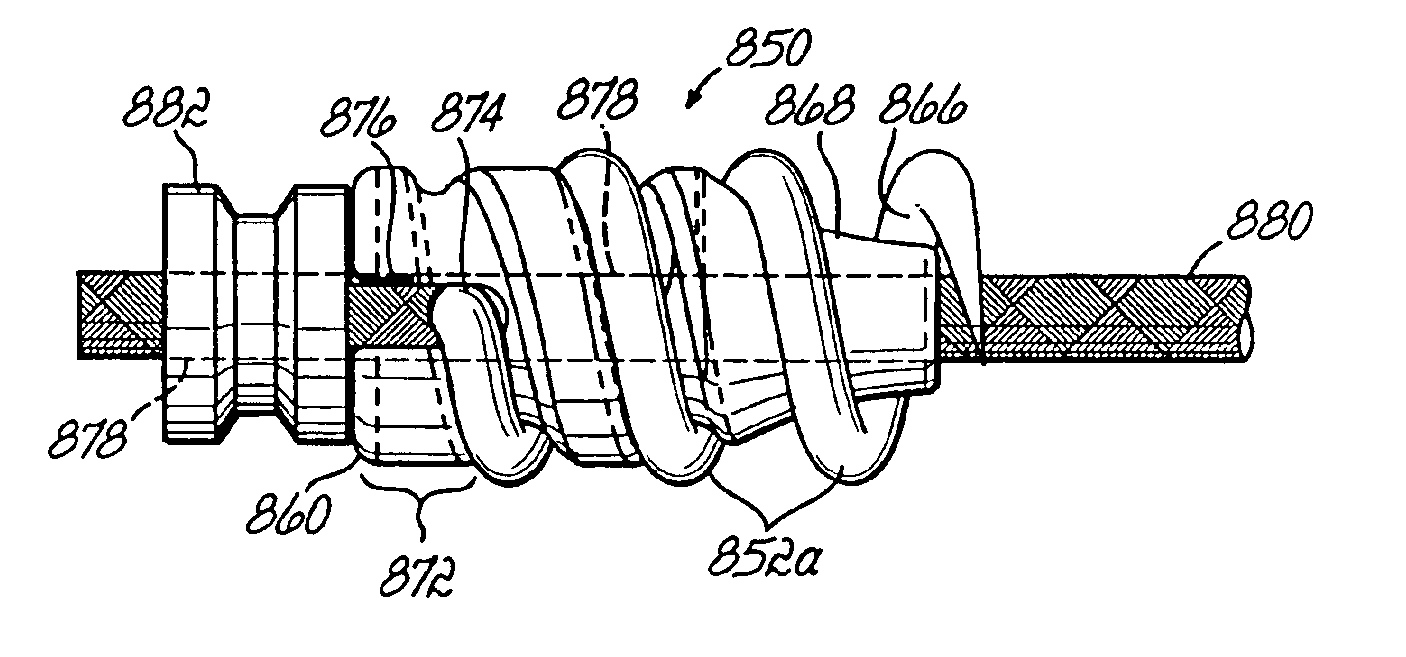
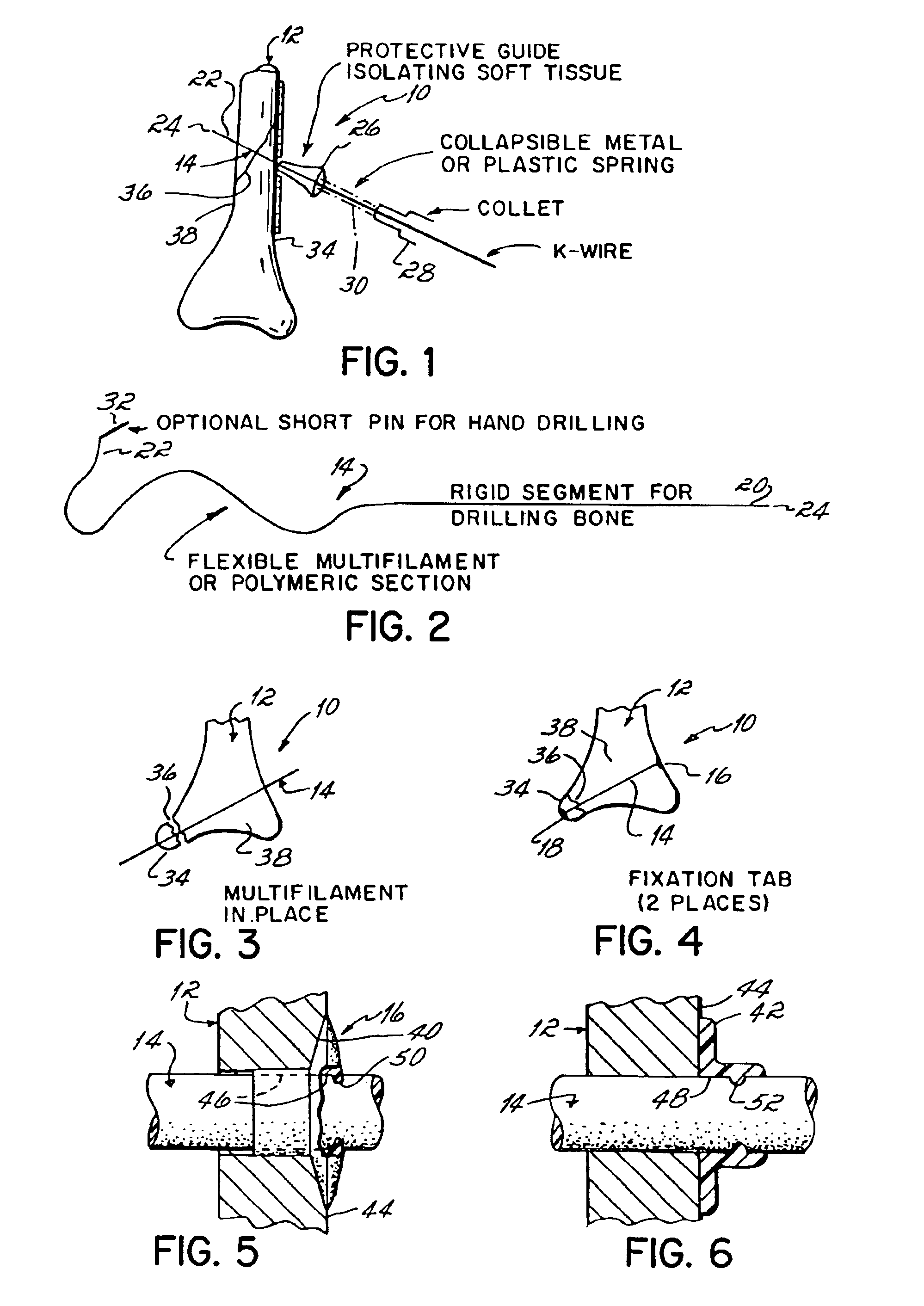
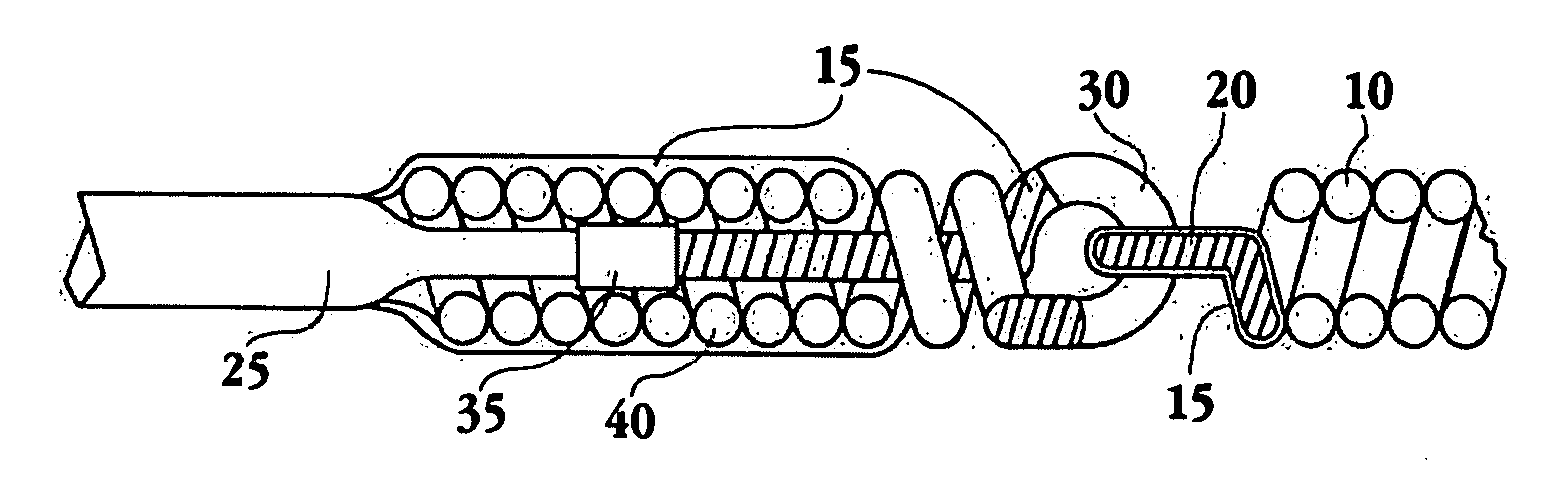
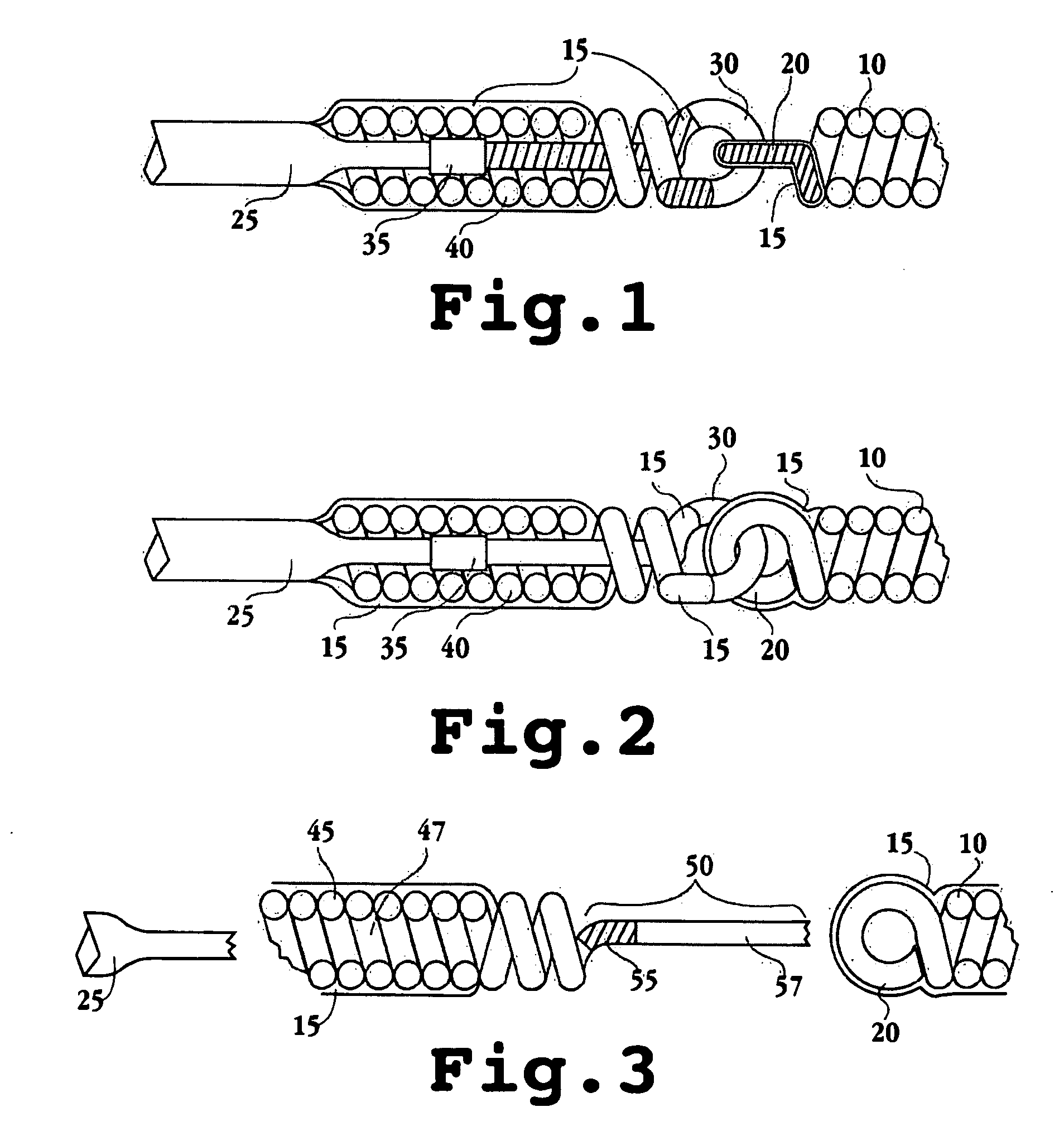
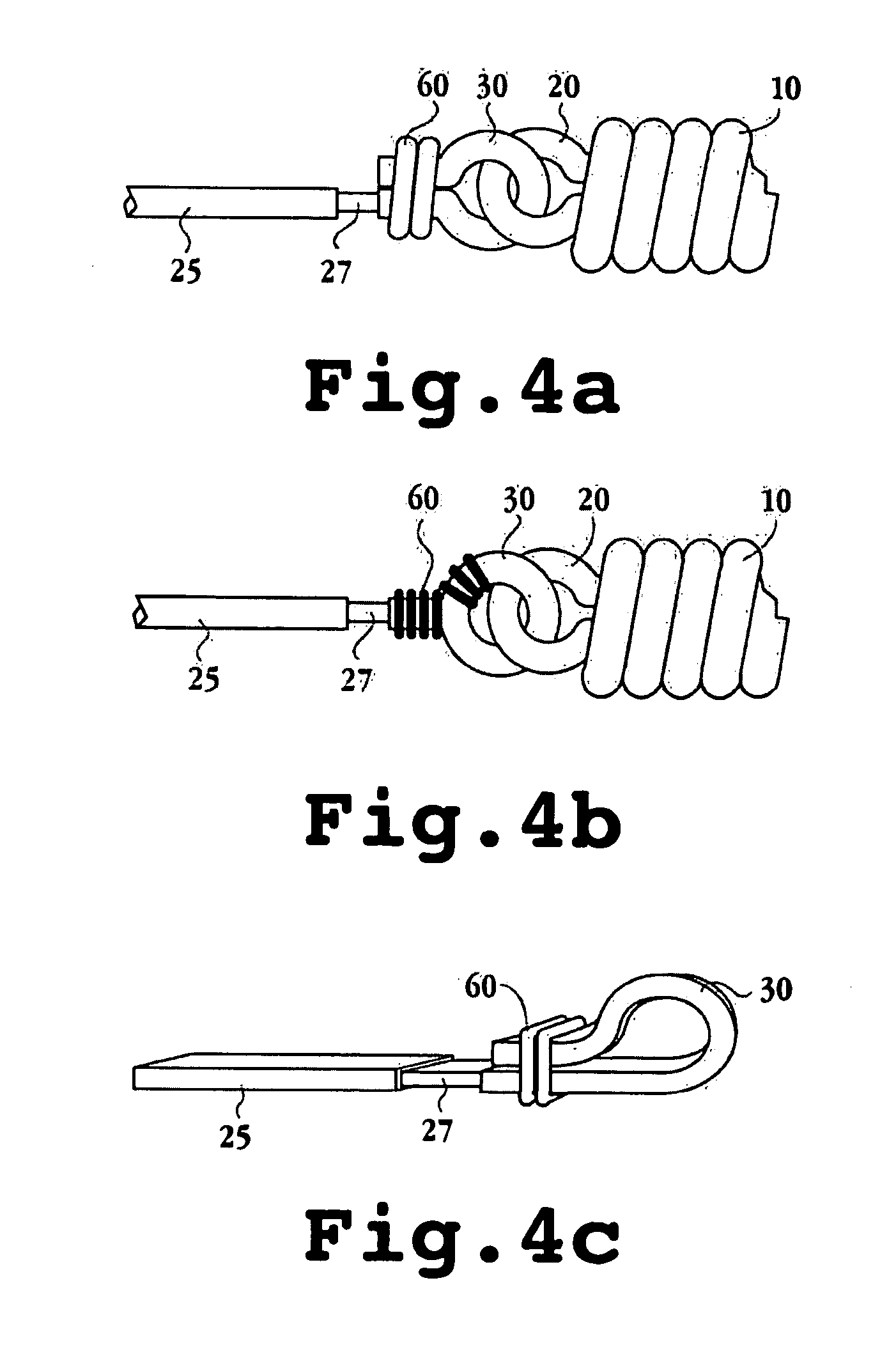
Apparatus and methods for tendon or ligament repair
InactiveUS6984241B2Ease of insertionReduce forceSuture equipmentsInternal osteosythesisLigament repairEngineering
Apparatus and methods for repairing damaged tendons or ligaments. Various repair apparatus include an elongate tensile member and a pair of anchor structures connected for movement along the tensile member on either side of a repair site, such as a tear or laceration. The anchor structures may take many forms, and may include barbed, helical, and crimp-type anchors. In the preferred embodiments, at least one anchor structure is movable along the elongate tensile member to assist with adjusting a tendon segment to an appropriate repair position and the anchor structure or structures are then lockable onto the elongate tensile member to assist with affixing the tendon at the repair position. The invention further provides tendon-to-bone repair apparatus and methods employing similar concepts. Tendon retrieval devices of the invention include helical members for rotating into a tendon end and subsequently moving the tendon to an appropriate operating position.
Owner:TENDON TECH
Flexible display device and method of manufacturing the same
ActiveUS20140183473A1Reduce tensile stressReduce resilient forceSolid-state devicesSemiconductor/solid-state device manufacturingEngineeringFlexible display
A flexible display device and a method of manufacturing the same are provided. The flexible display device comprises a first flexible substrate including a display area including an organic light emitting layer, and a peripheral circuit area, and a second flexible substrate coming in contact with the first flexible substrate and including a pattern for facilitating bending thereof, wherein the second flexible substrate has a certain shape according to the pattern, and the first flexible substrate has a shape corresponding to the certain shape. Various embodiments of the present invention provide a flexible display device capable of realizing a narrow bezel-type or bezel-free display device and simultaneously realizing improved types of design, facilitating bending of a bezel area so as to realize a narrow bezel-type or bezel-free display device, and minimizing damage to an area to be bent.
Owner:LG DISPLAY CO LTD
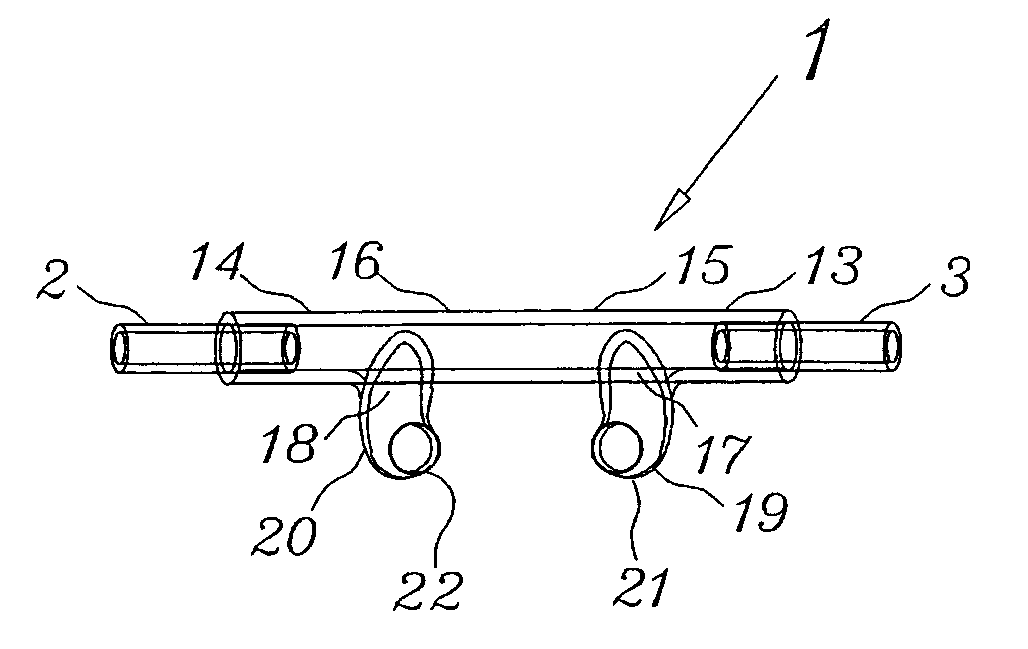
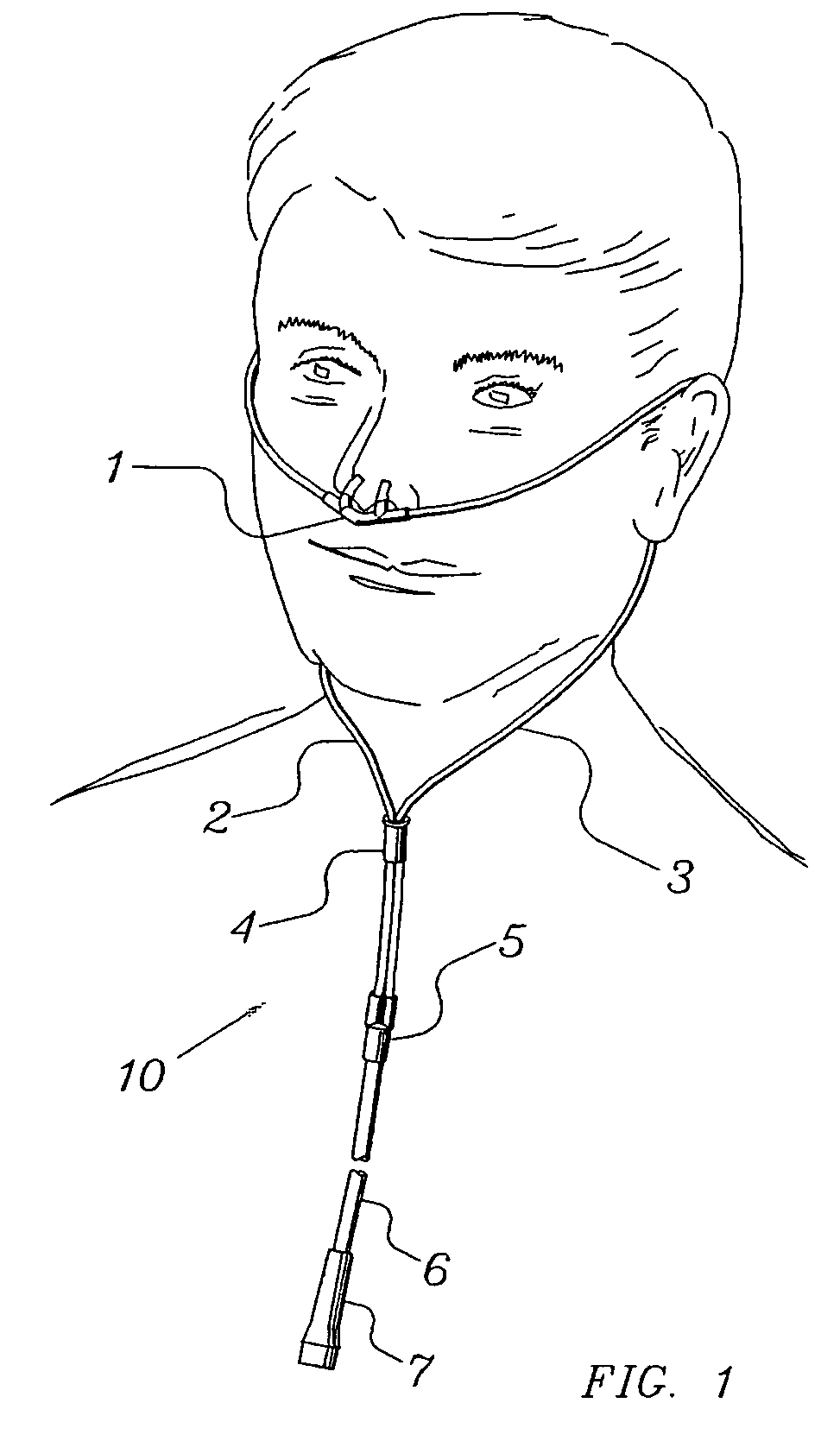
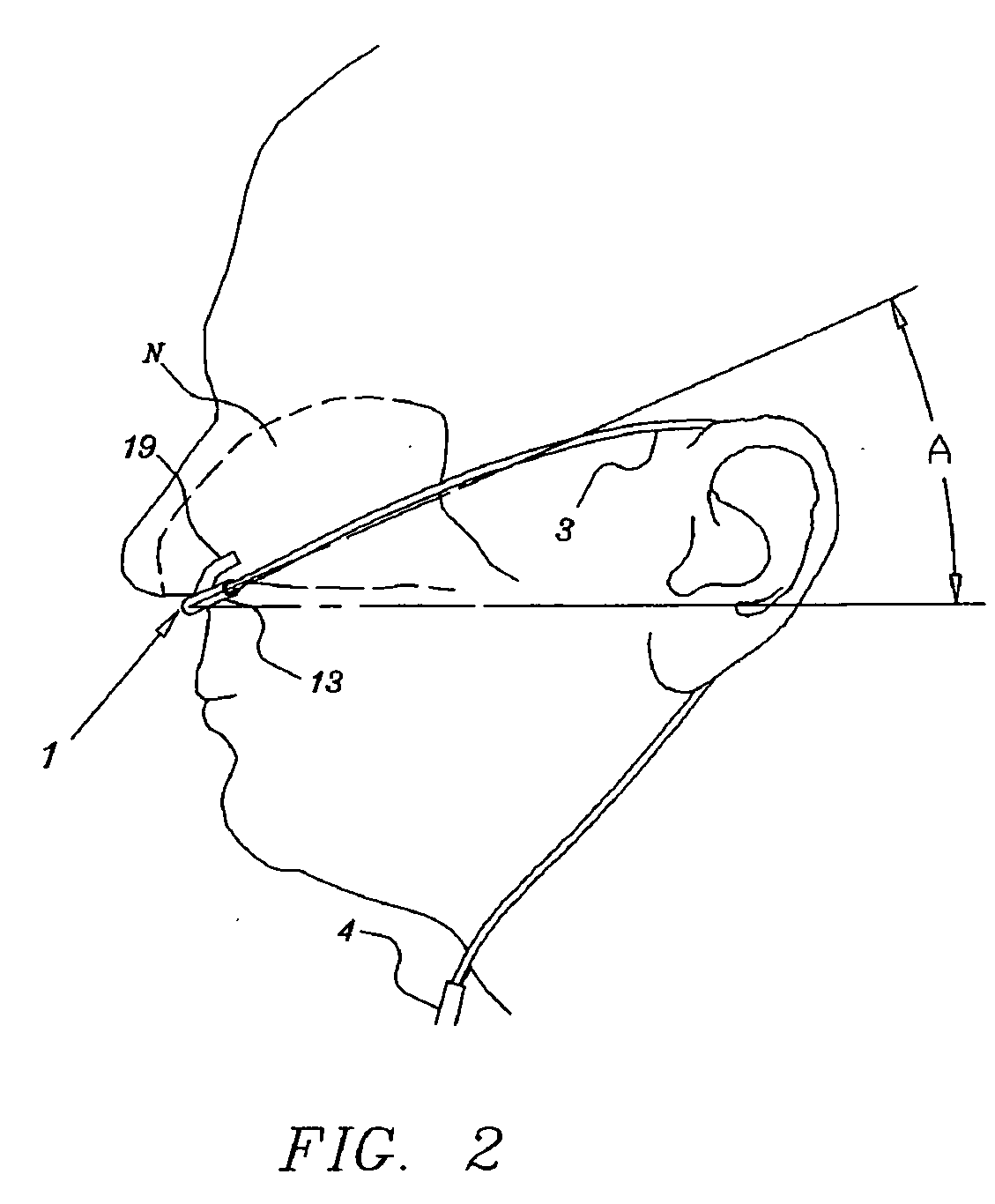
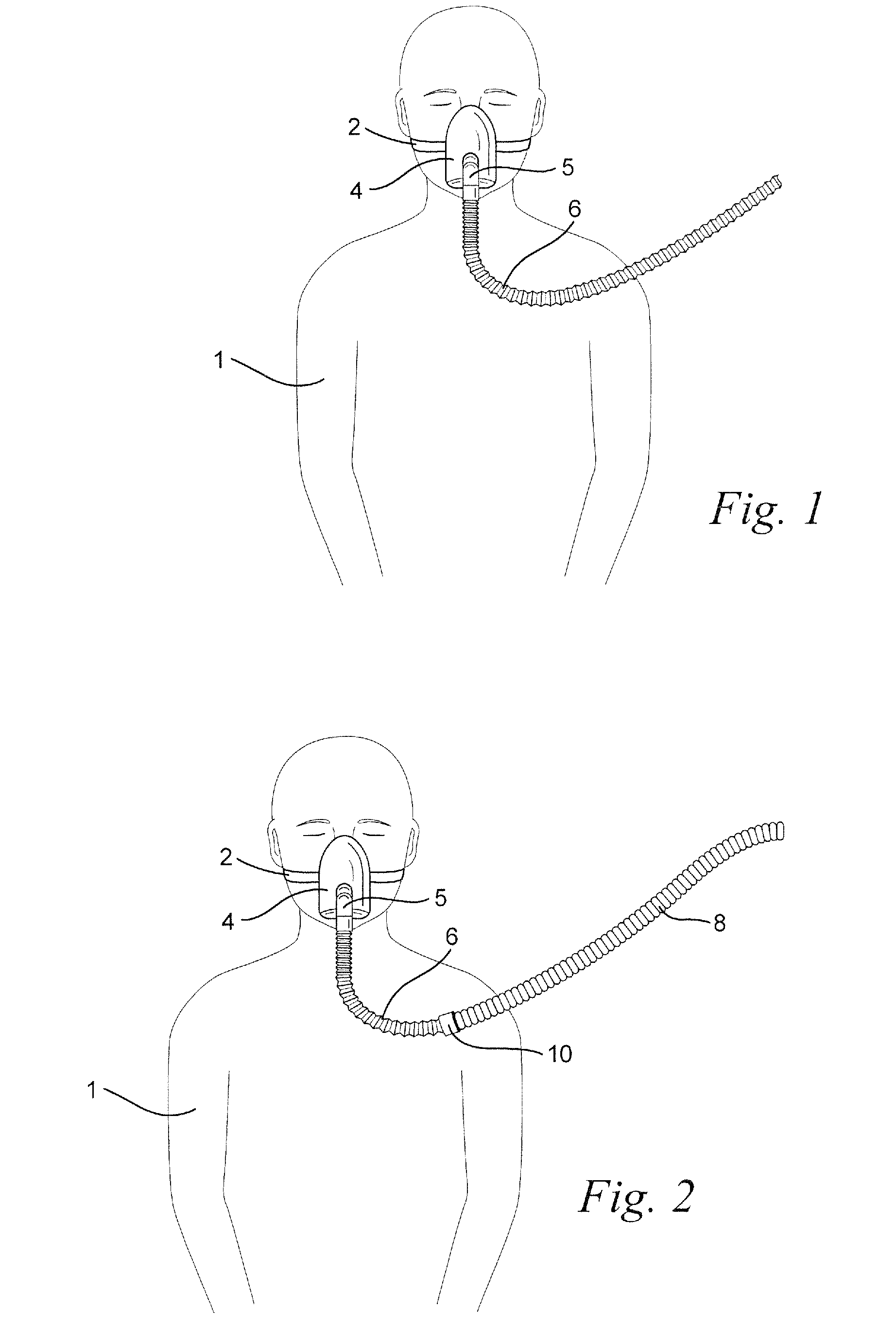
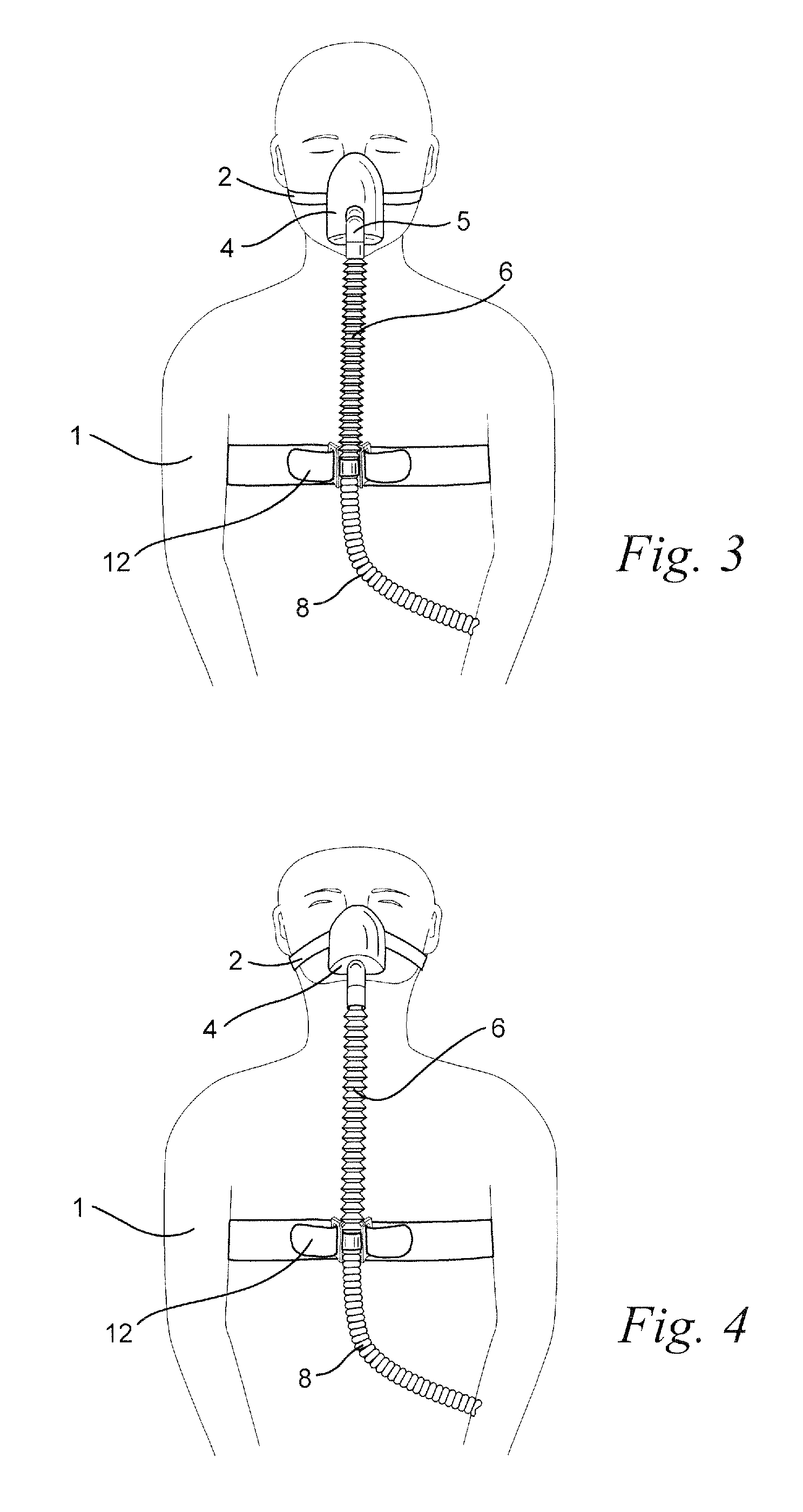
Nasal cannula assembly
A nasal cannula assembly designed for contact with the nasalabidial area of a patient's face comprising a nasal cannula, a pair of oxygen supply tubes connected to opposite ends of the nasal cannula and a main oxygen supply line. The nasal cannula is made of a flexible plastic material molded into a light-weight hollow tubular member having a main body portion formed at an acute angle in the center and having a pair of spaced exterior orifices projecting from the body at an angle and curved upwardly and inwardly for directing gas flow into a patient's nostrils. Attachment points for oxygen supply tubes are above center of gravity of the nasal cannula to make it self-righting thus eliminating need for stiff supply tubing to orient cannula. Oxygen supply tubes made from ultra-high molecular weight PVC possess superior flexibility and low compression set so that little tension on the tubing is required to hold cannula in proper position. A main oxygen supply line made from ultra-high molecular weight PVC resists the formation of twisted loops that tend to block oxygen flow.
Owner:THOMPSON PAUL S
Retractable tube for cpap
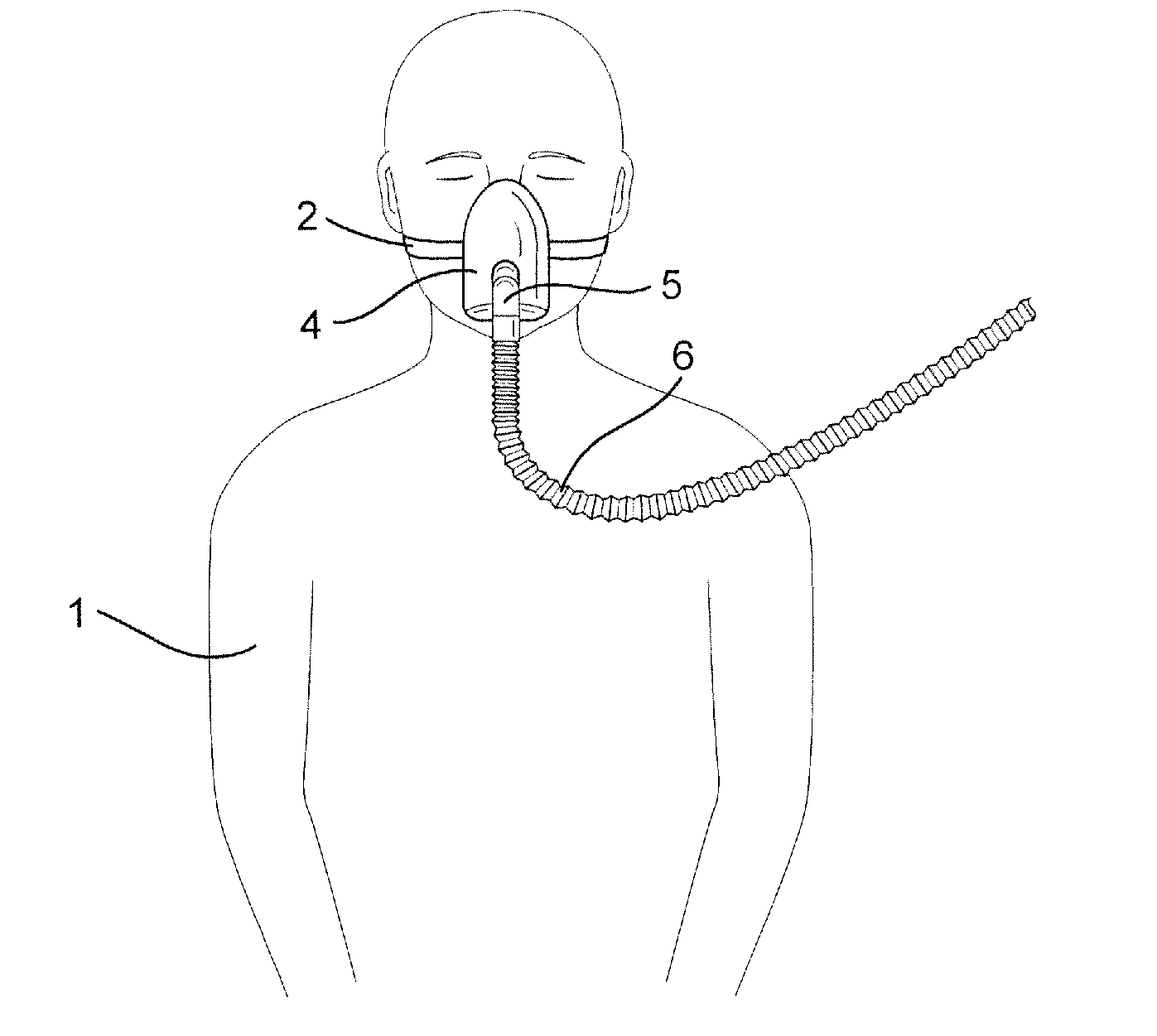
ActiveUS20090078259A1Reduces, or eliminates, the application of tube drag forces on the patient interfaceReduce tanglingRespiratorsPipe supportsEngineering
A retractable tube for use in a respiratory apparatus for delivering a pressurized flow of breathable gas to a patient has an internal diameter of about 30 mm or less, a weight of about 500 g / m or less, and an unextended length of about 2 m or less. The retractable tube includes a portion that is extensible in a range of about 40%-400% in response to force applied to the tube, and the extensible portion is configured to return the tube to its unextended length in the absence of force applied to the tube. A respiratory apparatus for delivering a flow of pressurized breathable gas includes a flow generator to generate the flow and a patient interface to deliver the flow to the patient's airways. The flow generator and the patient interface are connected by a retractable tube. A method of delivering a flow of pressurized breathable gas includes connecting the flow generator and the patient interface using a retractable tube.
Owner:RESMED LTD
Heparin barrier coating for controlled drug release
ActiveUS20050004663A1Reduce frictional forceReduce forceSuture equipmentsStentsCompound (substance)Antioxidant
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations as well as other therapeutic agents may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:WYETH
Jacketed bullet
ActiveUS9389052B2Reduced energy storageVelocity increasesAmmunition projectilesBarrelsEngineeringMechanical engineering
A jacketed bullet having a penetrator constructed of a hard material in line with a slug having a lower modulus. At least a portion of both the slug and the penetrator are then encased by a metal jacket. A plurality of circumferentially spaced and axially extending flutes are formed along the slug and possibly the penetrator. These flutes receive deformation of the jacket upon firing of the bullet into a rifled gun bore to thereby reduce friction between the bullet and the gun bore during operation.
Owner:ARMY US SEC THE
Shielded septum trocar seal
InactiveUS20050131349A1Reduce exerciseReduce forceInfusion syringesSurgical needlesPlastic materialsEngineering
The invention is directed to a trocar assembly having a channel defined along an elongate axis, the trocar assembly being adapted to receive a surgical instrument, the trocar assembly comprising a septum seal disposed in the channel including a seal tip having a proximal facing surface, the seal tip including portions defining an orifice; and a septum shield including a tubular member having a proximal end and a distal end, and a plurality of blades or leaflets protruding distally from the distal end of the tubular member, the septum shield being placed inside the septum seal such that the blades engage the proximal facing surface of the seal tip. The trocar assembly may further comprise a zero closure seal such as a double duckbill valve disposed in the channel outside of the septum seal. The septum shield operates to reduce the drag force and to minimize axial movement of the septum shield and the instrument during insertion and removal of the instrument through the septum seal. The septum shield may be formed from a rigid plastic material, and the septum seal may be formed of an elastomeric material. The blades or leaflets may overlap or offset one another. The orifice may be expandable to accommodate the instrument having a diameter of about 5-15 mm. The blades or leaflets have distal tips that glide or roll against the instrument during placement of the instrument. The distal tips may comprise of a combination of material, durometer and shield geometry to control the behavior of the septum shield. The trocar assembly may further comprise a second septum shield disposed outside of the septum seal.
Owner:APPL MEDICAL RESOURCES CORP
Smooth muscle controller
InactiveUS6947792B2Promote healingIncrease in motilityInternal electrodesHeart stimulatorsSmooth muscleMedicine
A method of promoting the healing of a lesion in a smooth muscle, comprising selecting a smooth muscle portion having a lesion and applying a non-excitatory electrical field to the portion, which field reduces the mechanical activity of the portion.
Owner:METACURE
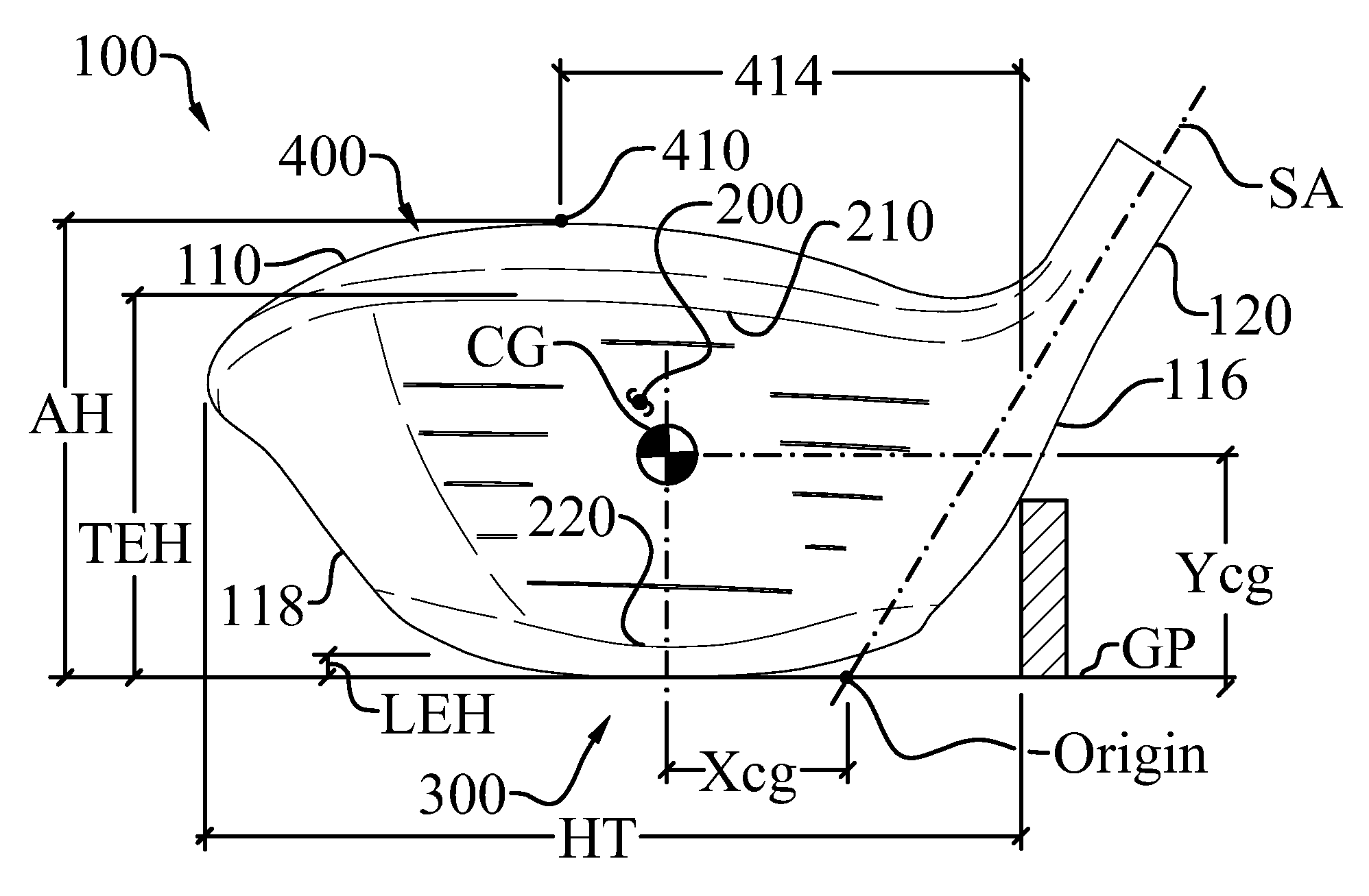
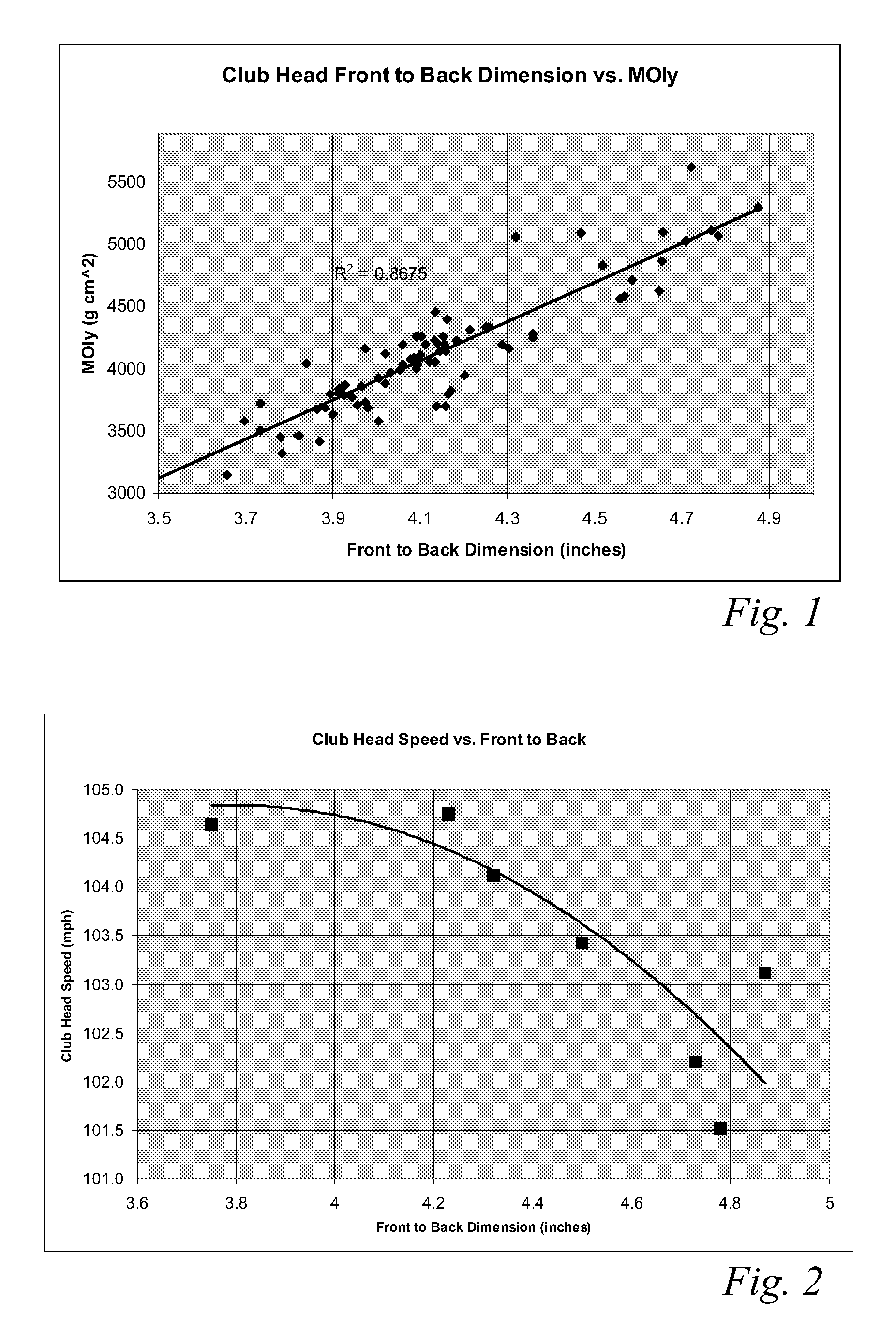
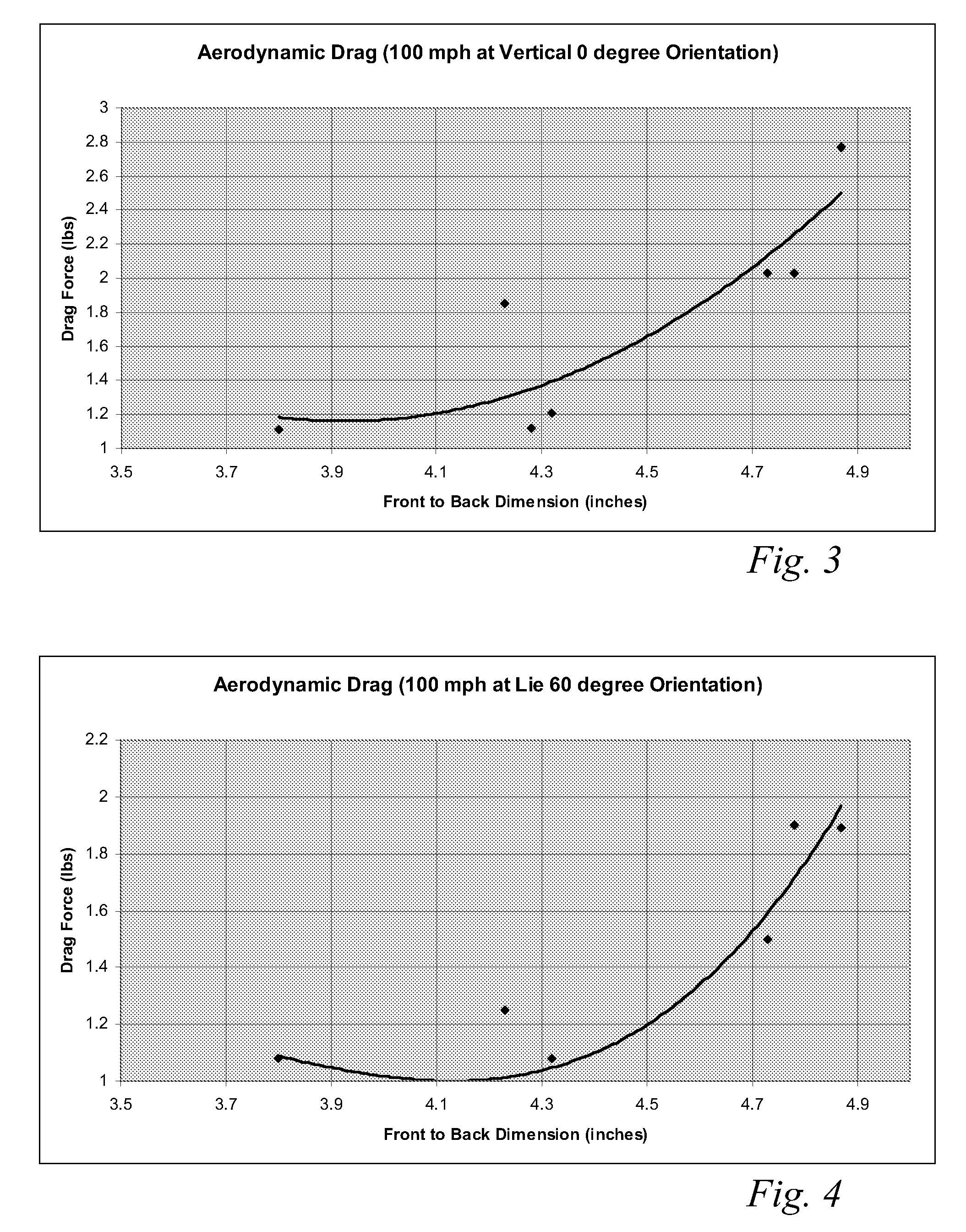
High volume aerodynamic golf club head
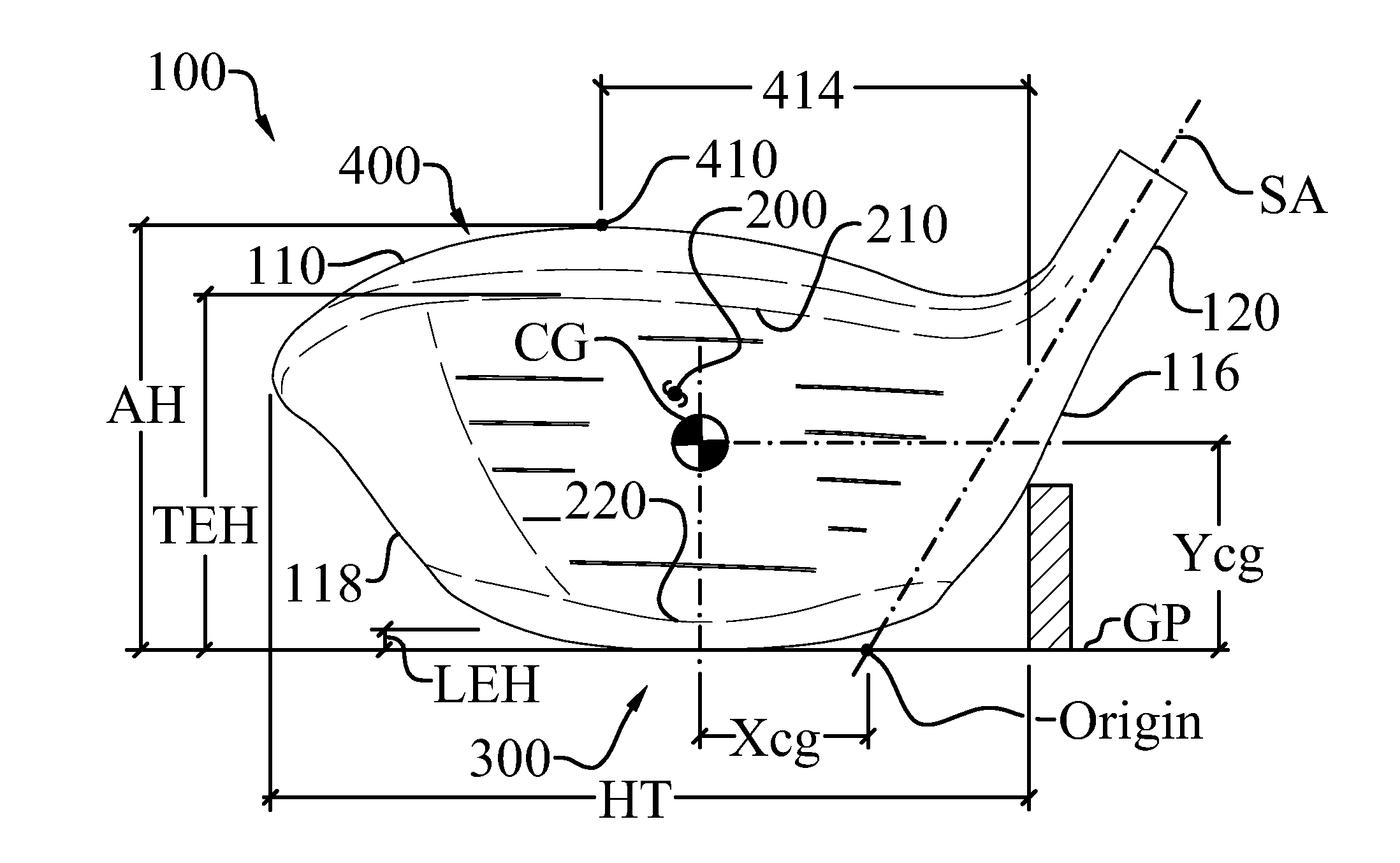
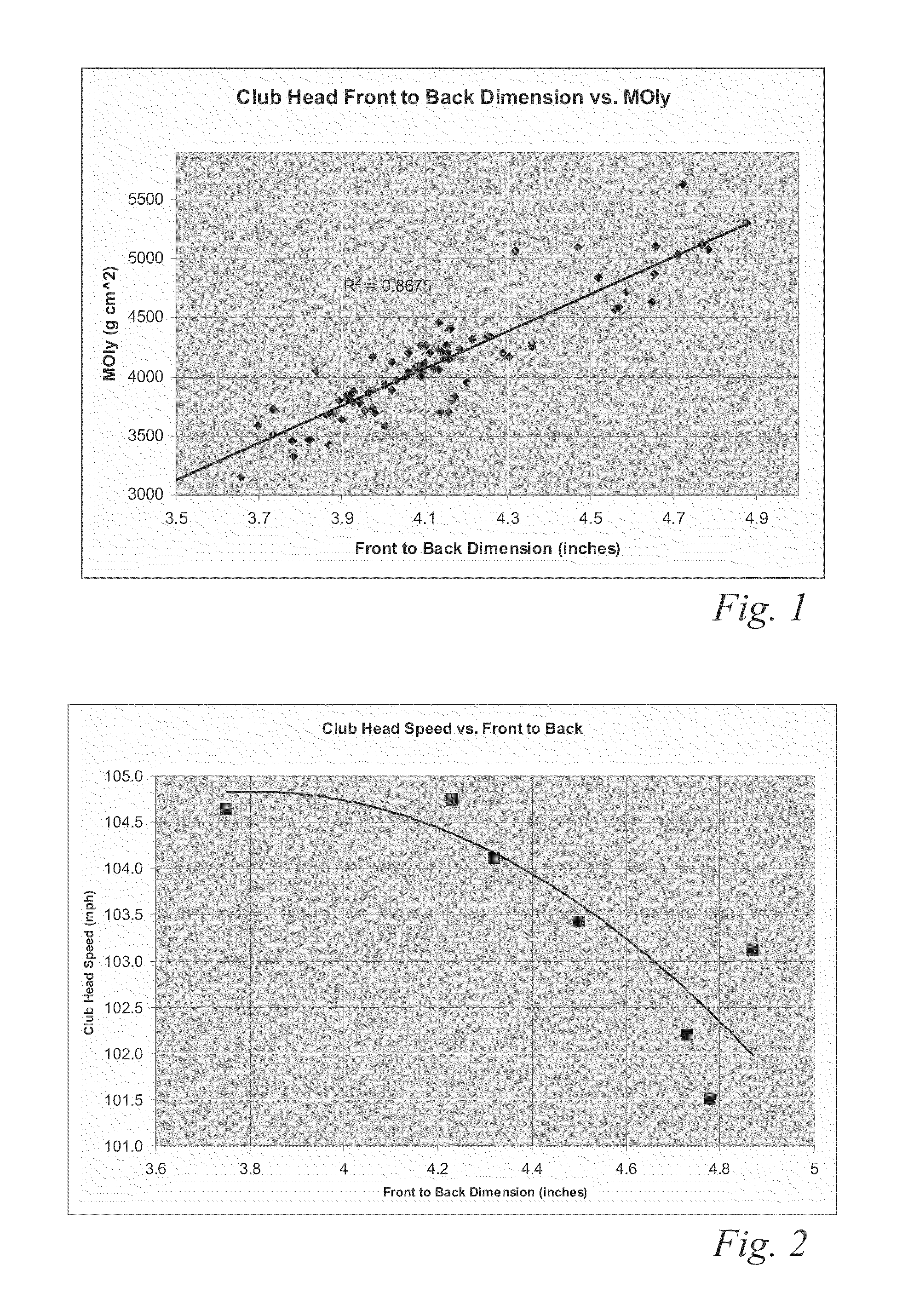
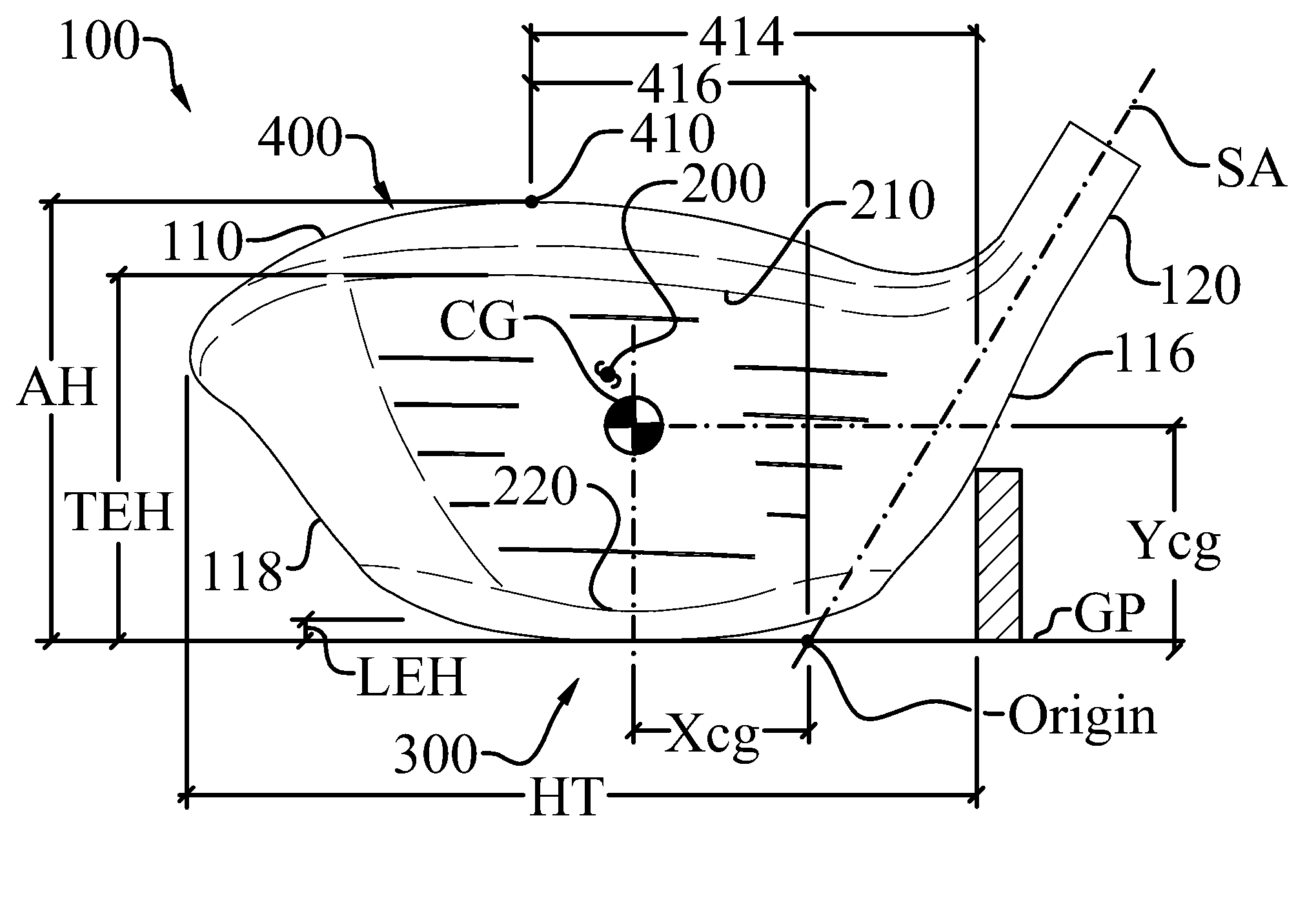
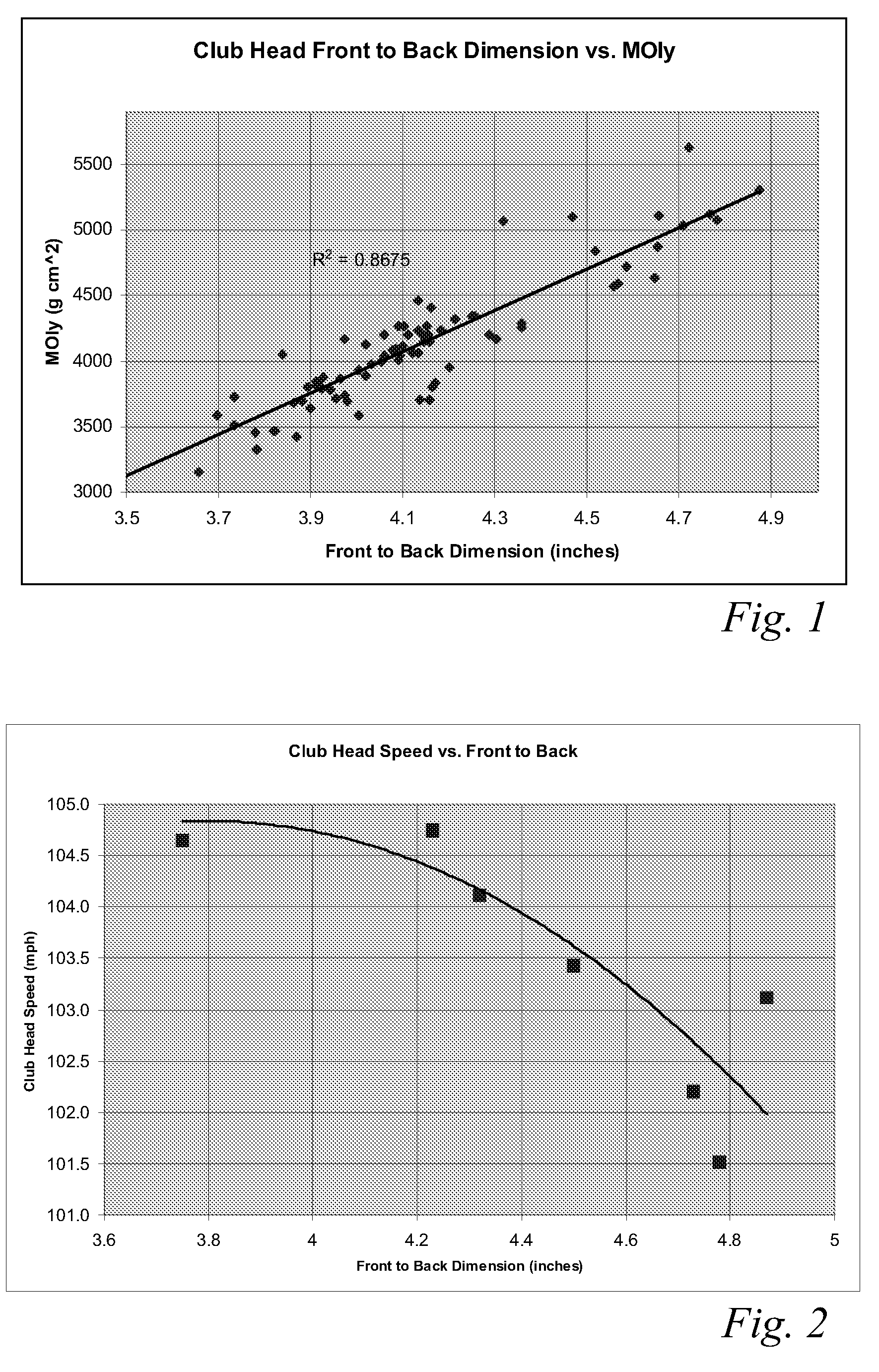
ActiveUS8083609B2Improve aerodynamic performanceIncrease volumeGolf clubsRacket sportsAerodynamic dragGround plane
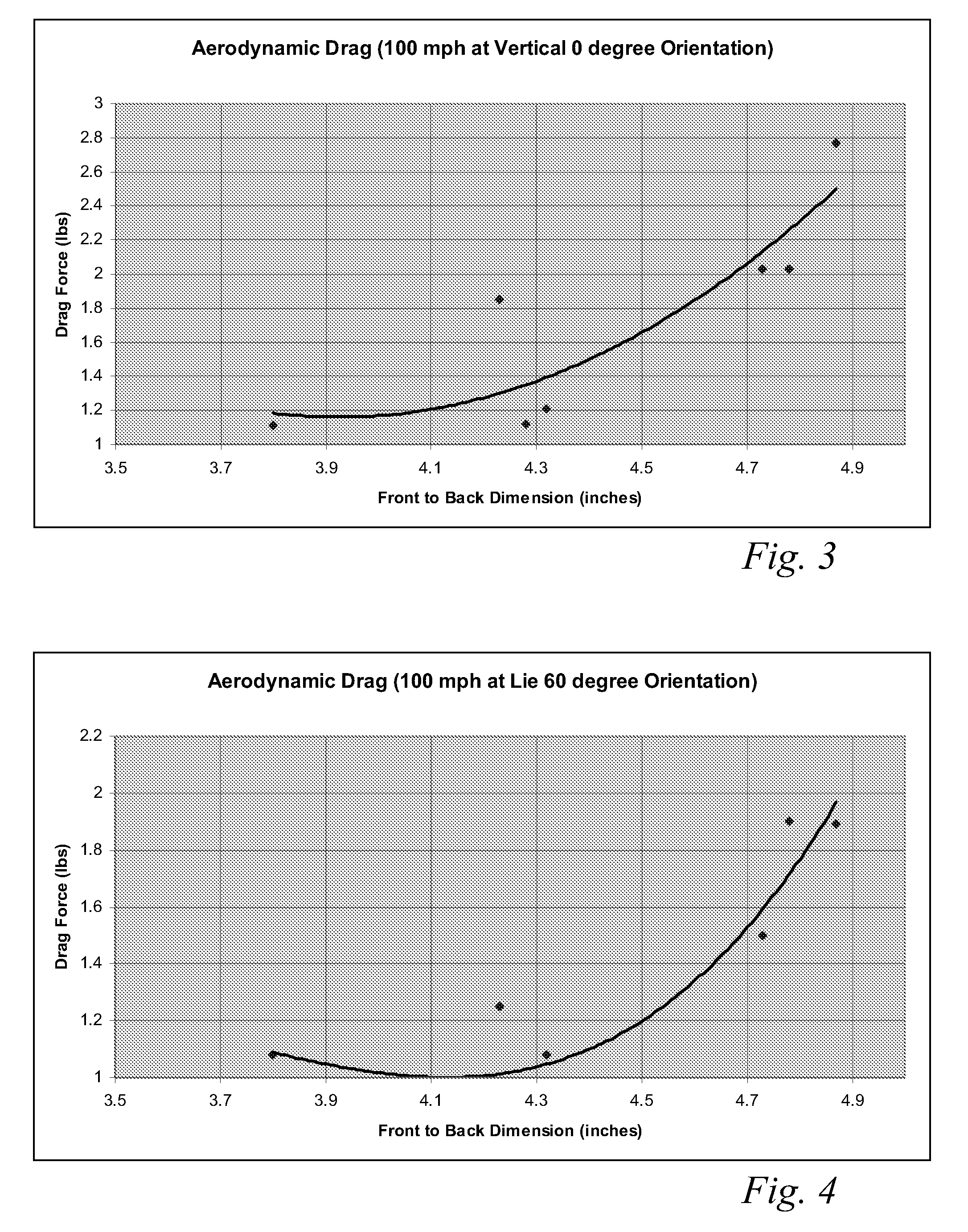
A high volume aerodynamic golf club head with a club head volume of at least 400 cc and a front-to-back dimension of at least 4.4 inches producing a face-on normalized aerodynamic drag force of less than 1.5 lbf when exposed to a 100 mph wind parallel to the ground plane and oriented at the front of the club head. The club head has a crown section having a crown apex located an apex height above a ground plane, wherein a portion of the crown section between the crown apex and the face has an apex-to-front radius of curvature that is less than 3 inches. An apex ratio of the apex height to a maximum face top edge height is at least 1.13. The apex height and location of the crown apex obtain desirable airflow reattachment close to the face and improve airflow attachment to the crown section.
Owner:TAYLOR MADE GOLF
Shingle assembly
InactiveUS7178295B2Reduce wind uplift forceReduce the temperaturePhotovoltaic supportsSolar heating energyEngineeringMechanical engineering
A barrier, such as a PV module, is secured to a base by a support to create a shingle assembly with a venting region defined between the barrier and base for temperature regulation. The first edge of one base may be interengageable with the second edge of an adjacent base to be capable of resisting first and second disengaging forces oriented perpendicular to the edges and along planes oriented parallel to and perpendicular to the base. A deflector may be used to help reduce wind uplift forces.
Owner:SUNPOWER CORPORATION
Resilient floor
Owner:VÄLINGE INNOVATION AB
Golf club head having trip step feature
InactiveUS20100016095A1Reduced aerodynamic drag forceLess aerodynamic dragGolf clubsRacket sportsHybrid typeAerodynamic drag
An aerodynamic golf club head incorporating a trip step feature located on the crown section. The benefits associated with the reduction in aerodynamic drag force associated with the trip step may be applied to drivers, fairway woods, and hybrid type golf club heads. The trip step is located between a crown apex and the back of the club head and may be continuous or discontinuous. The trip step enables a significant reduction in the aerodynamic drag force exerted on the golf club head by forcing the air passing over the club head from laminar flow to turbulent flow just before the natural separation point of the airstream from the crown. This selectively engineered transition from laminar to turbulent flow over the crown section slightly increases the skin friction but results in less aerodynamic drag than if the air were to detach from the crown section at the natural separation point.
Owner:TAYLOR MADE GOLF
Balloon catheter system for treating vascular occlusions
InactiveUS20070088380A1Increase the lengthShorten the lengthStentsBalloon catheterBalloon catheterBlood vessel
The present invention provides a balloon catheter comprising: a hollow inner shaft disposed within a hollow outer shaft; a balloon attached at its proximal end to said outer shaft and at its distal end to said inner shaft; wherein the inner shaft is constructed such that following radial expansion of the balloon to a first expanded state, said inner shaft is capable of responding to further longitudinal expansion of the balloon to a second expanded state by increasing its length from a resting value, and of responding to subsequent partial deflation back to said first expanded state by reducing its length back to said resting value.
Owner:ENDOCROSS
Electrolytically detachable implantable devices
Owner:STRYKER CORP +1
Void-containing polyester shrink film
InactiveUS20050119359A1Improve performanceHigh opacitySynthetic resin layered productsLabelsPolyesterVolumetric Mass Density
Disclosed are polyester shrink films comprising a voiding agent dispersed within a continuous polyester phase. The voiding agent comprises at least one first polymer and at least one second polymer, in which the polymer components have selected physical properties such as glass transition temperature, melting point, tensile modulus, surface tension, and melt viscosity. The resulting shrink films have high opacity, a low coefficient of friction, lower density, low shrink force, and good printability. The films are useful for sleeve label and other shrink film applications, and their lower density allows them to be readily separated from soft drink bottles, food containers and the like during recycling operations. Also disclosed is a process for separating a void-containing polyester from a mixture of polymers.
Owner:SHELBY MARCUS DAVID +2
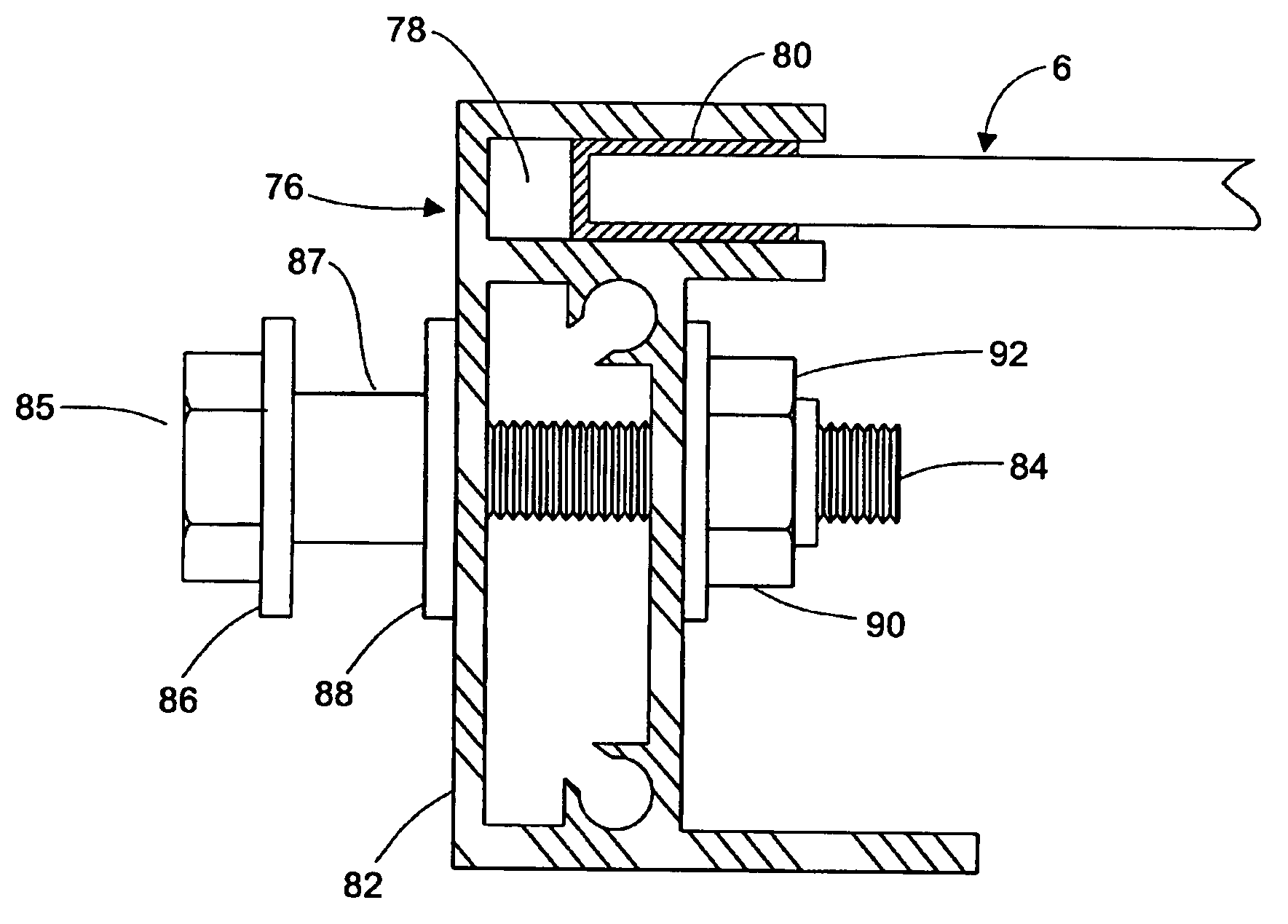
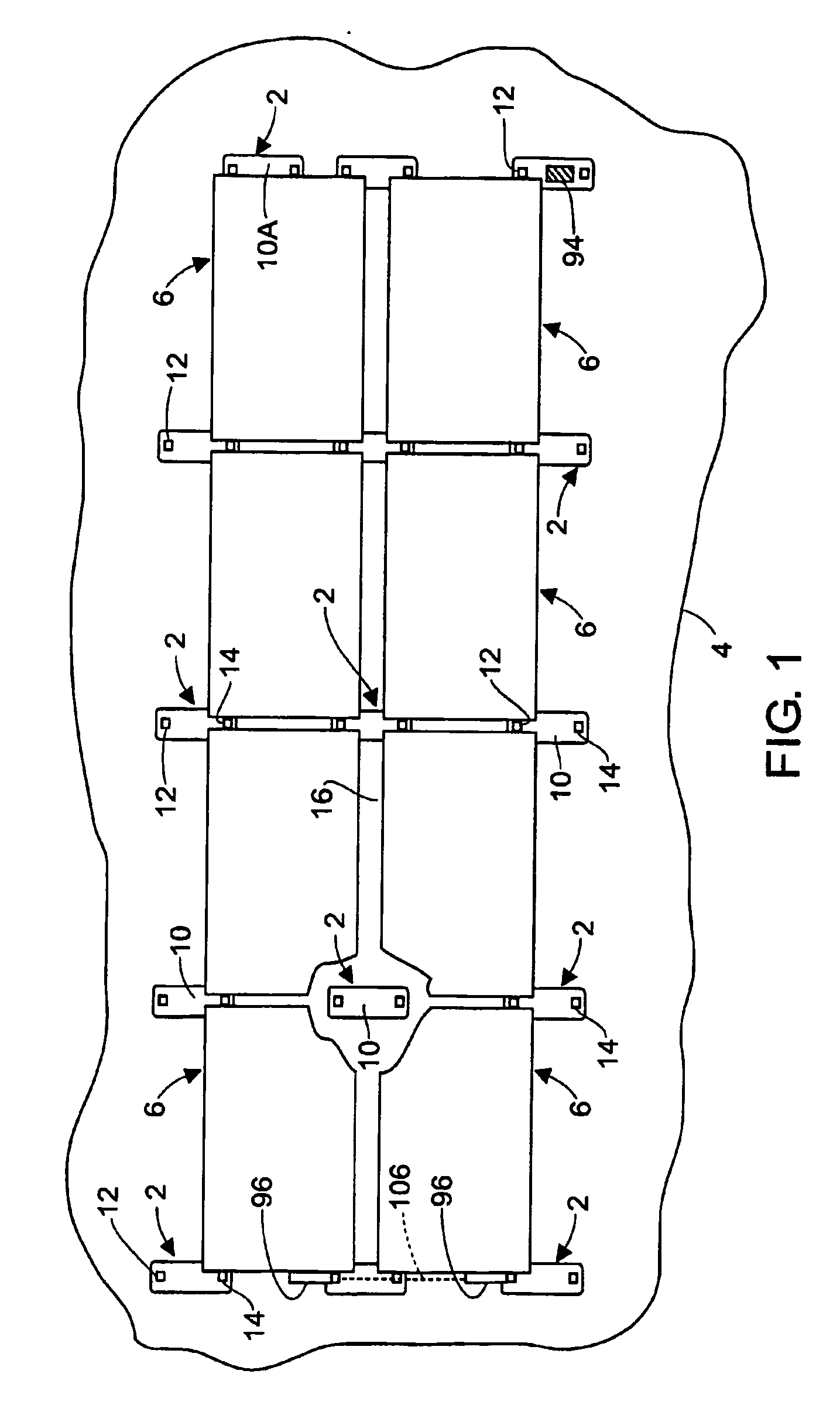
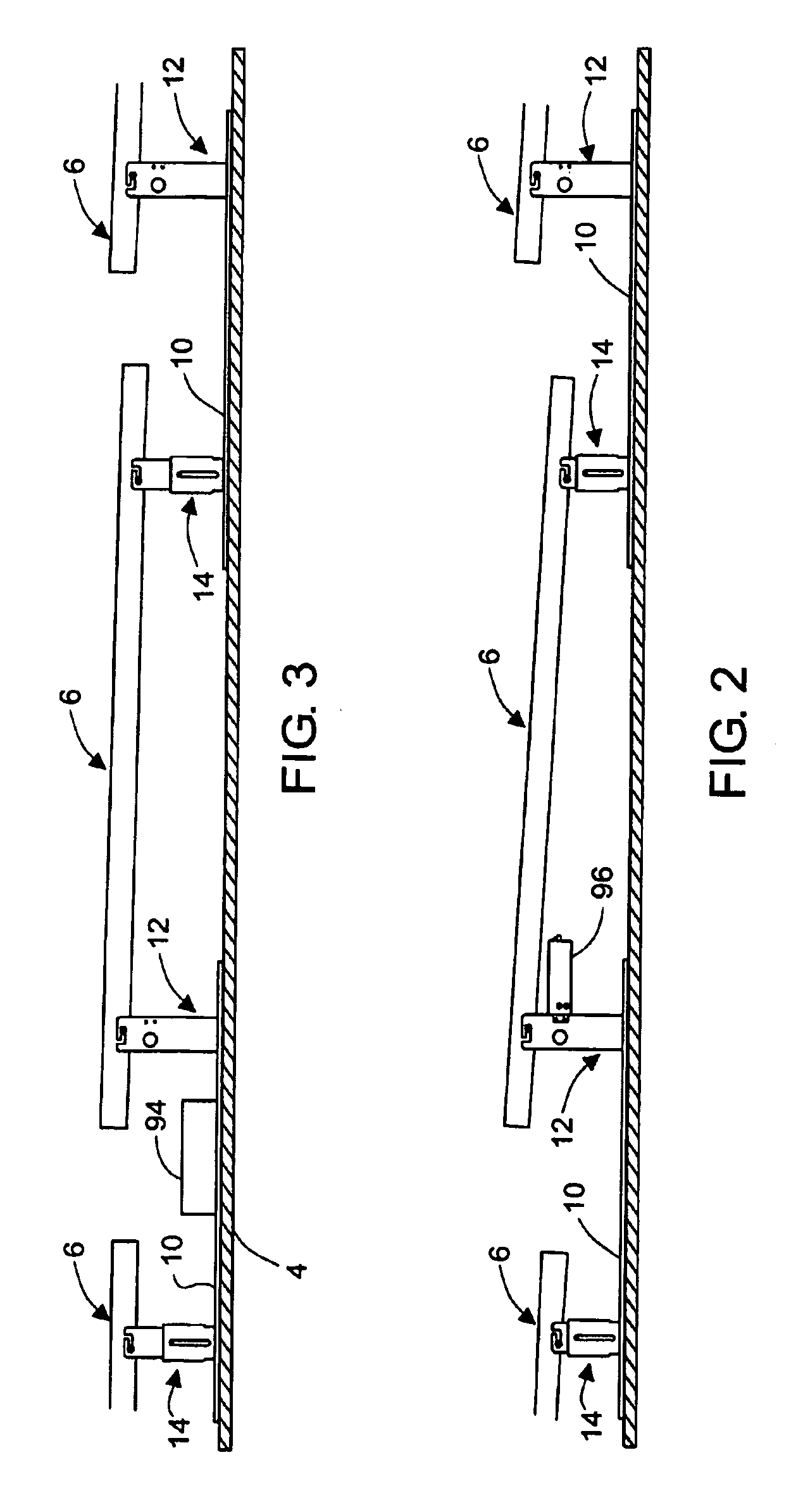
Apparatus and method for mounting photovoltaic power generating systems on buildings
InactiveUS20050115176A1Preserve integrityPreserve waterproof characteristicPhotovoltaic supportsSolar heating energyEffective heightEngineering
Rectangular PV modules (6) are mounted on a building roof (4) by mounting stands that are distributed in rows and columns. Each stand comprises a base plate (10) that rests on the building roof (4) and first and second brackets (12, 14) of different height attached to opposite ends of the base plate (10). Each bracket (12, 14) has dual members for supporting two different PV modules (6), and each PV module (6) has a mounting pin (84) adjacent to each of its four corners. Each module (6) is supported by attachment of two of its mounting pins (84) to different first brackets (12), whereby the modules (6) and their supporting stands are able to resist uplift forces resulting from high velocity winds without the base plates (10) being physically attached to the supporting roof structure (4). Preferably the second brackets (14) have a telescoping construction that permits their effective height to vary from less than to substantially the same as that of the first brackets (12).
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Wear resistant vapor deposited coating, method of coating deposition and applications therefor
ActiveUS20070284255A1Stable cutting edgeReduce torsion fatiguePigmenting treatmentSurgeryWear resistantShape-memory alloy
A low friction top coat over a multilayer metal / ceramic bondcoat provides a conductive substrate, such as a rotary tool, with wear resistance and corrosion resistance. The top coat further provides low friction and anti-stickiness as well as high compressive stress. The high compressive stress provided by the top coat protects against degradation of the tool due to abrasion and torsional and cyclic fatigue. Substrate temperature is strictly controlled during the coating process to preserve the bulk properties of the substrate and the coating. The described coating process is particularly useful when applied to shape memory alloys.
Owner:G&H TECH LLC
High volume aerodynamic golf club head having a post apex attachment promoting region
ActiveUS8088021B2Improve aerodynamic performanceIncrease valueGolf clubsRacket sportsAerodynamic dragGround plane
A high volume aerodynamic golf club head having a post apex attachment promoting region with a club head volume of at least 400 cc and a front-to-back dimension of at least 4.4 inches producing a face-on normalized aerodynamic drag force of less than 1.5 lbf when exposed to a 100 mph wind parallel to the ground plane and oriented at the front of the club head. The post apex attachment promoting region is on the surface of the crown section at an elevation above a maximum face height and begins at the crown apex and extends toward the back of the club head. The post apex attachment promoting region is a relatively flat portion of the crown section that is behind the crown apex, yet above the maximum top edge plane, and aides in keeping airflow attached to the club head once it flows past the crown apex.
Owner:TAYLOR MADE GOLF
Rigid articulated Pointe shoe
InactiveUS7036244B1Reduce downward forceRelieves high load of forceUpperBootlegsTransverse axisEngineering
The invention is a Pointe shoe for ballet. It has a rigid mid-foot section and a rigid toe loop connected by a transverse axis joint located at the metatarsal-phalange joint (M-P). With the foot in Pointe position, the weight of the dancer is supported by the rigid mid-foot section. The downward force is passed through the M-P joint to the front of the toe loop. None of the weight of the dancer needs to be supported by the toes. In contrast, prior art Pointe shoes have a rigid shank and toe cup to assist the toes in supporting the weight of the dancer. The toes have small bones, muscles, and ligaments. This often results in pain and injury to the toes. The shoe of the invention has a mid-foot section that is shaped with support surfaces for the sole of the heel bone and the dorsal side of the cuneiform and metatarsal bones. These bones are larger and stronger than the bones of the toes. This shoe provides a larger area of bone and tissue to support the weight of the dancer on Pointe. It is more comfortable to use and results in fewer injuries.
Owner:FINCH DENNIS
Device and method for delivering an endovascular stent-graft having a longitudinally unsupported portion
InactiveUS20060020319A1Reduce forceAvoid graft kinking and wrinklingStentsEar treatmentStent graftingInsertion stent
An endoluminal prosthesis having an unsupported or flexible region and a delivery system for delivering the endoluminal prosthesis is provided. The delivery system includes a prosthesis delivery catheter with stiffening elements that provide longitudinally rigid support to a flexible or unsupported portion of an endoluminal prosthesis as the prosthesis is being deployed. A prosthesis is removably coupled to the stiffening elements. The endoluminal prosthesis can be a stent or a stent graft or graft.
Owner:MEDTRONIC VASCULAR INC
Catheter with electrode strip
InactiveUS20050033136A1Reduce forceElectrocardiographyTransvascular endocardial electrodesElectrical conductorDistal portion
Apparatus for medical treatment or diagnosis in a body cavity of a mammalian subject includes an elongate probe, having an outer surface and comprising a distal portion, which is adapted for insertion into the body cavity. An electrode strip includes an elongate insulating substrate, which is wrapped around the distal portion of the probe so as to define a helix having distal and proximal ends and a length therebetween, the substrate being fixed to the outer surface of the probe over substantially all of the length of the helix. A plurality of electrodes are disposed along the length of the helix and fixed to the substrate. Electrical conductors are coupled to the electrodes and run along the substrate over the length of the helix so as to communicate with circuitry in a location proximal to the distal portion of the probe.
Owner:BIOSENSE WEBSTER INC
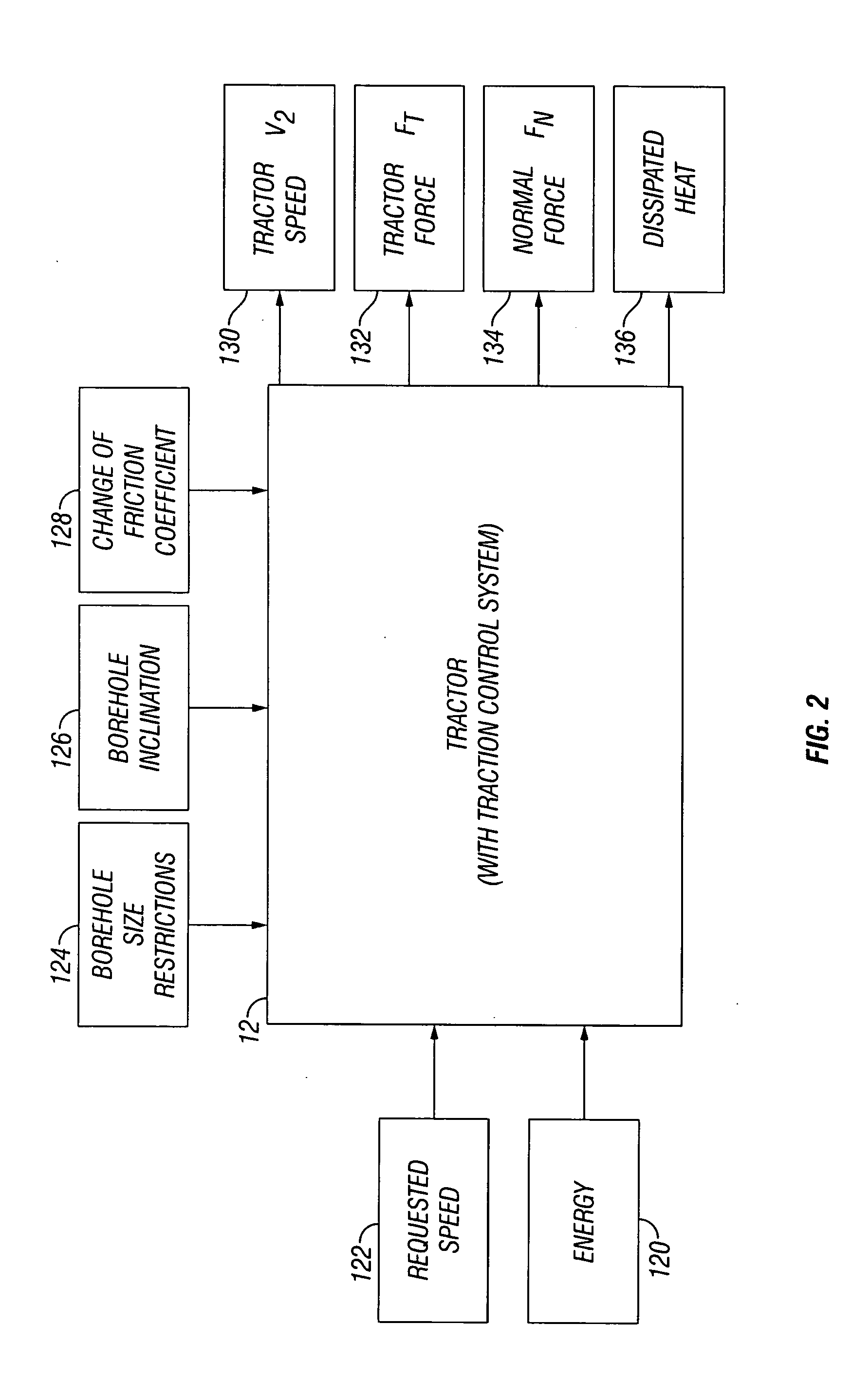
Traction control for downhole tractor
Apparatus, system and methods useful for controlling the traction of a downhole tractor in a borehole include the capability of repeatedly adjusting the normal force applied to at least one component that causes movement of the tractor in the borehole.
Owner:SCHLUMBERGER TECH CORP
Dispenser and cosmetic or dermatological preparation comprising an auxiliary for use with dispenser
InactiveUS20050189377A1Improves haptic propertyReduce forceCosmetic preparationsPower operated devicesPolyolSURFACTANT BLEND
The present invention is a cosmetic or dermatological preparation for use with a dispenser that includes an auxiliary to keep the dispenser operating smoothly. The auxiliary is selected from the group consisting of (i) polyols having 2 to 6 carbon atoms and 2 to 6 hydroxyl or alkoxy groups and (ii) surfactants that reduce the surface tension of the preparation to less than 30 mN / m. The preparation is particularly suitable with a dispenser comprising a container and an inner container wall for housing a cosmetic or dermatological preparation; a follow-up plunger on a base side of the dispenser, which is capable of being slidably displaced on the inner container wall under the pressure of the ambient atmosphere; a head section on a top end of the dispenser that can be slidably displaced in relation to the container and that comprises a dispensing channel, the dispensing channel capable of being connected in a communicating manner to the container; a manually actuable delivery device comprising a variable-volume delivery chamber, a delivery element that can be displaced longitudinally in relation to the container and the head section, comprising a delivery plunger that can be slidably displaced within the delivery chamber and a delivery stem connected to the delivery plunger, and a delivery channel circumferentially enclosed by the delivery stem and comprising a delivery-channel inlet opening communicating with the delivery chamber and a delivery-channel outlet opening. The delivery channel outlet opening is capable of being moved into an open position relative to the dispensing channel by displacing the delivery element.
Owner:BEIERSDORF AG
Floorboards, flooring systems and method for manufacturing and installation thereof
InactiveUS20080005998A1Easy to handleSmall floorboardWallsOrnamental structuresSurface layerEngineering
Floorboards with a format corresponding to a traditional parquet block for laying of mechanically joined floating flooring. Rectangular floorboards include a surface layer and a core with two long sides and two short sides, for making a floating flooring, which floorboards are mechanically lockable and which along their four sides have pairs of opposing connectors for locking similar, adjoining floorboards to each other both vertically and horizontally wherein the long sides have a length not exceeding 80 cm and the short sides have a width not exceeding 10 cm.
Owner:VÄLINGE INNOVATION AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com