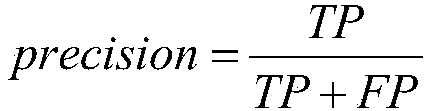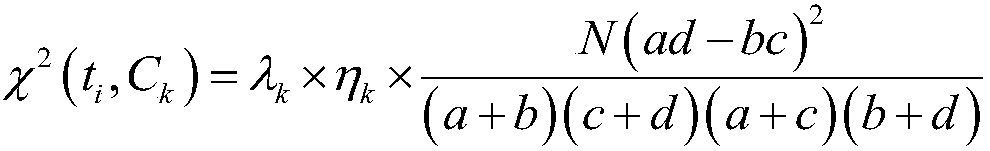Patents
Literature
44 results about "Adverse drug reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An adverse drug reaction (ADR) is an injury caused by taking medication. ADRs may occur following a single dose or prolonged administration of a drug or result from the combination of two or more drugs. The meaning of this expression differs from the meaning of "side effect", as this last expression might also imply that the effects can be beneficial. The study of ADRs is the concern of the field known as pharmacovigilance. An adverse drug event (ADE) refers to any injury occurring at the time a drug is used, whether or not it is identified as a cause of the injury. An ADR is a special type of ADE in which a causative relationship can be shown. ADRs are only one type of medication-related harm, as harm can also be caused by omitting to take indicated medications.
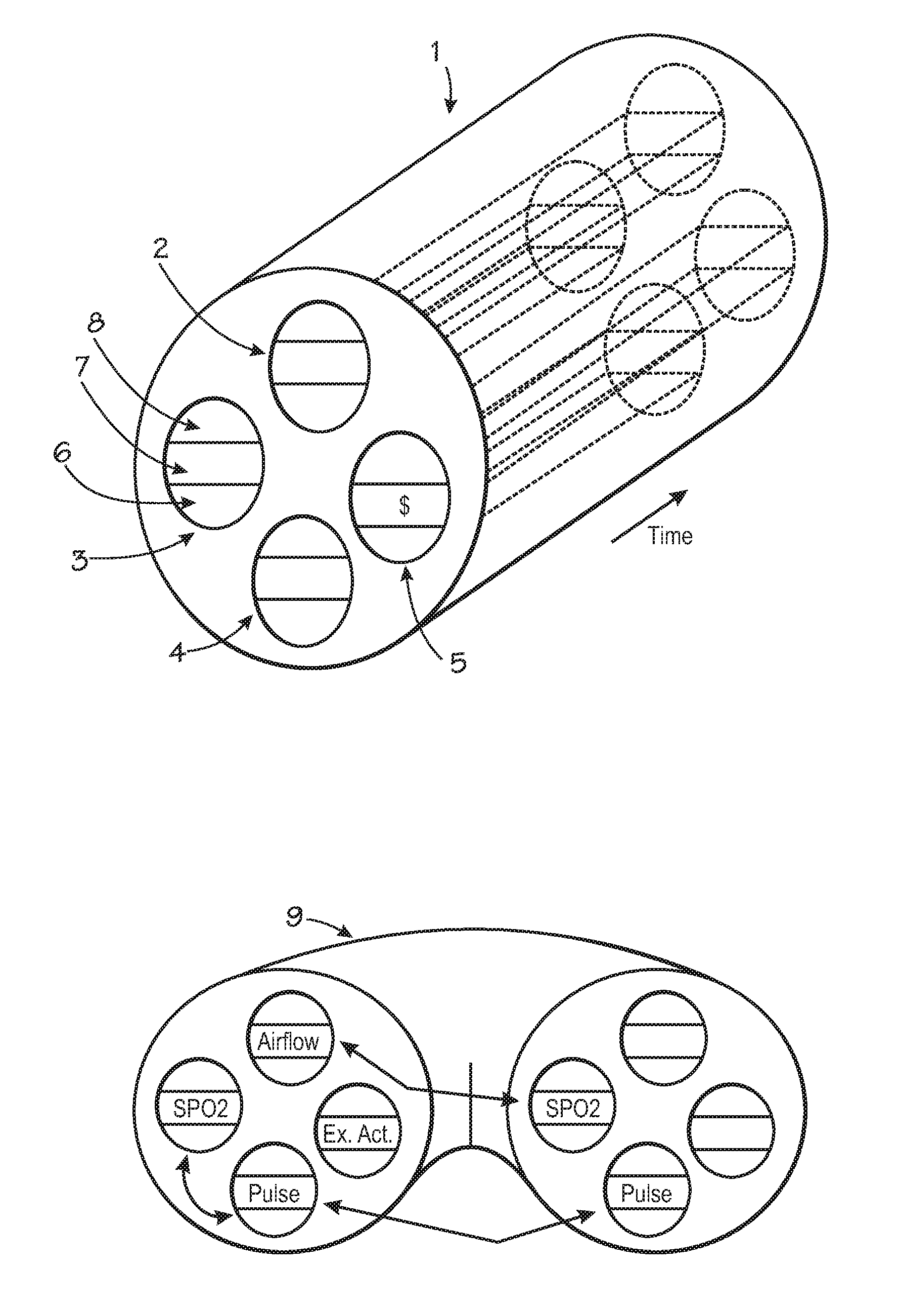
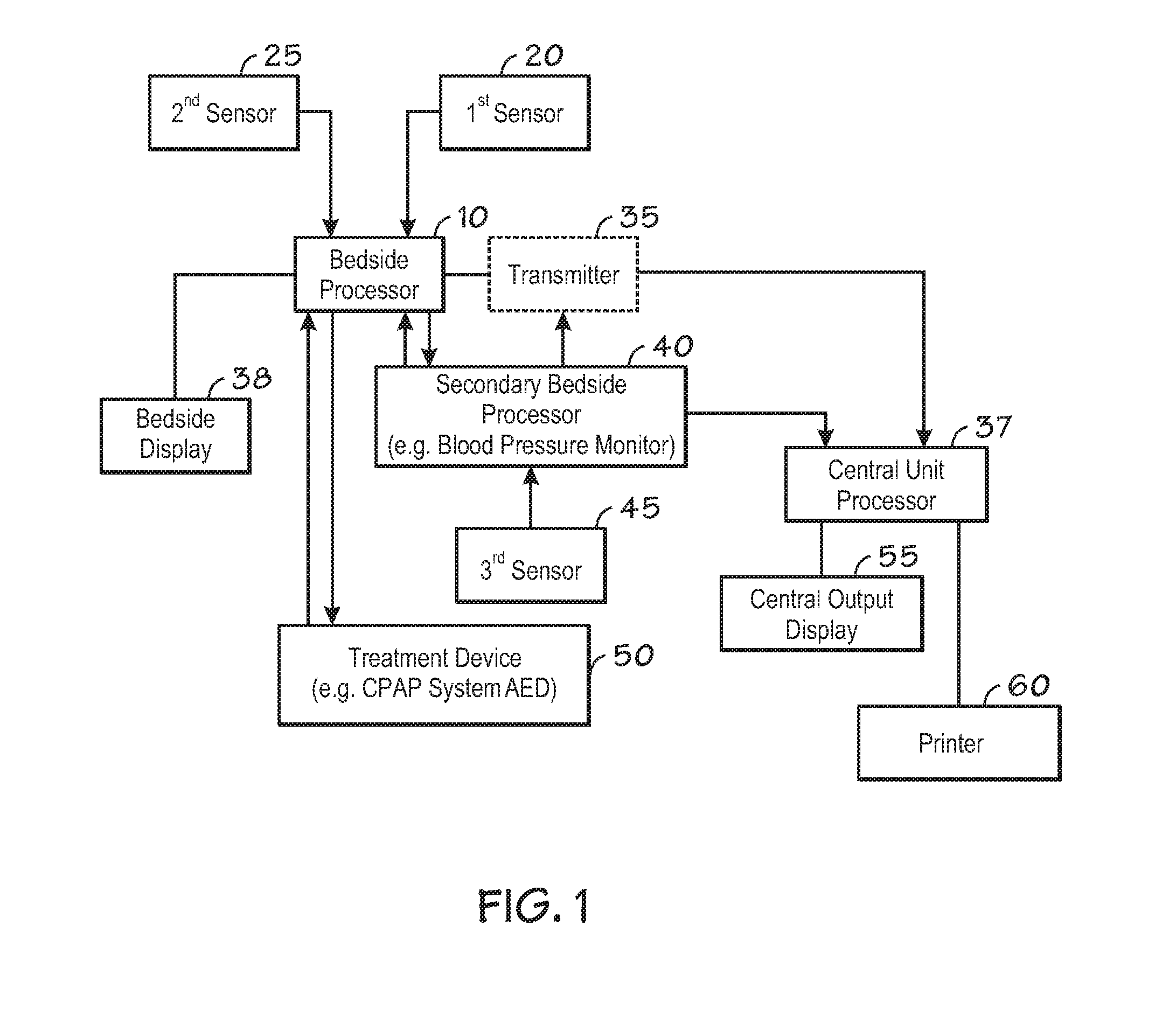
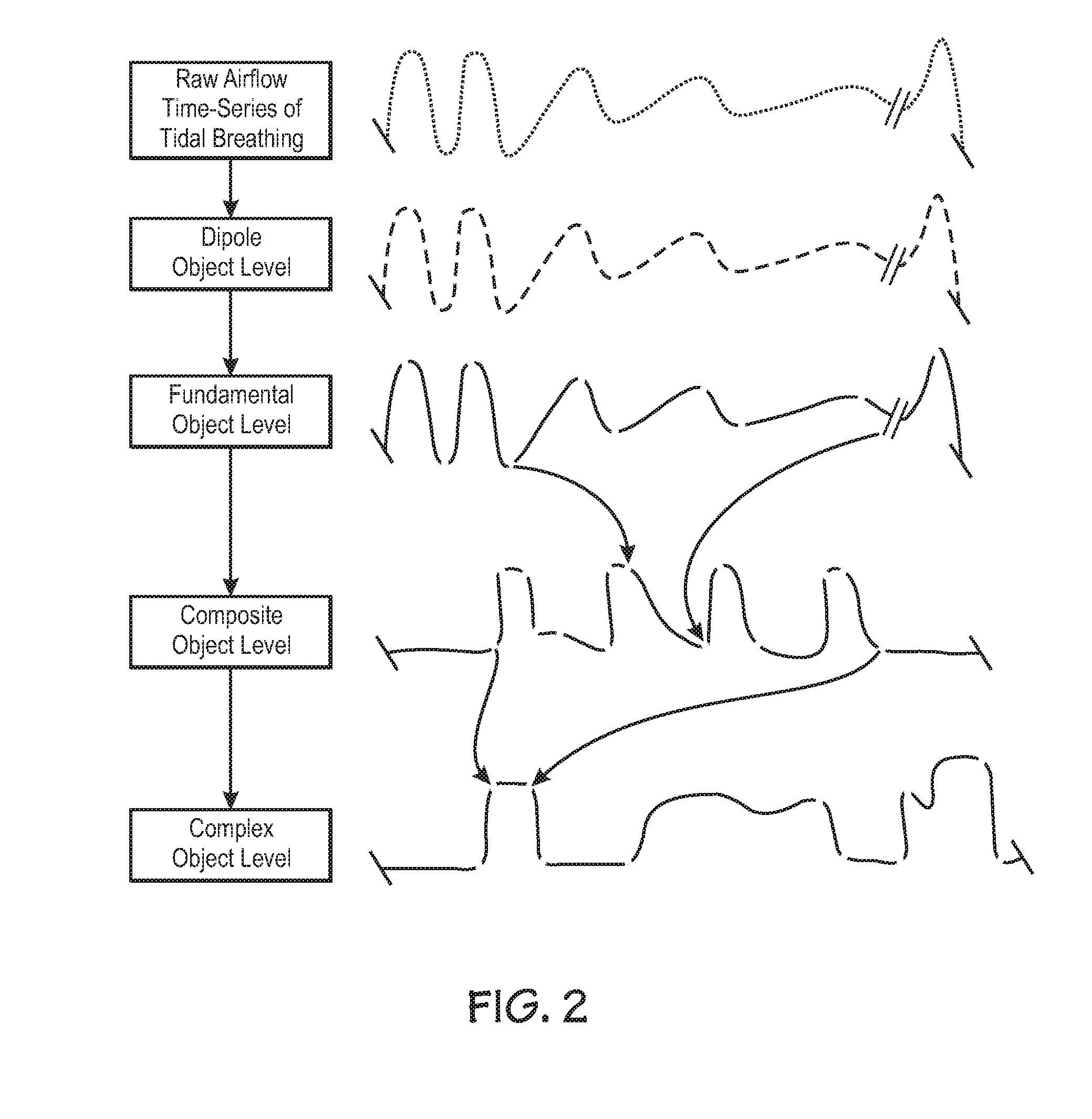
Centralized hospital monitoring system for automatically detecting upper airway instability and for preventing and aborting adverse drug reactions
InactiveUS20120330118A1Improve instabilityVigilance of hospital personnel may diminishDrug and medicationsSensorsAdverse drug reactionInstability
A system and method for the automatic diagnosis of obstructive sleep apnea in a centralized hospital critical care monitoring system for the monitoring of a plurality of patients in at least one of a critical care, step down, and cardiac ward by telemetry. The system includes a central processor having a display, and a plurality of telemetry units for mounting with patients, each of the telemetry units has a plurality of sensors for connection with each patient, the telemetry unit is capable of the transmission of multiple signals derived from the sensors to the central processor, in one preferred embodiment the method comprising steps of programming the system to analyze the signals and to automatically identify the presence and severity of obstructive sleep apnea and to provide an indication of the identification.
Owner:LYNN LAWRENCE A
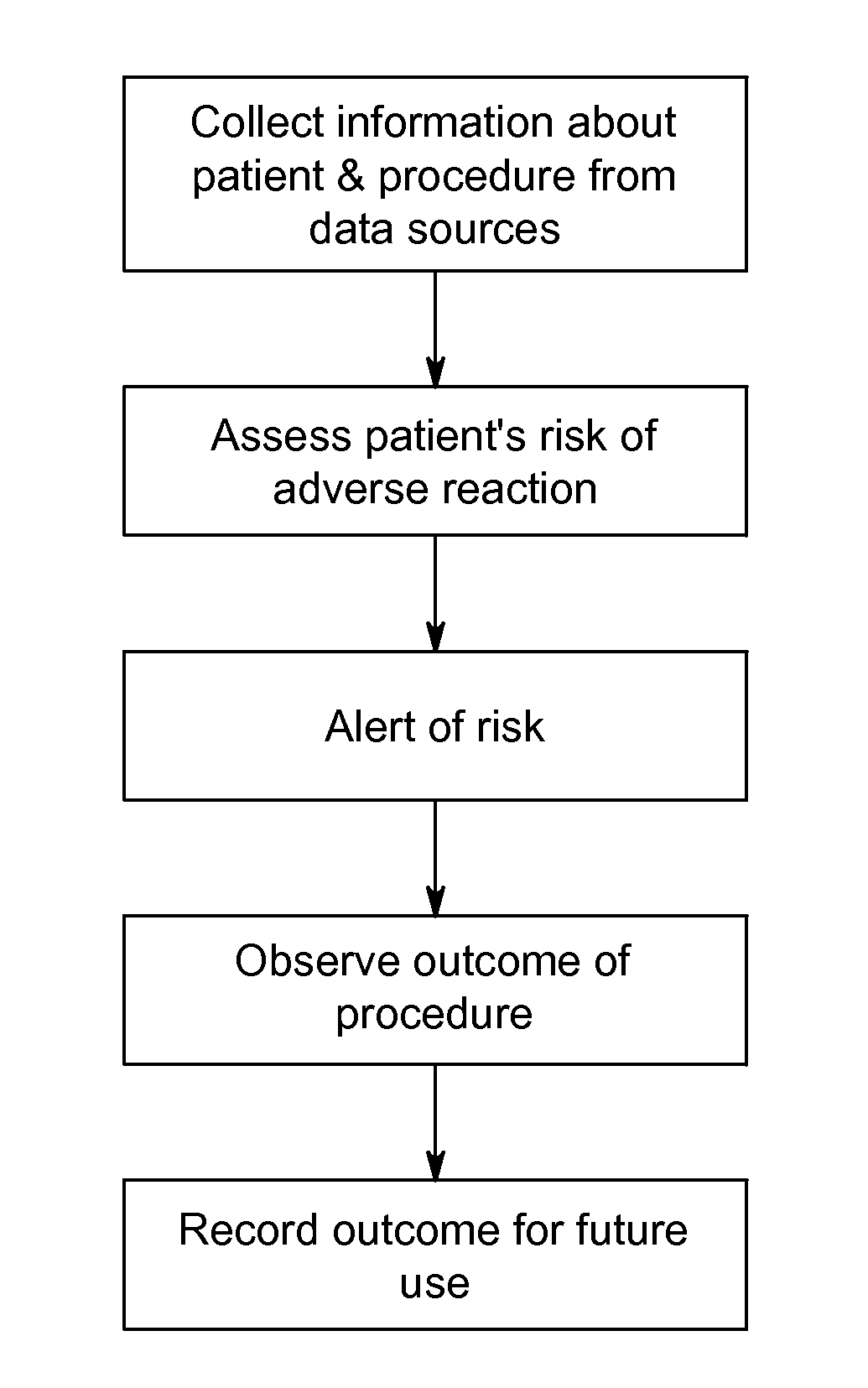
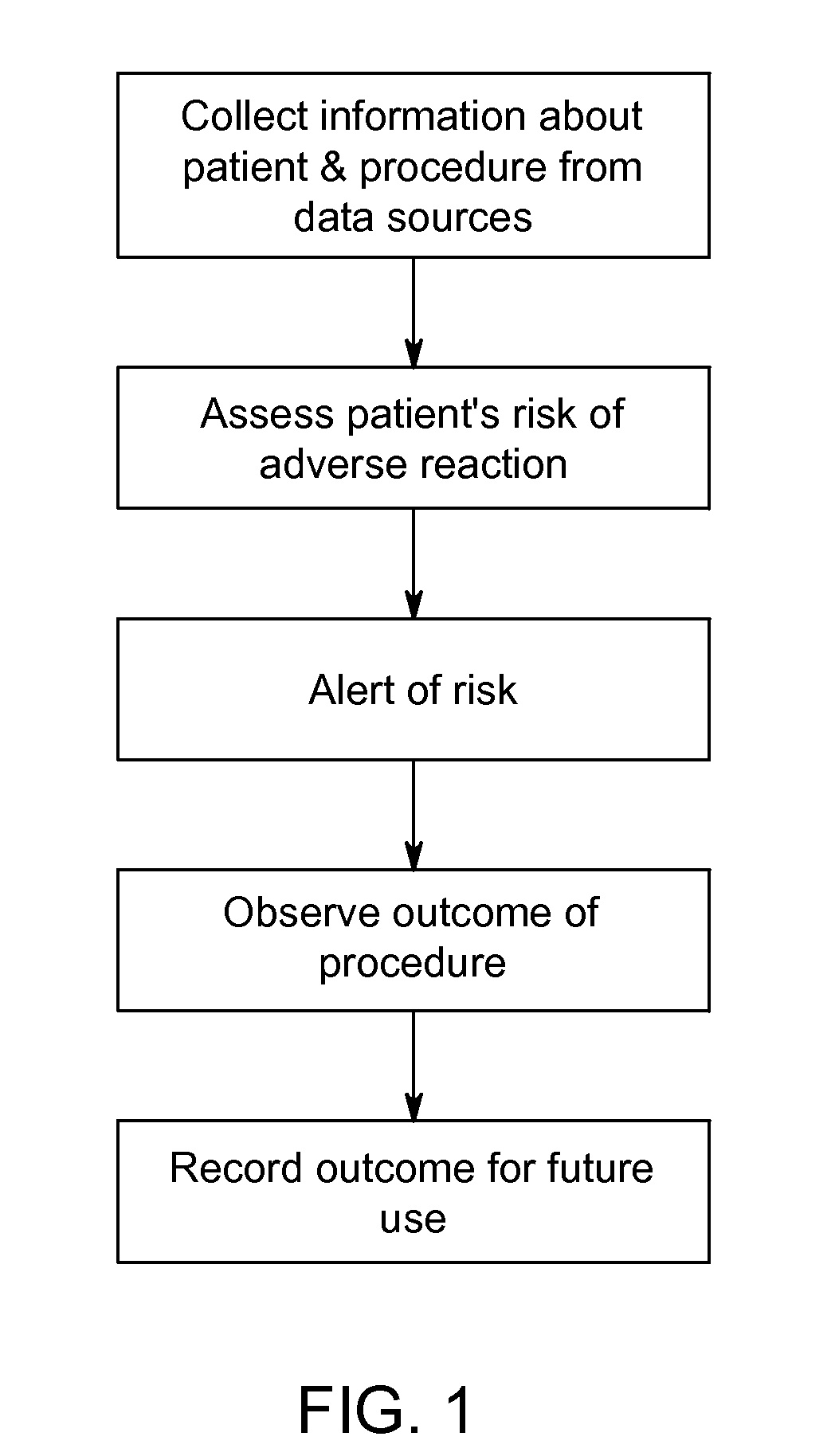
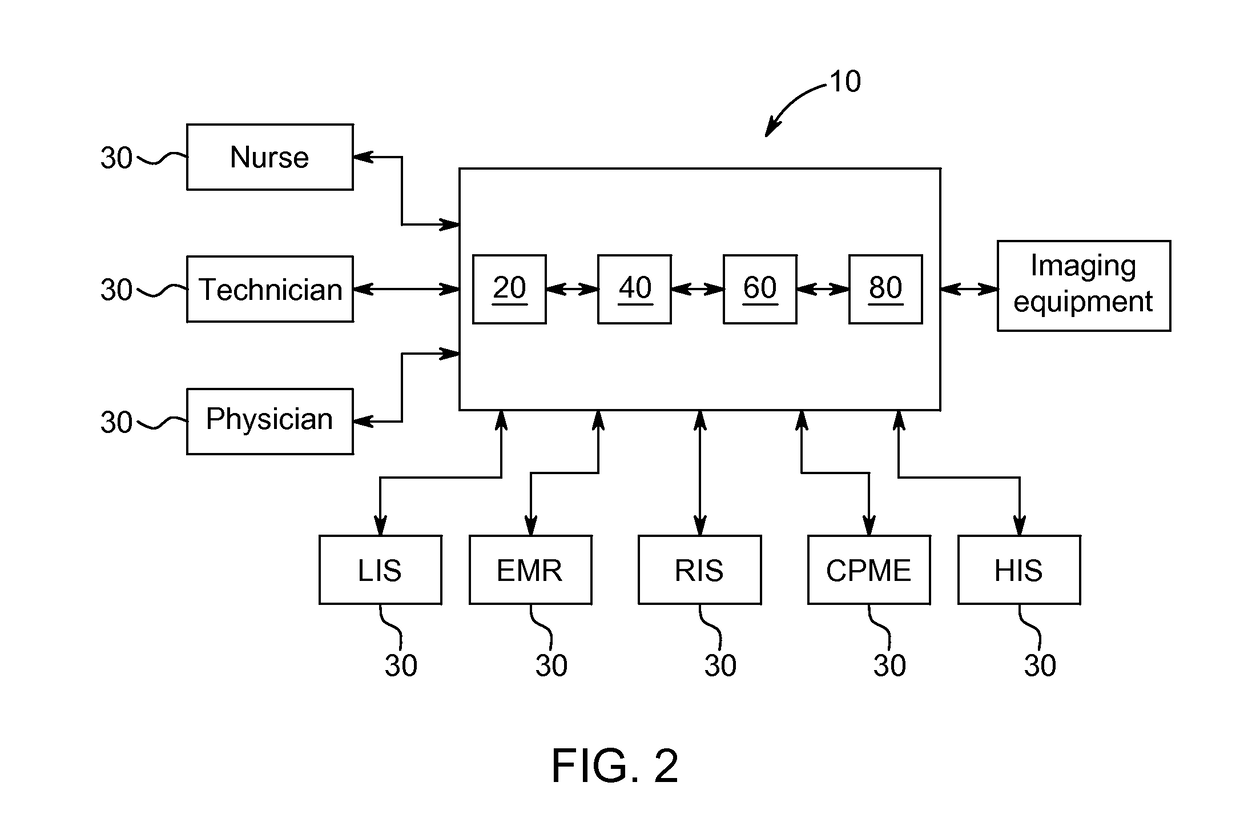
Systems and methods for managing adverse reactions in contrast media-based medical procedures
Described is a method of managing adverse reactions that may occur during a medical procedure that involves the administration of contrast media. The method includes acquiring, by a data acquisition unit, information about a patient and an upcoming medical procedure that involves the administration of contrast media, computing in advance of the medical procedure, by a risk assessment unit, a prediction of a risk that the patient will experience an adverse reaction to the contrast media based on the information about the patient and the upcoming medical procedure, and presenting to one or more medical personnel in advance of the medical procedure, by a risk alert unit, an indication of the risk in a visually perceptible form. Also described are systems and software that implement methods of managing adverse reactions.
Owner:BAYER HEALTHCARE LLC
Benzimidazole derivative, and preparation method and pharmaceutical applications thereof
ActiveCN105237527AEnhanced antihypertensive activityEnhance antihypertensive effectOrganic chemistryCardiovascular disorderBenzimidazole derivativeLiver and kidney
The invention belongs to the technical field of pharmaceutical chemistry, and more specifically discloses a benzimidazole derivative, and a preparation method and pharmaceutical applications thereof. The benzimidazole derivative comprises ligustrazine and a NO donor derivative. In vivo, ligustrazine or NO can be released from the benzimidazole derivative quickly, so that effective synergistic effect with Azilsartan is achieved, anti-hypertension curative effect is improved, adverse reaction is reduced, ideal protection effect on livers and kidneys of patients is achieved, and the blank of the prior art is filled.
Owner:WUHAN LL SCI & TECH DEV
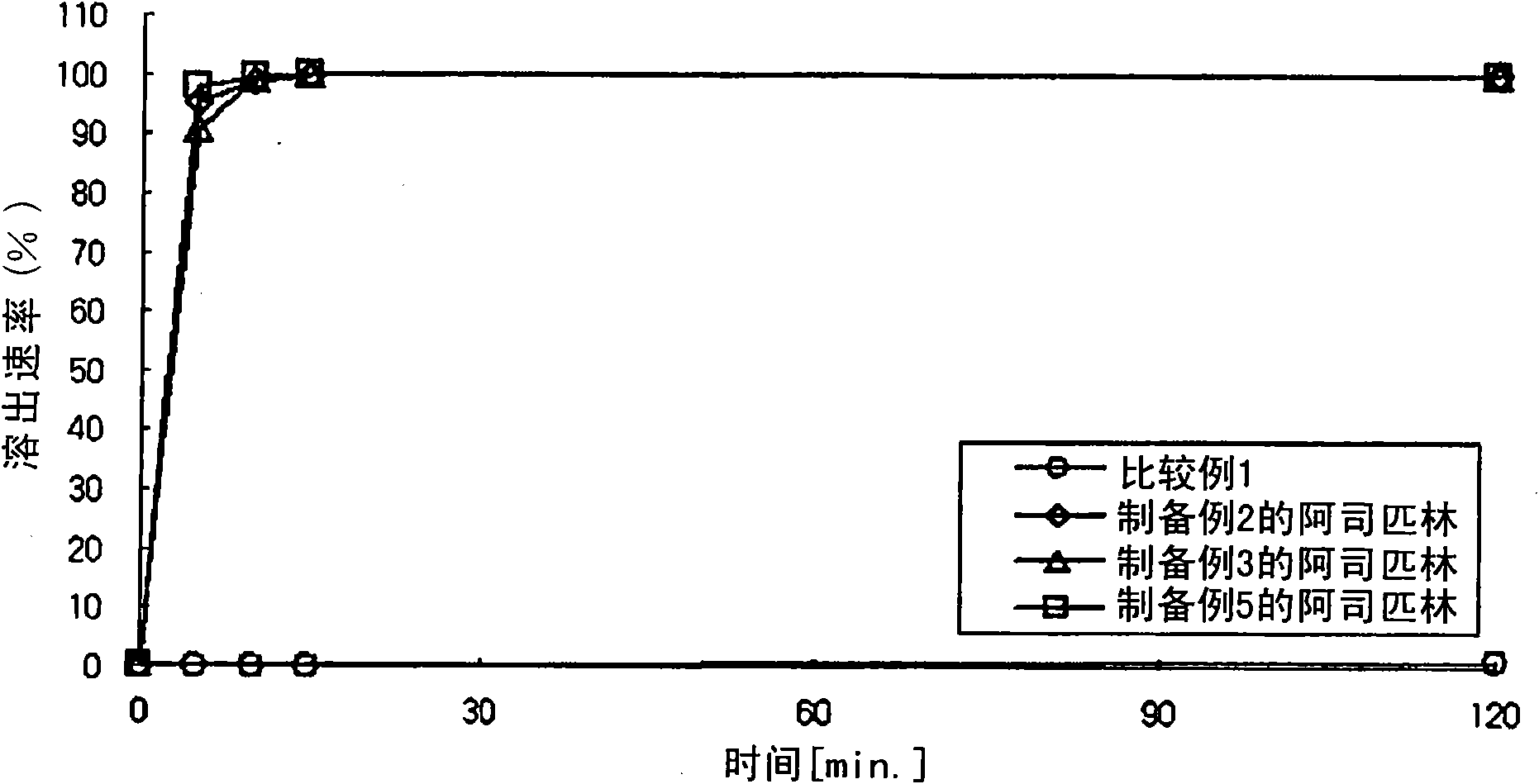
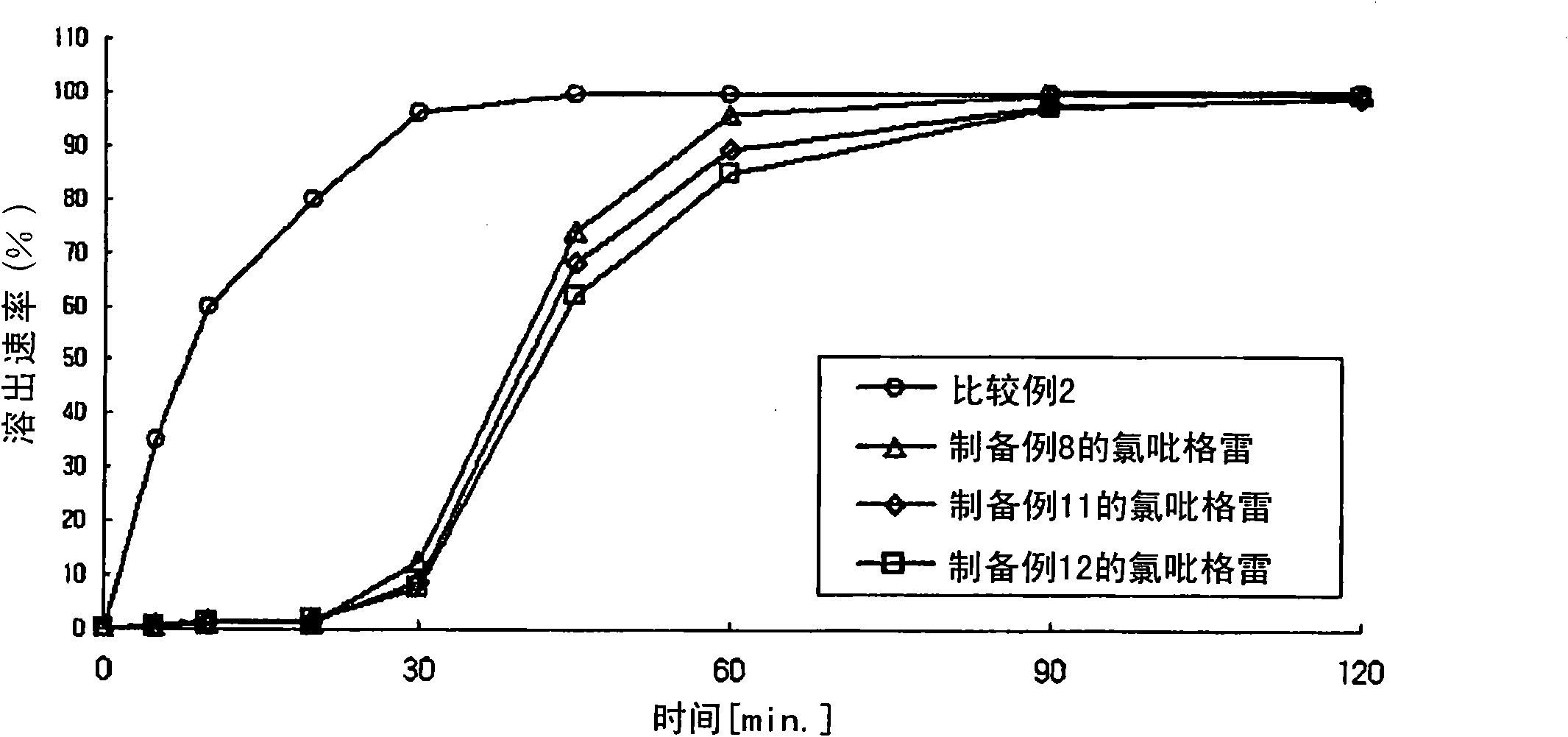
Composite preparation
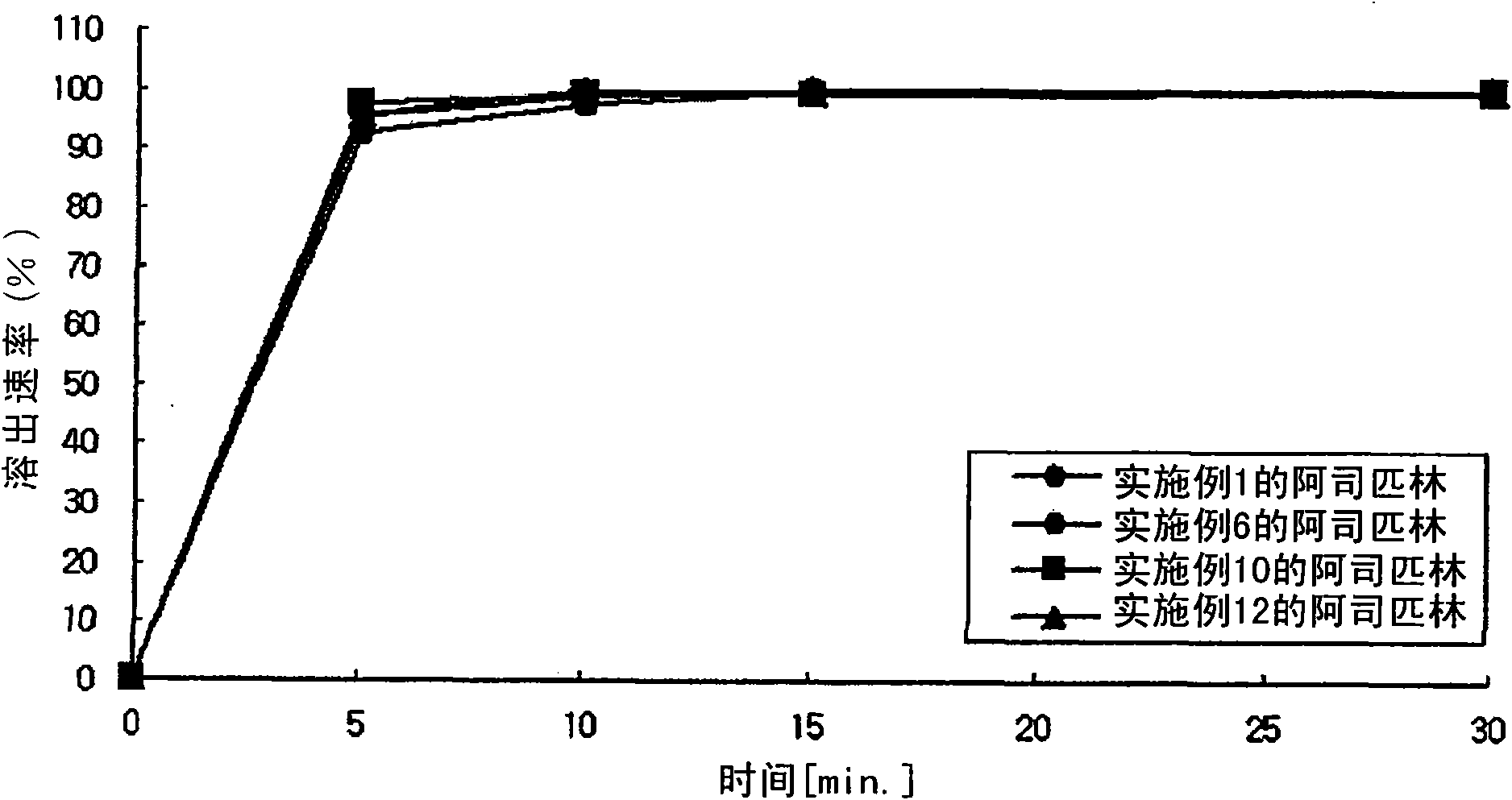
InactiveUS20110038931A1Excellent anti-platelet aggregation effectExcellent in clinical therapeutic effectPowder deliverySalicyclic acid active ingredientsClopidogrel resistanceAdverse drug reaction
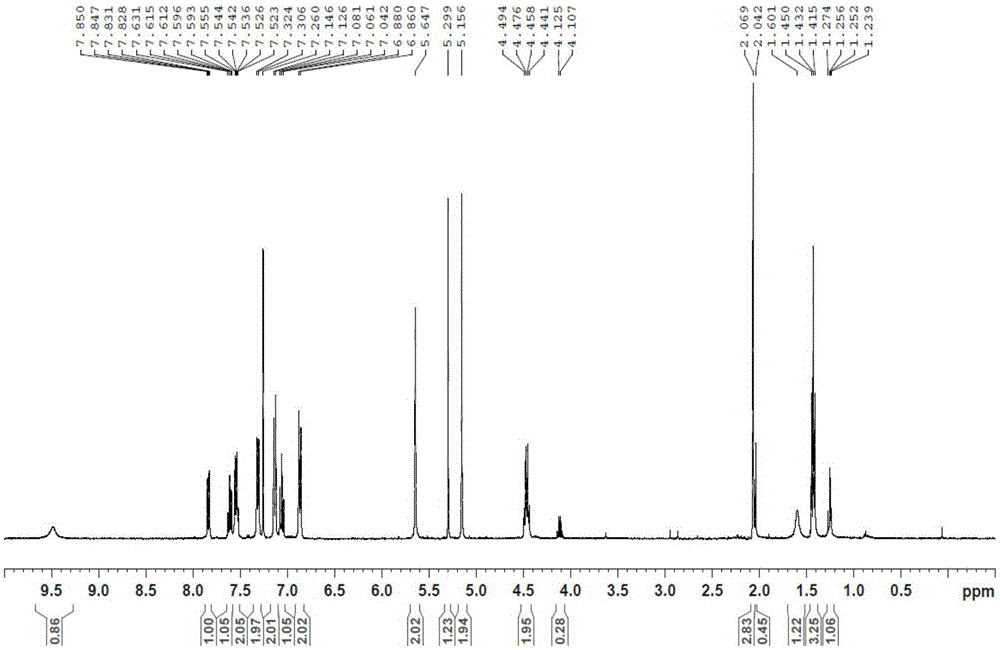
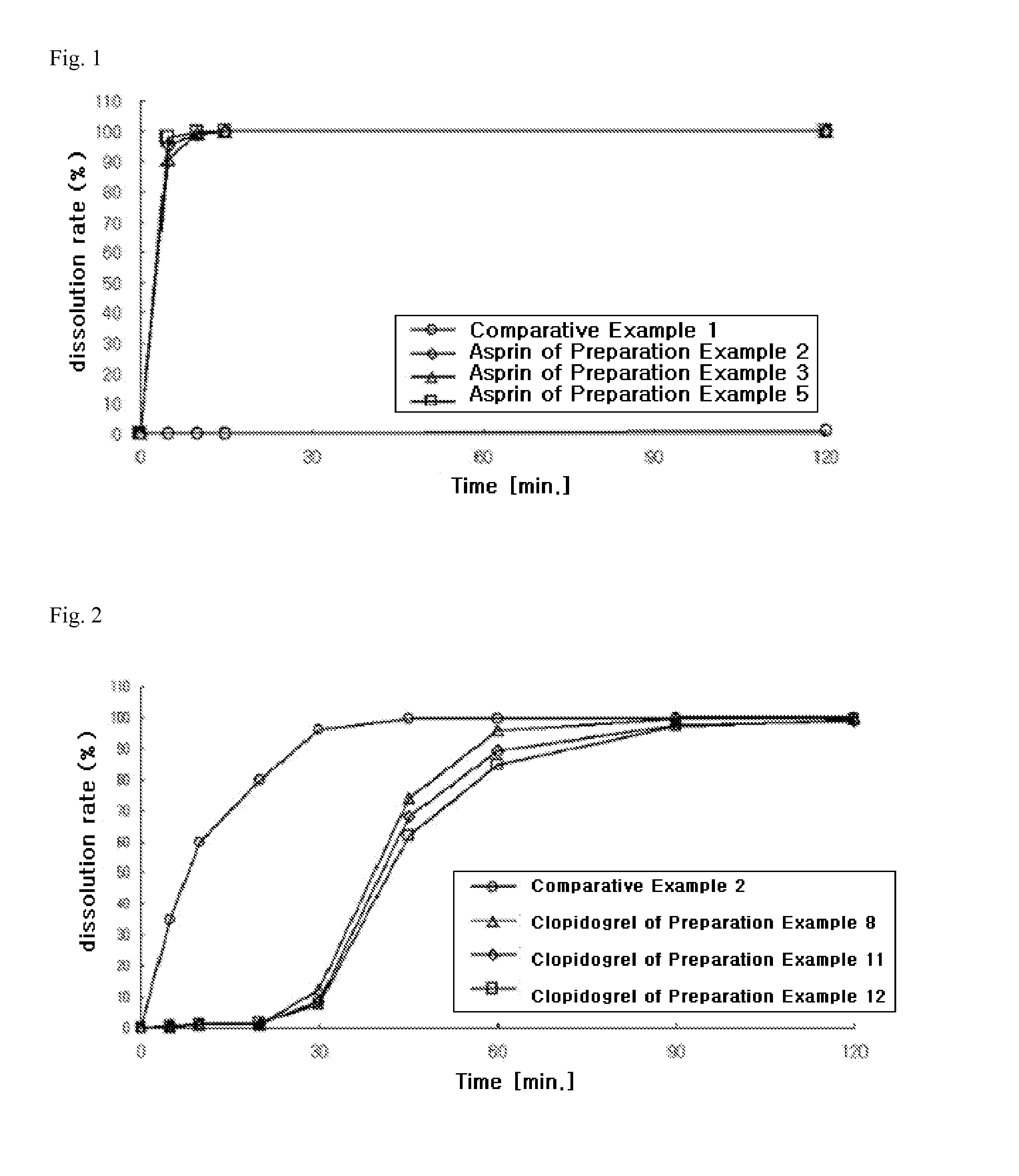
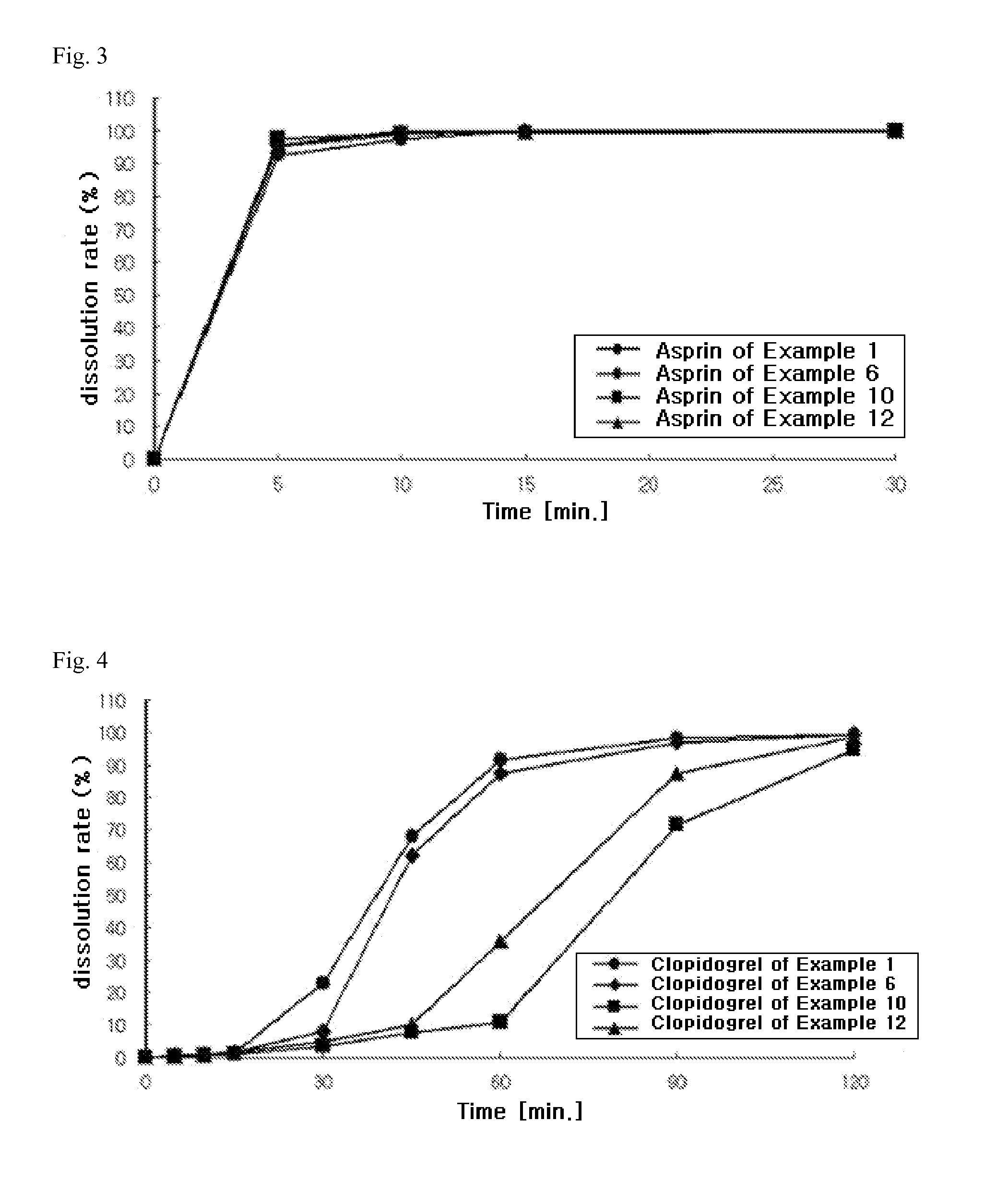
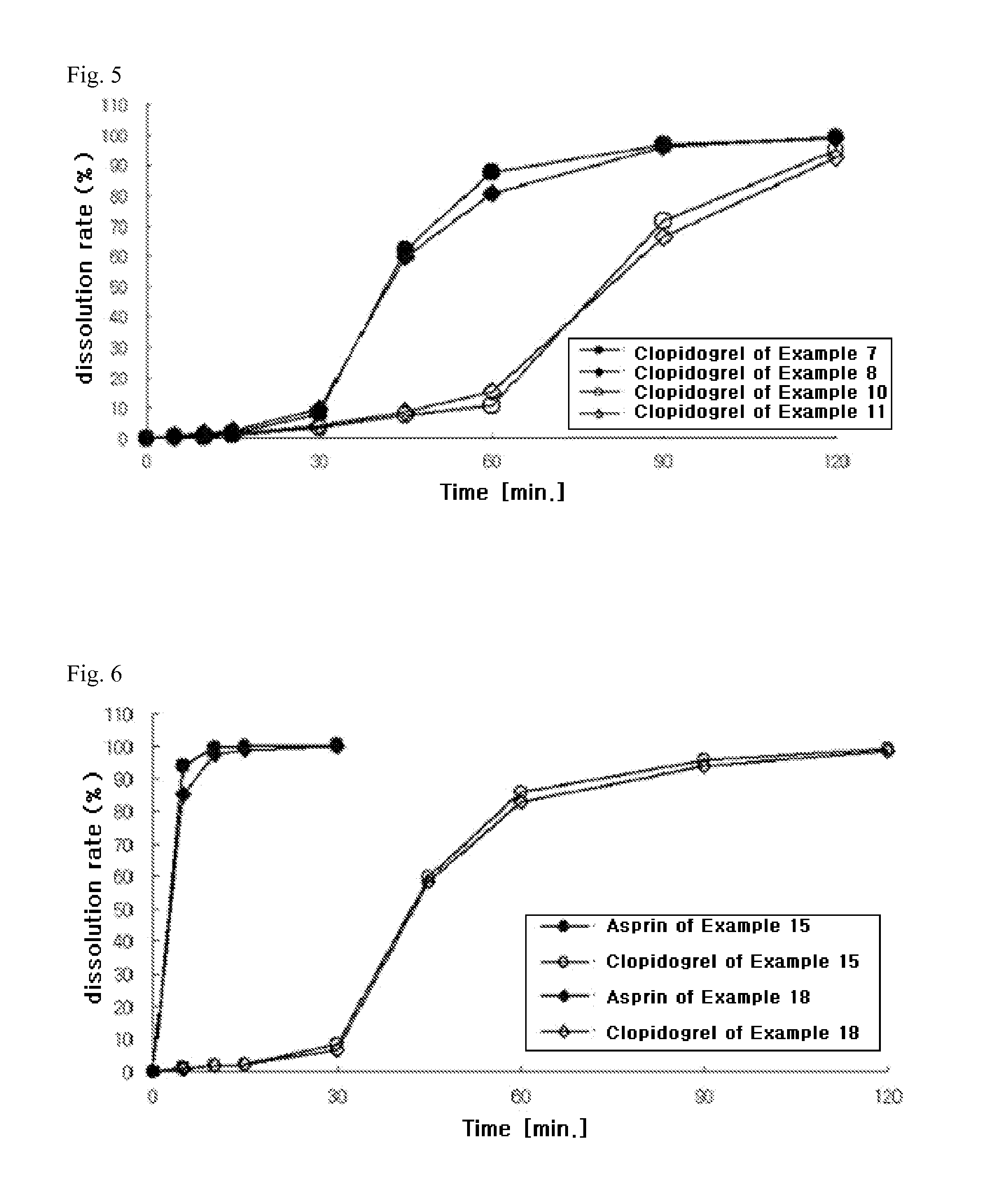
The present invention provides a combination preparation which comprises: a prior-release section comprising aspirin or a pharmaceutically acceptable salt thereof as a pharmacologically active component; and a delayed-release section comprising clopidogrel, an isomer thereof or a pharmaceutically acceptable salt thereof as a pharmacologically active component. The combination preparation of the present invention exhibits a far better effect in preventing platelet aggregation than does simultaneous oral therapy or treatment with the respective single preparations, and not only can it improve the patient's drug-taking compliance by administration once a day but it can also reduce the adverse reactions which follow long-term administration of aspirin. The combination preparation of the present invention is also advantageous in that it exhibits an outstanding effect in inhibiting blood platelet aggregation despite a reduction in the amount of aspirin ingested, and in that it converts clopidogrel resistance into susceptibility and prevents serious adverse reactions caused by clopidogrel resistance and in that it can be stored over the longer term since it is stable under common storage conditions.
Owner:HANALL PHARMA CO LTD
Genetic testing for risk of causing serious adverse reactions of skin of carbamazepine
InactiveCN102108382AMicrobiological testing/measurementFluorescence/phosphorescenceAdverse drug reactionAntiepileptic drug
The invention discloses a genetic testing method for risk of causing serious adverse reactions of carbamazepine. The genetic testing method comprises the following steps: collecting oral mucosa cells of a subject, extracting genome DNA (deoxyribonucleic acid) of the oral mucosa cells, testing HLA (human leukocyte antigen)-B*1502 allelotype of the genome DNA and evaluating the risk of causing the serious adverse reactions of the skin of an anti-epileptic medicament-carbamazepine, thereby providing reference basis for clinical individual medication.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
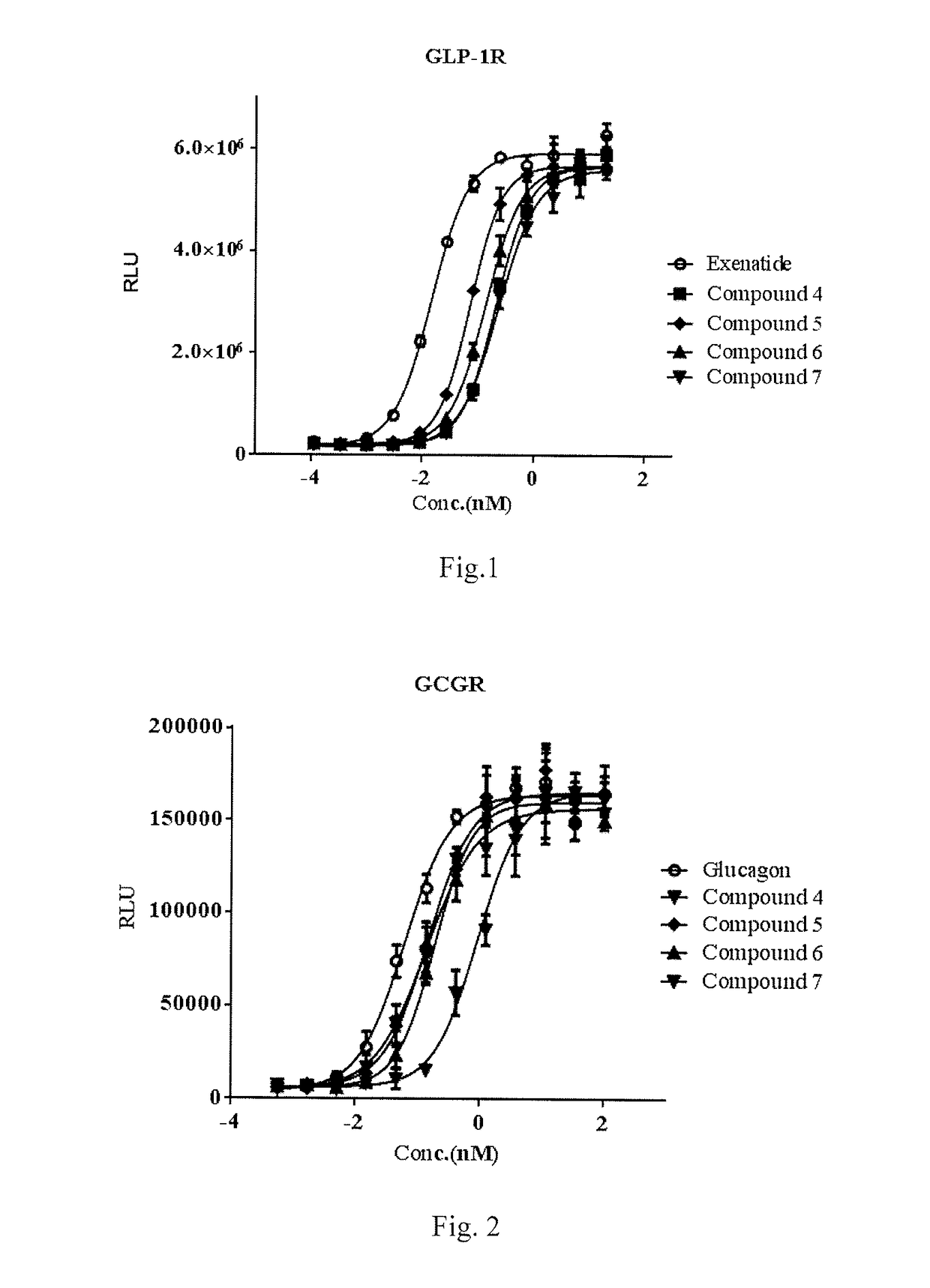
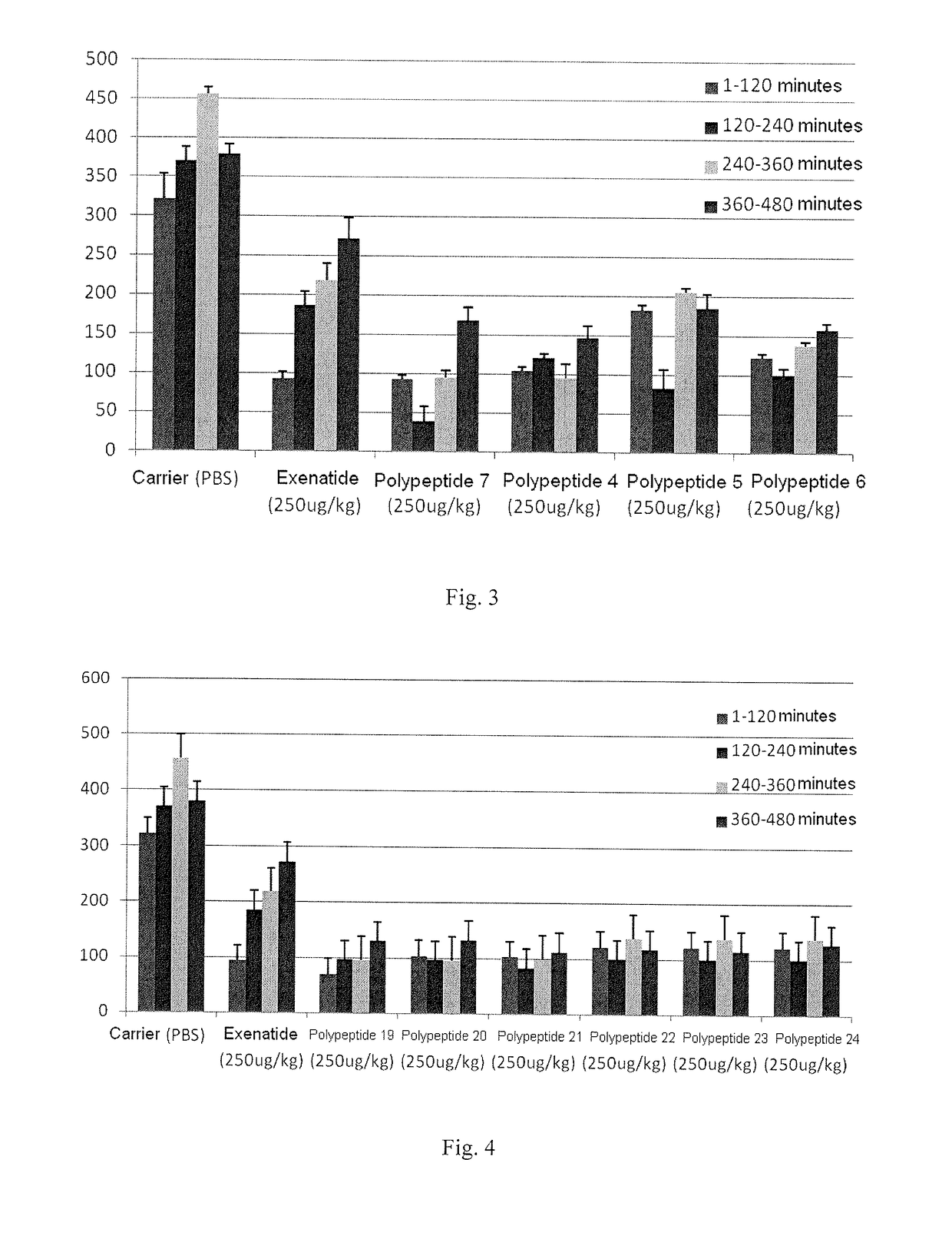
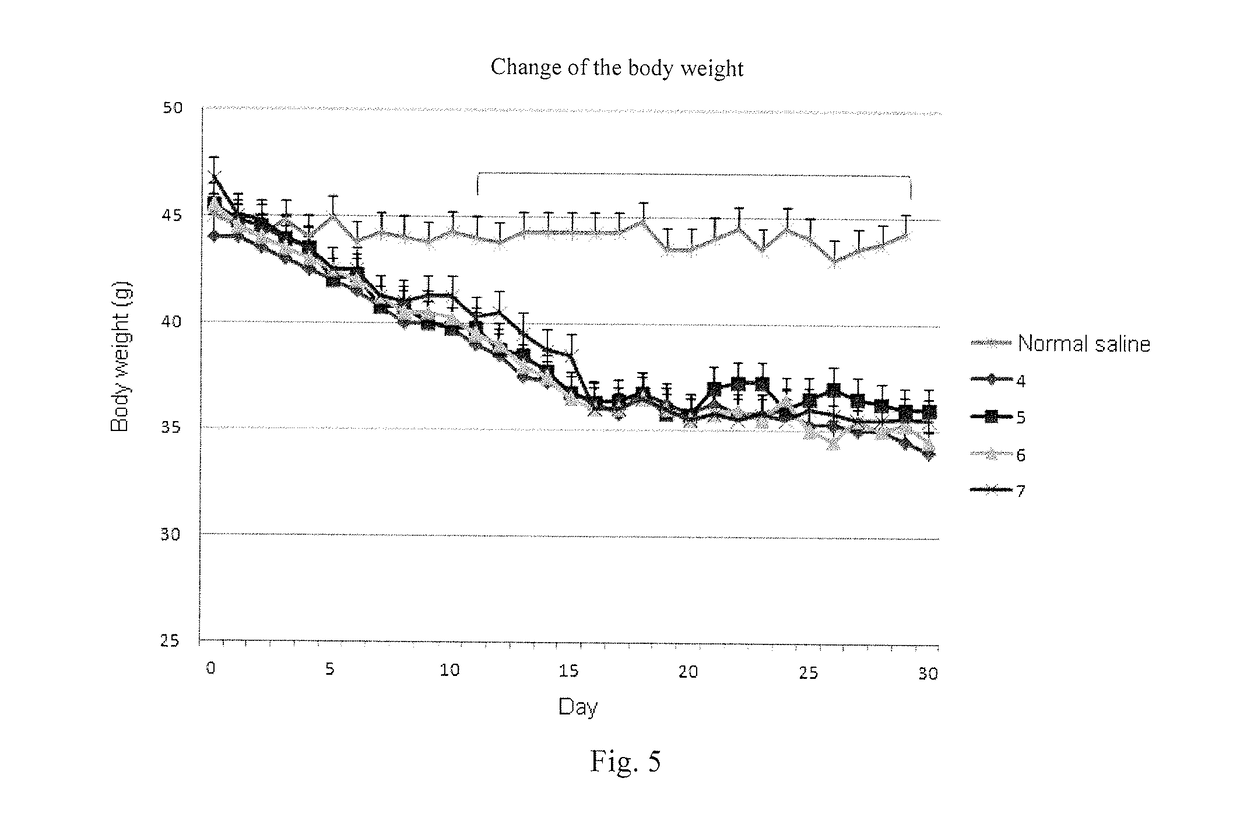
Oxyntomodulin analogue
ActiveUS20170183383A1Cosmetic preparationsPeptide-nucleic acidsDiabetes mellitusAdverse drug reaction
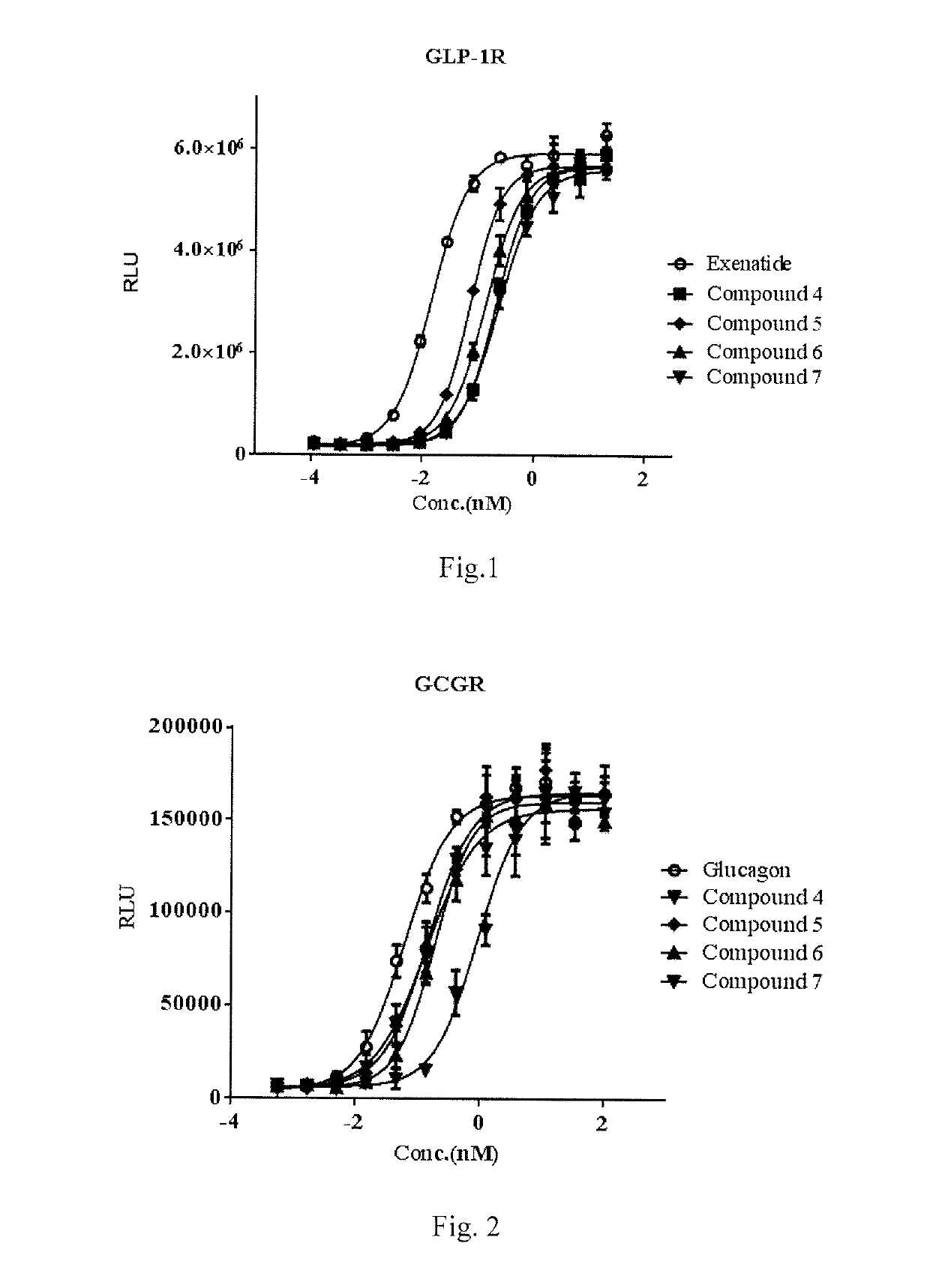
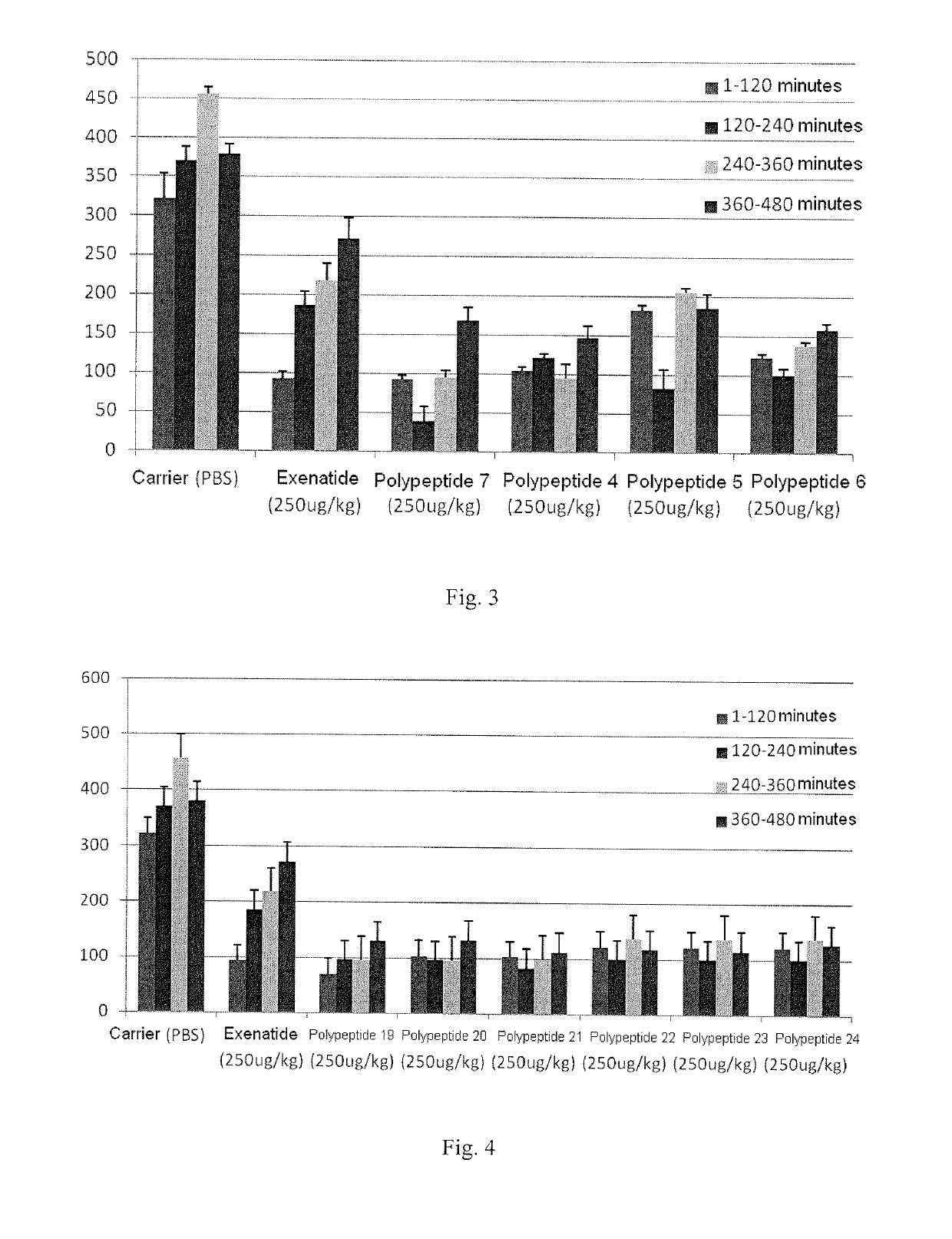
Provided is an oxyntomodulin analogue. The analogue comprises GCGR and GLP-1R dual agonist activity, improved enzymolysis stability and biological activity, and no adverse reactions. The analogue can be used to prepare medication for treating hyperphagia, obesity and diabetes.
Owner:JIANG XIANXING
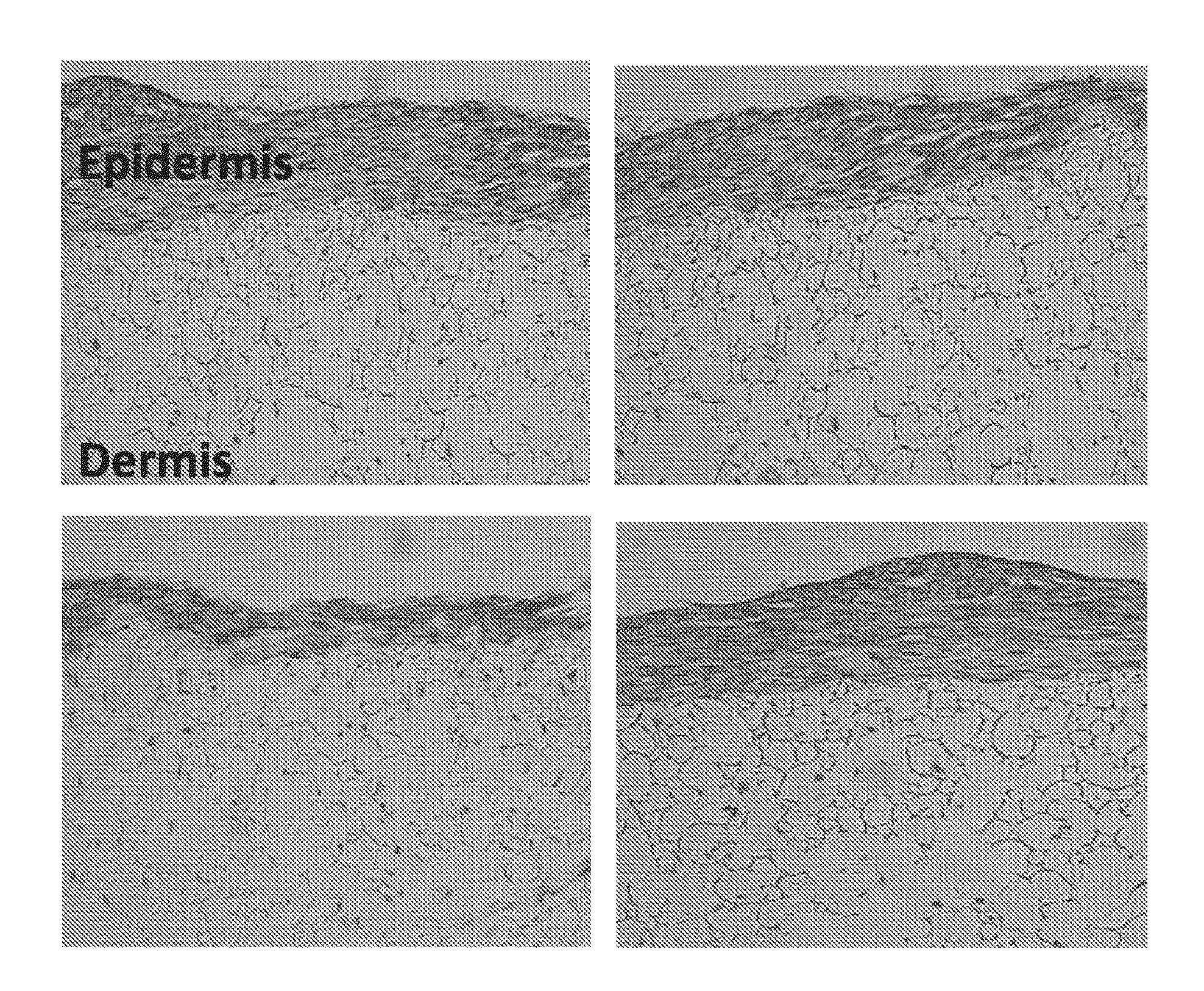
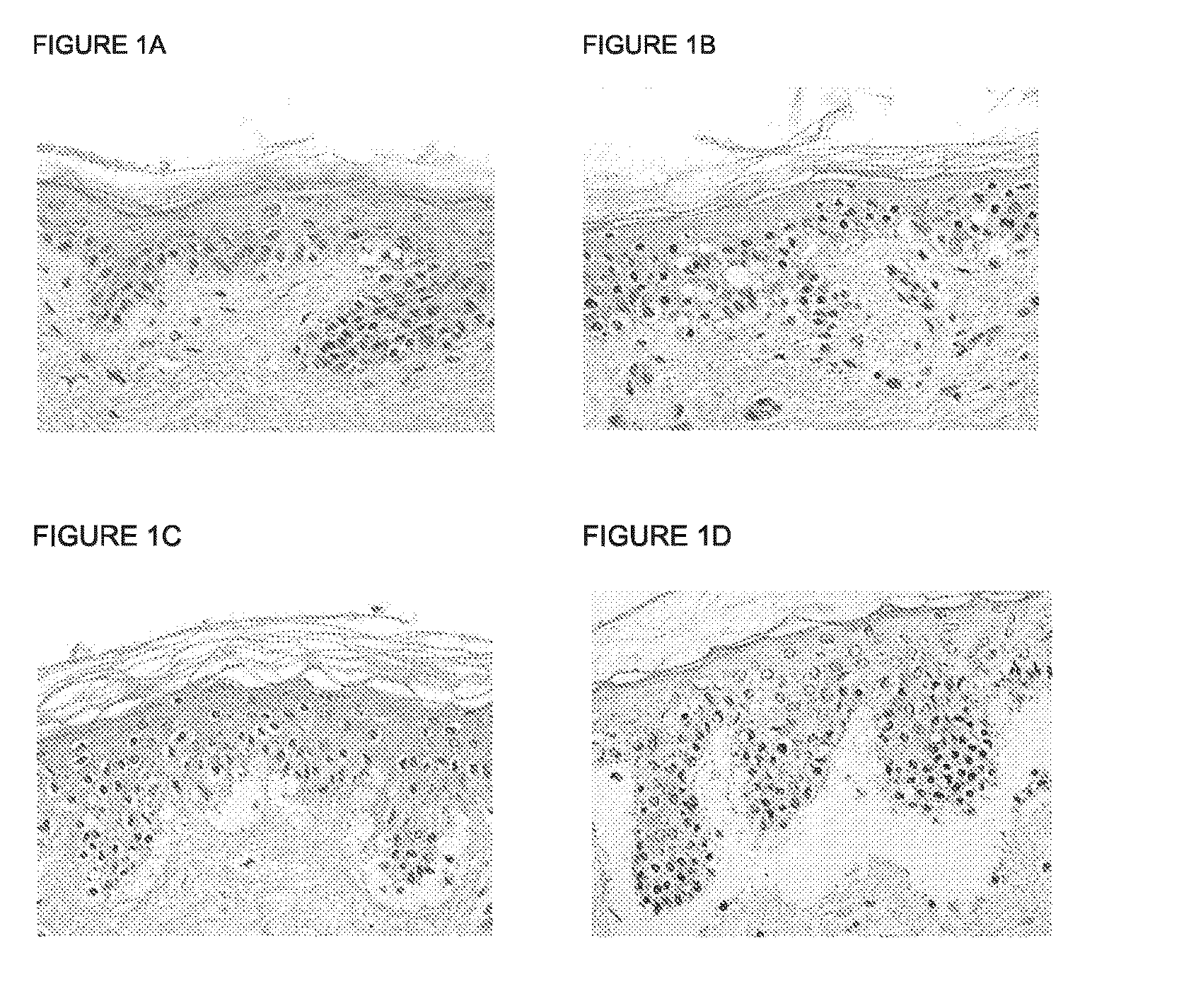
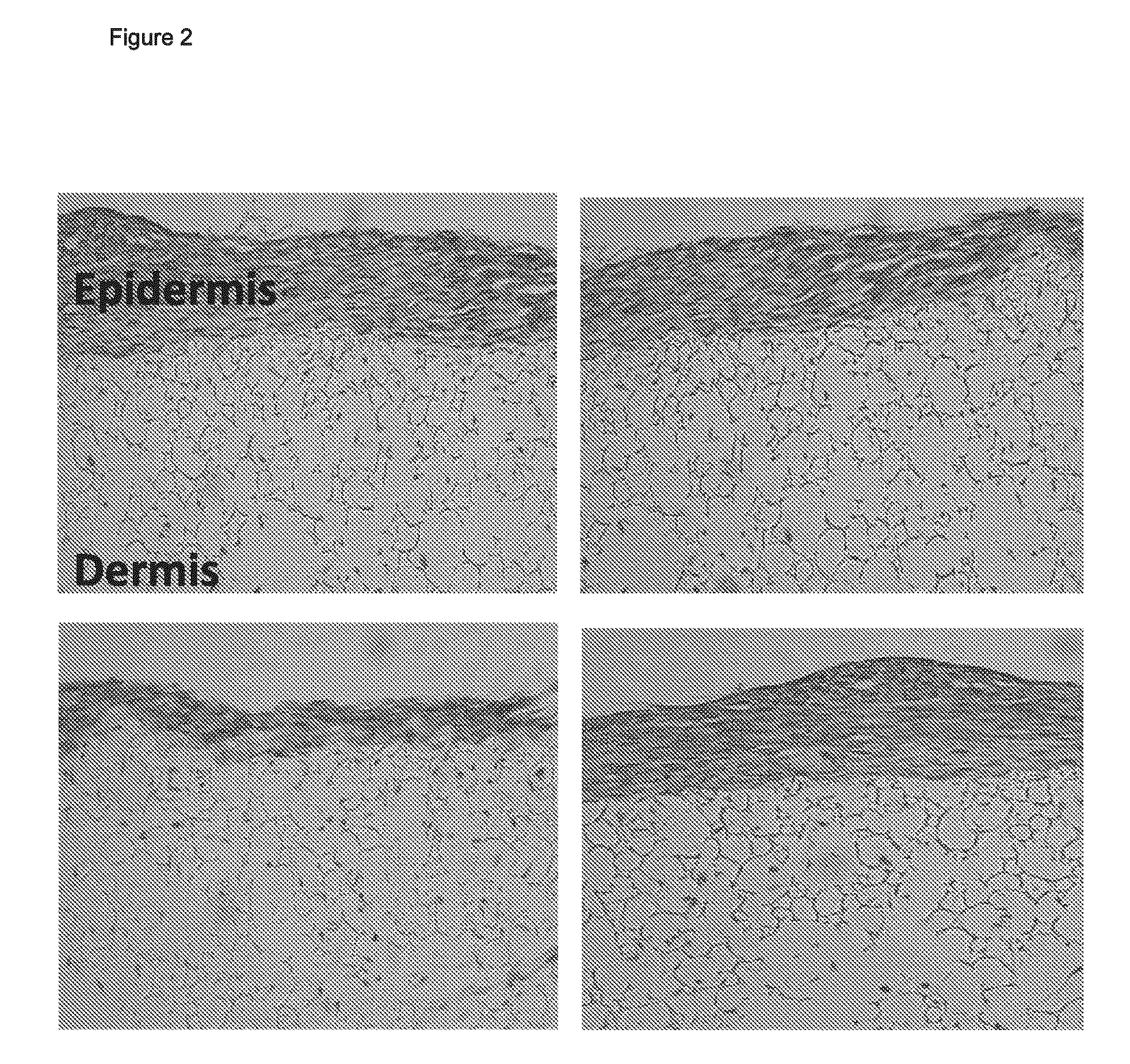
Skin model
ActiveUS20160003856A1Extend your lifeMicrobiological testing/measurementEpidermal cells/skin cellsContact dermatitisAdverse drug reaction
A three dimensional (3-D) model comprising a scaffold and autologous skin cells, the invention also provides methods of predicting immunogenicity and hypersensitivity or allergic or adverse immune reactions to potential therapeutic compounds, biologies, cosmetics and chemical sensitizers using the 3-D model of skin cells. The methods provide an in vitro assay employing autologous blood derived cells in the 3-D skin equivalent model and is of particular utility in the identification and prediction of skin sensitizers and in particular agents that may cause allergic contact dermatitis. The assay of the present invention provides inter alia methods of screening library compounds for sensitizing activity, identifying optimal therapeutics, especially but not exclusively, monoclonal antibodies and kits therefor.
Owner:NEWCASTLE UNIV +1
Composite preparation
ActiveCN101951896AExcellent anti-platelet aggregation effectSignificant clinical effectSalicyclic acid active ingredientsPharmaceutical non-active ingredientsClopidogrel resistanceAdverse drug reaction
The present invention provides a composite preparation which comprises: a prior-release section comprising aspirin or a pharmaceutically acceptable salt thereof as a pharmacologically active component; and a delayed-release section comprising clopidogrel, an isomer thereof or a pharmaceutically acceptable salt thereof as a pharmacologically active component. The composite preparation of the present invention exhibits a far better effect in preventing platelet aggregation than does simultaneous oral therapy or treatment with the respective single preparations, and not only can it improve the patient's drug-taking compliance by administration once a day but it can also reduce the adverse reactions which follow long-term administration of aspirin. The composite preparation of the present invention is also advantageous in that it exhibits an outstanding effect in inhibiting blood platelet aggregation despite a reduction in the amount of aspirin ingested, and in that it converts clopidogrel resistance into susceptibility and prevents serious adverse reactions caused by clopidogrel resistance and in that it can be stored over the longer term since it is stable under common storage conditions.
Owner:HANALL PHARMA CO LTD
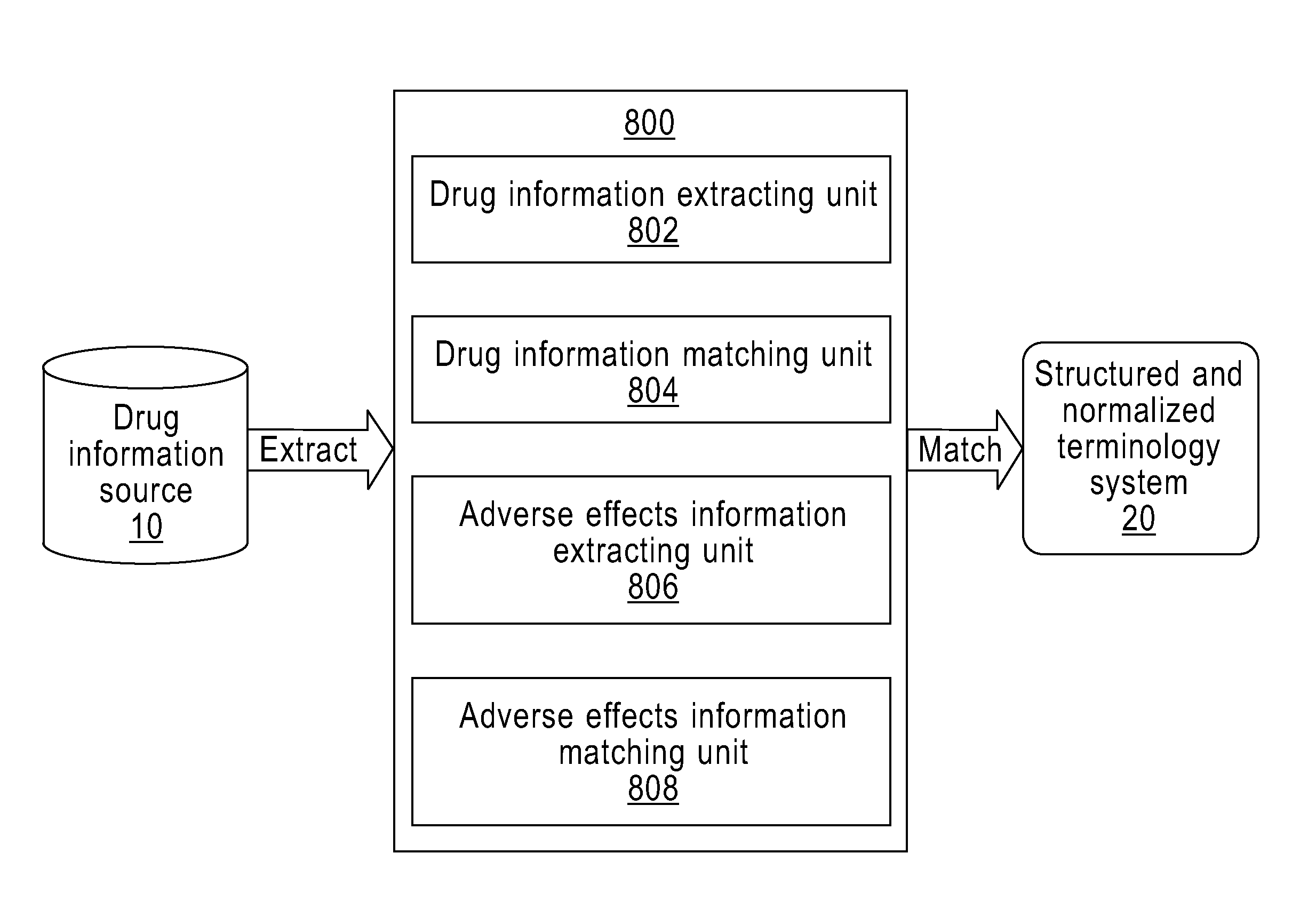
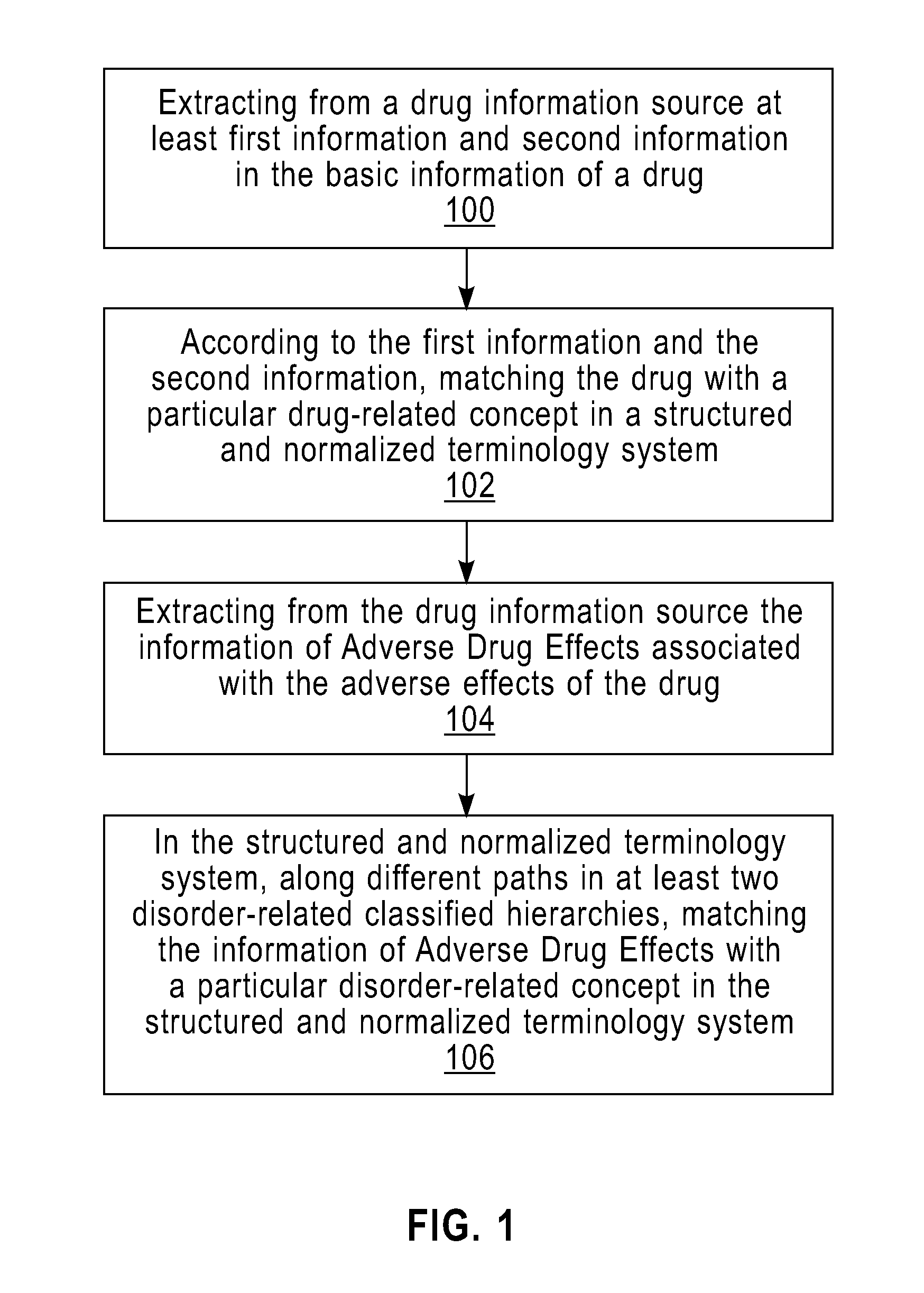
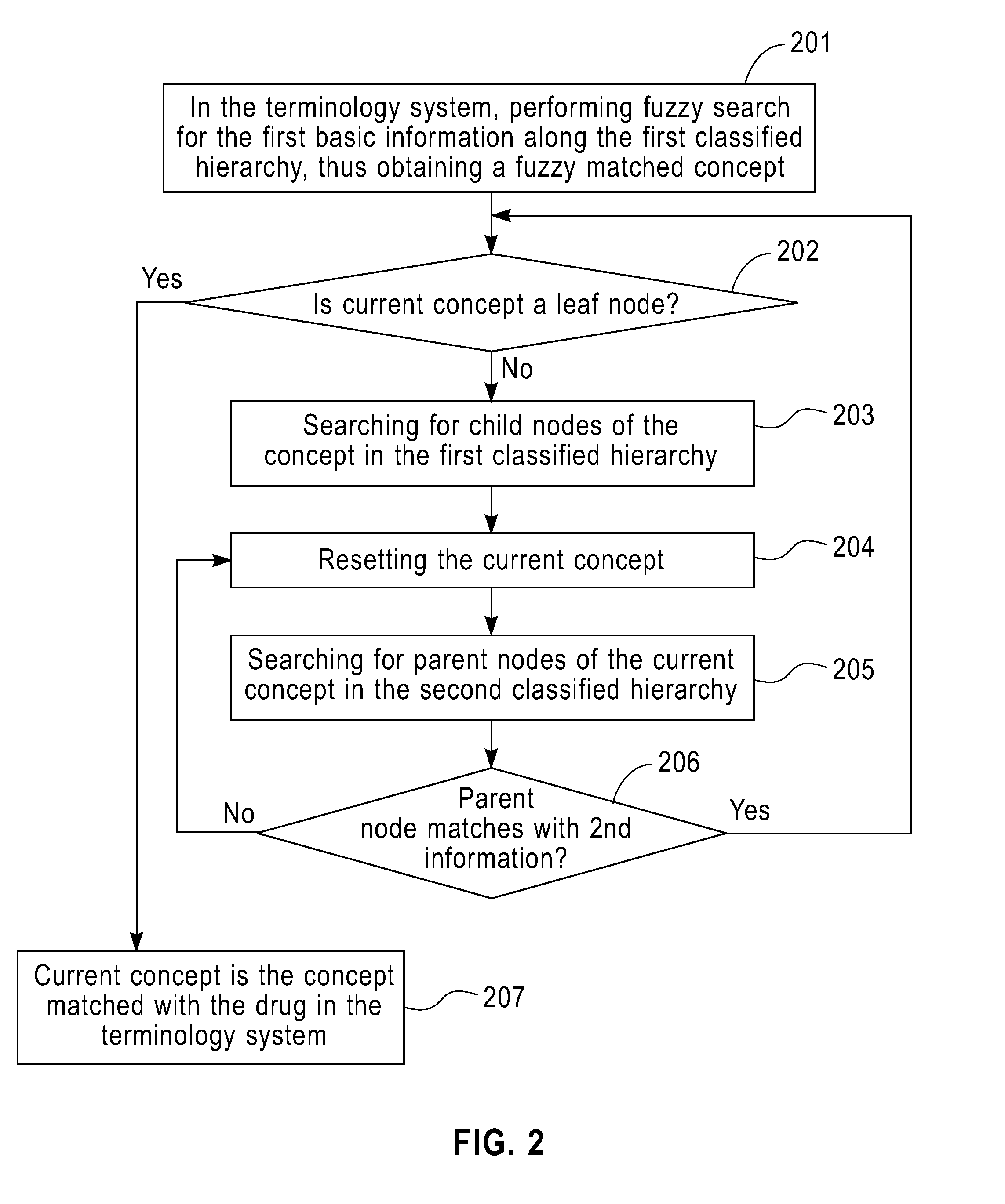
Method and Apparatus for Providing the Information of Adverse Drug Effects
InactiveUS20110258231A1Easily integrated into each otherFacilitate collection and integration and search and calculation and propagationDigital data processing detailsDrug referencesMedication informationMedicine
A method and apparatus for providing the information of adverse drug effects. The method includes: extracting at least a first information and a second information in basic information of a drug from a drug information source; matching the drug with a particular drug-related concept in a structured and normalized terminology system according to the first and the second information; extracting, from the drug information source, the information of Adverse Drug Effects associated with the drug; and matching the information of Adverse Drug Effects with a particular disorder-related concept in the structured and normalized terminology system; wherein the matching is along different paths in at least two disorder-related classified hierarchies. The invention can extract, standardize, and normalize information relating to adverse drug effects to help the integration, search, calculation, and propagation thereof.
Owner:IBM CORP
Glossy ganoderma and grass jelly soft extract and preparation method thereof
PendingCN108477565AThe primary and secondary collocations are orderly and appropriateUnique flavorFood scienceFlavorAdverse drug reaction
The invention relates to a glossy ganoderma and grass jelly soft extract and a preparation method thereof. Glossy ganoderma is used as a main raw material, and through cooperation of an appropriate quantity of Chinese mesona herbs, extractability raw materials are added or not added, fruit and vegetable juice is added or not added, nutrients are added or not added, during extract collection, an appropriate extract collecting agent is selected for cooperation of extract collection, an optimal recipe combination can be obtained, namely the glossy ganoderma and grass jelly soft extract being balanced, comprehensive and reasonable in nutrient components, orderly and appropriate in collocation of main materials and auxiliary materials, unique in flavor, and fine and smooth in mouth feel can beobtained. The food does not have requirements for eating crowds and eating amount limiting, the glossy ganoderma and grass jelly soft extract can be suitable for broad consumption groups, is free fromadverse drug reactions after being eaten, and does not have efficacy requirements of health-care foods. The eating scope of conventional glossy ganoderma products is enlarged, new vitality is provided for food markets, and higher economic value is created for society.
Owner:山东承御堂药业有限公司
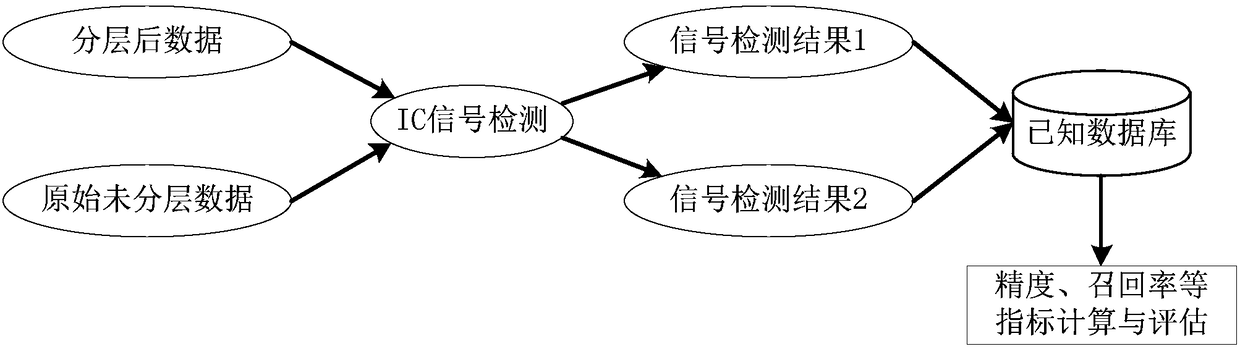
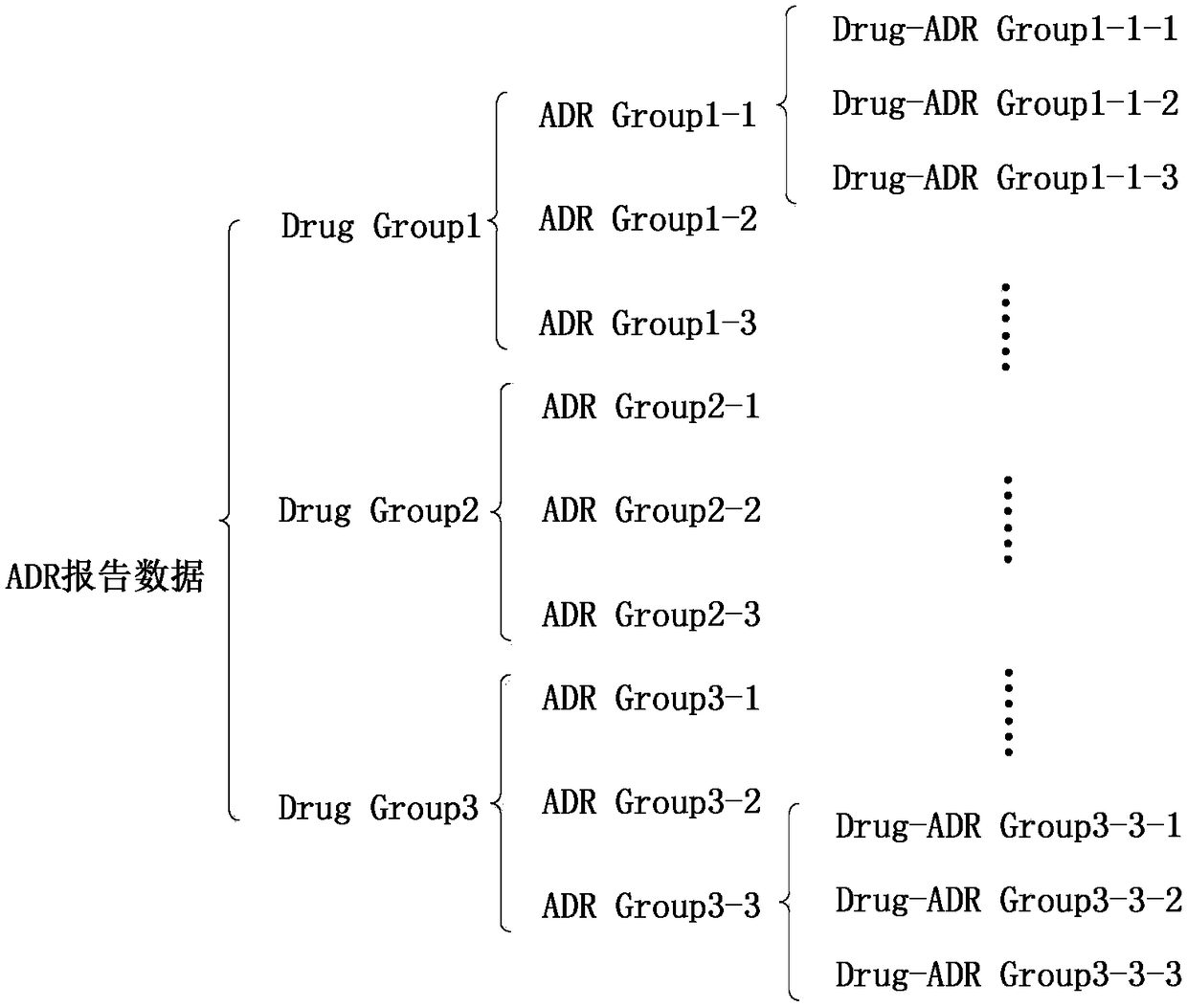
Layering strategy-based adverse drug reaction data shadowing effect eliminating method
The invention discloses a layering strategy-based adverse drug reaction data shadowing effect eliminating method. According to the method, a data shadowing effect eliminating method is researched in adverse drug reaction signal detection on the basis of the adverse drug reaction report data of China, so that a layering strategy-based adverse drug reaction data shadowing effect eliminating model isdesigned; the data are layered on the whole according to the frequencies of the presence of drugs, adverse reactions and drug-adverse reaction pairs in the report data; and data detection is performed on the layered data through adopting an IC method. According to the invention, reports in which the frequencies of the presence of the drugs, adverse reactions and drug-adverse reaction pairs belongto the same order of magnitude are grouped into the same layer, so that the problem of data shadowing effects caused by excessively large difference of the frequencies of the presence of the drugs, adverse reactions and drug-adverse reaction pairs can be solved, and therefore, the data shadowing effects can be eliminated. With the method of the invention adopted, suspicious signals in the adversedrug reaction reports can be the accurately and reliably detected. The method has important reference significance in clinical care and drug supervision links.
Owner:NANJING UNIV OF POSTS & TELECOMM
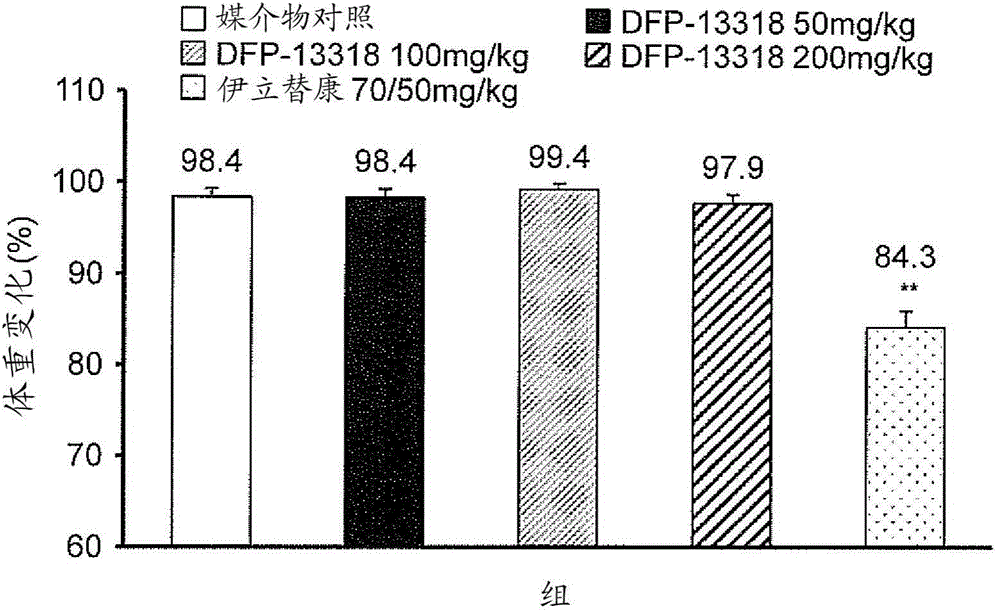
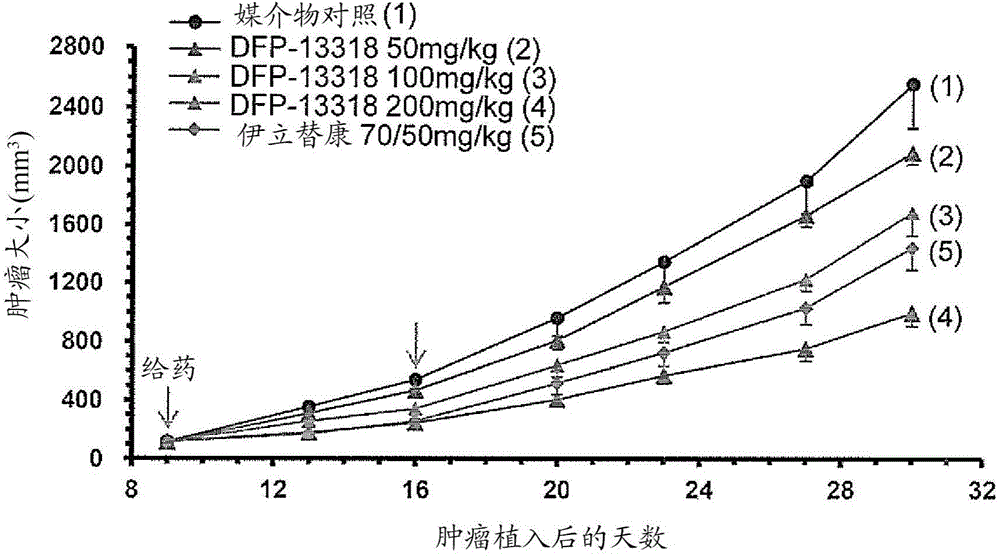
Anticancer agent free of adverse reactions
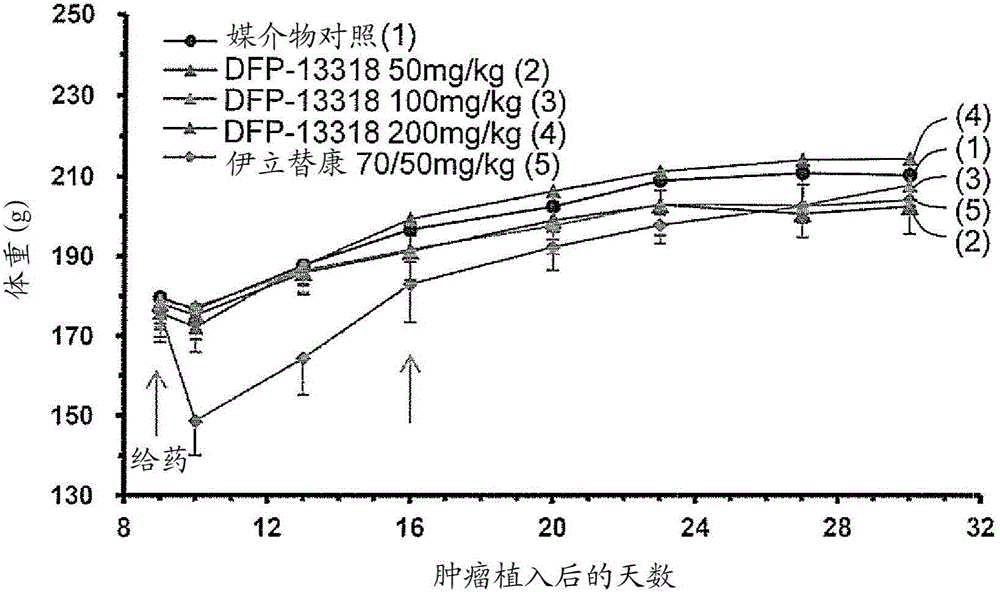
InactiveCN104981245APhysical strength is not wastedPhysical wasteOrganic active ingredientsPharmaceutical non-active ingredientsRegimenAnticarcinogen
The present invention provides a pharmaceutical composition for treating cancer, the composition comprising as the active ingredient a compound represented by general formula I: C-[-CH2-O-(-CH2CH2-O-)n-X1-CHR1-O-CO-NR2-CH2-X2]4 (I) (where X1-CHR1-O-CO-NR2 is a linker, X1 is a spacer, R1 is an optionally substituted C1-4 alkyl, R2 is a phenyl or substituted phenyl group, X2 is SN-38, and n is an integer of 200 to 1,000). The composition is characterized by the following: that the composition can be used in a subject having cancer at a dosage regimen that calls for parenteral administration of a single dose of approximately 0.01 to 11 µmol / kg body weight at a frequency of once a week every other week for at least two administrations; and that the composition does not have irinotecan-like adverse reactions but does impart the same or better cancer proliferation-inhibiting activity as irinotecan at a dose that is 1 / 10 (molar ratio) or less that of irinotecan. Also provided is a method for treating cancer.
Owner:DELTA FLY PHARMA
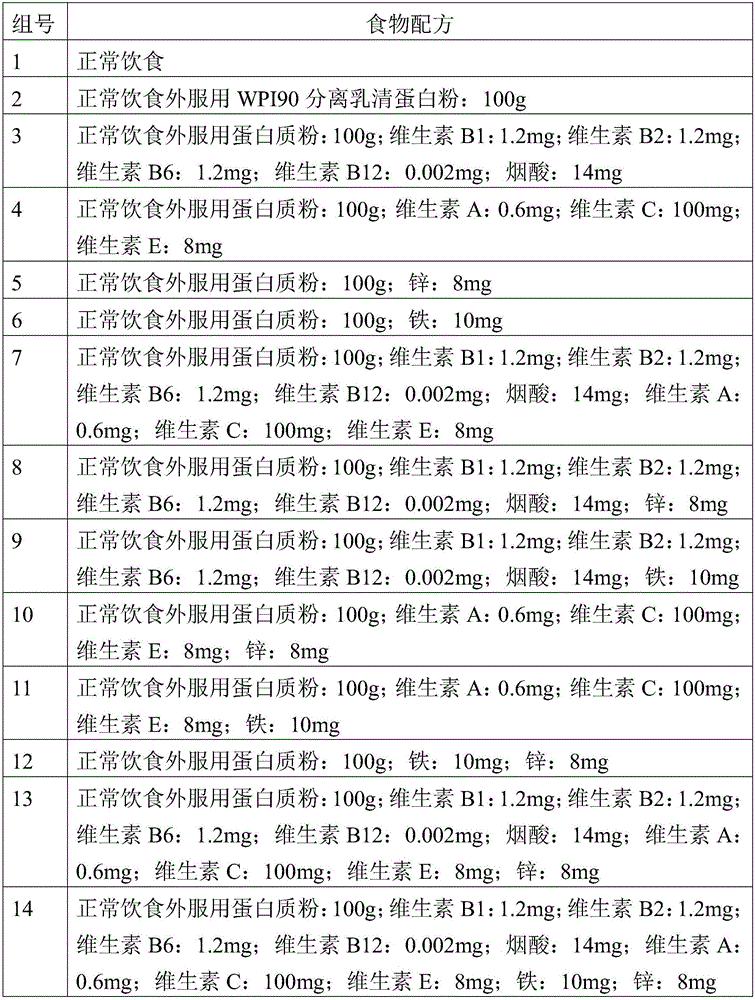
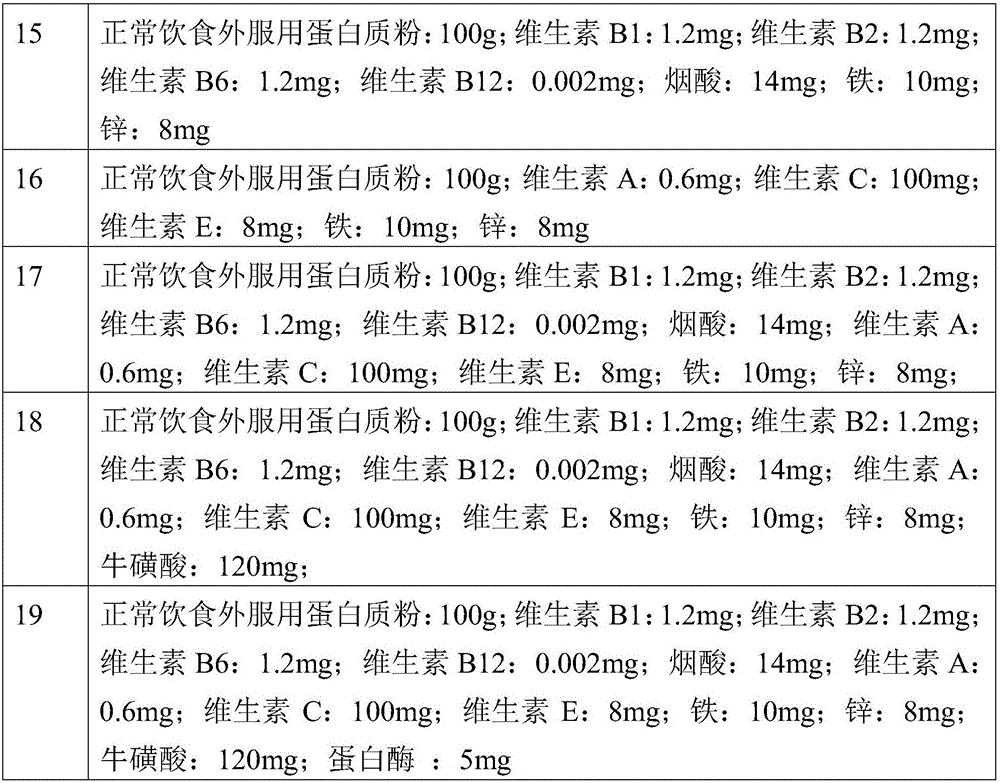
Composition with muscle gaining function and adverse reaction eliminating function
ActiveCN105815791APromote growthGuaranteed efficient growthFood ingredient functionsVitamin CLife quality
The invention provides a composition with a muscle gaining function and an adverse reaction eliminating function. The composition is prepared from, by weight, 80,000-120,000 parts of protein powder, 0.4-0.8 part of vitamin A, 1-1.4 parts of vitamin B1, 1-1.4 parts of vitamin B2, 1-1.4 parts of vitamin B6, 0.001-0.003 part of vitamin B12, 90-110 parts of vitamin C, 6-10 parts of vitamin E, 12-16 parts of nicotinic acid, 8-12 parts of iron and 6-10 parts of zinc. The composition can rapidly and stably assist bodybuilding people in increasing the muscle ratio and strength, and the uncomfortable symptoms such as dryness heat, dental ulcers, throat pain, acne and dry stool from which the bodybuilding people easily suffer are effectively relieved. The living quality of bodybuilding fans is improved while muscles are rapidly gained.
Owner:刘超
Compound multi-component injection
InactiveCN109893500ATherapeutic effect hasNo side effects such as sensitizationOrganic active ingredientsPharmaceutical delivery mechanismMedicineAdverse drug reaction
The invention belongs to the technical field of medicines, and particularly relates to a compound multi-component injection and a preparation process and application thereof. The compound multi-component injection is used for treating sepsis, cannot cause adverse reactions such as allergy and is good in stability.
Owner:董贵雨
Direct dispensing type double-pipe infusion soft bag
InactiveCN104161667AReduce entryReduce the risk of medical malpracticePharmaceutical containersMedical packagingDouble tubeAdverse drug reaction
The invention relates to a direct dispensing type double-pipe infusion soft bag which comprises an integrally formed bag body, an infusion pipe port and a medicine filling port. The outer end of the medicine filling port is connected with a dispensing device in a sealed mode, the connection portion of the medicine filling port and the dispensing device is provided with a rubber plug, the dispensing device is a cylindrical container with an opening in the lower end, the dispensing device comprises a puncture device, the two ends of the puncture device are provided with sharp ends, the wall of the dispensing device is provided with an annular protrusion, the annular protrusion limits downward sliding of the puncture device, and the middle lower portion of the lower sharp end is provided with a protrusion. By means of the soft bag, puncture times during dispensing are reduced, stability of the puncture device in the sliding process is guaranteed, sealing performance of the overall structure is improved, entering of pollutant bacteria is avoided, the probability that puncture fragments enter infused medicine is lowered, the risk that medical accidents are caused due to adverse reactions of medicine in infusion is reduced, the structure is simple, secondary assembling is not needed, the medicine filling port and the infusion pipe port are both downward, and falling of a penicillin bottle is avoided.
Owner:QINGDAO HUAREN PHARMA PACKAGING MATERIAL SCI & TECH
Lidocaine hydrochloride gel and preparation method thereof
ActiveCN112263544AAnti-inflammatoryFunction increaseOrganic active ingredientsAntipyreticAdverse drug reactionActive agent
The invention discloses lidocaine hydrochloride gel and a preparation method thereof. The lidocaine hydrochloride gel comprises the following components in parts by weight of 0.2-3.7 parts of lidocaine hydrochloride, 0.4-7 parts of a gel matrix, 0.01-3 parts of 0.1 M sodium hydroxide, 8-100 parts of water for injection, 0.1 to 1.8 parts of vitamin P; 0.2-5 parts of a volatile oil transdermal absorption enhancer, 0.2-5 parts of a surfactant and 0.4-3.5 parts of a cosurfactant. Vitamin P is added, the elasticity and permeability of capillary vessels are improved by improving the functions of thecapillary vessels, and adverse reactions such as vascular bleeding and the like caused by long-term use of traditional lidocaine drugs are avoided. By using the volatile oil transdermal absorption enhancer, the transdermal absorption rate is increased, the functions of sterilization, inflammation resistance and the like can be achieved, and the possibility of adverse reactions of medicines is reduced.
Owner:北京中泰邦医药科技有限公司
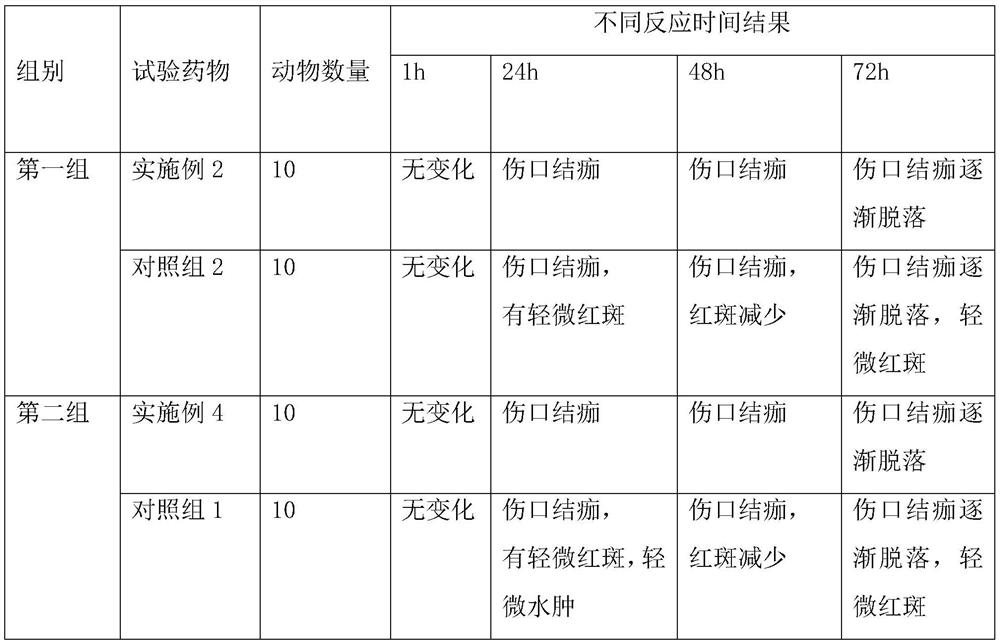
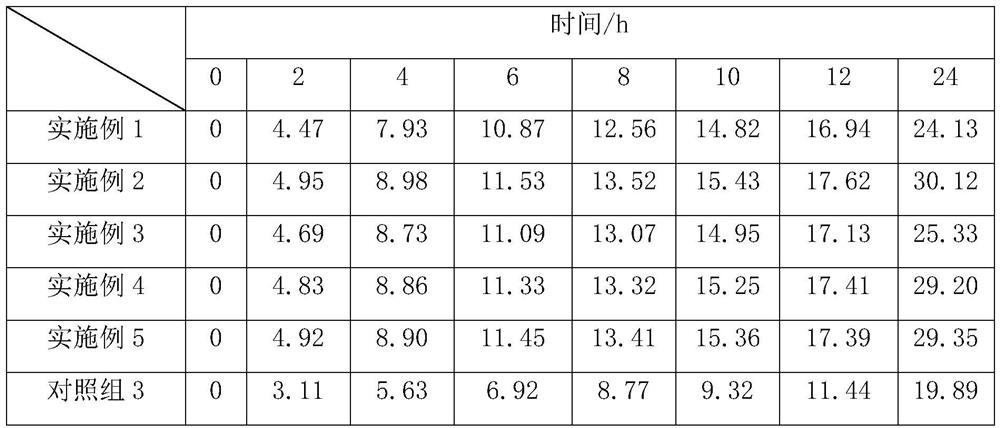
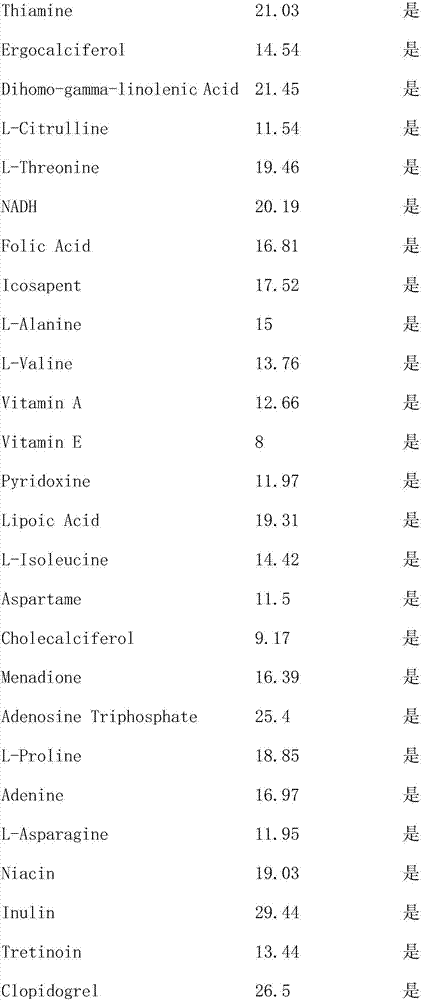
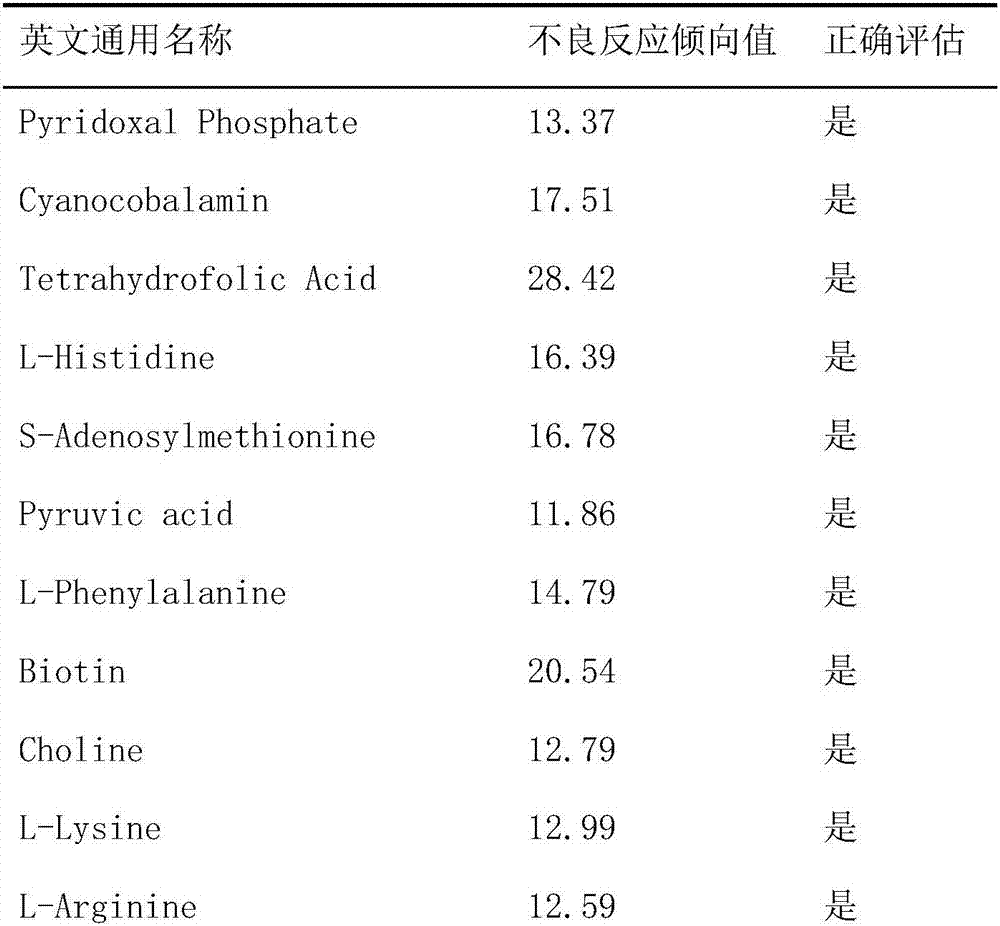
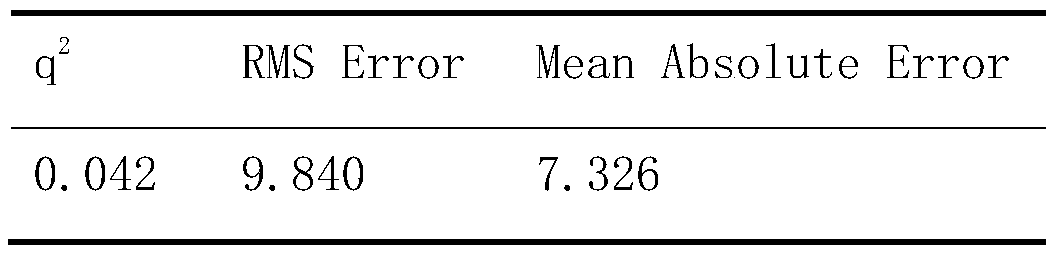
Method of evaluating tendency of adverse reactions of drug
ActiveCN107145735AReduce consumptionReduce blindnessChemical property predictionSpecial data processing applicationsMedicineAdverse drug reaction
The invention provides a method of evaluating tendency of adverse reactions of a drug. The method comprises the following steps of: excluding interference information according to structural characteristics of the drug and quantitative characteristics of an adverse reaction tendency value and establishing an evaluated quantitative relation model between the structural characteristics and the adverse reaction tendency value; and applying the model to evaluate safety of the drug, wherein the accuracy of evaluating the adverse reaction tendency value of the drug can be effectively increased and the consumption of scientific research resources is lowered. The method is targeted, can remarkably reduce the blindness of a common method of evaluating the adverse reaction tendency value, provides evaluated drug safety risk warn for safe monitoring in a research process of the drug and after the drug is marketed, and provides pre-warning to adverse reactions of the drug, so as to improve the foreseeability and the medical safety level of drug monitoring, and provide reference to personal medication.
Owner:CHINA PHARM UNIV
Drug risk assessment method based on ADR monitoring report and outlier detection technology
InactiveCN111415758AMedical data miningDigital data information retrievalPattern recognitionCluster algorithm
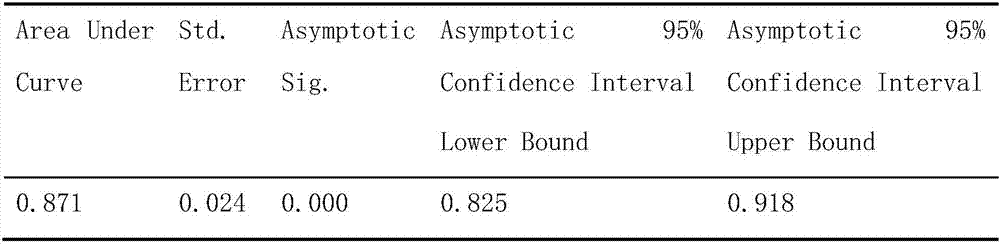
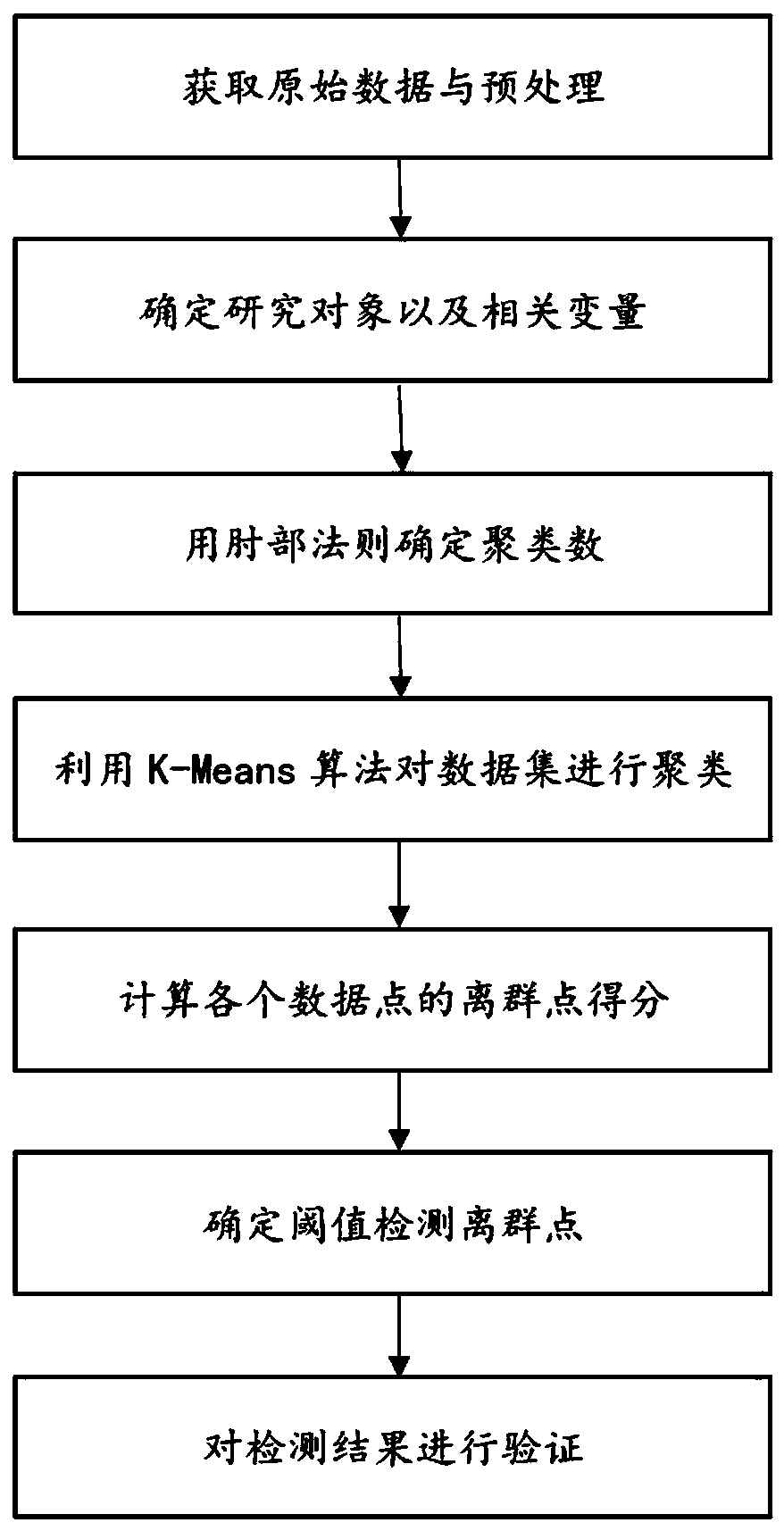
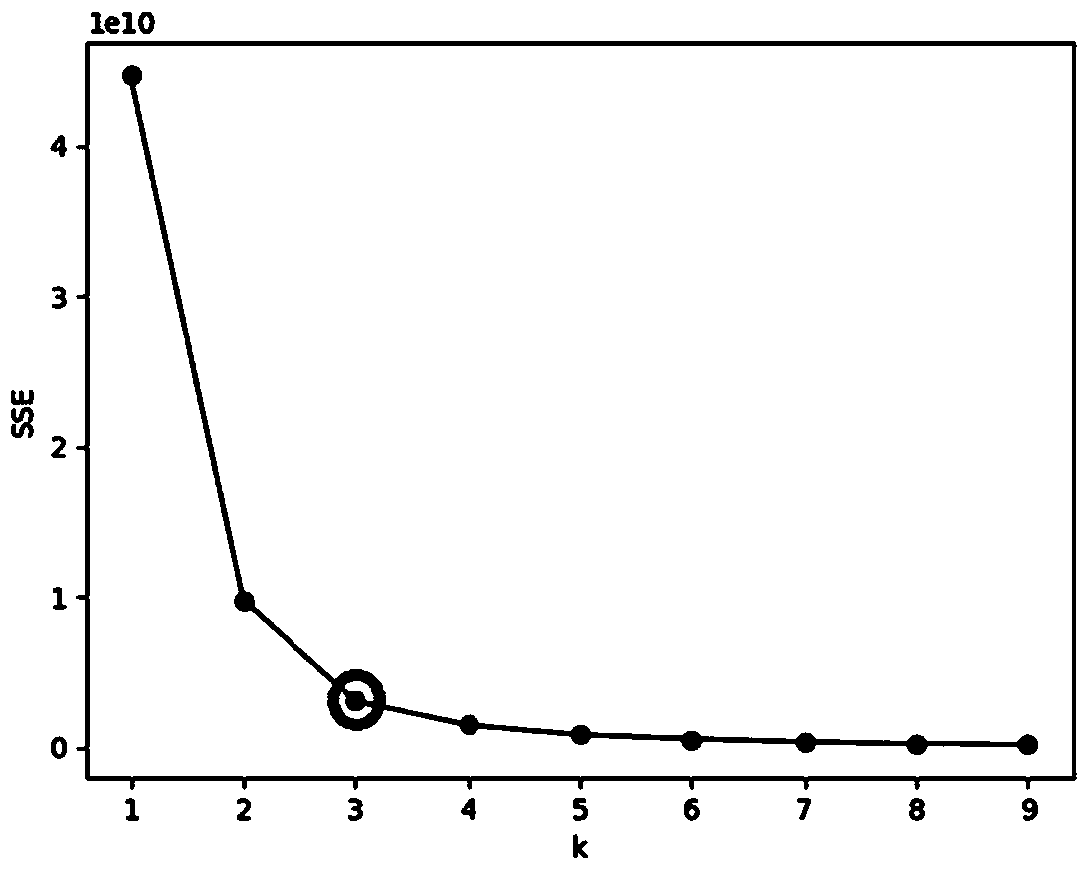
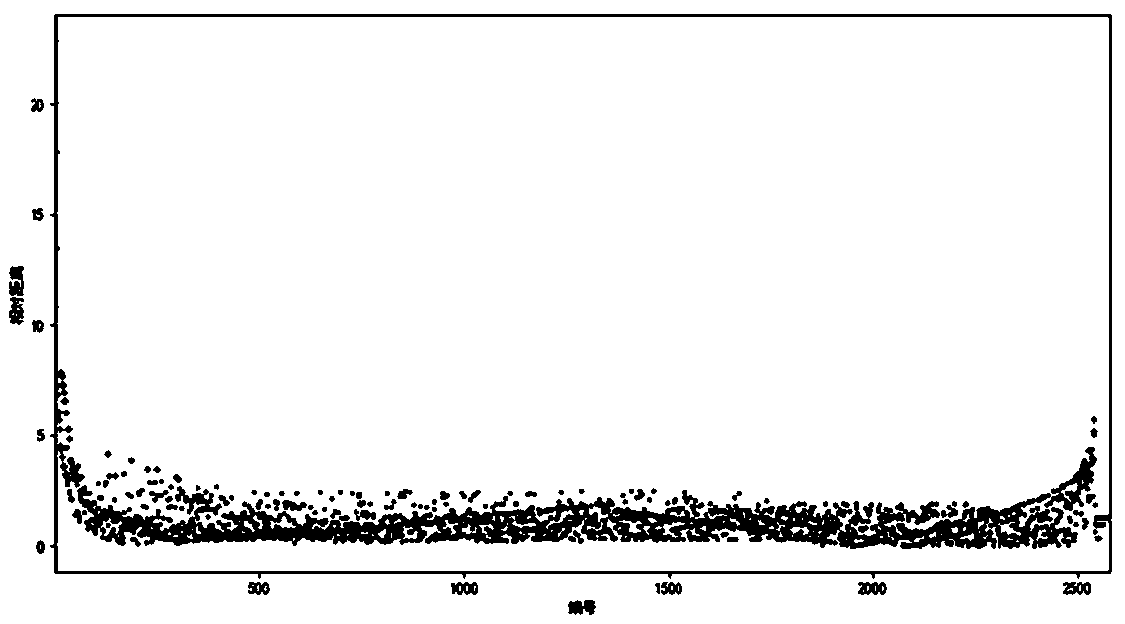
The invention provides a drug risk assessment method based on an ADR monitoring report and an outlier detection technology, and the method comprises the following steps: obtaining an original ADR database, and carrying out the normalization processing of drug names and adverse reaction names in the original database; determining outlier detection research objects and parameters: calculating a PRRvalue and an IC value of each drug-adverse reaction combination by adopting a proportional imbalance method, and constructing a spatial vector model by taking the PRR value and the IC value as features; determining a clustering number by utilizing an elbow rule, and finding a parameter k for minimizing a cost function; clustering by adopting a K-Means clustering algorithm, and randomly selecting kdata points as an initial centroid for clustering iteration according to a clustering number k determined by an elbow rule; calculating outlier scores of all the data points; and determining a threshold value and carrying out outlier detection on each cluster: setting the threshold value according to outlier score conditions, and determining points exceeding the threshold value as outliers. The invention provides an abnormal signal detection method for drug alert.
Owner:NANJING UNIV OF POSTS & TELECOMM
System and method for improving adverse reaction reporting rate of clinical drugs
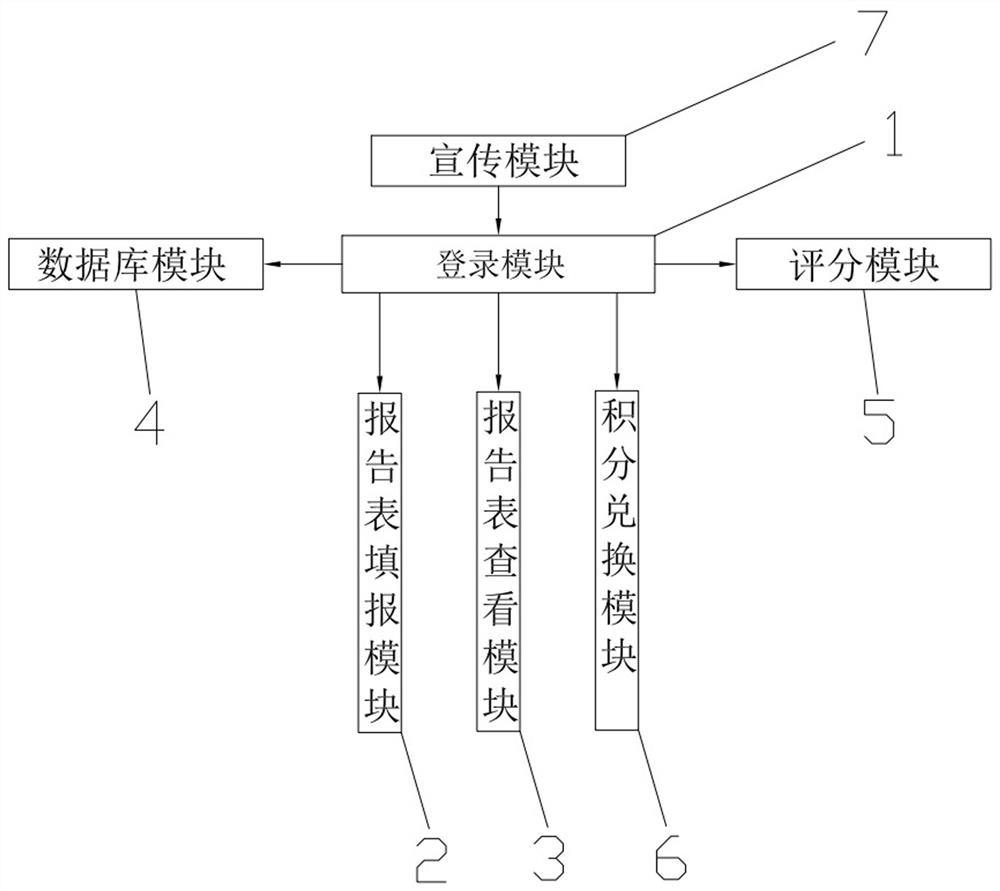
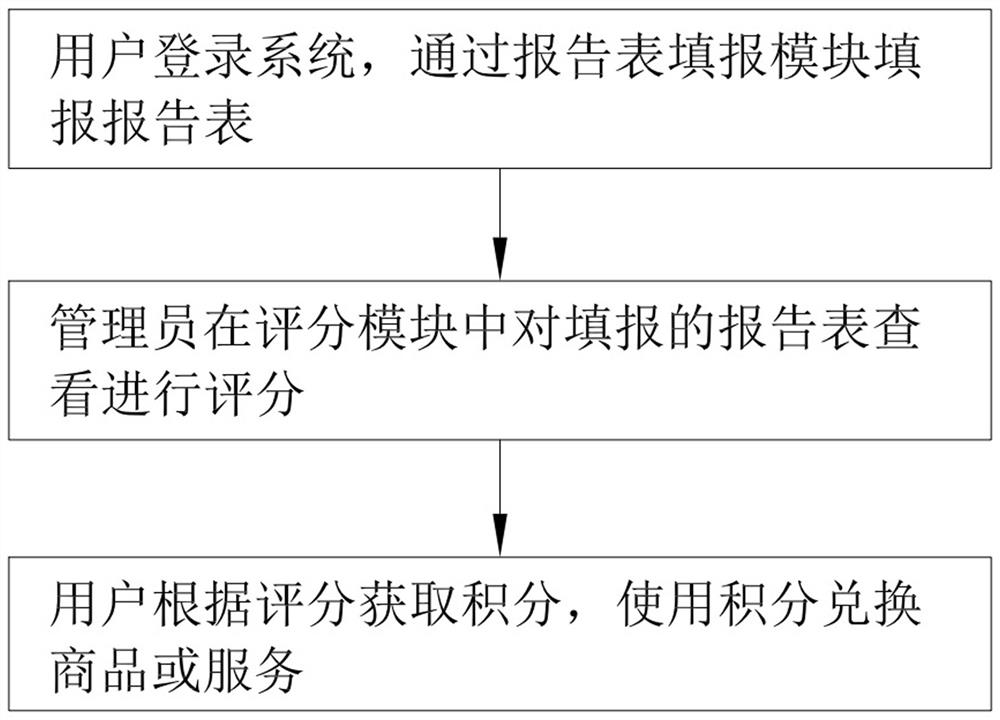
PendingCN112216403ANot very enthusiasticEnthusiasm to fill inDiscounts/incentivesDrug referencesData packAdverse drug reaction
The invention relates to the technical field of medical systems, in particular to a system and method for improving the adverse reaction report rate of clinical drugs. The system comprises a login module used for allowing a user to login the system, a report form filling module used for filling a report form after login, a report form viewing module used for viewing the filled report form, a database module used for storing related data including login information data and report table information data, a scoring module used for scoring the filled report form so as to enable a related user toobtain scores according to the scores and used for evaluating the filled report form and allowing the user to change the report form subsequently and obtain more points after the change, and a creditexchange module used for exchanging commodities or services by using the credits. The invention has the beneficial effects that by simplifying the process and awarding mechanism of the report form, the interest of the masses and all institutions in reporting the adverse reaction report form is stimulated, and the problem that the report missing rate of adverse reaction reports is high is solved.
Owner:WENZHOU PEOPLES HOSPITAL
Oxyntomodulin analogue
ActiveUS10479819B2No adverse eventsLong half-lifeCosmetic preparationsPeptide-nucleic acidsDiabetes mellitusAdverse drug reaction
Provided is an oxyntomodulin analogue. The analogue comprises GCGR and GLP-1R dual agonist activity, improved enzymolysis stability and biological activity, and no adverse reactions. The analogue can be used to prepare medication for treating hyperphagia, obesity and diabetes.
Owner:JIANG XIANXING
Patient medicine taking tracking management system, electronic medicine box and device
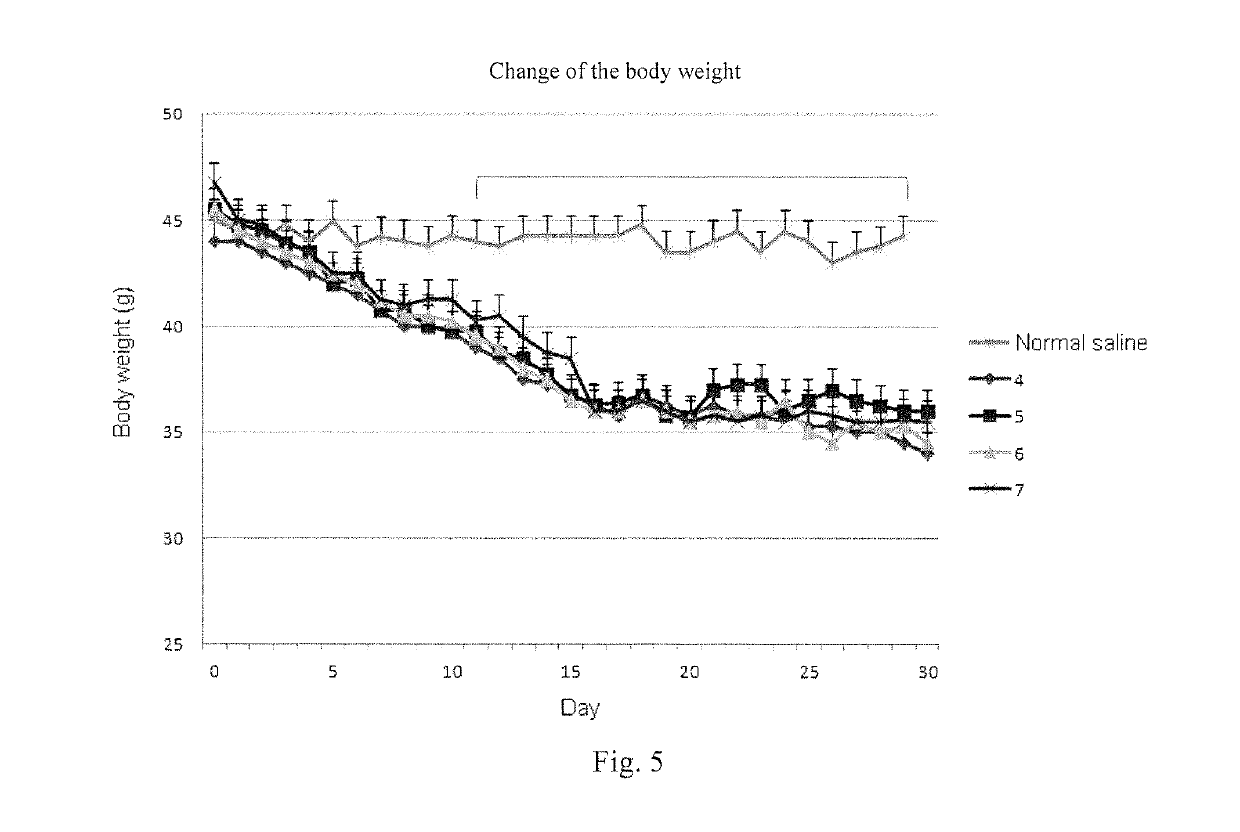
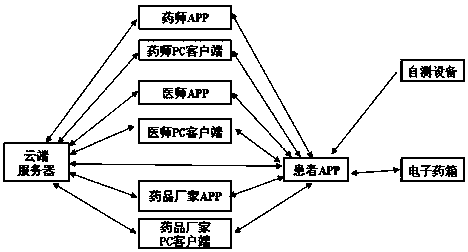
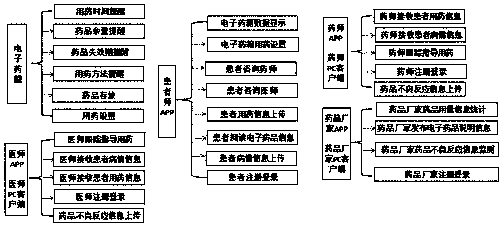
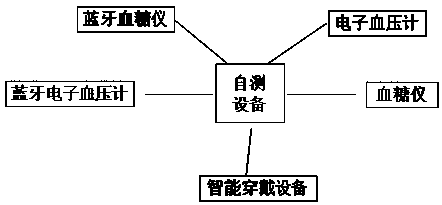
InactiveCN110339066AMedication on timeAccurate medicationOral administration deviceDiseaseTreatment effect
The invention provides a patient medicine taking tracking management system, an electronic medicine box and a device. The system is composed of a cloud server, a pharmacist APP, a pharmacist PC clientside, a physician APP, a physician PC client side, a medicine manufacturer APP, a medicine manufacturer PC client side, a patient APP, the electronic medicine box and self-examination equipment. Thepharmacist APP, the pharmacist PC client side, the physician APP, the physician PC client side, the medicine manufacturer APP, the medicine manufacturer PC client side and the patient APP exchange data information through the cloud server to achieve a patient medicine taking tracking management function. A patient can take medicines timely and correctly in the whole process, and the medicine taking compliance of the patient is improved; a physician and a pharmacist can track the diseases condition and medicine taking condition of the patient and dynamically grasp medicine taking information and disease condition information of the patient in real time, tracking and guiding services are provided for the patient in the whole process, and the treatment effect on diseases is improved; a medicine manufacturer can monitor adverse reaction information of medicines and count dosage information of the medicines, and a reference is provided for guiding the production of the medicines.
Owner:王晓利
Pharmaceutical composition for treating adverse reactions due to administration of spiegelmers
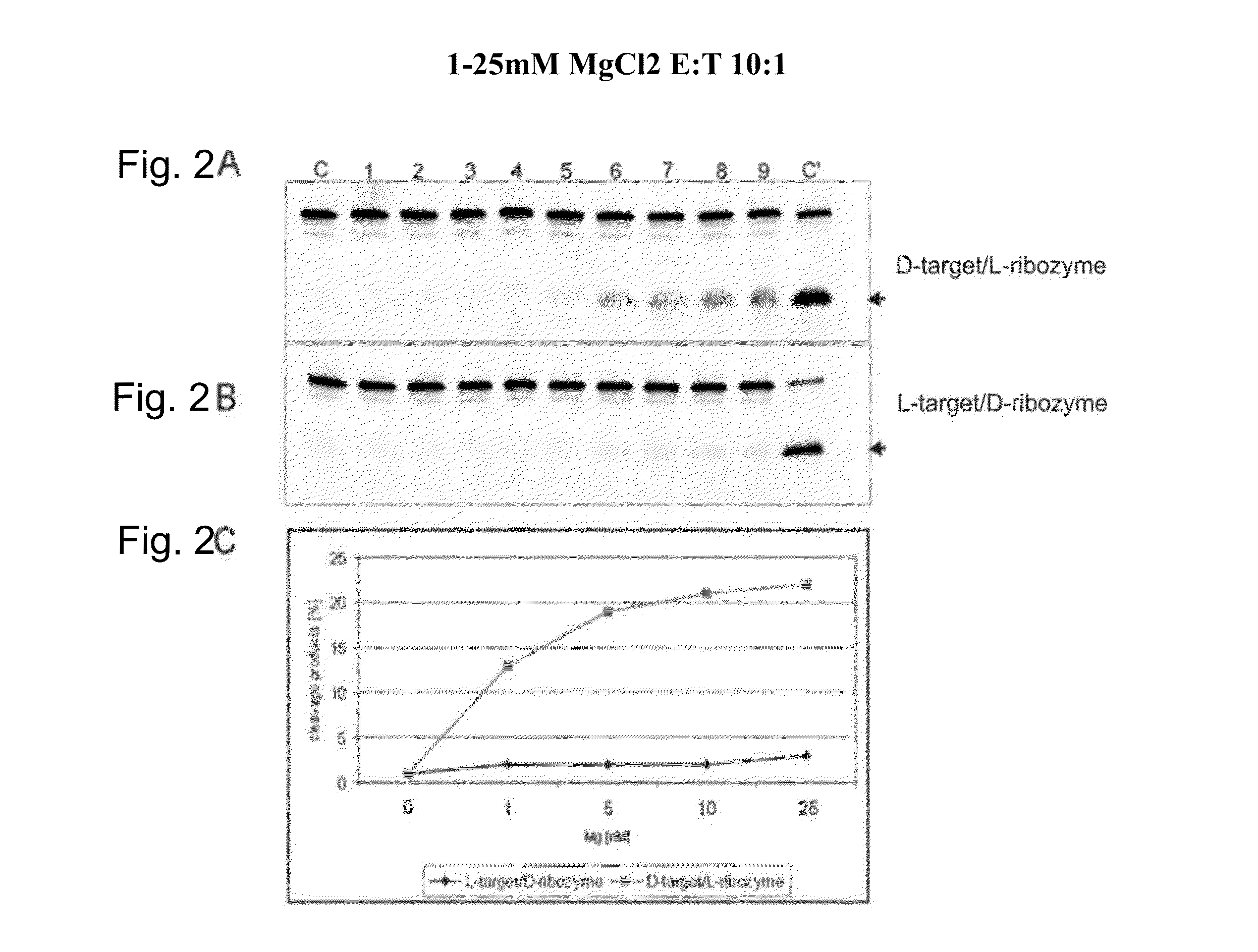
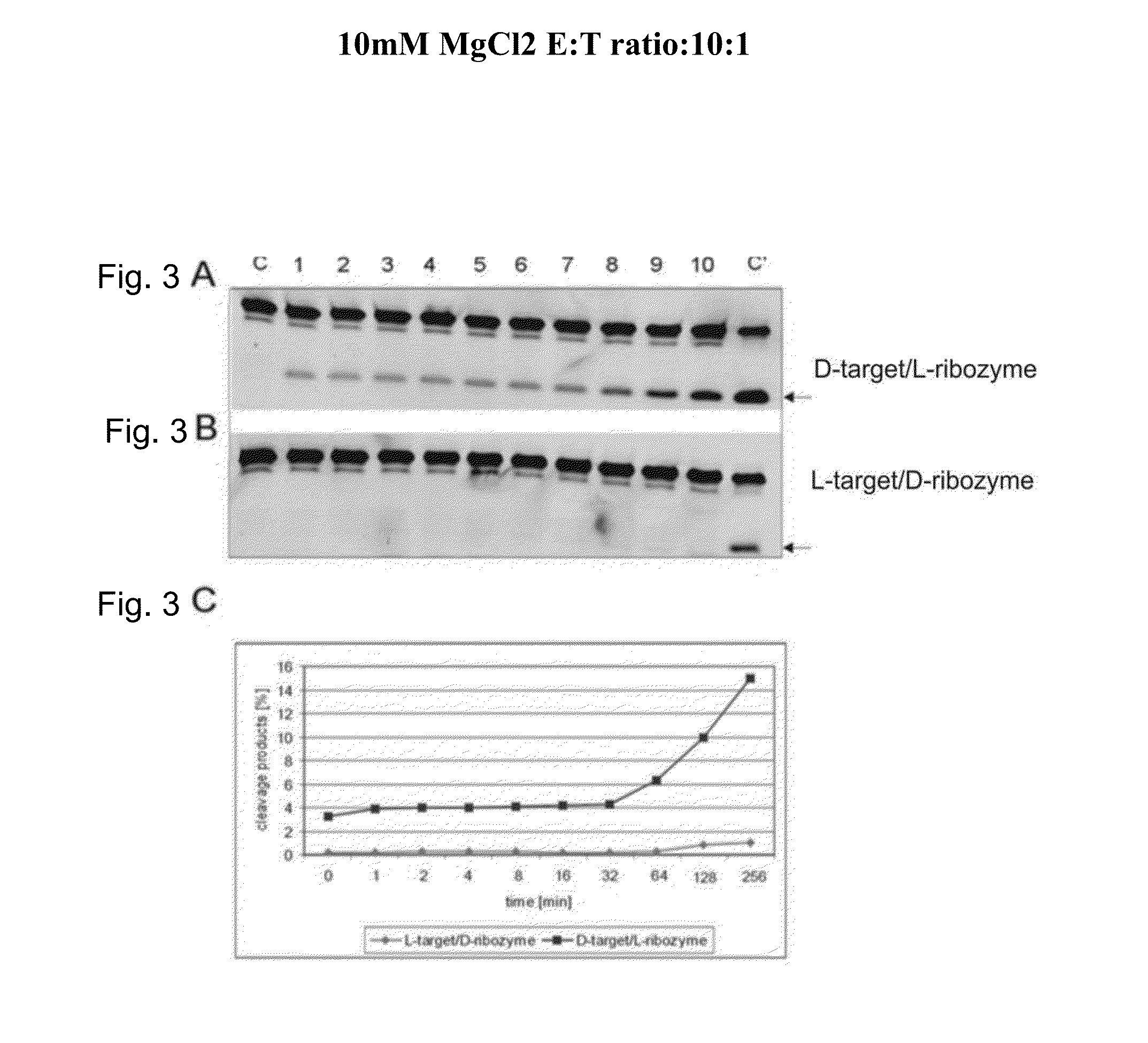
InactiveUS20120149763A1Strong specificityDestroy efficacyOrganic active ingredientsSugar derivativesAdverse drug reactionPharmacology
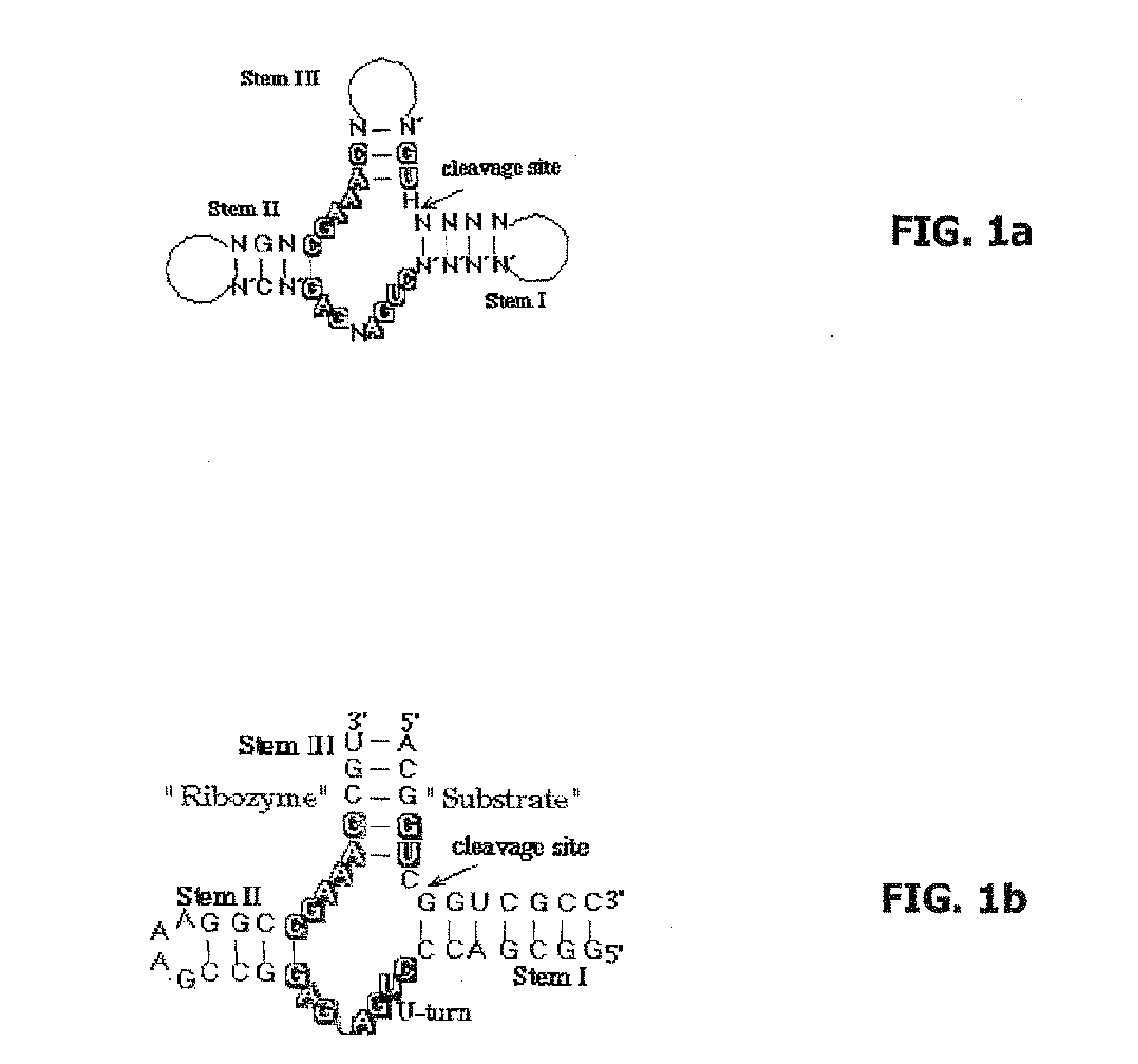
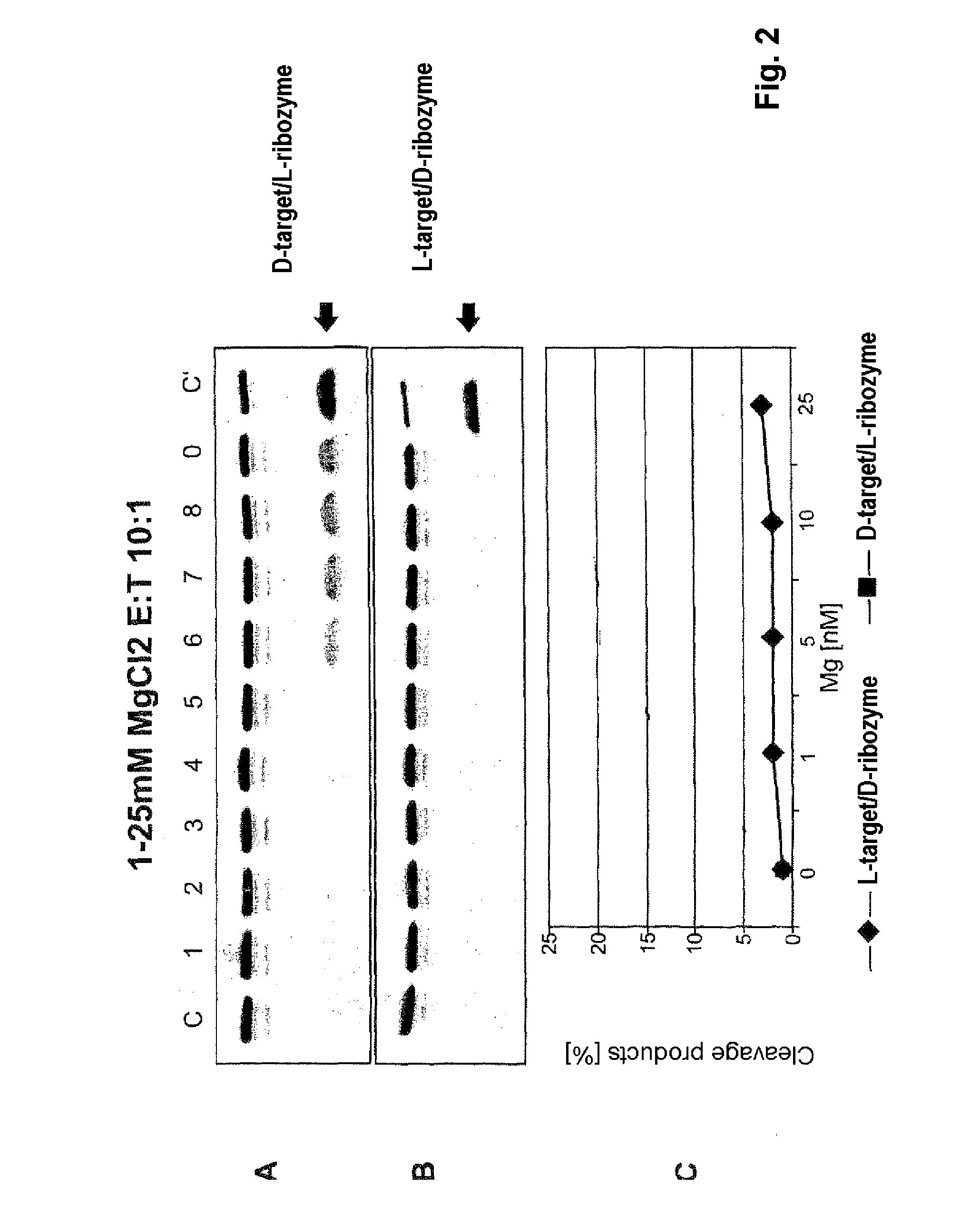
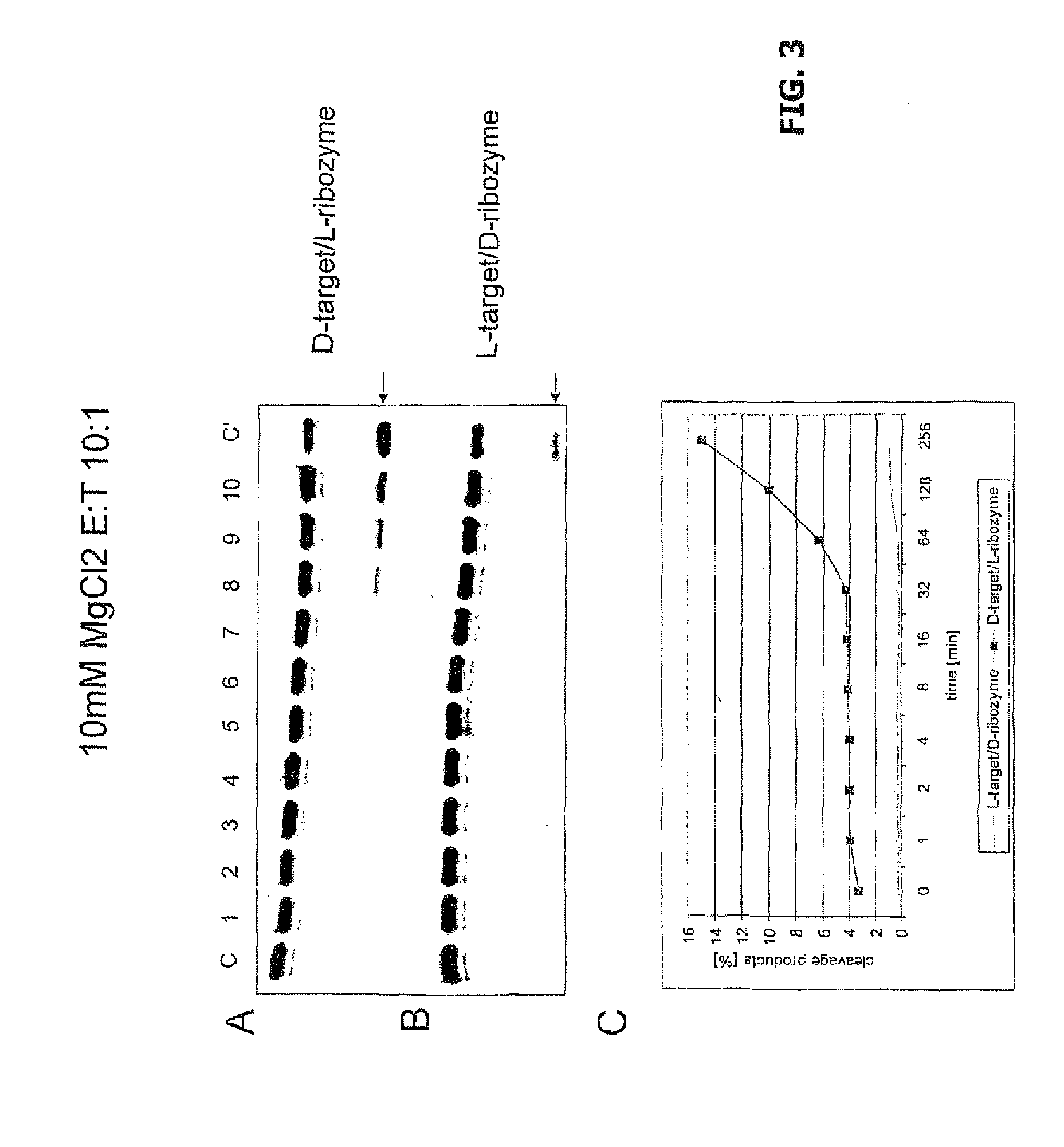
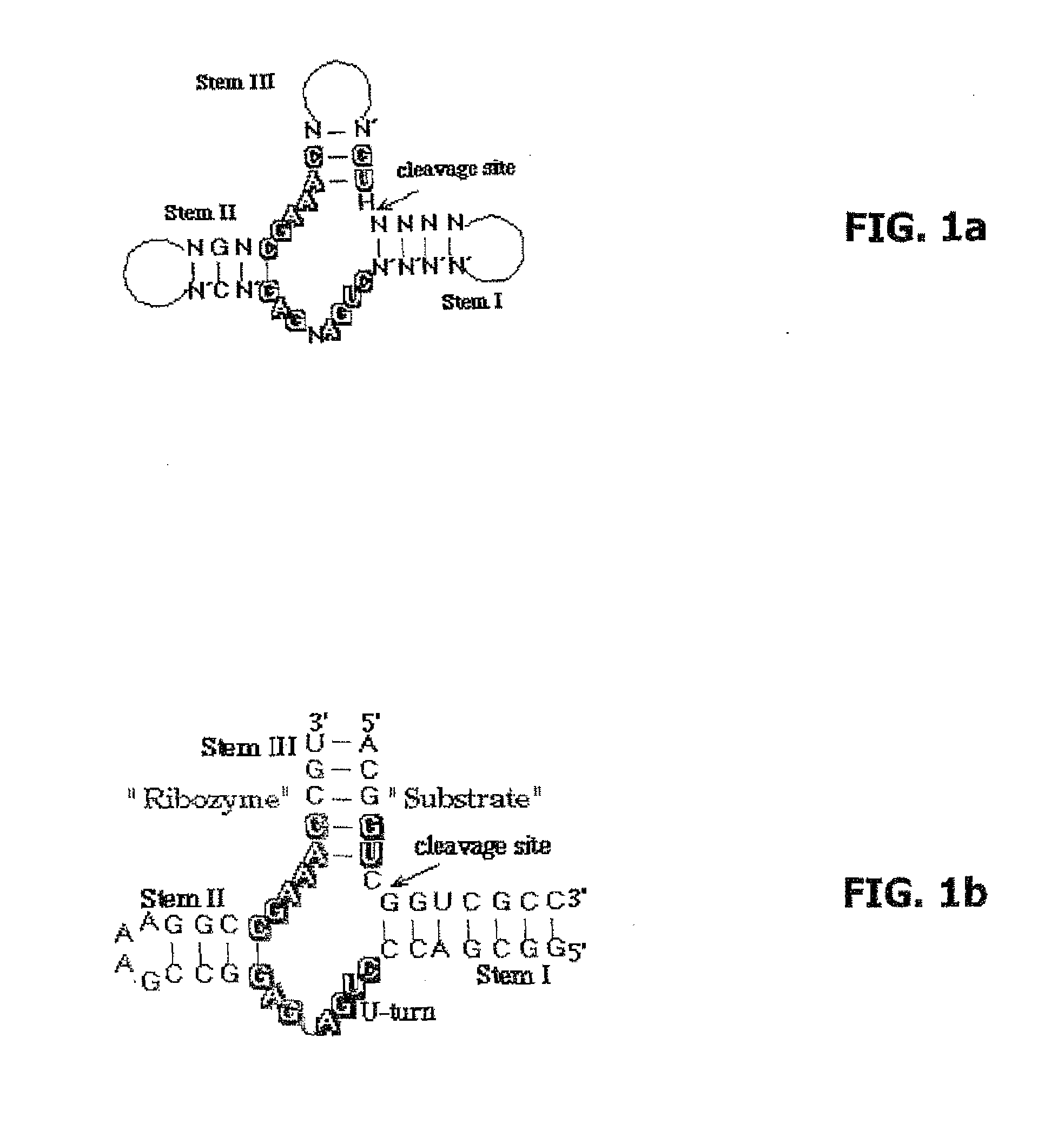
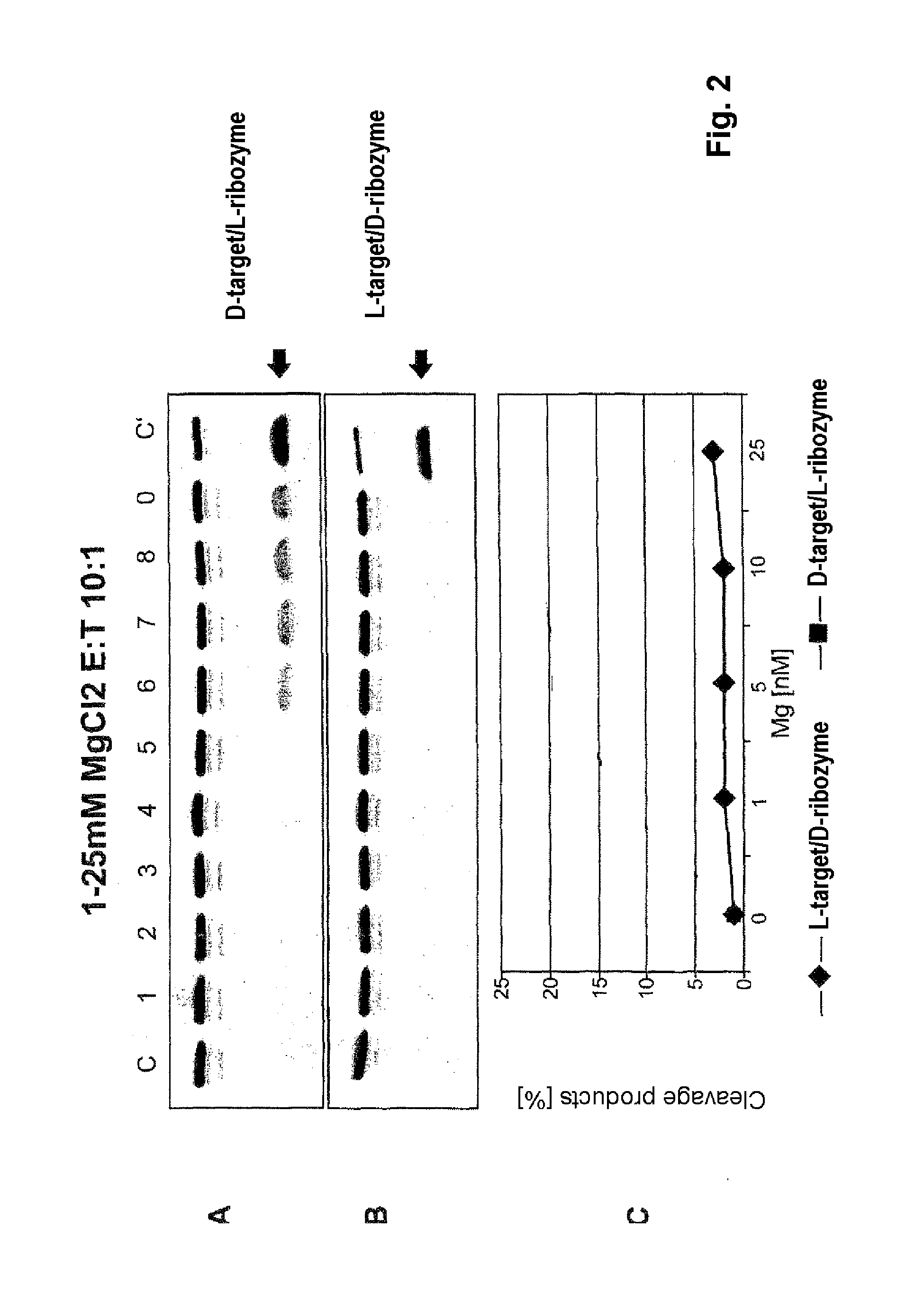
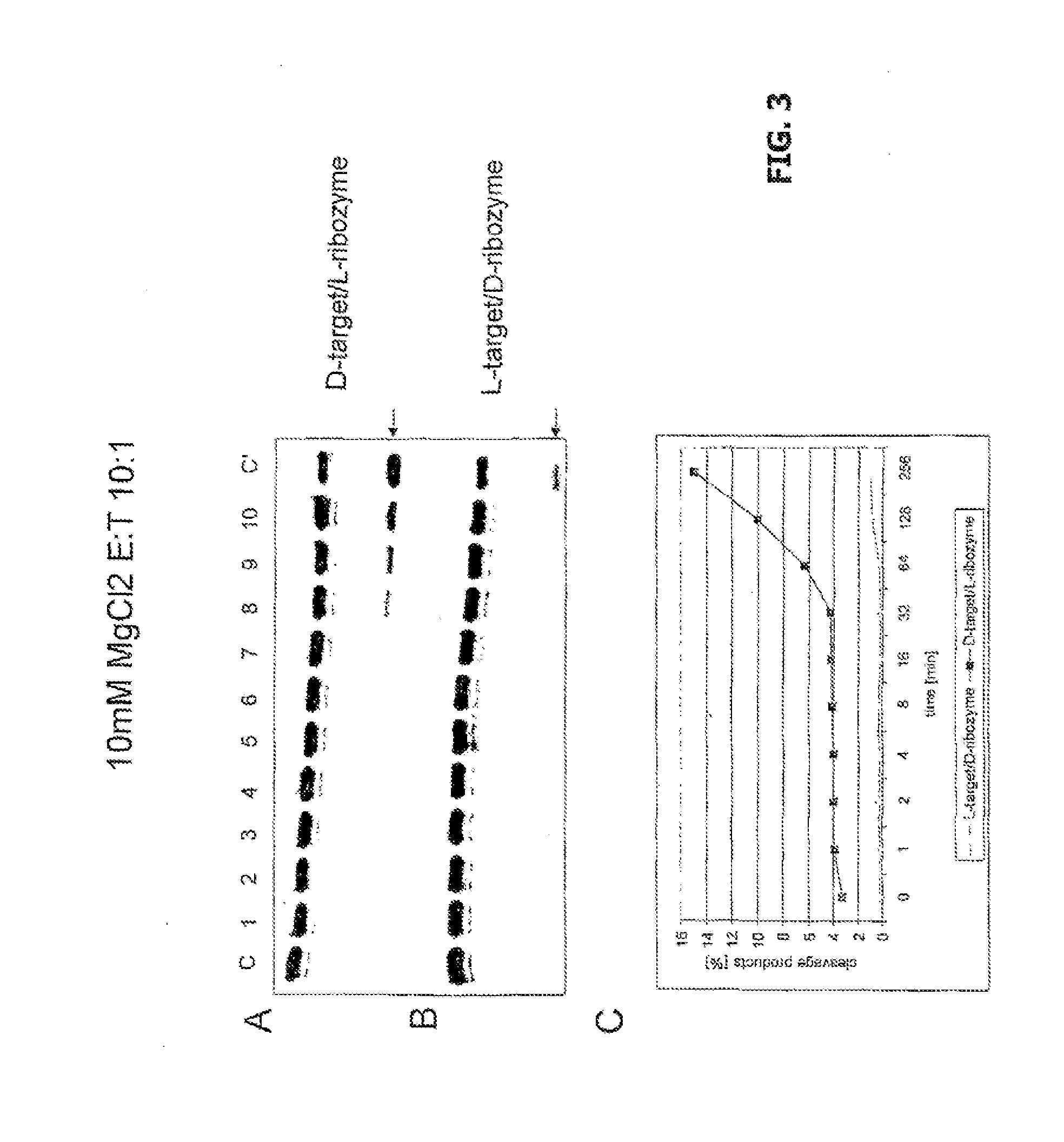
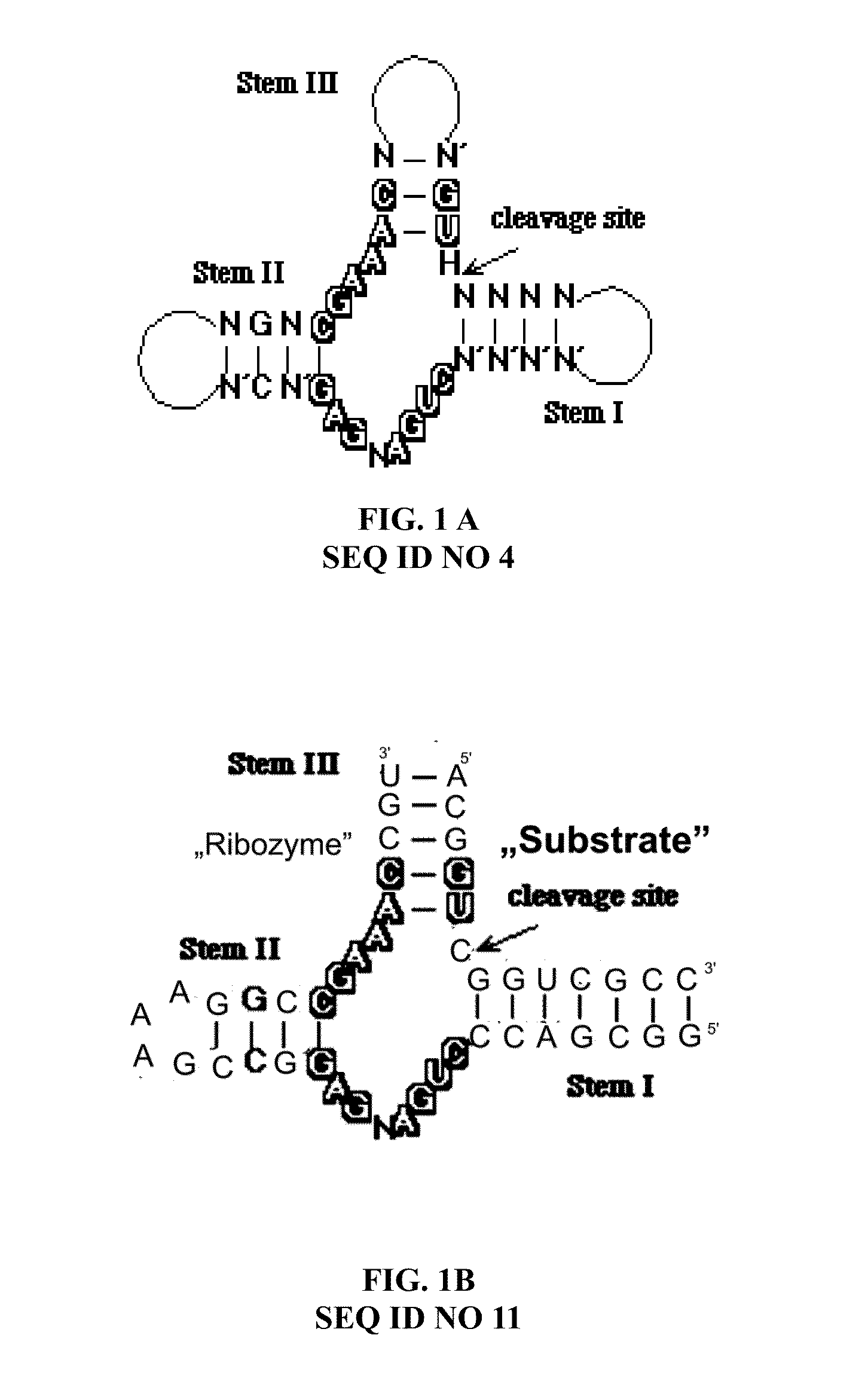
The invention relates to the use of an L-ribozyme, which is capable of splitting an L-RNA in the region of a target sequence of the L-RNA, in order to produce a pharmaceutical composition for treating undesired physiological adverse reactions due to the administration of a therapeutic molecule containing the L-RNA. Alternatively, an endogenous target RNA may also be split by the L-ribozyme.
Owner:FREE UNIV OF BERLIN
Application of polymorphism of retinoid X receptor alpha (RXRA) genes to adverse reactions of antituberculous drugs
The invention discloses application of polymorphism of retinoid X receptor alpha (RXRA) genes to adverse reactions of antituberculous drugs, and relates to the field of screening of adverse reactionsof tuberculous drugs. The invention discloses application of the polymorphism of the RXRA genes to tuberculosis treatment, it is found that through detection of the polymorphism of the RXRA genes, therisk of generating the adverse reactions after tuberculous patients are treated by the antituberculous drugs can be predicted, certain support is provided for avoiding the adverse reactions caused bythe related antituberculous drugs, and certain guidance is also provided for drug use of the tuberculous patients.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
A method of assessing the propensity of a drug to develop an adverse reaction
ActiveCN107145735BReduce consumptionReduce blindnessChemical property predictionDrug referencesAdverse drug reactionMedicine
The invention provides a method of evaluating tendency of adverse reactions of a drug. The method comprises the following steps of: excluding interference information according to structural characteristics of the drug and quantitative characteristics of an adverse reaction tendency value and establishing an evaluated quantitative relation model between the structural characteristics and the adverse reaction tendency value; and applying the model to evaluate safety of the drug, wherein the accuracy of evaluating the adverse reaction tendency value of the drug can be effectively increased and the consumption of scientific research resources is lowered. The method is targeted, can remarkably reduce the blindness of a common method of evaluating the adverse reaction tendency value, provides evaluated drug safety risk warn for safe monitoring in a research process of the drug and after the drug is marketed, and provides pre-warning to adverse reactions of the drug, so as to improve the foreseeability and the medical safety level of drug monitoring, and provide reference to personal medication.
Owner:CHINA PHARM UNIV
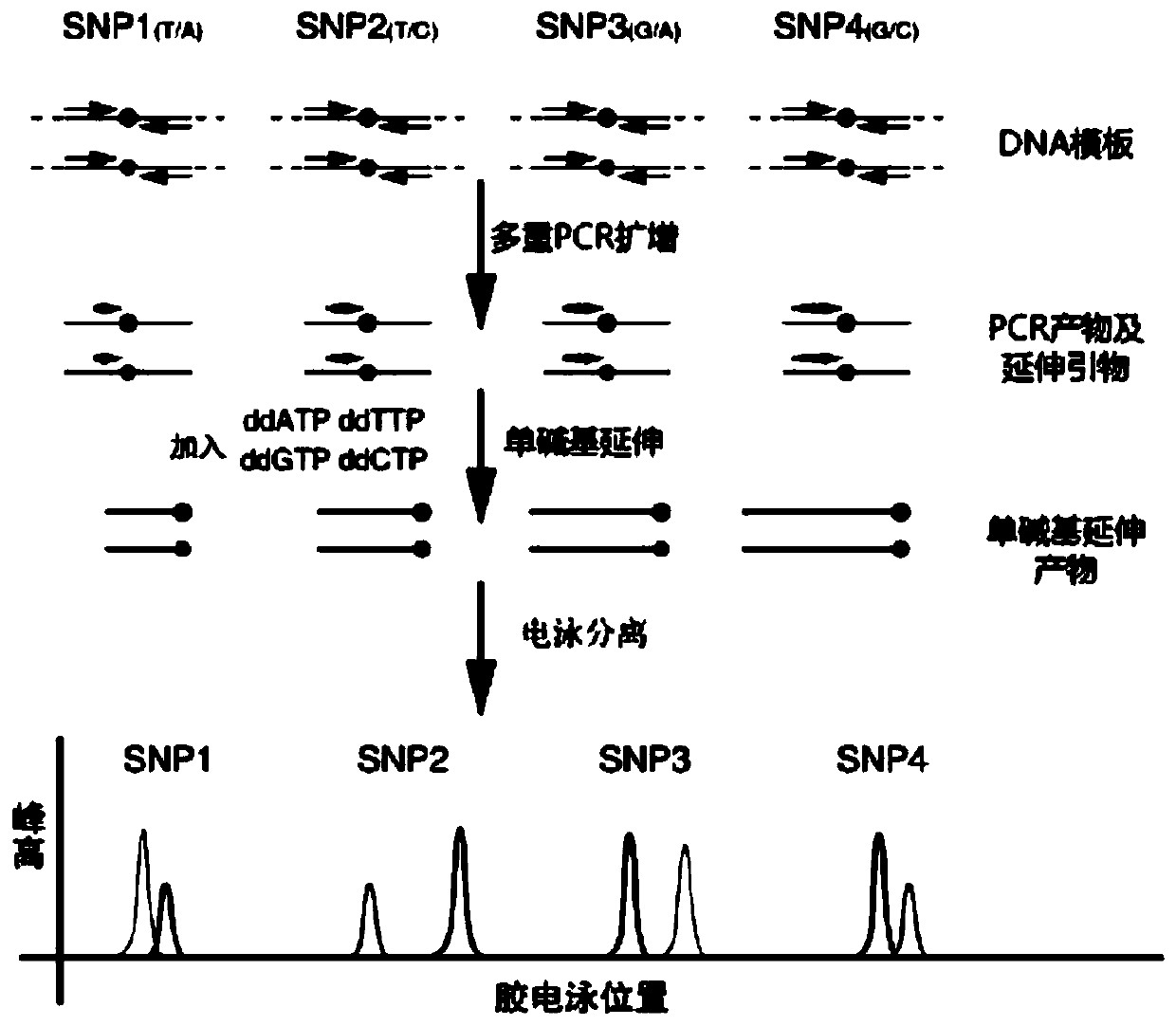
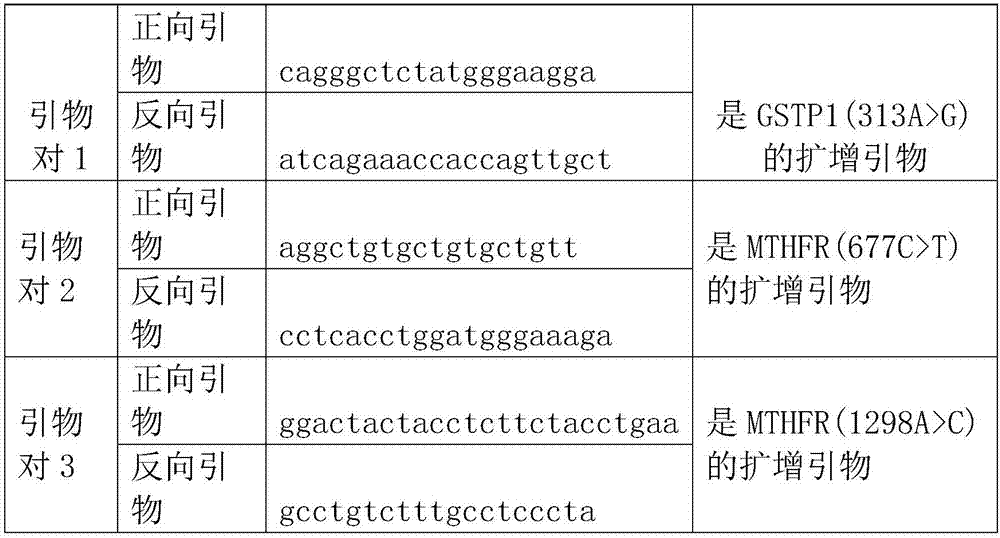
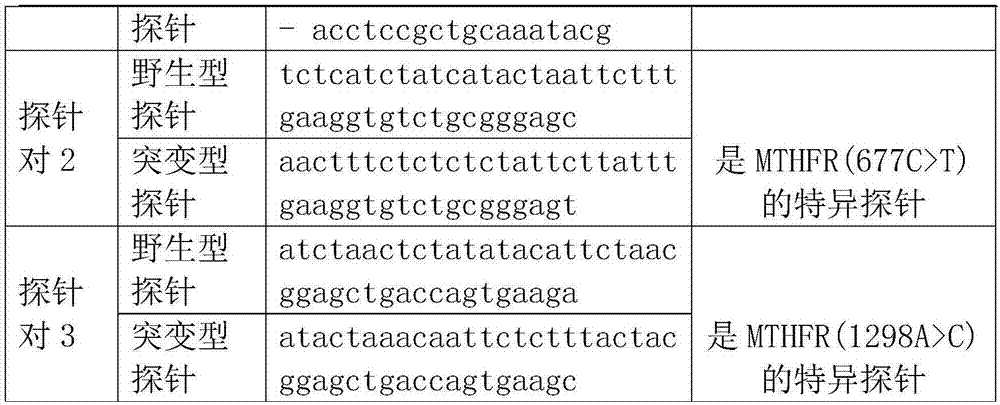
Method and kit for detecting SNP locus genotypes having adverse reactions to CTX drugs
InactiveCN108004306ASensitive detectionQuick checkMicrobiological testing/measurementMagnetic beadAdverse drug reaction
The invention discloses a method and kit for detecting SNP locus genotypes having adverse reactions to CTX drugs. The invention relates to the fields of molecular biology and medicine; according to the kit provided by the invention, aiming at SNP loci with cyclophosphamide adverse reaction risks and in human genomic DNA, namely a GSTP1 gene rs1695, specific primers and probes are designed, a hybridization reaction with MagPlex-TAG magnetic beads with corresponding report genes is performed, and detection is performed on a luminex 200 instrument through chromogenic reaction; a base type of eachSNP locus is judged through reading and interpretation of signal values. The kit has the beneficial effects that the specific primers, probes and report gene probe sequences are designed by using theSNP loci related to the adverse reaction risks of cyclophosphamide used by patients with systemic lupus erythematosus and other diseases, and the types of the SNP loci can be detected in a typing manner. The detection kit provided by the invention can detect the types of the SNP sensitively and quickly, and can provide reliable experimental evidence for predicting medication adverse reaction risks of the patients with systemic lupus erythematosus, and the clinical treatment selection is guided.
Owner:GUANGZHOU HEKANG MEDICAL TECH CO LTD
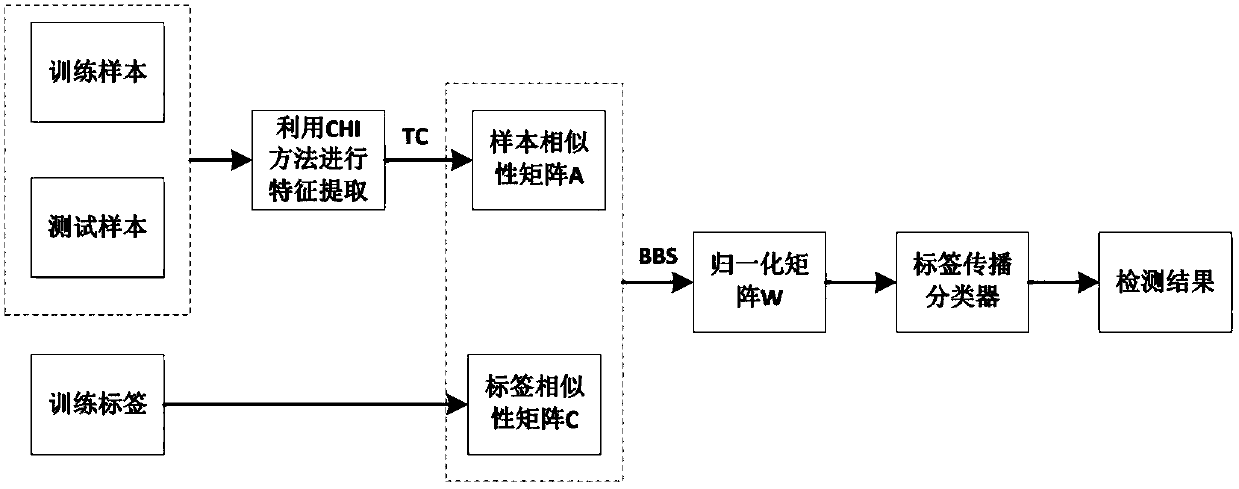
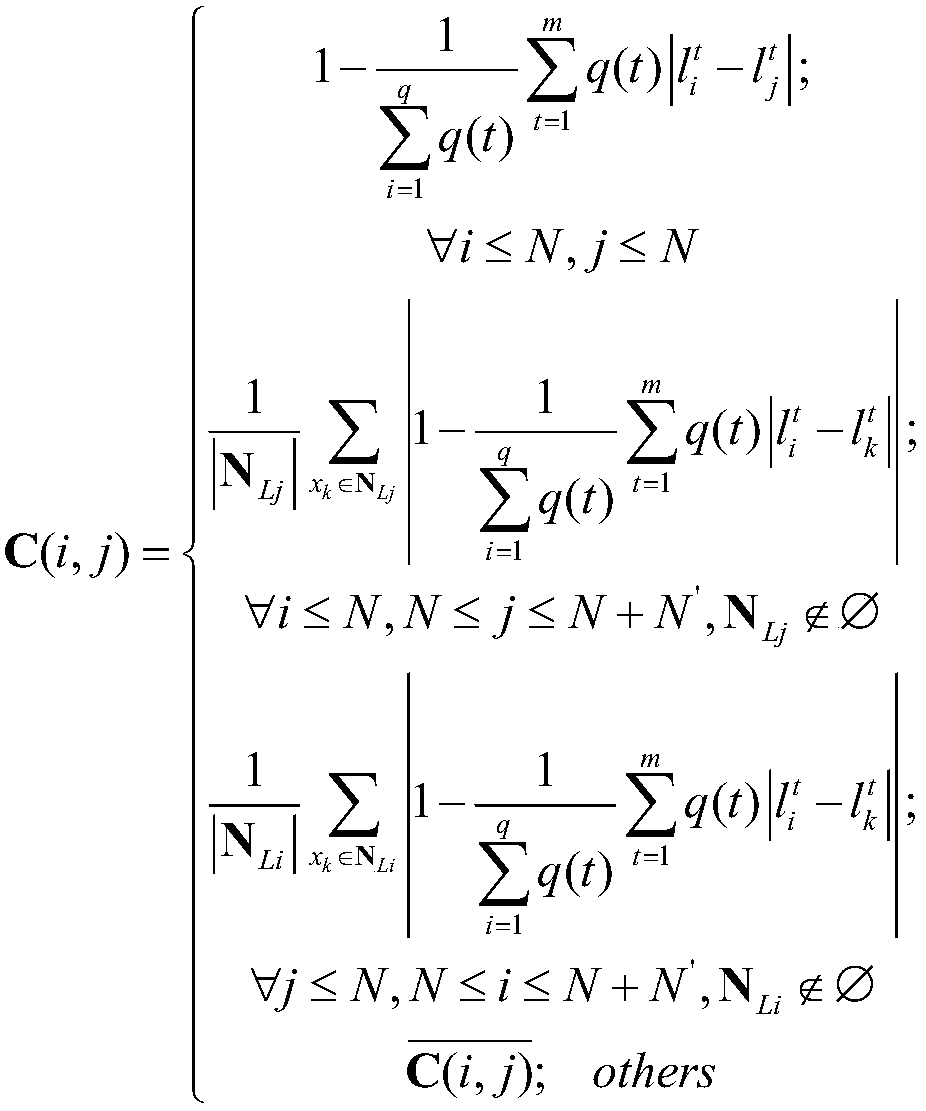
Clinical drug-drug adverse reaction detection method based on label propagation algorithm
ActiveCN108376567ADrug and medicationsCharacter and pattern recognitionDrug adverse reactionsAdverse drug reaction
The invention relates to a clinical drug-drug adverse reaction detection method based on a label propagation algorithm. The drug-drug adverse reaction is further detected based on novel similarity based on a given drug sample set and label initialization reconstitution of a label propagation mode. The method comprises the steps of firstly, filtering drug characteristics by adopting a CHI method, selecting the characteristic that the information amount is large; secondly, constructing a new sample similarity according to the sample label similarity and the sample similarity adjusted by the laplace operator; thirdly, establishing the initialization information of the unknown label sample based on the known label sample information; finally, detecting the adverse reaction of the drug throughlabel propagation. According to the method, the drug similarity calculation mode and the label propagation mode are reconstructed, so that the similarity between drugs is more accurate, the label propagation mode is smoother, and the detection of drug-drug adverse reactions in the clinical stage can be effectively improved.
Owner:DALIAN UNIV
Human leukocyte antigen gene detection kit for screening skin adverse reactions caused by sulfasalazine
ActiveCN104818316BBlocking interactionMicrobiological testing/measurementBiological testingMetaboliteAdverse drug reaction
The invention provides a human leukocyte antigen gene detection kit for screening skin drug adverse reaction caused by salazosulfapyridine, belongs to the technical field of biomedicine, and relates to a detection kit of human leukocyte antigen gene HLA-B*13:01, wherein the HLA-B*13:01 gene can be adopted as the marker gene of salazosulfapyridine-induced DRESS. According to the present invention, DNA is extracted from the peripheral blood of a patient, and the HLA-B*13:01 gene is detected by using the current method, such as PCR-SSO (sequence-specific oligonucleotide probe) method; and the HLA-B*13:01 gene detection kit of the present invention can be used for screening before the salazosulfapyridine use and screening the target drug for treatment of salazosulfapyridine-induced DRESS, wherein the screening before the salazosulfapyridine use can guide the clinical medication so as to reduce the occurrence of the salazosulfapyridine-induced DRESS, and the drug screening mainly act on the HLA-B*13:01 molecules in a targeting manner so as to block the interaction between the HLA-B*13:01 molecules and the salazosulfapyridine or the metabolites thereof in the pathogenesis.
Owner:FUDAN UNIV +1
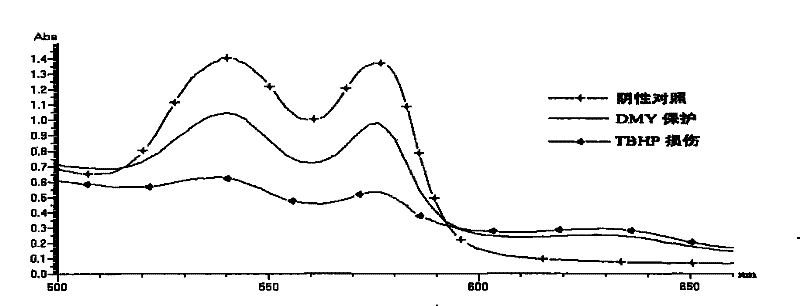
Application of dihydromyricetin in preparing medicament for preventing and treating adverse reaction of tumor chemoradiotherapy
InactiveCN101485655BDamage suppressionInhibition of poisoningOrganic active ingredientsAntinoxious agentsAdverse drug reactionOncology
Owner:SOUTH CHINA UNIV OF TECH
Pharmaceutical composition for treating adverse reactions due to administration of spiegelmers
InactiveUS20130237591A1Destroy efficacyEffectively and highly selectivelyOrganic active ingredientsSugar derivativesAdverse drug reactionRibozyme
The invention relates to the use of an L-ribozyme, which is capable of splitting an L-RNA in the region of a target sequence of the L-RNA, in order to produce a pharmaceutical composition for trating undesired physiological adverse reactions due to the administration of a therapeautic modecule containing the L-RNA. Alternatively, an endogeneous target RNA may also be split by the L-ribozyme.
Owner:FREE UNIV OF BERLIN
Pharmaceutical composition for treating adverse reactions due to administration of spiegelmers
InactiveUS20150140020A1Destroy efficacyEffectively and highly selectivelyOrganic active ingredientsSugar derivativesAdverse drug reactionRibozyme
The invention relates to the use of an L-ribozyme, which is capable of splitting an L-RNA in the region of a target sequence of the L-RNA, in order to produce a pharmaceutical composition for trating undesired physiological adverse reactions due to the administration of a therapeautic modecule containing the L-RNA. Alternatively, an endogeneous target RNA may also be split by the L-ribozyme.
Owner:FREE UNIV OF BERLIN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com