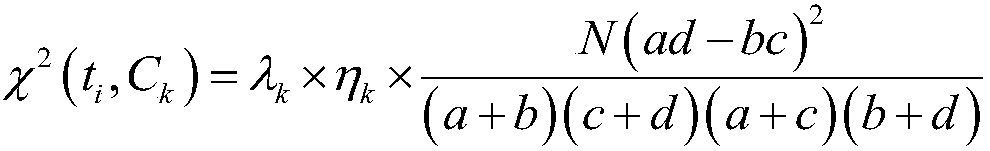Clinical drug-drug adverse reaction detection method based on label propagation algorithm
A label propagation algorithm and adverse reaction technology, applied in the field of clinical drug-adverse drug reaction detection based on label propagation algorithm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
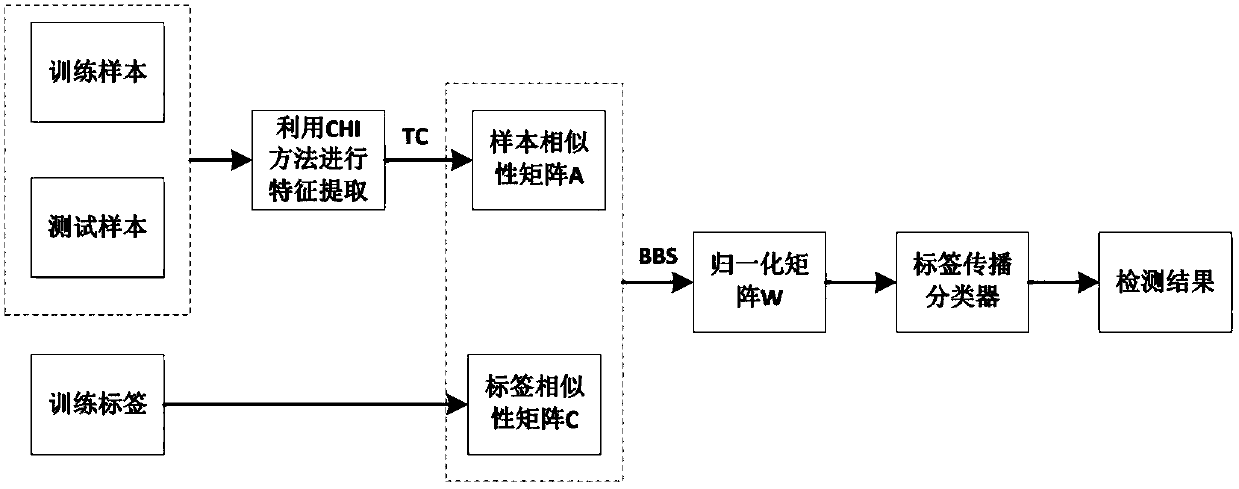
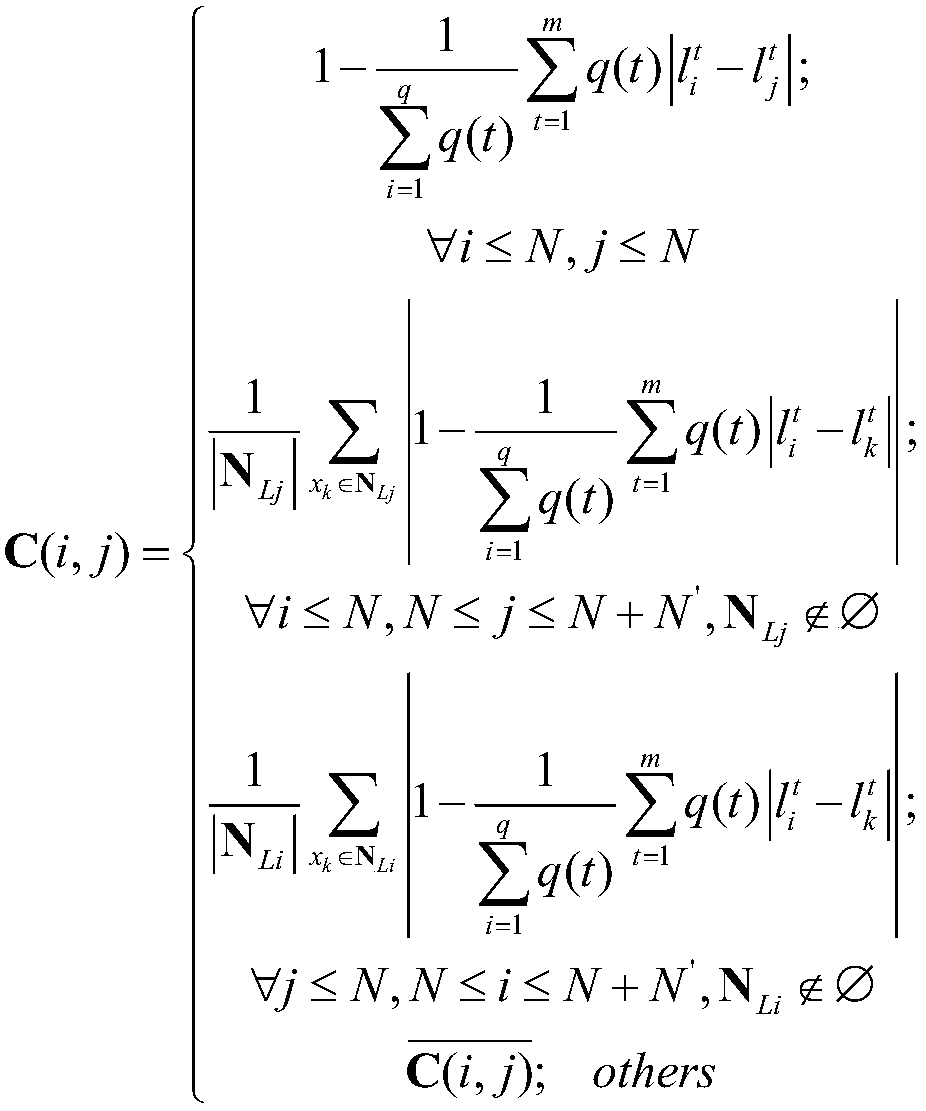
[0032] Such as figure 1 As shown, in order to achieve the improvement of the theoretical label propagation algorithm of the present invention and the purpose of effective experimental drug detection, firstly, obtain the drug data set, use the CHI feature extraction method to filter the sample features in the drug sample data set, and select The feature with a large amount of information; secondly, the Laplacian algorithm is used to improve the Jaccard correlation coefficient (TC) method to calculate the sample similarity of the drug, and calculate the label similarity of the drug according to the label similarity method, according to the drug The sample similarity of the drug and the label similarity of the drug are used to reconstruct the similarity of the drug; then the BBS algorithm is used to normalize the similarity matrix of the drug to obtain the similar normalization matrix of the drug; finally, the label information of the training drug is initialized The label inform...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com