Patents
Literature
102 results about "Drug adverse reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oligonucleotide for detecting cytochrome P450 enzyme series mutation site and gene chip
InactiveCN101054601AGood practical valueMicrobiological testing/measurementGenotypeCytochrome p450 enzyme
The present invention provides a set of oligonucleotide probe for detecting CYP450 enzyme gene hot mutant site and its uses, belonging to clinical molecular diagnosis field. The probe is designed at whole gene sequence of each subtype enzyme of CYP450 and has relative high sensitivity and specifity. The present invention also provides a gene chip for detecting cytochrome P450 enzyme series mutant sites and can detect gene typing of DNA specimen. The inventive probe can be used in P450 genetype diagnosi, clinical medicament and preventing drug adverse reaction.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Kukoline intravenous transfusion preparation
ActiveCN101347408AOvercome the bias that IV route of administration is not possibleSolve the clinical problems prone to anaphylactic shockOrganic active ingredientsAntipyreticClinical efficacyRheumatism
The invention provides a sinomenine injection special for intravenous administration. The sinomenine injection is an injection which comprises 0.005-0.3wt% of sinomenine and an aqueous solvent for injection, or an injection which comprises sterile injection powder or lyophilized injection powder used for preparation just before injection to cause the sinomenine concentration to be 0.005-0.3wt% in the injection and the aqueous solvent for injection. The sinomenine injection of the invention is used for treating rheumatism, chronic pain and other chronic inflammatory diseases. Compared with other existing injection forms, the sinomenine injection special for intravenous injection has lower drug adverse reaction, and the clinical curative effect is obviously improved.
Owner:李蕴麟
Method and system for registration, identifying and processing of drug specific data
InactiveUS20020095261A1Increase probabilityEasy to useBiostatisticsProteomicsDrug specific IgEScreening method
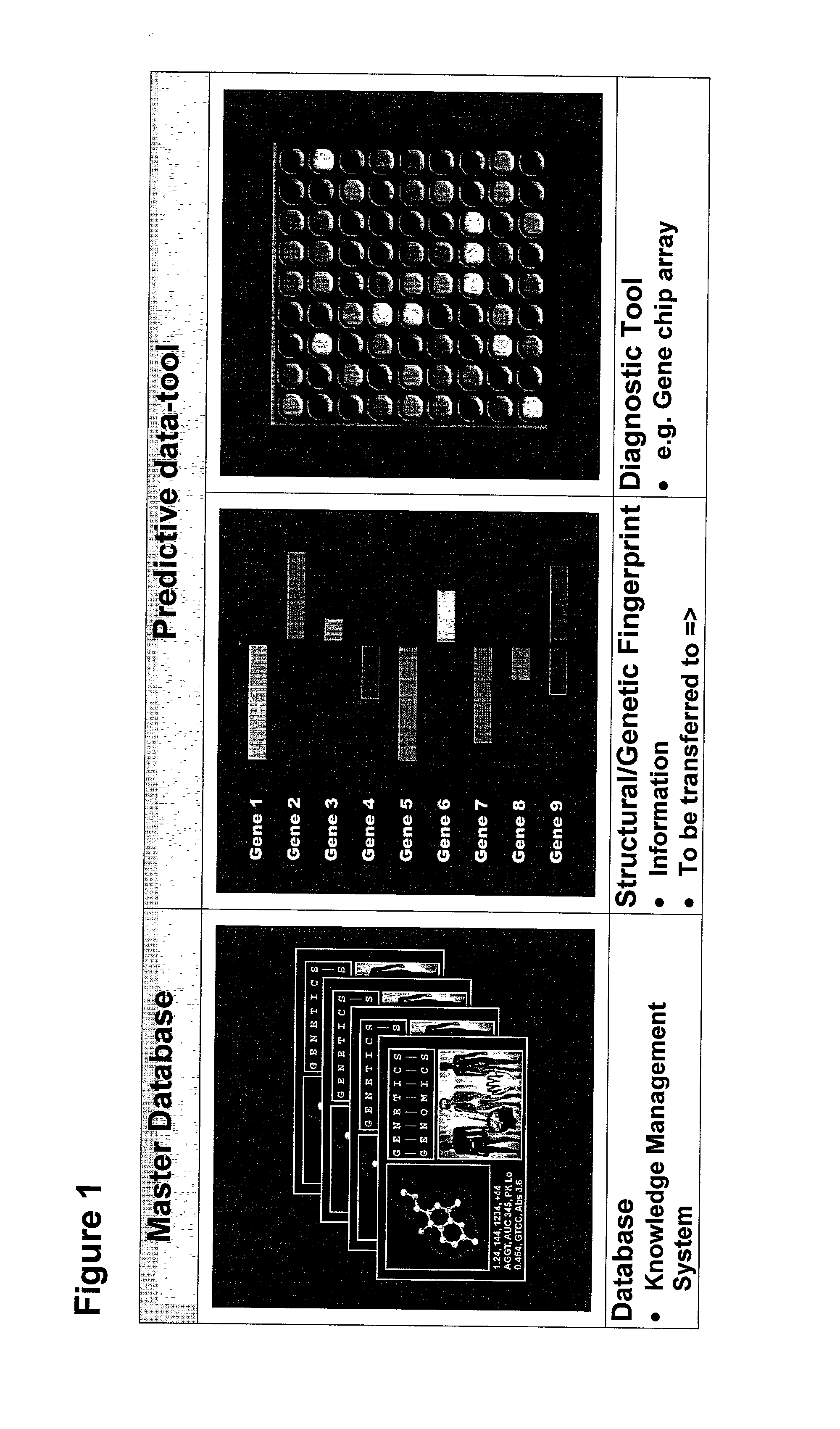
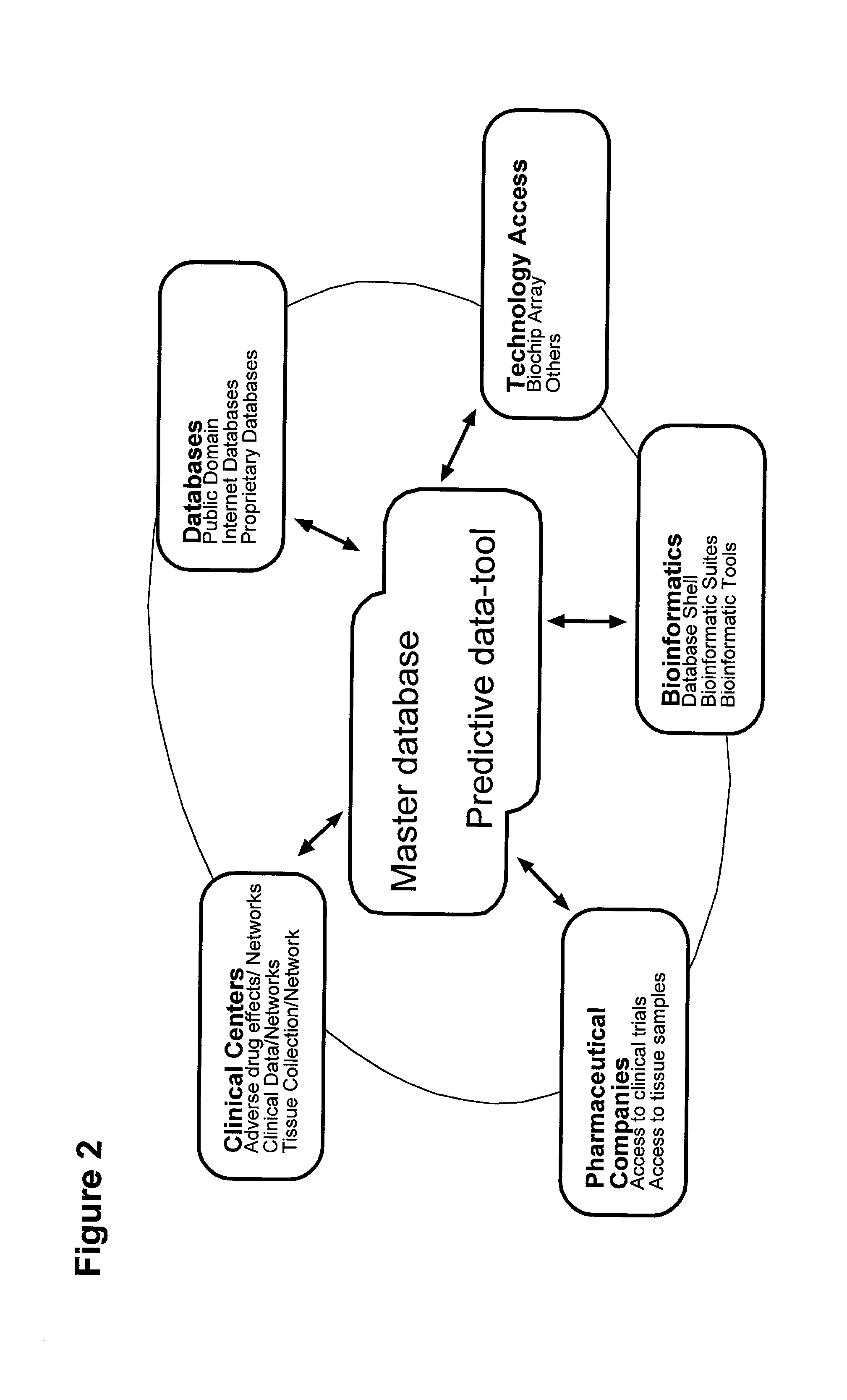
The invention relates to a method for registration, identifying and processing of drug specific data and for making drugs available to individual patients without severe adverse drug reactions. The invention relates also to a system for carrying out such a method. According to the present invention, the system comprises a master database correlating patterns of gene expression and genetic polymorphisms with drug-induced i.e. drug-related adverse effects and drug structure. The system also comprises a predictive data-tool. In this tool the structural and genetic fingerprints predictive for adverse effects in individual patients due to treatment with a selected drug are stored. The method according the invention is characterized in that the master database being coupled to the database of the predictive data-tool in such a way that a user of the system can develop and carry out different screening approaches either to verify the sociability of drugs for a specific selected category of patients or to search a specific drug for a selected category of patients which do not have adverse drug reactions or to make risk-analyses.
Owner:THERASTRAT
17BetaHSD TYPE 5 INHIBITOR
InactiveUS20090181960A1Suppress synthesisSuppress intracrine androgen synthesisBiocideOrganic chemistrySelective inhibitionBenign prostatic hyperplasia (BPH)
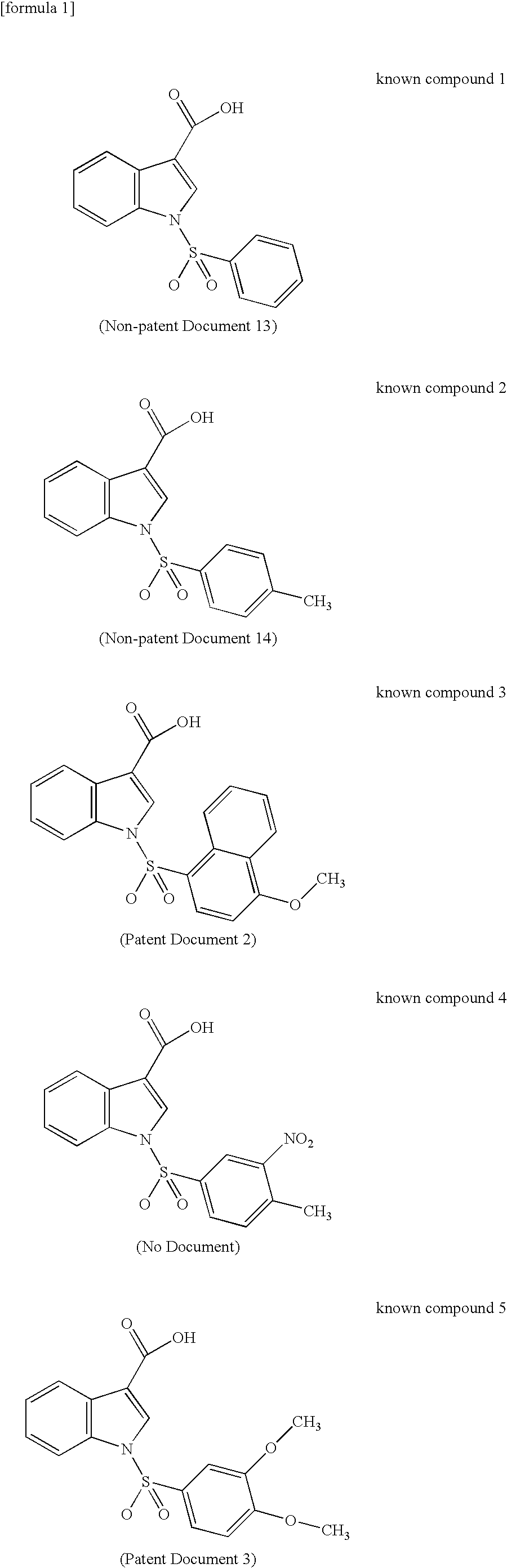
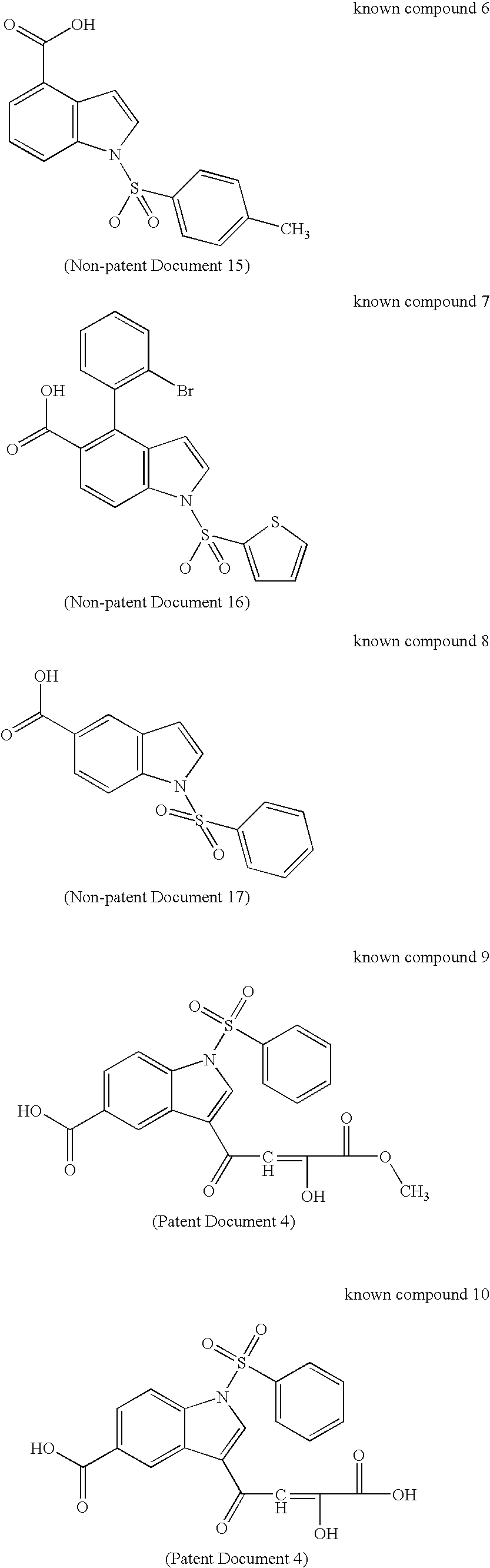
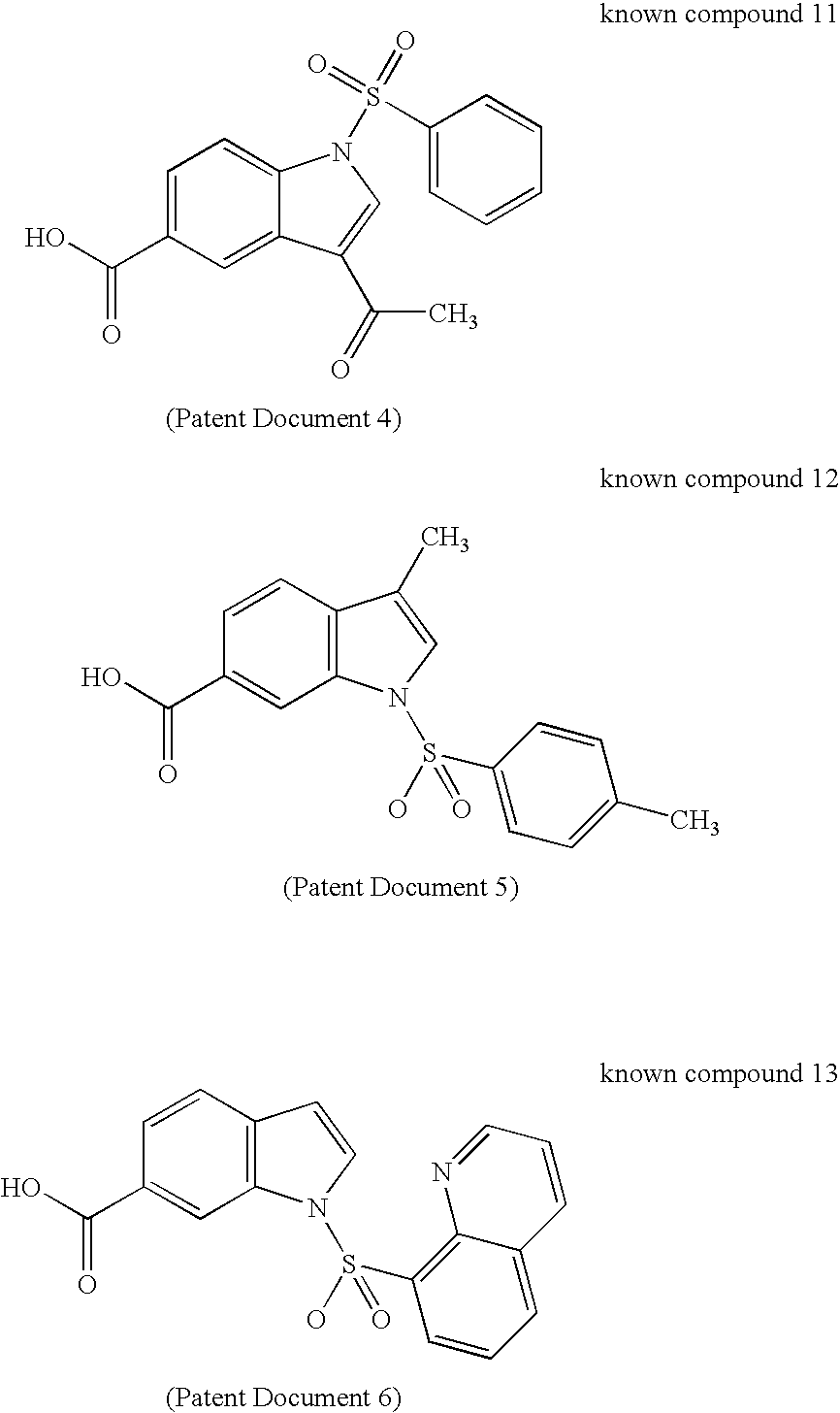
To provide a novel and excellent method for treating and / or preventing prostatic cancer, benign prostatic hyperplasia, acne, seborrhea, hirsutism, baldness, alopecia, precocious puberty, adrenal hypertrophy, polycystic ovary syndrome, breast cancer, lung cancer, endometriosis, leiomyoma and the like based on selective inhibitory activity against 17βHSD type 5.It was found that an N-sulfonylindole derivative, where the indole ring is substituted by a carboxy group, a carboxy-substituted lower alkyl group or a carboxy-substituted lower alkenyl group at its carbon atom, has potent selective inhibitory activity against 17βHSD type 5 and may become a therapeutic agent and / or preventive agent for benign prostatic hyperplasia, prostatic cancer and the like without accompanying adverse drug reactions due to a decrease in testosterone, and the present invention has thus been completed.
Owner:ASTELLAS PHARMA INC
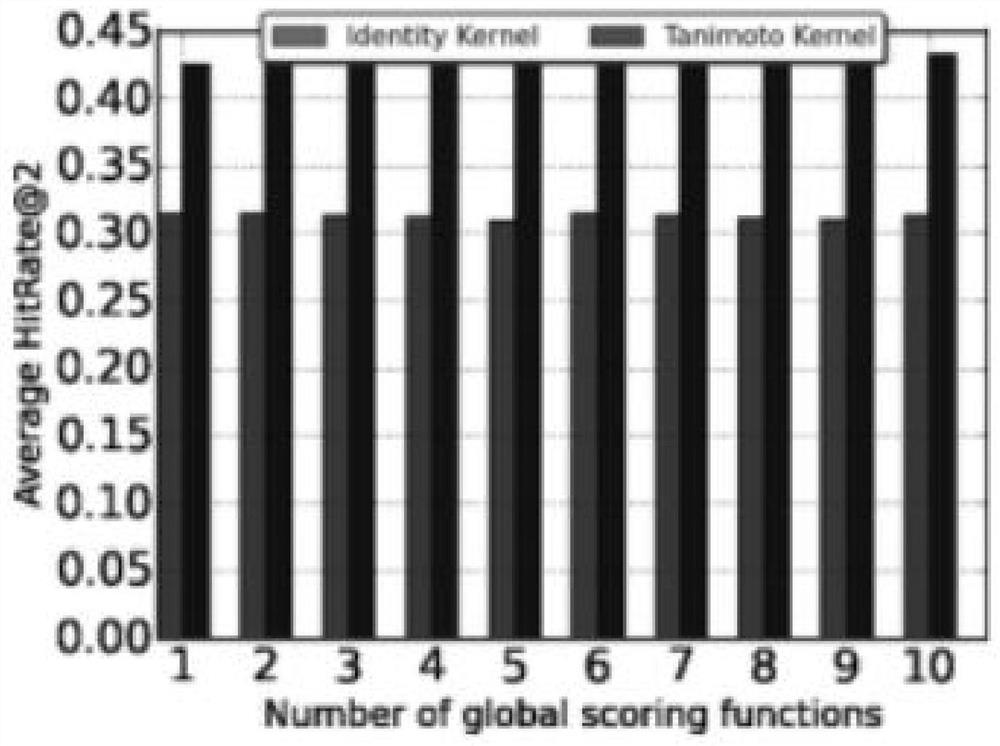
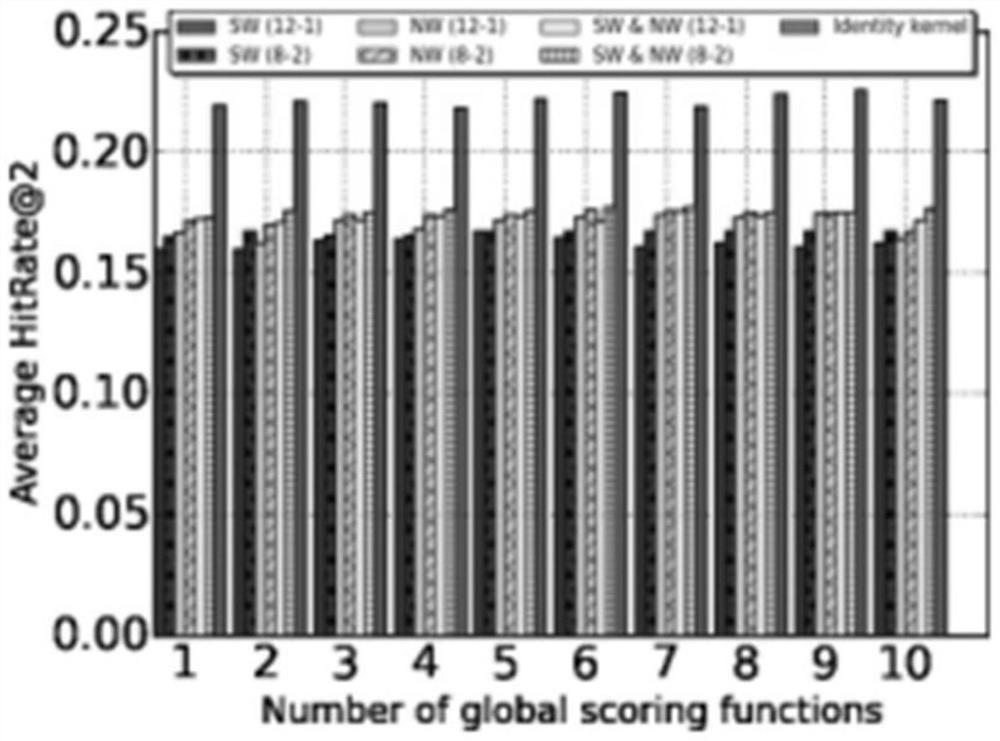
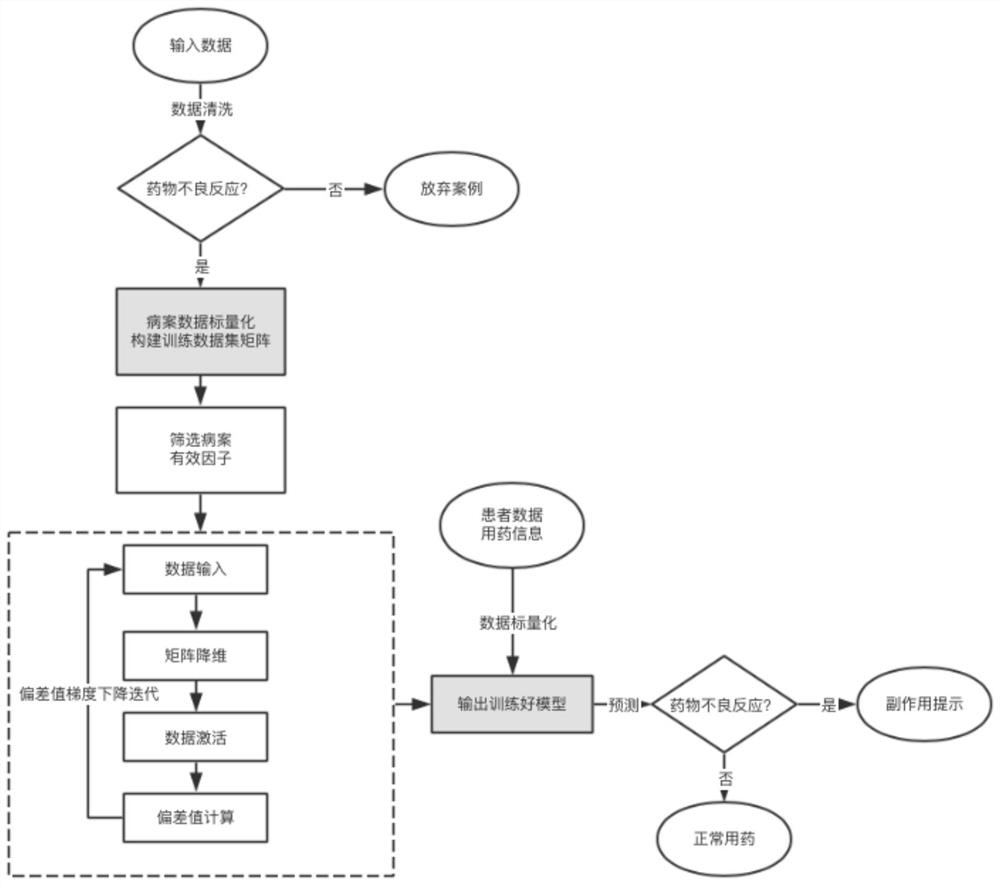
Personalized adverse drug reaction prediction method, system and device and medium
ActiveCN111863281AShorten the clinical trial cycleThe value of good practical applicationDrug referencesDrug adverse reactionsMedicine
The invention provides a personalized adverse drug reaction prediction method, system and device and a medium, and belongs to the technical field of biomedicine. The invention provides a multi-task learning model (KEMULA) based on multi-kernel function learning so as to replace traditional learning methods of universal application and complete individuation. More specifically, the model calculatesand ranks the risks of ADR development of patients by learning a constrained personalized ADR ranking function by assuming a sharing function of the model. This function is referred to as a personalized ADR ranking function, which is a linear combination of several scoring functions that calculate the risk of developing related ADR of patients. In addition, the model is also combined with Laplacian regularization to ensure that variable information trained by personADRank functions of similar patients is close, so that the causal relationship (true positive) of the model to the association between a given patient and the corresponding ADR can be improved. Therefore, the method has good practical application value.
Owner:SHANDONG UNIV
Systems and Methods for Evaluating Enzyme Competency
InactiveUS20090005270A1Assessing abilityRapidly and accurately determineBioreactor/fermenter combinationsBiological substance pretreatmentsChemical reactionFluorescence
The present invention provides systems and methods for determining enzymatic competency, which is important in determining whether a patient may suffer an adverse drug reaction, has a disease associated with defects in specific enzymatic function, and / or has an enzyme defect that is likely to cause pathophysiology. As contemplated herein, a parent molecular entity is administered to a patient in whom enzymatic competency is to be determined. A sample of the patient's bodily fluid is exposed to a sensor of the invention to distinguish, detect, and quantify a detectable entity in the bodily fluid. Sensor-acquired data regarding the detectable entity is used to determine enzymatic competency. Preferably, a sample of a patient's exhaled breath is collected and exposed to the sensor of the invention. Types of sensor systems of the invention include, but are not limited to, surface resonance arrays; microelectromechanical sensors (such as microcantilever-based technology); molecularly imprinted polymer sensors; amplifying fluorescent sensor technology; aptamer-based sensor technology; SAW sensors; infrared sensors; fuel cells; chemical reactors; and pH sensitive sensors.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
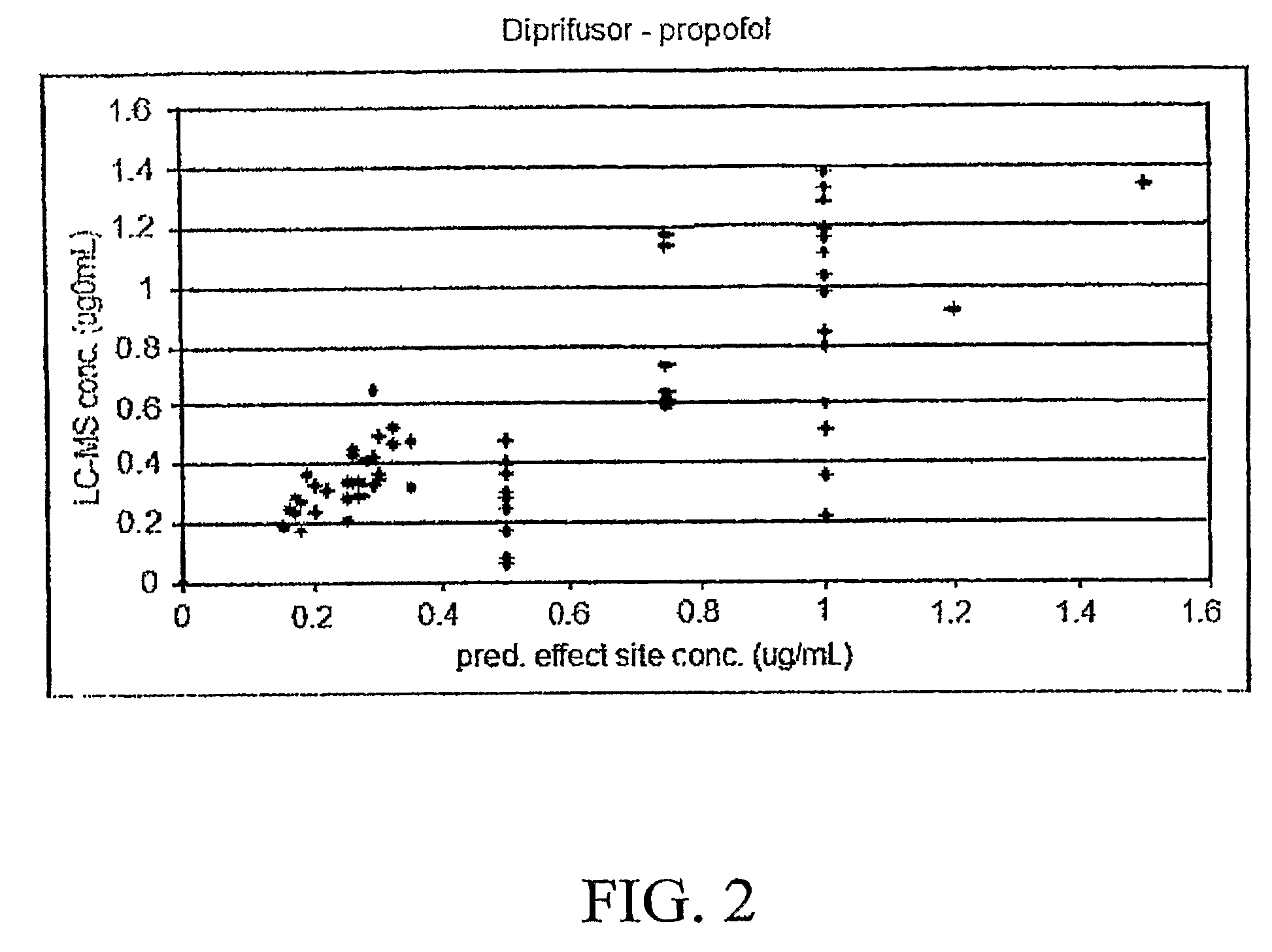
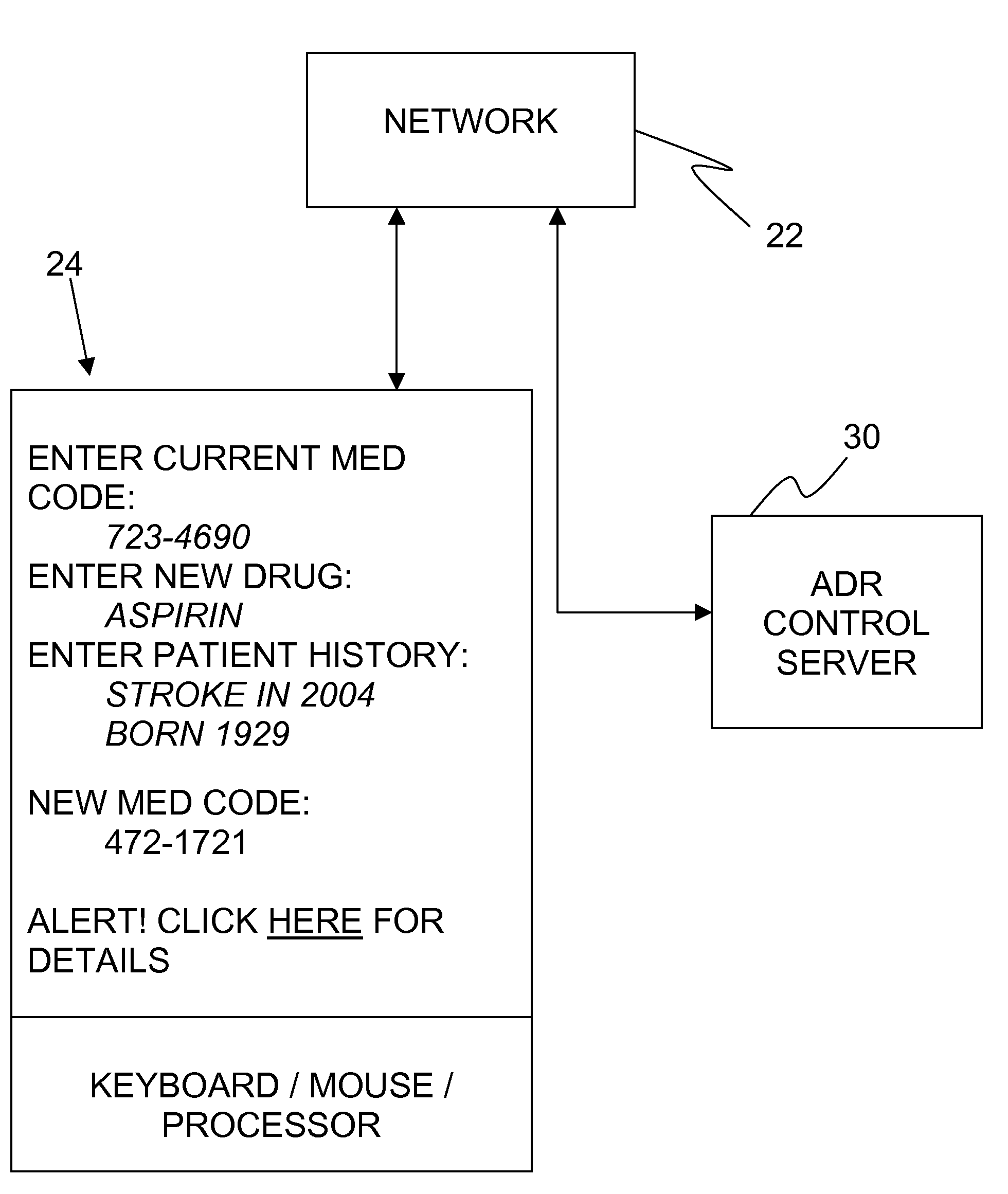
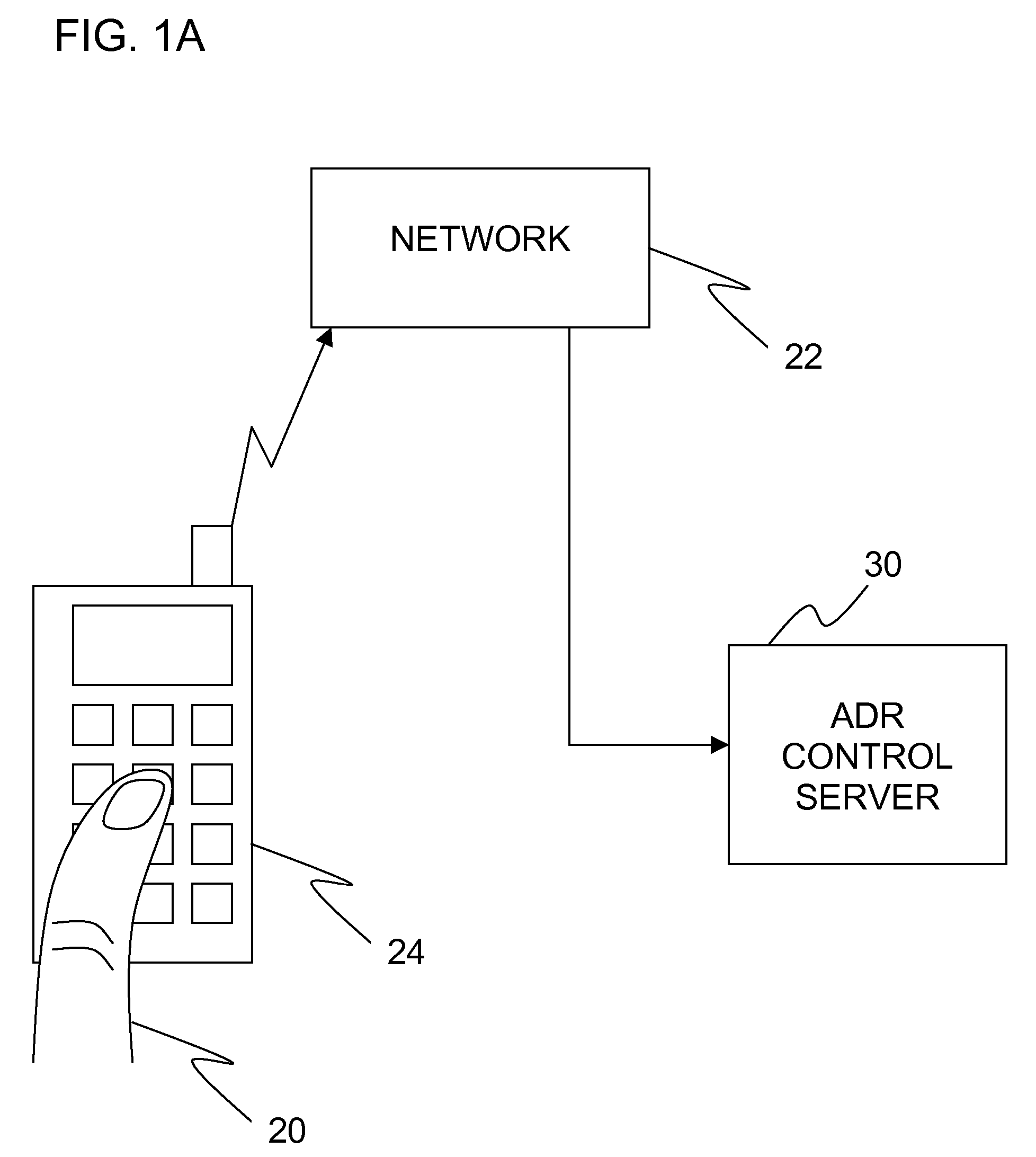
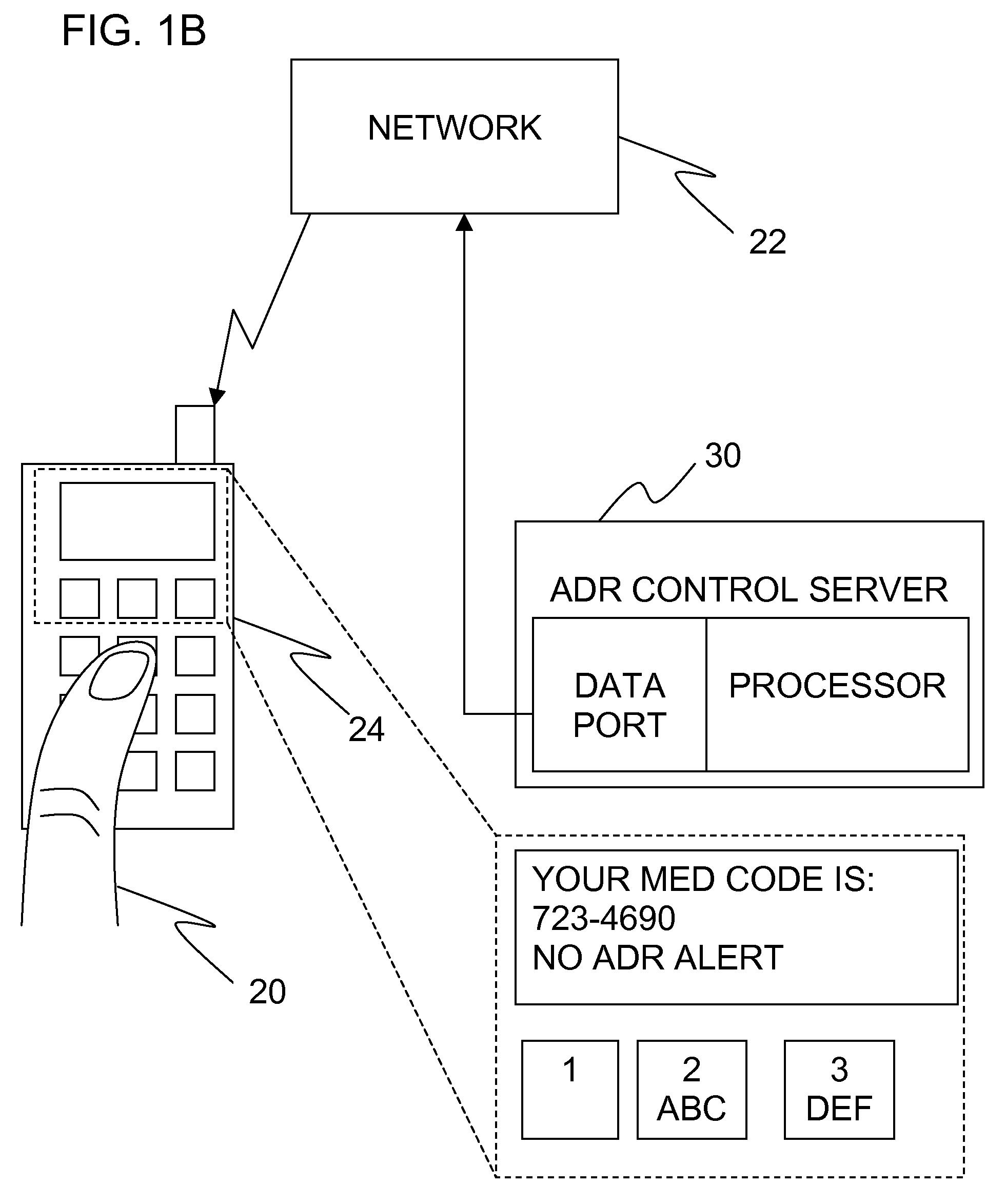
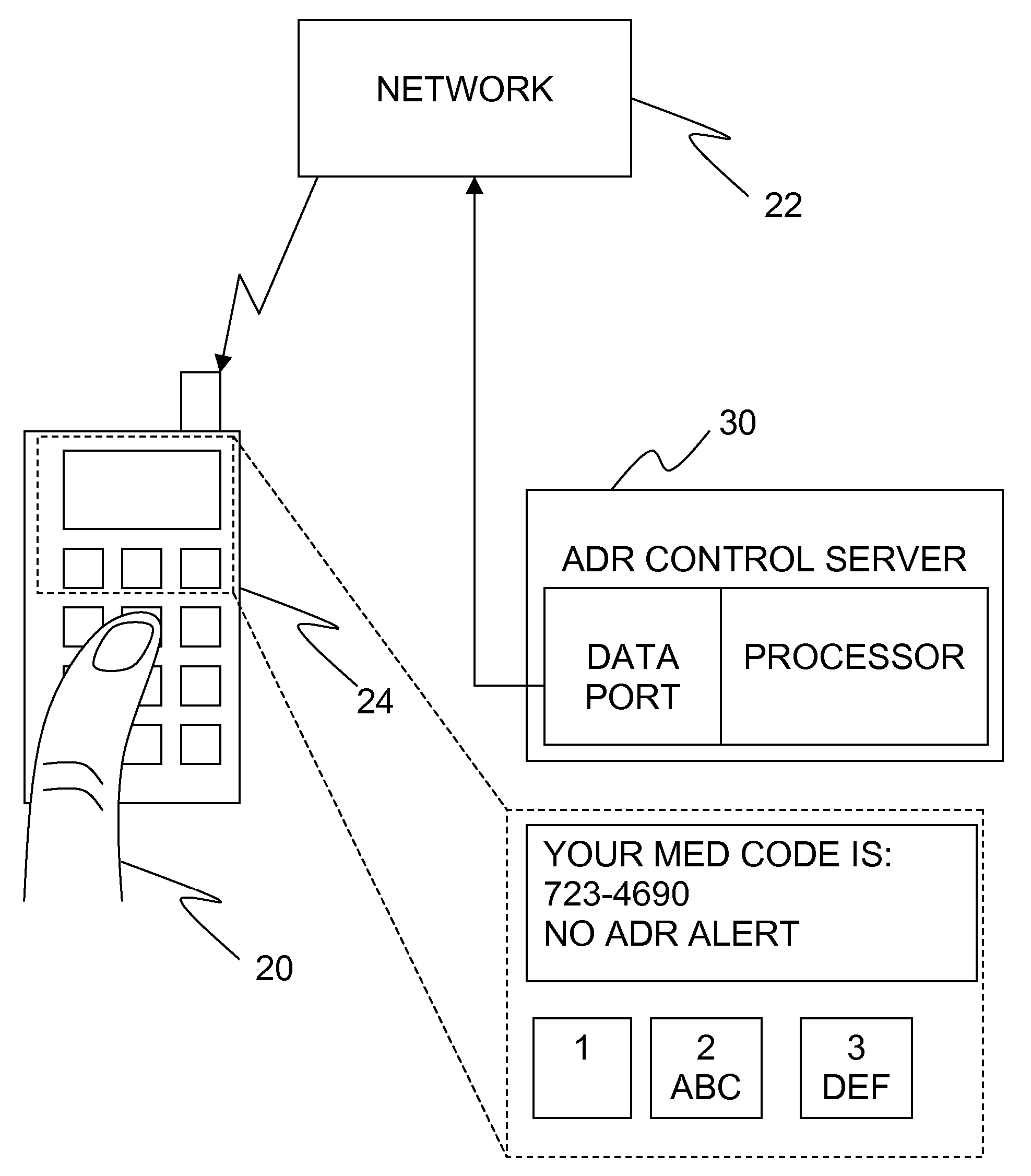
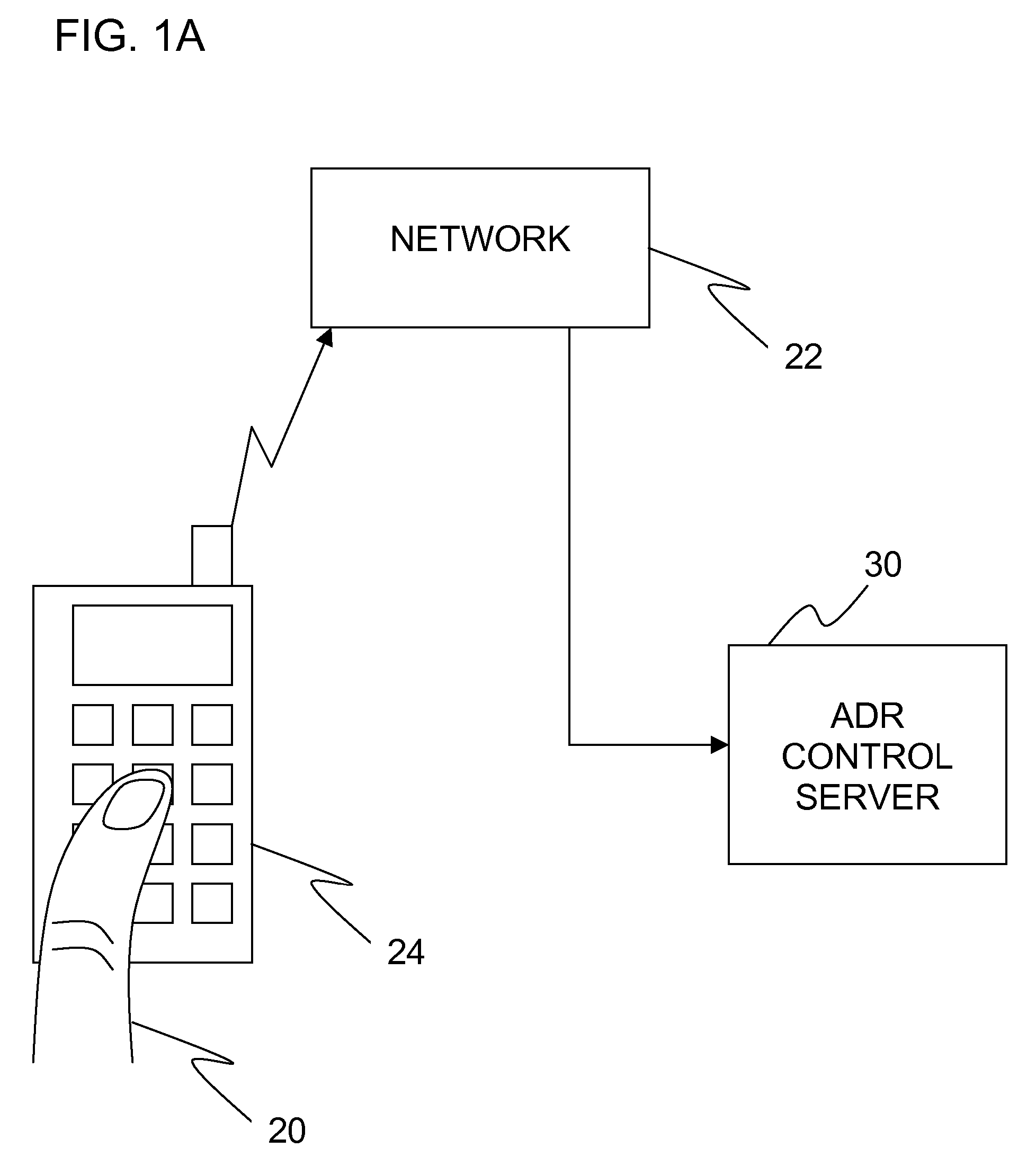
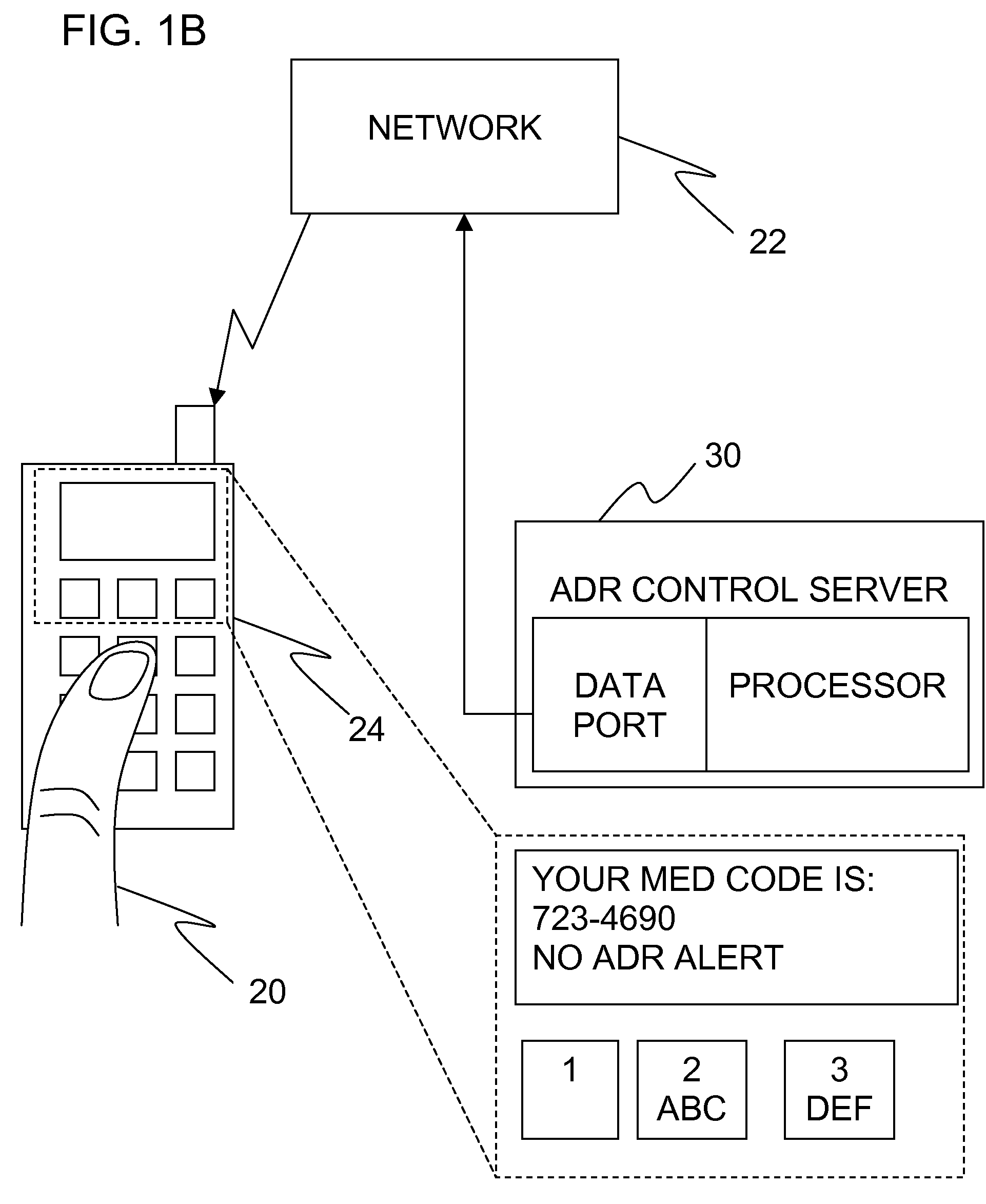
Adverse drug reaction reduction
Apparatus is provided, including an adverse drug reaction (ADR) control server (3) including a processor and a data port. The server is configured to receive from a user (20), via the data port, in a first interaction with the user, a list of one or more current medications, and to send to the user a first medication code responsively to the list. In a second interaction with the user, the server receives the first medication code from the user and an indication of a new medication. The server accesses, by the processor, the list of one or more current medications based on the received first medication code, and generates an adverse drug reaction (ADR) warning based on the list of one or more current medications and the new medication. The server sends the user a new medication code based on the list of one or more current medications and the new medication. Other embodiments are also described.
Owner:MIRIK MEDICAL
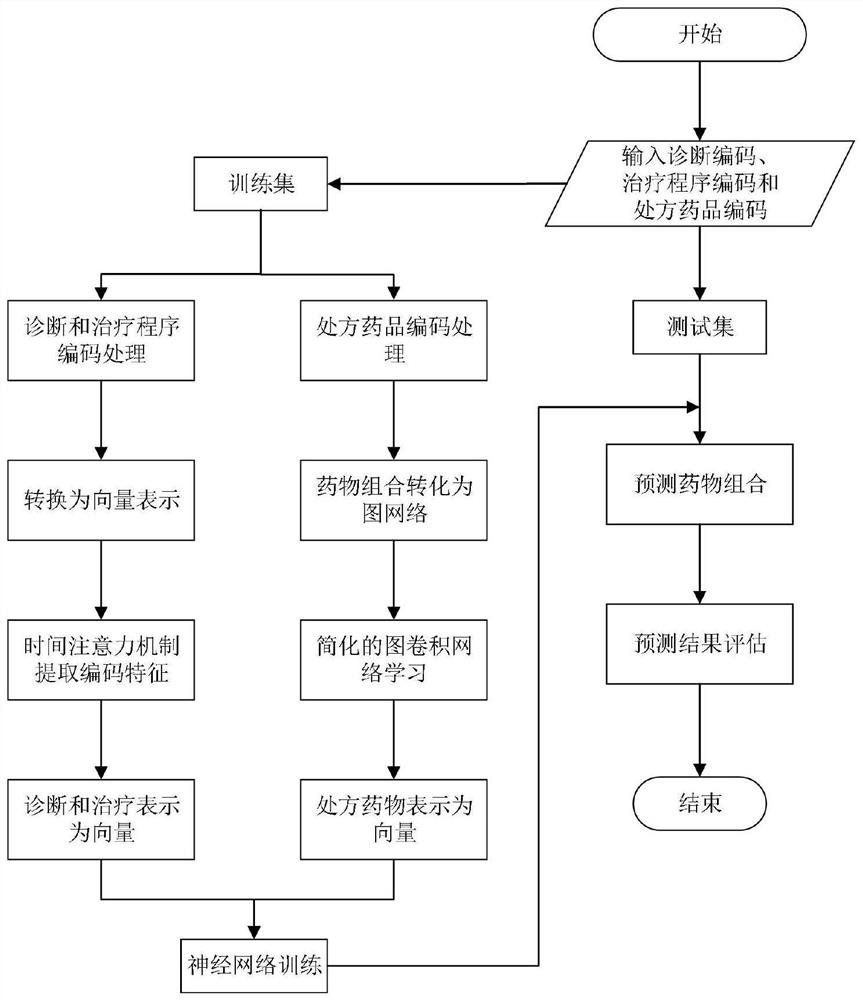
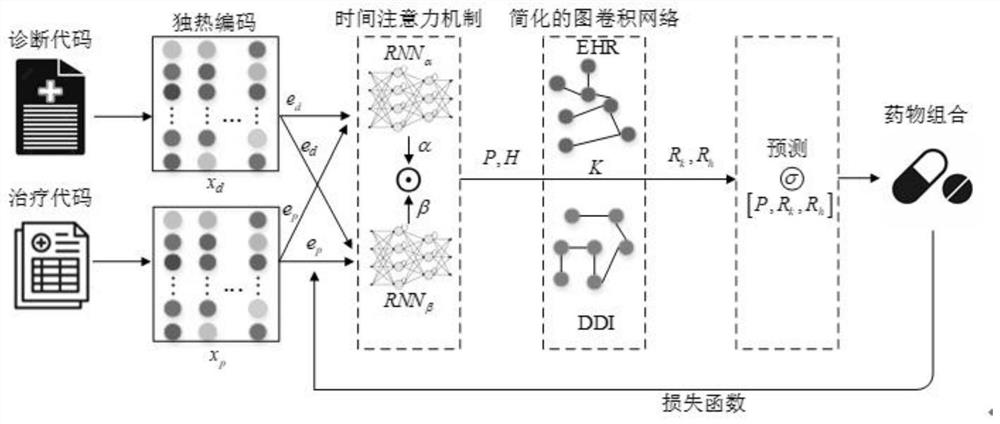
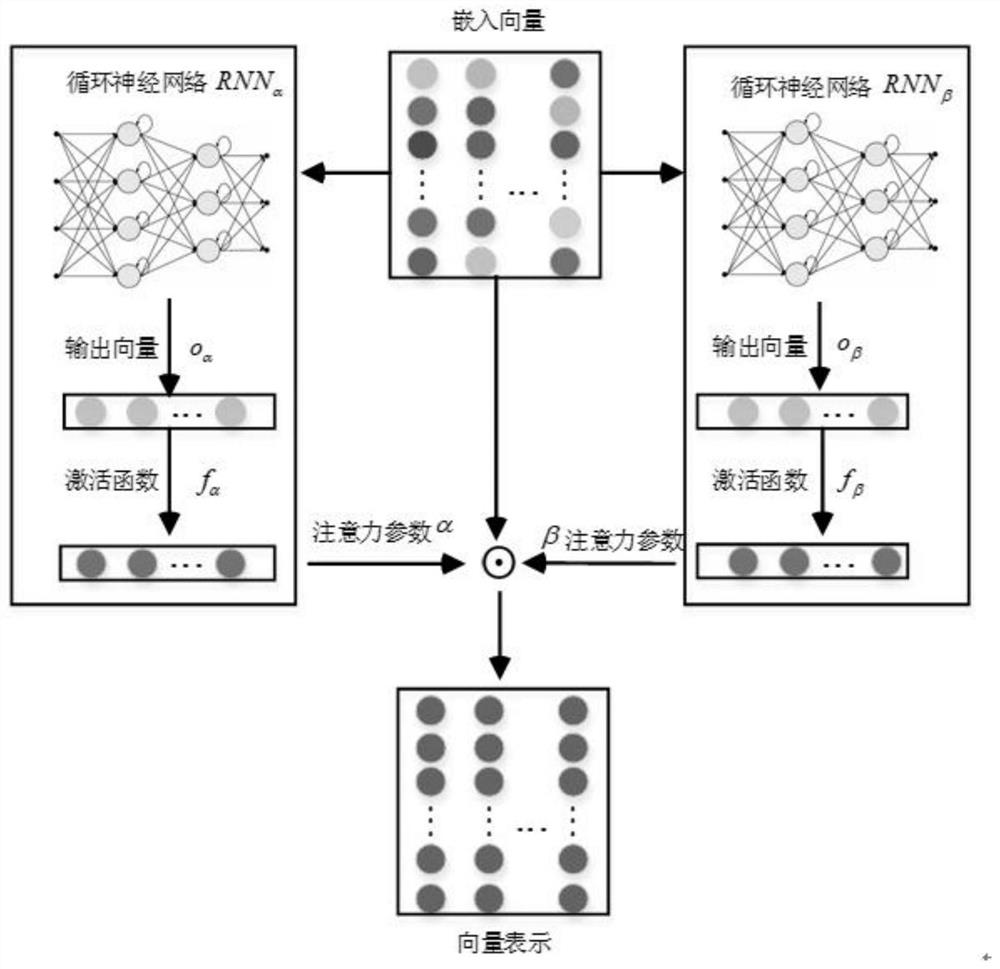
Drug combination recommendation method based on time attention mechanism and graph convolutional network
PendingCN111798954AImprove accuracyFewer calculation parametersDrug and medicationsNeural architecturesNetwork modelArtificial intelligence
The invention provides a drug combination recommendation method based on a time attention mechanism and a graph convolutional network. According to the invention, reasonable drugs can be recommended for treatment of critical patients in a complex medical environment, and clinicians can be helped to treat patients. Diagnosis and treatment in electronic health records are coded in a unified coding format, time sequence information in diagnosis and treatment is stored, codes are converted into vectors, and the time attention mechanism composed of two layers of recurrent neural networks is used for capturing the time sequence information. The method aims at medicines of prescriptions issued by doctors in the electronic health records and medicines having adverse reactions with known medicines,graph network structure data are converted to describe the relation between different medicine combinations, and medical medication knowledge in a medicine graph network is learned by utilizing the graph convolutional network. Compared with the prior art, the simplified graph convolutional network reduces the calculation parameters of the neural network model and reduces the training and learningtime under the condition of maintaining the prediction accuracy unchanged.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Adverse drug reaction reduction
Apparatus is provided, including an adverse drug reaction (ADR) control server (3) including a processor and a data port. The server is configured to receive from a user (20), via the data port, in a first interaction with the user, a list of one or more current medications, and to send to the user a first medication code responsively to the list. In a second interaction with the user, the server receives the first medication code from the user and an indication of a new medication. The server accesses, by the processor, the list of one or more current medications based on the received first medication code, and generates an adverse drug reaction (ADR) warning based on the list of one or more current medications and the new medication. The server sends the user a new medication code based on the list of one or more current medications and the new medication. Other embodiments are also described.
Owner:MIRIK MEDICAL
Betahistine hydrochloride liposome and preparation method thereof
ActiveCN102631320AImprove efficacyImprove antioxidant capacityOrganic active ingredientsSenses disorderRotary evaporatorMethylene Dichloride
The invention relates to a betahistine hydrochloride liposome and a preparation method of the betahistine hydrochloride liposome. The preparation method comprises the steps of: firstly dissolving lipide ingredients in methylene dichloride, and carrying out reduced-pressure evaporation in water bath until a film is formed; dissolving the film in diethyl ether, and then adding phosphate buffer containing betahistine into the solution; carrying out ultrasonic treatment on the mixed suspension liquid, placing the treated liquid on a rotary evaporator, and carrying out reduced-pressure vaporization until all the organic reagent is evaporated; and finally, obtaining the betahistine hydrochloride liposome. The betahistine hydrochloride is made into liposome, so that the medicine effect is obviously improved, the permeability of blood capillary can be improved, and the dosage of the medicine reaching blood vessels of the brain can be increased; the betahistine hydrochloride liposome is used for improving the treatment effect of Meniere syndrome, reduces the gastrointestinal irritation and the adverse drug reaction, and is beneficial to reducing the side effects such as stomach upset, nausea, dizziness and the like.
Owner:黑龙江亿达鸿药业有限公司
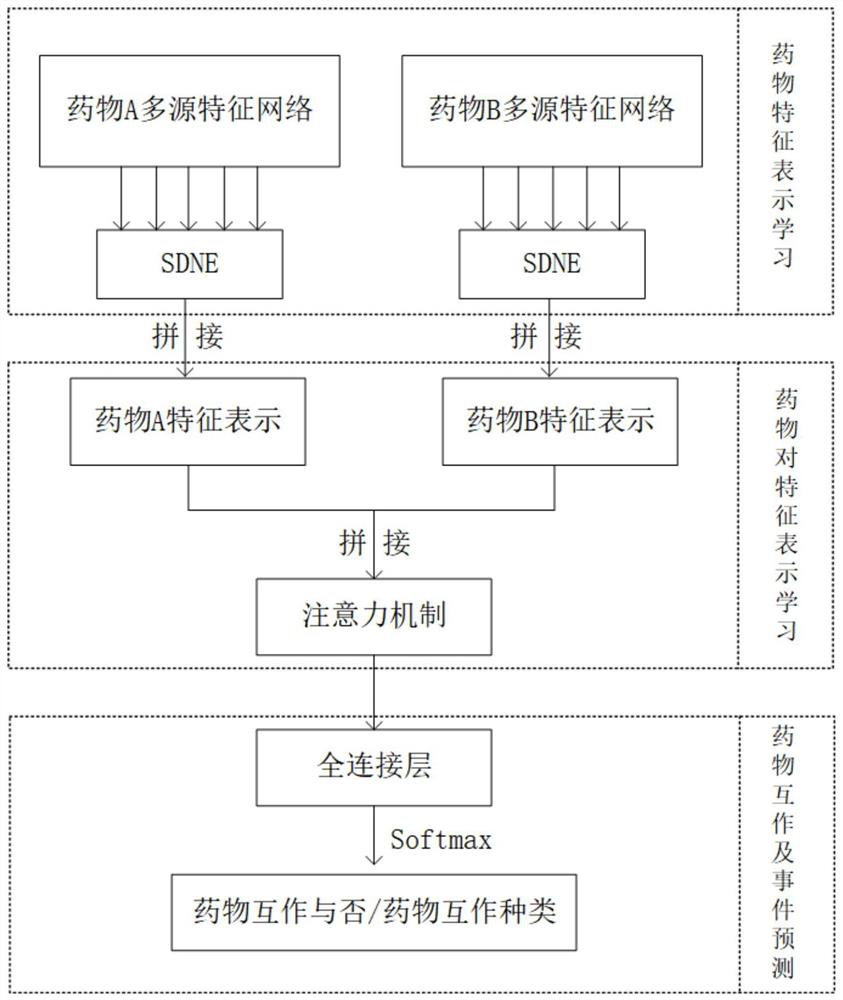
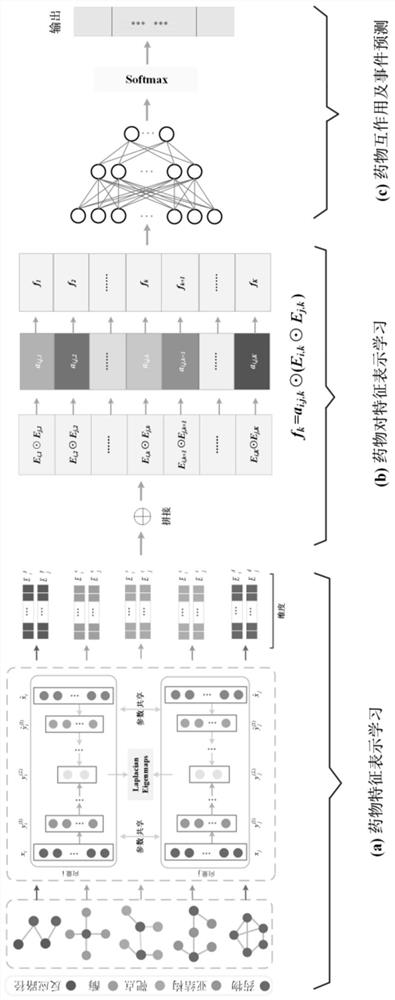
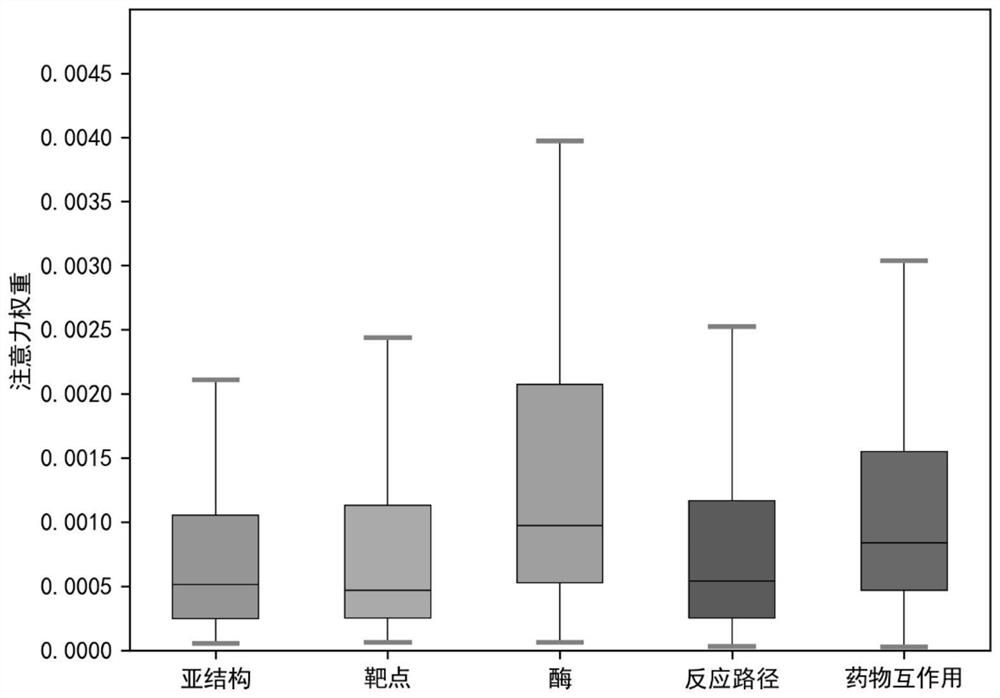
Drug interaction and event prediction method and model based on attention neural network
PendingCN112037856AImprove robustnessInterpretableNeural architecturesHybridisationDrug adverse reactionsDrug utilisation
The invention provides a drug interaction and event prediction method and a model based on attention neural network. According to the method, more comprehensive feature vector representation is obtained by integrating heterogeneous drug feature networks on the basis of a deep attention neural network framework, and a graph representation learning method is combined, the difference of the influenceof drugs on other drugs in different characteristics and dimensions is analyzed, and the accuracy of drug interaction prediction and the robustness of the model are improved under the condition of adopting multiple data sources, so that the model has interpretability, the function of predicting a drug interaction event is realized, the understanding of a mechanism hidden behind adverse drug reactions is facilitated, meanwhile, reasonable combined medication can be guided, and medication safety is promoted.
Owner:HUAZHONG AGRI UNIV
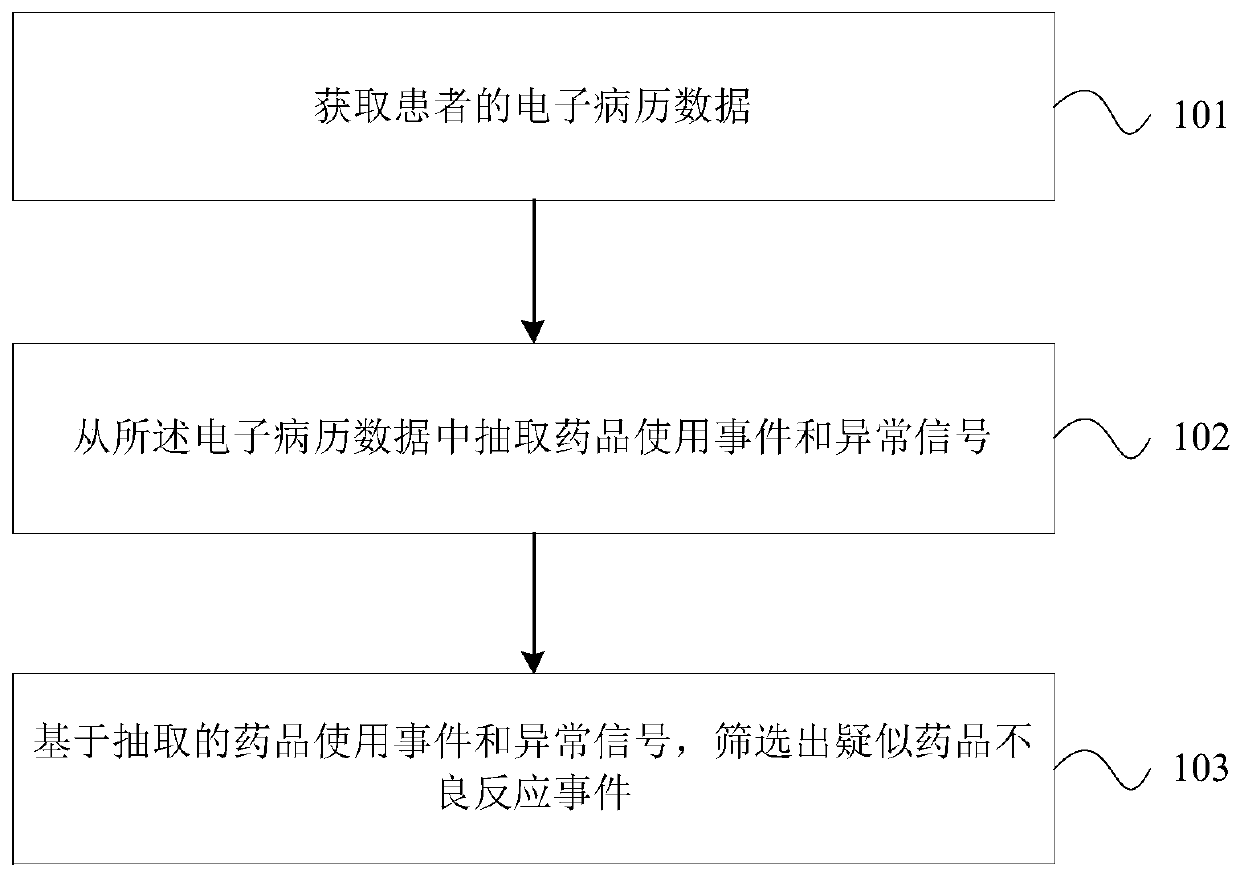
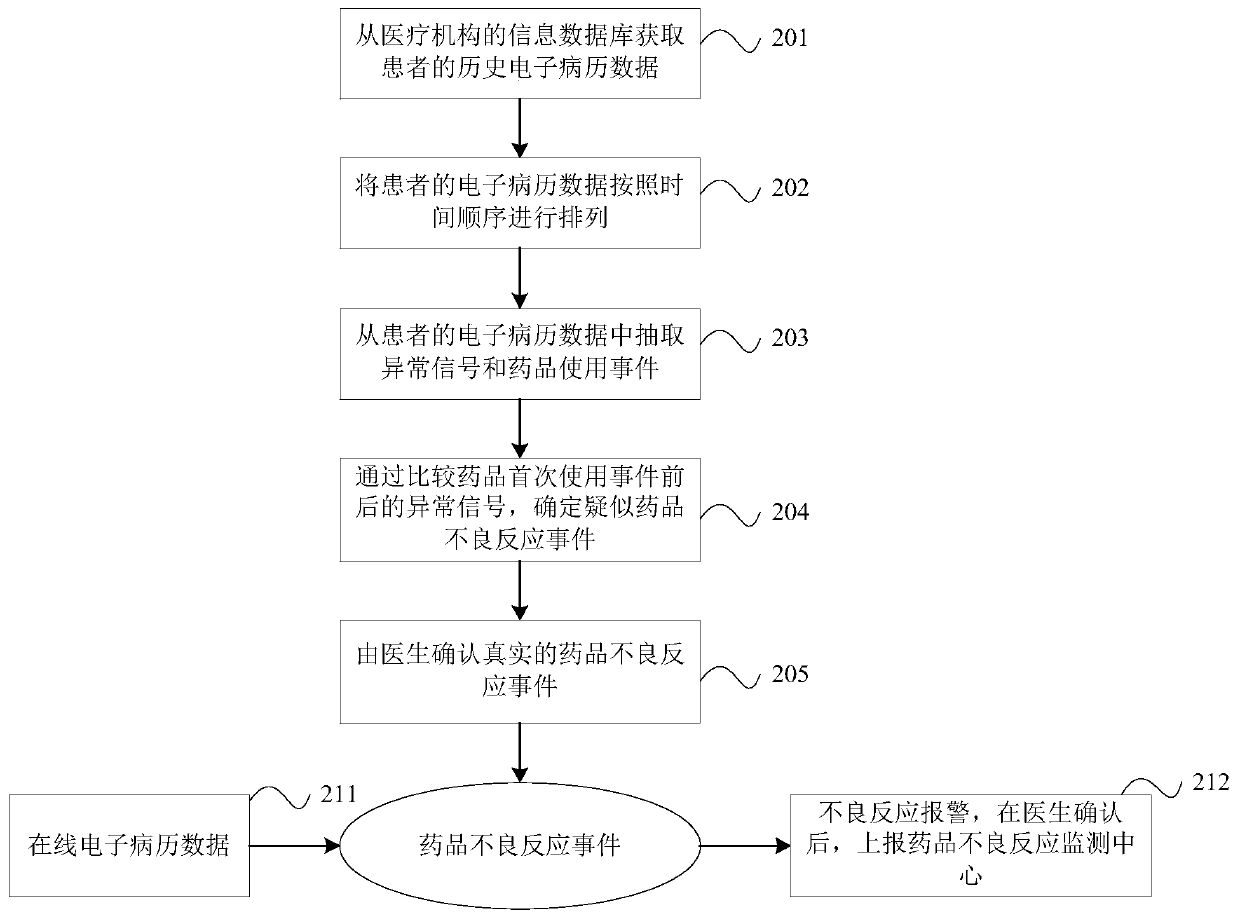
Method and device for monitoring adverse drug reaction and readable medium
PendingCN111599481AIncrease the number of reportsReduce false negative rateMedical data miningDrug referencesMedical recordDrug adverse reactions
The invention discloses a method for monitoring adverse drug reaction. The method comprises the following steps: acquiring electronic medical record data of a patient; extracting a medicine use eventand an abnormal signal from the electronic medical record data; and screening out suspected drug adverse reaction events based on the extracted drug use event and the abnormal signal.
Owner:国家药品监督管理局药品评价中心
Rapid identification method and system for adverse reactions of drugs based on big data
ActiveCN111402971ADetect adverse reaction signalsDigital data information retrievalCharacter and pattern recognitionDrug adverse reactionsPharmaceutical drug
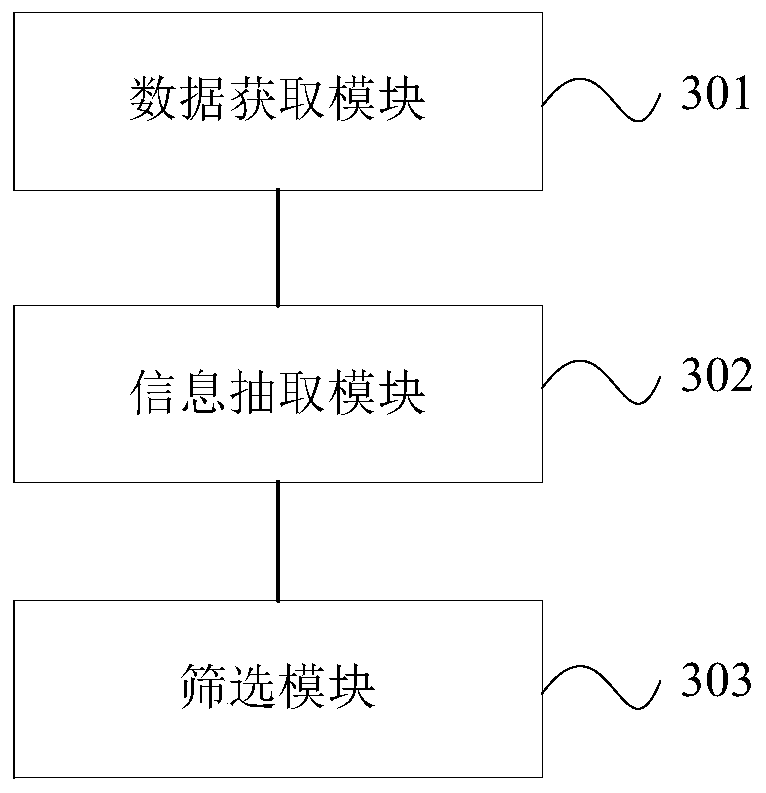
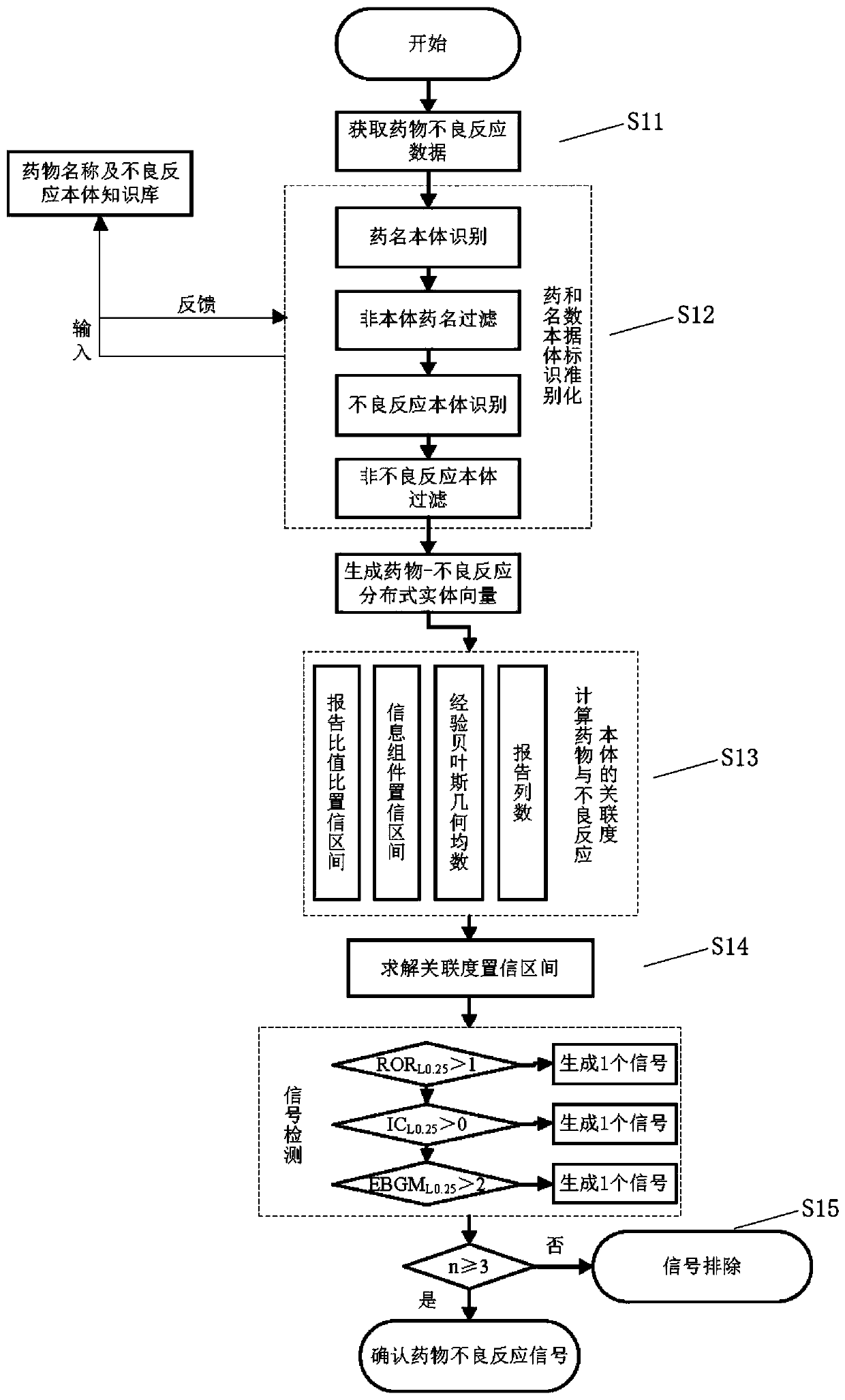
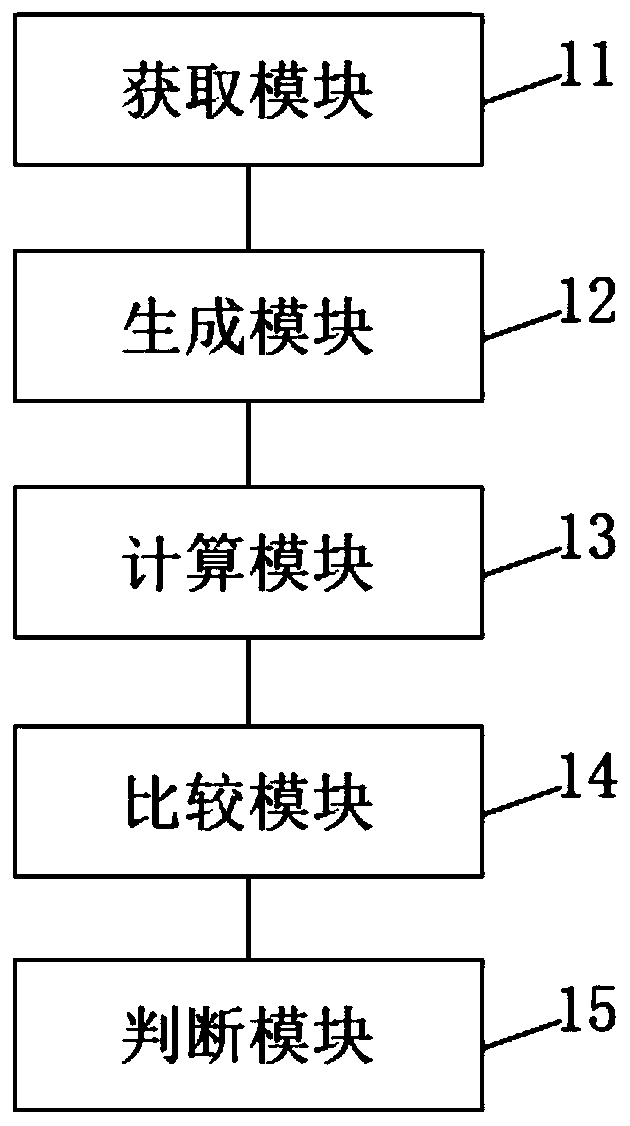
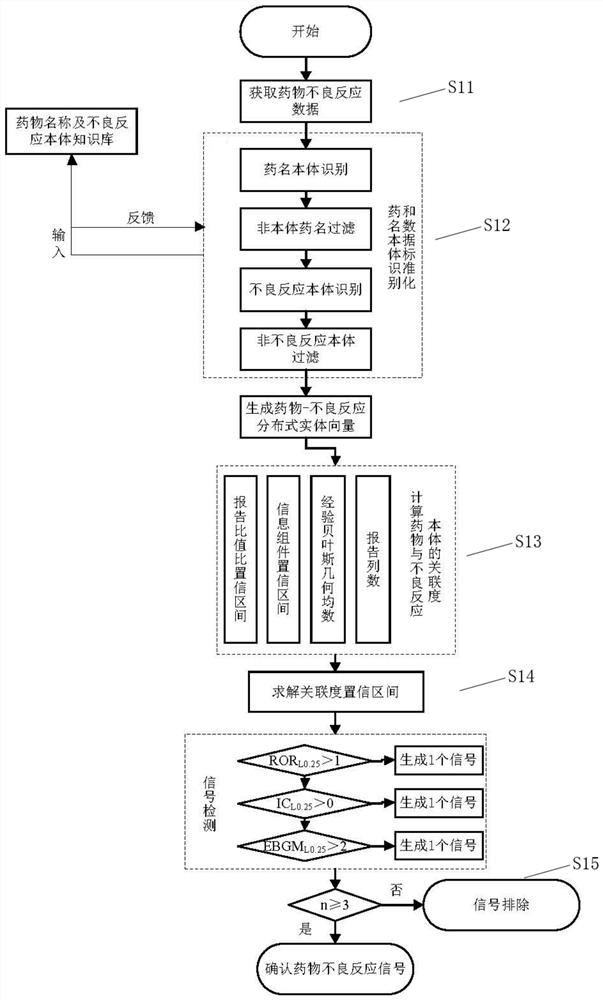
The invention discloses a rapid identification method and system for adverse reactions of drugs based on big data. The rapid identification method for adverse reactions of drugs based on big data in the invention comprises the following steps: S11, acquiring adverse reaction data of a drug; S12, comparing the obtained adverse drug reaction data with a pre-stored drug name ontology knowledge base and an adverse reaction name ontology knowledge base to generate drug-adverse reaction distributed entity vectors; S13, according to the generated drug-adverse reaction distributed entity vectors, calculating a plurality of association degree values of the drug and an adverse reaction body; S14, solving a confidence interval of each association degree value according to the plurality of associationdegree values obtained by calculation, and comparing the confidence interval of each association degree value obtained by solving with a preset reference value to obtain a comparison result; and S15,judging whether the comparison result is greater than a preset threshold value or not, and if so, determining that the comparison result is an adverse drug reaction signal, or if not, conducting exclusion.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
A method and system for rapid identification of adverse drug reactions based on big data
ActiveCN111402971BDetect adverse reaction signalsDigital data information retrievalCharacter and pattern recognitionDrug adverse reactionsPharmaceutical drug
The invention discloses a method and system for rapid identification of adverse drug reactions based on big data. Among them, a large data-based rapid identification method for adverse drug reactions involved in the present invention includes the steps of: S11. Acquiring adverse drug reaction data; S12. Comparing the knowledge base and the adverse reaction name ontology knowledge base to generate drug-adverse reaction distributed entity vectors; S13. According to the generated drug-adverse reaction distributed entity vectors, calculate several correlation degrees between drugs and adverse reaction ontology value; S14. According to the calculated several correlation degree values, the confidence interval of each correlation degree value is solved, and the confidence interval of each correlation degree value obtained by the solution is compared with a preset reference value to obtain a comparison result; S15 . Judging whether the comparison result is greater than a preset threshold, if yes, it is an adverse drug reaction signal; if not, it is excluded.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
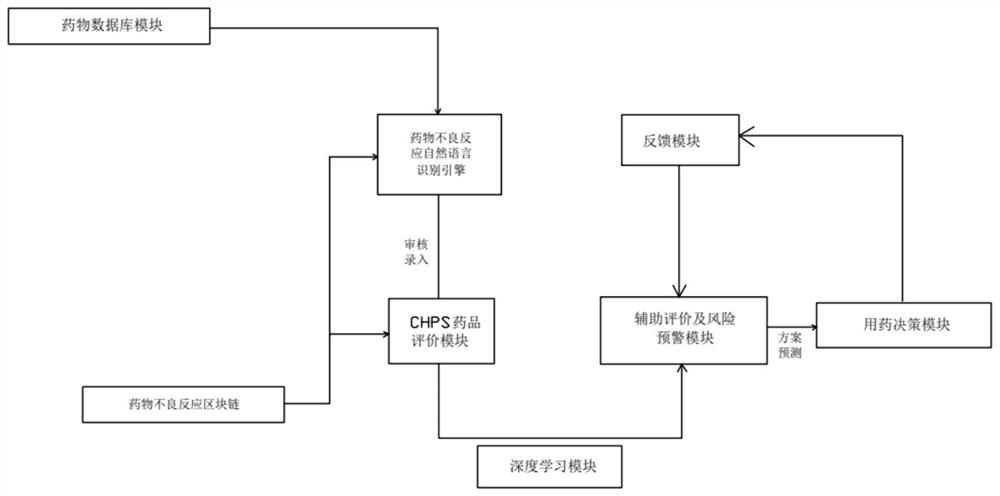
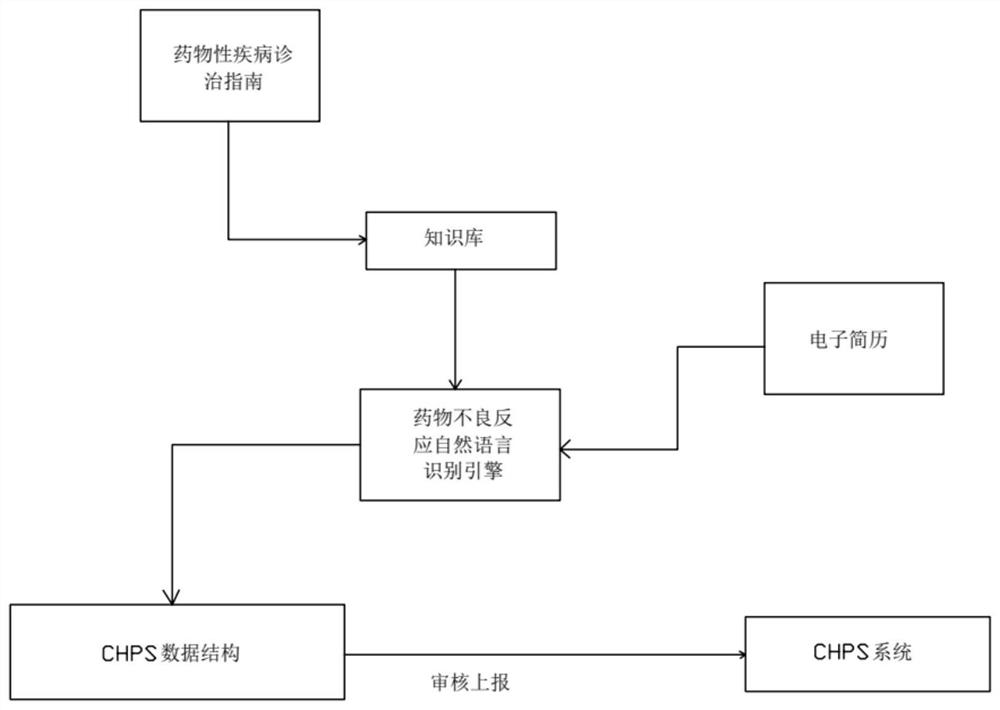
ADR auxiliary decision-making system based on deep learning
PendingCN114283949AIncrease the value of dataImprove securityDrug and medicationsMedical automated diagnosisMedication informationDrug Databases
The invention discloses an ADR auxiliary decision-making system based on deep learning, and the system comprises a drug database module which is used for obtaining and storing drug information and related adverse drug reaction information; the CHPS drug evaluation module is used for exporting perfect adverse drug reaction data on the basis of a CHPS system; the deep learning module is used for learning adverse drug reaction data exported from the CHPS drug evaluation module; the auxiliary evaluation and risk early warning module is used for comparing the input certain medicine information by using the deep learning module; the medication decision-making module is used for carrying out medication decision-making judgment after passing through the auxiliary evaluation and risk early warning module and giving information about whether medication is carried out or not; the feedback module is used for feeding back the related physical sign data of the patient after medication and the dosage and frequency data of medication to the deep learning module; the system can make decision analysis and risk management on medication based on deep learning while improving and perfecting adverse drug reaction data.
Owner:湖南云视数据科技有限责任公司
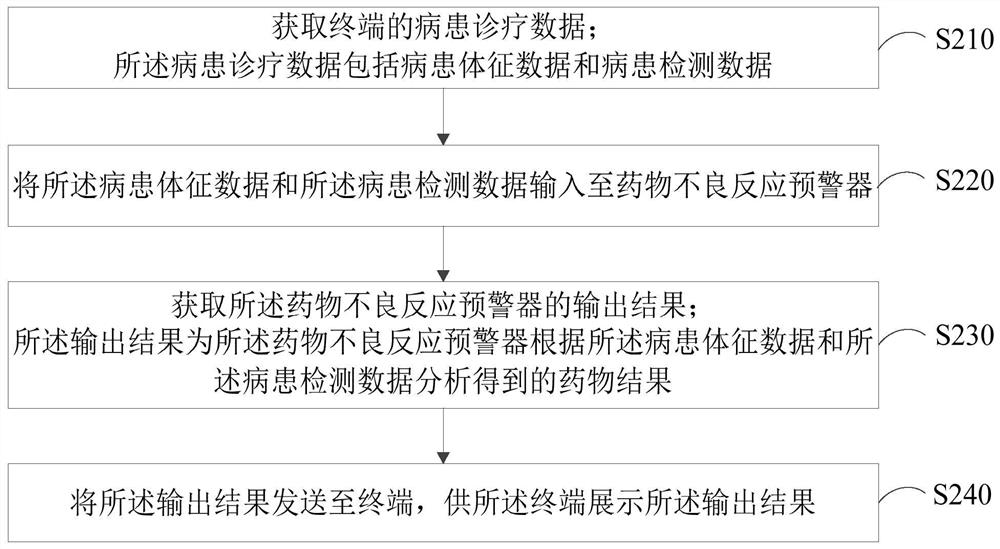
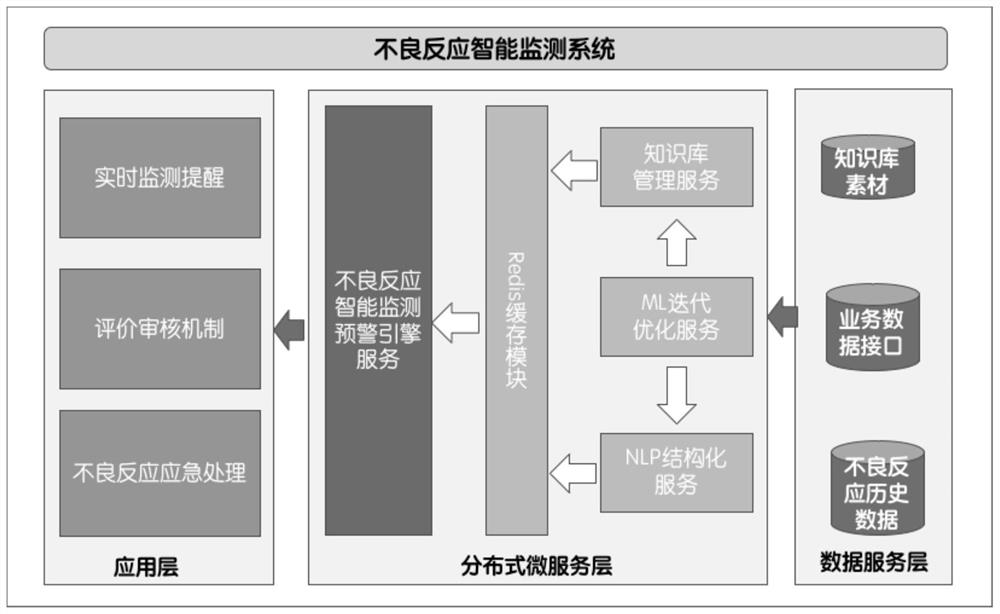
Intelligent monitoring method, device, system and computer equipment for adverse drug reactions
ActiveCN110322944BImprove accuracyPerfect comprehensivenessMedical data miningDrug and medicationsData packEmergency medicine
The invention relates to an intelligent monitoring method, device and system for adverse drug reactions and computer equipment. The method comprises the following steps: acquiring patient diagnosis and treatment data of a terminal, wherein the patient diagnosis and treatment data comprises patient sign data and patient detection data; inputting the physical sign data of the patient and the detection data of the patient into an adverse drug reaction early-warning device; acquiring an output result of the adverse drug reaction early-warning device, wherein the output result is a drug result obtained by the drug adverse reaction early-warning device through analysis according to the patient sign data and the patient detection data; and sending the output result to the terminal for the terminal to display the output result. By adopting the scheme, the accuracy of intelligent monitoring of the adverse drug reactions can be improved, and the comprehensiveness of an intelligent monitoring system of the adverse drug reaction can be perfected.
Owner:ZHEJIANG UNIV
Applications of traditional Chinese medicine composition in preparation of drug used for inhibiting angiogenesis
ActiveCN103417907AAvoid diversionSkeletal disorderAntineoplastic agentsDiabetic retinopathyRadix Astragali seu Hedysari
The invention discloses applications of a traditional Chinese medicine composition in preparation of a drug used for inhibiting angiogenesis. The traditional Chinese medicine composition comprises traditional Chinese medicine ingredients such as radix astragali and ligustrum lucidum. The traditional Chinese medicine composition is capable of benefiting qi and nourishing yin, invigorating spleen and tonifying kidney, and removing stasis and relaxing vein; and the applications of the traditional Chinese medicine composition are right focused on pathomechanism. It is confirmed by experiments that the traditional Chinese medicine composition is capable of inhibiting angiogenesis effectively, and can be used for treating malignant tumor, rheumatoid arthritis, diabetic retinopathy and unstable arterial plaque in clinic. The traditional Chinese medicine composition is reasonable in compatibility of medicines, small in adverse drug reaction and taken by patients for a long term.
Owner:SHIJIAZHUANG YILING PHARMA
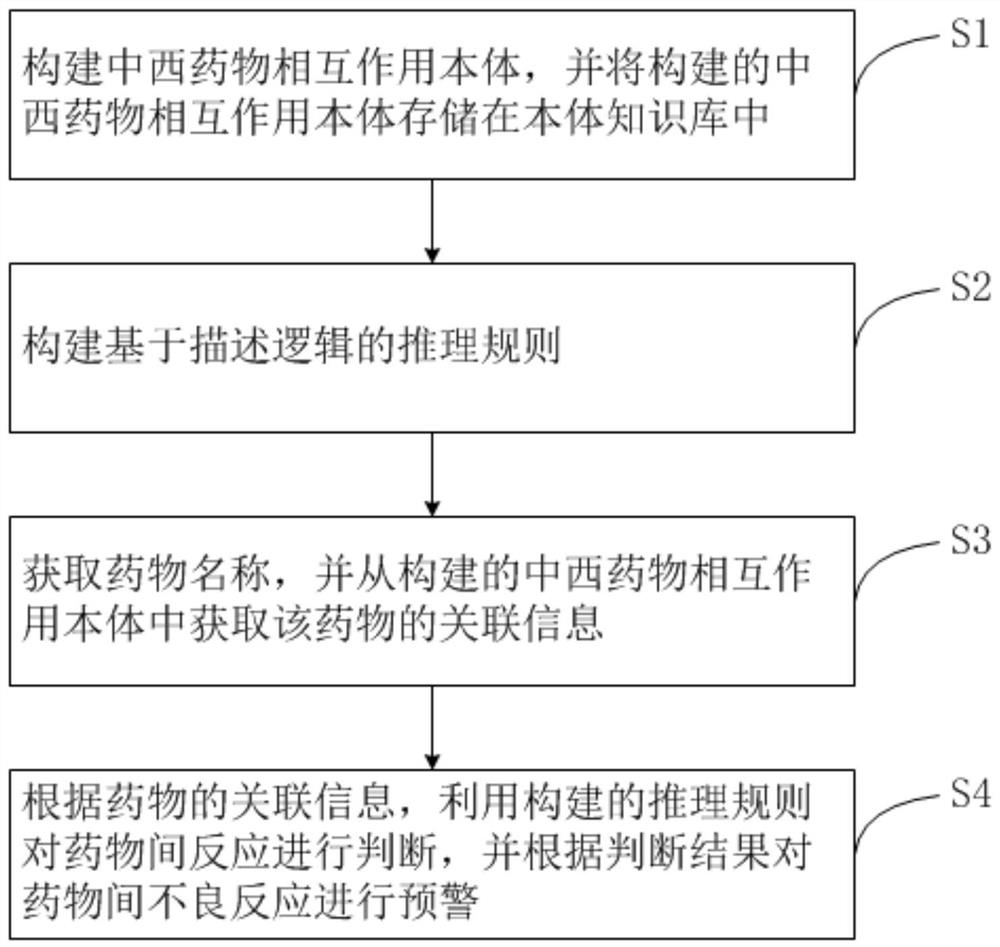
Inter-drug adverse reaction early warning method, early warning device and early warning system
PendingCN113161011AImprove early warning efficiencyImprove recallDrug referencesDrug adverse reactionsWestern medicine
The invention provides an inter-drug adverse reaction early warning method, early warning device and early warning system, and the early warning method comprises the following steps: constructing a Chinese and Western drug interaction ontology, and storing the constructed Chinese and Western drug interaction ontology in an ontology knowledge base; the Chinese and western medicine interaction ontology comprises a semantic type, an instantiated entity and a semantic relationship; constructing an inference rule based on description logic; the inference rule is constructed based on a corresponding interaction result in the interaction of the Chinese and western medicine types and the medicine components; acquiring a drug name, and acquiring associated information of the drug from the constructed Chinese and western drug interaction ontology; and judging the reactions between the drugs by using the constructed inference rule according to the association information of the drugs, and performing early warning of the adverse reactions between the drugs according to the judgment result. According to the method, the early warning efficiency of the adverse reactions between the drugs can be greatly improved, and the recall ratio and the precision ratio of the adverse reactions between the drugs are improved.
Owner:INST OF INFORMATION ON TRADITIONAL CHINESE MEDICINE CACMS
Application of traditional Chinese medicine composition in preparation of drug for inhibiting rheumatoid arthritis angiogenesis
ActiveCN103830662AAvoid diversionAnthropod material medical ingredientsAntipyreticRadix Astragali seu HedysariYiqi yangyin
The invention discloses an application of a traditional Chinese medicine composition in preparation of drugs for inhibiting rheumatoid arthritis angiogenesis. The traditional Chinese medicine composition comprises traditional Chinese medicines such as radix astragali, ligustrum lucidum and the like. The traditional Chinese medicine composition has the effects of tonifying qi and nourishing yin, invigorating spleen and tonifying kidney, dissipating binds and dredging collaterals, and focuses on the pathogenesis; experiments confirm that the traditional Chinese medicine composition of the invention can effectively inhibit angiogenesis, and can be clinically used for rheumatoid arthritis. The traditional Chinese medicine composition of the invention is reasonable in combination, has less adverse drug reaction, and is suitable for long-term use by patients.
Owner:SHIJIAZHUANG YILING PHARMA
Application of Chinese medicine composition in preparation of medicine for treating atypical hyperplasia of gastric mucosa
ActiveCN101579496APrevent stomach cancerDefinite curative effectAnthropod material medical ingredientsDigestive systemDiseaseOmodysplasia
The invention discloses an application of a Chinese medicine composition in preparation of a medicine for treating the atypical hyperplasia of the gastric mucosa. The medicine is formed by combining Chinese medicines such as radix astragali, glossy privet fruit, and the like. The Chinese medicine composition has the efficacy of supplementing qi and nourishing yin, spleen kidney nourishing and eliminating stagnation activate meridians, is tangential to pathogenesis and is clinically used for treating the disease to obtain a better effect. The invention has rational proportion and small adverse reaction of the medicine, and can be used by patients for a long time.
Owner:HEBEI YILING MEDICINE INST
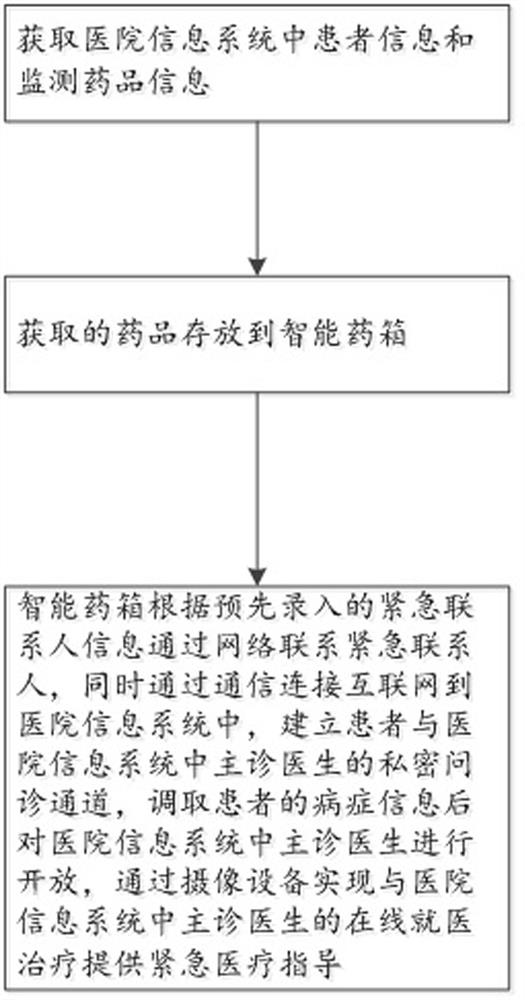
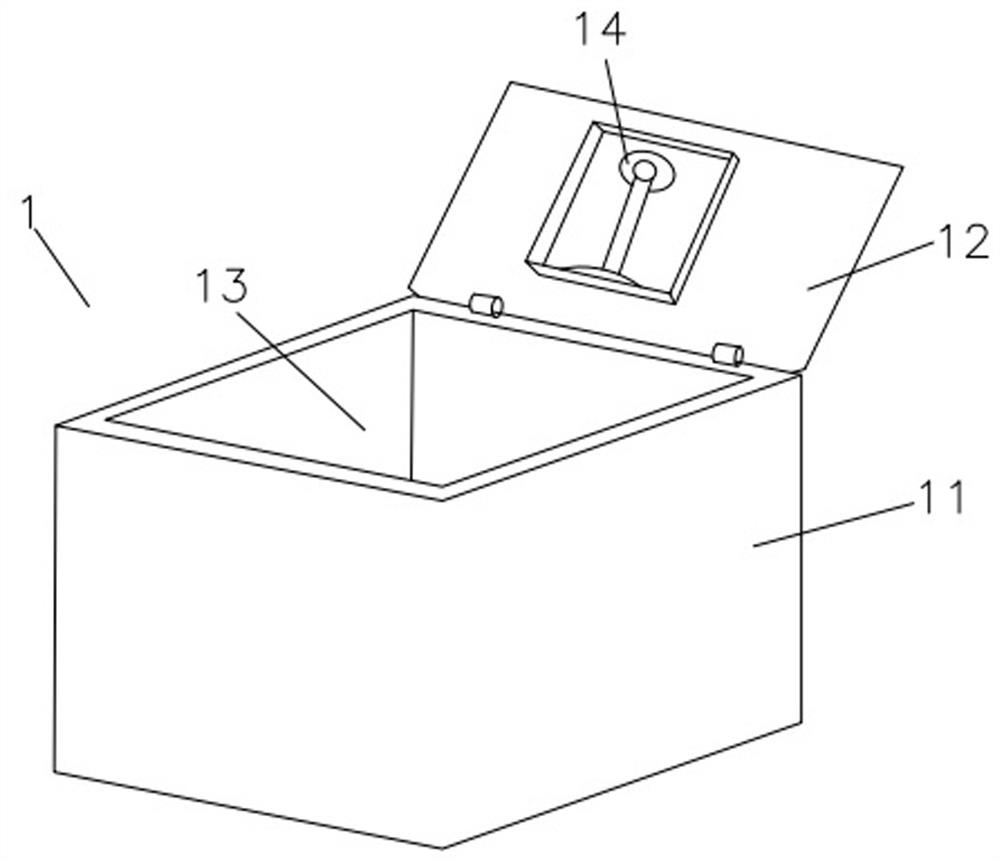
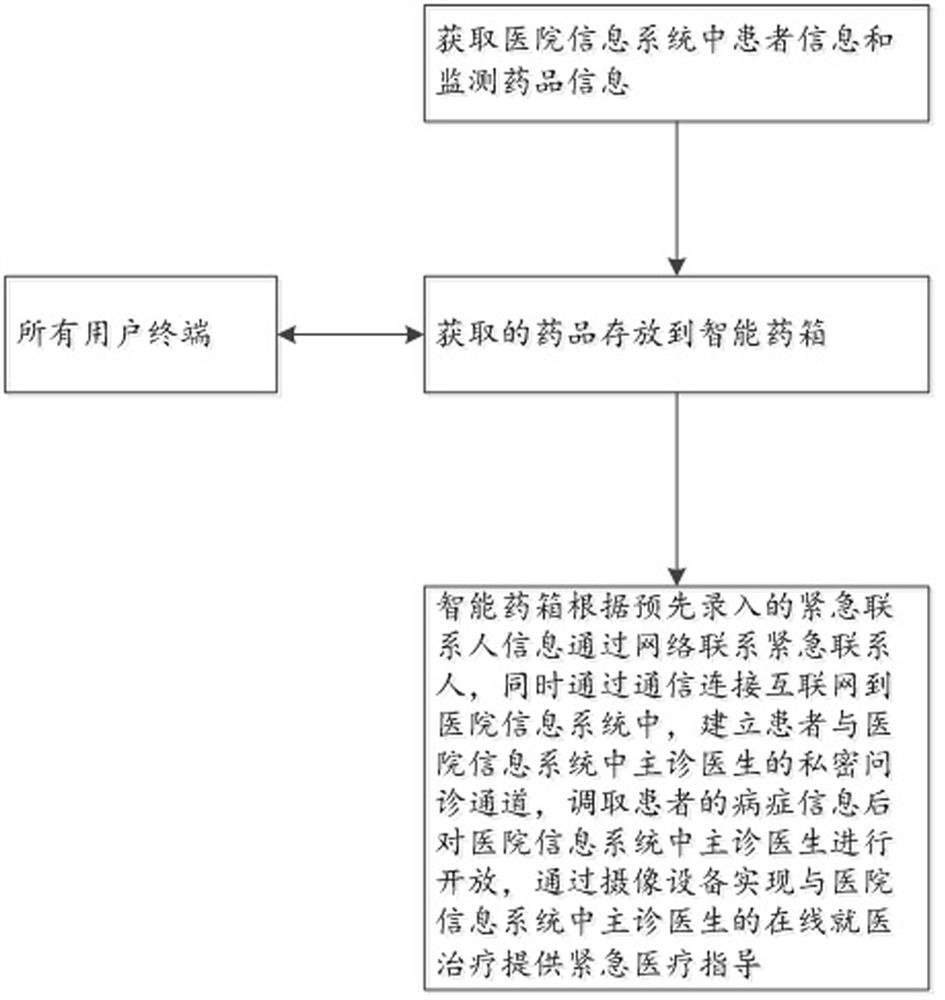
Adverse drug reaction treatment method and drug storage medium device
PendingCN112349432AConvenient treatmentPromote recoveryMedical communicationDrug and medicationsEmergency medicineHospital information system
The invention discloses a drug adverse reaction processing method and a drug storage medium device. The drug adverse reaction processing method comprises the following steps that patient information and monitoring drug information in a hospital information system are acquired; the obtained medicine is stored in an intelligent medicine box, the intelligent medicine box comprises camera equipment, astorage chamber and a processor, the camera equipment sends shot and scanned information to the processor, and patient information and monitored medicine information are sent to the processor throughthe Internet; the intelligent medical box contacts an emergency contact person through a network according to pre-input emergency contact person information, is connected to a hospital information system through communication, establishes a private inquiry channel between a patient and a main diagnosis doctor, calls disease information of the patient, opens for the main diagnosis doctor, emergency medical guidance is provided for online medical treatment of the main diagnosis doctor through camera equipment. According to the invention, adverse reactions of drugs of patients can be detected, and more importantly, a solution can be provided rapidly, which is more beneficial to the health and safety of the patients.
Owner:WENZHOU PEOPLES HOSPITAL
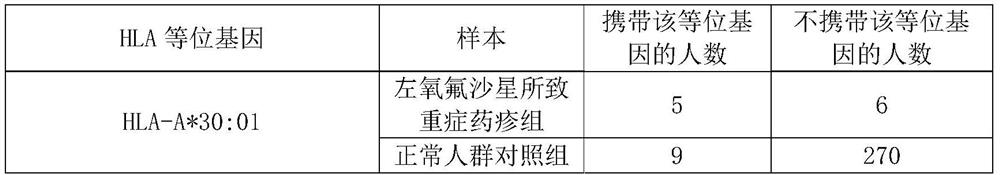
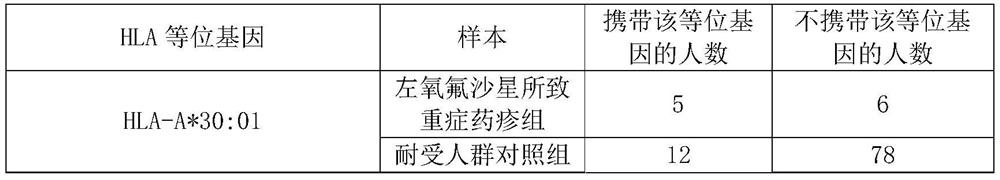
Application of substance for detecting HLA-A*30:01 allele in evaluating risk of severe drug rash caused by levofloxacin
ActiveCN112029847AReduce generationMicrobiological testing/measurementAgainst vector-borne diseasesWhite blood cellBiology
The invention discloses an application of a substance for detecting an HLA-A*30:01 allele in evaluating the risk of human severe drug rash caused by levofloxacin. The invention also discloses an application of the substance for detecting the HLA-A*30:01 allele in preparation of a product for detecting or evaluating the risk of human adverse drug reaction responding to the levofloxacin. Experimentsprove that the human leukocyte antigen gene-HLA-A*30:01 allele is related to the risk of the severe drug rash caused by the levofloxacin. The HLA-A*30:01 allele can be used as a marker gene for predicting the occurrence risk of the severe drug rash caused by the levofloxacin.
Owner:FUDAN UNIV +1
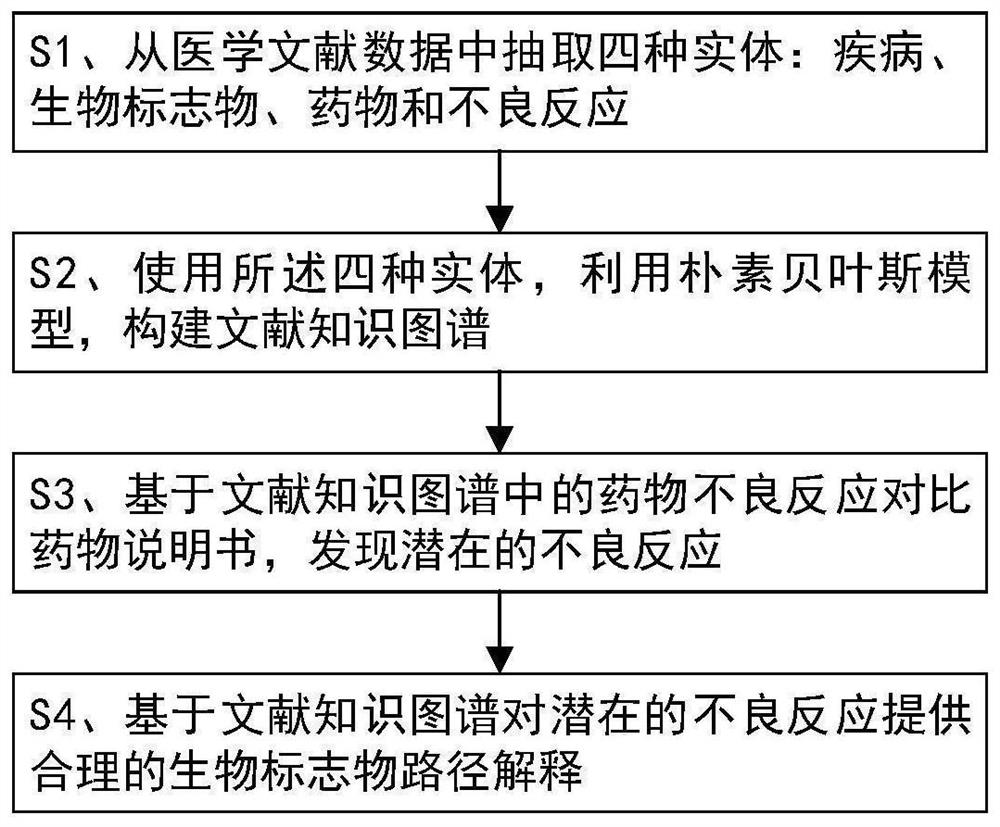
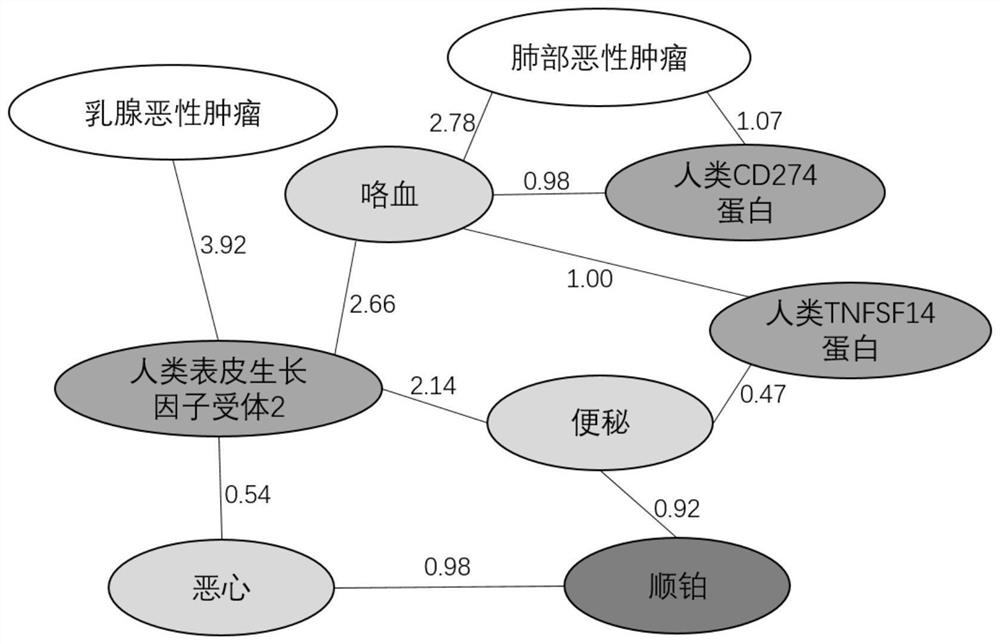
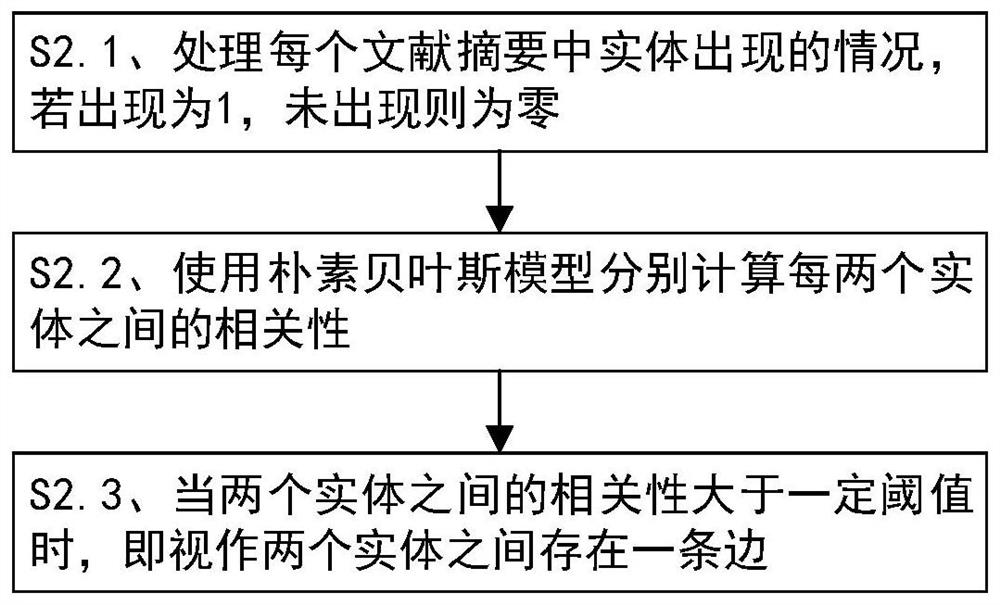
Interpretable adverse drug reaction discovery method based on literature knowledge graph
PendingCN113161013AAdverse reaction foundReliable methodMedical data miningDrug referencesDiseaseDrug adverse reactions
The invention discloses an interpretable adverse drug reaction discovery method based on a literature knowledge graph. The method comprises the following steps: S1, extracting four entities from medical literature data: a disease, a biomarker, a drug and an adverse reaction; s2, constructing the literature knowledge graph by using the four entities and utilizing a naive Bayesian model: the literature knowledge graph comprises vertexes and edges, the vertexes comprise vertexes of four entity types, the edges represent the relationship between the two vertexes, the weight on the edges represents the correlation between the two vertexes, and the correlation is described through importance indexes; s3, based on the adverse drug reaction in the literature knowledge map, comparing a drug specification, and discovering potential adverse reactions; and S4, providing reasonable biomarker path interpretation for the potential adverse reaction based on a literature knowledge graph. Potential adverse reactions of drugs are explored through a technical means of computational medicine, and power is provided for mechanism research of the adverse reactions.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
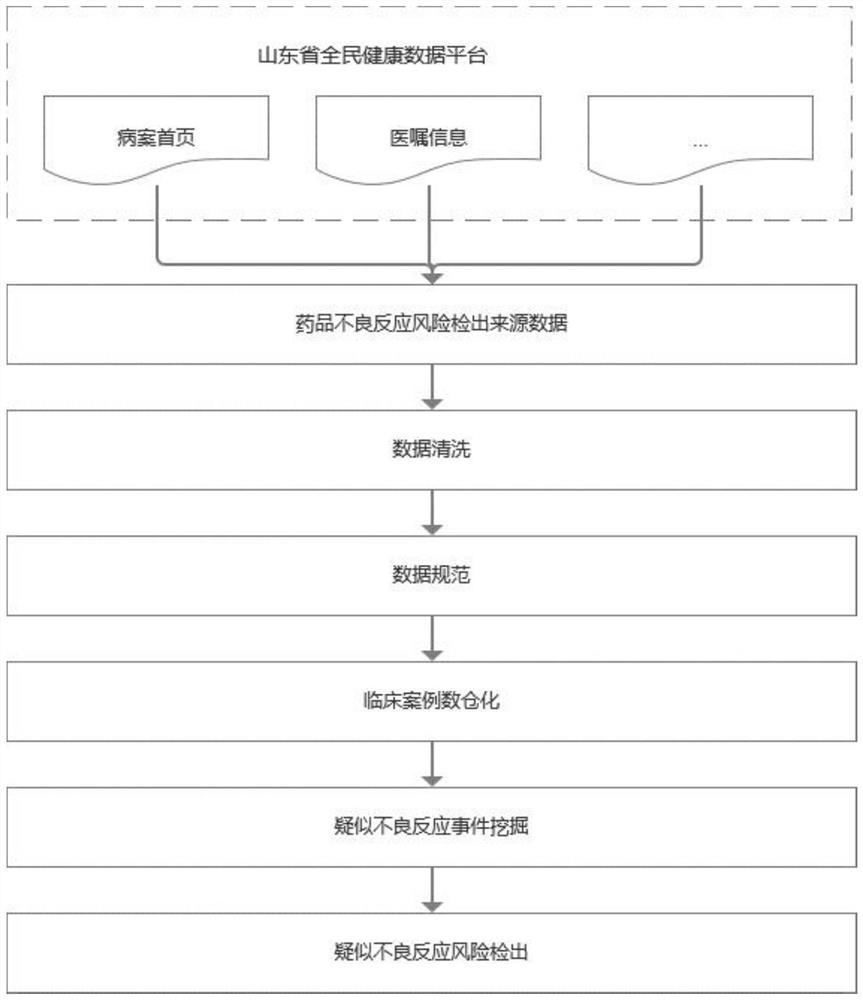
Method for detecting adverse drug reaction signal
PendingCN112669991AAccurate and safe monitoringReduce riskDrug referencesInformatizationData warehouse
The invention discloses a method for detecting adverse drug reaction signal, and belongs to the technical field of computer software and medical informatization. According to the drug adverse reaction signal detection method, data related to adverse drug reactions in a national health information platform is obtained, the data is cleaned and normalized and then integrated into a clinical case data warehouse again, and adverse reaction risk signals are detected according to a preset mining algorithm and a signal detection model. The method for detecting the adverse drug reaction signals can timely, comprehensively and accurately monitor the safety of medical instruments on the market, processes the detected signals in time, reduces the risk of adverse drug reactions of patients, and has good popularization and application values.
Owner:山东健康医疗大数据有限公司
Christina loosestrife herb polysaccharide with immunity-strengthening effect and preparation method and application thereof
InactiveCN107011455AImmunity is effectiveReduce adverse reactionsOrganic active ingredientsImmunological disordersIon-exchange resinWeak base
The invention discloses a christina loosestrife herb polysaccharide with an immunity-strengthening effect and solves the problems that a drug for strengthening the human immunity is obvious in adverse reaction and inaccurate in effect in the prior art. The christina loosestrife herb polysaccharide is prepared through degreasing christina loosestrife herb, carrying out water extraction and alcohol precipitation, intercepting a component of which the molecular weight is greater than 10,000Dalton through dialysis and separating through macroporous weak-base anion-exchange resin. The preparation method comprises the following steps of degreasing, water extraction, alcohol precipitation, dialysis, macroporous weakly basic anion exchange resin column chromatography and drying. The christina loosestrife herb polysaccharide is applied to preparation of foods and health foods or drugs with the immunity-strengthening effect. The christina loosestrife herb polysaccharide is accurate in immunity-strengthening effect, small in adverse reaction, simple in preparation method, simple in operation and suitable for industrial production.
Owner:成都众宜坊农业开发有限公司
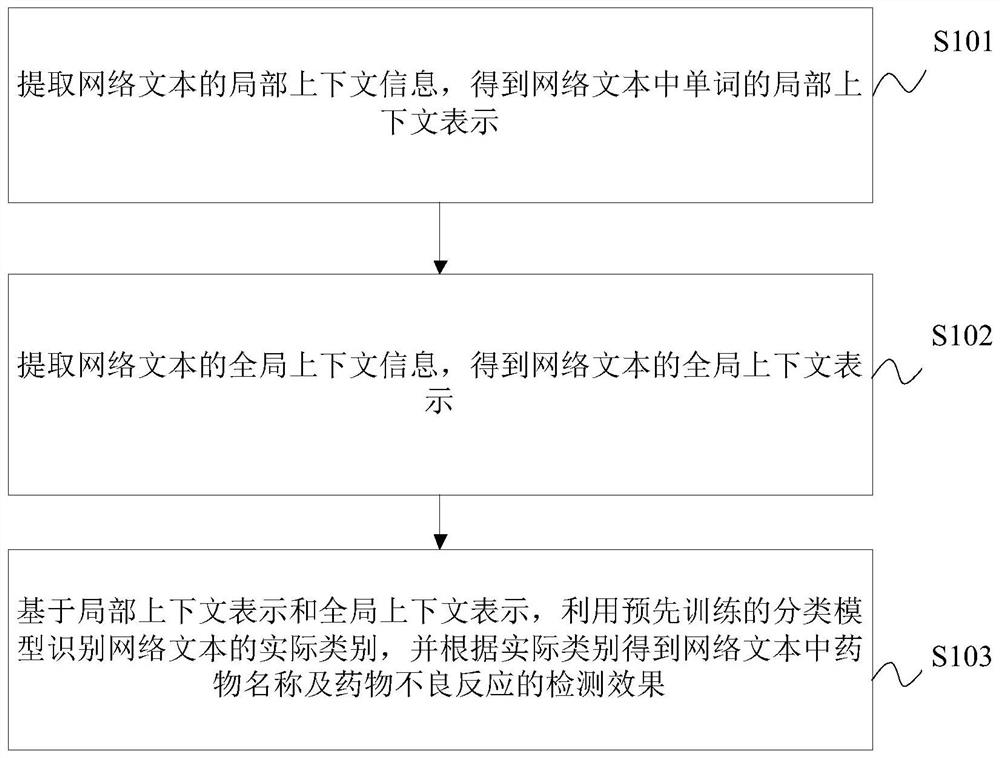
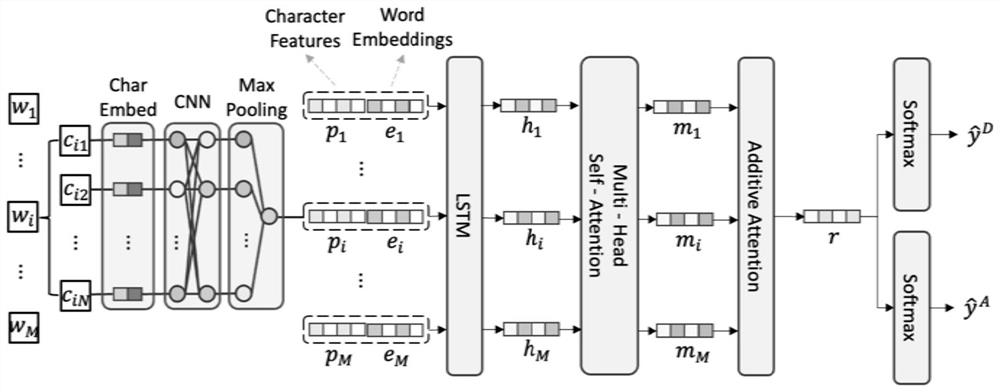
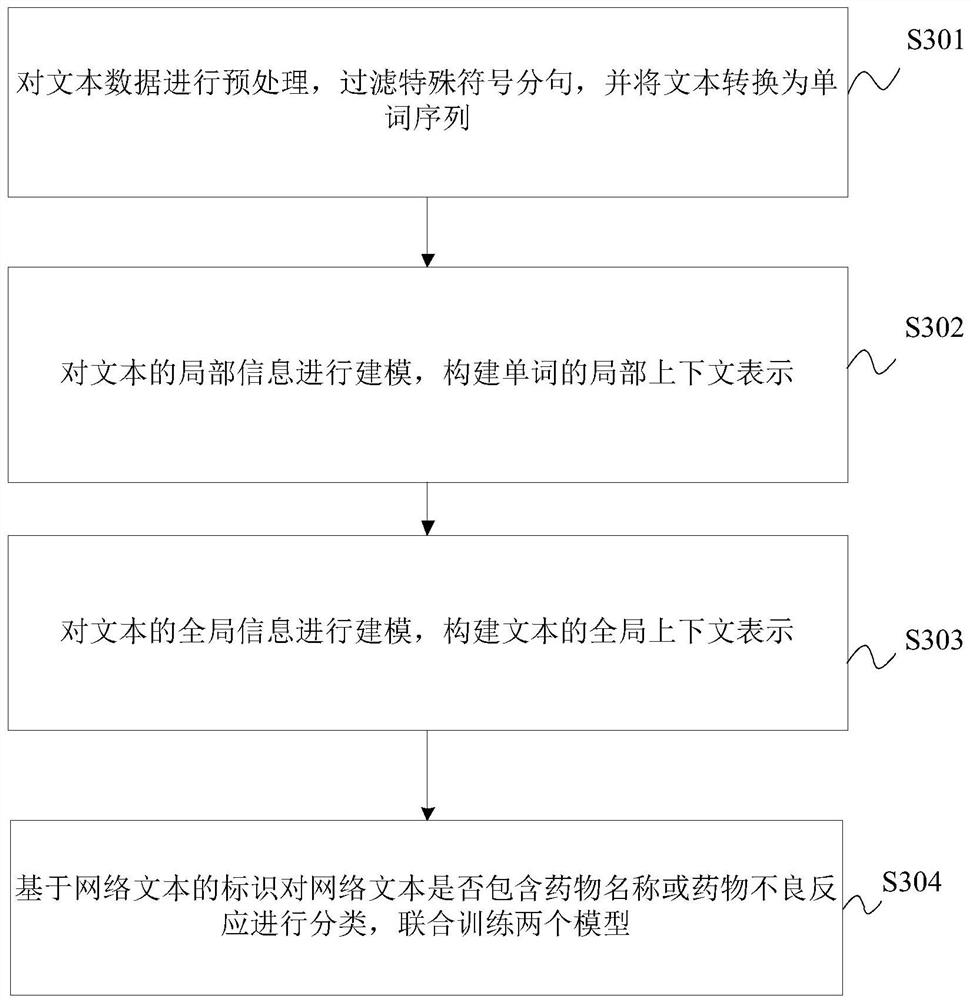
Joint detection method for drug name and adverse drug reaction in web text
PendingCN114492386ANatural language data processingNeural architecturesDrug adverse reactionsCybertext
The invention relates to the technical field of text processing, in particular to a joint detection method for drug names and adverse drug reactions in web texts, and the method comprises the following steps: extracting local context information of the web texts to obtain local context representations of words in the web texts; extracting global context information of the web text to obtain global context representation of the web text; and based on the local context representation and the global context representation, utilizing a pre-trained classification model to identify an actual category of the web text, and obtaining a detection effect of the drug name and the adverse drug reaction in the web text according to the actual category. Therefore, the detection effect of the drug name and the adverse drug reaction in the web text is effectively improved.
Owner:TSINGHUA UNIV
Knowledge graph-based adverse drug reaction prediction system and method
PendingCN114743692AAvoid harmRemind drug safetyDrug referencesKnowledge representationDrug adverse reactionsTheoretical computer science
The invention relates to an adverse drug reaction prediction system and method based on a knowledge graph, the system comprises a database module, a knowledge graph module and a prediction module, the database module is connected with the knowledge graph module, and the knowledge graph module is in interactive connection with the prediction module. The database module is used for constructing a database containing a plurality of drug names and corresponding adverse reaction data; the knowledge graph module is used for inquiring the adverse reaction of the to-be-detected medicine from the database and drawing a corresponding knowledge graph; judging whether adverse reaction conflicts exist among the plurality of to-be-detected medicines or not, and drawing a corresponding knowledge graph; the prediction module is used for predicting the potential adverse reaction of the to-be-detected medicine. Compared with the prior art, the method has the advantages that the known adverse reaction of the to-be-detected medicine can be quickly and accurately inquired, the potential adverse reaction of the medicine can be predicted, and meanwhile, the adverse reaction conflict among multiple medicines can be judged, so that the harm possibly generated by multi-medicine combination is avoided.
Owner:SHANGHAI UNIV OF ENG SCI
Composition for prevention and/or improvement of adverse drug reaction, symptom associated with adverse drug reaction, and/or adverse reaction associated with medical treatment
PendingUS20220023335A1Prevent and improve adverse drug reactionIncrease adverse drug reactionInorganic active ingredientsAntinoxious agentsDrug adverse reactionsPharmaceutical drug
The present invention provides a composition for prevention and / or improvement of an adverse drug reaction, a symptom associated with an adverse drug reaction, and / or an adverse reaction associated with medical treatment. More specifically, the present invention provides a composition for prevention and / or improvement of an adverse drug reaction, a symptom associated with an adverse drug reaction, and / or an adverse reaction associated with medical treatment in a subject, comprising molecular hydrogen as an active ingredient.
Owner:MIZ CO LTD
Method of using animal models to predict adverse drug reactions
InactiveUS20050005309A1Disease diagnosisBiological testingPharmaceutical industryADR - Adverse drug reaction
The invention relates generally to the process of target validation in the pharmaceutical industry. A process for validating molecular targets is disclosed.
Owner:CANTOR GLENN H +1
Method of Dosing a Patient with Multiple Drugs Using Adjusted Phenotypes of CYP450 Enzymes
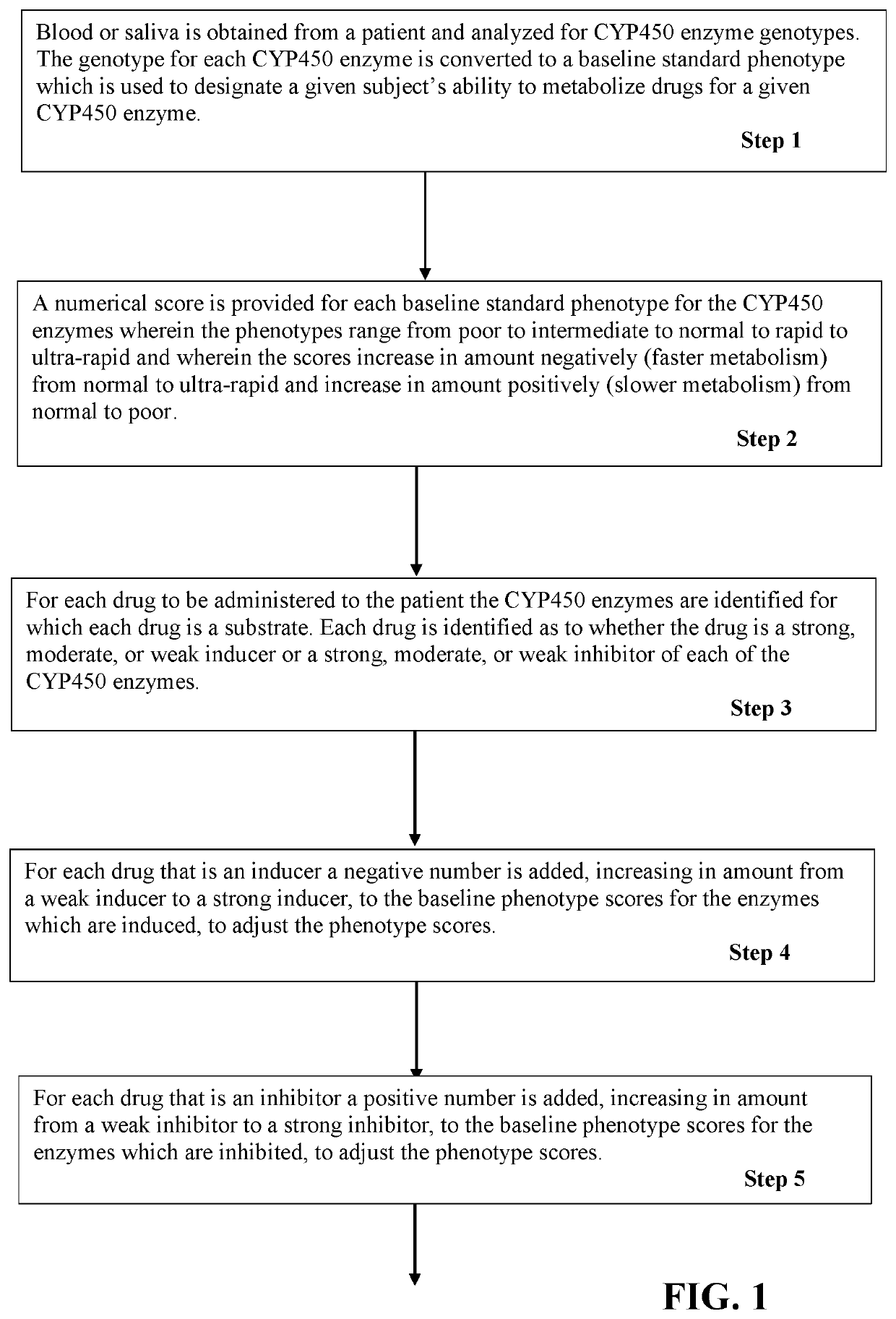
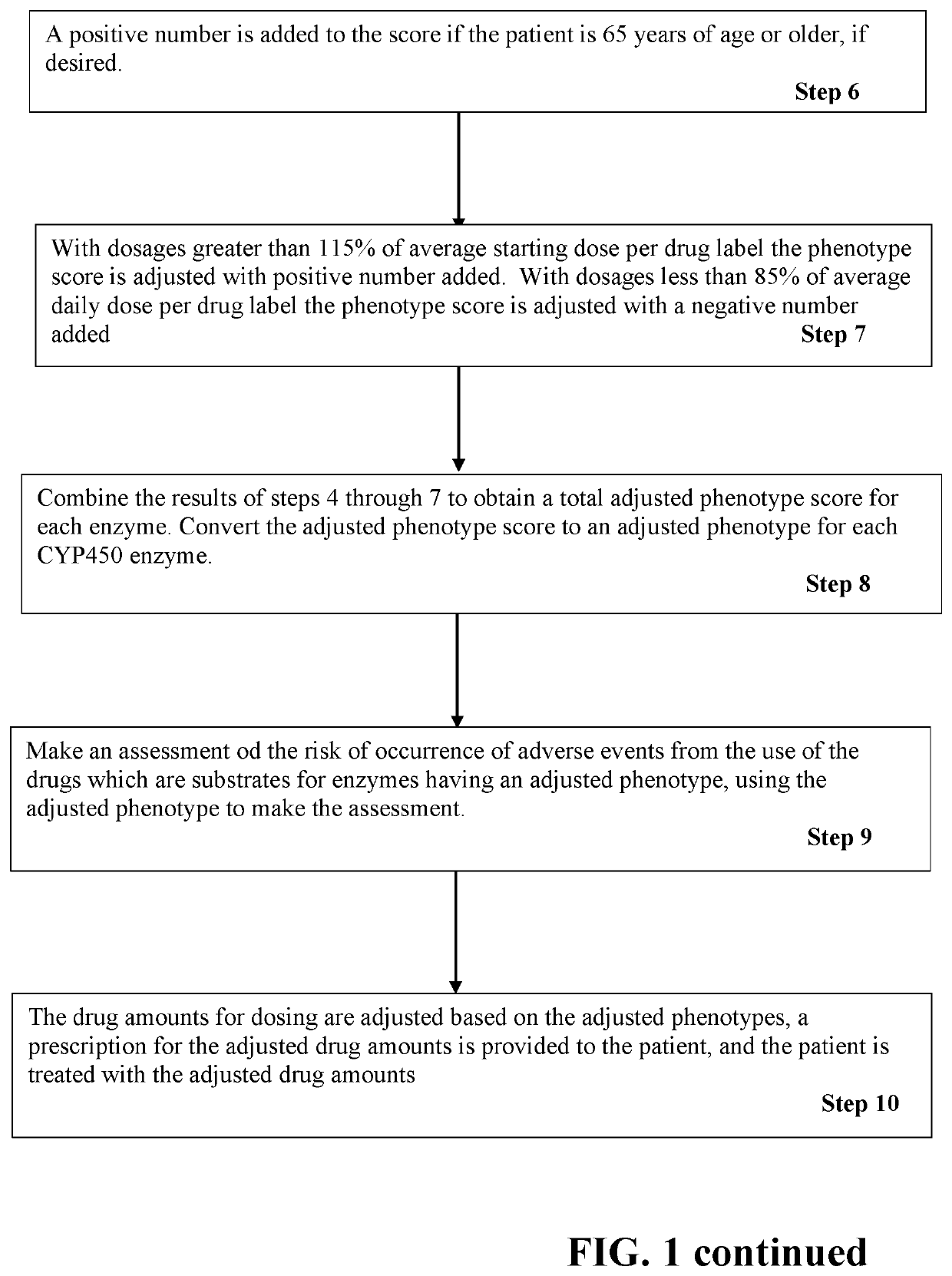
PendingUS20200199659A1Accurate rapid risk assessmentEasy to adjustHealth-index calculationMicrobiological testing/measurementDrug adverse reactionsPharmaceutical drug
A method of treating a patient with multiple drugs using adjusted phenotypes of CYP450 enzymes to assess the risk of adverse drug reactions occurring due to drug-enzyme interactions. CYP450 enzyme genotypes and phenotypes are measured in a patient. The phenotypes are scored numerically. The drugs intended for treatment are scored numerically for their ability to induce or inhibit the CYP450 enzymes. The drug scores are used to adjust the CYP450 phenotype scores relative to inducing or inhibiting the enzymes. An accurate adjusted phenotype score for a given CYP450 enzyme is converted to an accurate adjusted phenotype. Any of the intended drugs for treatment that are substrates for the given CYP450 enzyme can be evaluated for risk of an adverse drug reaction based on the adjusted phenotype. This method of rapid risk assessment provides an accurate basis for decisions regarding changes in dose, eliminating a drug, or replacing a drug.
Owner:STODDARD JOHN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com