Kukoline intravenous transfusion preparation
A technology for sinomenine and intravenous administration, which is applied in the direction of anti-inflammatory agents, drug delivery, non-central analgesics, etc., can solve problems such as hindering application and drug efficacy, improve clinical application safety, avoid adverse reactions, The effect of promoting widespread application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
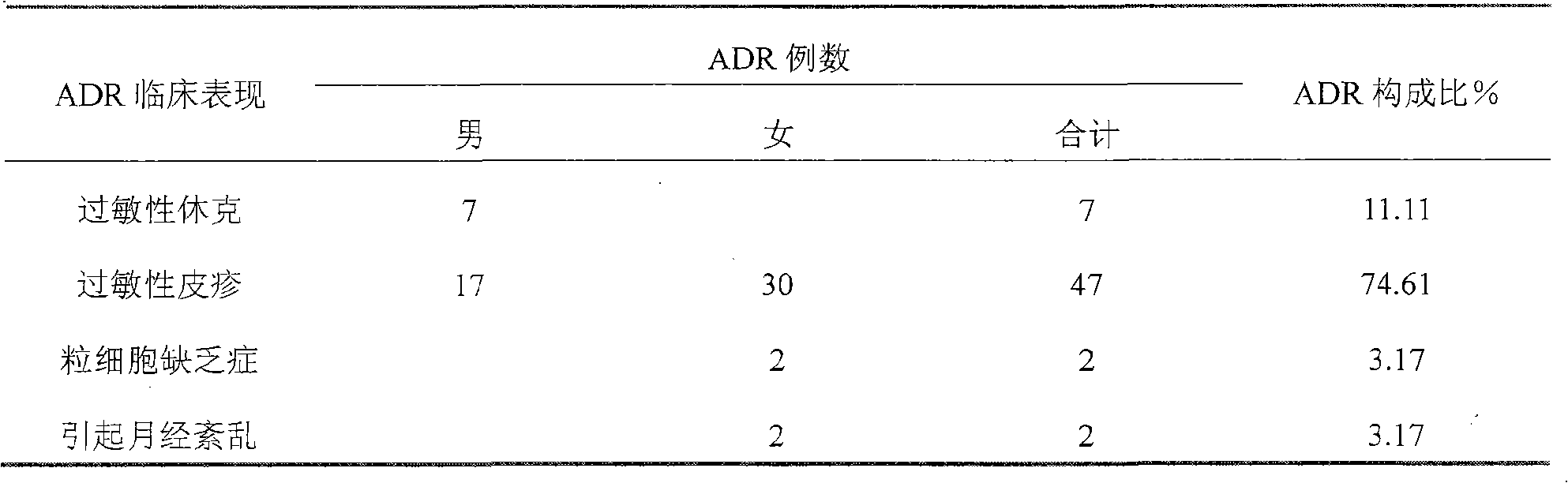
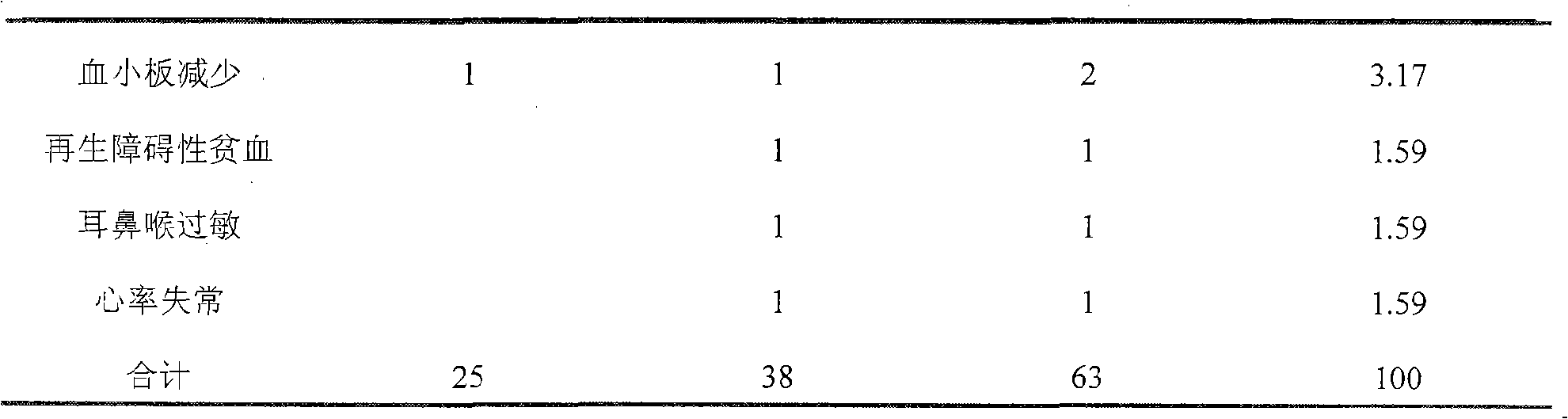
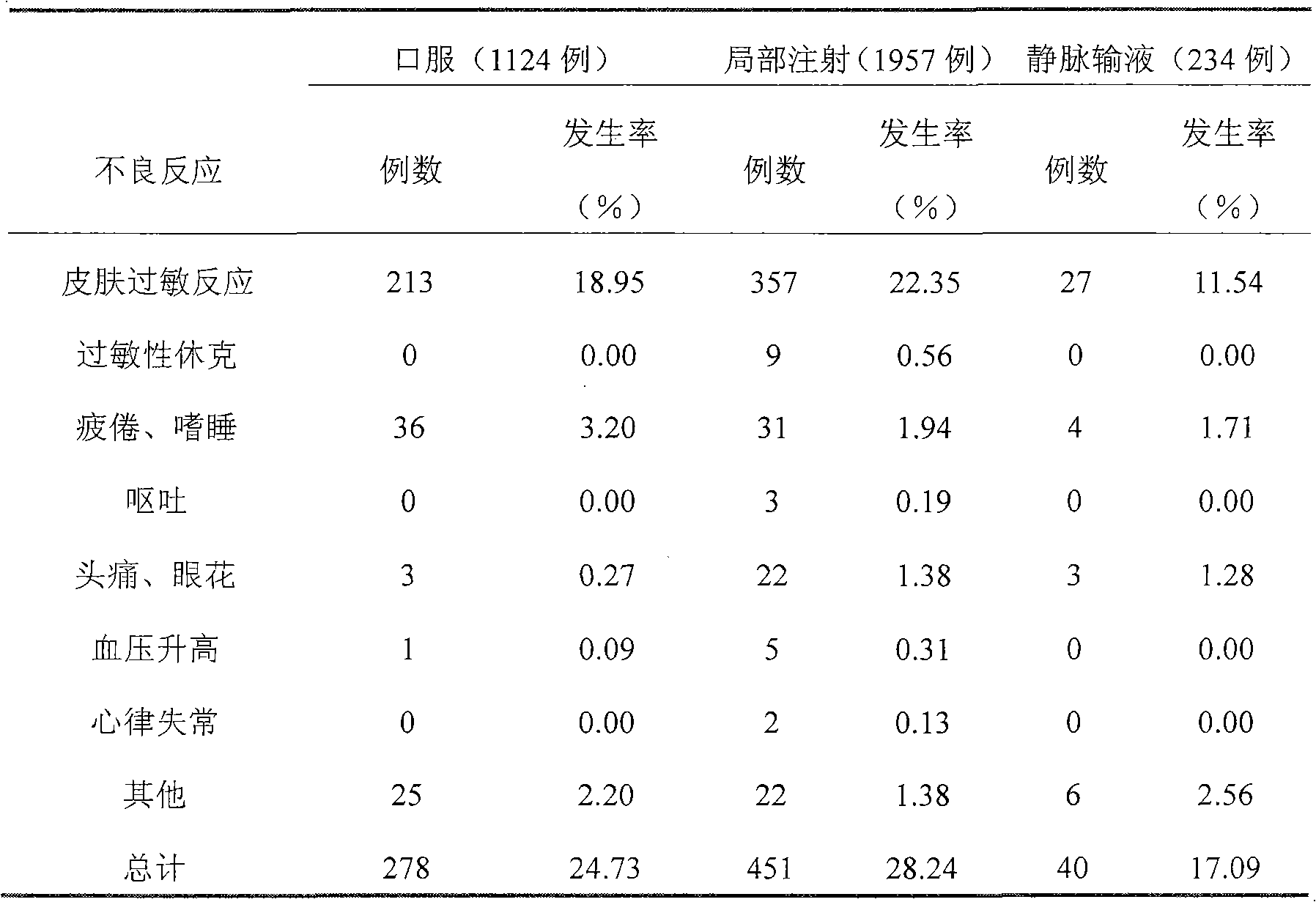
[0042] Sample preparation method: Take 0.3g of sinomenine hydrochloride, 0.0005g of disodium edetate, 0.016g of sodium sulfite, and 9g of sodium chloride, dissolve them in an appropriate amount of water for injection, heat to 80°C, add an appropriate amount of activated carbon for injection, and keep warm After 15 minutes, filter out the activated carbon, and after fine filtration with a microporous membrane, add water for injection from the filter to 1000ml, fill it into 250ml / bottle, and sterilize it to obtain. A total of 200 bottles were prepared. The concentration of sinomenine is 0.03wt%
[0043] Usage: Intravenous infusion, 250ml each time, 1-2 times a day.
Embodiment 2
[0045] Sample preparation method: take 0.6g of sinomenine hydrochloride, 0.0005g of disodium edetate, 0.016g of sodium sulfite, and 9g of sodium chloride, dissolve them in an appropriate amount of water for injection, heat to 80°C, add an appropriate amount of activated carbon for injection, and keep warm After 15 minutes, filter out the activated carbon, and after fine filtration with a microporous membrane, add water for injection from the filter to 1000ml, fill it into 250ml / bottle, and sterilize it to obtain. A total of 200 bottles were prepared. The concentration of sinomenine is 0.06wt%.
Embodiment 3
[0047] Sample preparation method: Take 1.0g of sinomenine hydrochloride and 50g of glucose, dissolve them in an appropriate amount of water for injection, heat to 80°C, add an appropriate amount of activated carbon for injection, keep warm for 20 minutes, filter out the activated carbon, and fine filter through a microporous membrane, Add water for injection from the filter to 1000ml, fill to 250ml / bottle, and sterilize to get ready. A total of 200 bottles were prepared. The concentration of sinomenine is 0.1wt%
[0048] Usage: Intravenous infusion, 250ml each time, 1-2 times a day or as directed by the doctor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com