Rapid identification method and system for adverse reactions of drugs based on big data
A technology of adverse reaction and identification method, applied in the field of emergency medicine, which can solve the problems of incomplete exposure of adverse drug reactions, long time-consuming, and difficulty in obtaining adverse drug reaction information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
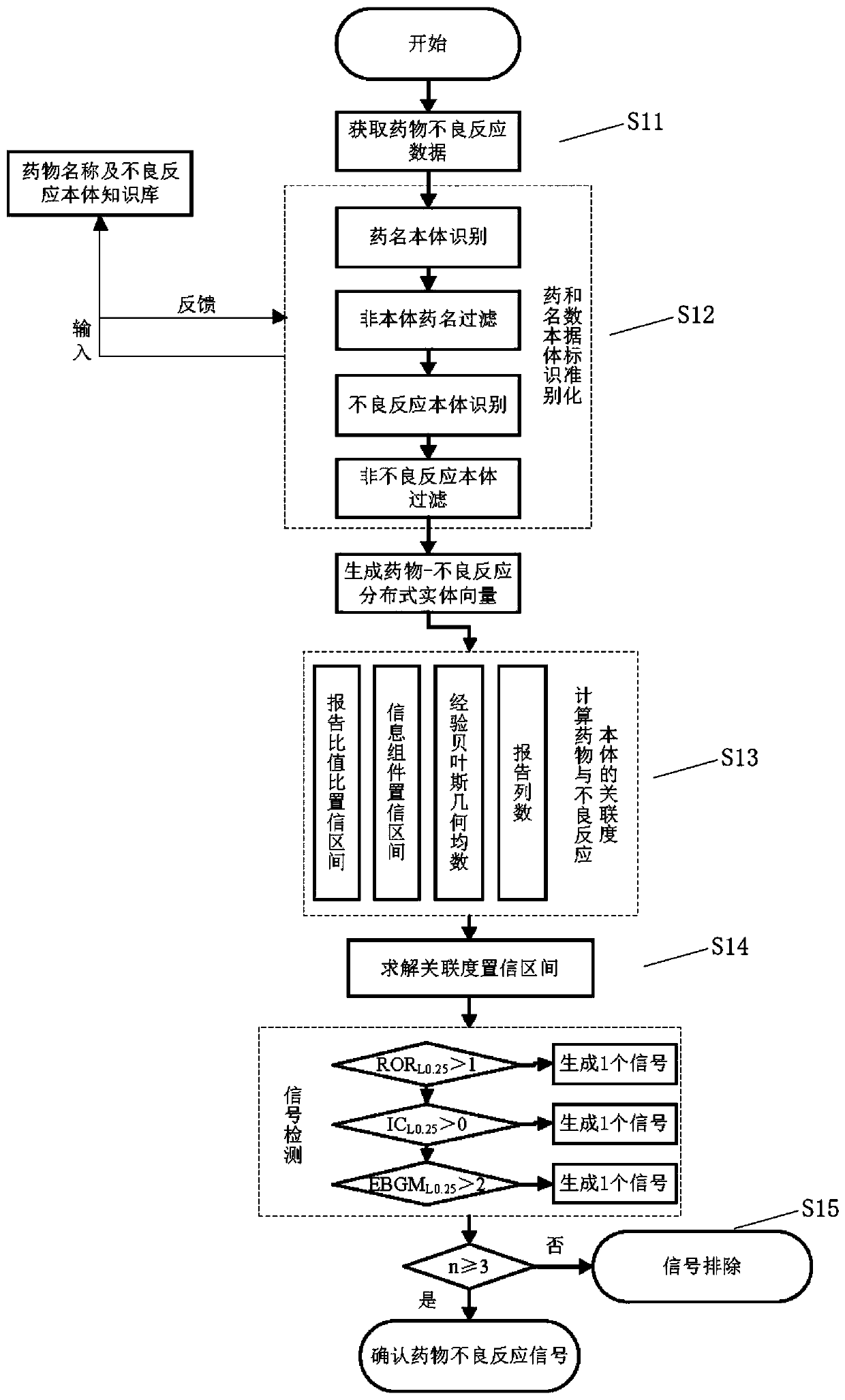
[0038] This embodiment provides a fast identification method for adverse drug reactions based on big data, such as figure 1 shown, including steps:
[0039] S11. Obtain adverse drug reaction data;
[0040] S12. Compare the obtained adverse drug reaction data with the pre-stored drug name ontology knowledge base and adverse reaction name ontology knowledge base respectively to generate drug-adverse reaction distributed entity vectors;
[0041] S13. According to the generated drug-adverse reaction distributed entity vector, calculate several correlation values between the drug and the adverse reaction ontology;
[0042] S14. Solve the confidence interval of each correlation value according to the calculated several correlation values, and compare the confidence interval of each correlation value obtained by the solution with a preset reference value to obtain a comparison result;
[0043] S15. Judging whether the comparison result is greater than a preset threshold, if yes, ...
Embodiment 2
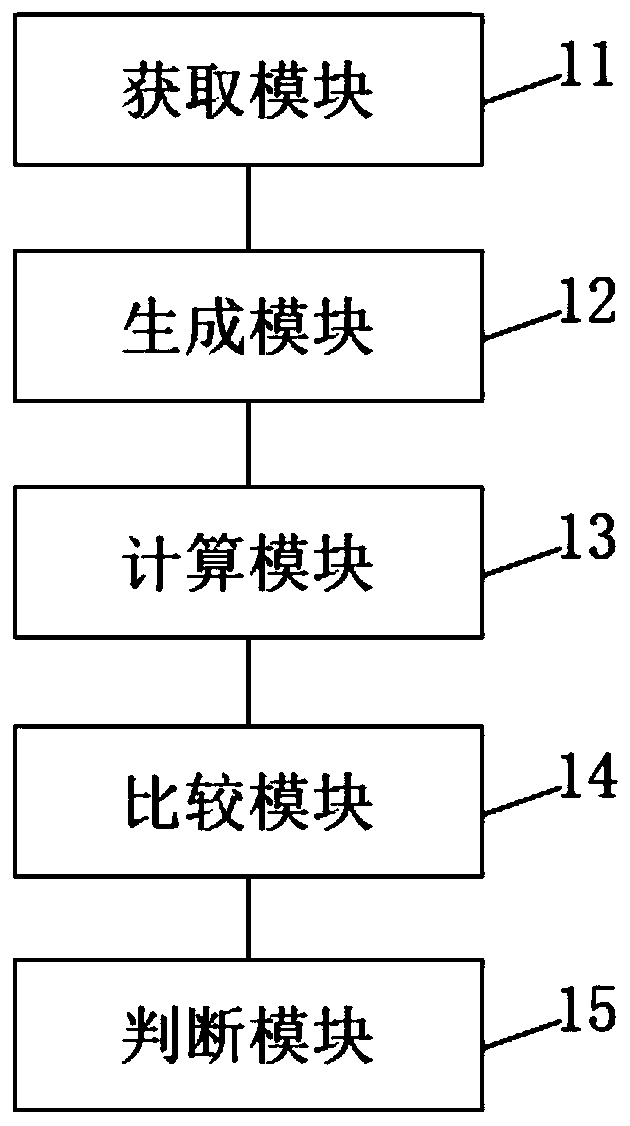
[0101] This embodiment provides a rapid identification system for adverse drug reactions based on big data, such as figure 2 shown, including:
[0102] An acquisition module 11, configured to acquire adverse drug reaction data;
[0103] Generating module 12, for comparing the obtained adverse drug reaction data with the pre-stored drug name ontology knowledge base and adverse reaction name ontology knowledge base respectively, to generate drug-adverse reaction distributed entity vector;
[0104] Calculation module 13, used to calculate several correlation values between the drug and the adverse reaction ontology according to the generated drug-adverse reaction distributed entity vector;
[0105] The comparison module 14 is used to solve the confidence interval of each correlation degree value according to the calculated several correlation degree values, and compare the confidence interval of each correlation degree value obtained by the solution with a preset reference va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com