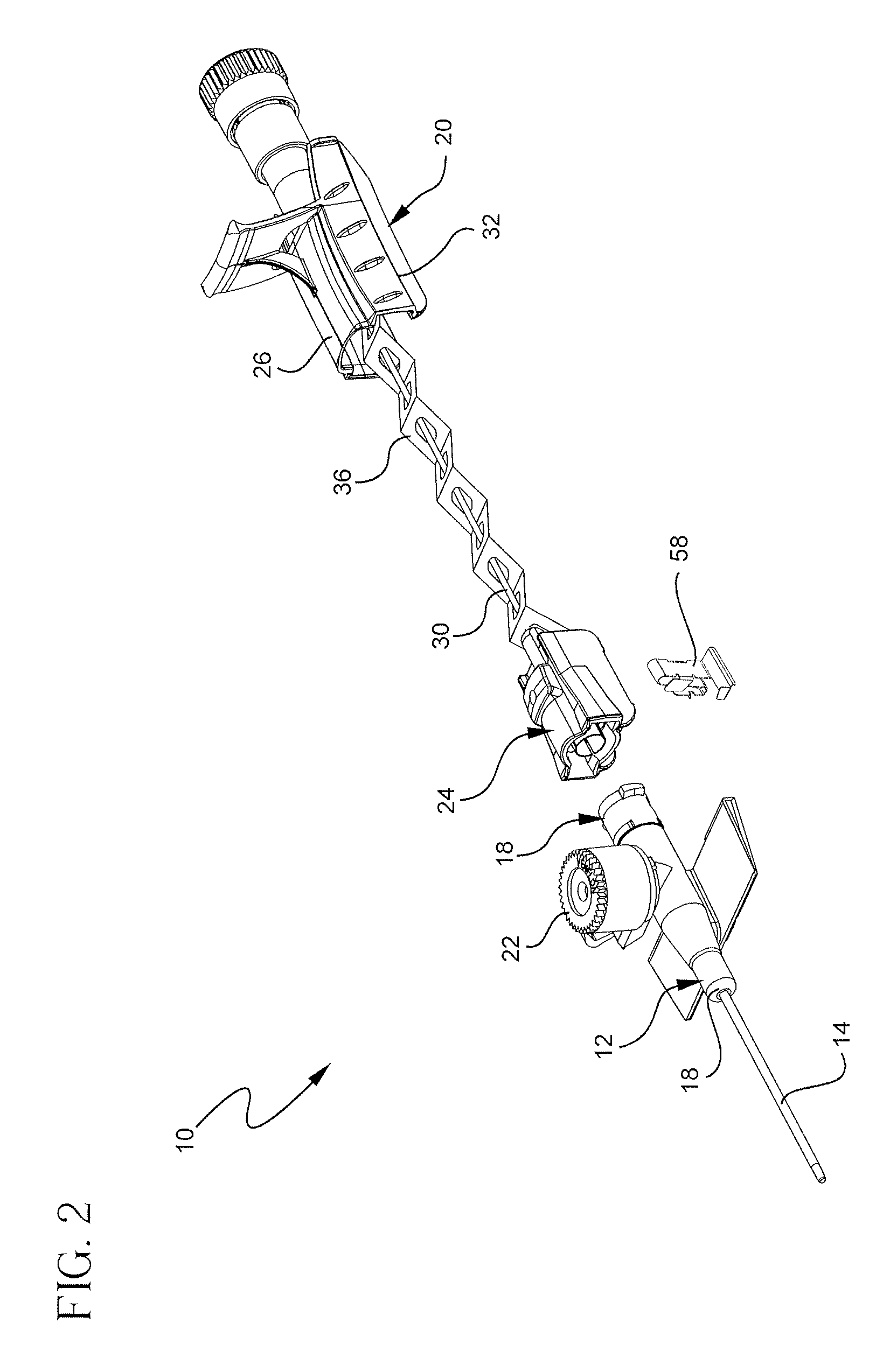
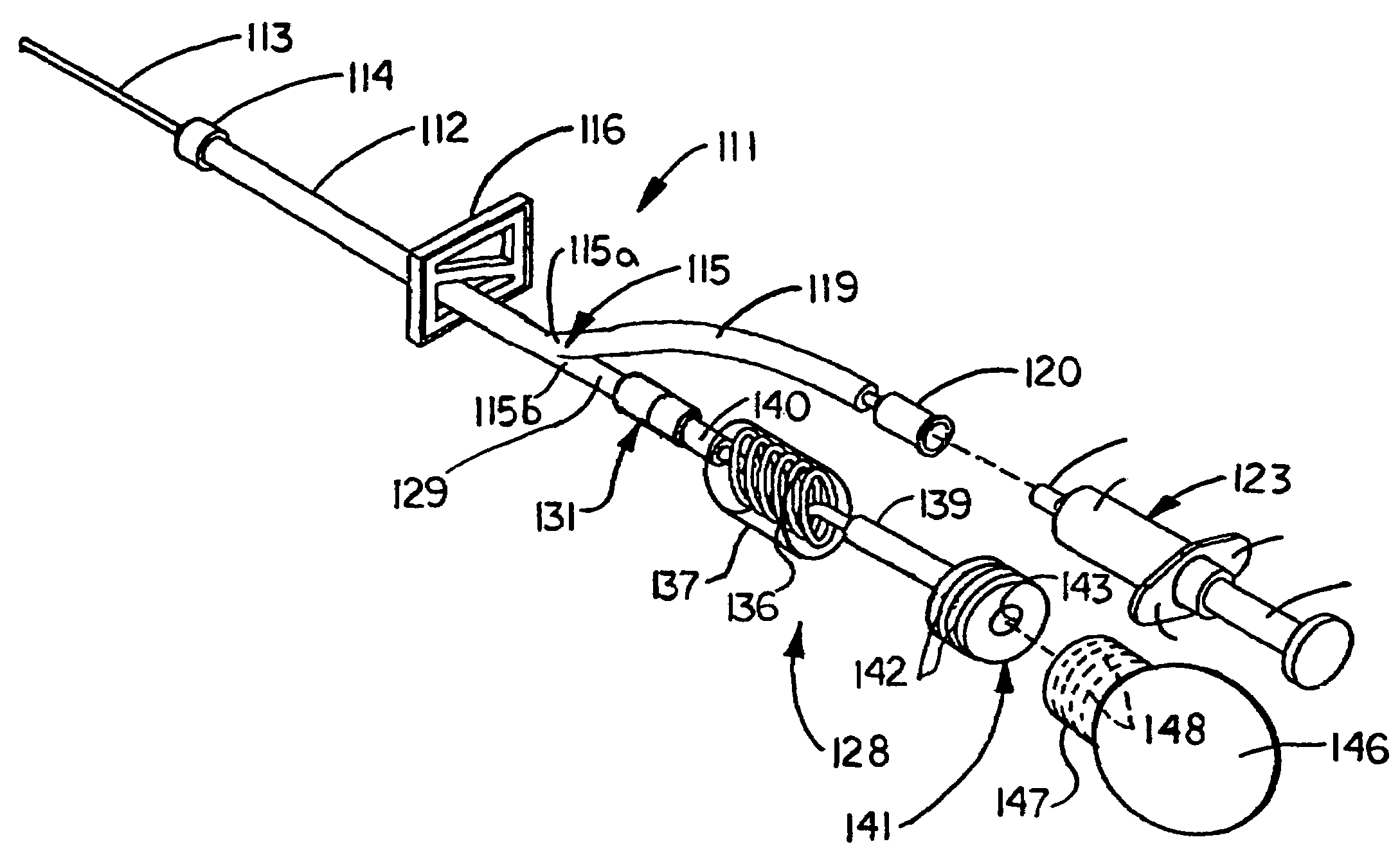
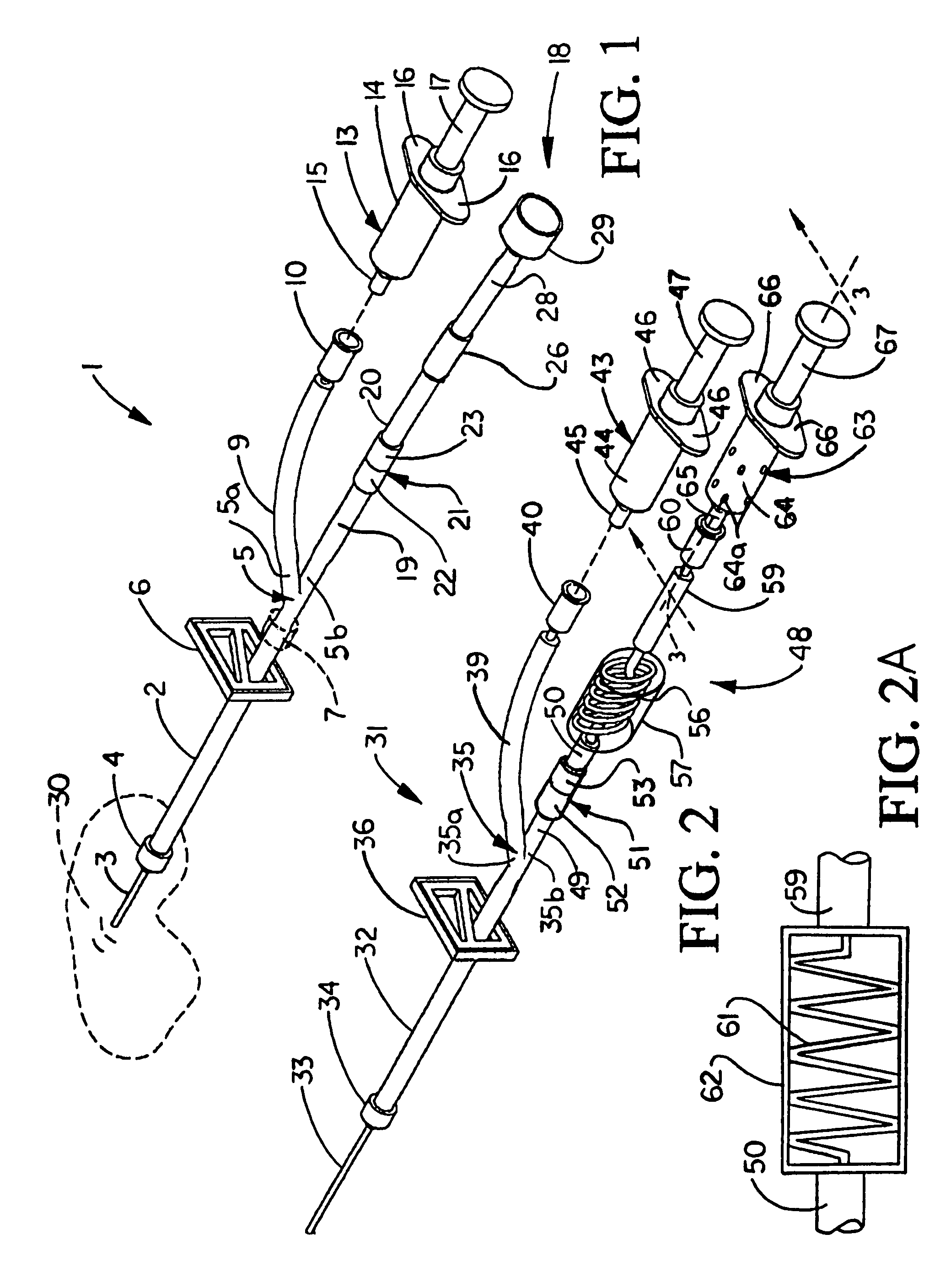
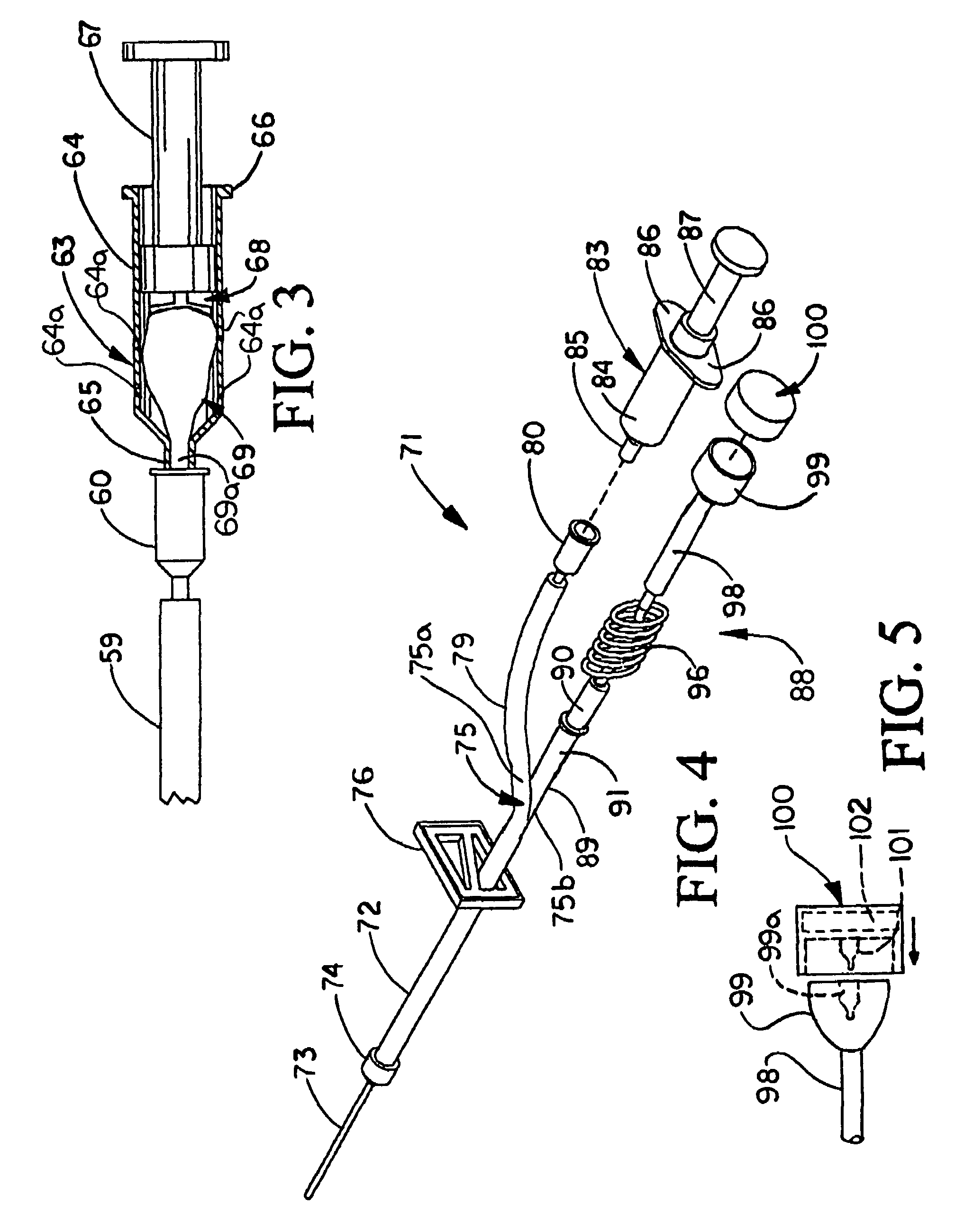
Patents
Literature
741 results about "Blood capillary" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Blood capillary. n. One of the minute blood vessels that connect arterioles and venules and are a part of an intricate network throughout the body for the interchange of oxygen, carbon dioxide, and other substances between blood and tissue cells.
Drug administration method
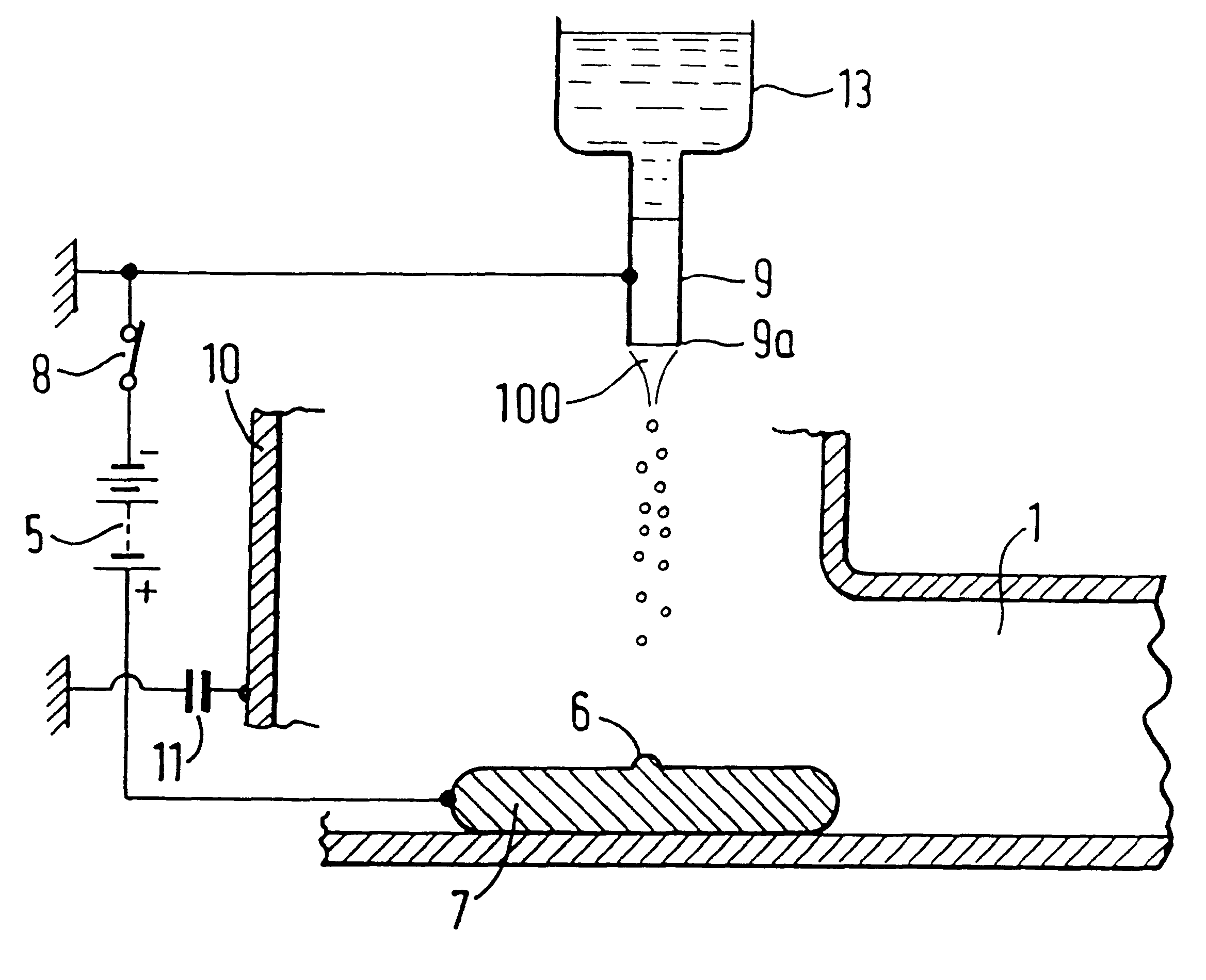
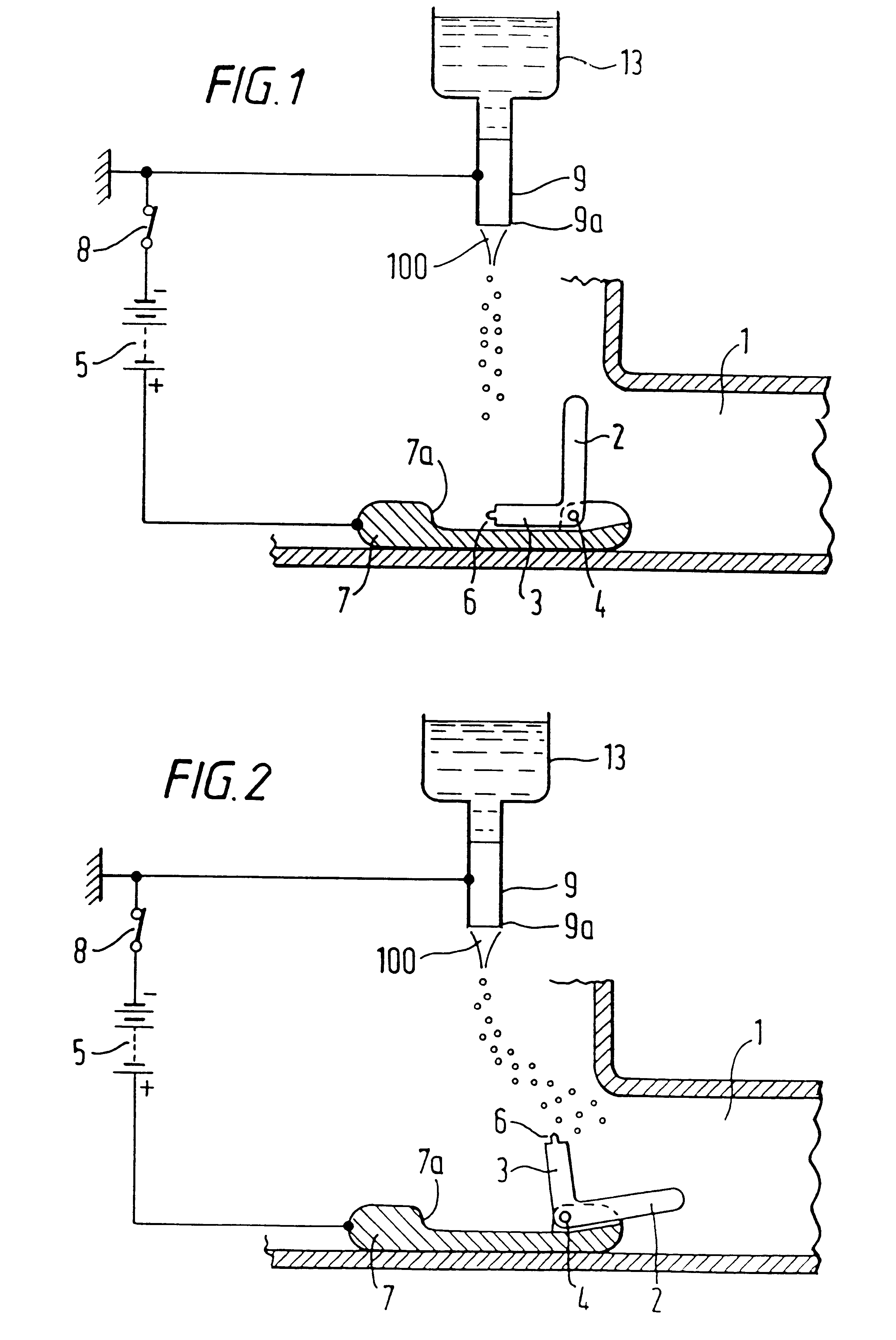
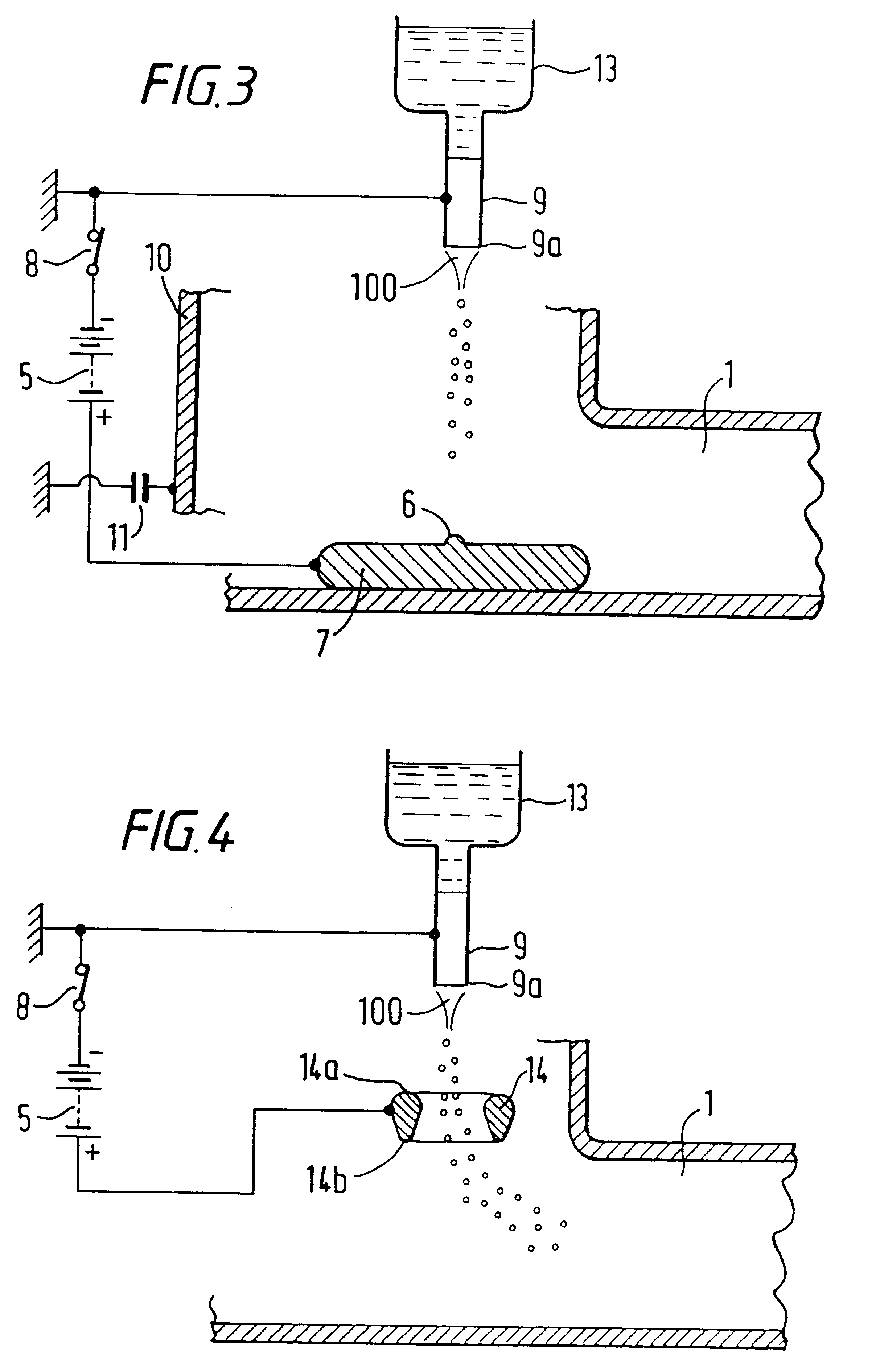
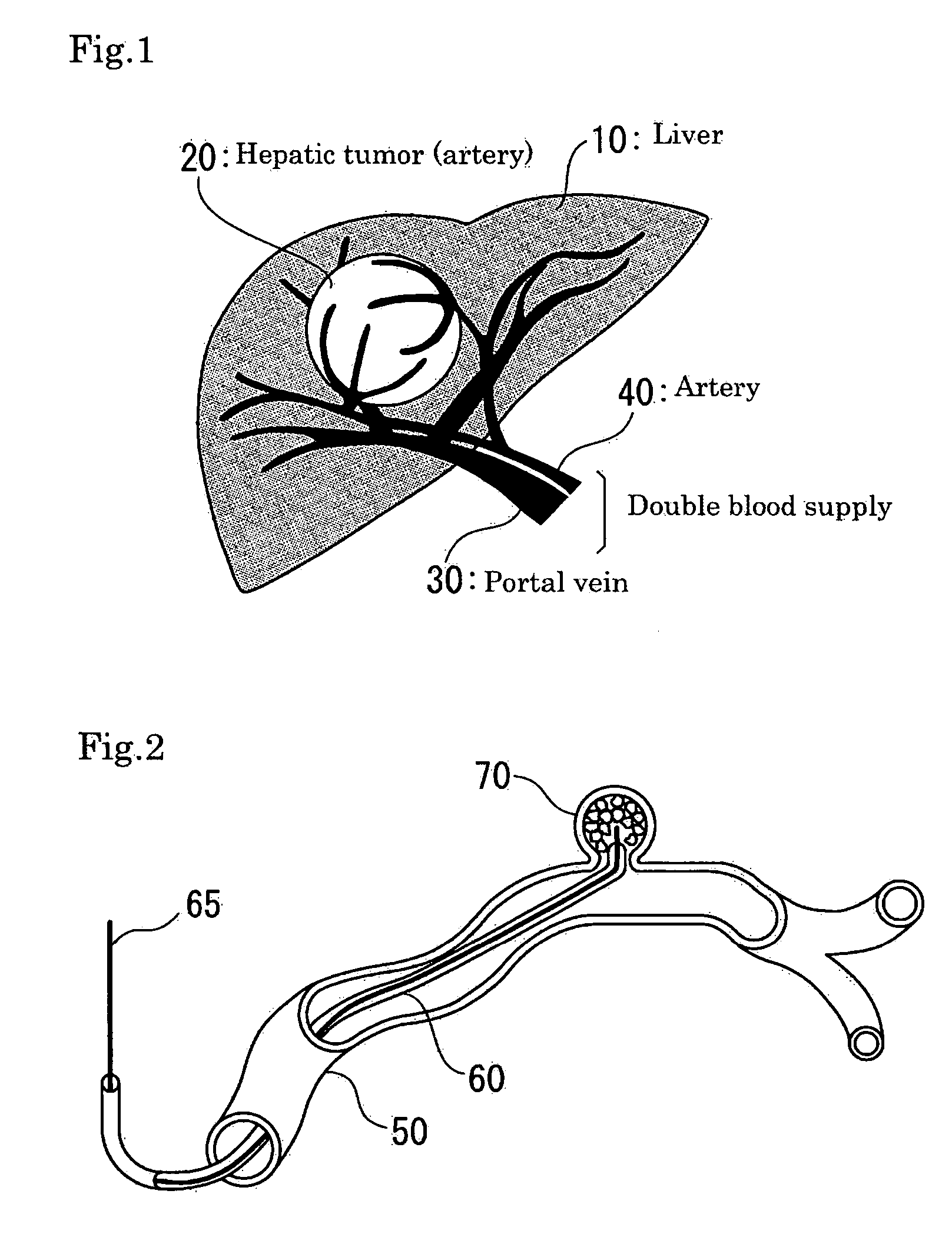
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
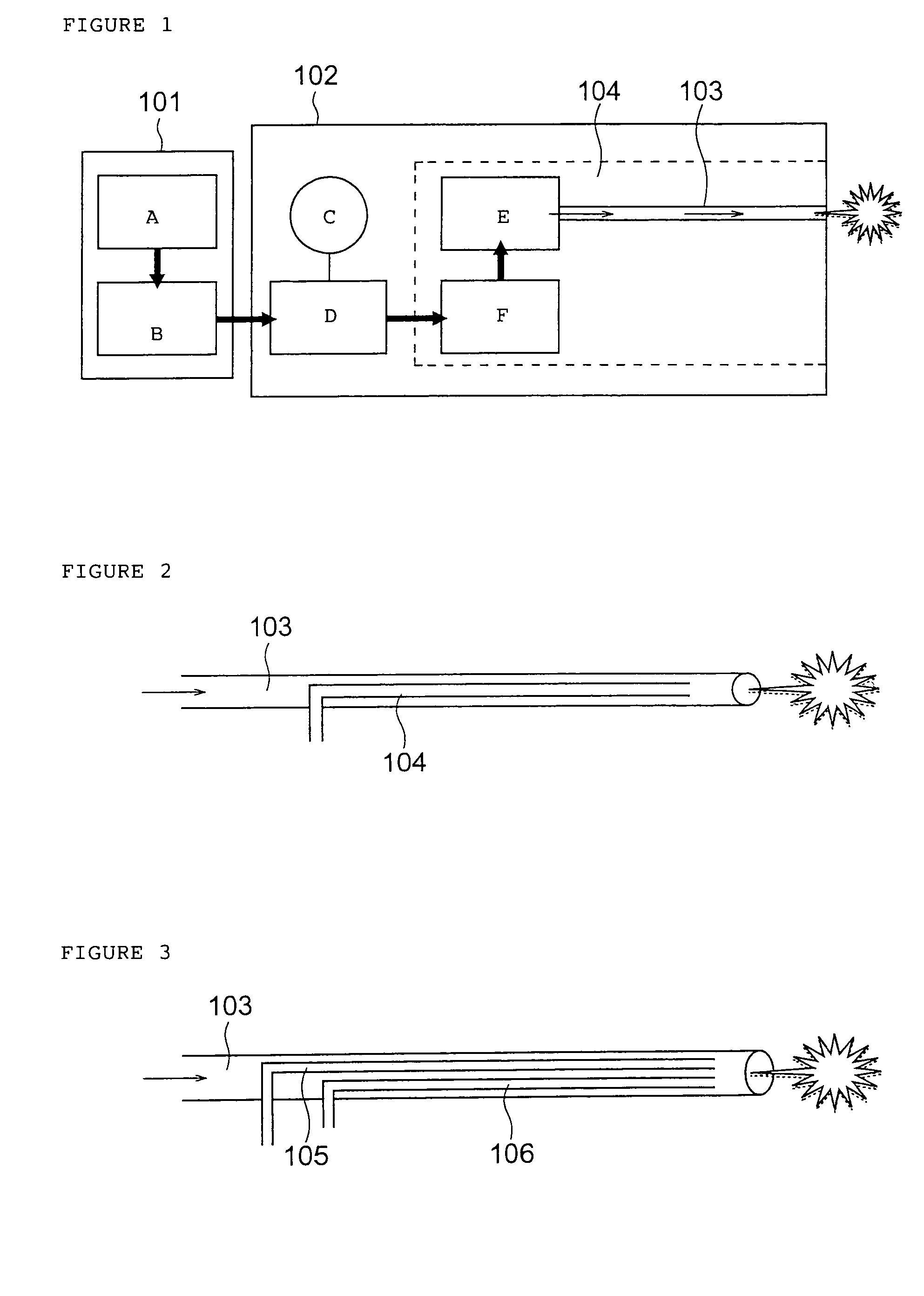
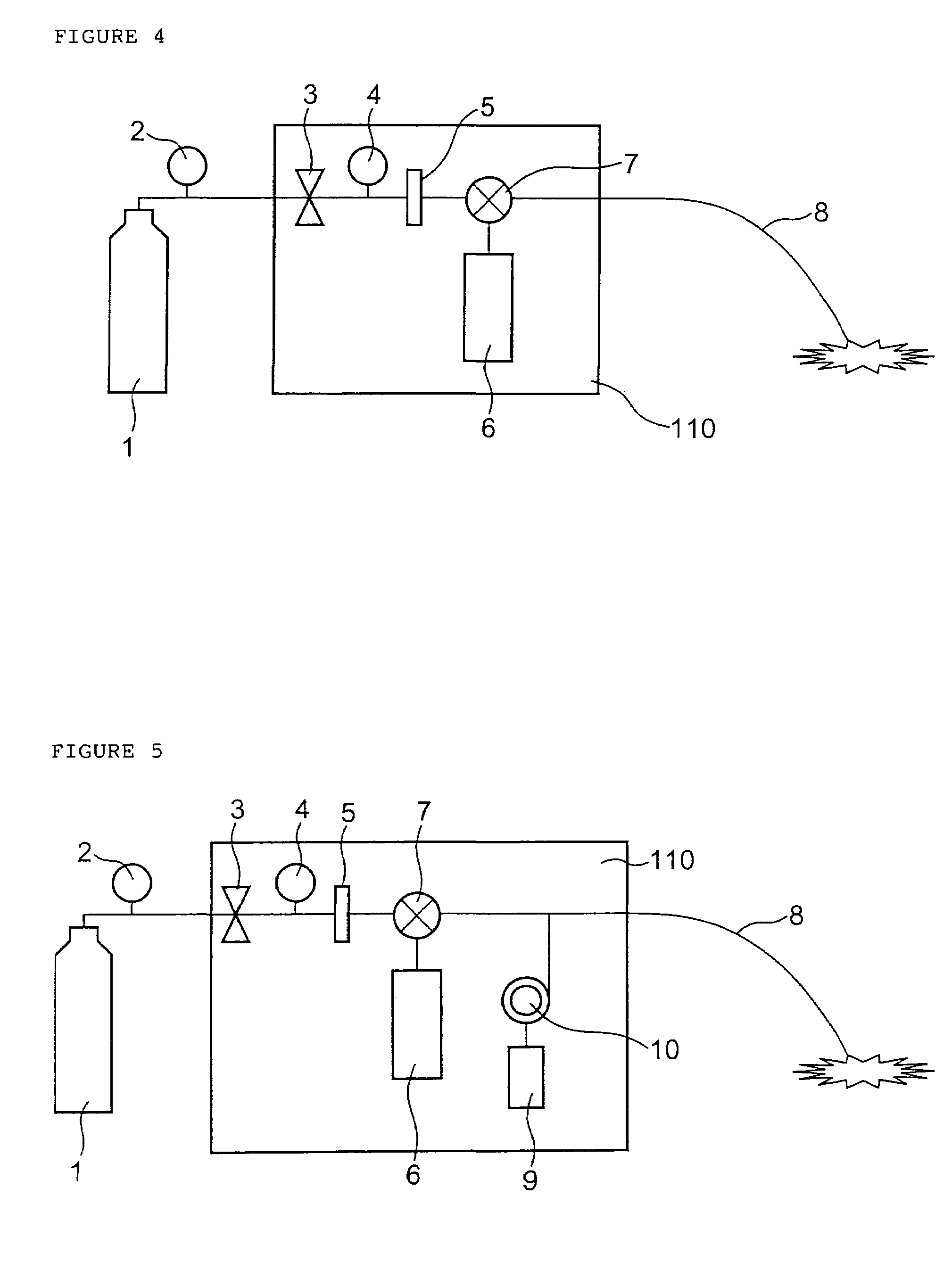
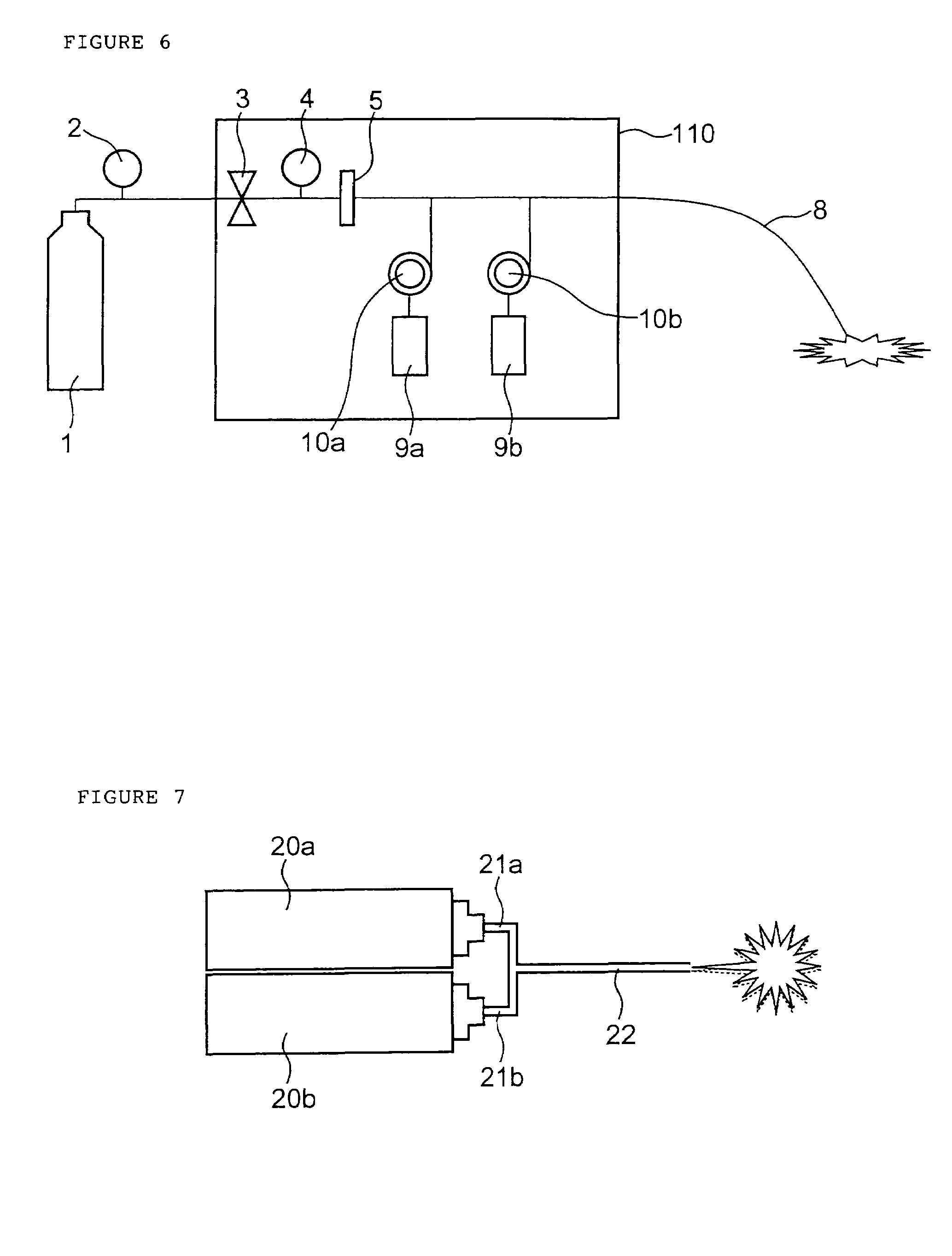
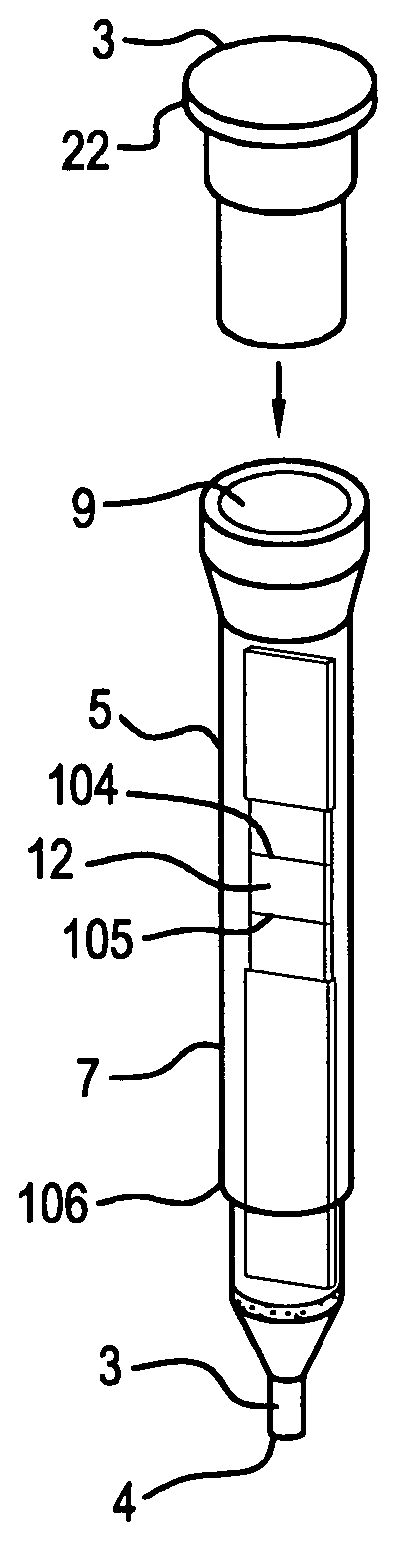
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
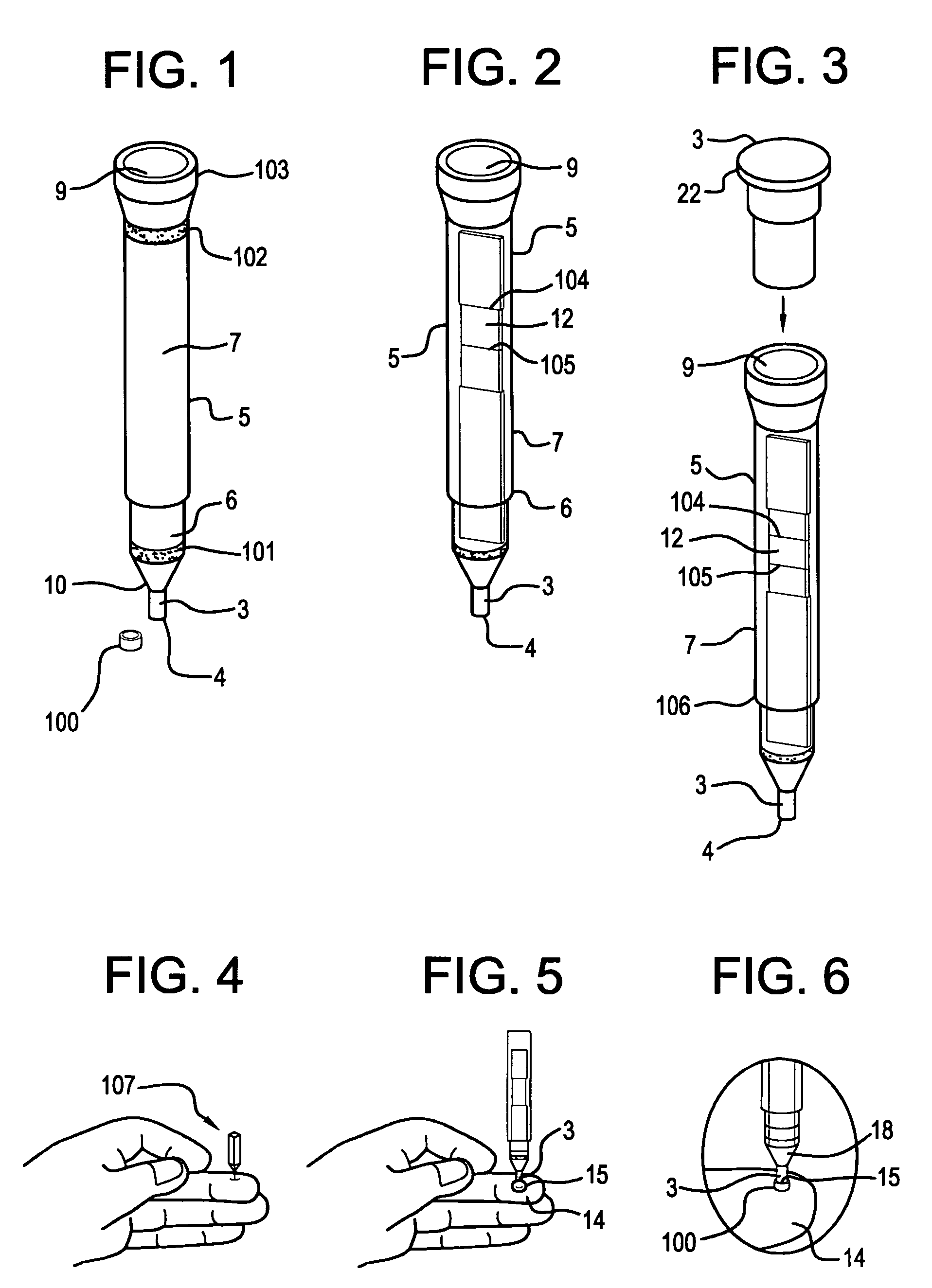
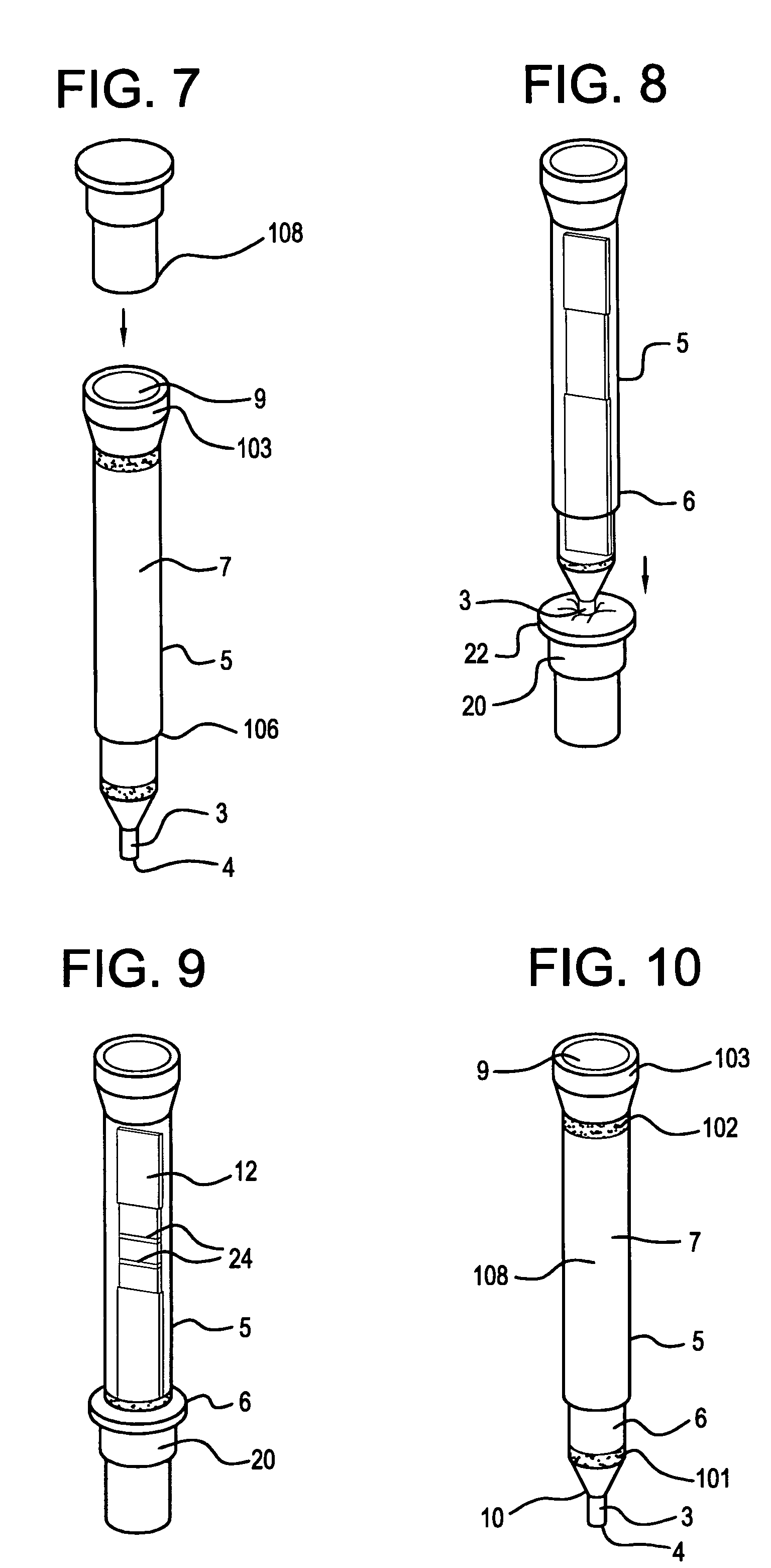
Specimen collecting, processing and analytical assembly
ActiveUS20050232813A1Prevent the biological marker from degradingEasy to storeAnalysis using chemical indicatorsSurgeryPreservativeBlood capillary
A specimen collecting, processing and analytical assembly, designed for testing small volumes of body fluids, substances and secretions, said assembly comprising a one piece barrel assembly with a volumetrically graduated capillary tube having an open end, and optionally coated internally with an anticoagulant, a stabilizer or a preservative. The barrel assembly includes a filter membrane fitted above the capillary end at the junction of the barrel assembly and the capillary tube, a support means at the barrel's second open end, and an analytical testing means disposed there between. The invention also provides a sealed vial containing an analytical testing reagent, the vial being substantially airtight and sealed with a pierceable material, a first tip cap for closing the open end of the capillary tube, a second cap to close sealably the second open end of the barrel container and a lancet to induce capillary skin punctures.
Owner:SAVVIPHARM INC
Blood testing tool
A blood testing tool is provided, which separates blood cells and can collect blood plasma or blood serum with a high yield. The blood testing tool includes an asymmetric porous membrane with a pore size distribution in which an average pore size varies to be reduced continuously or discontinuously in a thickness direction. The porous membrane includes a blood supply portion, a development portion, and a blood-cell blocking portion formed between the blood supply portion and the development portion and pores in the blood cell blocking portion include only pores through which blood cells cannot pass. When blood is supplied to one side with larger pores of the blood supply portion, the blood moves in a direction parallel to a surface of the porous membrane by a capillary phenomenon, but only blood plasma or blood serum moves into the development portion to develop.
Owner:ARKRAY INC
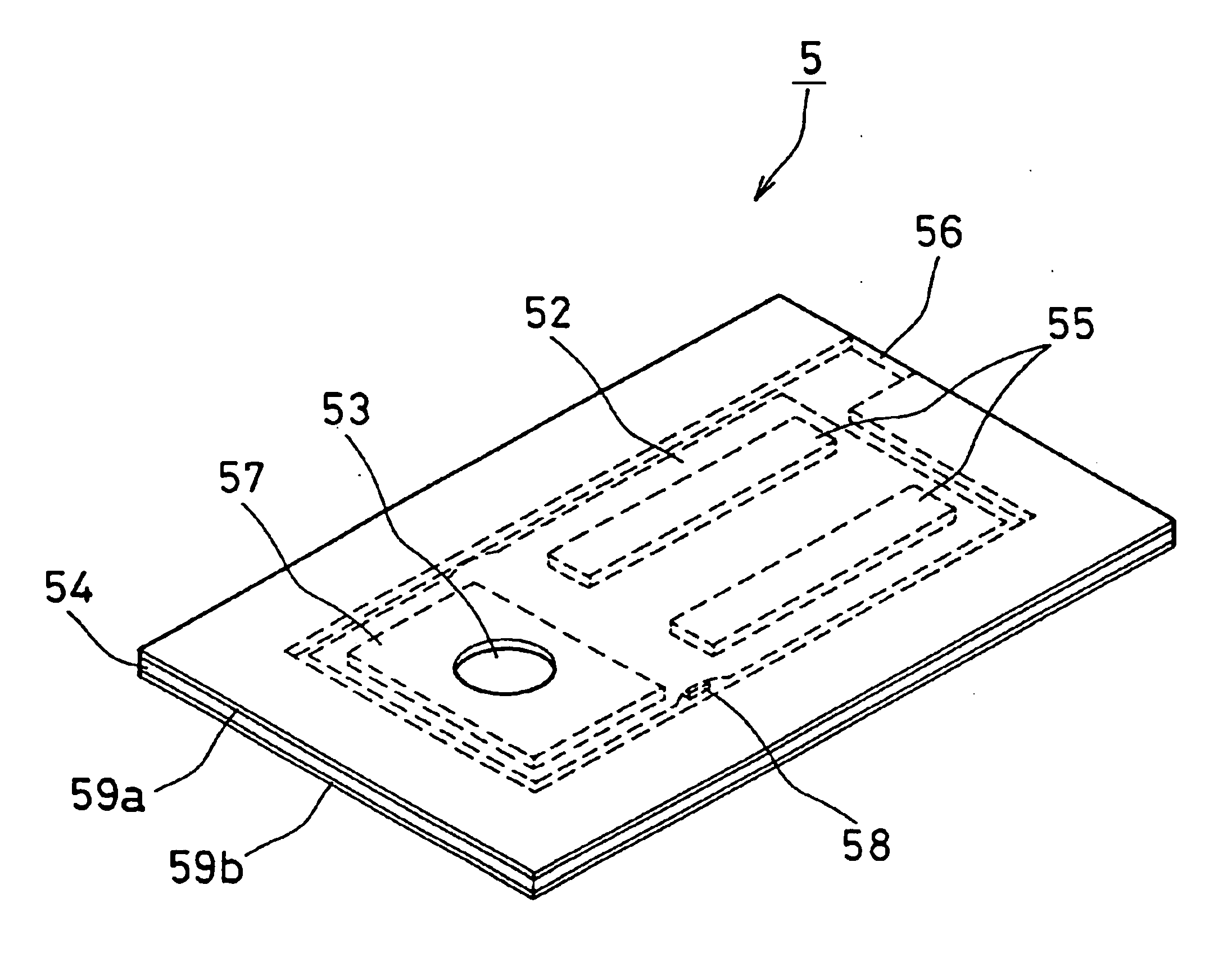
Electrochemical test sensor
Disclosed is an electrochemical sensor for the determination of analytes in body fluids, e.g. glucose in blood. The sensor involves a non-conductive base which provides a flow path for the body fluid with the base having a working and counter electrode on its surface which are in electrical communication with a detector of current. The base and a cover therefore provide a capillary space containing the electrodes into which the body fluid is drawn by capillary action. The counter electrode has a sub-element which contains an electroactive material and is configured in the system (sensor and meter) to provide an error signal when insufficient body fluid is drawn into the capillary.
Owner:ASCENSIA DIABETES CARE HLDG AG
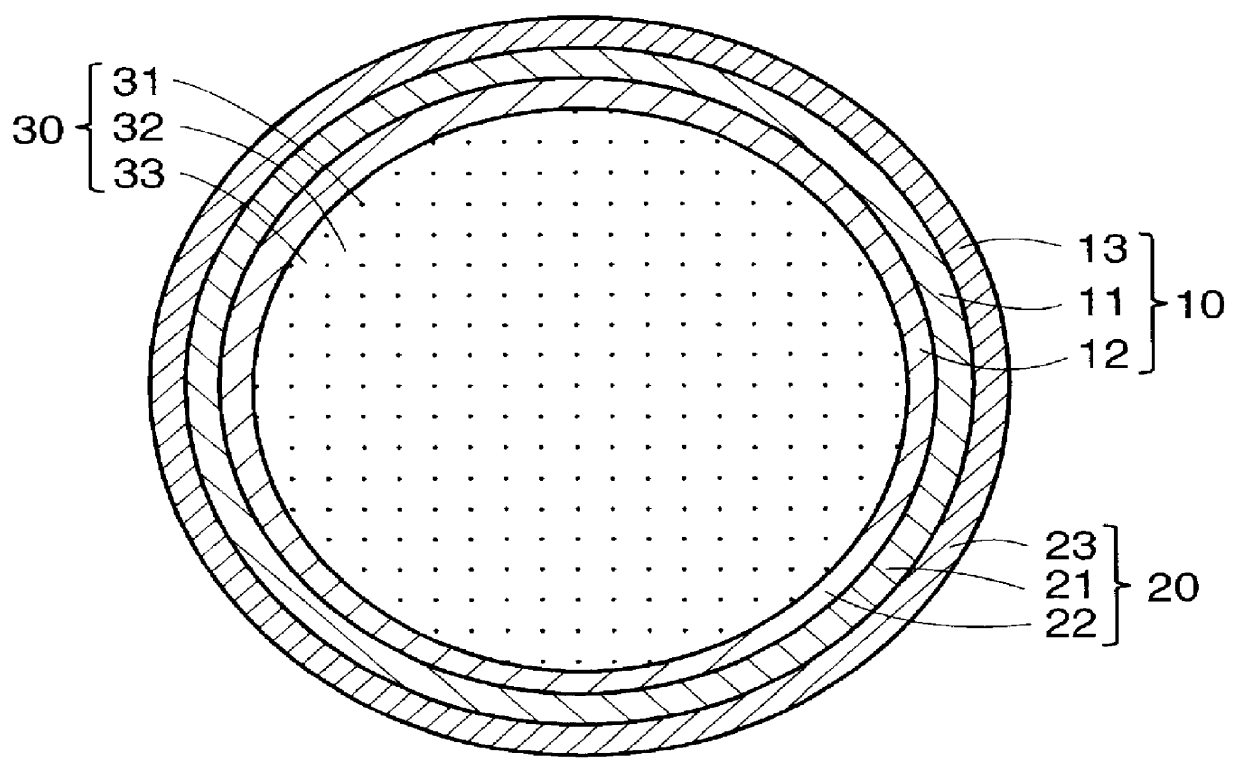
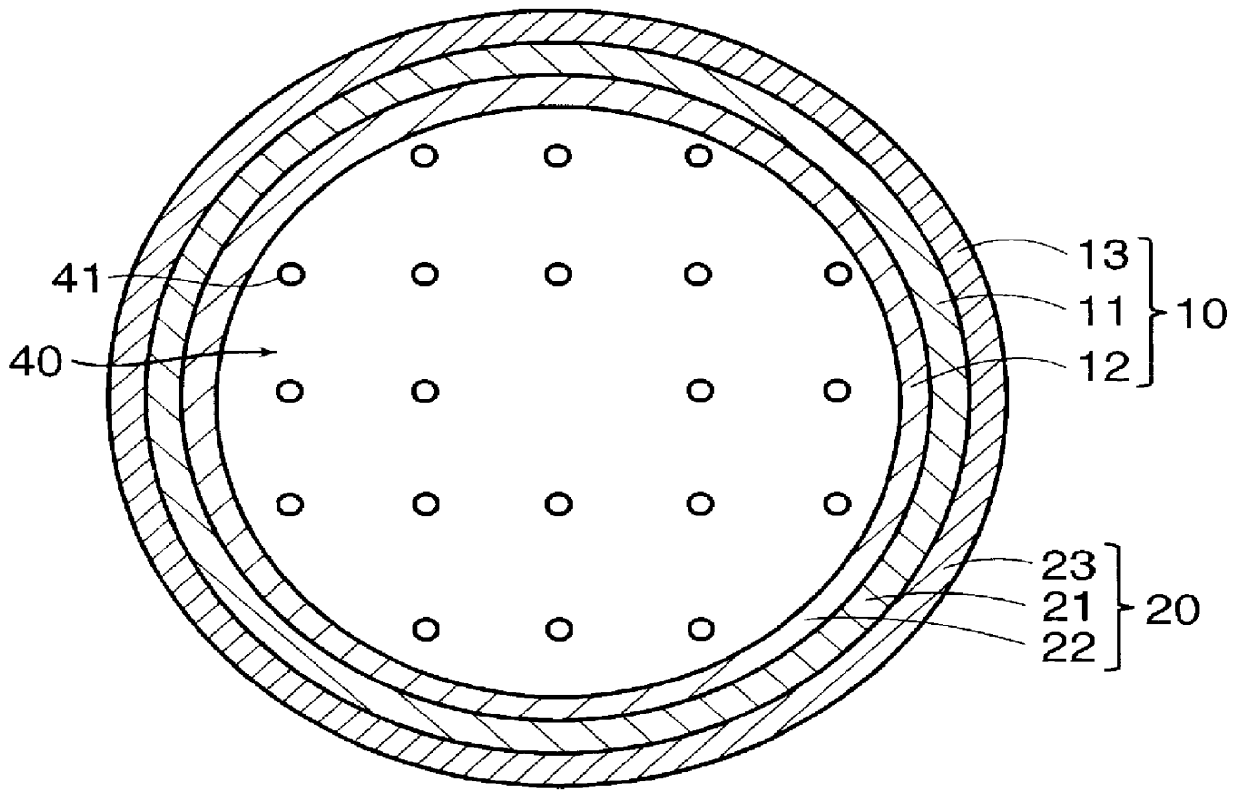
Artificial neural canal
InactiveUS6090117AStay in shapePrevent intrusionTubular organ implantsTissue regenerationMedicineBlood capillary
PCT No. PCT / JP97 / 04203 Sec. 371 Date May 20, 1999 Sec. 102(e) Date May 20, 1999 PCT Filed Nov. 19, 1997 PCT Pub. No. WO98 / 22155 PCT Pub. Date May 28, 1998The present invention offers an artificial tube for nerve which can remain in the body until the nerve regenerates while does not remain as a foreign body in the body following nerve regeneration, and which induces axons regenerated from severed nerve stumps, can promote infiltration of blood capillaries from the body and regeneration of nerve tissue. The present invention comprises a tube 10 or 20 having coating layers 12, 13 or 22, 23 composed of gelatin or collagen on the inner and outer surfaces of a tube 11 or 21 composed of a material being biodegradable and absorbable in vivo, and a collagen body 30 or 40 having cavities 32, 33 or 41 which pass through said tube so as to be substantially parallel to the axis of said tube; wherein, said cavities are filled with a matrix gel.
Owner:TAPIC INT
Method and apparatus for analyte measurement test time
ActiveUS20080210574A1Improving analyte measurement test timeImprove convenienceMicrobiological testing/measurementVolume/mass flow measurementMeasurement testConcentrations glucose
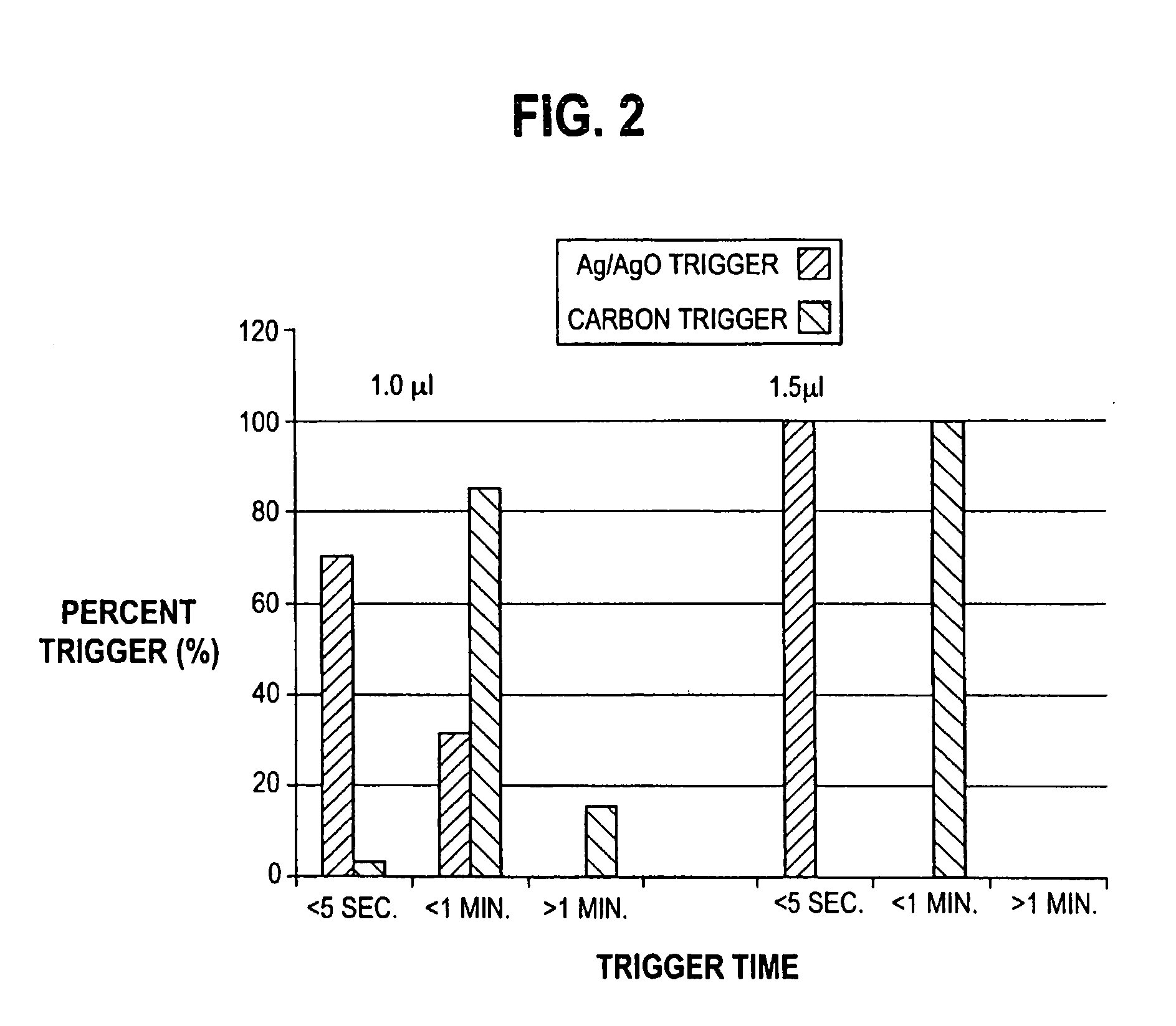
A disposable biosensor test strip is provided that includes a plurality of penetrating members. Each penetrating member is associated with a capillary chamber that has a depth suitable for capillary flow of blood and holds a volume of less than about 1.0 μl of the blood sample. A working electrode and a counter or reference electrode are disposed within the capillary chamber. A reagent is proximal to or in contact with at least the working electrode. The reagent includes an enzyme and a mediator. The reagent reacts with glucose to produce an electroactive reaction product. A blood sample, containing glucose, is applied into the capillary chamber. The capillary chamber directs capillary flow of the blood sample into contact with the reagent to cause the blood sample to at least partially solubilize or hydrate the reagent. The blood sample is detected in the capillary chamber. The electroactive reaction product is electro-oxidized or electro-reduced at the working electrode. Within 10 seconds after detecting, a determination is made of glucose concentration and a readout of the measurement is provided. The glucose determination is made by correlating the electro-oxidized or electro-reduced electroactive reaction product to the concentration of glucose in the blood sample.
Owner:SANOFI AVENTIS DEUT GMBH
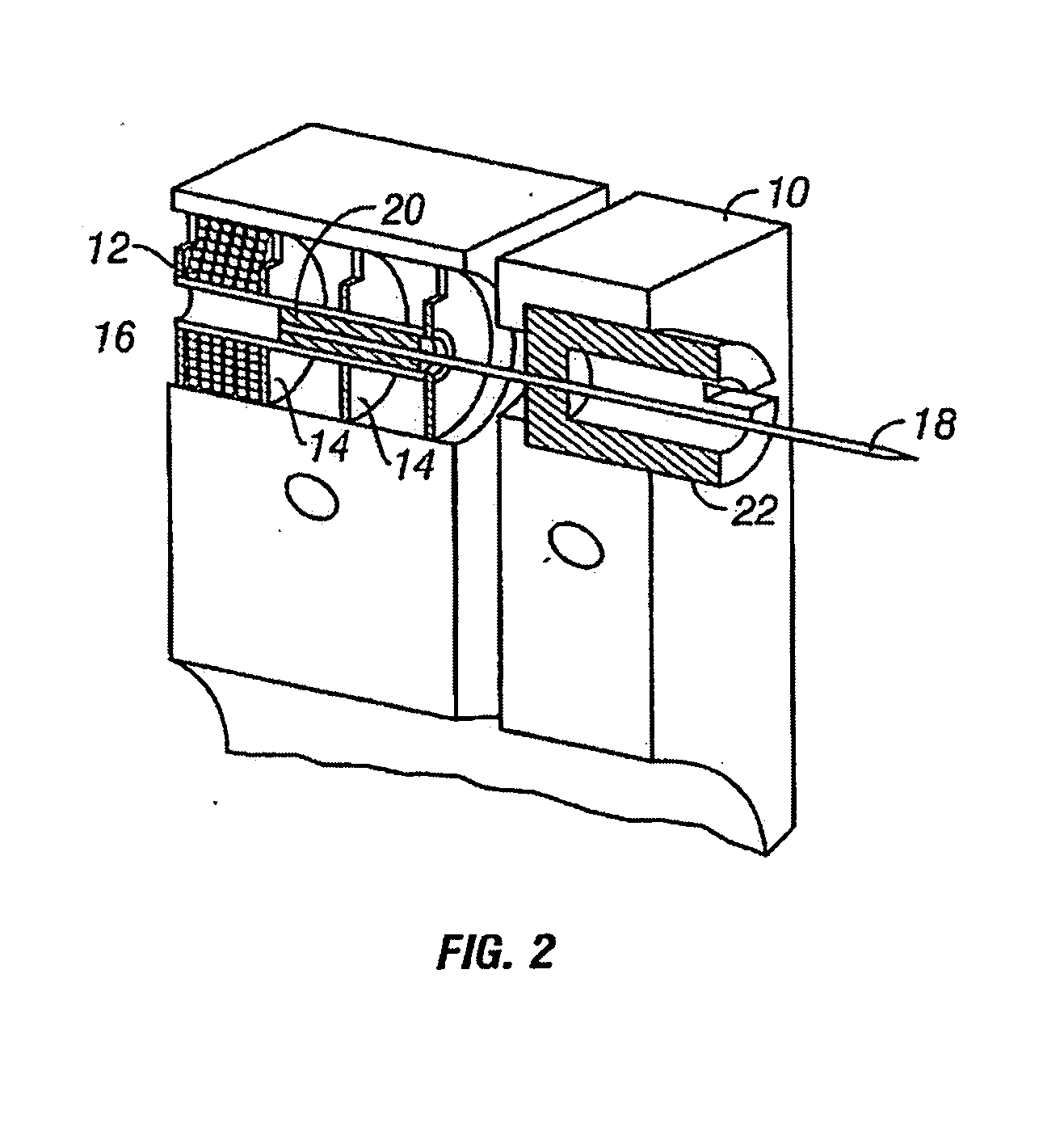
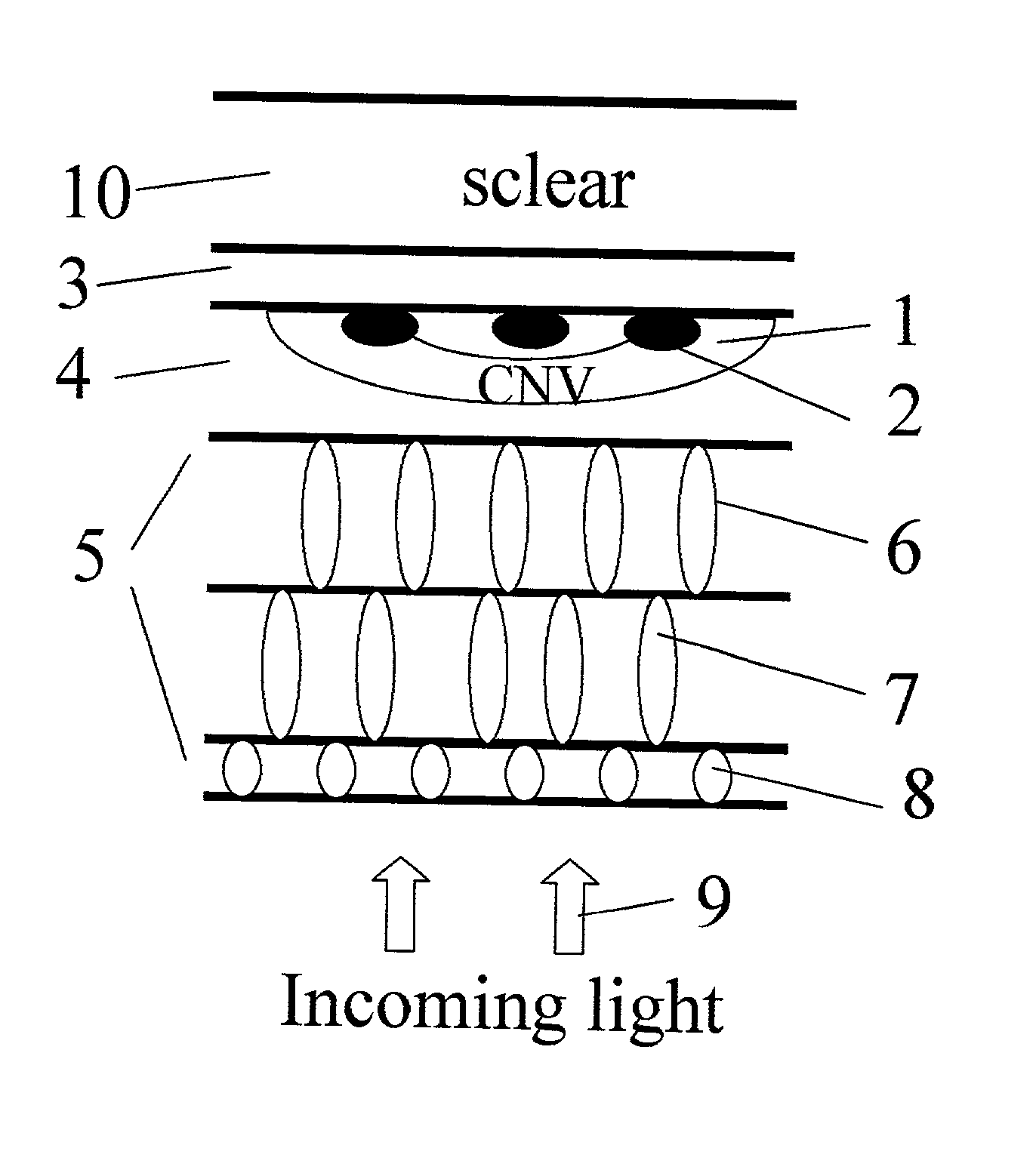
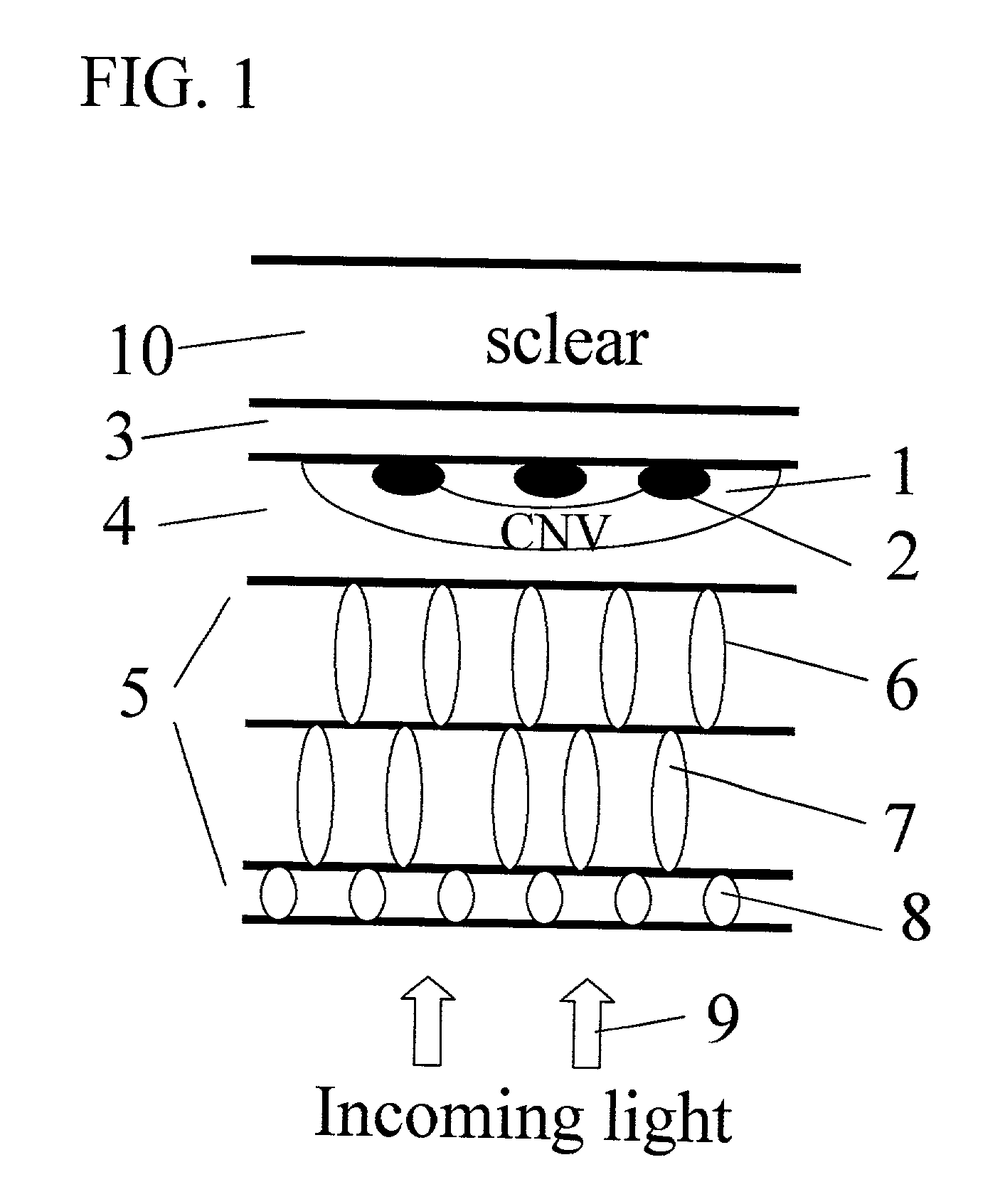
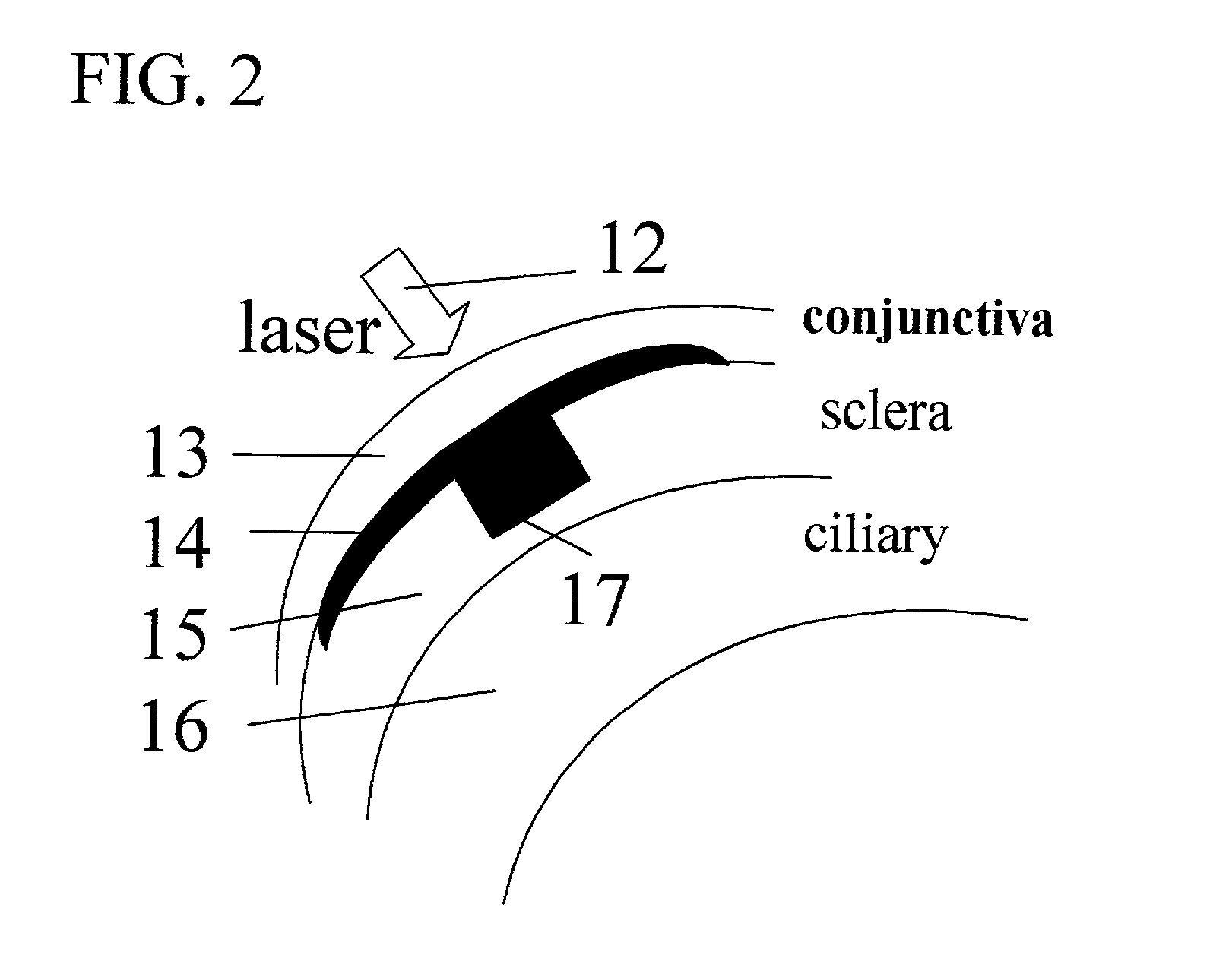
Apparatus and methods for prevention of age-related macular degeneration and other eye diseases
InactiveUS20030105456A1Minor side effectsIncrease flexibilityLaser surgerySurgical instruments for heatingRadio frequencyLaser beams
Surgical apparatus and surgical methods are proposed for the prevention of age-related macular degeneration (AMD) and choroidal neovascularization (CNV), and other eye diseases such as glaucoma by removal of the sclera tissue to reduce its rigidity and increase the flood flow and decrease pressure in the choriocapillaris. The disclosed preferred embodiments of the system consists of a tissue ablation means and a control means of ablation patterns and a fiber delivery unit. The basic laser beam includes UV lasers and infrared lasers having wavelength ranges of (0.15-0.36) microns and (0.5-3.2) microns and diode lasers of about 0.98, 1.5 and 1.9 microns. AMD and CNV are prevented, delayed or reversed by using an ablative laser to ablate the sclera tissue in a predetermined patterns outside the limbus to increase the elasticity of the sclera tissue surrounding the eye globe The surgery apparatus also includes non-laser device of radio frequency wave, electrode device, bipolar device and plasma assisted device
Owner:LIN J T
Dispensing device
An inhaler for enabling inhaled administration, has a housing having an outlet duct defining a passageway through which air can be drawn by inhalation on the part of a user. The housing contains a capillary nozzle; a container for containing a quantity of liquid to be supplied to the capillary nozzle; and an electric field controller for applying a voltage to the liquid prior to issue from the container via the capillary nozzle to expose the liquid to an electric field to cause comminution of the liquid emerging from the capillary nozzle to product a spray of electrically charged droplets such that upon inhalation by the user, electrically charged droplets for the deposition in the user's airways.
Owner:BATTELLE MEMORIAL INST
Wireless respiratory and heart rate monitoring system
InactiveUS7740588B1High light transmittanceHigh sensitivityCatheterRespiratory organ evaluationOptical radiationAnimal science
A combined respiratory and heart rate sensor for a mammal has a flexible support with a surface adapted to be positioned over the skin of a mammal. A pair of inductive coil or capacitive plate elements, spaced apart by a foam spacer having openings therein, are attached to the support surface and are capable of independently sensing respiratory movement of the mammal in a direction perpendicular to the support surface and producing an output signal proportional thereto. The heart rate sensor includes an LED attached to the flexible support capable, and at least two light-detecting elements spaced apart from the LED, each capable of independently sensing light radiation reflected through the skin and capillaries from the light-emitting element and producing an output signal proportional thereto. A barrier layer attached to the support shield from the light-detecting elements light radiation that is not emitted from the LED.
Owner:SCIARRA MICHAEL
Novel non-selective cation channel in neuronal cells and methods for treating brain swelling
The present invention is directed to therapeutic compounds, treatment methods, and kits affecting the NCCa-ATP channel of neural tissue, including neurons, glia and blood vessels within the nervous system, and methods of using same. The NCCa-ATP channel is newly expressed in neural tissue following injury such as ischemia, and is regulated by the sulfonylurea receptor SUR1, being inhibited by sulfonylurea compounds, e.g., glibenclamide and tolbutamide, and opened by diazoxide. Antagonists of the NCCa-ATP channel, including SUR1 antagonists, are useful in the prevention, diminution, and treatment of injured or diseased neural tissue, including astrocytes, neurons and capillary endothelial cells, that is due to ischemia, tissue trauma, brain swelling and increased tissue pressure, or other forms of brain or spinal cord disease or injury. Agonists of the NCCa-ATP channel may be are useful in the treatment neural tissue where damage or destruction of the tissue, such as a gliotic capsule, is desired.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
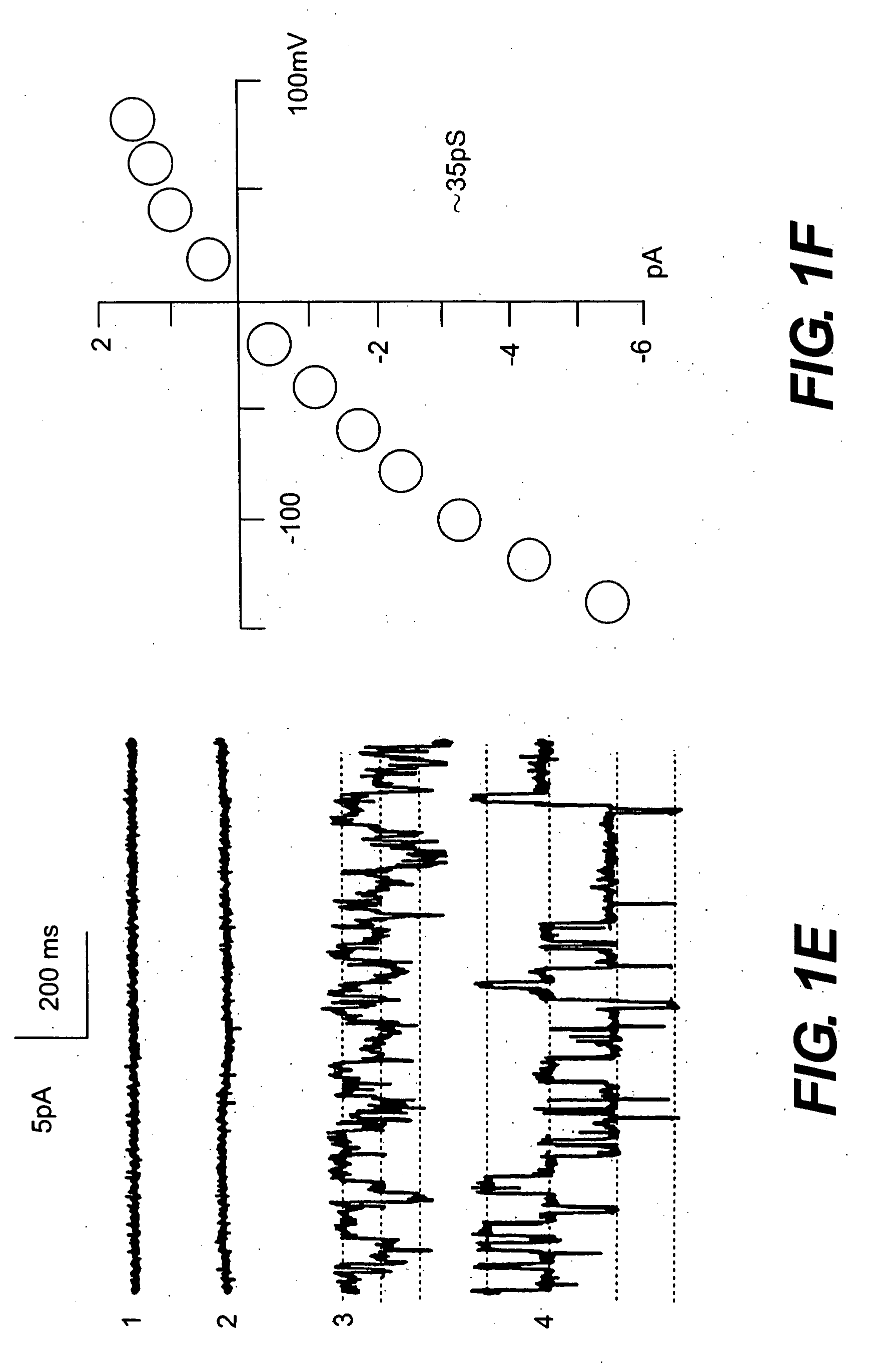
Apparatus and method for analyzing a liquid in a capillary tube of a hematology instrument
InactiveUS7207939B2Ultrasonic/sonic/infrasonic diagnosticsAnalysing fluids using sonic/ultrasonic/infrasonic wavesBlood capillaryHematology
An apparatus and method for determining the density and fluid-type of a fluid flowing in a capillary tube, the velocity and viscosity of a blood sample flowing in a capillary tube, the erythrocyte sedimentation rate (ESR) of a blood sample after flow has been brought to an abrupt stop in a capillary tube, and / or the zeta sedimentation rate (ZSR) of a blood sample after flow has been brought to an abrupt stop in a capillary tube. These measurements are accomplished by directing a waveform pulse, such as an ultrasound pulse, at a pre-determined frequency transversely across the capillary tube and sample fluid, and by determining the flight of time of the pulse through the capillary tube and sample fluid and / or the Doppler shift of the echo signals reflecting off cells moving forwardly or transversely within a flowing, or stationary, blood sample.
Owner:COULTER INTERNATIONAL CORPORATION
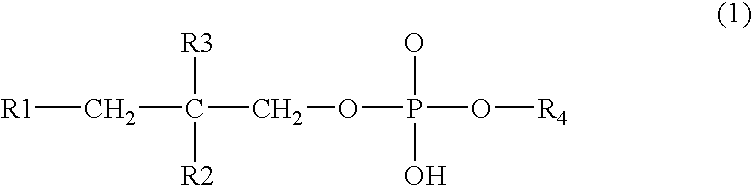
NSAID formulations, based on highly adaptable aggregates, for improved transport through barriers and topical drug delivery
The invention describes novel formulations of nonsteroidal anti-inflammatory drugs (NSAIDs) based on complex aggregates with at least three amphipatic components suspended in a suitable, e.g. pharmaceutically acceptable, polar liquid medium. A suitably ionised NSAID is one of the two, amongst said three, components that tends to destabilise lipid membranes, the other system component with such activity being typically a surfactant. In contrast, the remaining amongst said at least three amphipatic components typically forms a stable lipid membrane on it's own. An essential characteristics of the resulting, relatively large, aggregates is an improved ability to penetrate pores, in a semi-permeable barrier, at least 30%, and often much smaller than the average diameter of the complex aggregate. This enables said aggregates to mediate NSAID transport through semi-permeable barriers including mammalian skin. As a result of the skin penetration by NSAID loaded large aggregates, the drug delivered transcutaneously with such carriers gets deeper into the tissue than the corresponding NSAID from a solution on the skin surface. This is believed to be due to the special ability of suitable large carriers to bypass the local sink of blood capillaries at the epidermal-dermal junction in the skin. The carrier-mediated delivery of locally applied NSAIDs thus allows therapy of deep tissues under the drug administration site, which is medically highly desirable.
Owner:IDEA AG
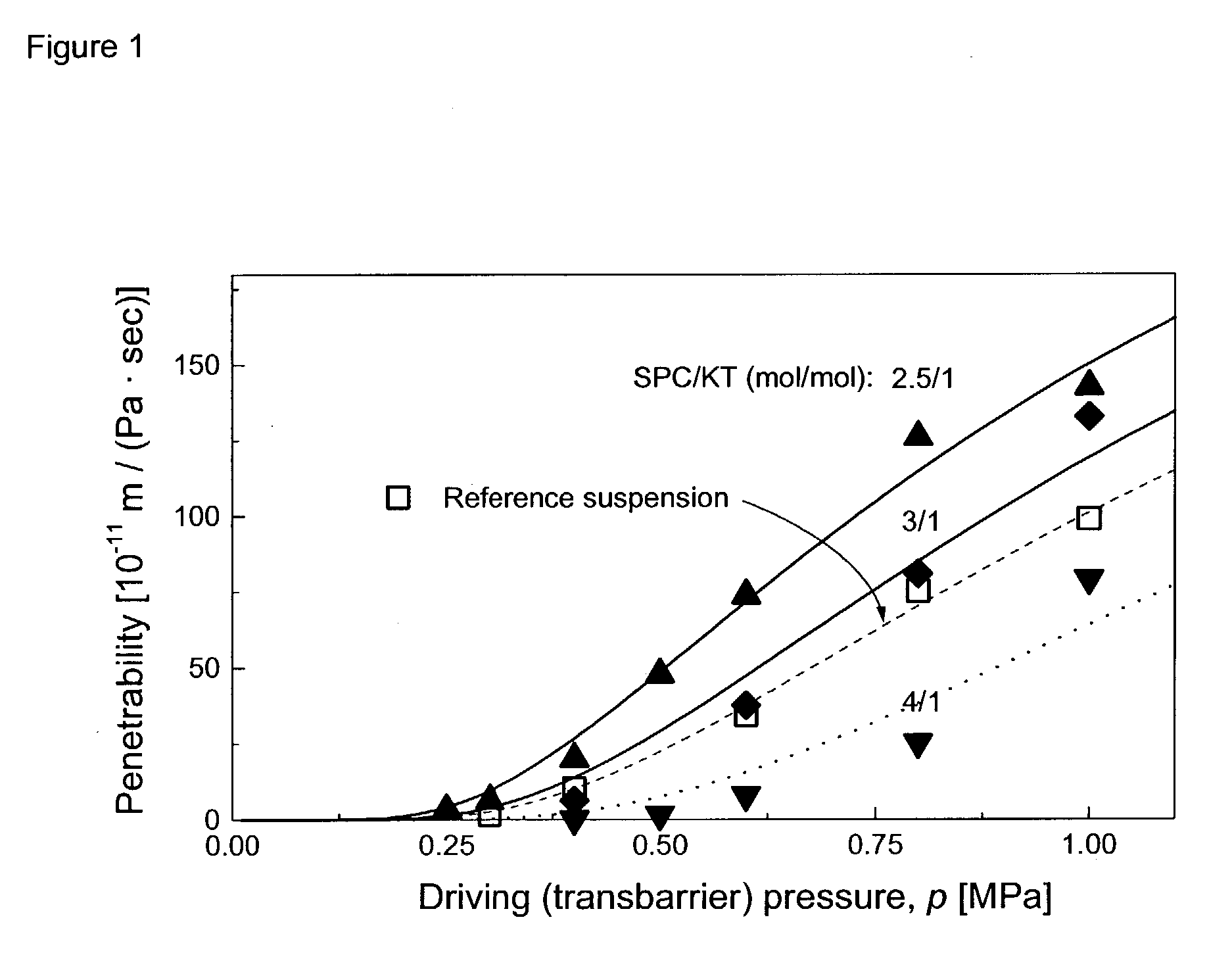
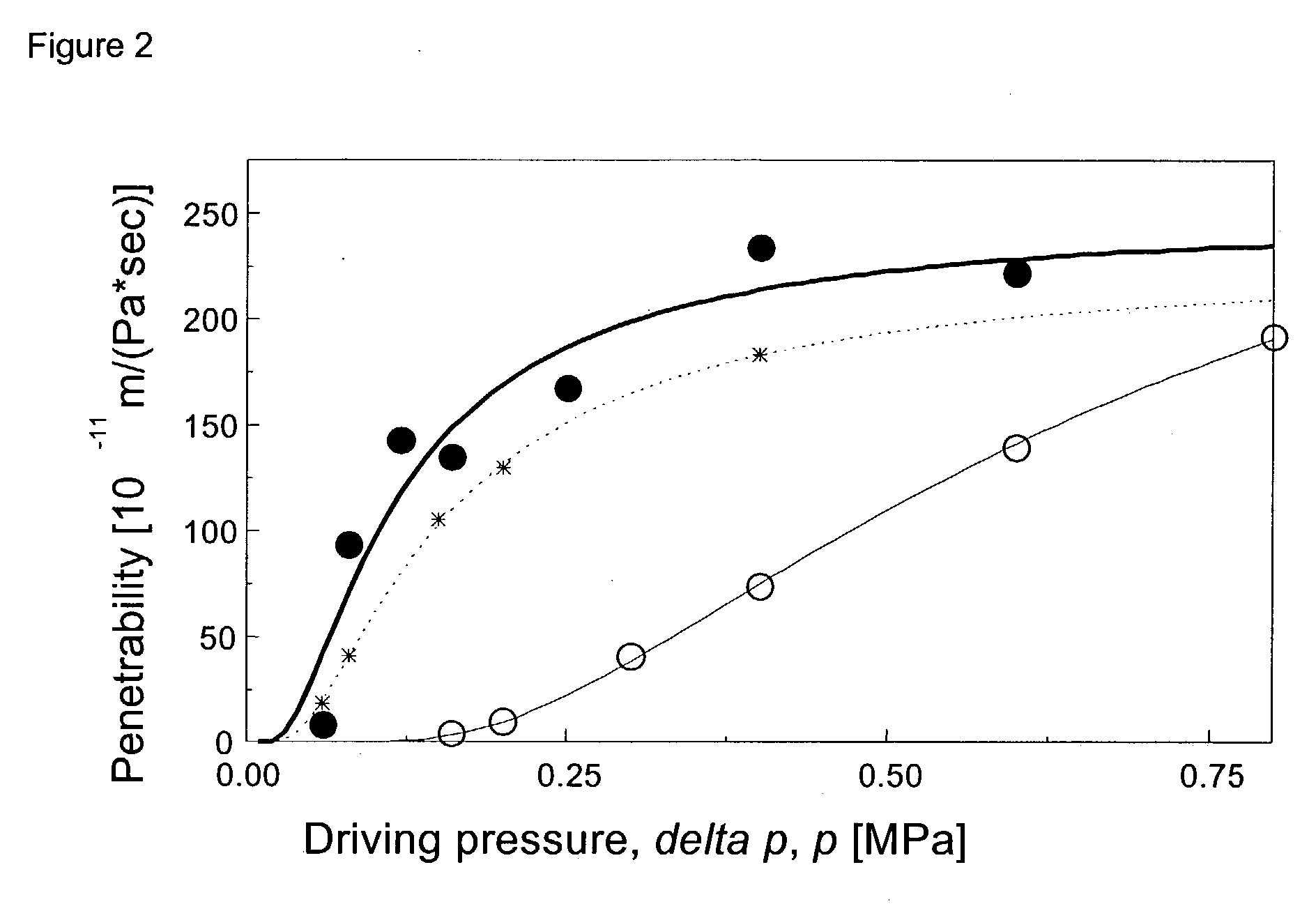
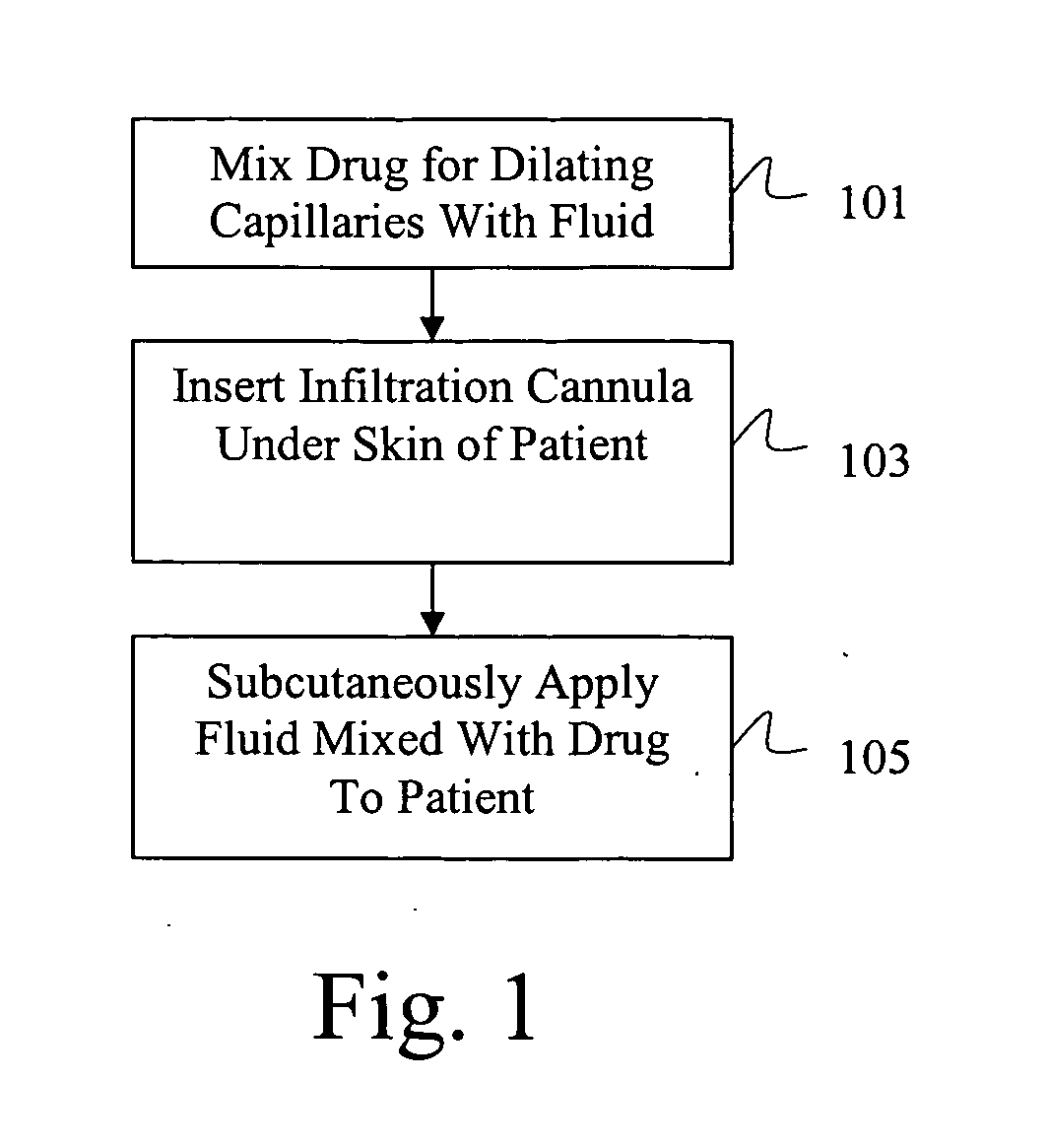
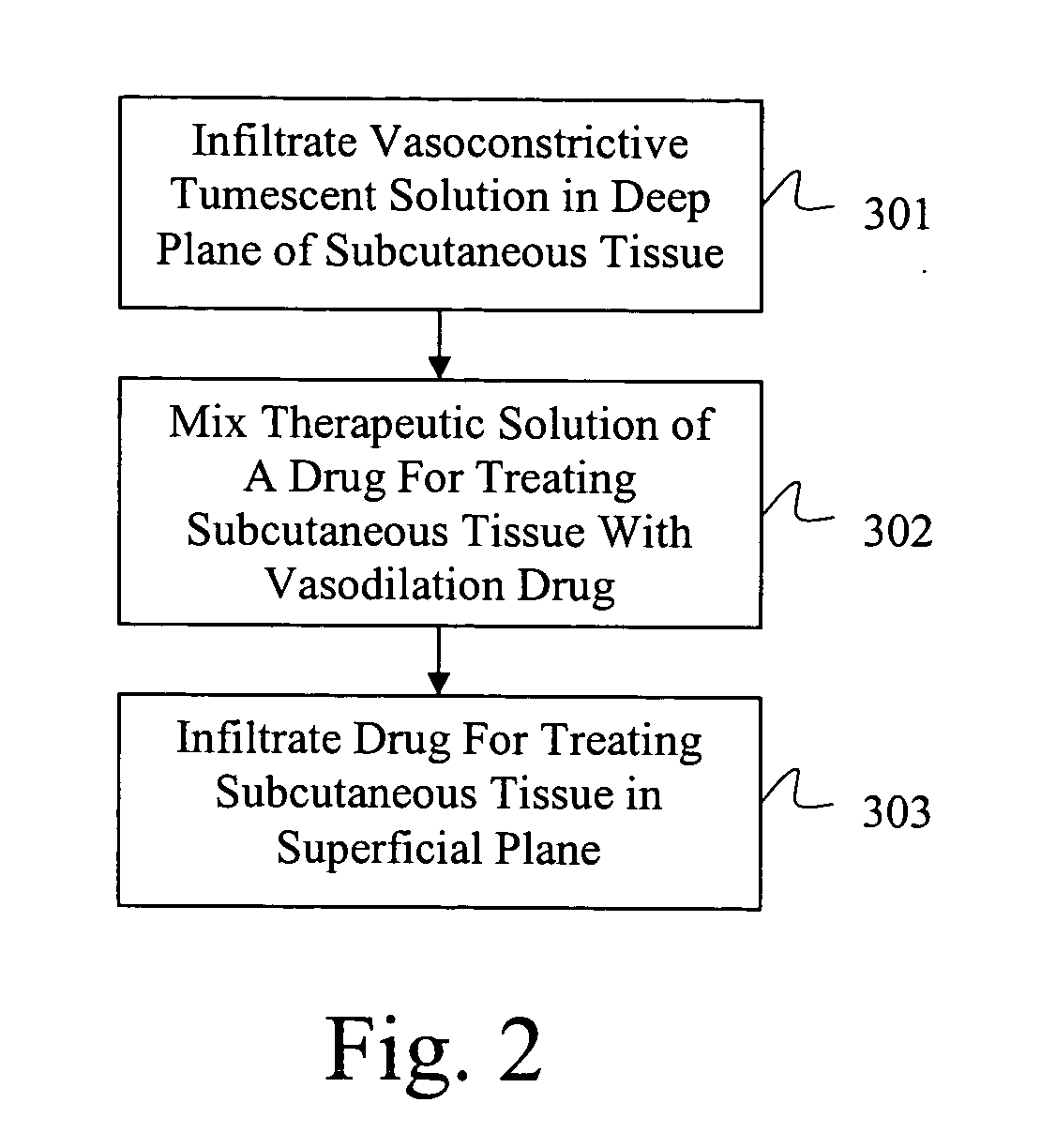
Drug delivery system for accelerated subcutaneous absorption
ActiveUS20050287134A1Prevent and delay absorptionImprove absorption rateBiocidePeptide/protein ingredientsWhole bodyVascular dilation
A method for accelerating subcutaneous absorption of a fluid or drug into the systemic circulation of a specific targeted tissue. A first drug operative to produce local capillary vasodilatation and / or increase the rate of bulk flow of solution through the interstitial space is mixed with a fluid. The fluid may contain a crystalloid solution or a dilute solution of a pharmacologic drug required for a routine or emergency therapeutic treatment for a patient. The first drug is substantially non-toxic to the patient. The fluid mixed with the first drug is then delivered subcutaneously or into deeper tissues of the patient, by use of a hyperdermic needle or infiltration cannula. As the capillaries are dilated, the fluid is efficiently absorbed and circulated systemically.
Owner:KLEIN JEFFREY A
Production process of high-quality ecological antibacterial health-care sock
ActiveCN103815555AGood health effectImprove antibacterial propertiesLiquid/gas/vapor article treatmentPanty-hoseYarnEscherichia coli
The invention discloses a production process of a high-quality ecological antibacterial health-care sock. The production process of the high-quality ecological antibacterial health-care sock comprises the following steps of yarn manufacturing, sock weaving, seam allowance processing, reinforcing, setting, pre-drying, water bathing, drying and package detection. The production process of the high-quality ecological antibacterial health-care sock adopts the method that natural cotton fibers, aloe fibers and modal fibers are interlaced and makes full use of the good antibacterial effect of the aloe fibers, and the bottom of the sock adopts a flat structure, so that the fabric can meet the requirements for high air permeability and comfort. The aloe fibers are used for replacing traditional common viscose, and aloe isocitric acid calcium and other matter have the functions of improving the constitution, strengthening the heart, promoting blood circulation, softening hardened arteries, lowering the cholesterol content and expanding the blood capillaries, and have a certain inhibition effect on escherichia coli and staphylococcus aureus. Compared with the prior art, the high-quality ecological antibacterial health-care sock has good health-care performance and antibacterial performance.
Owner:浙江丰悦针纺有限公司
Three-Dimensional Apertured Film for Transmitting Dynamically-Deposited and Statically-Retained Fluids
ActiveUS20080114317A1Many timesReduce the possibilityLayered productsBaby linensBlood capillaryCapillary action
A three-dimensional film for use as an acquisition distribution layer in an absorbent article comprising a first surface with drains extending downward from the first surface, the drains being capable of transmitting fluid by gravity; and protrusions extending upward from the first surface to an upper surface with at least one capillary extending downward from the upper surface, the capillaries being capable of transmitting fluid in contact with the upper surface by capillary action. The drains rapidly transmit fluid through the film, particularly fluid that is dynamically-deposited. The capillaries transmit statically-retained fluid that is in contact with the upper surface of the protrusions.
Owner:TREDEGAR FILM PROD CORP
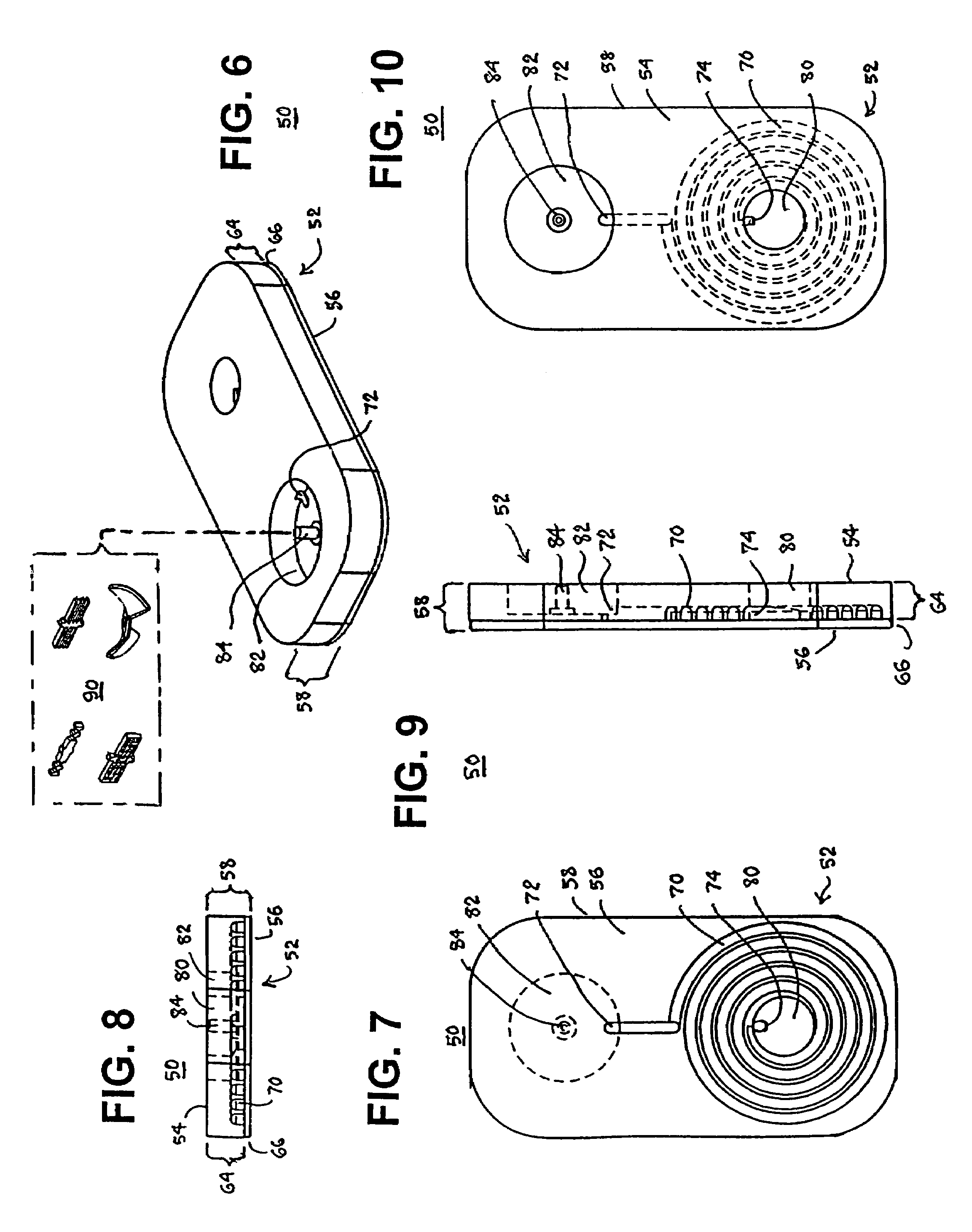
Specimen collecting, processing and analytical assembly
ActiveUS7378054B2Prevent the biological marker from degradingEasy to storeAnalysis using chemical indicatorsSurgeryPreservativeBlood capillary
A specimen collecting, processing and analytical assembly, designed for testing small volumes of body fluids, substances and secretions, said assembly comprising a one piece barrel assembly with a volumetrically graduated capillary tube having an open end, and optionally coated internally with an anticoagulant, a stabilizer or a preservative. The barrel assembly includes a filter membrane fitted above the capillary end at the junction of the barrel assembly and the capillary tube, a support means at the barrel's second open end, and an analytical testing means disposed there between. The invention also provides a sealed vial containing an analytical testing reagent, the vial being substantially airtight and sealed with a pierceable material, a first tip cap for closing the open end of the capillary tube, a second cap to close sealably the second open end of the barrel container and a lancet to induce capillary skin punctures.
Owner:SAVVIPHARM INC
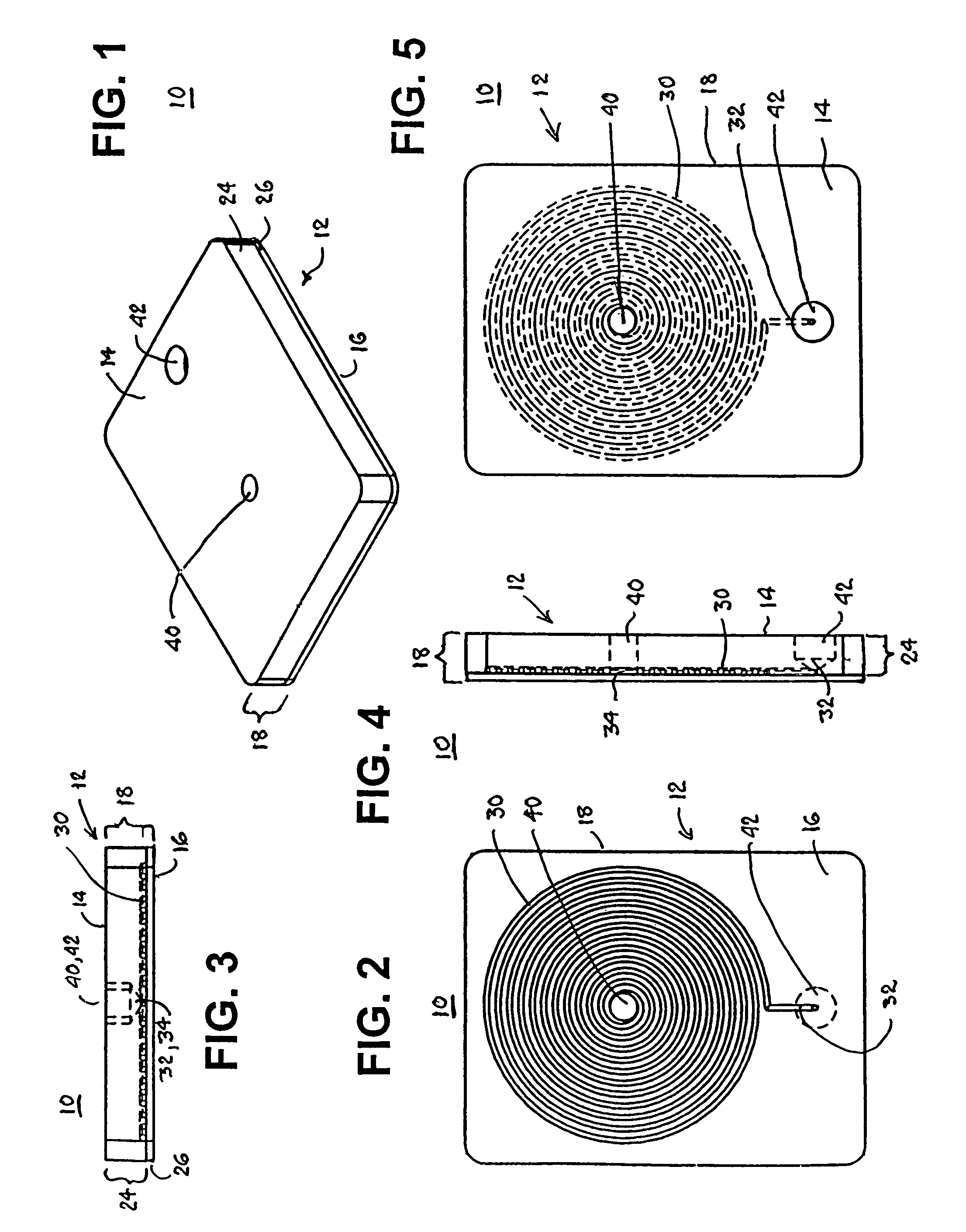
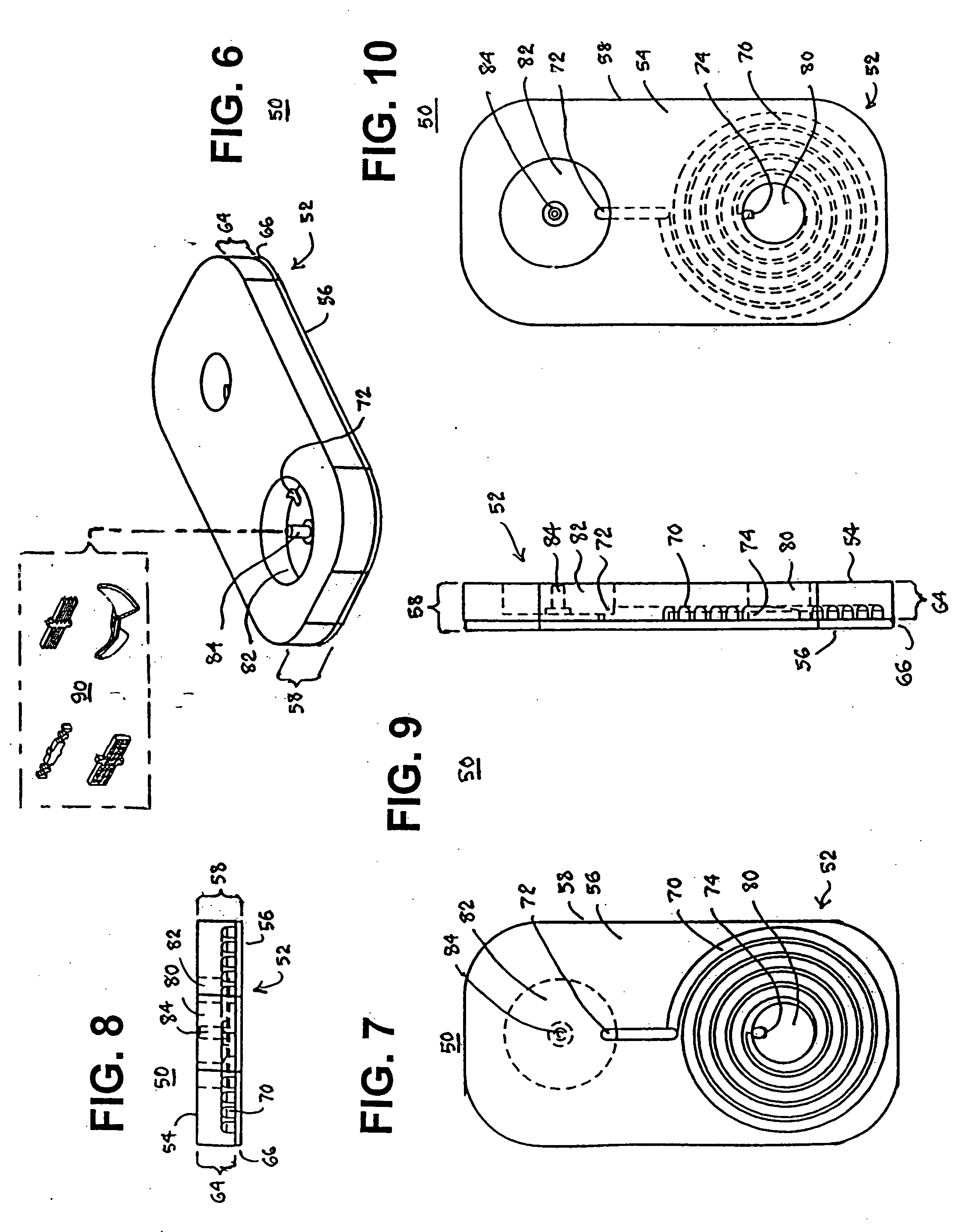
Blood coagulation test cartridge, system, and method
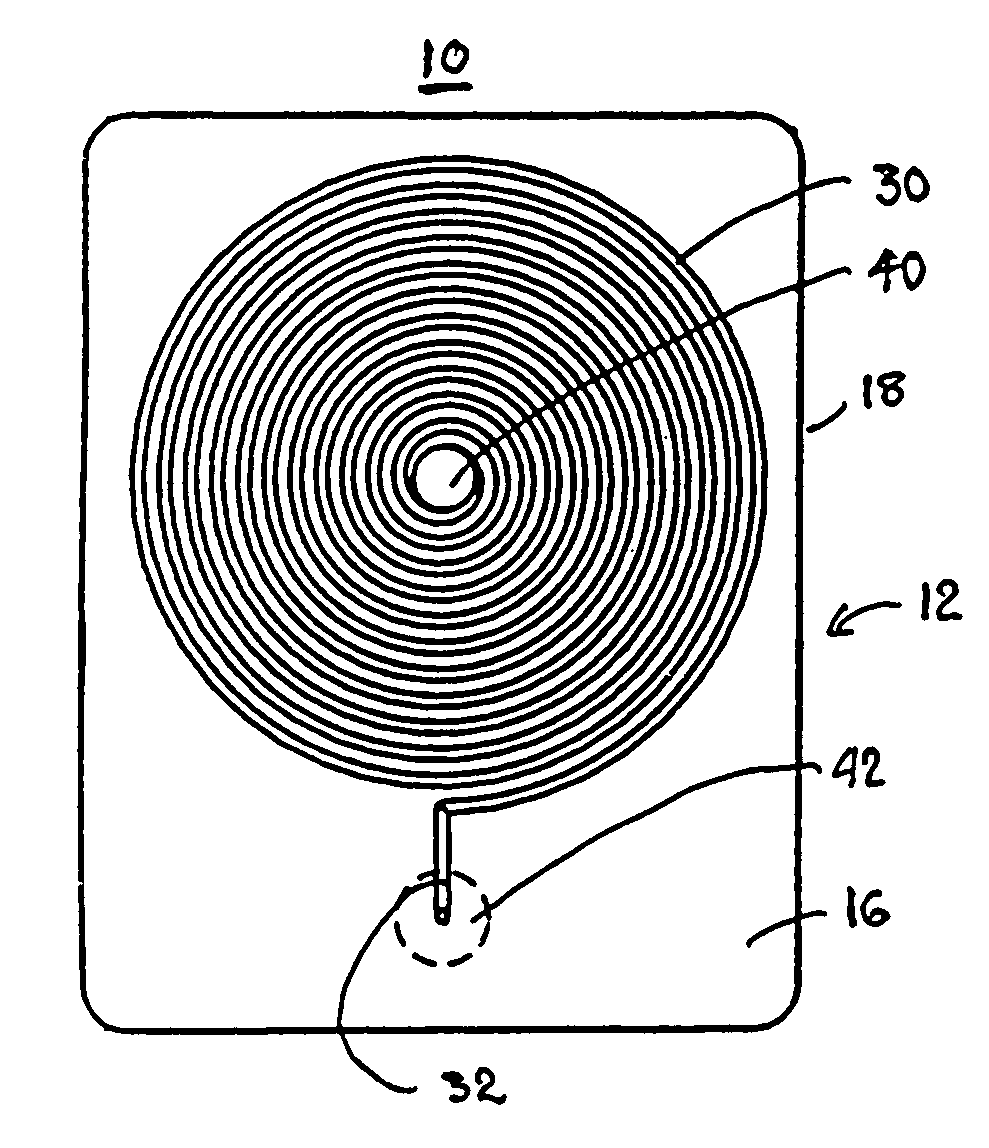
InactiveUS7439069B2Practical and convenientRapid and inexpensiveMicrobiological testing/measurementWithdrawing sample devicesBlood capillaryEngineering
A system and method for determining a coagulation time, e.g., thrombin time, PT, aPTT, and ACT, of a blood sample deposited in a test cartridge is disclosed. The test cartridge includes a blood receptacle that is open to the atmosphere into which a blood sample is to be deposited, a vacuum port that is open to atmosphere, and a spiral capillary within the test cartridge having a capillary length and cross-section area, a first capillary end of the spiral capillary open to the blood receptacle and a second capillary end of the spiral capillary open to the vacuum port, whereby the spiral capillary is closed to atmosphere. When a blood sample is deposited in the blood receptacle, a vacuum is drawn through the vacuum port and the blood is drawn through the spiral capillary until coagulation occurs. A pressure change is detected, and the coagulation time is measured.
Owner:MEDTRONIC INC
Controlled release endoprosthetic device
InactiveUS20050192664A1Overcome problemsVascular smooth cell proliferation caused by stents can be reducedOrganic active ingredientsStentsBULK ACTIVE INGREDIENTPerfusion
The invention relates to improved drug-delivery endoprosthetic device for insertion at a vascular site via catheter placement at the site, comprising: (a) a structural member into the upper and / or lower surface of which one or more micro-deepenings are engraved and / or on which a polymer member is carried, for co-expansion with the polymer member from a contracted state to an expanded state when the device is exposed to said stimulus, (b) optionally a polymer member capable of expanding from a contracted to a stable, expanded state when the polymer member is exposed to a selected stimulus, wherein the device can be delivered from a catheter, with the structural and the optional polymer members in their contracted states, and is adapted to be held in a vessel at the vascular target site by radial pressure against the wall of the vessel, with the structural and the optional polymer members in their expanded states; and wherein the micro-deepenings of said structural member and / or said polymer member comprise a pharmaceutical composition containing one or more active ingredients selected from the group consisting of agents to inhibit or at least reduce excessive proliferation of vessel wall cells, agents to enhance the downstream perfusion of tissue, agents to promote and / or to enhance the neo-formation of capillaries, agents designed to modulate the amount or activity of coagulation factors, agents to reduce the amount of Thrombin- and / or Fibrin-formation, embedded therein for release from the member, with such in its expanded state.
Owner:BOEHRINGER INGELHEIM PHARM KG
Blood testing tool
InactiveUS20010005488A1Analysis using chemical indicatorsWithdrawing sample devicesRed blood cellBlood capillary
A blood testing tool is provided, which separates blood cells and can collect blood plasma or blood serum with a high yield. The blood testing tool includes an asymmetric porous membrane with a pore size distribution in which an average pore size varies to be reduced continuously or discontinuously in a thickness direction. The porous membrane includes a blood supply portion, a development portion, and a blood-cell blocking portion formed between the blood supply portion and the development portion and pores in the blood cell blocking portion include only pores through which blood cells cannot pass. When blood is supplied to one side with larger pores of the blood supply portion, the blood moves in a direction parallel to a surface of the porous membrane by a capillary phenomenon, but only blood plasma or blood serum moves into the development portion to develop.
Owner:ARKRAY INC
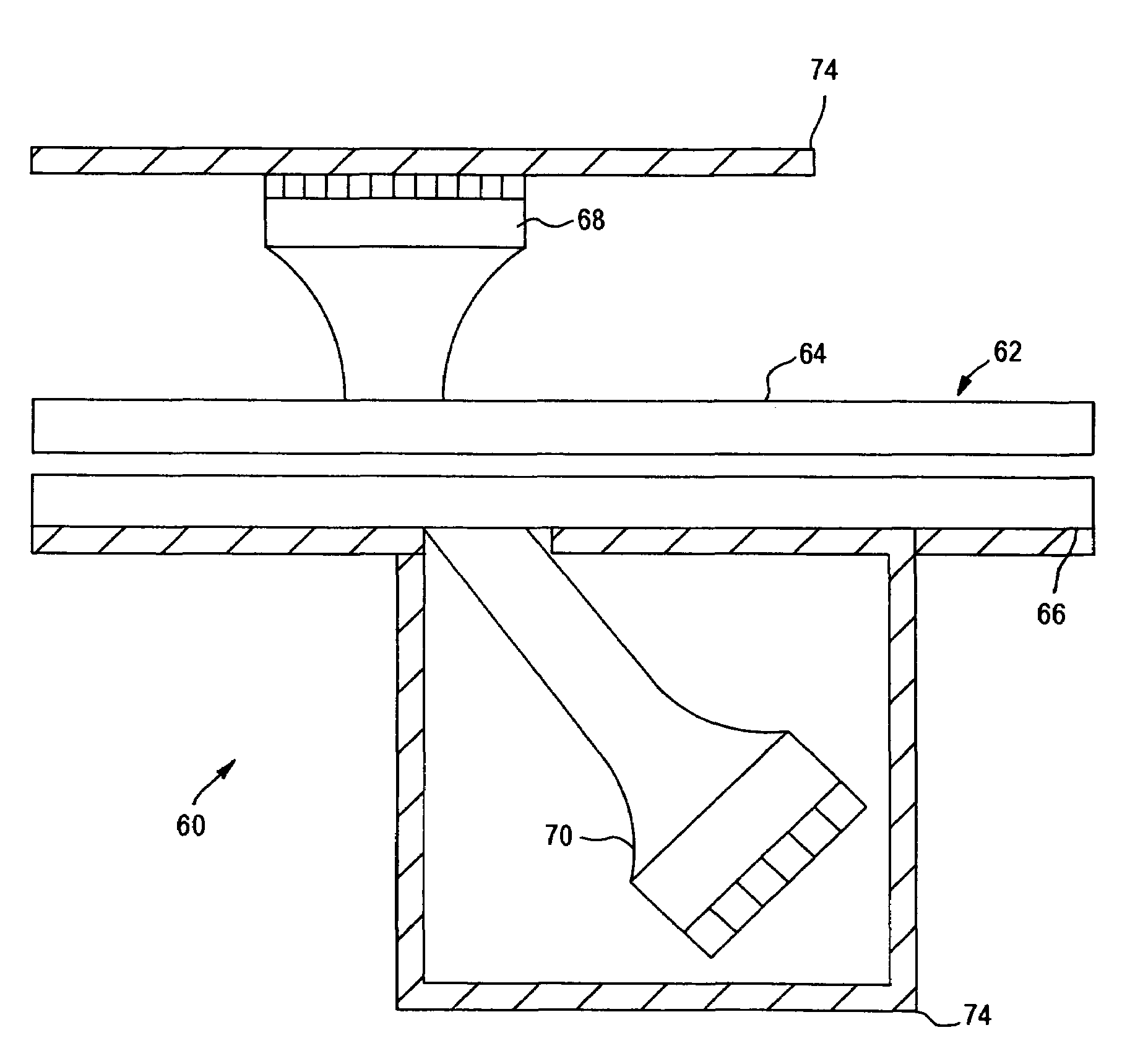
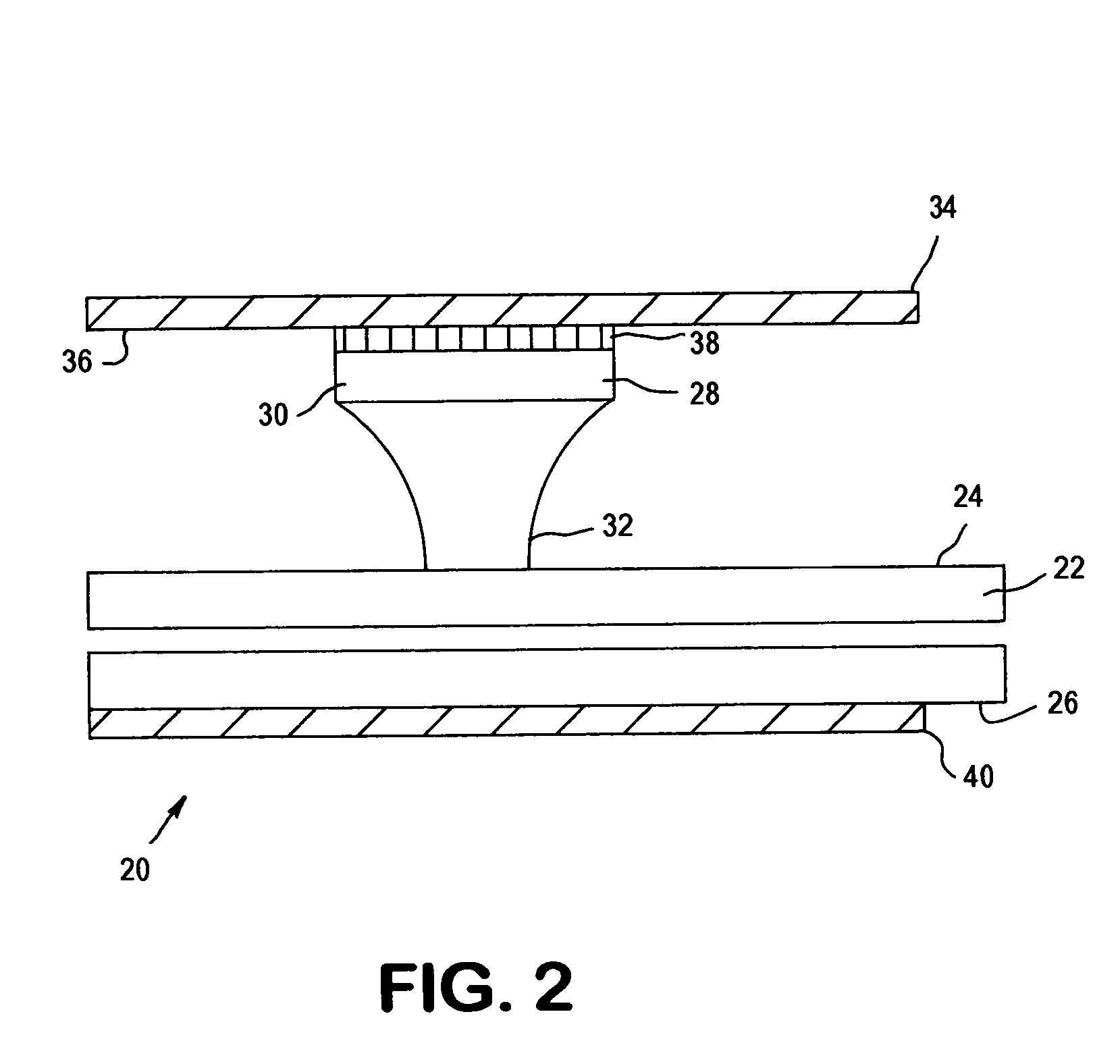
Fluidic capillary chip for regulating drug flow rates of infusion pumps
InactiveUS20090326517A1Avoid erosionValve arrangementsSynthetic resin layered productsDrugs solutionInfusion pump
An erosion-resistant capillary chip for use with in an infusion pump that is made from a silicon substrate having a first surface that includes a micro groove etched therein and a glass plate laminated to the first surface. The glass plate covers the micro groove so that a micro fluid conduit is created. The glass plate includes an inlet bore that connects with the micro fluid conduit and the silicon substrate includes an outlet bore that connects with the micro fluid conduit so that a drug solution entering the inlet bore from the infusion pump may pass through the micro fluid conduit at a restricted flow rate to the outlet bore and thereafter to a target site of a patient. The micro groove includes a passivation layer made from silicon nitride or silicon carbide that protects the micro groove against erosion from passing fluids having high basic or high acidic pH levels. A method for making the capillary chip is disclosed, as well as an infusion pump incorporating the improved capillary chip.
Owner:CODMAN NEURO SCI
Systems and methods for collecting fluid from a subject
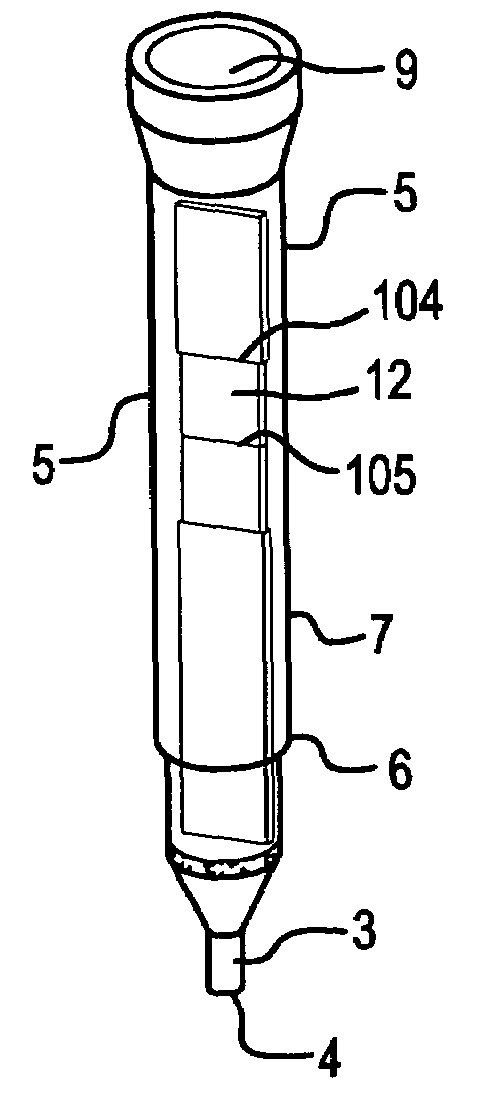
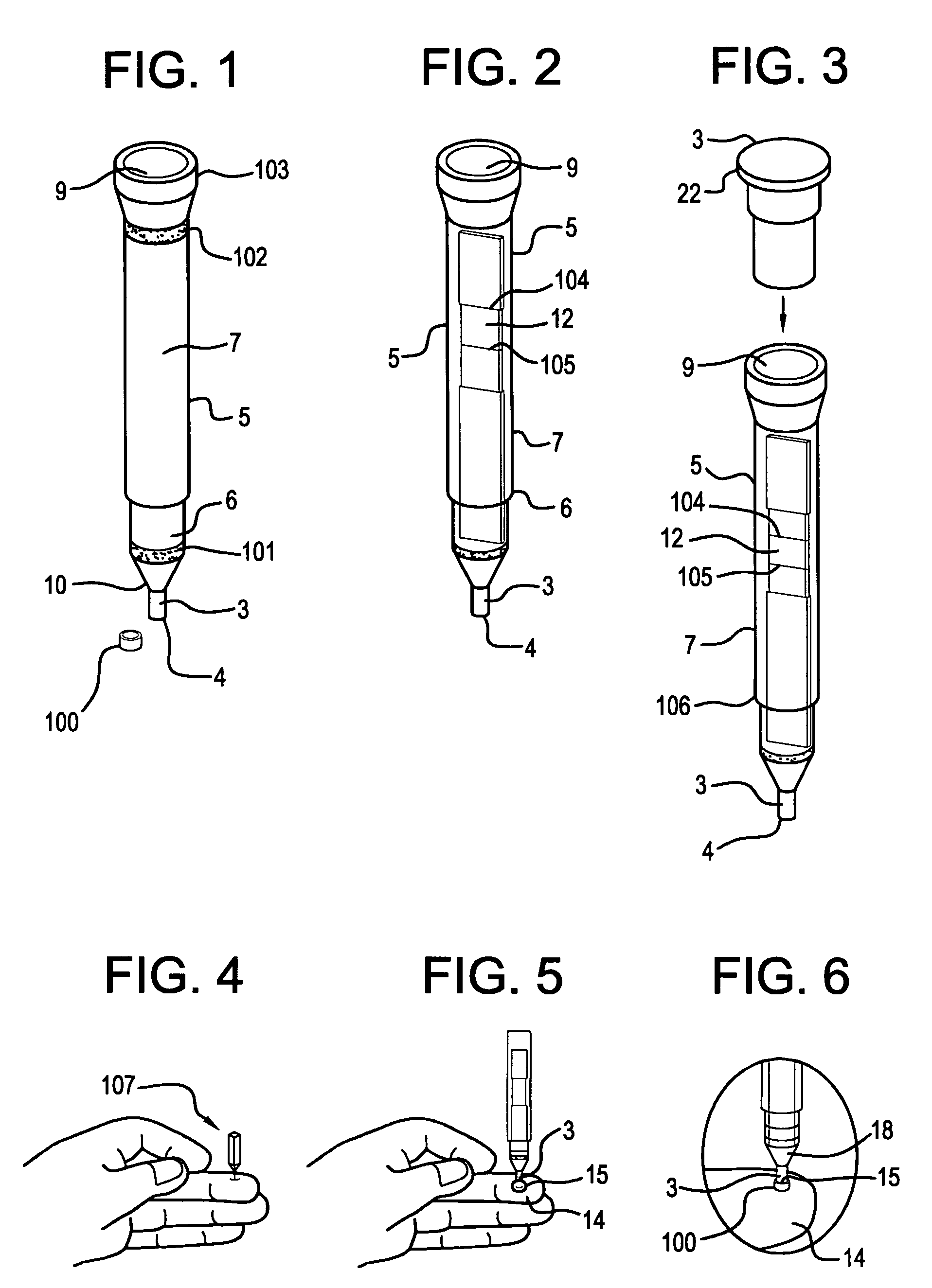
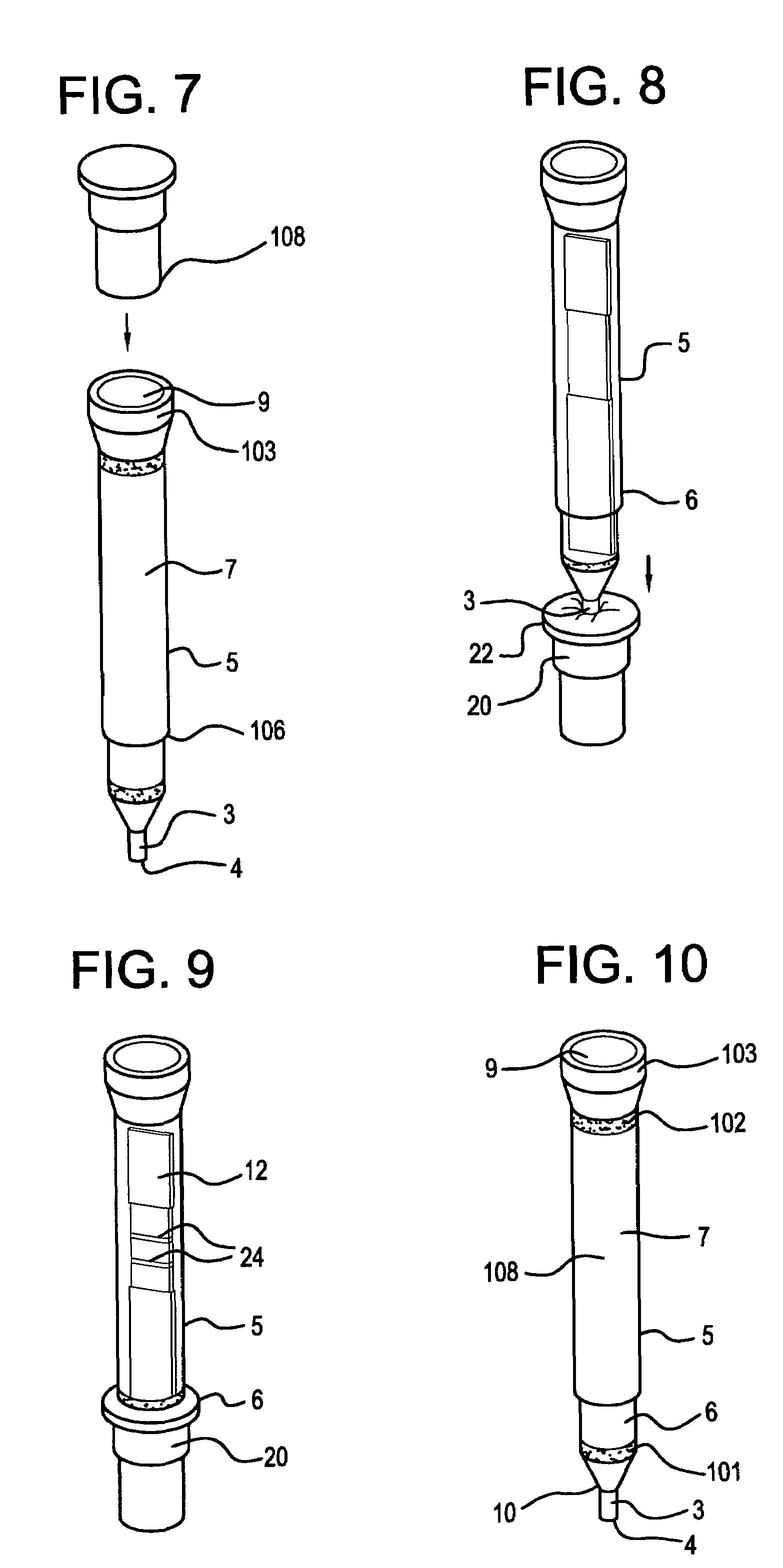
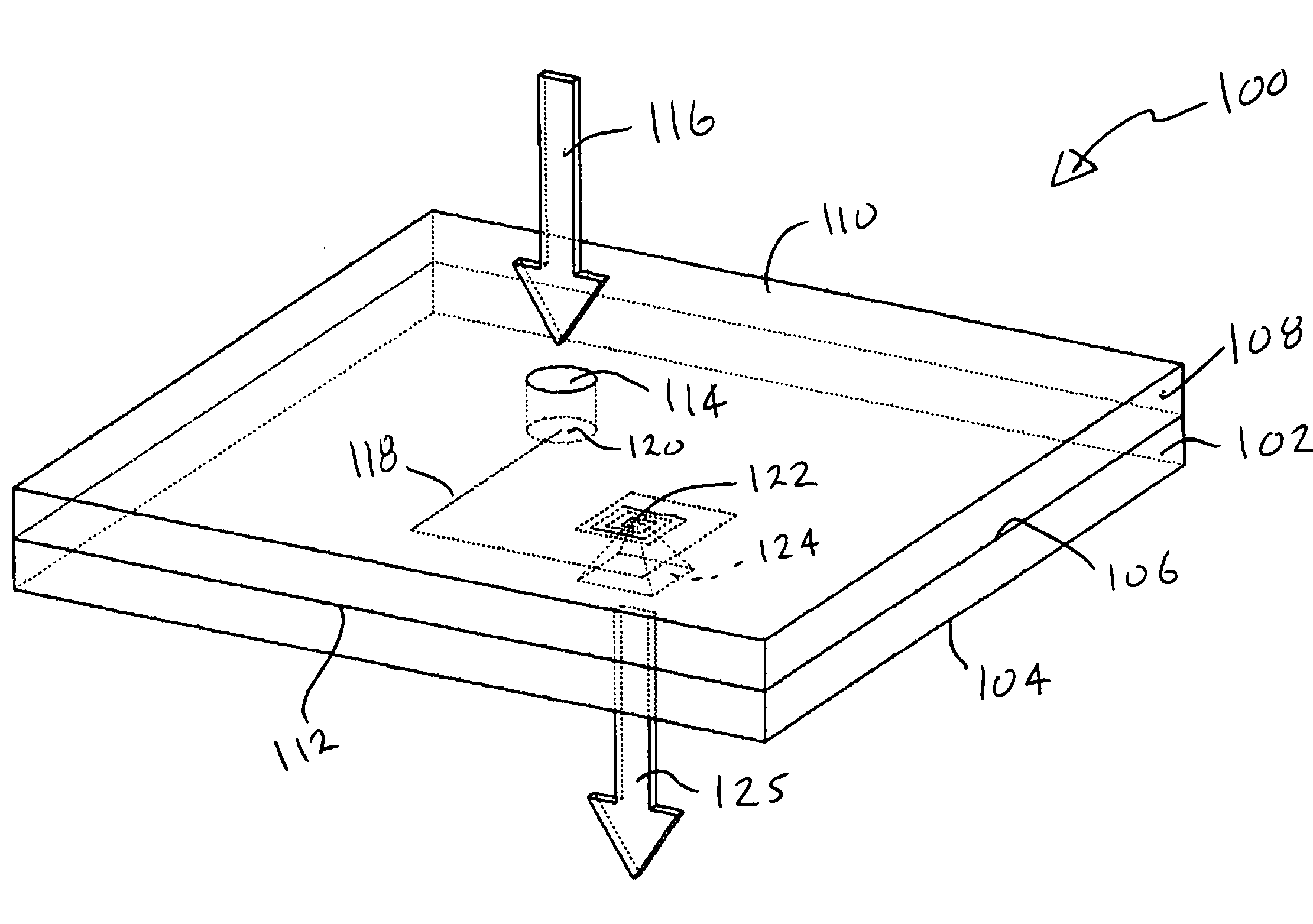
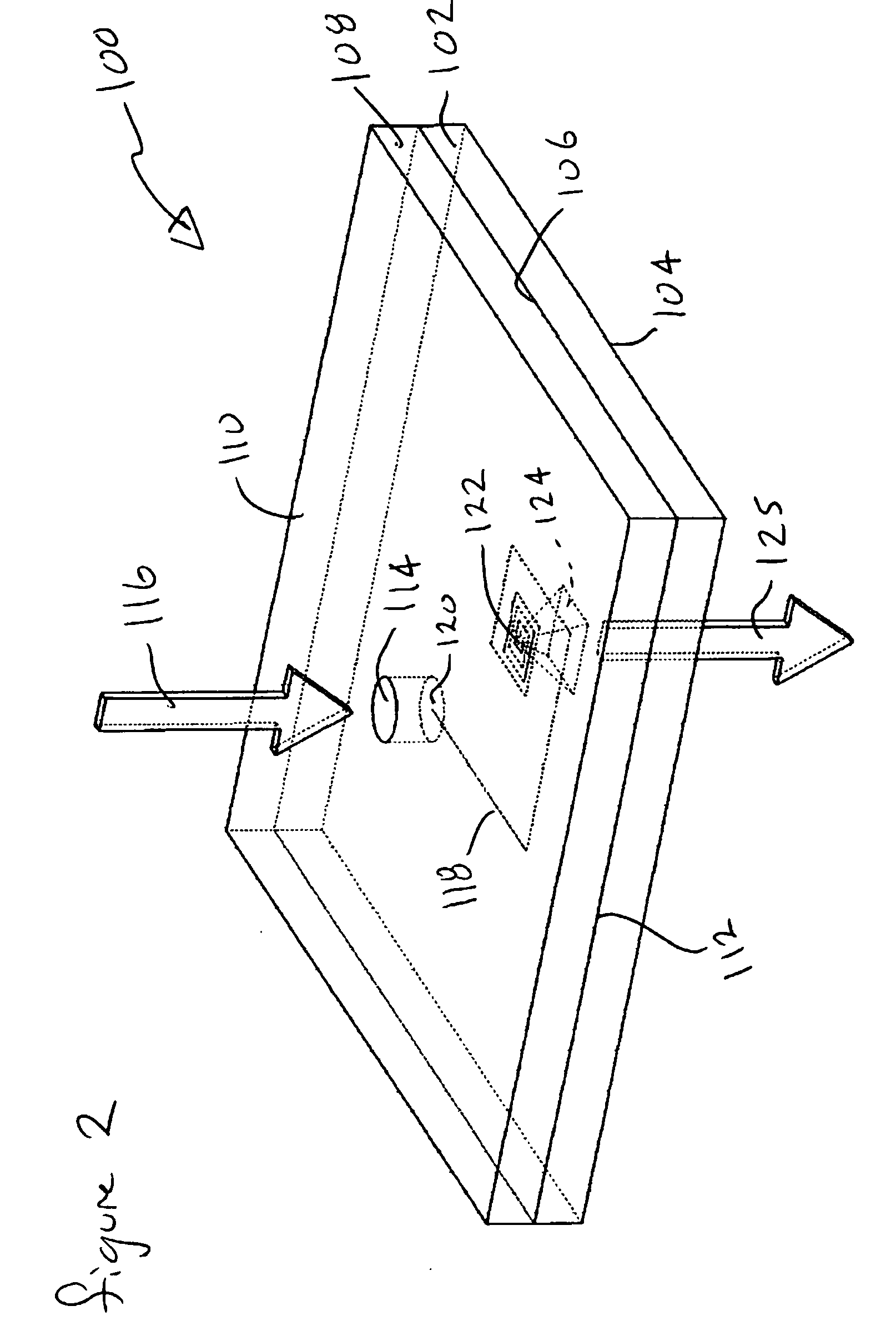
ActiveUS9295417B2Small sizeConvenient handling and useMicroneedlesVaccination/ovulation diagnosticsBlood capillaryReady to use
Owner:YOURBIO HEALTH INC
Vascular access device blood sealing and exposure prevention
ActiveUS20080147009A1Minimize exposureMinimizing to contaminationInfusion syringesCatheterVascular Access DevicesBlood capillary
An extravascular system for accessing the vasculature of a patient including a catheter having an interior surface, a needle disposed within the catheter, and / or a needle cap defining at least one capillary space and including a flexible seal surrounding the at least one capillary space. The flexible seal may engage the interior surface of the catheter. A method of controlling exposure to a liquid from an extravascular system including providing a wicking material positioned adjacent to a flow channel such that any fluid located external to the flow channel is retained by the wicking material. The wicking material may be incorporated into any extravascular system where exposure to liquids is undesirable.
Owner:BECTON DICKINSON & CO
Devices for collecting blood and administering medical fluids
InactiveUS7662110B2Preventing vacuum-induced collapseFacilitating active flow of bloodSamplingInfusion syringesBlood collectionVein
Novel devices which can be used to both collect blood samples from and administer medical fluids to a patient on a repeated and continual basis using one rather than multiple needle insertions. The device typically includes a main tubing segment confluently connected to a cannula for insertion in the patient's vein. A syringe port and a volumeter for collecting blood branch separately from the main tubing segment. The device is used to collect blood by attaching an empty blood collection syringe to the syringe port, inserting the cannula in the patient's vein, allowing passive flow of blood from the main tubing segment into the volumeter under intrinsic venous blood pressure and capillary action, and then facilitating active flow of blood from the volumeter into the blood collection syringe by extending the syringe plunger. The device may be used to administer medical fluids to the patient through the main tubing segment from a medical fluid syringe or catheter attached to the syringe port.
Owner:ONE STICK LLC
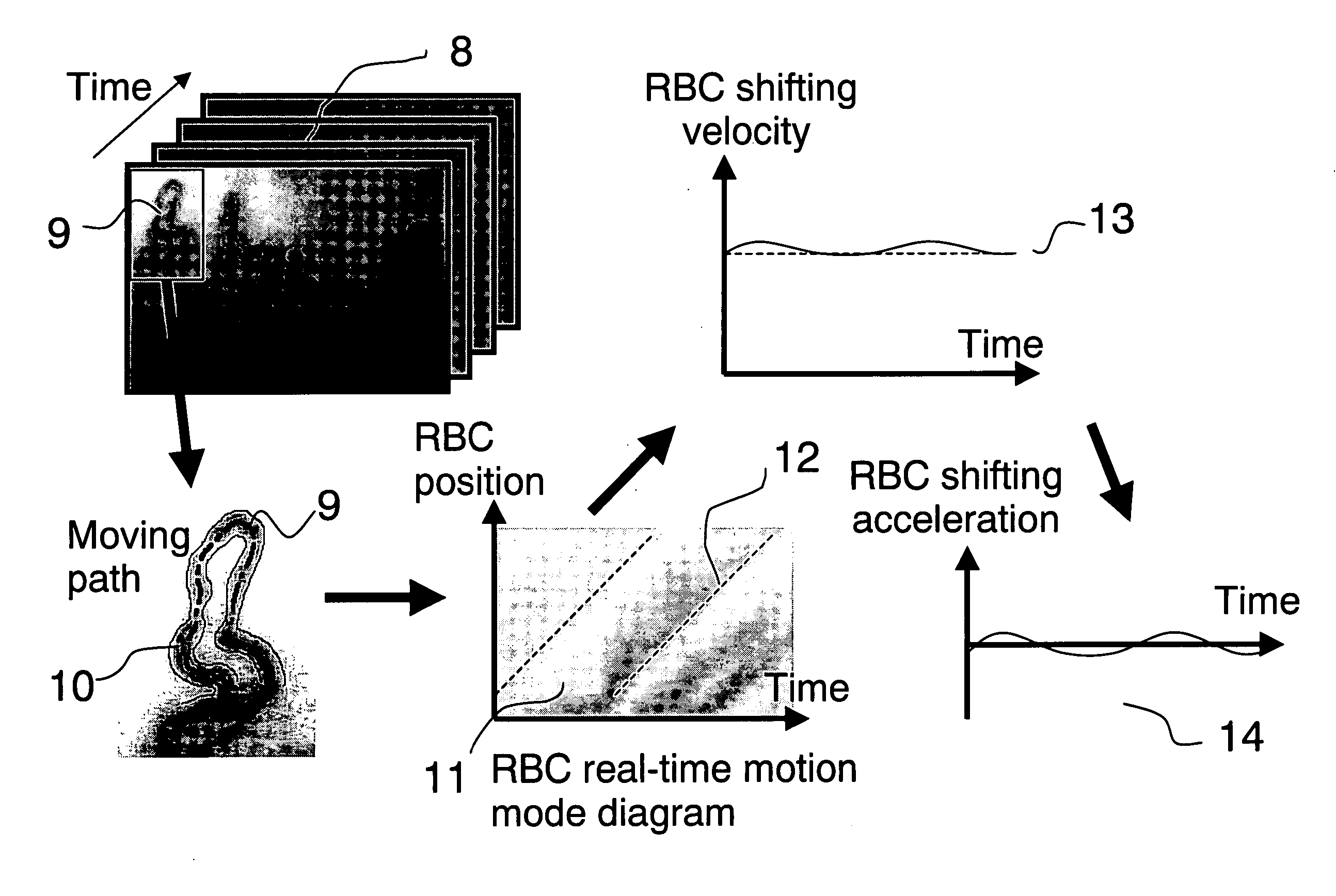
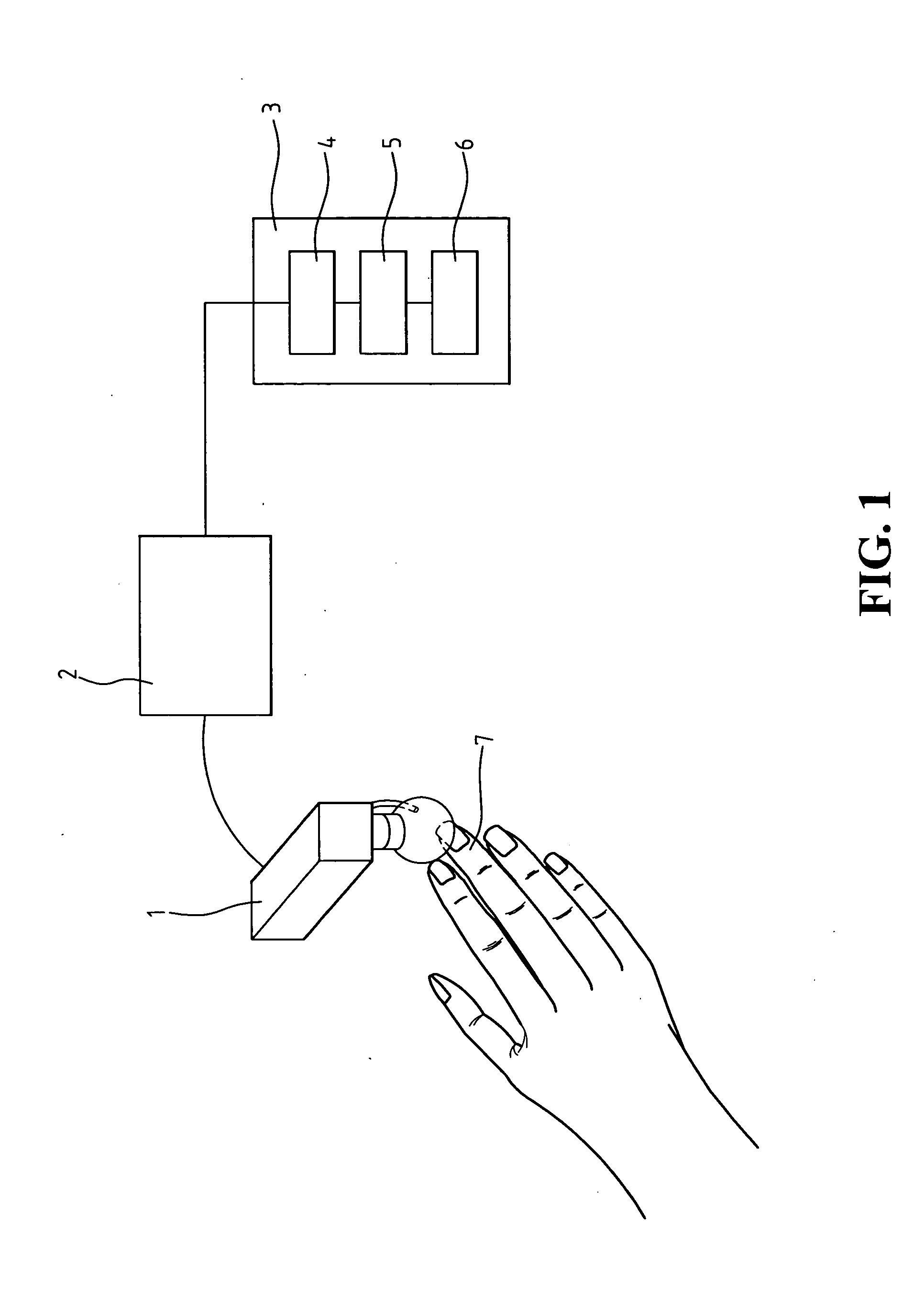
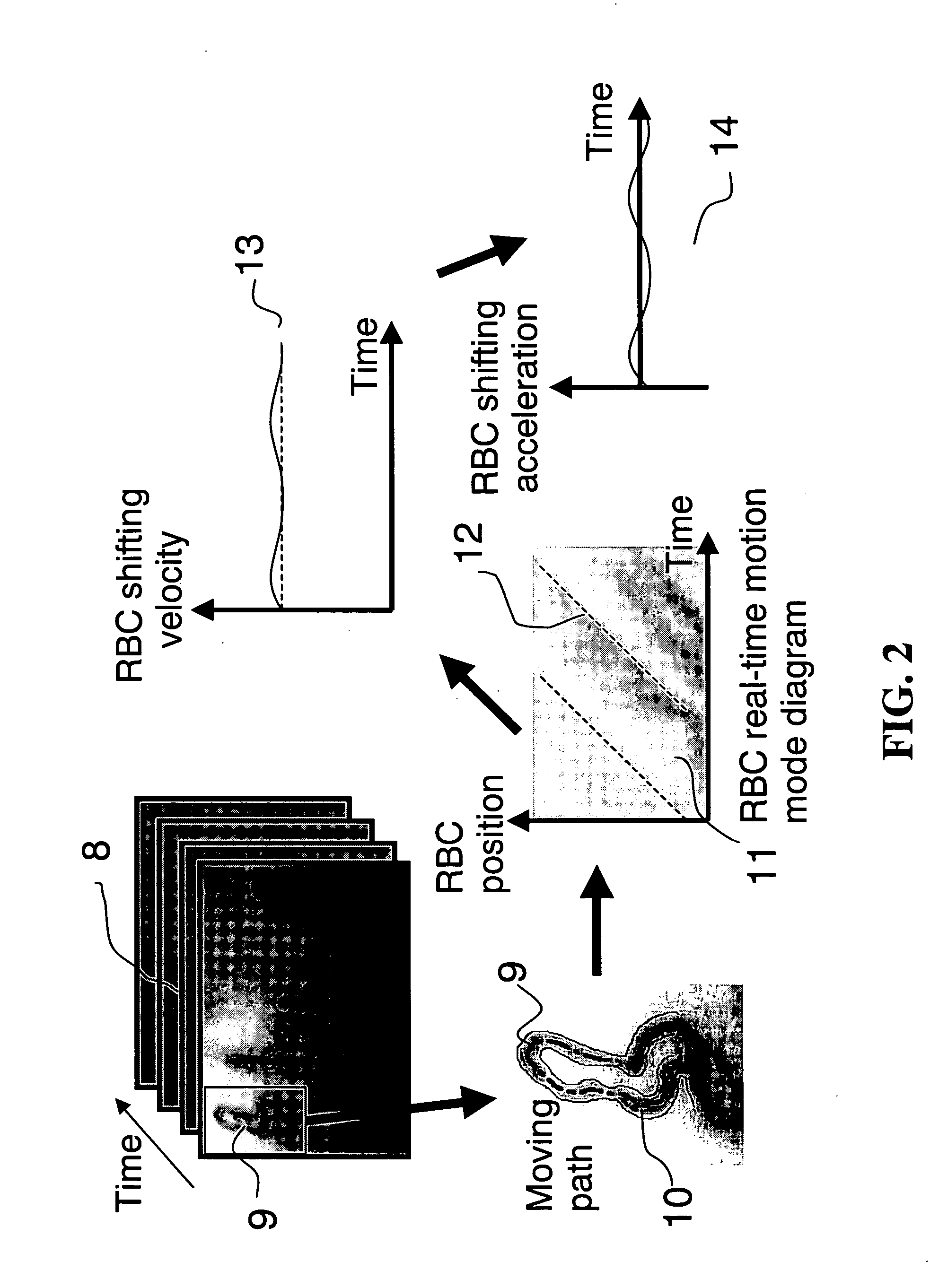
System and method for real-time microcirculation diagnosis
InactiveUS20060161063A1Accurate assessmentShifting velocityImage enhancementImage analysisAnalysis dataRed blood cell
Disclosed is a system and method for real-time microcirculation diagnosis. The system comprises a capillary photographic device, an image capturing device, and a data processing unit. By way of the system, informations about movement behavior of a single red blood cell and the situation of a single capillary are obtained. The method of the present invention comprises the steps of analyzing gray scale of capillary imagine pictures, and producing analytical data and diagram for monitoring movement behavior of a single red blood cell and the situation of a single capillary. The present invention improves the disadvantages of the conventional systems or methods, which only provide an average velocity of red blood cells in a microcirculation system. The system of the present invention does not need a hardware or device for Doppler analysis, and thus decreasing a hardware cost. The present invention can also provide more data and more valuable physiological informations.
Owner:SHAU YIO WHA
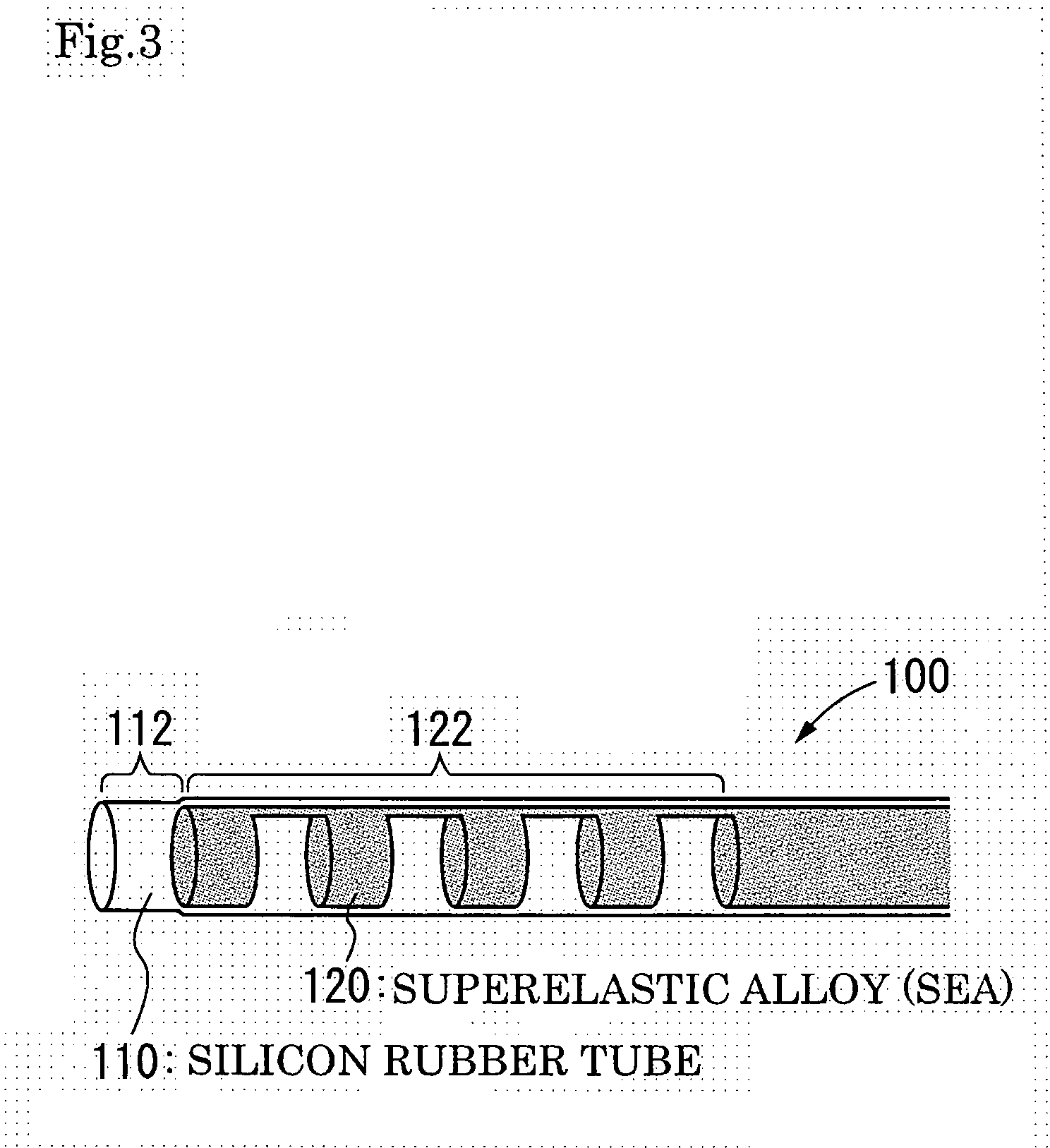
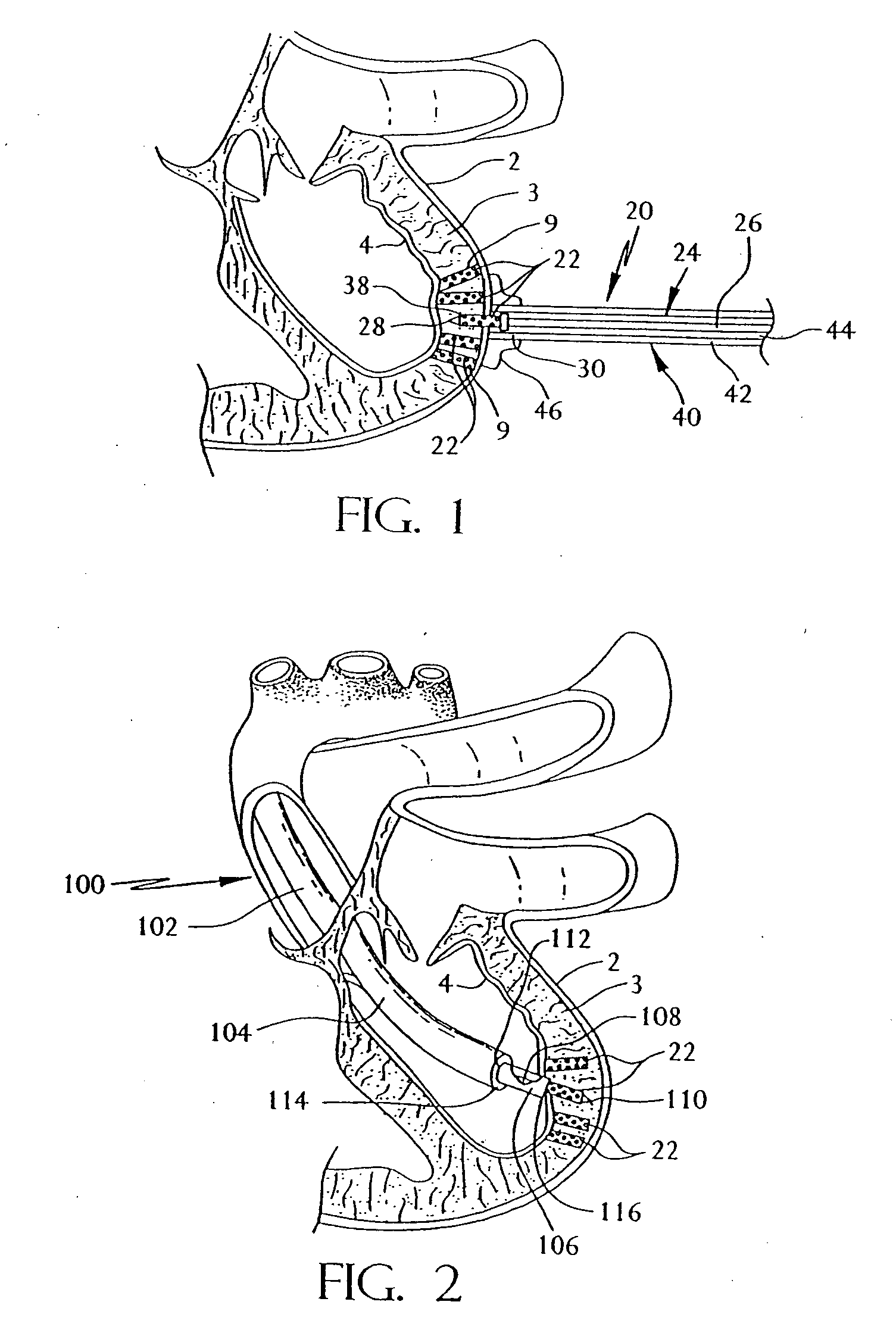
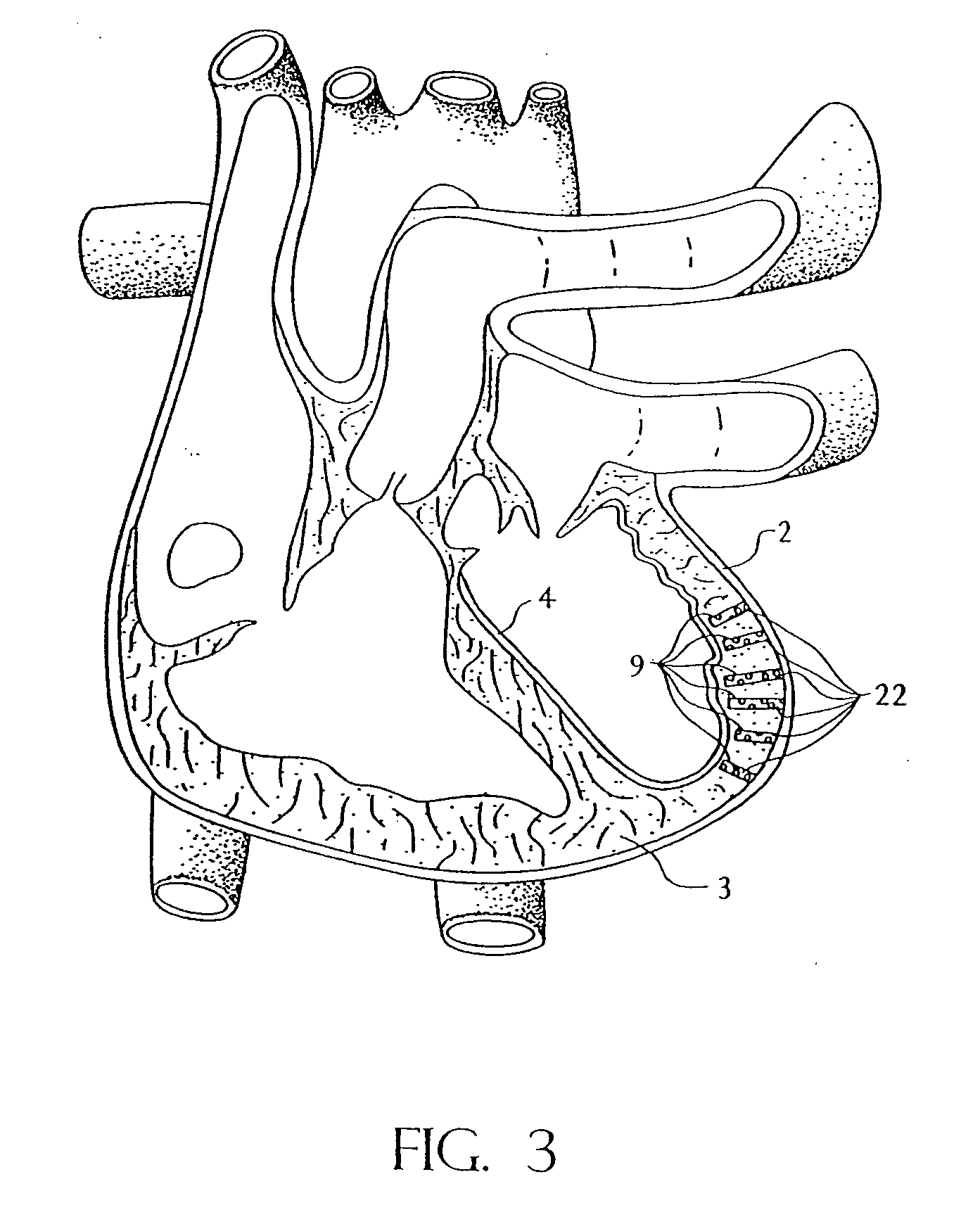
Active capillary
Catheter of such a structure that Ti—Ni superelastic allow (SEA) tube has its outside covered with thin-film silicone rubber tube. The SEA tube at part whose flexion is desired is wrought to cut off multiple grooves (crenas) with thin joints left. The covering with the silicone rubber tube is effected without the use of its front end portion in the covering. When a negative pressure is applied to physiological saline placed therein, the front end portion works as a valve, and the silicone rubber tube at the wrought part yields inward so that flexion of the catheter occurs at that part.
Owner:TOHOKU TECHNO ARCH CO LTD
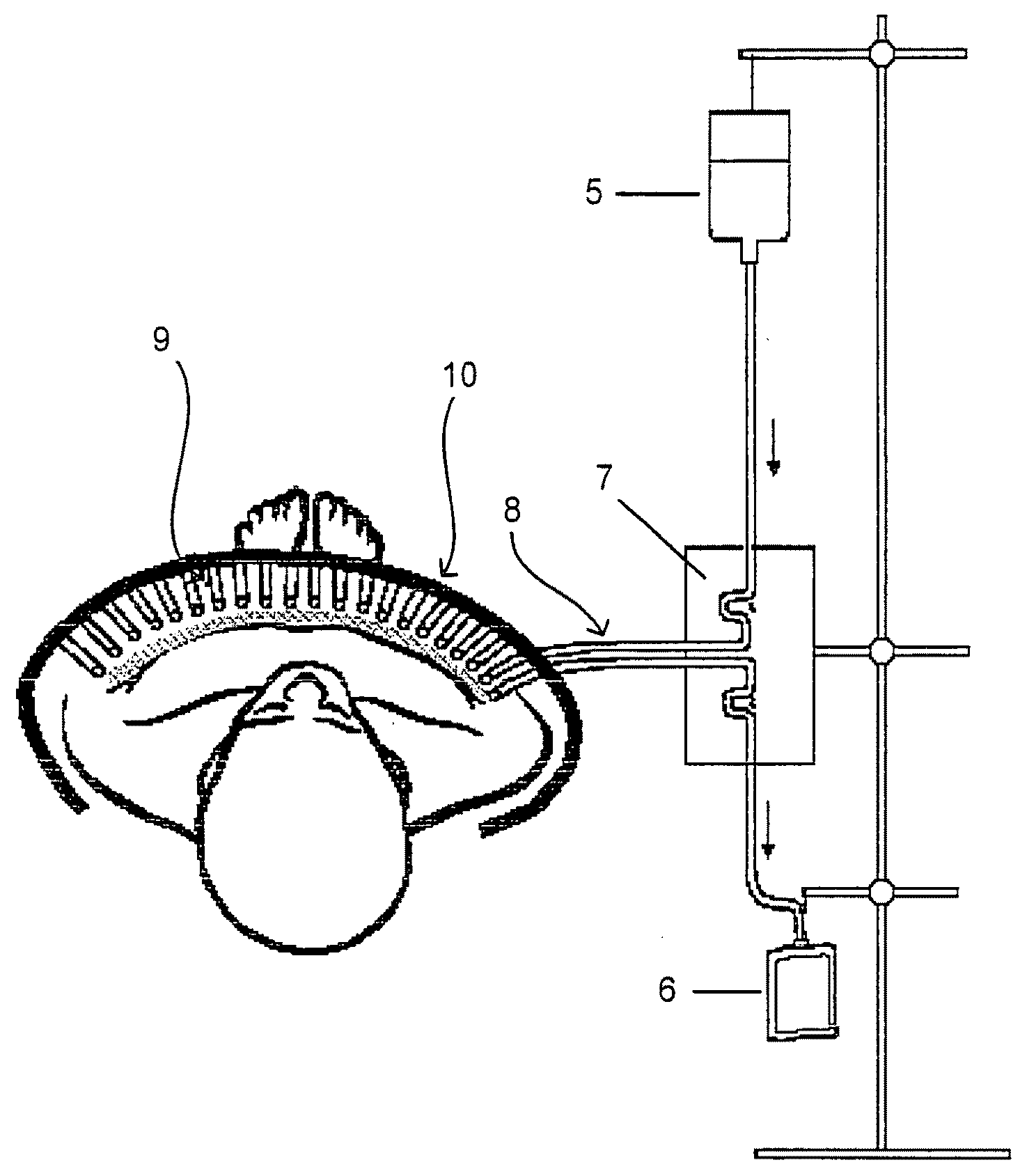
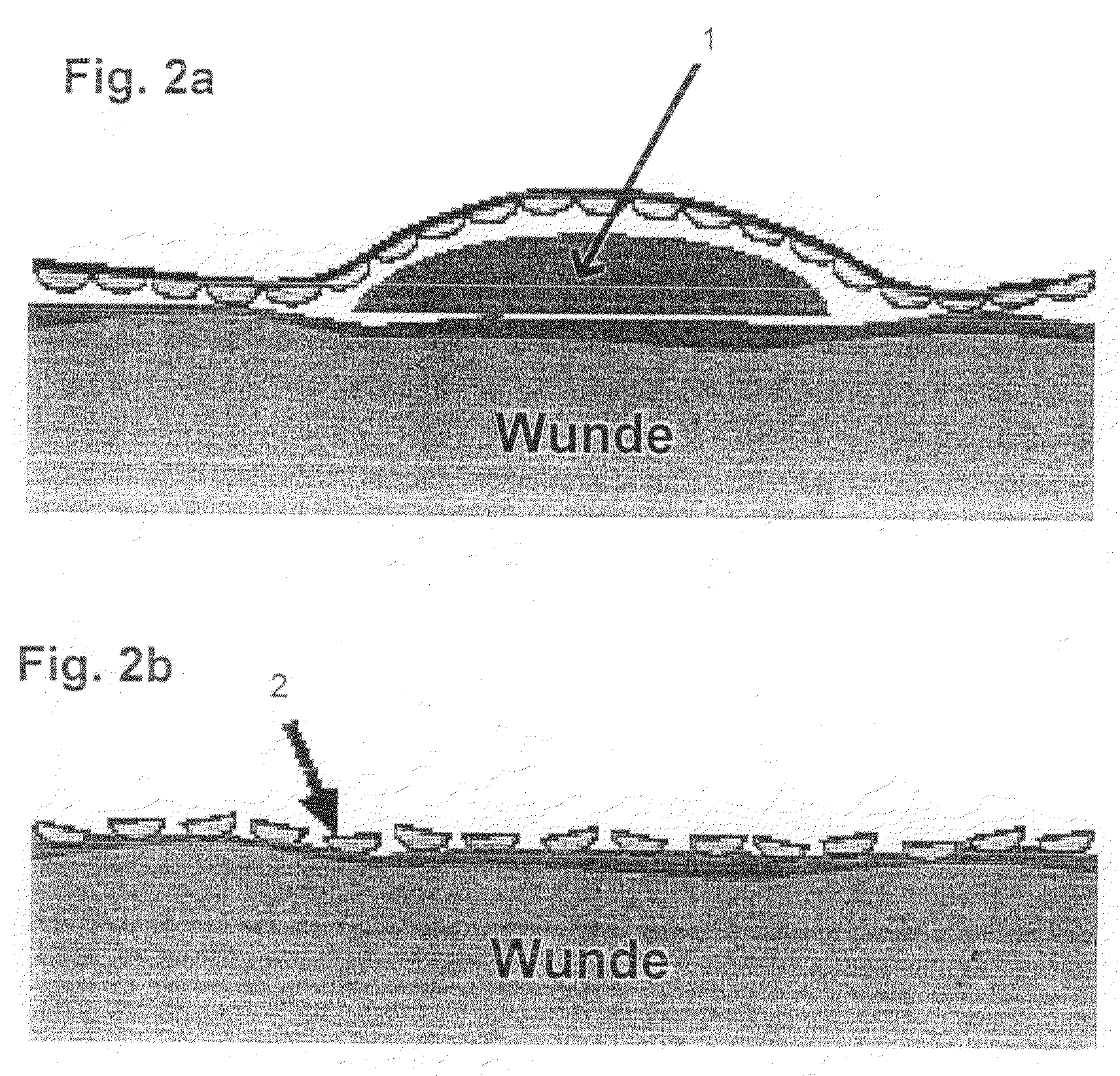
Artificial "arterio-venous" permeable hollow fiber capillary system
ActiveUS20090196855A1Improves mass exchangeModerate negative pressureBiocideSemi-permeable membranesFiberWound dressing
An artificial “arterio-venous” permeable hollow fiber capillary membrane system for wound treatment is described, that can be placed onto a wound, under the wound dressing, to enable fluid- / mass exchange, and thus enables pH- and electrolyte regulation, the supply of factors / mediators / medications, the removal of secretion- and / or waste material, and the support of therapeutically applied cells in the wound.
Owner:BORNEMANN REINHARD
Method and apparatus for carrying out the controlled heating of tissue in the region of dermis
InactiveUS20070135880A1Improve protectionIncrease heatSurgical instrument detailsTherapeutic coolingAdjuvantCapillary malformation
Owner:APSARA MEDICAL
System for assessing tissue substance extraction
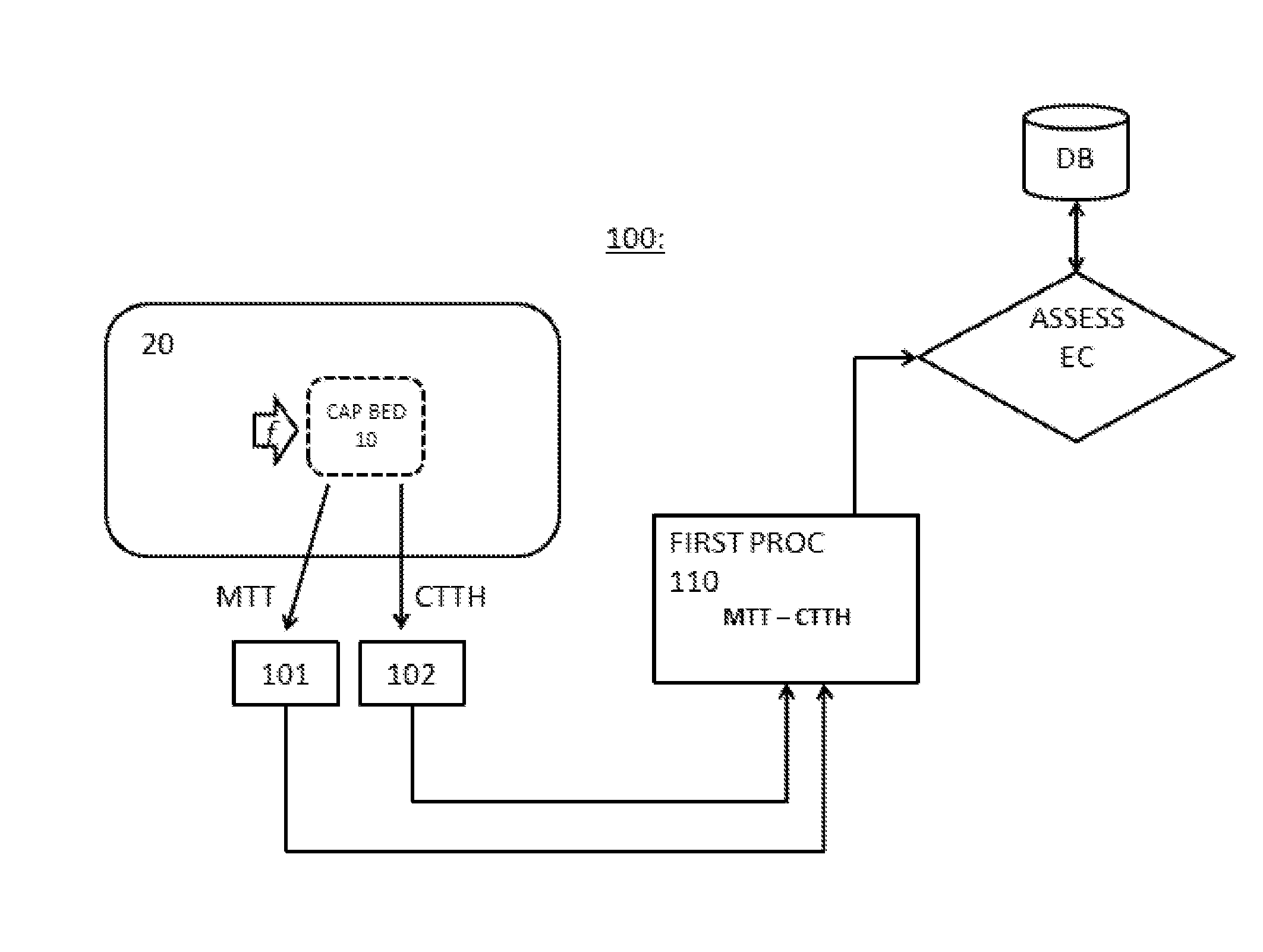
The present invention relates to a system for measuring a micro-vascular flow distribution of a tissue portion of a mammal comprising means (101) for measuring a first indicator (MTT) for the blood flow through a capillary bed, means (102) for measuring a second indicator (CTTH) of heterogeneity of the blood flow in said capillary bed, and a first processor (110) arranged for using the first and the second indicator to estimate an extraction capacity (EC) of a substance from the blood in said capillary bed.
Owner:AARHUS UNIV +1
Transmyocardial revascularization system and method of use
InactiveUS20050159726A1Increase perfusionMinimizing pain sensationStentsHeart valvesActive agentCardiac cycle
A transmyocardial revascularization system including a plurality of inserts formed of a material to elicit a healing response in tissue of the myocardium and deployment instruments and associated components for deploying the inserts into the wall of the myocardium. The inserts are arranged to be disposed within respective lumens or channels in the wall of the myocardium. The inserts can take various forms, e.g., be solid members, tubular members, or porous members, and may be resorbable, partially resorbable or non-resorbable. In some embodiments the inserts are arranged to be left in place within the channels in the wall of the myocardium to result in plural lumens which enable blood to flow therethrough and into contiguous capillaries. The deployment instruments are arranged to pierce the tissue of the myocardium from either the endocardium or the epicardium to insert the inserts into the myocardium, depending on the particular deployment instrument used. The deployment instruments may make use of a stabilizing device to stabilize them during the procedure of inserting the inserts into the myocardium. A controller may also be provided as part of the system to coordinate the operation of the deployment instrument with the cardiac cycle. The formation of the lumens can be achieved either by the inserts or by some other means, such as a piercing tip or an energy applicator forming a portion of the instrument. The inserts may include one or more of pharmaceuticals, biologically active agents, radiopaque materials, etc.
Owner:KENSEY NASH CORP
Blood coagulation test cartridge, system, and method
InactiveUS20050255601A1Practical and convenientRapid and inexpensiveMicrobiological testing/measurementWithdrawing sample devicesBlood capillaryEngineering
A system and method for determining a coagulation time, e.g., thrombin time, PT, aPTT, and ACT, of a blood sample deposited in a test cartridge is disclosed. The test cartridge comprises a blood receptacle that is open to the atmosphere into which a blood sample is to be deposited, a vacuum port that is open to atmosphere, and a spiral capillary within the test cartridge having a capillary length and cross-section area, a first capillary end of the spiral capillary open to the blood receptacle and a second capillary end of the spiral capillary open to the vacuum port, whereby the spiral capillary is closed to atmosphere. When a blood sample is deposited in the blood receptacle, a vacuum is drawn through the vacuum port and the blood is drawn through the spiral capillary until coagulation occurs. A pressure change is detected, and the coagulation time is measured.
Owner:MEDTRONIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com