A method of assessing the propensity of a drug to develop an adverse reaction
A technology of adverse reactions and drugs, applied in the fields of toxicology and information processing, can solve problems such as affecting the final accuracy of evaluation, unable to filter information interference, and wrong evaluation results, so as to improve predictability and drug safety, reduce blindness, Evaluate effects with high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
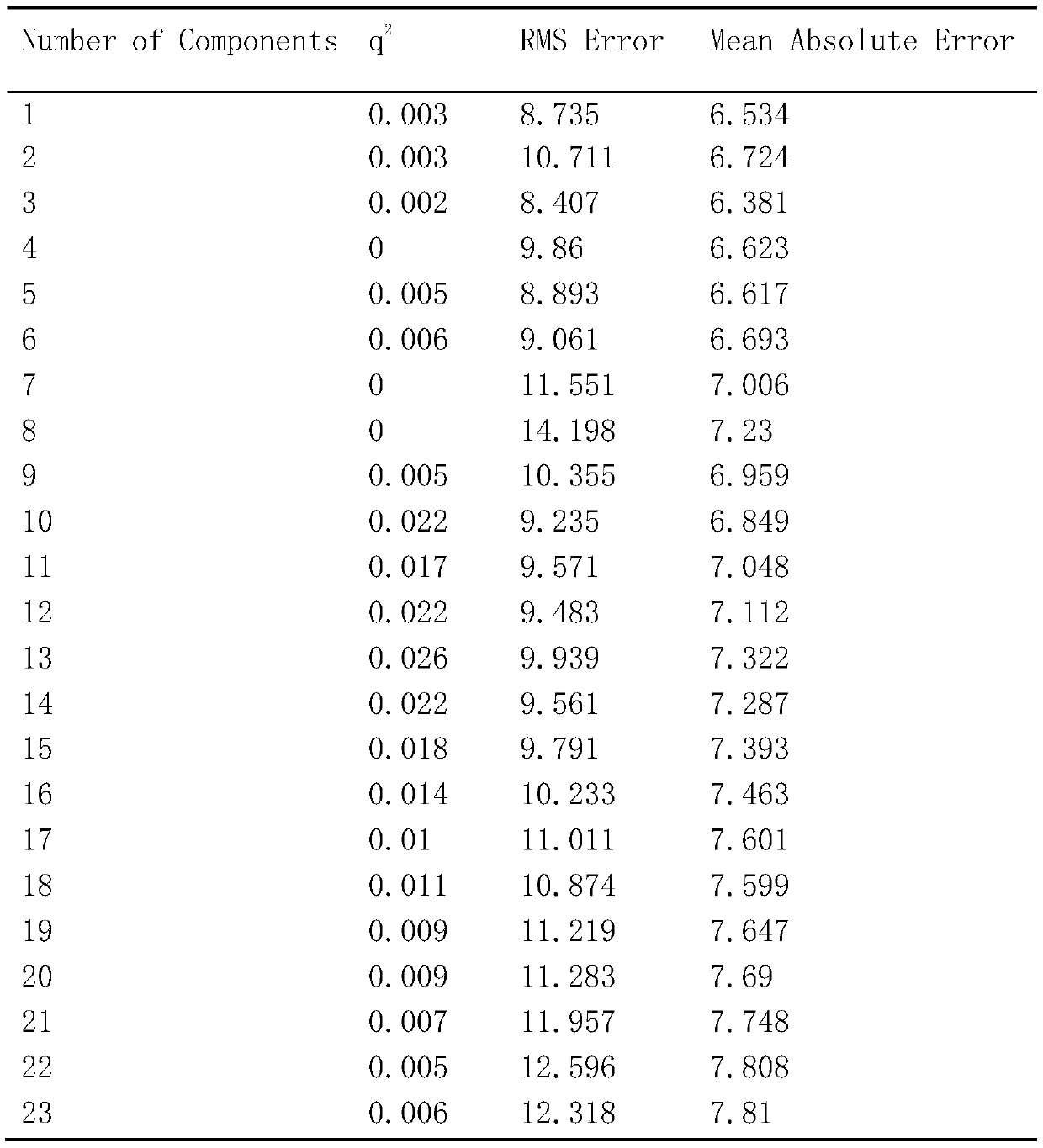
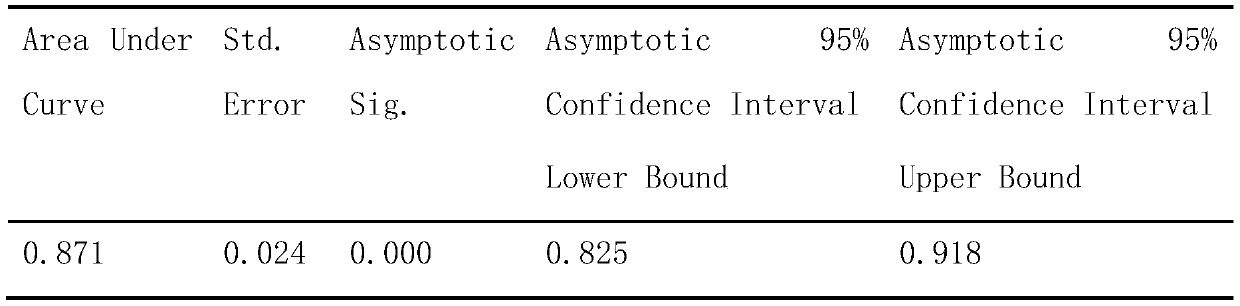
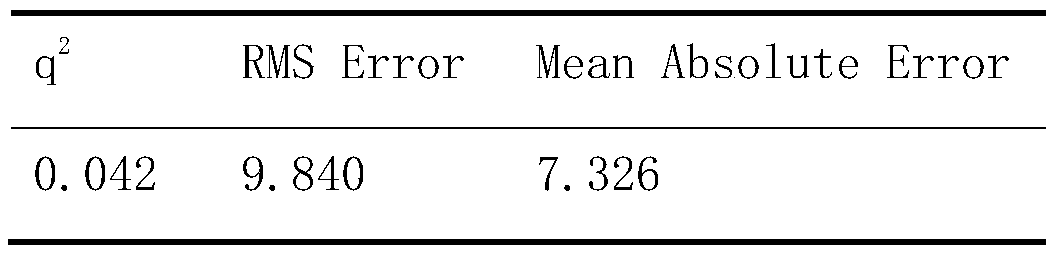
Image
Examples
Embodiment Construction
[0035] Embodiments of the present invention are described in detail below.
[0036] The present invention proposes a method for evaluating the tendency of adverse drug reactions, which specifically includes the following steps:
[0037] Step 1: Measure and collect relevant information of patients who have adverse reactions, and form an adverse reaction report. The relevant information includes: basic personal information of patients, adverse reactions that have occurred, and medications they are taking.
[0038] This example takes the adverse reaction of "Angina Pectoris" as an example. According to the report of the patient who is taking the treatment drug, it is confirmed that the patient has angina pectoris after taking the treatment drug by means of electrocardiogram examination or coronary artery CT examination. For this adverse reaction, the patient's basic personal information, adverse reactions occurred, and the treatment drugs being taken are recorded in the record, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com