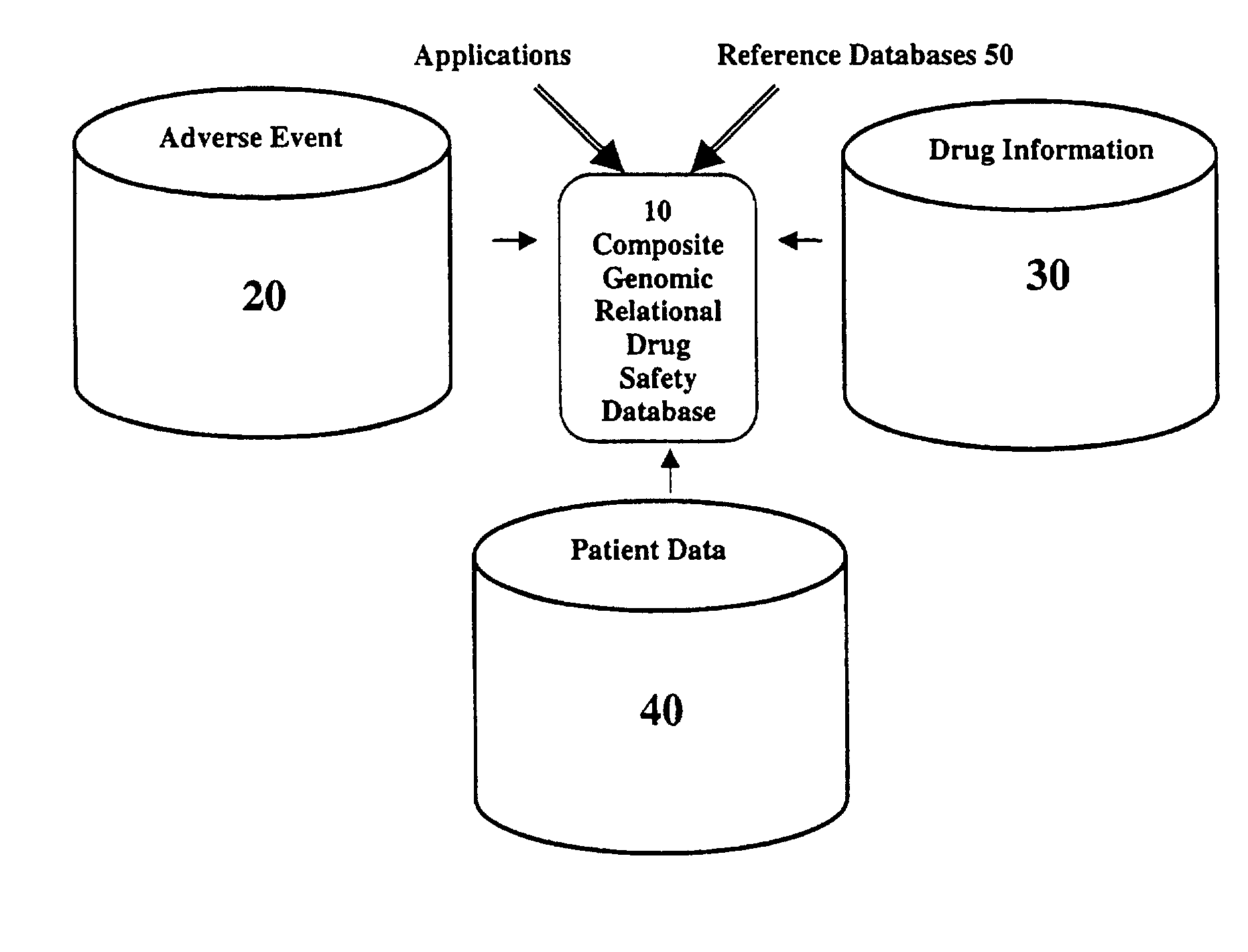
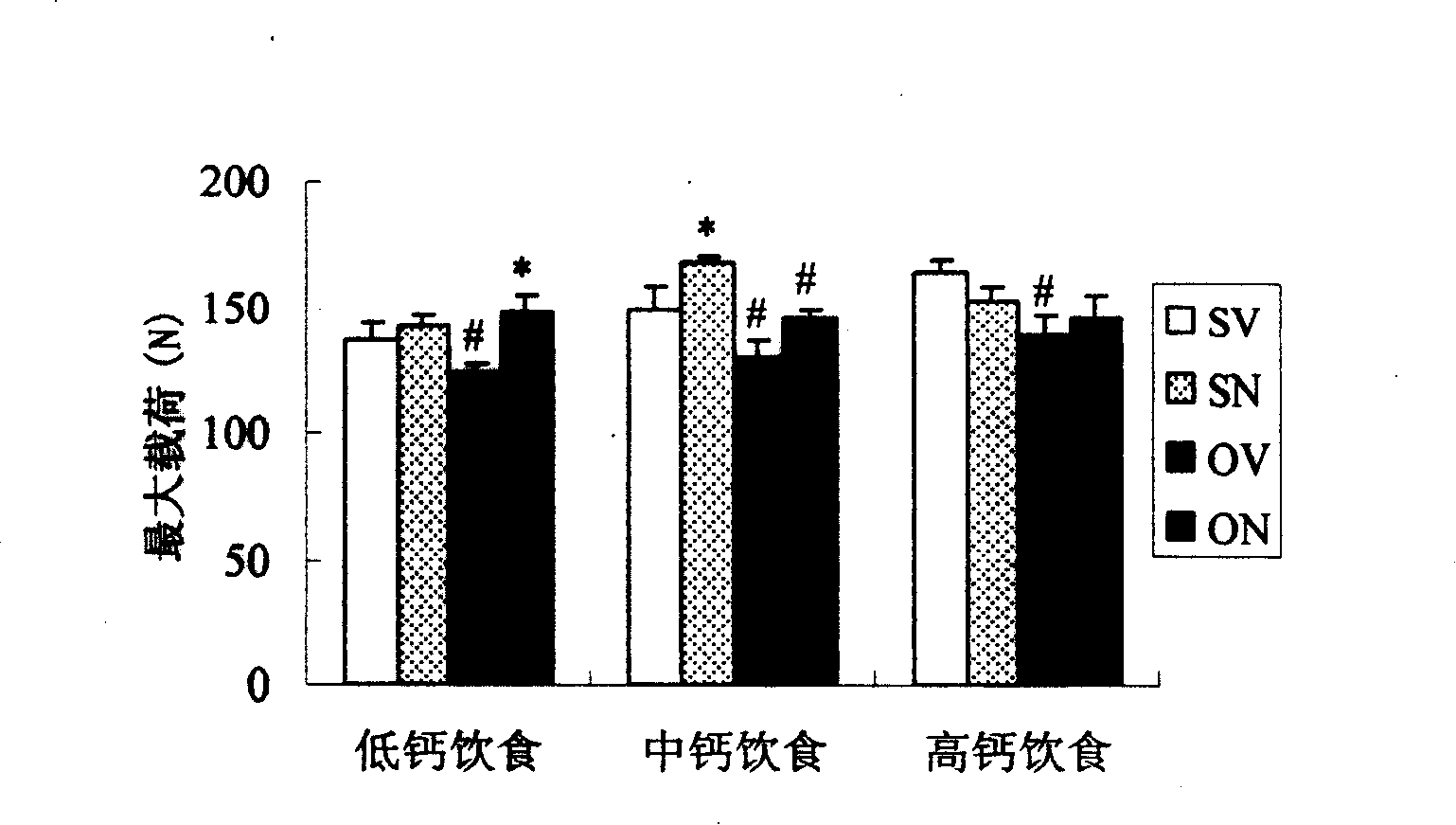
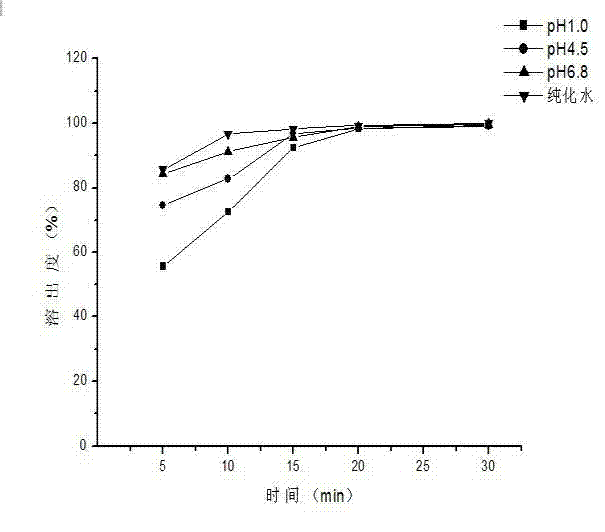
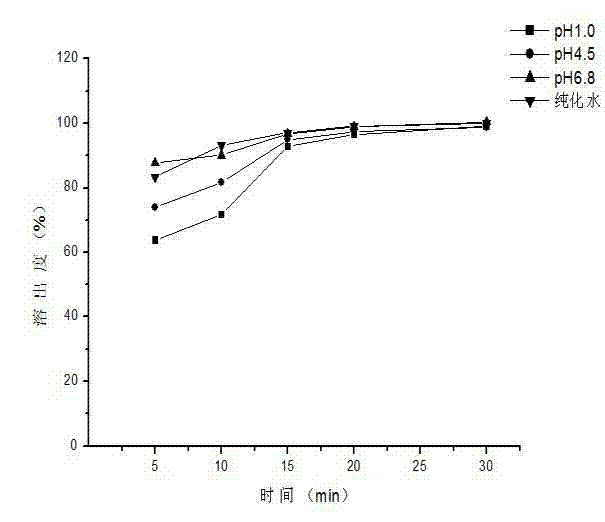
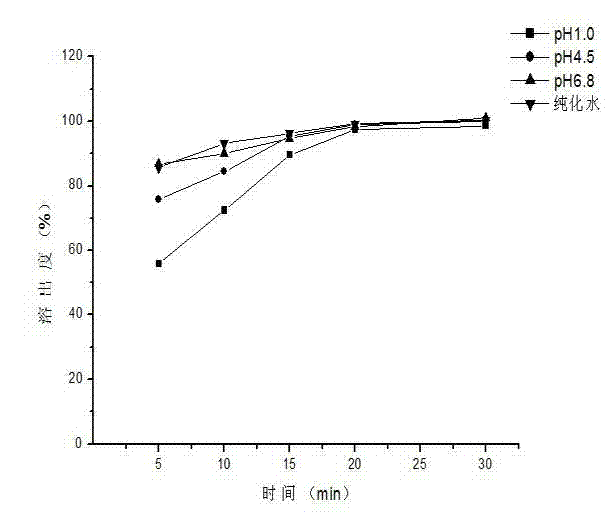
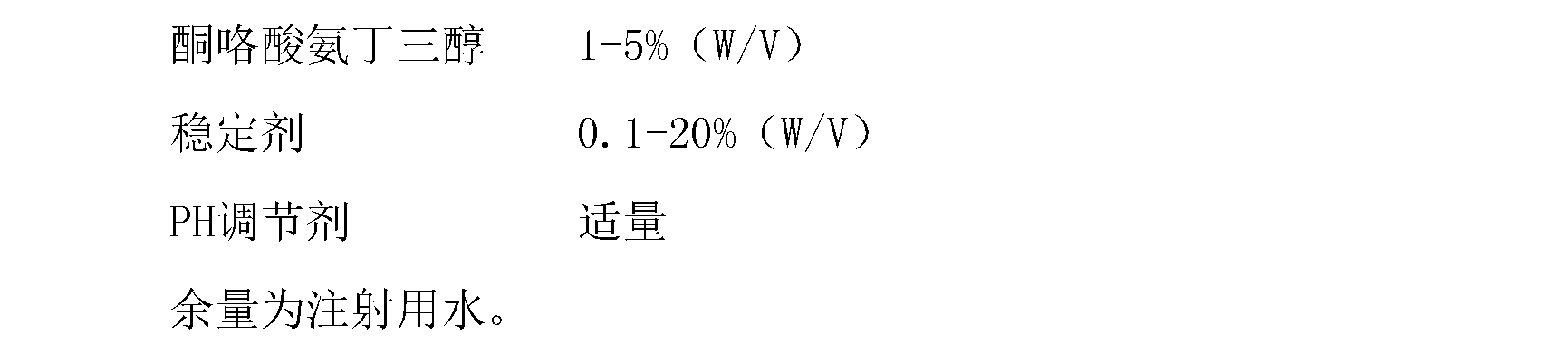Patents
Literature
375 results about "Drugs safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In pharmaceutical research and production, a drug safety associate is responsible for tracking and investigating safety issues and adverse reactions of drugs.
Medication security apparatus and method
Owner:HERRERA HECTOR J
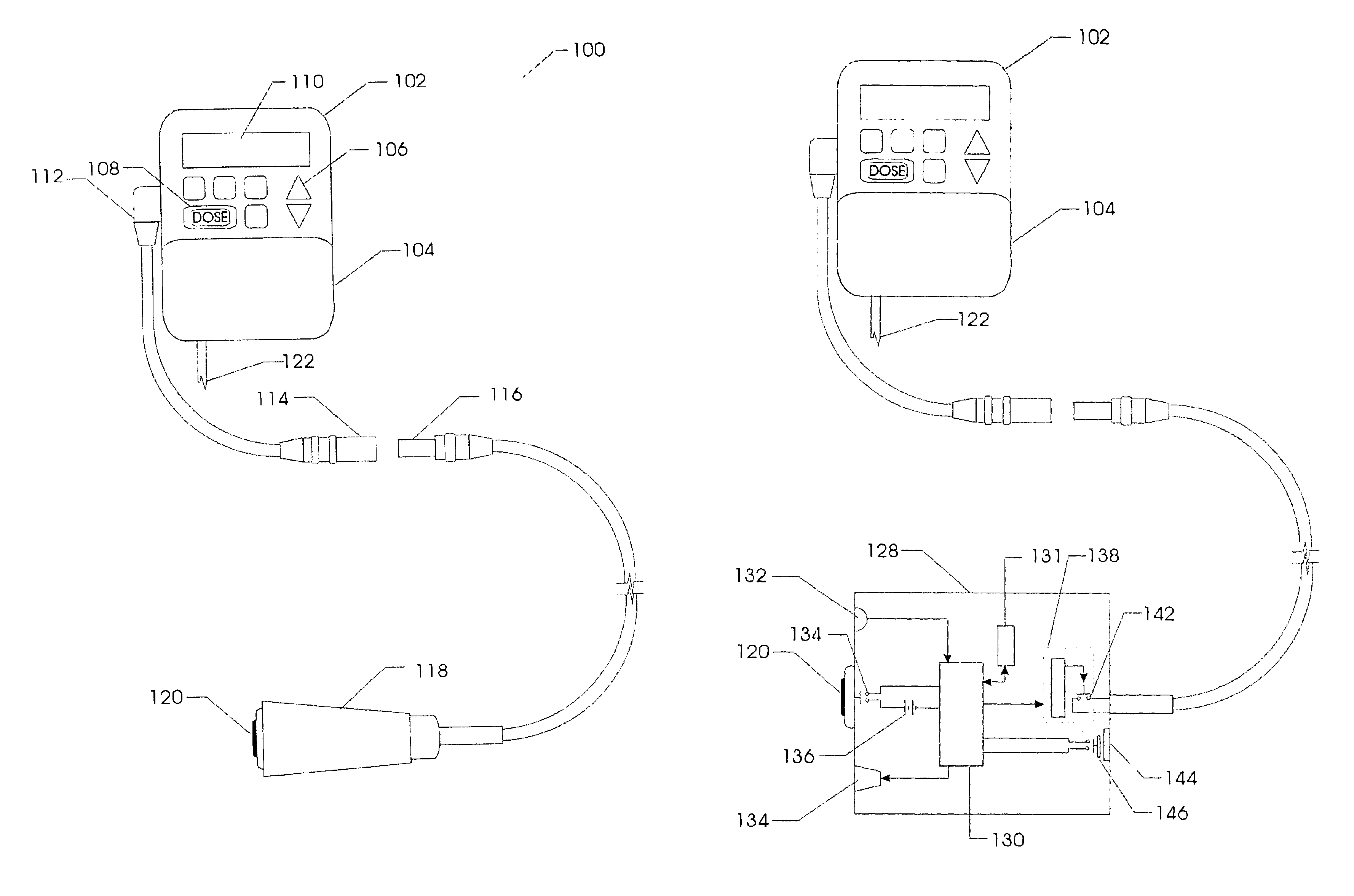
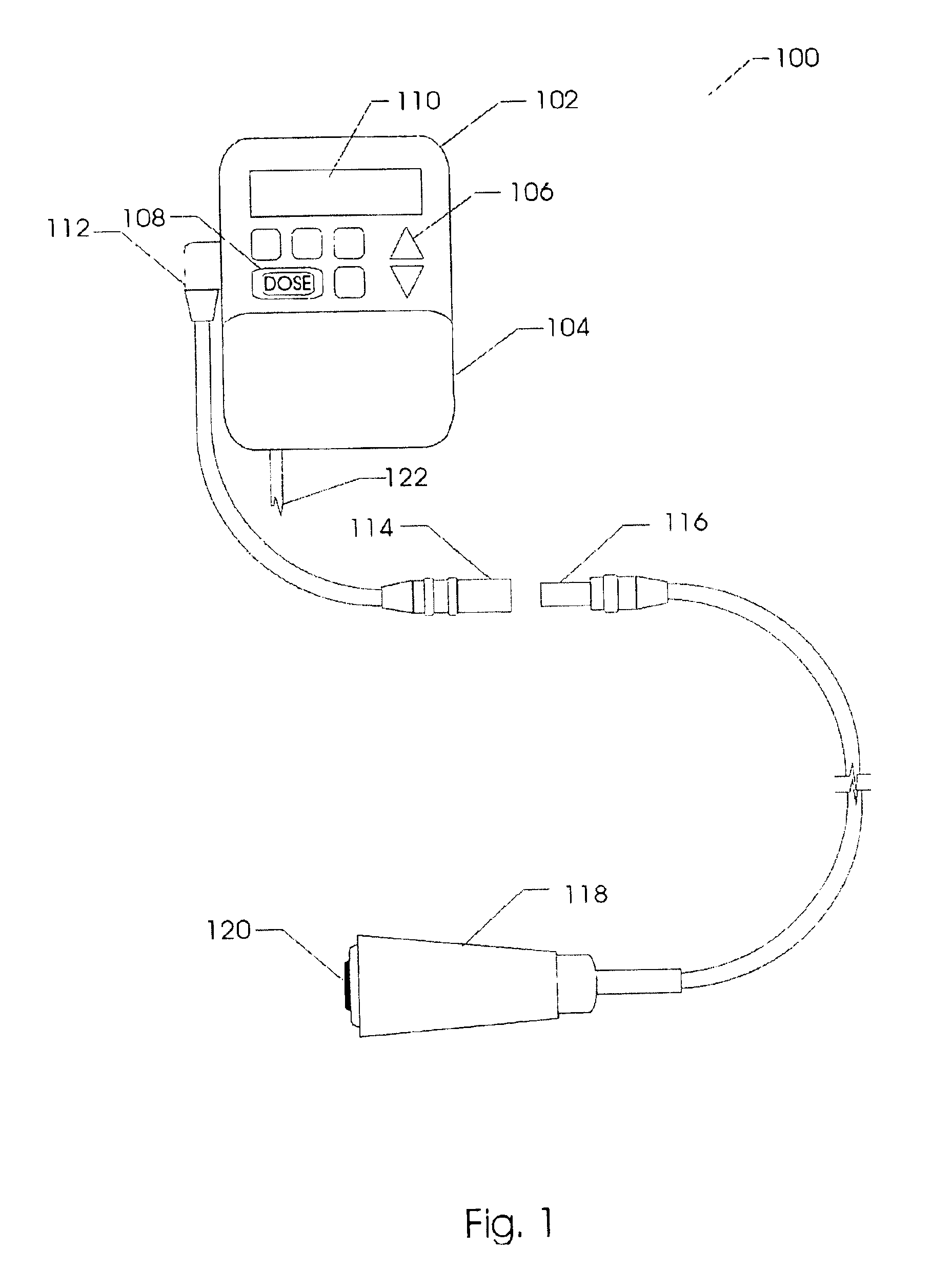
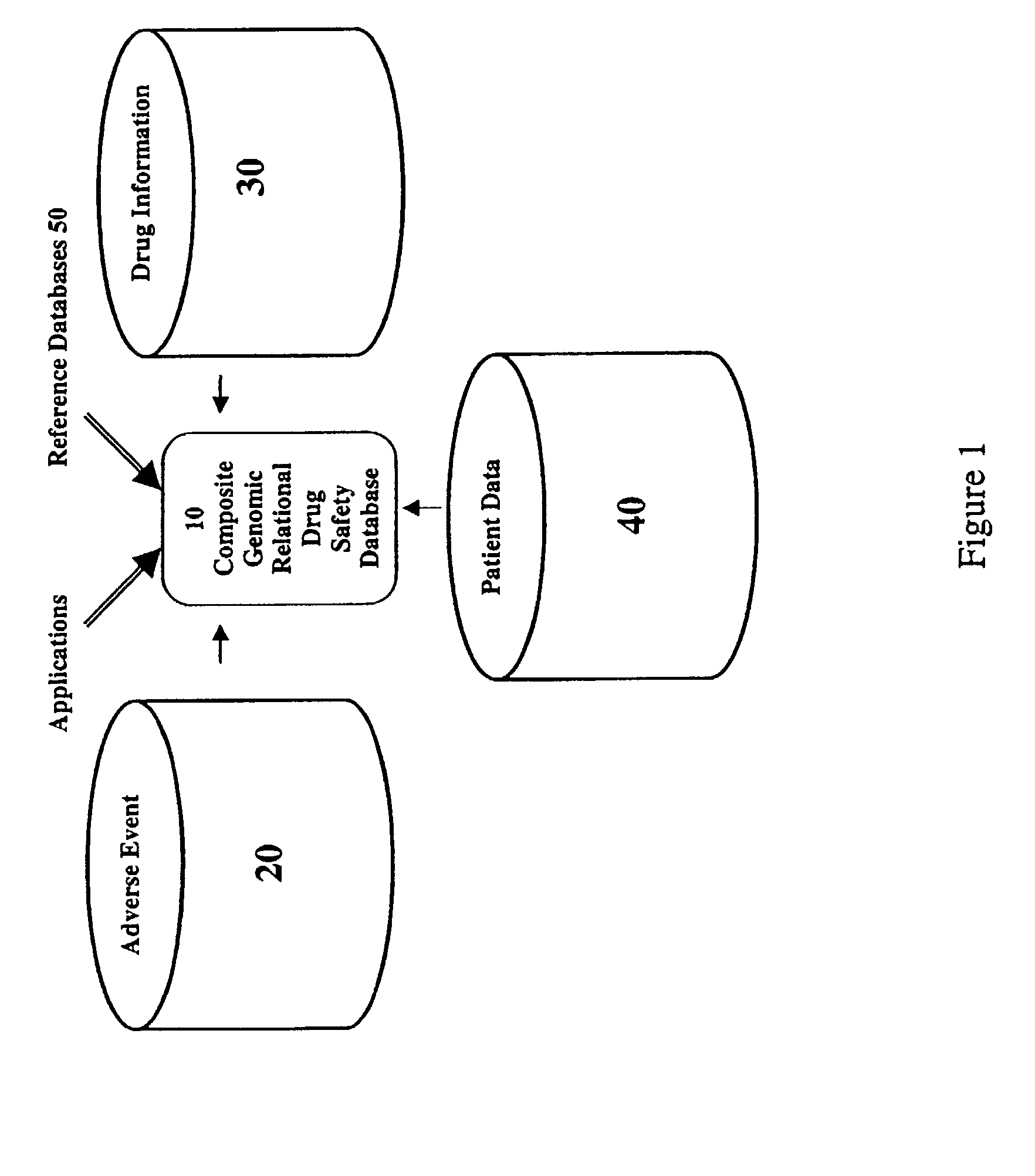
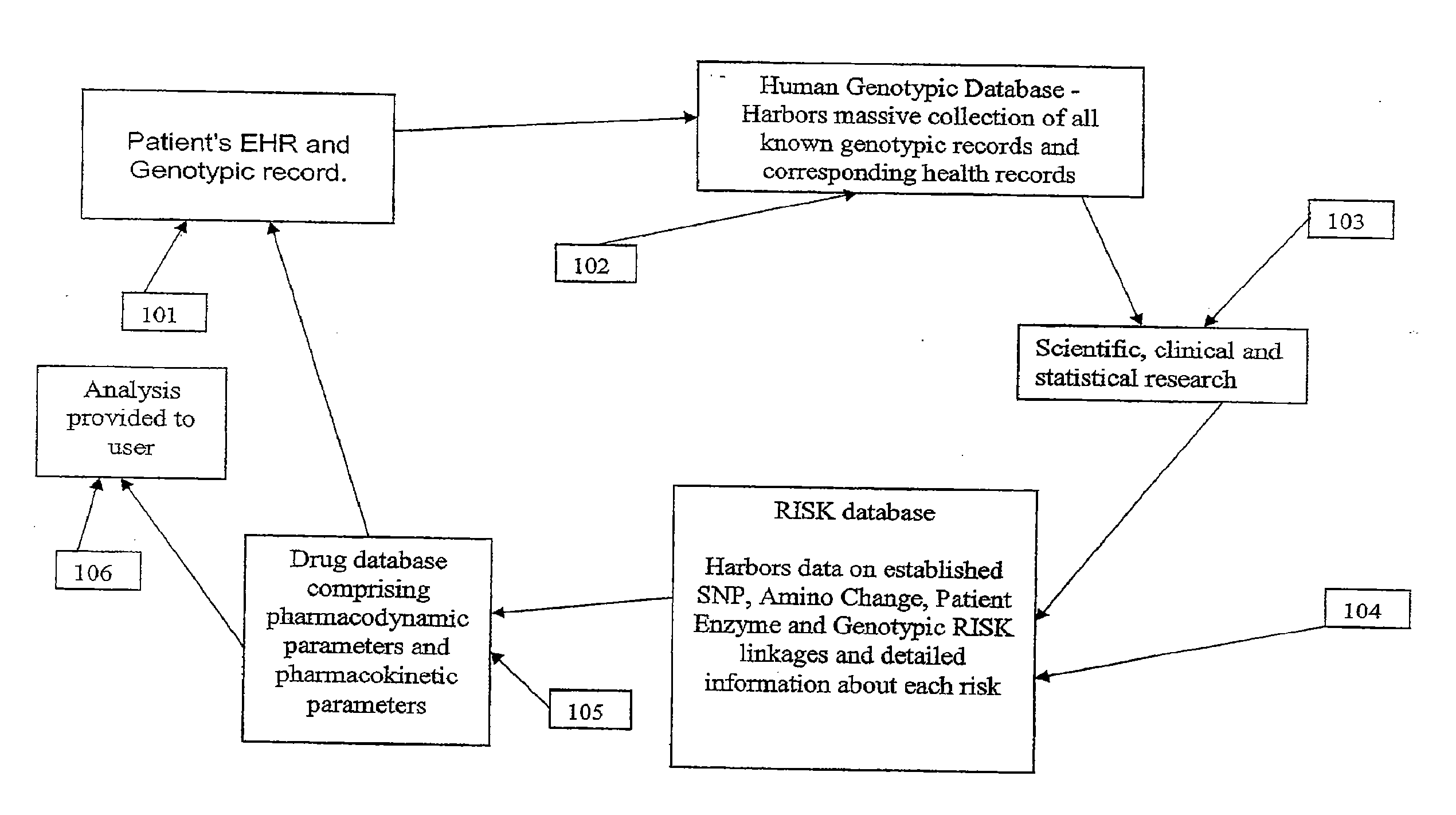
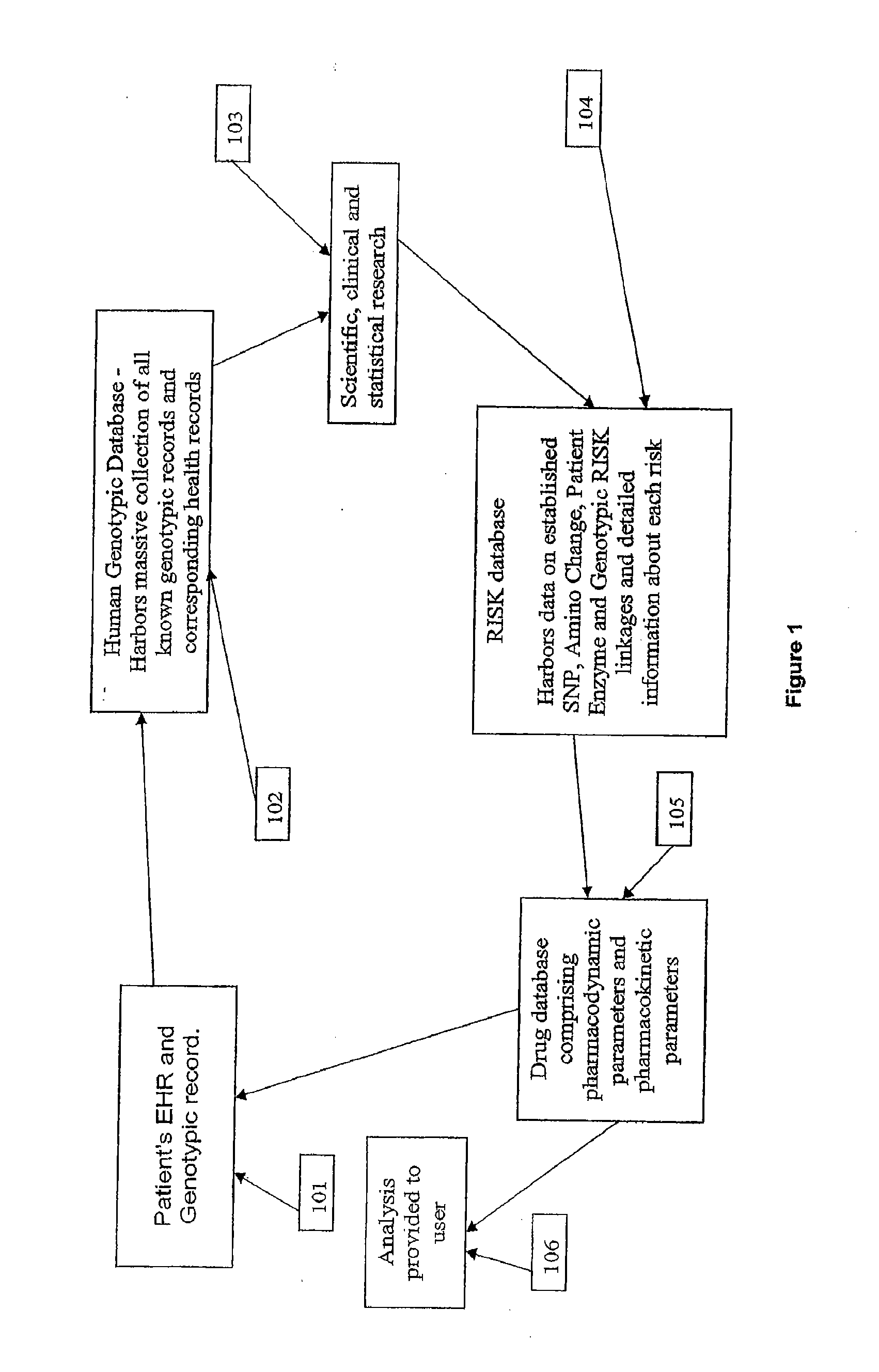
Method and system for the analysis and association of patient-specific and population-based genomic data with drug safety adverse event data
ActiveUS7461006B2Risk of adverse drug reactionIncreased and decreased chanceBiostatisticsComputer-assisted medical data acquisitionPatient characteristicsGenomic DNA
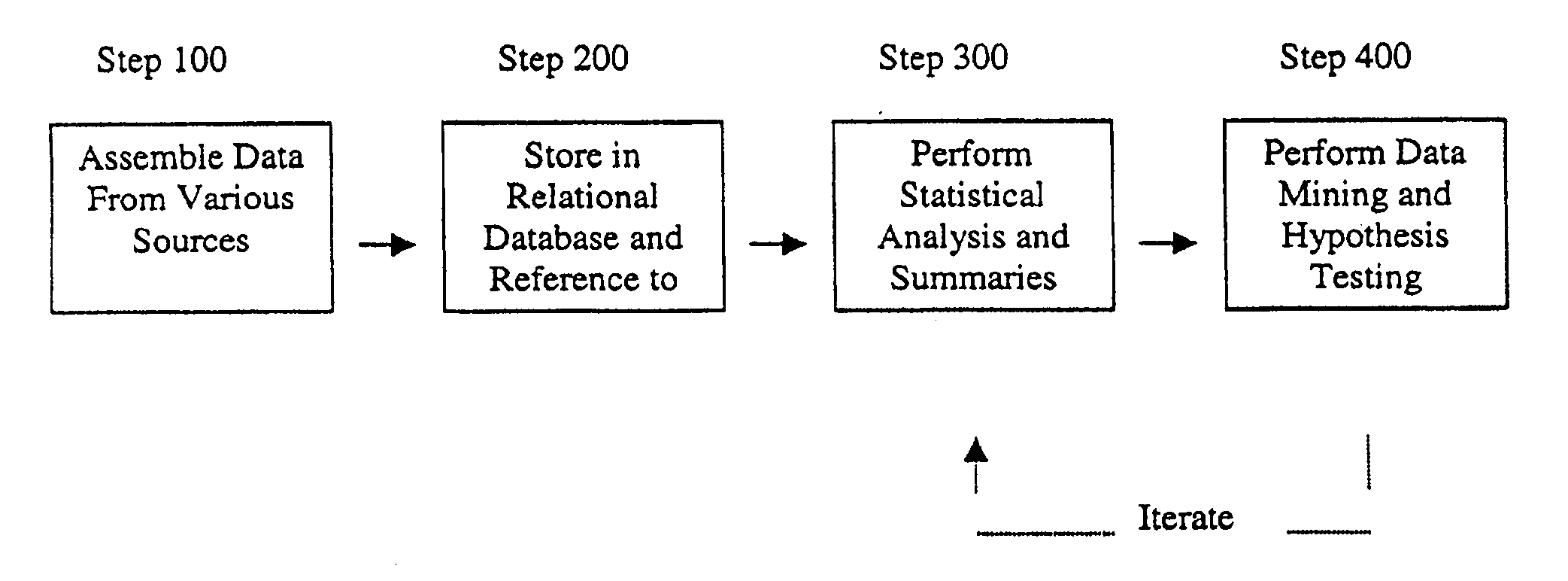
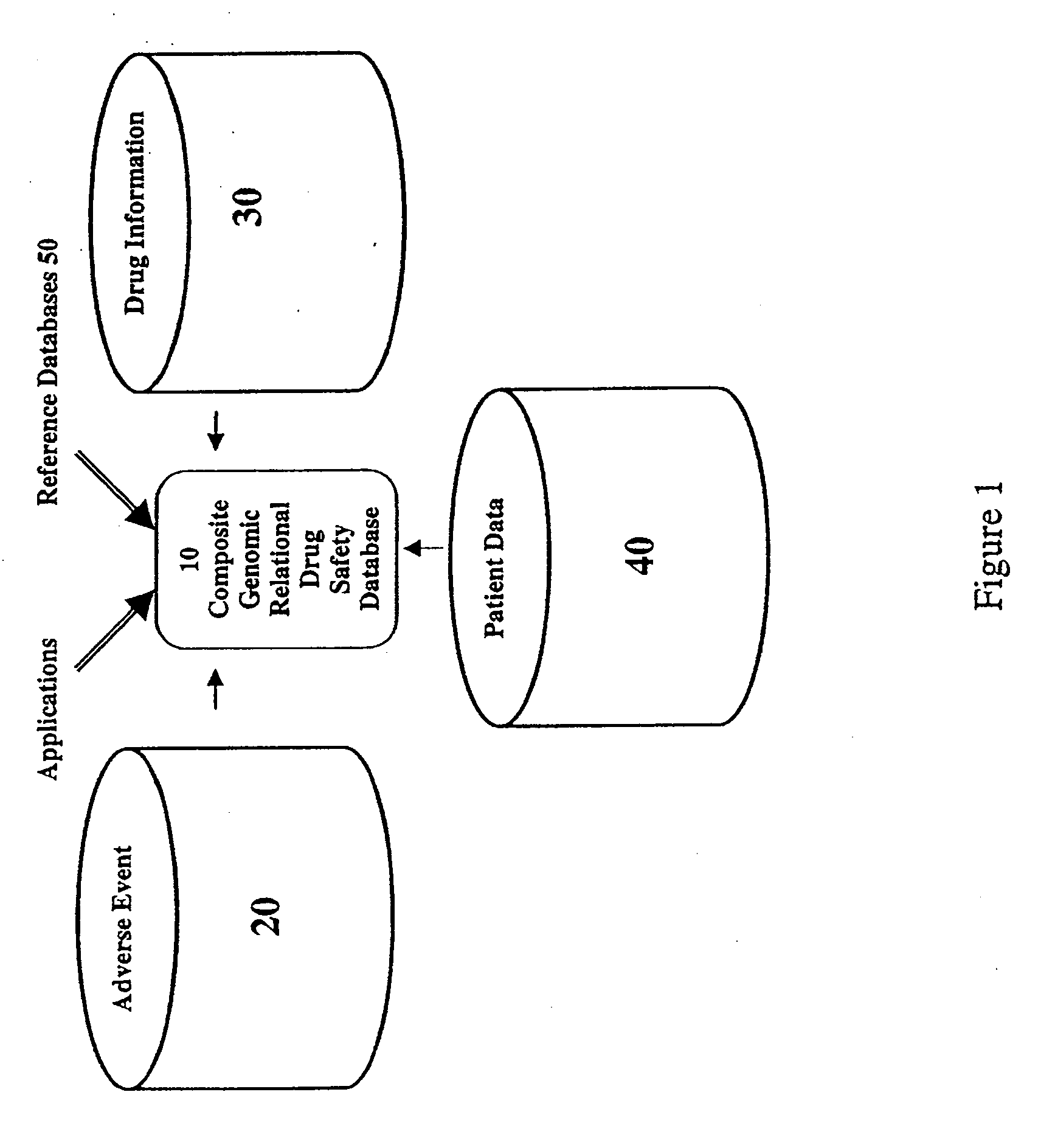
A method for assessing and analyzing one or more drugs, adverse effects and associated risks, and patient characteristics resulting from the use of at least drug of interest is disclosed. The method comprises the steps of selecting one or more cases for analysis, said cases describing the behavior between at least one drug of interest and a patient genotype; profiling statistically derived values from multiple cases related to the safety of the at least one drug, wherein at least one filter is employed for deriving said values; at least one data mining engine; and an output device for displaying the analytic results from the data mining engine. A system for performing the method is likewise disclosed.
Owner:DRUGLOGIC
Two-part capsule to accept pharmaceutical preparations for powder inhalers
InactiveUS20010008637A1Releasing their contentPowder deliveryMedical devicesPowder InhalerWater insoluble
The present invention relates to capsules for holding pharmaceutical preparations for powder inhalers with increased drug safety and capsules for pharmaceutical preparations for powder inhalers with improved adaptation to their use in powder inhalers. The capsules consist of water-insoluble hydrophobic synthetic materials which do not significantly affect the pharmaceutical quality of the contents themselves, but which improve the usability of the filled capsules with regard to their function, their longevity and / or the geographic location of their use, and are advantageous at various stages from manufacture up to utilisation.
Owner:HOCHRAINER DIETER +1
Two-part capsule to accept pharmaceutical preparations for powder inhalers
InactiveUS20040131668A1Releasing their contentPowder deliveryMedical devicesPowder InhalerWater insoluble
The present invention relates to capsules for holding pharmaceutical preparations for powder inhalers with increased drug safety and capsules for pharmaceutical preparations for powder inhalers with improved adaptation to their use in powder inhalers. The capsules consist of water-insoluble hydrophobic synthetic materials which do not significantly affect the pharmaceutical quality of the contents themselves, but which improve the usability of the filled capsules with regard to their function, their longevity and / or the geographic location of their use, and are advantageous at various stages from manufacture up to utilisation.
Owner:BOEHRINGER INGELHEIM PHARM KG
Methods and compositions for improving drug safety
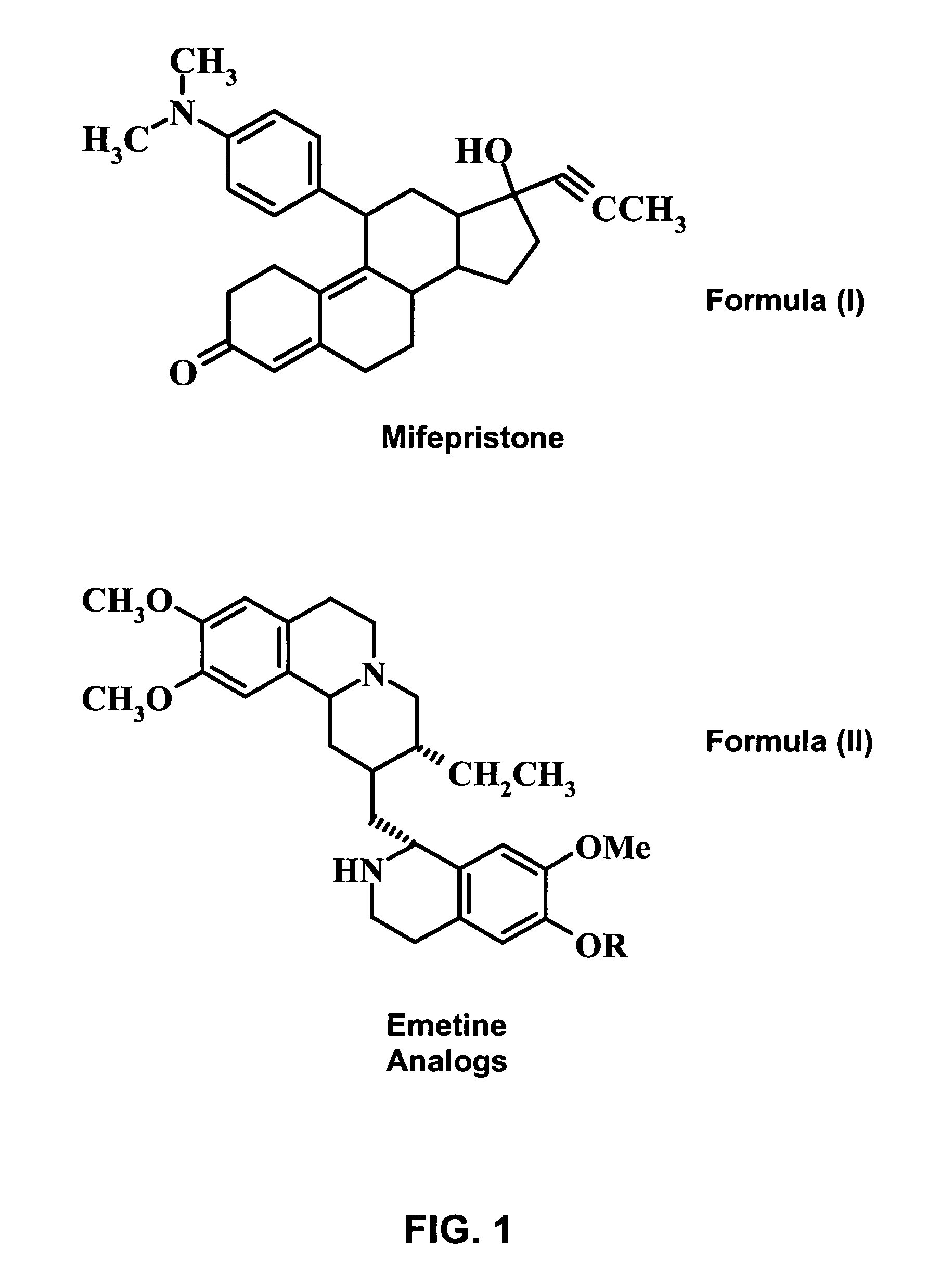
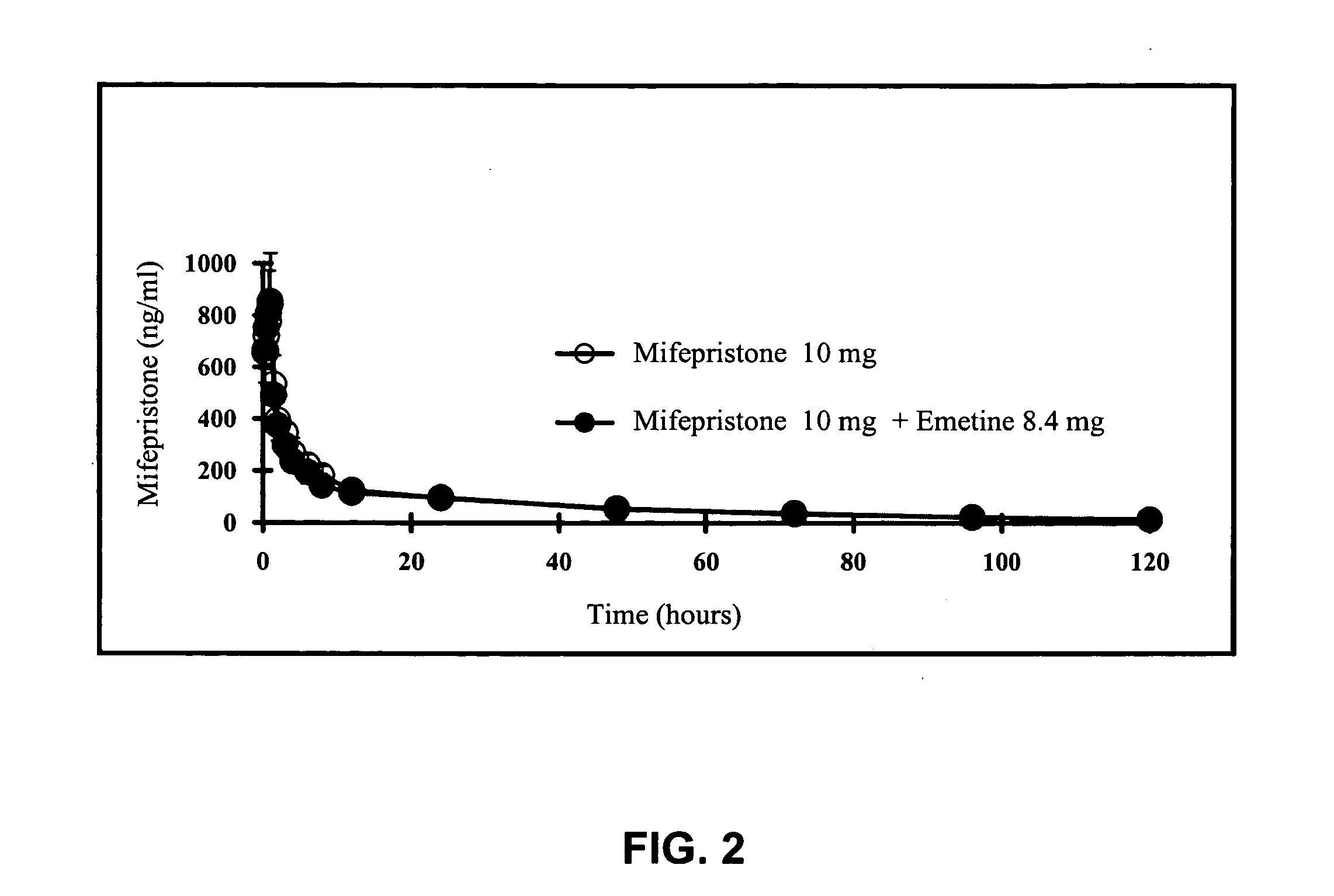
A pharmaceutical composition with improved safety includes a selected amount of a vomit-inducing agent, wherein the selected amount is less than an amount needed to induce vomit in a user; and a therapeutic agent. The therapeutic agent may be selected from a sleeping pill, an anxiolytic, a hypnotic, a contraceptive agent. The therapeutic agent may also be selected from diazepam, flunitrazepam, alprazolam, triazolam, fludiazepam, midazolam, estazolam, zopiclone, and a combination thereof. The vomit-inducing agent may be selected from emetine, cephaeline, and a combination thereof.
Owner:LOTUS PHARMA CO LTD
Detection integrated chip and detection method
ActiveCN102580797AEasy to operateShort detection cycleLaboratory glasswaresMaterial analysisEngineeringReagent
The invention discloses a detection integrated chip and a detection method. The chip is divided into an upper layer, a middle layer and a lower layer, wherein the upper layer of the chip is connected with the middle layer of the chip, the middle layer of the chip is connected with the lower layer of the chip; the upper layer or middle layer of the chip is provided with a group of through holes used for loading samples; the lower layer of the chip is provided with a group of grooves; and each layer of the chip is provided with micro-channels used for connecting the grooves. By adopting the chip, the pretreatment, quantitative delivery and reaction of the sample solution can be automatically completed once and the contents of the components of the solution can be detected, thus the detection of the multi-index single-reagent method can be satisfied and the chip can be used in the detection of the multi-index two-reagent method and the multi-reagent method. The chip disclosed by the invention can be used in the analysis and detection fields such as biological analysis, medical detection, environmental pollutant detection, and food and drug safety.
Owner:TIANJIN MNCHIP TECH CO LTD +1
Intelligent-algorithm-based drug safety confidence index system and construction method
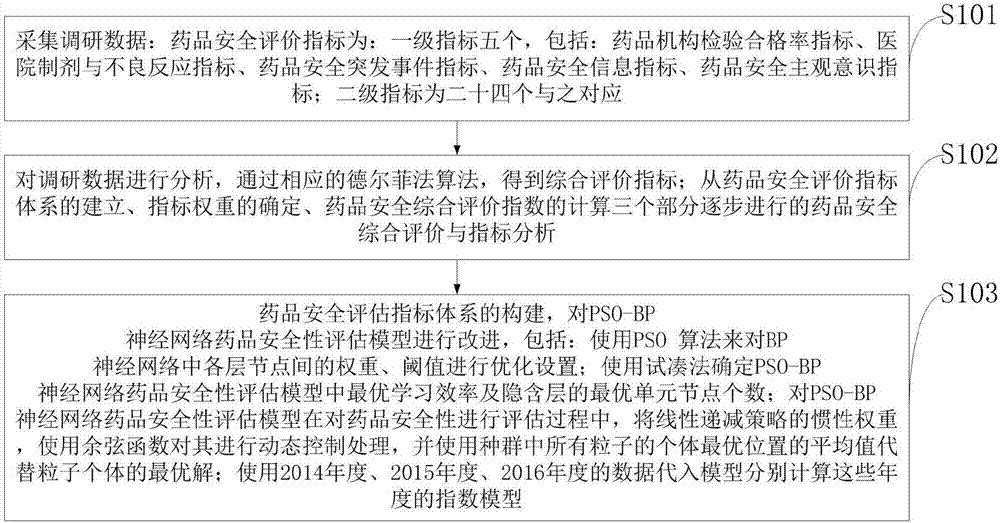
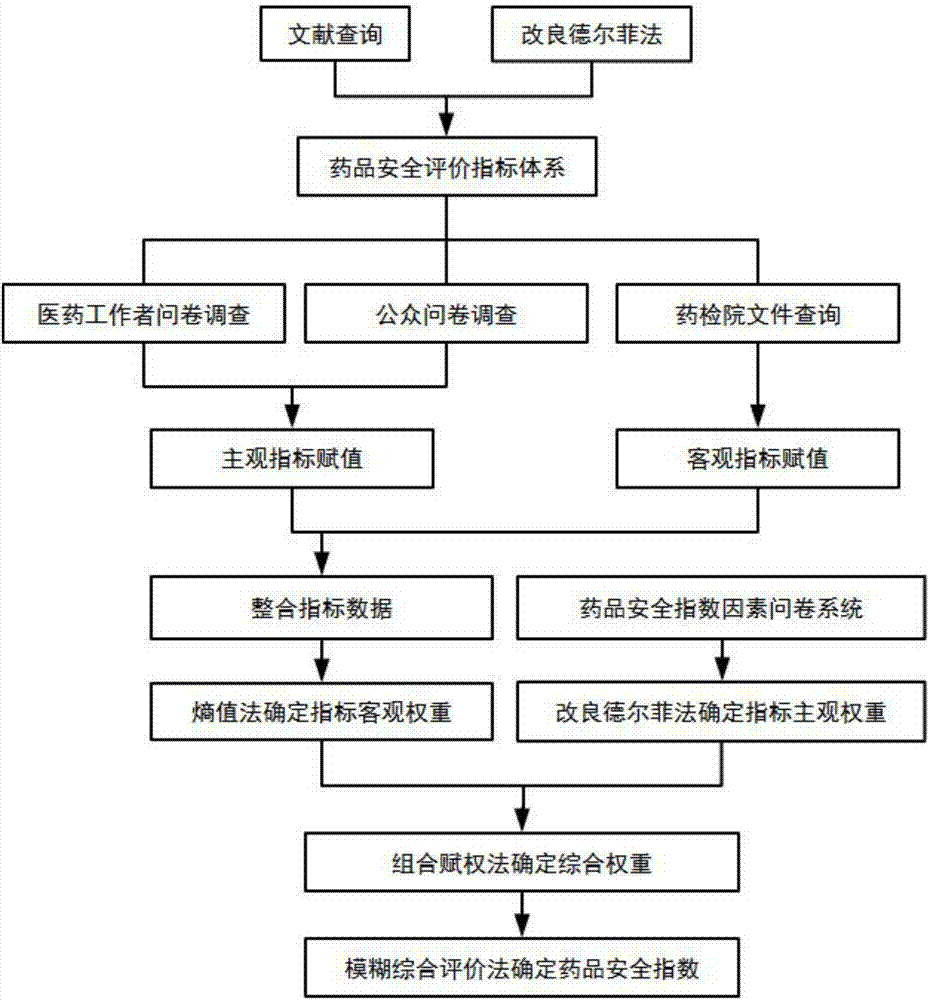
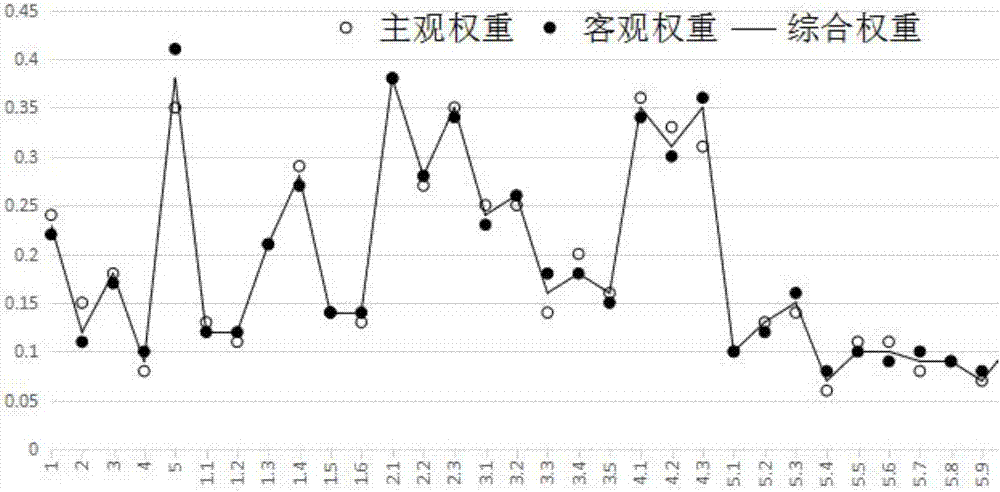
The invention belonging to the technical field of medical data processing discloses an intelligent-algorithm-based drug safety confidence index system and a construction method. The construction method comprises: research data are collected; the research data are analyzed and a comprehensive evaluation index is obtained by a corresponding delphi method; and a drug safety evaluation index system is constructed and a PSO-BP-neural-network drug safety evaluation model is improved. The system is composed of a target layer, a project layer, a factor layer, and an index layer. According to the invention, a corresponding evaluation mode needs to be constructed in a future drug safety index analysis and the algorithm, data, and various factor weight ratios of drug safety evaluation index are analyzed in the evaluation result, so that the selected data source becomes accurate, the used algorithm meets the practical situation, and the constructed drug safety evaluation index is based on scientific, people-oriented, truth-seeking, rigorous, innovative principles.
Owner:湖北省食品药品监督检验研究院
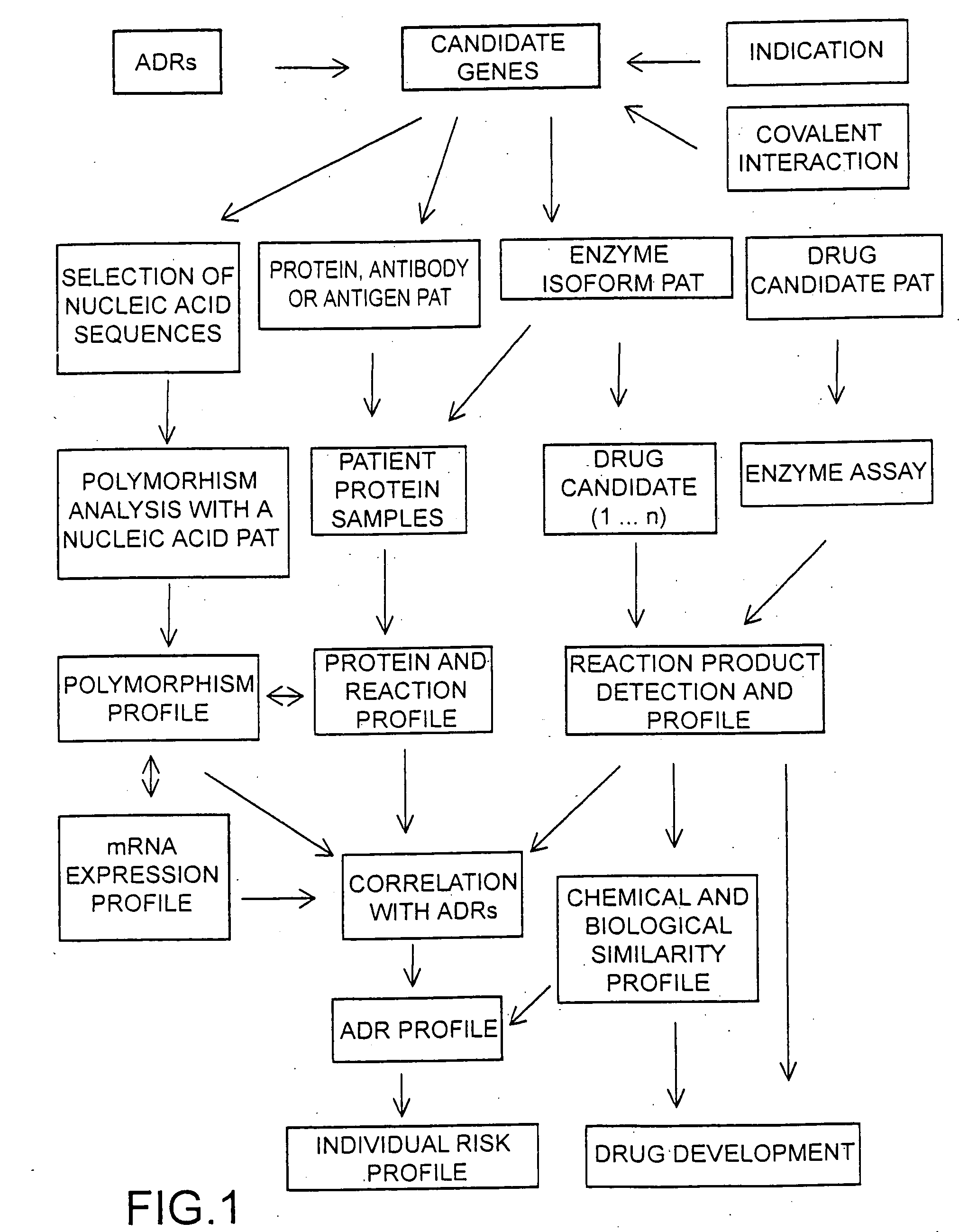
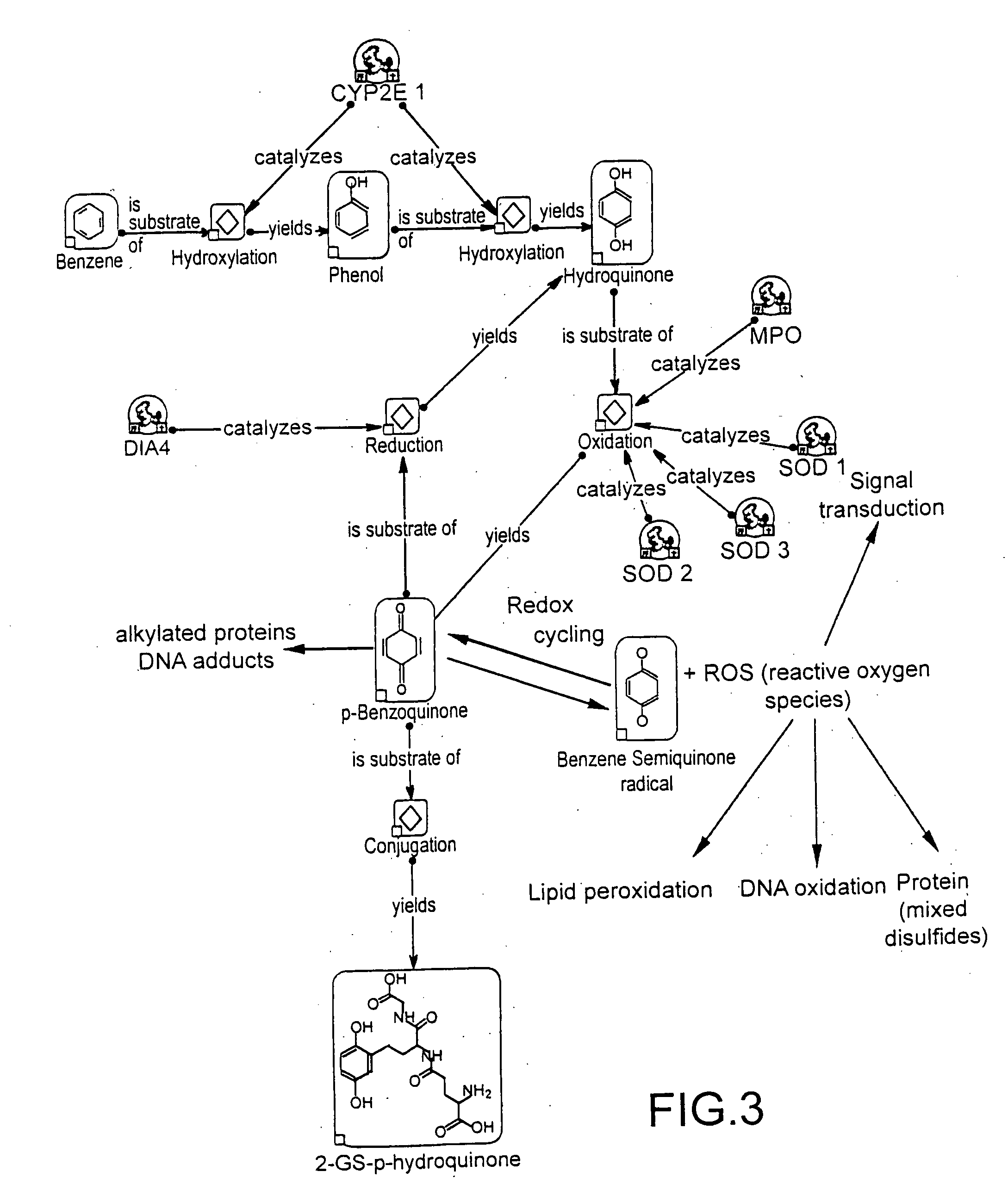
Individual drug safety
InactiveUS20050037366A1Microbiological testing/measurementBiostatisticsProtein profilingActivity profiling
The invention provides means to determine the predisposition of individuals to adverse drug reactions (ADRs). The methods are based on genotyping or parallelized enzyme and protein profiling or both. Parallelized enzyme activity profiling can be used for drug screening and development. As examples of the invention we show the prediction of adverse drug reactions of pulmonary hypertension patients by identifying genes and alleles linked to known ADRs and liver enzyme reaction profiling with ADR correlation.
Owner:THERASTRAT
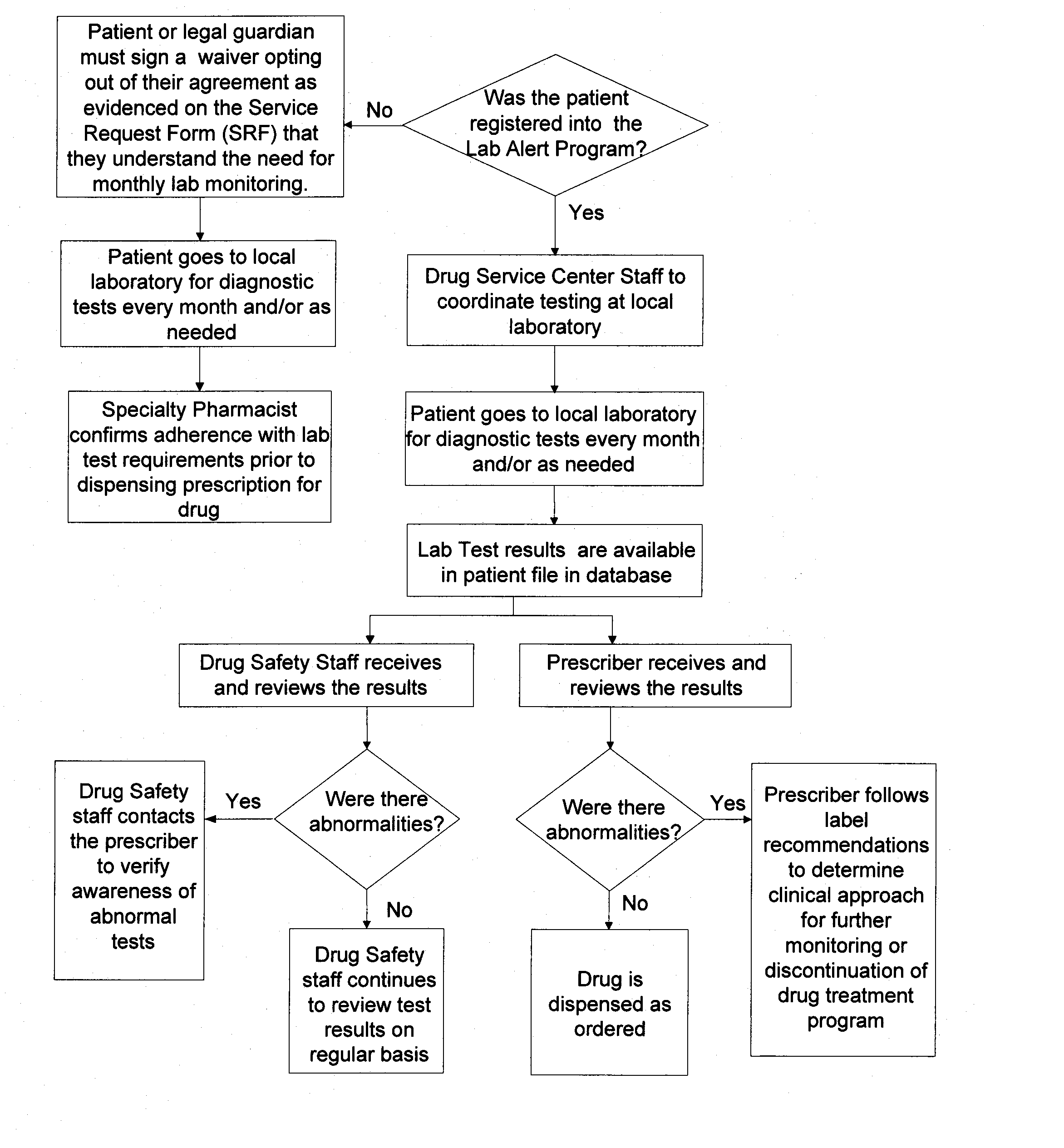
Method of managing and reducing side effects associated with exposure to a drug
InactiveUS20070219825A1Minimize occurrenceData processing applicationsHealth-index calculationHealth related informationPharmacy
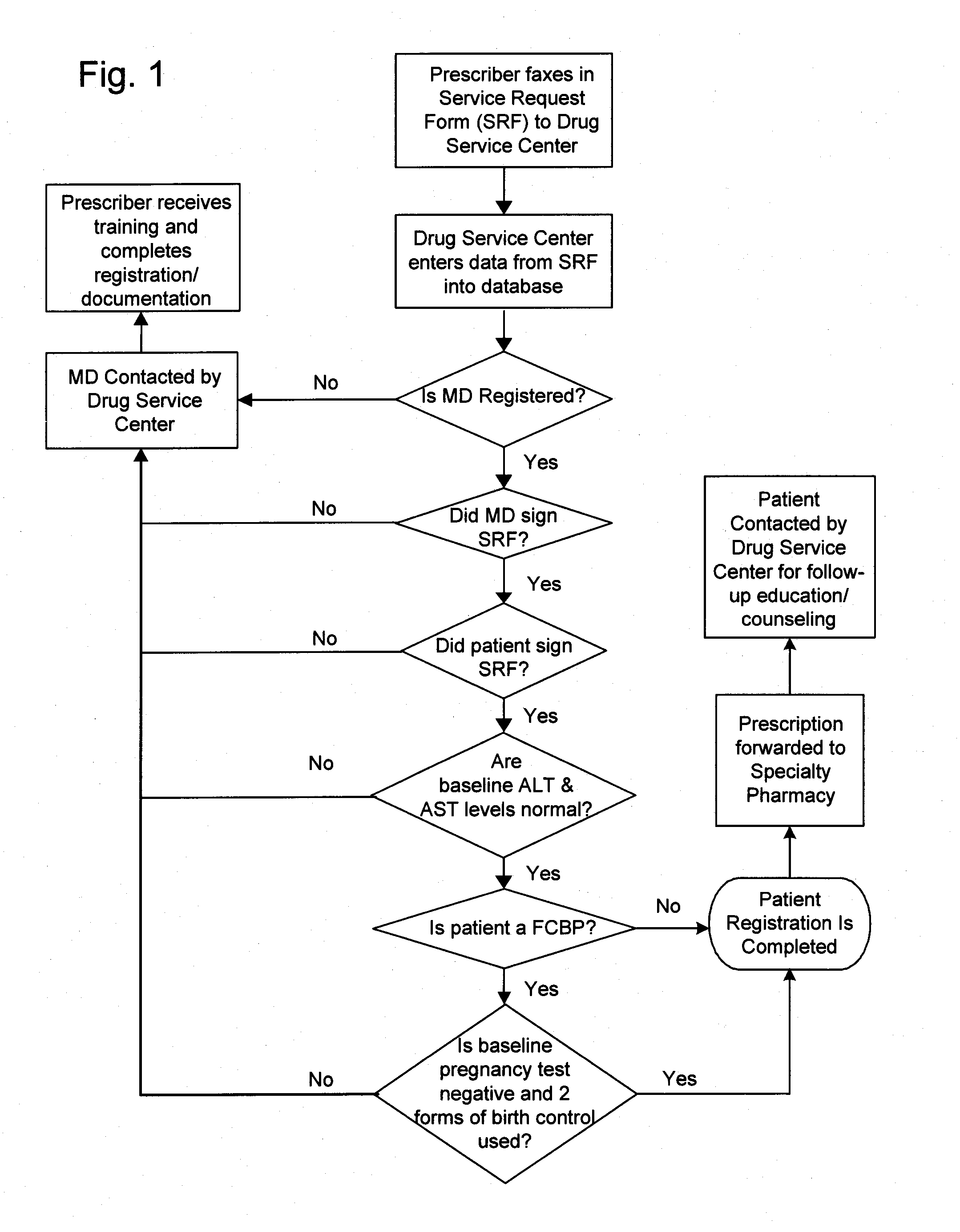
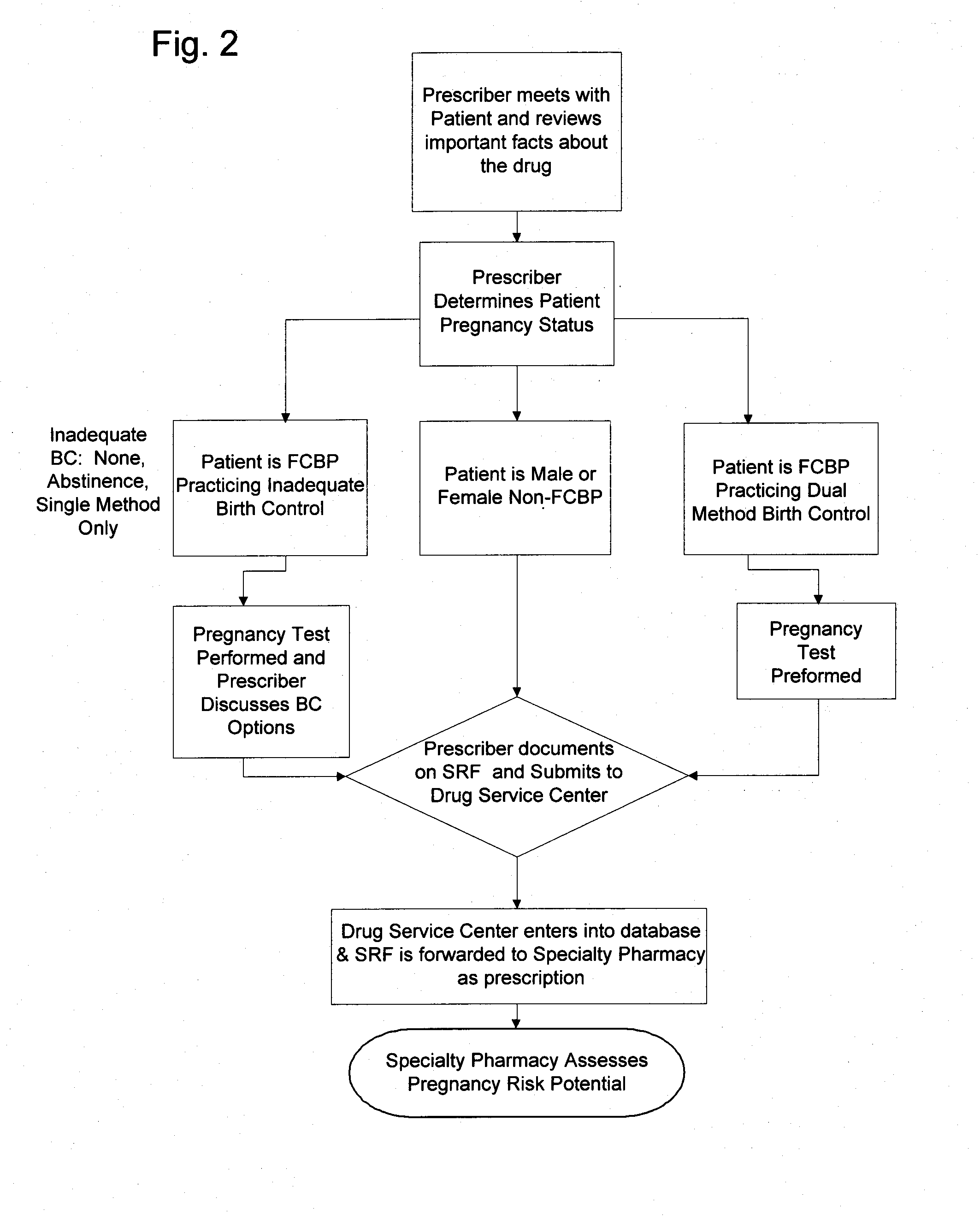
A method for restricted distribution of a drug to a patient while minimizing the occurrence of adverse side effects. The method restricts access to the drug by preventing distribution to certain patients for whom the drug may be contraindicated. The method permits a prescription to be filled by a pharmacy only after the pharmacy has received an approval for release of the prescription. Generation of the prescription approval depends on registration of a prescriber, pharmacy and patient in a central database, and the determination that the risk is acceptable that the patient will experience an adverse side effect. The database contains a patient profile that may include additional patient-specific health-related information that is probative of the risk that an adverse side effect is likely to occur if the patient is exposed to the drug. Based in part on the information in the patient profile, the registered prescriber makes a determination as to the patient's risk of adverse side effects. If the registered prescriber determines that the risk is acceptable, a prescription request is generated. At the time that the prescription is to be dispensed to the patient, the pharmacy also makes a determination as to the patient's risk of adverse side effects. If the pharmacy determines that the risk is acceptable, the drug is dispensed to the patient. Additional steps may be taken to ensure compliance with the drug delivery method, such as education, oversight by a drug safety analyst, and / or regular contact with a representative.
Owner:ENCYSIVE PHARMA INC
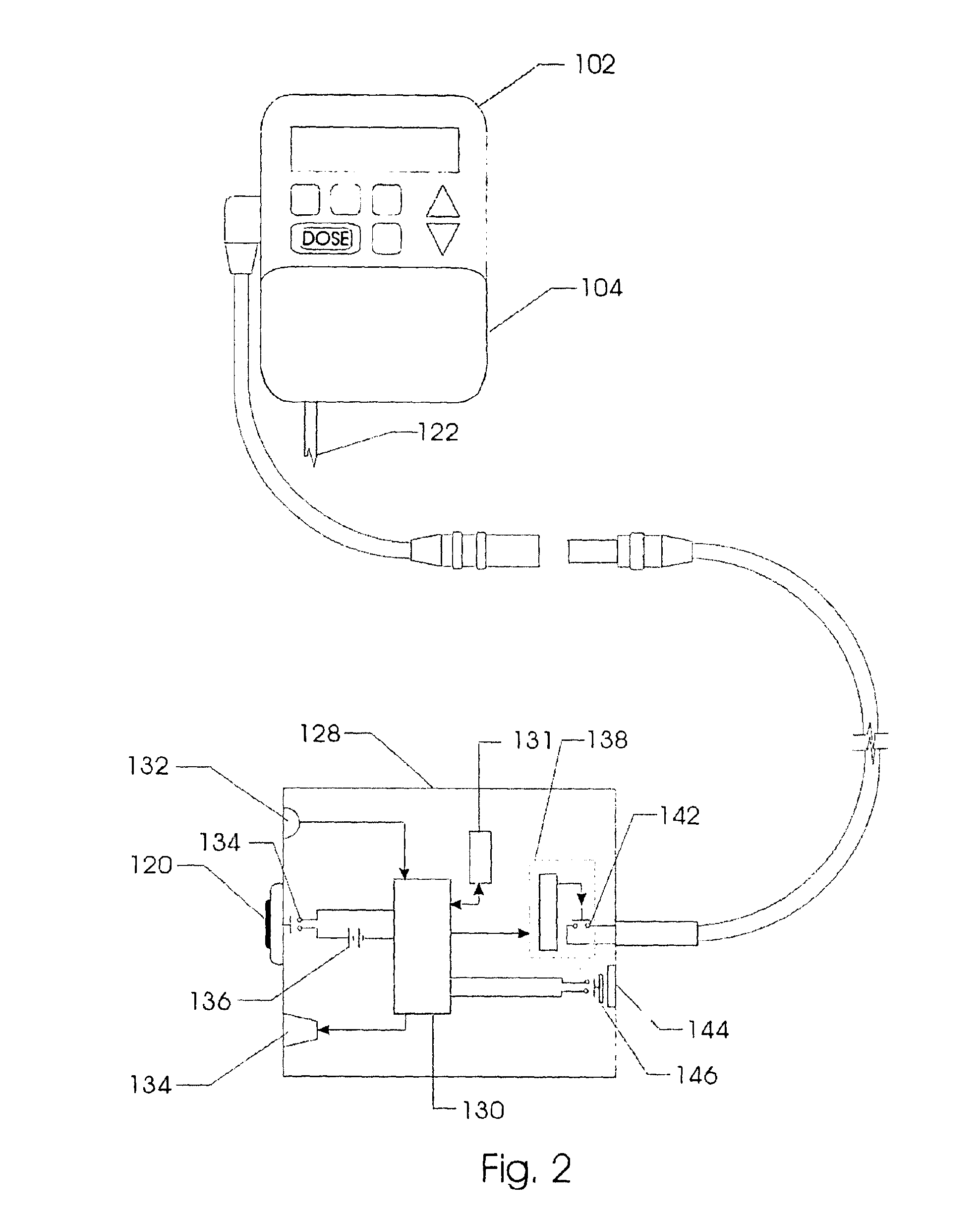
Method and system for the analysis and association of patient-specific and population-based genomic data with drug safety adverse event data
InactiveUS20090076847A1Increased and decreased chanceMedical data miningBiostatisticsPatient characteristicsGenomic DNA
A method for assessing and analyzing one or more drugs, adverse effects and associated risks, and patient characteristics resulting from the use of at least drug of interest is disclosed. The method comprises the steps of selecting one or more cases for analysis, said cases describing the behavior between at least one drug of interest and a patient genotype; profiling statistically derived values from multiple cases related to the safety of the at least one drug, wherein at least one filter is employed for deriving said values; at least one data mining engine; and an output device for displaying the analytic results from the data mining engine. A system for performing the method is likewise disclosed.
Owner:DRUGLOGIC
Tacrolimus sustained-release preparation and preparation method thereof
InactiveCN101664394AOvercome the problems of poor water solubility and narrow therapeutic windowImprove securityOrganic active ingredientsGranular deliverySolubilityCyclodextrin
The invention provides a tacrolimus sustained-release preparation and a preparation method thereof. The invention adopts a solid dispersion technology, or cyclodextrin inclusion technology or a solubilizing method for micronizing drugs and then adding one or more types of surfactants and the like, and obviously improves solubility thereof so as to improve the bioavailability thereof, then adds oneor more types of skeleton materials and other auxiliary materials so as to prepare a skeleton type sustained-release preparation, or adopts a sustained-release material to carry out coating so as toprepare a diaphragm-controlled type or osmotic pump type sustained-release preparation. The tacrolimus sustained-release preparation has better solubility and dissolution rate, high bioavailability and sustained-controlled-release effect, thus maintaining stable blood and drug concentration, reducing the occurrence rate of adverse reaction, and improving clinical drug safety; in addition, the invention has easy obtaining for materials, simple and feasible preparation technique, high yield and low cost, can realize large-scale industrialized production and has obviously economical benefit.
Owner:宋洪涛
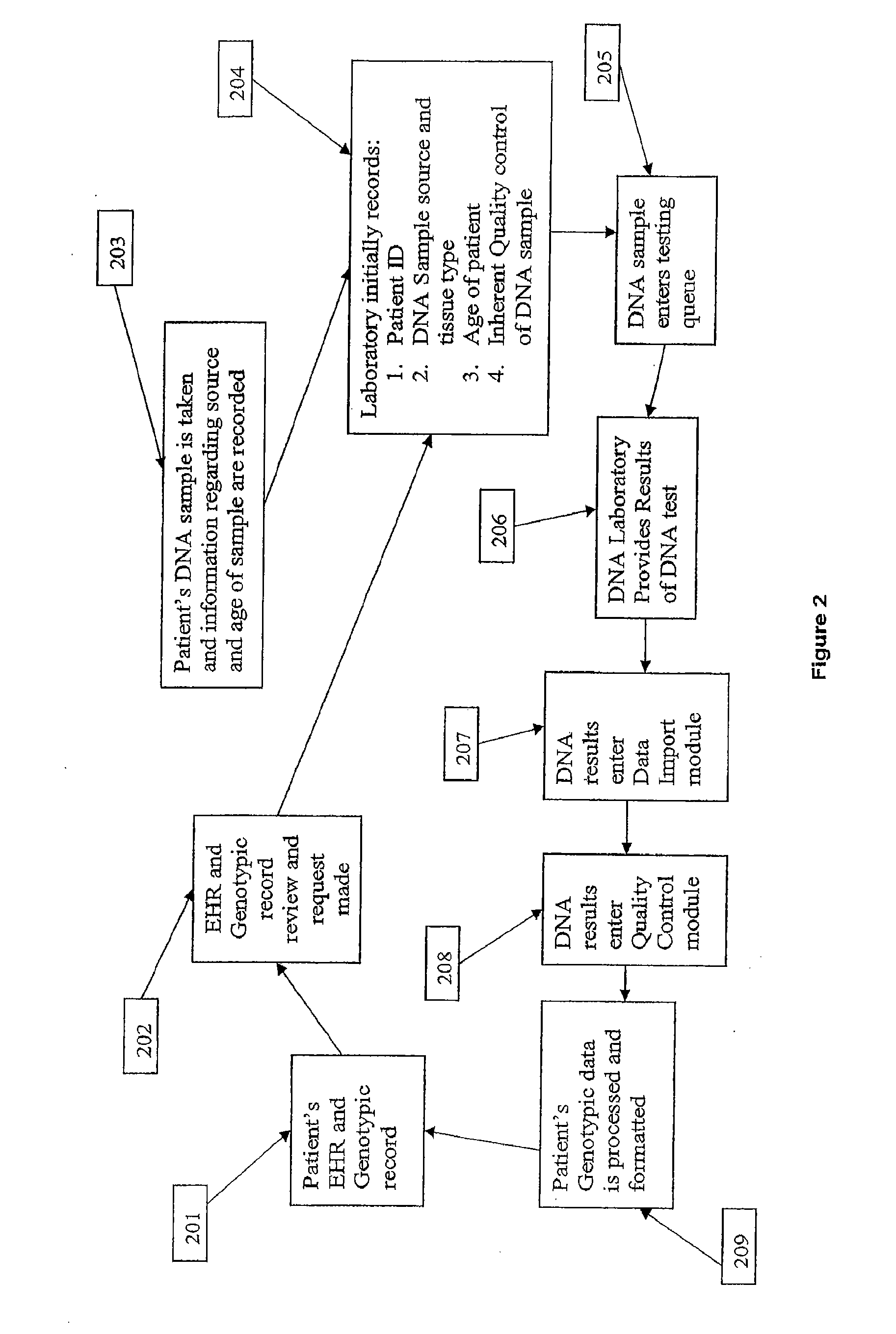
Method for patient genotyping
The present invention is a system and method for utilizing human genetic and genomic information to guide prescription dispensing and improved drug safety in a pharmacy setting. The system and method of the present invention utilizes a dedicated information management system and software to utilize patient-specific genetic information to screen for increased risk of adverse drug reactions and therapeutic responses at the time of drug dispensing.
Owner:KANE MICHAEL D +3
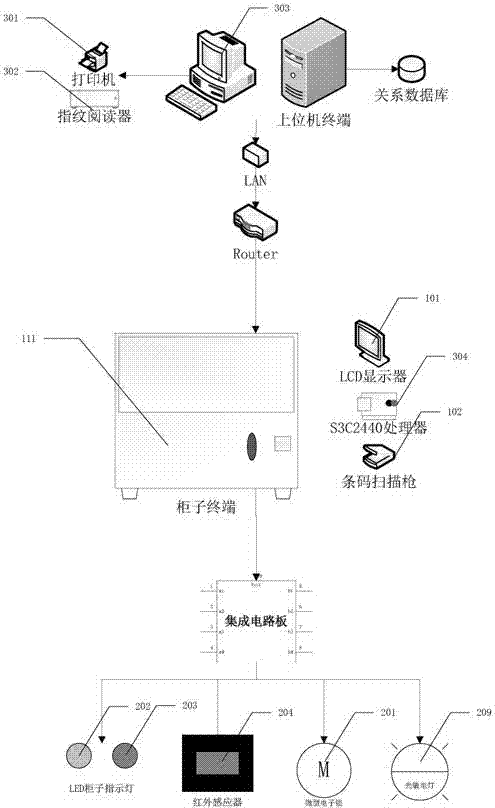
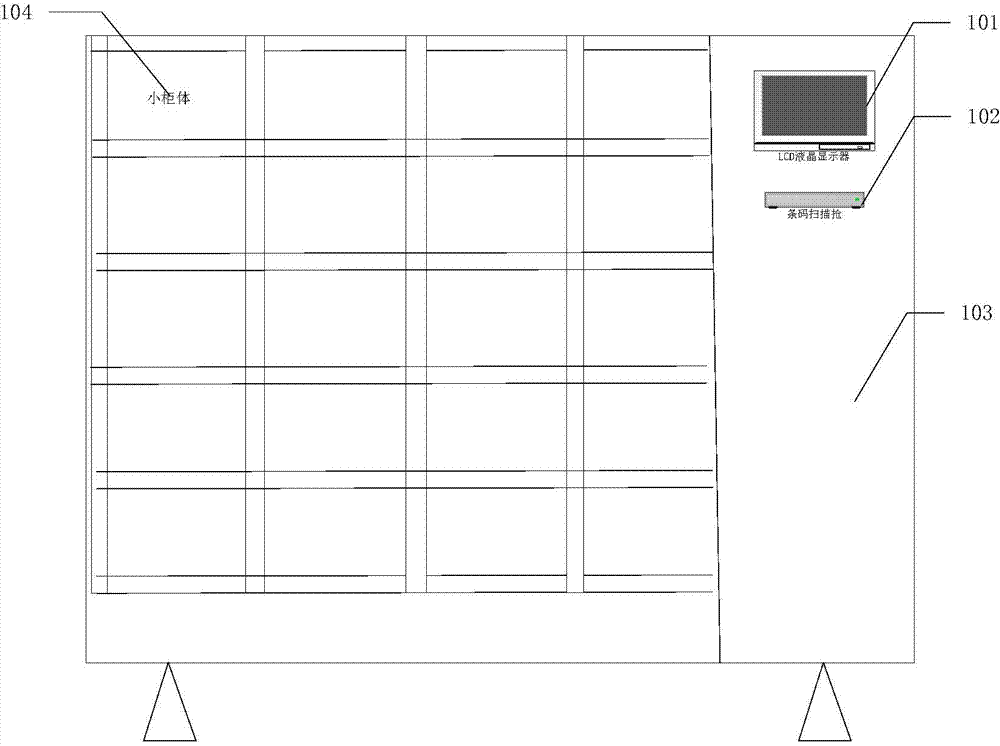
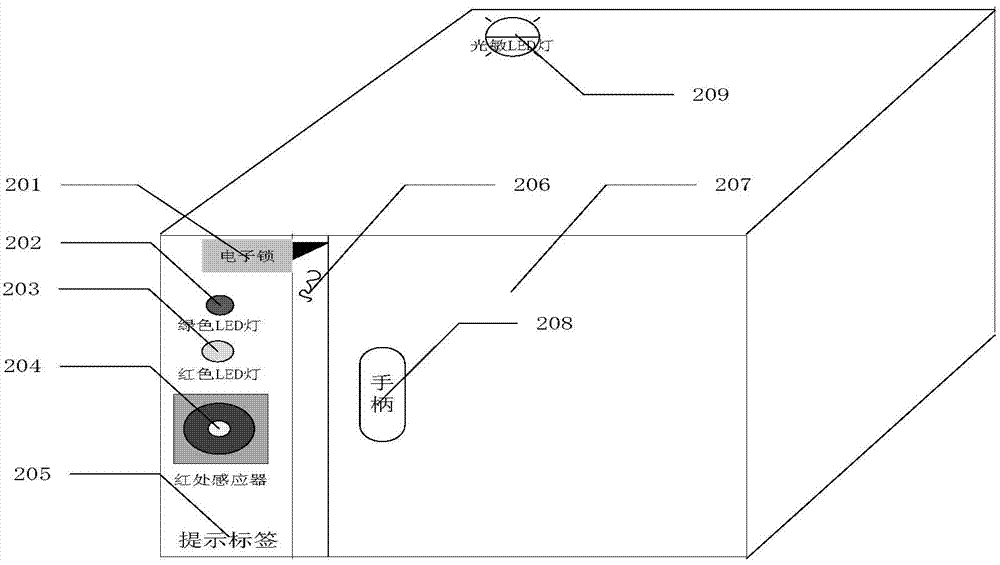
Distributed intelligent drug safety management system and using method
ActiveCN103886414AImprove securityImprove convenienceResourcesTransmissionInformatizationDistributed intelligence
The invention discloses a distributed intelligent drug safety management system. The distributed intelligent drug safety management system comprises an upper computer terminal and an intelligent cabinet terminal. A laboratory information management system is installed in the upper computer terminal, and the upper computer terminal is connected with and has communication with a fingerprint reader and a POS miniature printer. The intelligent cabinet terminal comprises a cabinet and an intelligent control terminal, and the intelligent control terminal is connected with a liquid crystal display and used for displaying drug list information, drug using information and cabinet using conditions. A plurality of small cabinet bodies are installed on the cabinet and distributed in an array mode. The upper computer terminal is connected with the intelligent control terminal through the TCP / IP, after mutual authentication succeeds, communication is achieved, and the intelligent control terminal is used for controlling the cabinet to be opened and closed. The system has the advantages of being safe in management, intelligent in control, user-friendly in operation, easy to extend and maintain and capable of effectively achieving safe and effective storage and informatization management of agents and drugs in the fields of materials, chemistry and medicines.
Owner:HUAZHONG UNIV OF SCI & TECH
Aptamer electrode for detecting terramycin, and manufacturing method thereof
InactiveCN104777206AConvenient amountIncrease concentrationMaterial analysis by electric/magnetic meansAptamerEngineering
The invention provides an aptamer electrode for detecting terramycin. The electrode is characterized in that a glassy carbon electrode is sequentially modified with a graphene-gold nanometer compound and a BSA-OTC compound from bottom to top. A signal layer is methylene blue modified aptamer having a G tetrad structure and folded under an RCA chain amplification reaction, and the content of a target product is reflected through the amount of methylene blue. The invention also provides a manufacturing method of the aptamer electrode. The aptamer electrode has the advantages of simple manufacturing method, stable performances, and good repeatability, and is suitable for terramycin detection in the medicine safety and the industrialized practical application of biosensors.
Owner:UNIV OF JINAN
Method of detecting mononucleotide pleomorphism of CYP2D6 gene ninth exon
InactiveCN101109018ASignificant practical valueSugar derivativesMicrobiological testing/measurementNucleotideExon
The invention relates to a testing method of CYP2D6 gene exon 9's single nucleotide polymorphism, and meanwhile relates to a separation nucleic acid and an allel-specific nucleic acid primer. The method comprises steps as described below: firstly the confirmation of the 1332 nucleic acid showed in the SEQ ID No: 1 in the human CYP2D6 gene exon 9, then test of the existence of the single nucleotide polymorphism, specifically a separation nucleic acid with the SEQ ID NO: 1 and the 1322 position is A, an allel-specific nucleic acid primer with the length of 15 to 50bp and specifically hybridizes and amplifies the amplified products of the 1322 nucleic acid polymorphism showed in the SEQ ID No: 1 in the human CYP2D6 gene exon 9. The invention can be used to research the relation between CYP2D6 gene polymorphism in Chinese people and the clinical drug safety, and provide guidance to the development of new drugs.
Owner:SHANGHAI JIAO TONG UNIV
Method for detecting CYP2C9 gene exon 9 mononucleotide polymorphism
The invention discloses a detection method for the CYP2C9 gene ninth exon single nucleotide polymorphism, belonging to the technical field of the genetic engineering. The method comprises the following steps: step one, by taking the CYP2C9 gene ninth exon in a GenBank database and the exon and intron joint part sequence as a template, a pair of allele-specific nucleic acid primers are designed; the primer pair is used for amplifying the DNA sequence of the CYP2C9 gene ninth exon; and then corresponding separated nucleic acids are obtained by conducting separation and purification on the amplified product; and step two, detection is carried out on the 1739-1740 nucleotides of the CYP2C9 gene ninth exon of the separated nucleic acids so as to fix whether single nucleotide polymorphism exists or not. The invention can be used for studying the relationship between the CYP2C9 gene polymorphism in the population of China and the clinical drug safety, and provides guiding basis for the research and development of new drugs.
Owner:SHANGHAI JIAO TONG UNIV
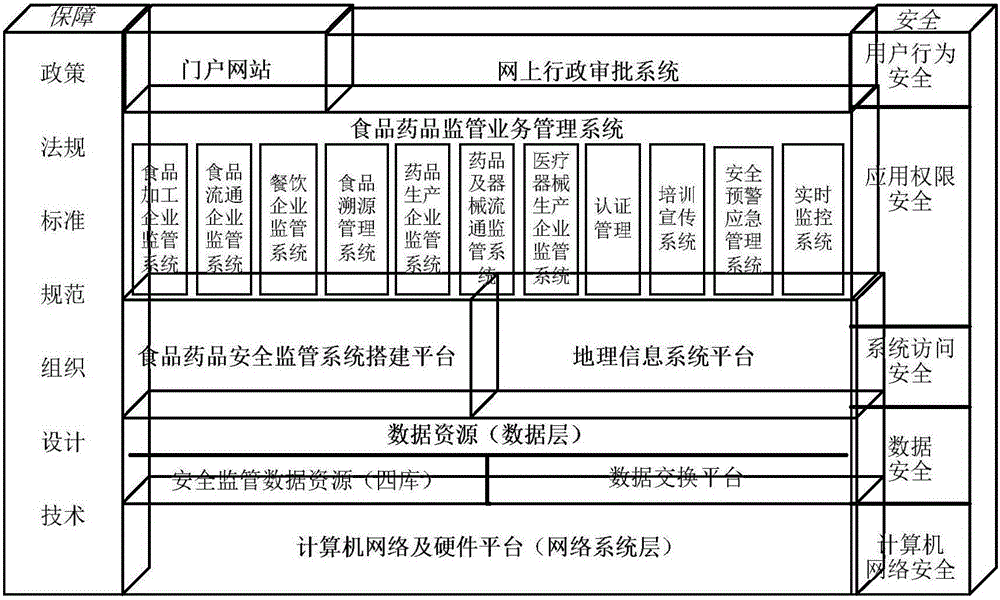
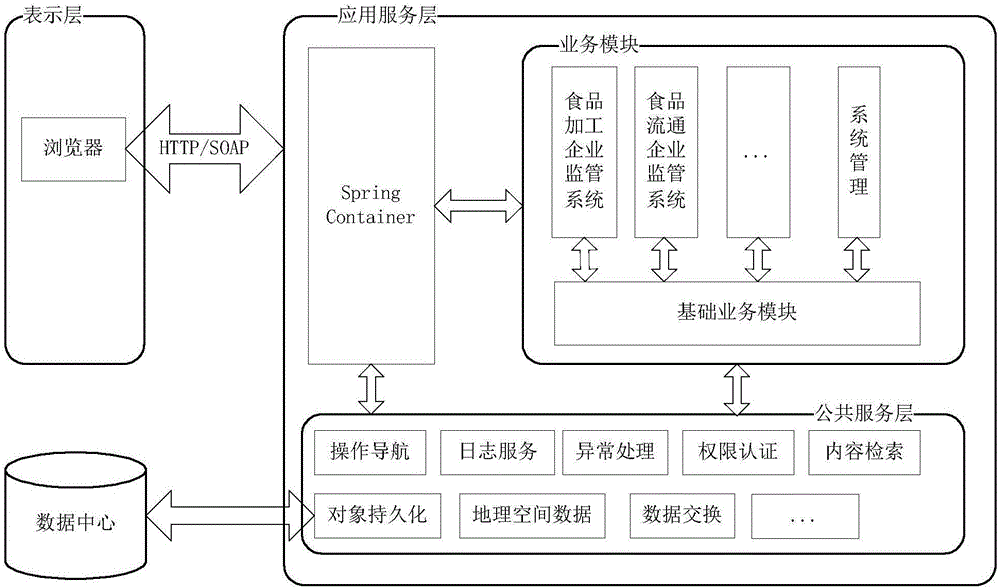
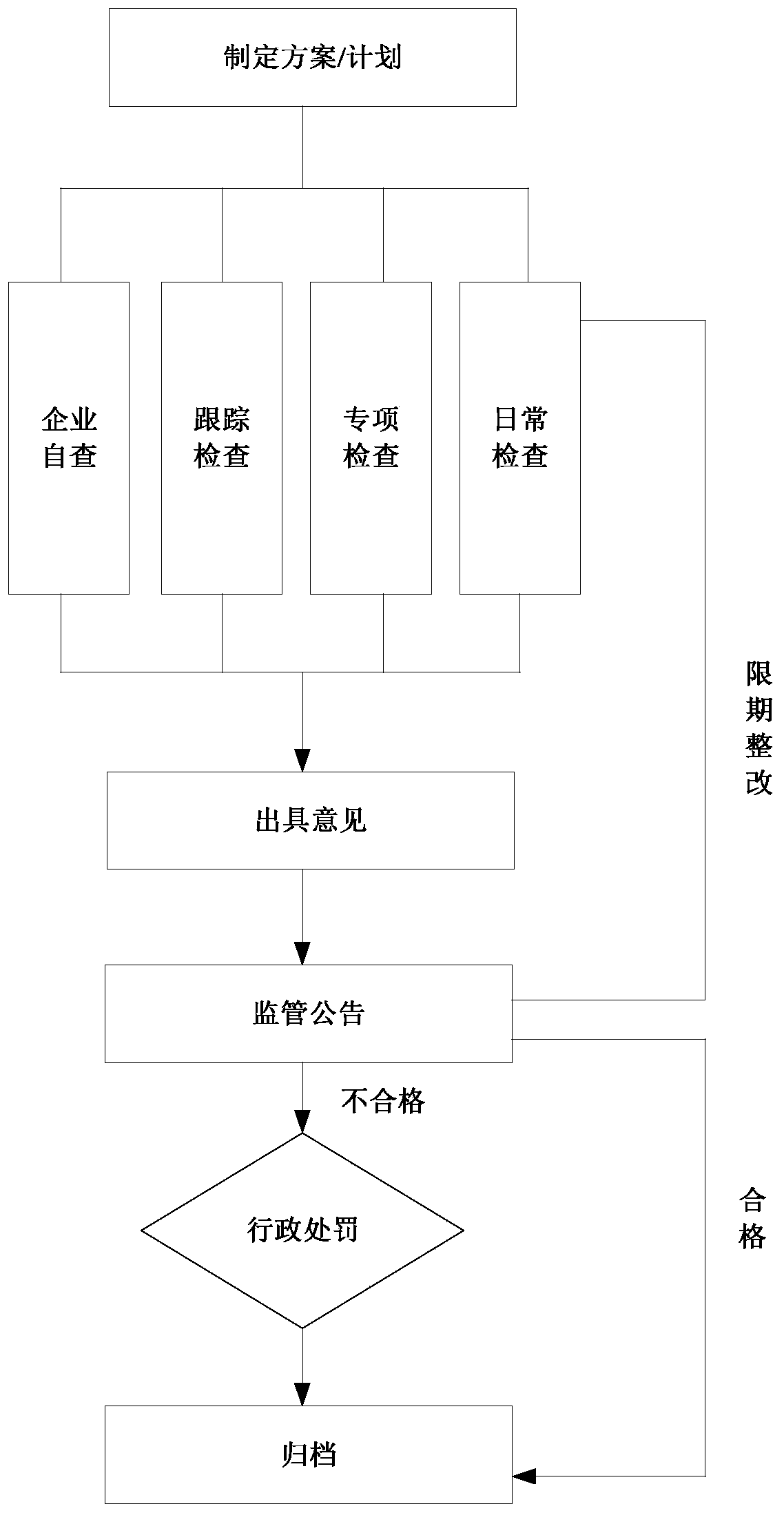
Food and drug safety supervisory system
InactiveCN106096973AGuarantee food safetyRealize chain managementCommerceInformatizationBusiness management
The invention discloses a food and drug safety supervisory system, which comprises a computer network and hardware platform, a data layer, a system building platform, a business management system and a web portal, wherein the data layer is used for establishing an organization, management, maintenance and update system of a business database according to a database management technology; the system building platform is the supporting environment of an information system and comprises a system permission management and message platform mechanism and a log management, unified certification and Gis building platform; the business management system is used for realizing the informatization of food and drug safety supervisory business under a network environment; and the web portal is used for finishing the communication and the management of information associated with the public through the web portal under the support of each business system. The invention provides the food safety supervisory system which is suitable for the situation of China and emphasizes the prevention, the whole process supervision of safe production and circulation of key food and drugs is realized, a food and drug quality safety situation is guaranteed to be constantly stable and good, a food and drug safety level is obviously improved, and the diet safety of urban and rural residents is exactly guaranteed.
Owner:库尔勒市食品药品监督管理局
Drug identification system based on deep learning and identification method thereof
InactiveCN107545150AAvoid instabilityCharacter and pattern recognitionSpecial data processing applicationsTest sampleDrug identification
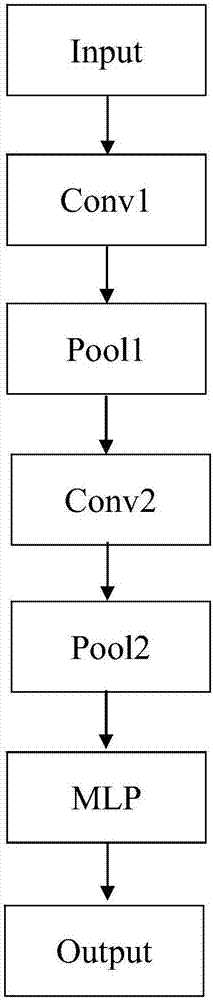
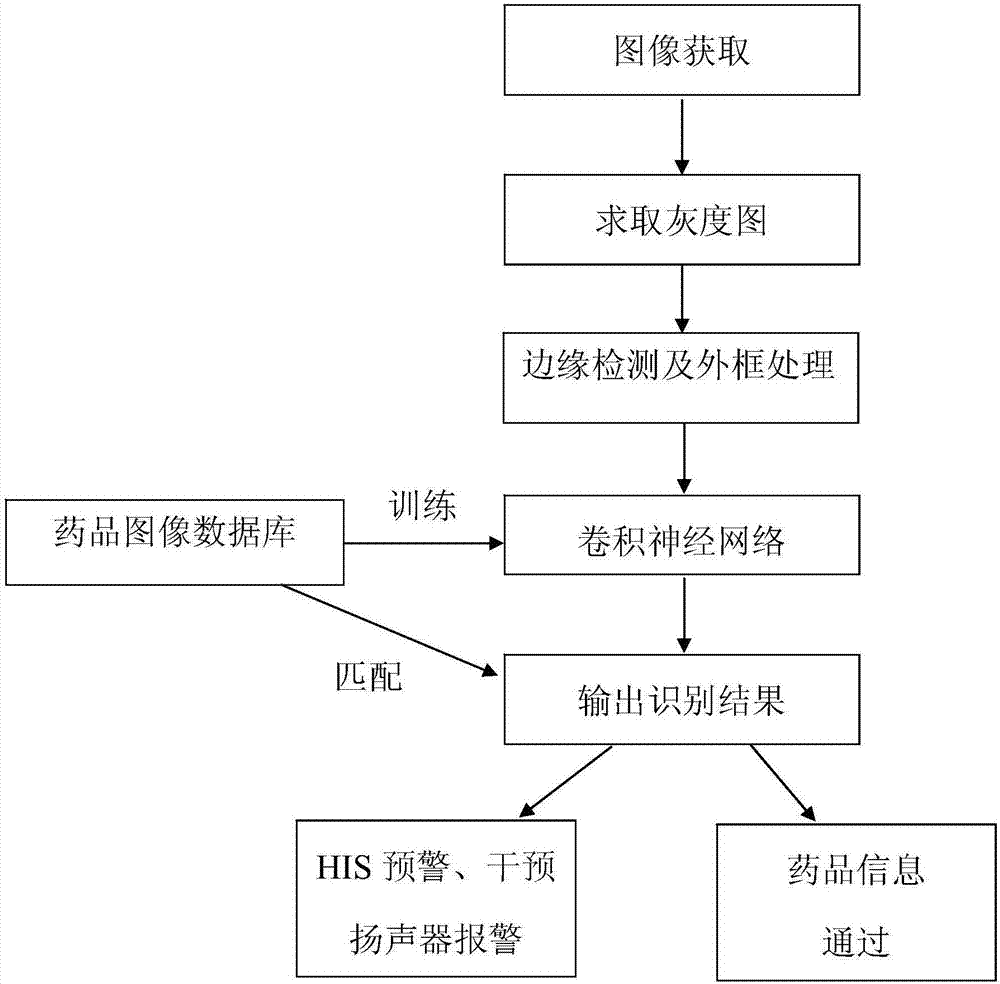
The present invention is a drug recognition system and its recognition method based on deep learning, including: 1. Preprocessing of drug image training samples, test samples, and target images. Including obtaining grayscale image, edge detection and frame processing. 2. Construct a model unit for establishing a drug recognition model based on deep learning. Including setting the initial parameters of each network layer and classifier of CNN, and inputting the above preprocessed image into CNN to obtain the features we need. 3. The identification unit is used to input the drug with identification, and then the above-mentioned established drug identification model based on deep learning obtains the convolution features of the image in the deep learning model, and cascades the obtained convolution features into the input The classifier finally outputs the recognition result. 4. Identify the hardware part of the system. It has a more accurate recognition rate and speed in various complex backgrounds and drug information, which can better reduce the error rate in drug application and the subsequent individual drug safety application platform.
Owner:张晨
Ticagrelor pharmaceutical composition and preparing method thereof
ActiveCN105998026AImprove complianceQuick effectOrganic active ingredientsInorganic non-active ingredientsPatient complianceTicagrelor
The invention relates to a sustained-release preparation composition of an anticoagulant drug ticagrelor (also called brilinta) and a preparing method thereof. The composition comprises ticagrelor and other pharmaceutical accessories. The composition is characterized in that the composition can be quick in acting, also can ensure persistent effectivity within 24 hours, and further can reduce Cmax based on guarantee of the effective plasma concentration, thereby improving patient compliance and reducing drug safety problem in the premise without reduction of curative effect.
Owner:SICHUAN HAISCO PHARMA CO LTD
Pharmaceutical composition containing mosapride citrate
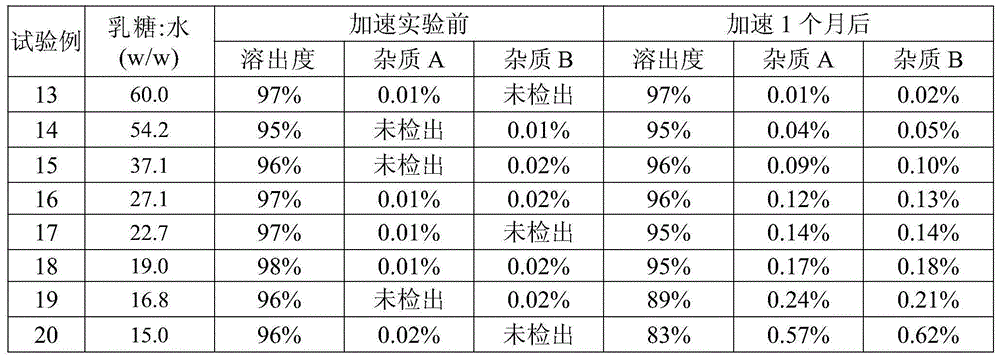
The invention relates to a pharmaceutical composition containing mosapride citrate. The pharmaceutical composition contains lactose and other pharmaceutically acceptable accessory materials. Through strict control of a mass ratio of lactose to water in the pharmaceutical composition, mosapride citrate long-term storage stability is greatly improved and related substance generation and increasing risk is reduced. The pharmaceutical composition provides guarantee for clinical drug safety and effectiveness.
Owner:CHENGDU KANGHONG PHARMA GRP
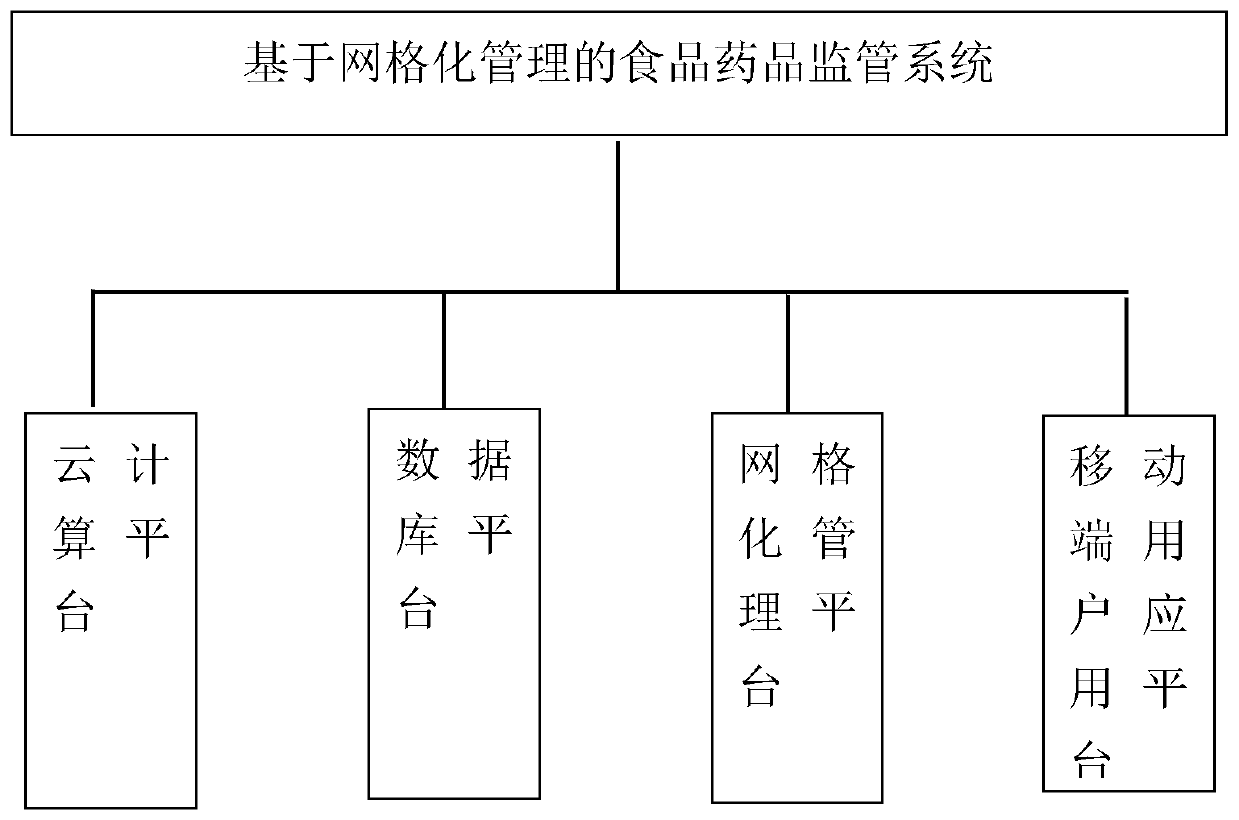
Food and drug supervision system and method based on gridding management
InactiveCN109801004ARealize intelligent serviceRealize refined managementResourcesFood safetyDrug product
The invention discloses a food and drug supervision system and method based on gridding management. The supervision system comprises a cloud computing platform, a database platform, a gridding management platform and a mobile terminal user application platform, the gridding management platform is used for dividing the supervision range into grids, and food and drug production and circulation information of all supervision enterprises in the grids is marked on a geographic map information module (GIS); The supervision method comprises the steps of administrative supervision, technical supervision and inspection case handling, and inspection case handling comprises the steps of grid division, information collection, task providing, task dispatching, task processing, processing feedback, casechecking and checking and comprehensive evaluation. According to the invention, a food safety supervision system based on a GIS professional map for prevention is established, food and drug safety production and circulation process supervision are realized, and food and drug quality safety is guaranteed.
Owner:郭承湘 +2
Protein-protein interactions for pharmacological profiling
InactiveUS20050221280A1Enable optimizationMicrobiological testing/measurementLibrary screeningToxicantEfficacy
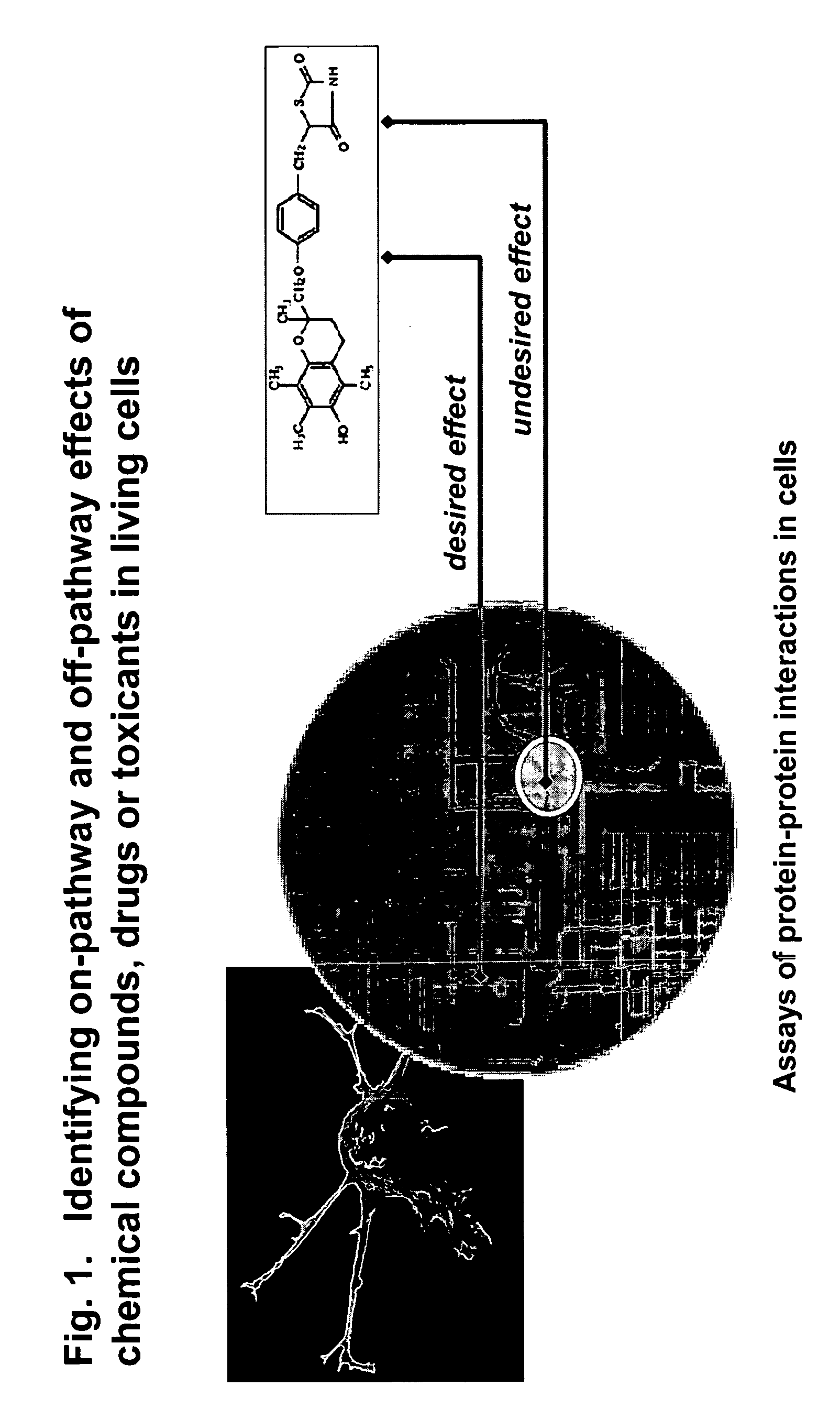
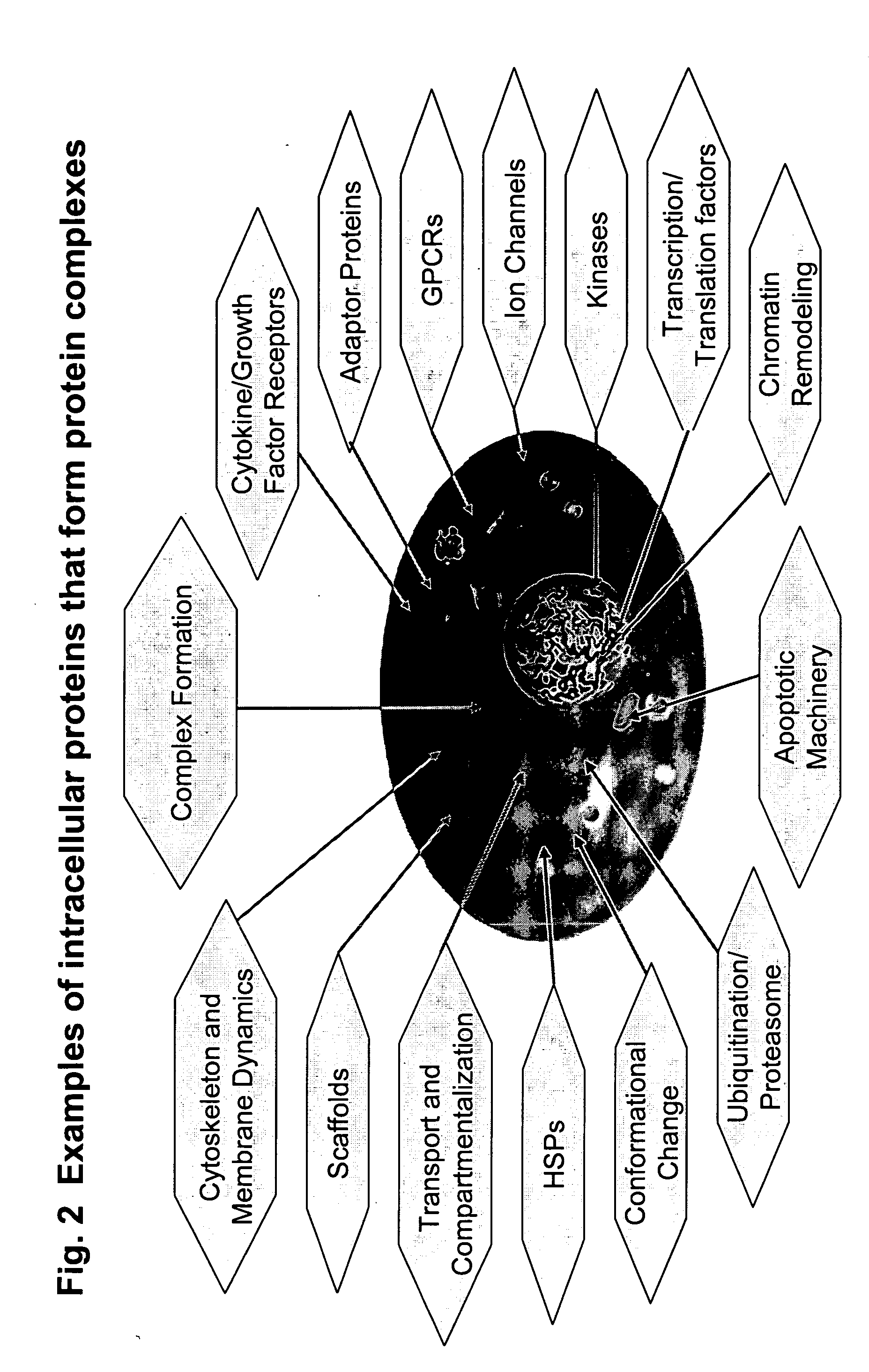
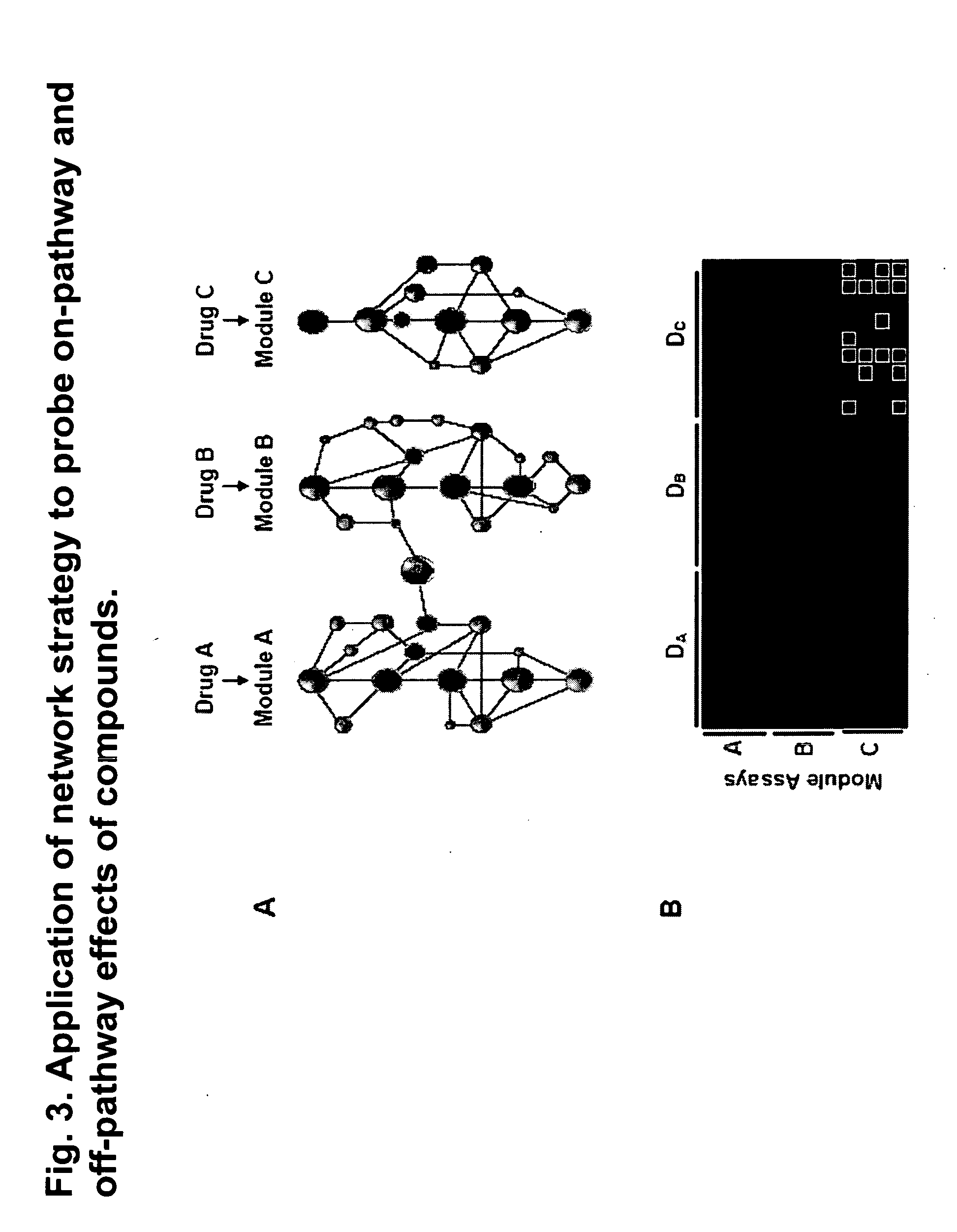
The present invention provides methods for performing pharmacological profiling of a chemical compound, in particular to improve drug safety and efficacy at an early stage in the drug development process. The chemical compound may be a test compound, drug lead, known drug or toxicant. The compound is profiled against a panel of assays. Preferred embodiments of the invention include high-content assays for protein-protein interactions. The compositions and methods of the invention can be used to identify pathways underlying drug efficacy, safety, and toxicity; and to effect attrition of novel compounds with undesirable or toxic properties. The compositions and methods of the invention can also be used to identify new uses of therapeutic agents, to screen libraries of chemical compounds, to perform lead optimization, and to perform studies of structure-activity relationships in the context of intact cells. The compositions and methods of the invention can be applied to any test compound, drug, drug target, pathway, and therapeutic indication.
Owner:ODYSSEY THERA INC
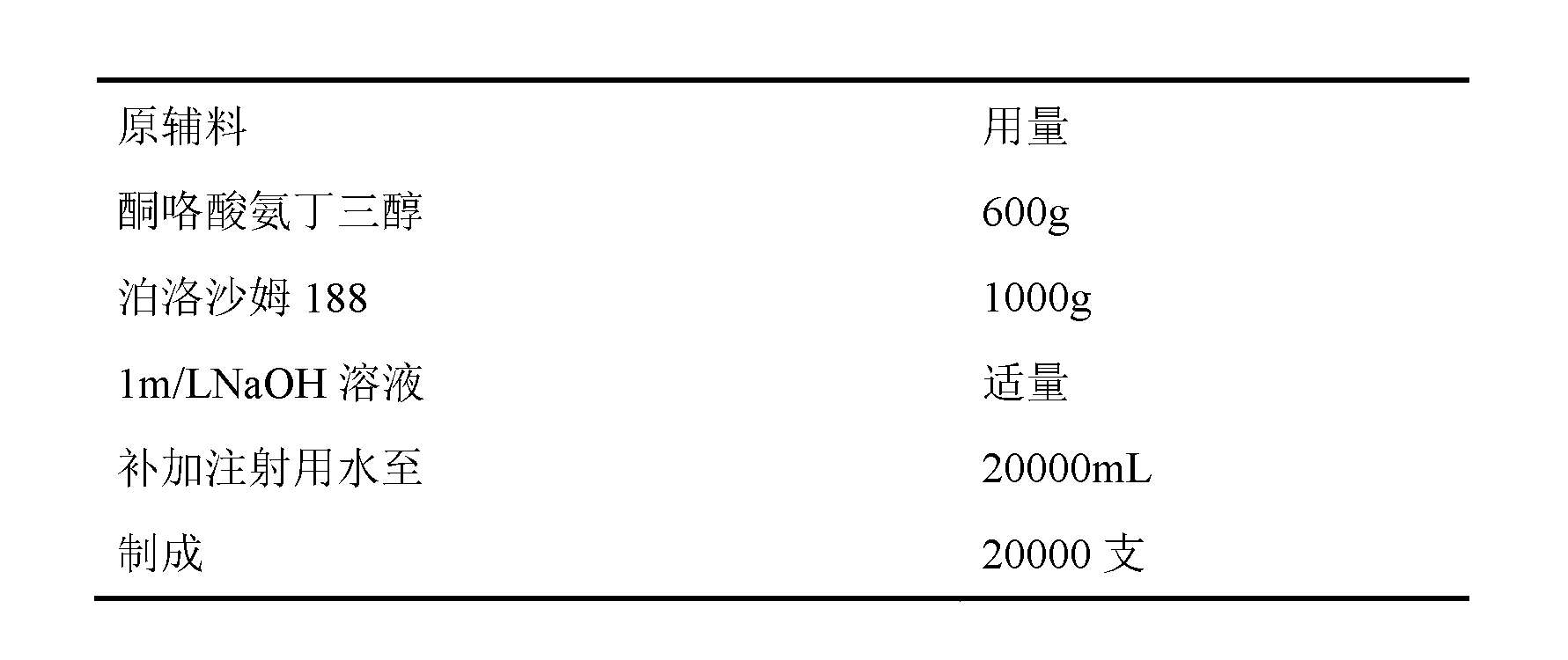
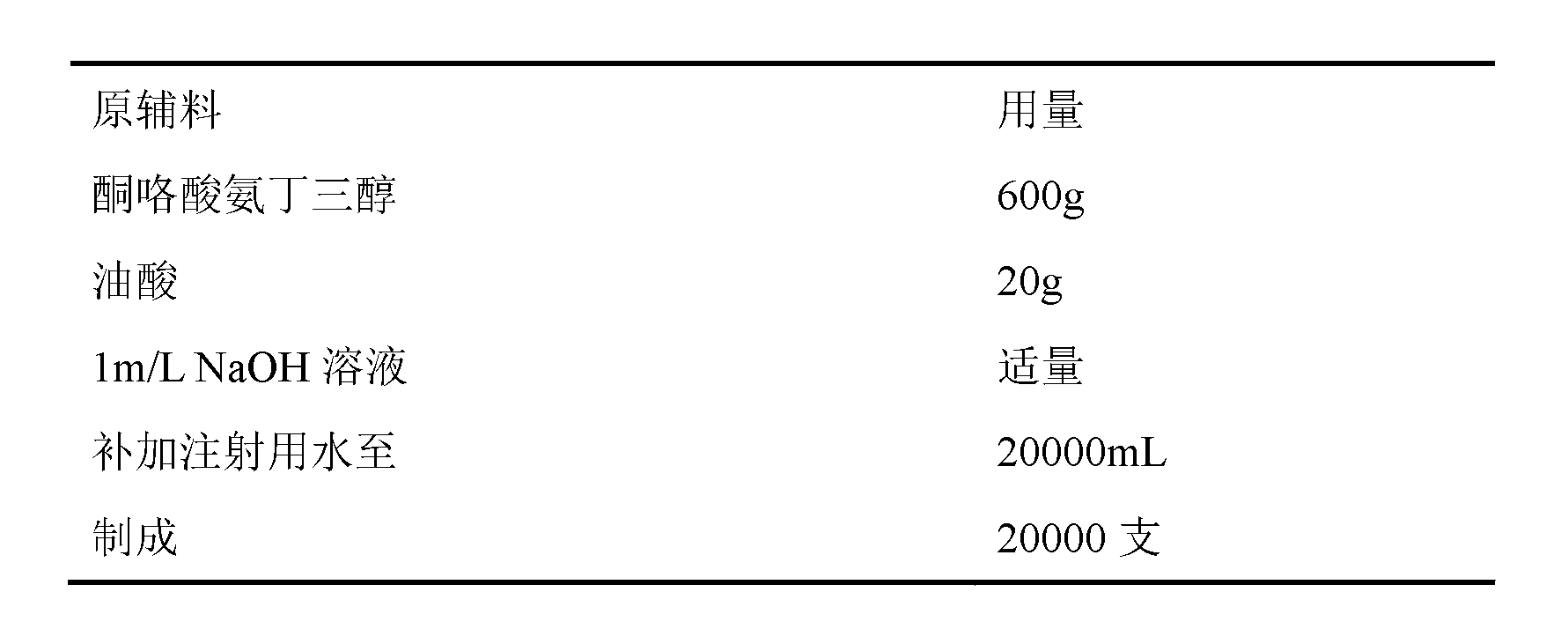
Ketorolac tromethamine injection
InactiveCN102846542ASolve easy discolorationConvenient amountOrganic active ingredientsAntipyreticUse medicationIrritation
The invention discloses a prescription of a ketorolac tromethamine injection and a preparation method. The injection provided by the invention can not only effectively solve the problem that the existing ketorolac tromethamine injection containing ethanol causes irritation while being injected and improve the safety of the drugs and the compliance of the drugs, but also completely avoid the white points caused by the traditional technology after sterilization treatment, and thus the ketorolac tromethamine injection is good in stability, high in safety, reliable in quality and significant in efficacy.
Owner:TIANJIN CHASE SUN PHARM CO LTD
New active clindamycin phosphate compound and medicinal composition thereof
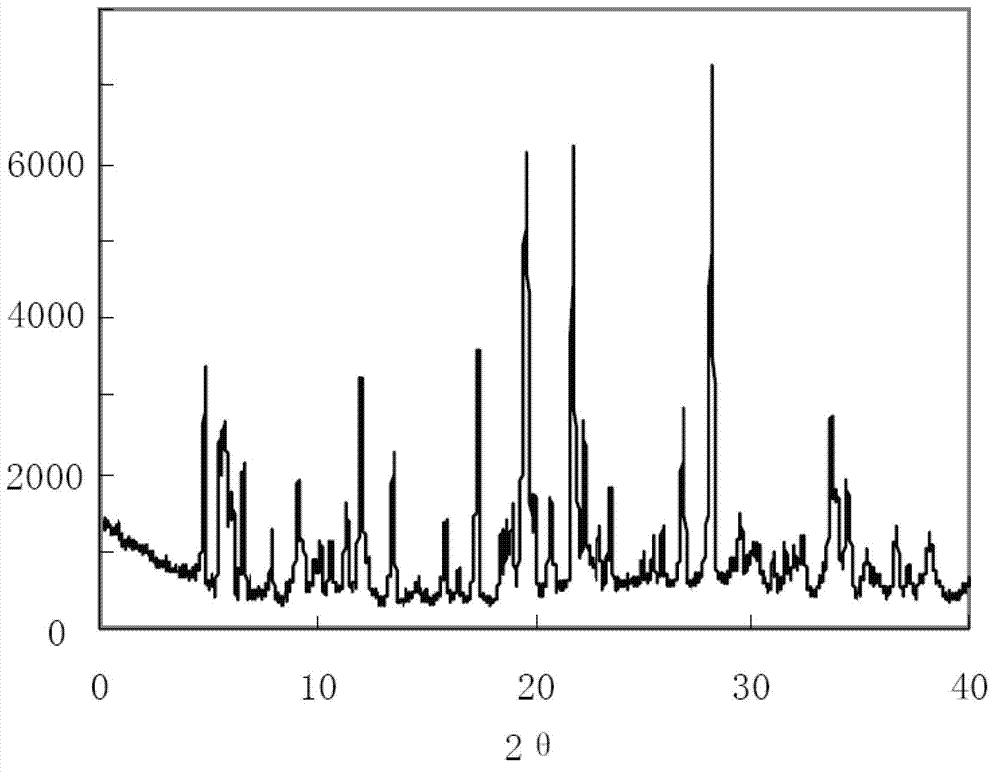
ActiveCN102731585ASimple manufacturing methodGood reproducibilityAntibacterial agentsOrganic active ingredientsClindamycin PhosphateX-ray
The invention relates a new active clindamycin phosphate compound and a medicinal composition of the compound. When the clindamycin phosphate compound is determined by using powder X-ray diffraction, characteristic diffraction peaks appear at the angels of 4.85 degrees, 5.73 degrees, 6.64 degrees, 9.12 degrees, 11.98 degrees, 13.51 degrees, 17.37 degrees, 19.61 degrees, 21.76 degrees, 22.23 degrees, 23.46 degrees, 26.82 degrees, 28.16 degrees, 33.68 degrees and 34.29 degrees in a X-ray diffraction pattern represented in 2theta + / -0.2 degree diffraction angles. The stability of the clindamycinphosphate compound provided by the invention is improved remarkably; the influences on the appearance, content and other aspects of the clindamycin phosphate compound caused by long-term storage can be ignored, thereby ensuring the drug safety for patients. In addition, the new clindamycin phosphate compound is suitable to be popularized and applied to various clindamycin phosphate preparations.
Owner:南城第二医院
Molecular identifying method for quickly identifying truth of beauveria bassiana in Chinese herbal medicine stiff silkworm
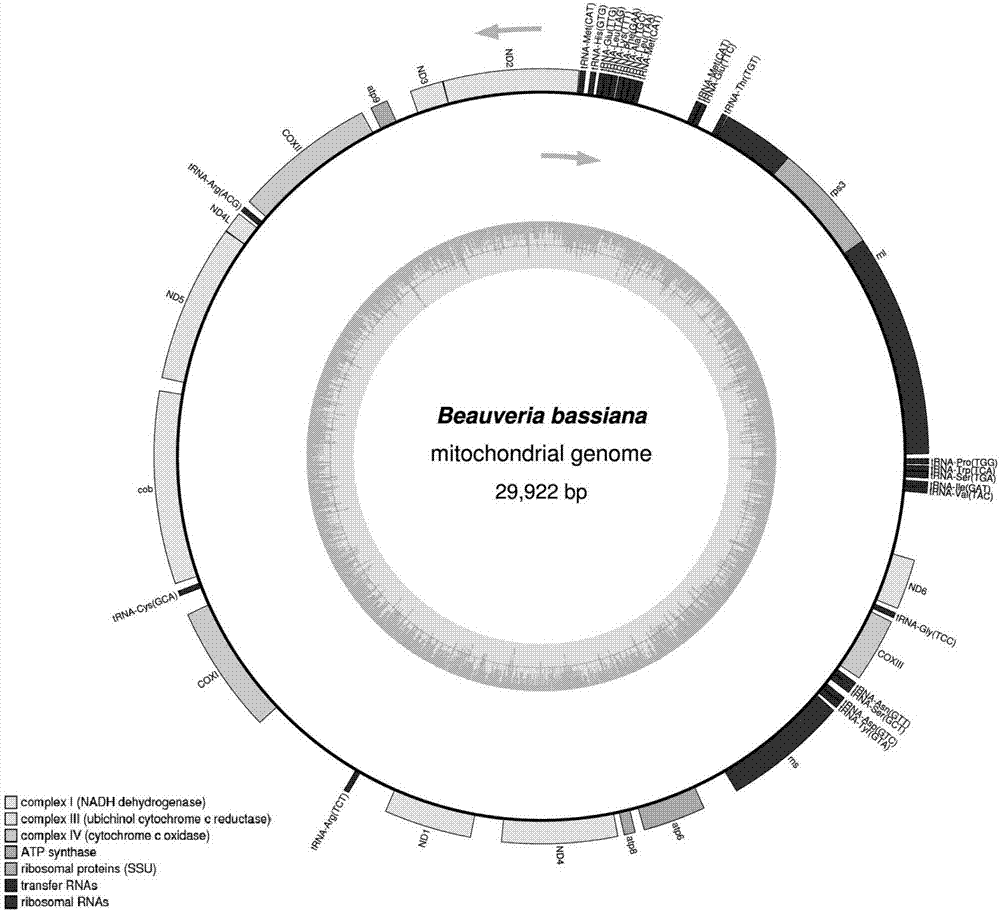
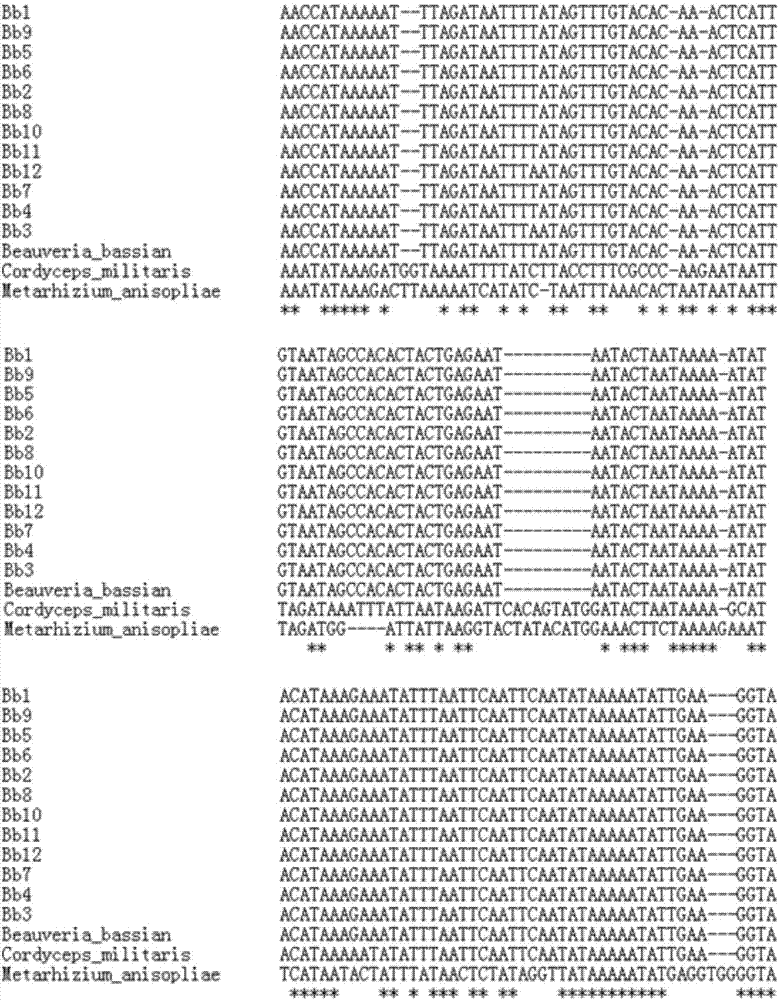
ActiveCN107164471AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesRibosomal protein E-L30Biotechnology
The invention discloses a primer set for quickly identifying truth of beauveria bassiana in Chinese herbal medicine stiff silkworm and a molecular identifying method. The sequence of the primer set is respectively shown as SEQ ID NO.1-2. According to the invention, on the basis of the completion of high-throughput sequencing of complete genome of Guangdong beauveria bassiana mitochondria and bioinformatic comparative analysis of mitochondria complete genome of multiple cordyceps species, the difference of the beauveria bassiana mitochondria gene sequences at the aspect of geographical species is compared and obvious variation of ribosomal protein gene of beauveria bassiana is founded; a pair of primers for specifically detecting beauveria bassiana are screened on the basis of the design; the molecular identifying method for quickly and efficiently identifying truth of beauveria bassiana in Chinese herbal medicine stiff silkworm is established; the method is simple, convenient and quick, has high specificity, high sensitivity and wide application scope and can realize the identification for the truth of the beauveria bassiana from different producing places; a technical support is supplied for quality standardization and normalization and clinic drug safety of the Chinese herbal medicine stiff silkworm.
Owner:SOUTH CHINA AGRI UNIV
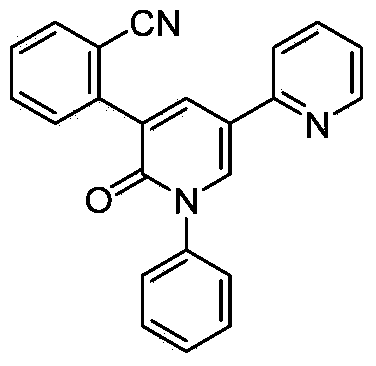
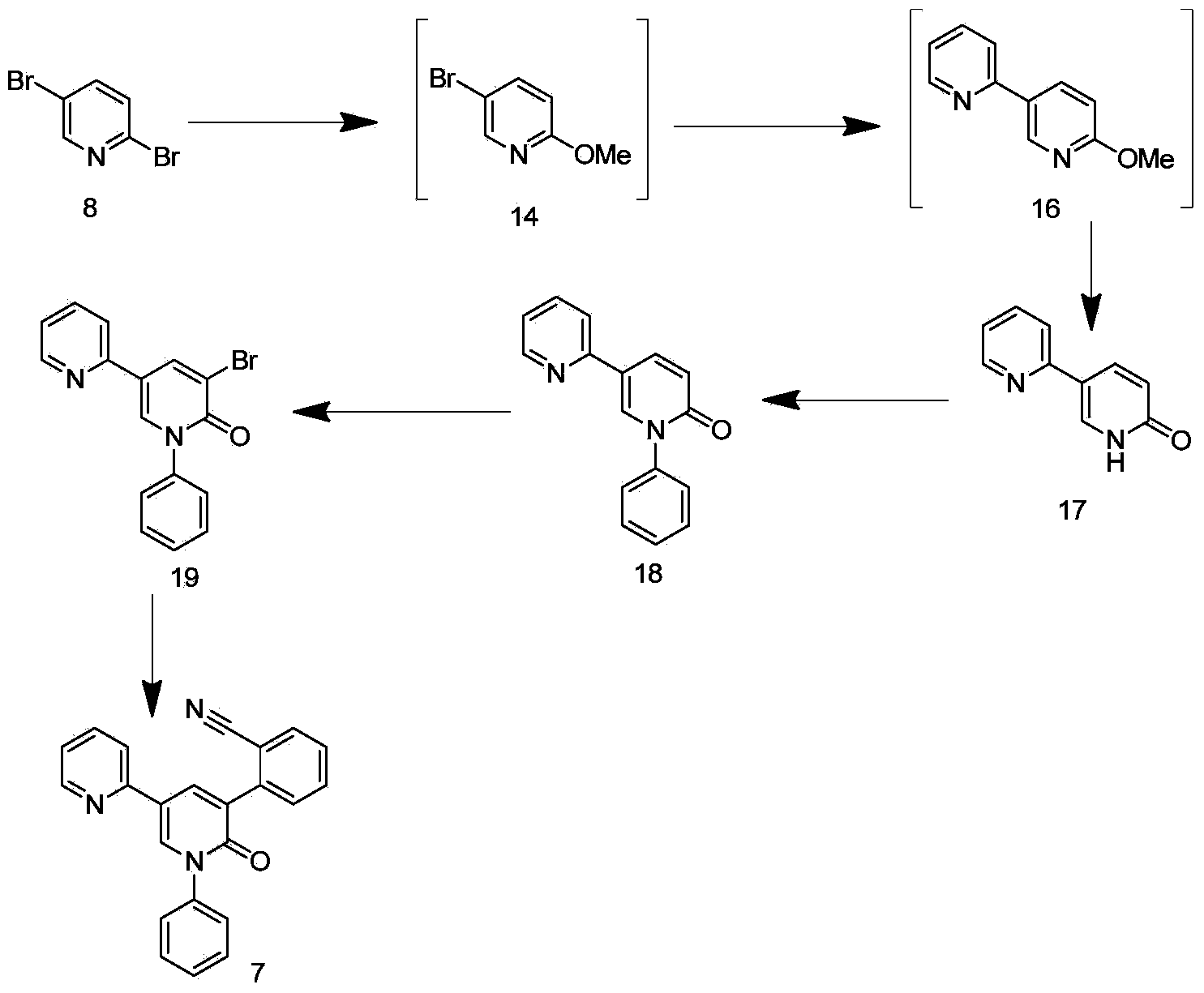
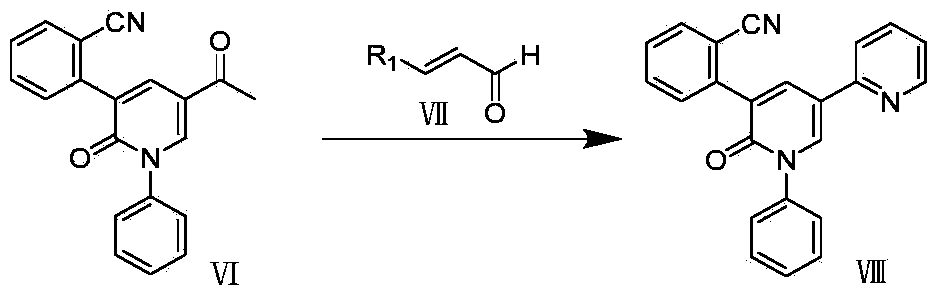
Synthetic method of perampanel, intermediate of perampanel and synthetic method of intermediate
The invention relates to the field of medicinal chemical synthesis and in particular relates to a synthetic method of perampanel, an intermediate of the perampanel and a synthetic method of the intermediate. The provided synthetic method of the perampanel comprises the following steps: carrying out a cyclization reaction on a compound shown in a formula V and a compound shown in a formula VI, so that a compound shown in a formula VII is obtained, wherein the formulas V, VI and VII are described in the specification, and R1 is alkyl amino, amino or alkoxy. The invention also aims at providing a synthetic method of an intermediate of the perampanel. In a synthetic process, phenyl, cyano phenyl and 2-pyridyl are sequentially introduced, compared with the prior art, a sequence of introducing substituent groups is changed, so that the problems that reaction yield is low and byproducts are many and can not be removed as 2-pyridyl is firstly introduced and then cyano phenyl is introduced can be solved; a route is changed, and a Pd containing catalyst is not used in reaction of the last step, so that Pd residue is reduced, drug safety is improved, and reaction yield and product purity are greatly improved, so that the synthetic method of the perampanel is applicable to industrial production.
Owner:ZHEJIANG YONGNING PHARMA
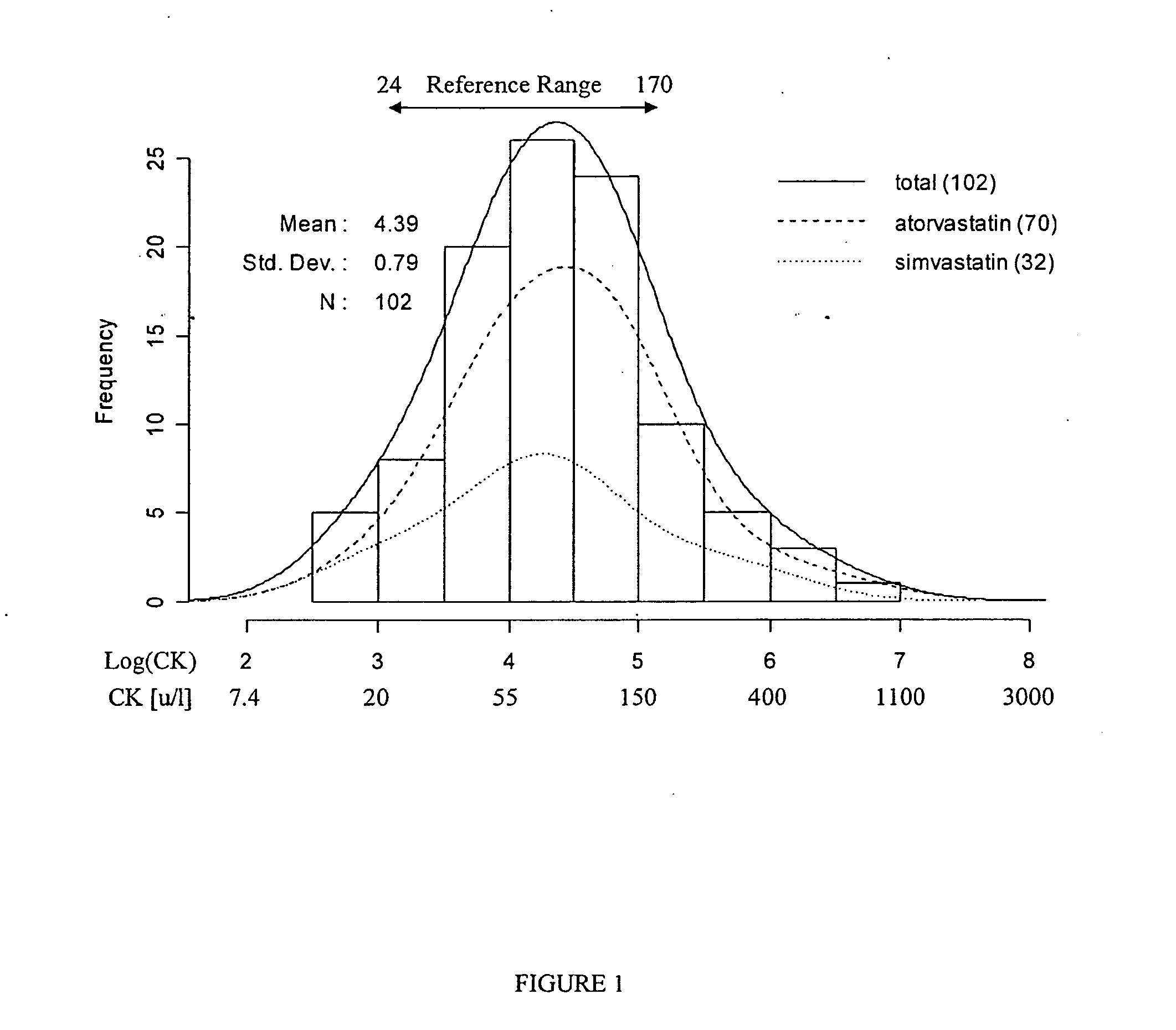
Physiogenomic method for predicting statin injury to muscle and muscle side effects
InactiveUS20070202518A1Reduce generationPrevent and reduce muscular side effectBioreactor/fermenter combinationsBiological substance pretreatmentsSide effectMedicine
The present invention relates to the use of genetic variants of associated marker genes to predict an individual's susceptibility to muscular injury and muscular side effects in response to statin therapy. The present invention further relates to analytical assays and computational methods using the novel marker gene set. The present invention has utility for personalized medical treatment, drug safety, statin compliance, and prophylaxis of muscle side effect.
Owner:GENOMAS
New medical purpose of privet and pharmaceutical formulation thereof
InactiveCN101239083ADefinite curative effectSmall toxicitySkeletal disorderPill deliverySide effectSucrose
The invention discloses a ligustrum lucidum and the new use for Chinese medicine of a ligustrum lucidum extractive for preparing medicines for prevention or treatment of osteoporosis, and also discloses a granule for prevention or treatment of osteoporosis, wherein, the granule comprises 25 to 35 percents of the ligustrum lucidum extractive and 65 to 75 percents of an excipient which chooses sucrose and dextrine, or is made into capsules. The capsules contain the ligustrum lucidum extractive. The ligustrum lucidum single-taste and the relative pharmaceutical preparation of the invention has the advantages of accurate curative effect, low side effects, more drug safety, simple preparation craft and lower cost in the application of prevention or treatment of osteoporosis.
Owner:普尔药物科技开发(深圳)有限公司 +1
Capecitabine granule and preparation method thereof
ActiveCN103356488AImprove stabilityMask bad tasteOrganic active ingredientsGranular deliverySide effectAdhesive
The invention relates to an oral anti-tumor drug capecitabine granule and a preparation method thereof. The capecitabine granule exists in the form of a cyclodextrin inclusion compound and is prepared from capecitabine serving as a raw material drug and auxiliary materials comprising cyclodextrin, a diluting agent, a disintegrating agent, an adhesive and a flavoring agent by wet granulation. The capecitabine granule disclosed by the invention not only covers the bitterness of a capecitabine drug and enhances the drug compliance of a cancerous person, but also enhances the stability of the capecitabine in gastrointestinal tracts and accelerates the digestion velocity, thereby preventing the peak valley phenomenon of the blood concentration of a drug, outstandingly reducing the adverse reactions of the drug, such as irritation and toxic side effect, on the gastrointestinal tracts due to the stable release of capecitabine in the gastrointestinal tracts, enhancing the drug safety and better taking the anti-tumor effect.
Owner:QILU PHARMA HAINAN
Method for making epileptic seizure animal model
The invention provides a method for making an epileptic seizure animal model. The method for making the epileptic seizure animal model comprises the step of applying kainic acid to primate animal sea horses by adopting a stereospecific technique. The made epileptic seizure animal model has the advantages of local injection administration, low dosage, little relation between epileptogenic dosage and genders and ages of animals, good repeatability, clear epileptogenic process and good drug safety.
Owner:MENG FAN'GANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com