Two-part capsule to accept pharmaceutical preparations for powder inhalers
a technology of powder inhaler and capsule, which is applied in the direction of powder delivery, medical preparations, other medical devices, etc., can solve the problems of not being able to guarantee the pharmaceutical quality of the contents, the material used is not very resistant to air humidity, and the material is not specially perfected for powder inhaler
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
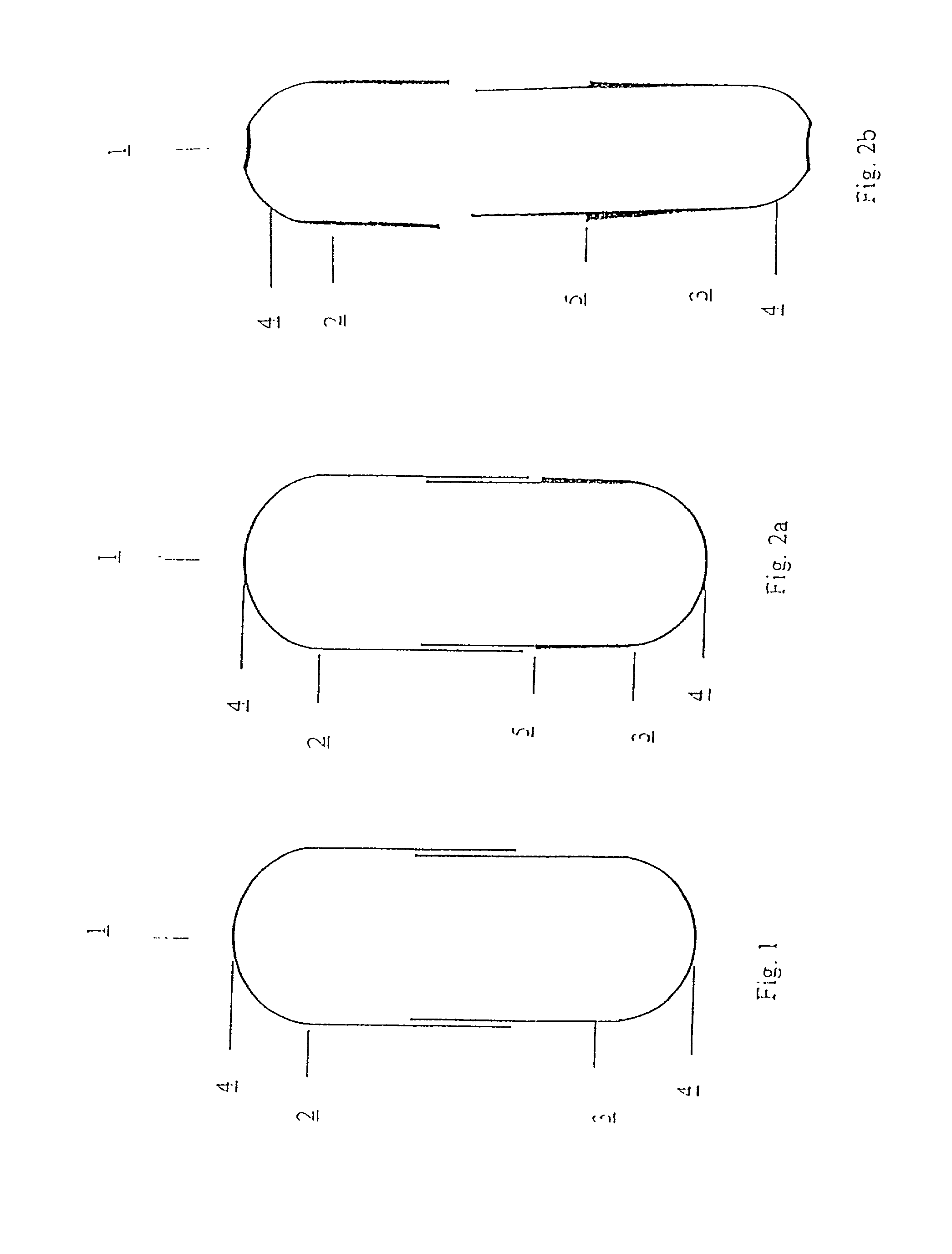
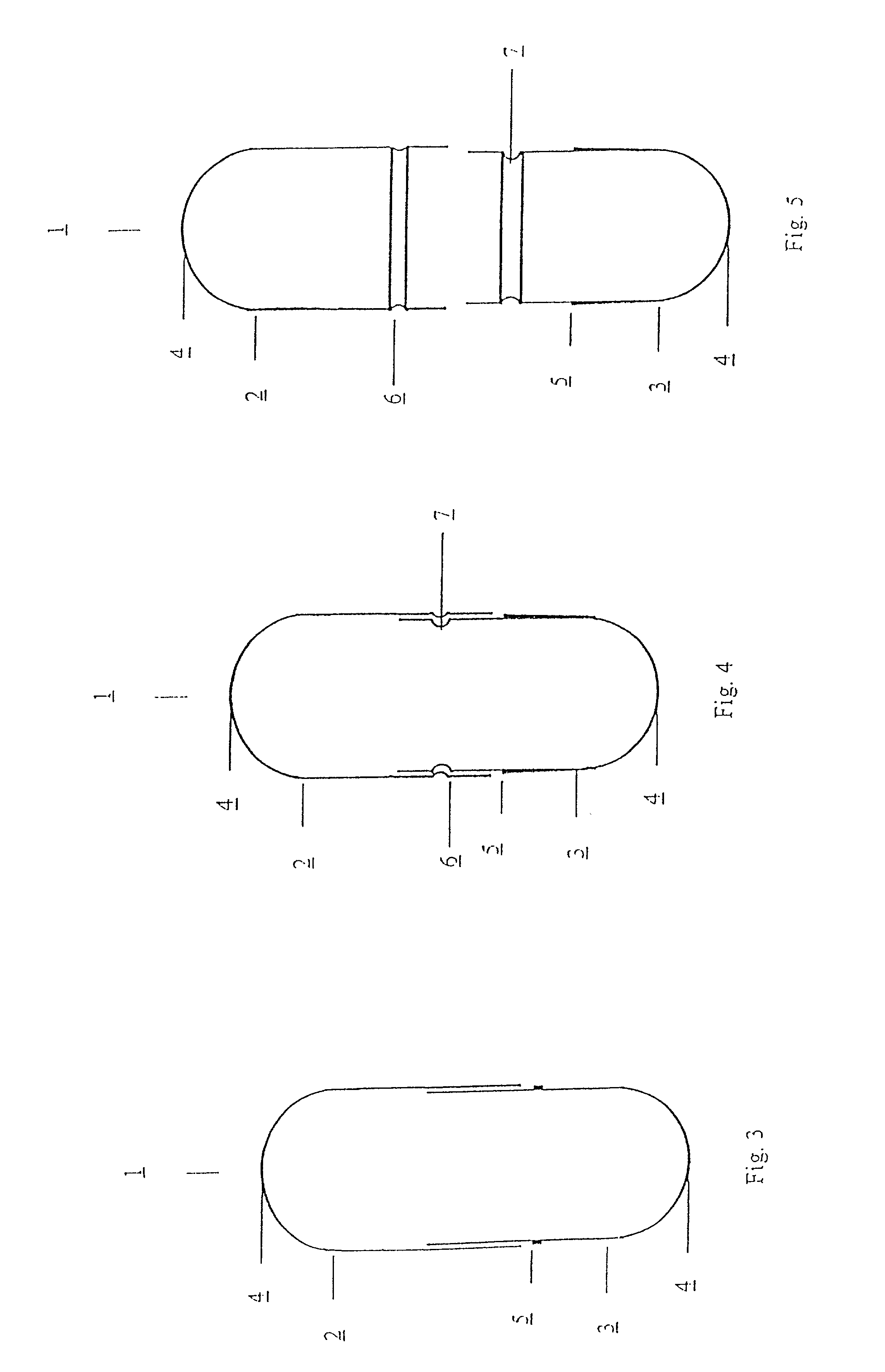
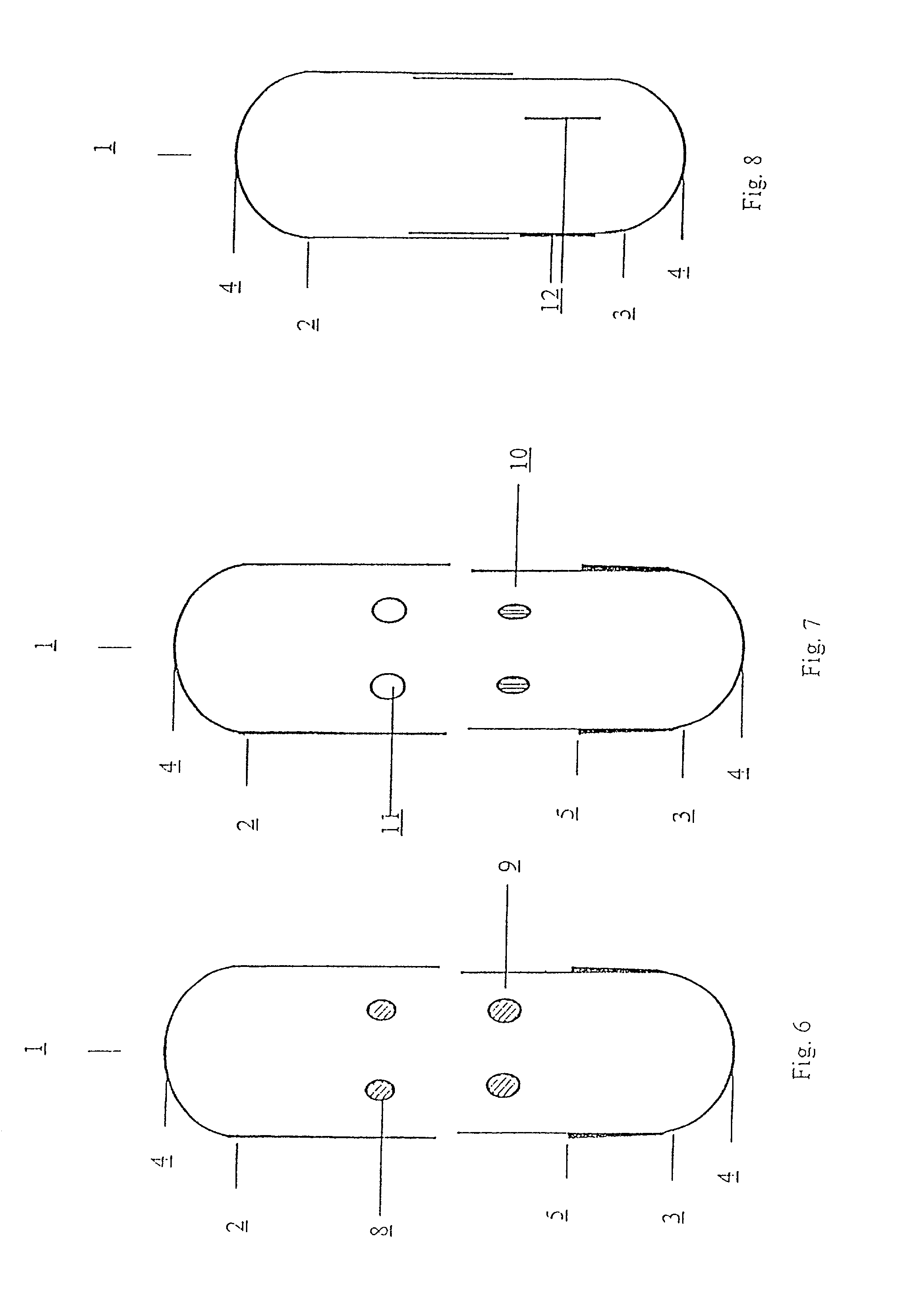
[0011] The present invention relates to a capsule for holding pharmaceutical preparations for powder inhalers with increased drug safety and capsules for pharmaceutical preparations for powder inhalers with improved adaptation to use in powder inhalers. The capsules consist of water-insoluble, hydrophobic synthetic materials, which do not themselves substantially influence the pharmaceutical quality of the contents, but which improve the usability of the filled capsules with regard to their function, their longevity and / or the climatic zone, and are advantageous at various stages from production through to utilisation.
[0012] The capsules, according to the invention, consist of two parts, a capsule body (body) and a capsule cap (cap), which can be connected together so as to form a stable enclosed hollow space of defined volume which contains the pharmaceutical formulation. The dimensions of the capsule are chosen so that the capsule can be used with common powder inhalers which are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com