Method and system for the analysis and association of patient-specific and population-based genomic data with drug safety adverse event data
a genomic data and data analysis technology, applied in the field of method and system, can solve problems such as extreme complexity, and achieve the effect of assessing the risk of an adverse drug reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
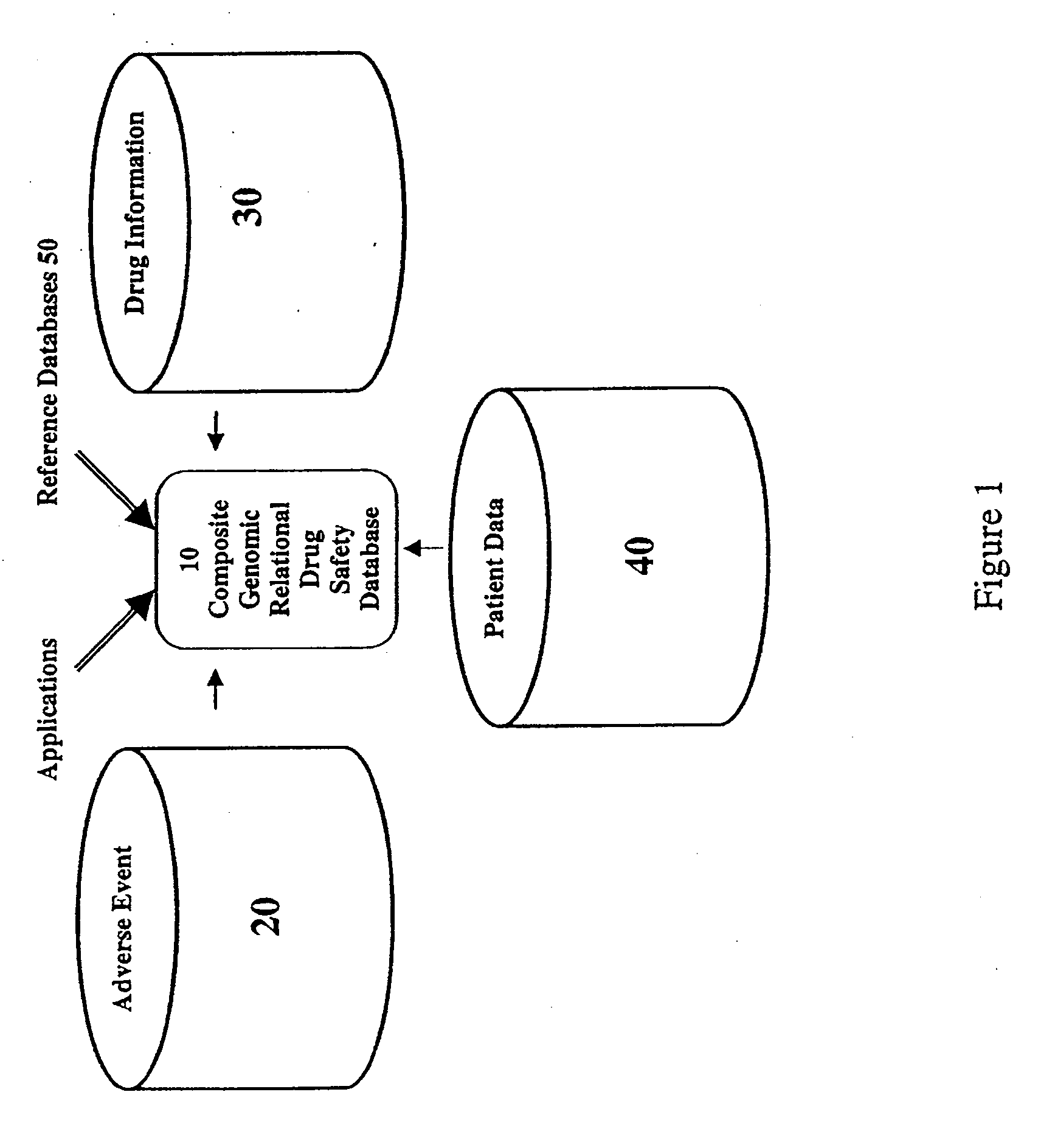
[0023]The present invention comprises a system and method for creating, storing and using patient-specific and population-based genomic drug safety data including at least one or more integrated databases; a selector for selecting at least one drug for analysis (based on the generic, brand name or therapeutic category); a profiler for displaying statistics that describe one or more behaviors of the drug in multiple dimensions; a series of at least two filters and the means to control the filters individually and in combination; at least one data mining engine. Preferably, the data mining engine is a correlator, a proportional analysis engine, and a comparator; and a graphical user interface for displaying the results of the analysis.
[0024]Dimensions such as age, sex, weight, diet, dates, reactions, doses, outcomes, illnesses, report source, and concomitant drugs can be analyzed in combinations of two dimensions, in combinations of three dimensions, in combinations of four dimensions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com