Tacrolimus sustained-release preparation and preparation method thereof
A technology of tacrolimus and controlled-release preparations, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, and medical preparations containing active ingredients. Predict and increase drug solubility and other issues to achieve the effects of reducing the incidence of adverse reactions, improving bioavailability, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
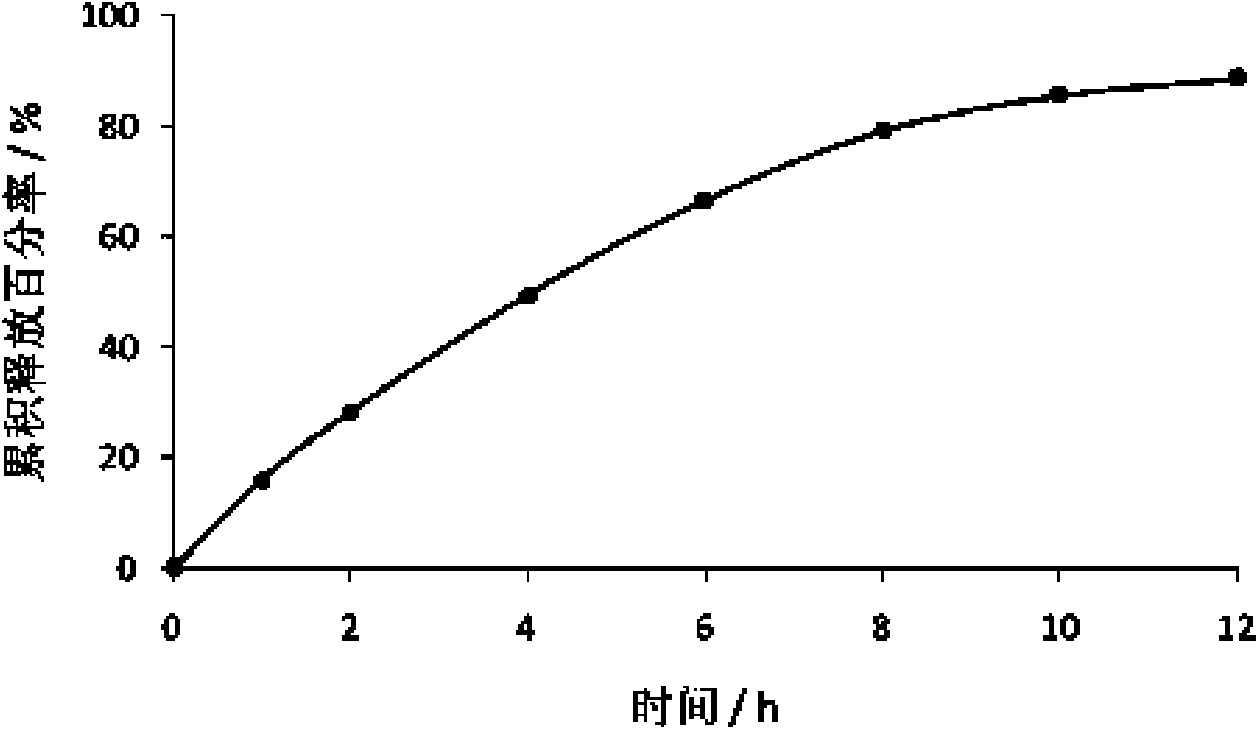
[0075] Example 1 Preparation of Tacrolimus Hydrophilic Gel Matrix Tablets
[0076] Tacrolimus
1g
Hydroxypropyl-β-cyclodextrin
20g
Hydroxypropyl Methyl Cellulose K4M
50g
92g
3.3g
0.8g
A total of 1000 pieces
[0077] Preparation Process:
[0078] After dissolving tacrolimus and hydroxypropyl-β-cyclodextrin with an appropriate amount of absolute ethanol, after ultrasonicating in an ultrasonic instrument for 20 minutes, transfer it to a rotary evaporator to evaporate the solvent to dryness at a temperature of 30-40°C, and precipitate Put the product in a vacuum drying oven at 25-40°C for 24 hours, take it out, grind it, pass through an 80-mesh sieve, and mix it with hydroxypropyl methylcellulose K4M, microcrystalline cellulose, lactose, and magnesium stearate in equal amounts Evenly, the powder is directly compressed ...
Embodiment 2
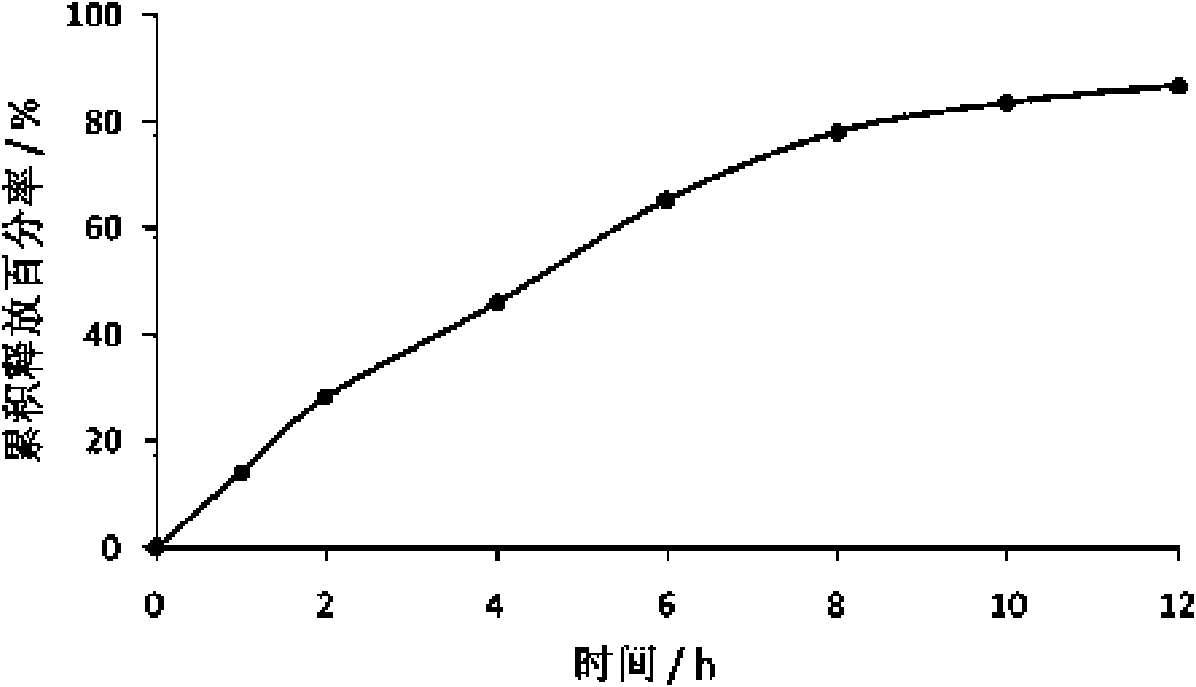
[0080] Example 2 Preparation of Tacrolimus Film Controlled Release Tablets
[0081]
[0082]
[0083] Preparation Process:
[0084] Dissolve tacrolimus in an appropriate amount of ethanol solution, heat polyethylene glycol 6000 in a water bath at 60-70°C until it melts, mix well, stir vigorously, and pour the melt on a stainless steel plate to form a thin layer, making Cool it quickly into a solid, then place the solid in a vacuum oven at 25-40°C for 24 hours, take it out, add a small amount of micropowder silica gel to grind (micropowder silica gel as a lubricant can reduce the loss of solid dispersion during the grinding process), and pass After an 80-mesh sieve, mix it with lactose evenly, add an appropriate amount of 1% polyoxyethylene pyrrolidone aqueous solution, make a soft material, granulate, dry, sieve, granulate, add magnesium stearate, mix evenly, and compress into tablets. The above-mentioned coating material is used for coating, and the release degree is c...
Embodiment 3
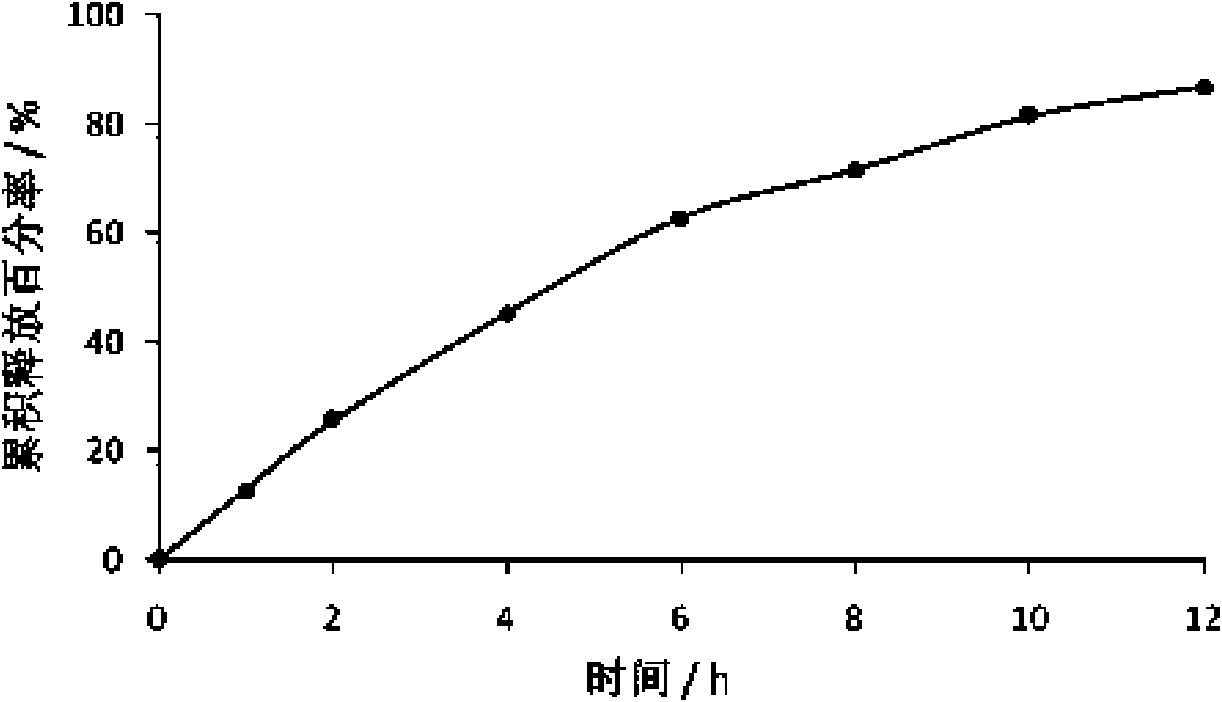
[0086] Example 3 Preparation of Tacrolimus Osmotic Pump Tablets
[0087] Chip
Tacrolimus
1g
Hydroxypropyl-β-cyclodextrin
15g
polyoxyethylene
28g
72g
Magnesium stearate
0.7g
Coating material
Cellulose acetate
12g
polyethylene glycol 400
1.5g
Acetone:water (100:1, v:v)
Appropriate amount
A total of 1000 pieces
[0088] Preparation Process:
[0089] After dissolving tacrolimus and hydroxypropyl-β-cyclodextrin with an appropriate amount of absolute ethanol, after ultrasonicating in an ultrasonic instrument for 20 minutes, transfer it to a rotary evaporator to evaporate the solvent to dryness at a temperature of 30-40°C, and precipitate Put the product in a vacuum oven at 25-40°C for 24 hours, take it out, grind it, pass through an 80-mesh sieve, mix it with polyoxyethylene, sodium chloride, and magnesium ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com