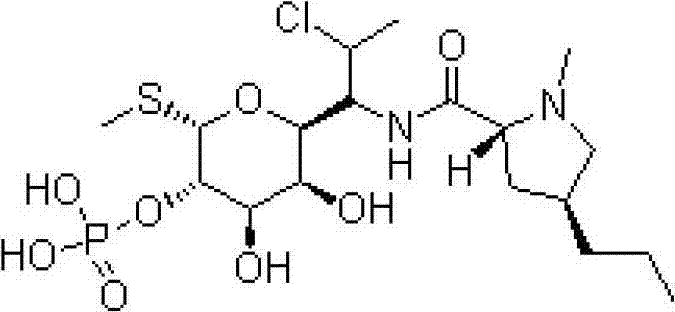
New active clindamycin phosphate compound and medicinal composition thereof
A technology of clindamycin phosphate and compounds, which is applied in the field of medicine, can solve the problems of unguaranteed quality, low crystal yield, and poor reproducibility of the preparation method, and achieve simple and feasible preparation methods, simple preparation methods, The effect of stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
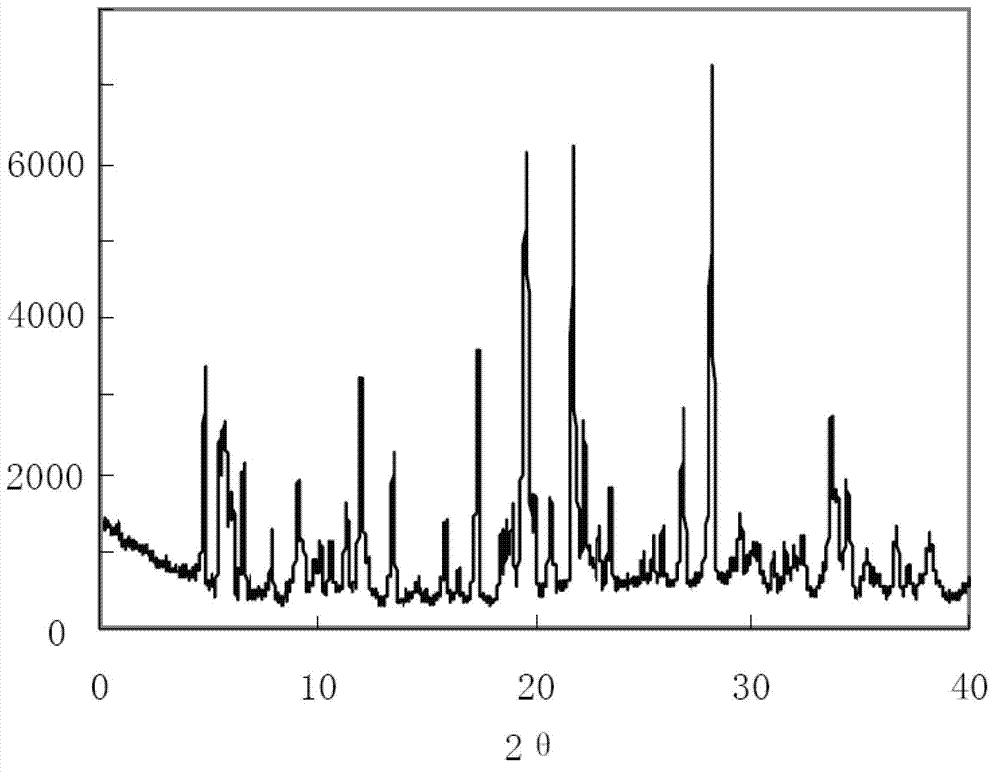
[0048] The preparation of embodiment 1 clindamycin phosphate compound
[0049] (1) Take the crude clindamycin phosphate and fully dissolve it in a mixed solution of isopropanol and n-butanol to obtain a clindamycin phosphate solution. The ratio of isopropanol to n-butanol in the mixed solution is 4 :3, the mass volume ratio of clindamycin phosphate and Virahol is 1:3;
[0050] (2) Add a mixture of ether and acetone dropwise to the solution obtained in step 1 under an ultrasonic field of 0.5KW. In the mixture, the amount of ether and acetone is 4:5, and the mass of clindamycin phosphate and the mixture The volume ratio is 3:7; the dropping speed is 8ml / min; the stirring is continued at a speed of 12rmp during the dropping process;
[0051] (3) Continue ultrasonication for 20 minutes at a stirring speed of 4rmp and an ultrasonic field power of 0.25kw, let the solution stand and cool down to 15°C at a speed of 0.06°C / min, grow crystals for 6 hours, filter, and use 2 times the am...
Embodiment 2
[0053] The preparation of embodiment 2 clindamycin phosphate compounds
[0054] (1) Take the crude product of clindamycin phosphate and fully dissolve it in a mixed solution of isopropanol and n-butanol to obtain a clindamycin phosphate solution. The ratio of isopropanol to n-butanol in the mixed solution is 2 :3, the mass volume ratio of clindamycin phosphate and Virahol is 1:1.5;
[0055] (2) Add a mixture of ether and acetone dropwise to the solution obtained in step 1 under an ultrasonic field of 0.4KW. In the mixture, the amount of ether and acetone is 2:5, and the mass of clindamycin phosphate and the mixture The volume ratio is 3:4; the dropping speed is 5ml / min; the stirring is continued at a speed of 10rmp during the dropping process;
[0056] (3) Continue ultrasonication for 10 minutes at a stirring speed of 2rmp and an ultrasonic field power of 0.2kw, let the solution stand and cool down to 12℃ at a speed of 0.02℃ / min, grow crystals for 4 hours, filter, and use 1 t...
Embodiment 3
[0058] The preparation of embodiment 3 clindamycin phosphate compounds
[0059] (1) Take the crude product of clindamycin phosphate and fully dissolve it in a mixed solution of isopropanol and n-butanol to obtain a clindamycin phosphate solution. The ratio of isopropanol to n-butanol in the mixed solution is 5 : 3, the mass volume ratio of clindamycin phosphate and Virahol is 1: 4.5;
[0060] (2) Add a mixture of ether and acetone dropwise to the solution obtained in step 1 under an ultrasonic field of 0.6KW. In the mixture, the amount of ether and acetone is 6:5, and the mass of clindamycin phosphate and the mixture The volume ratio is 3:11; the dropping speed is 10ml / min; the stirring is continued at a speed of 16rmp during the dropping process;
[0061] (3) Continue ultrasonication for 25 minutes at a stirring speed of 5rmp and an ultrasonic field power of 0.3kw, let the solution stand and cool down to 18°C at a speed of 0.1°C / min, grow crystals for 8 hours, filter, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com