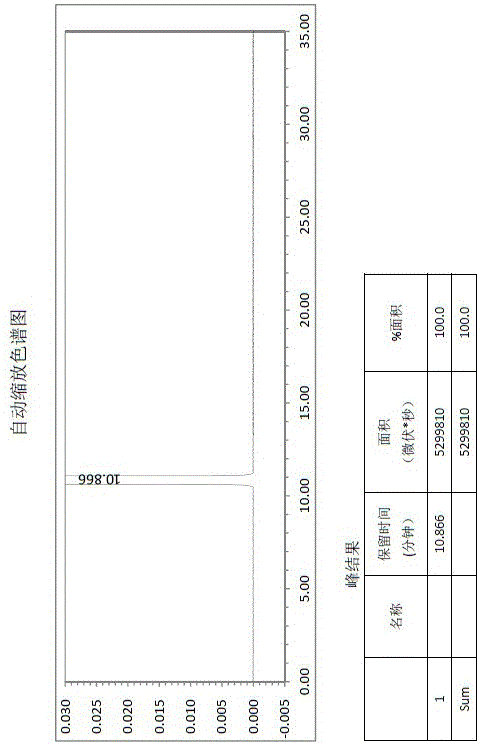
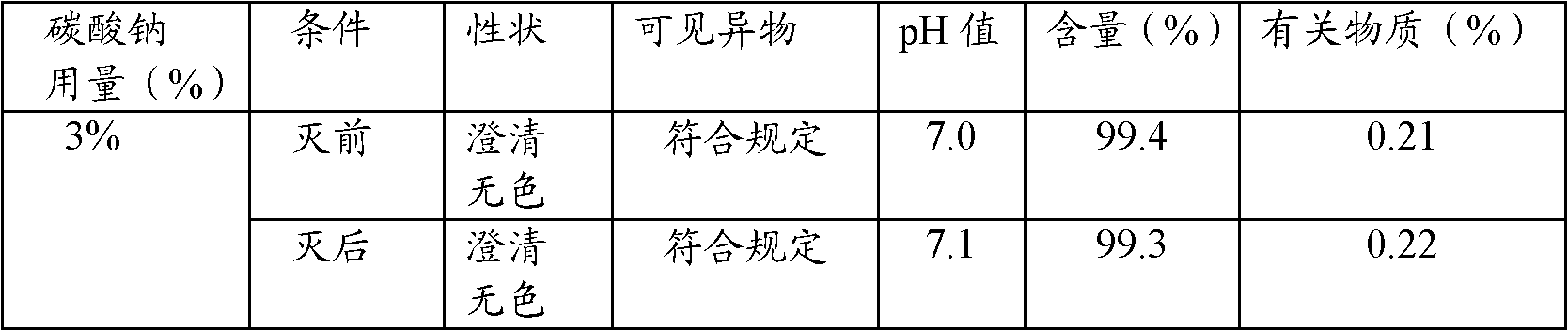
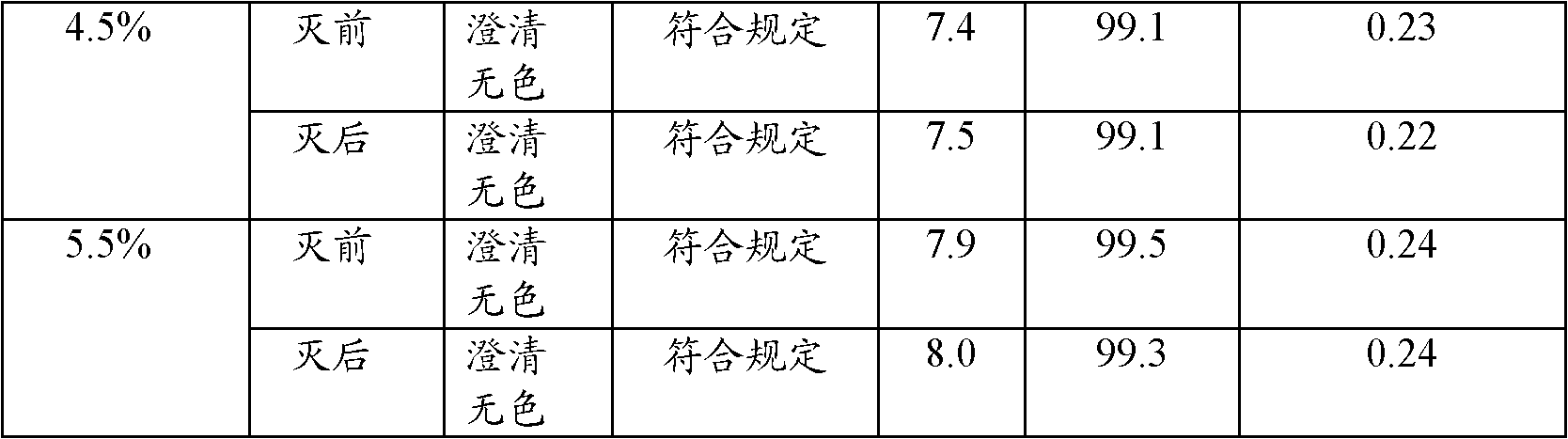
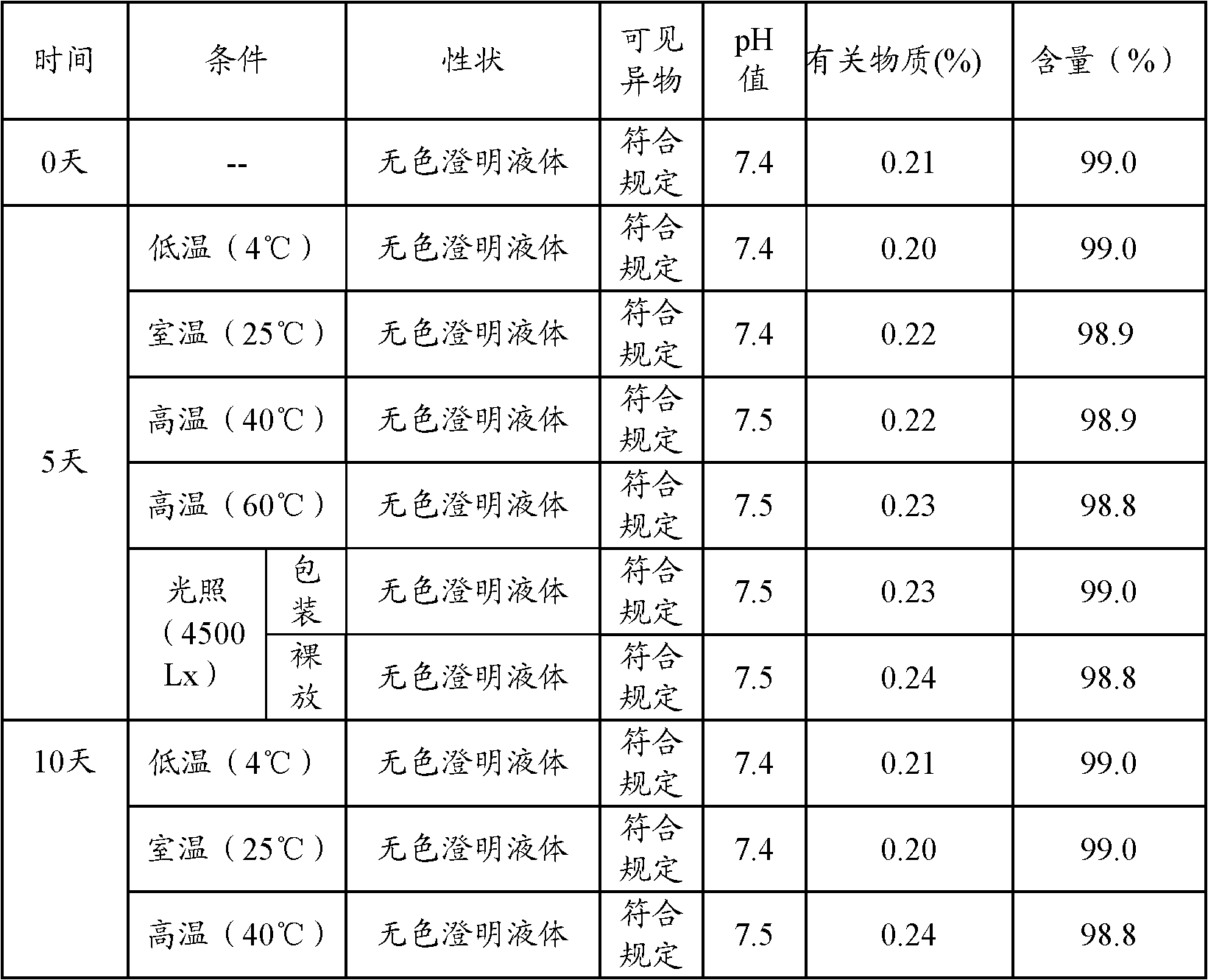
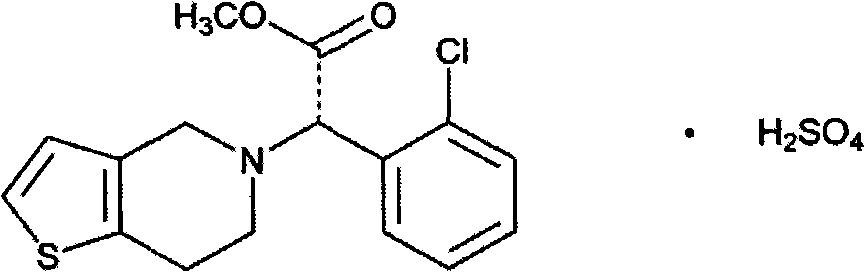
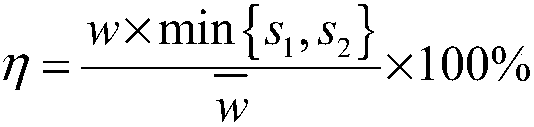Patents
Literature
482results about How to "Ensure medication safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
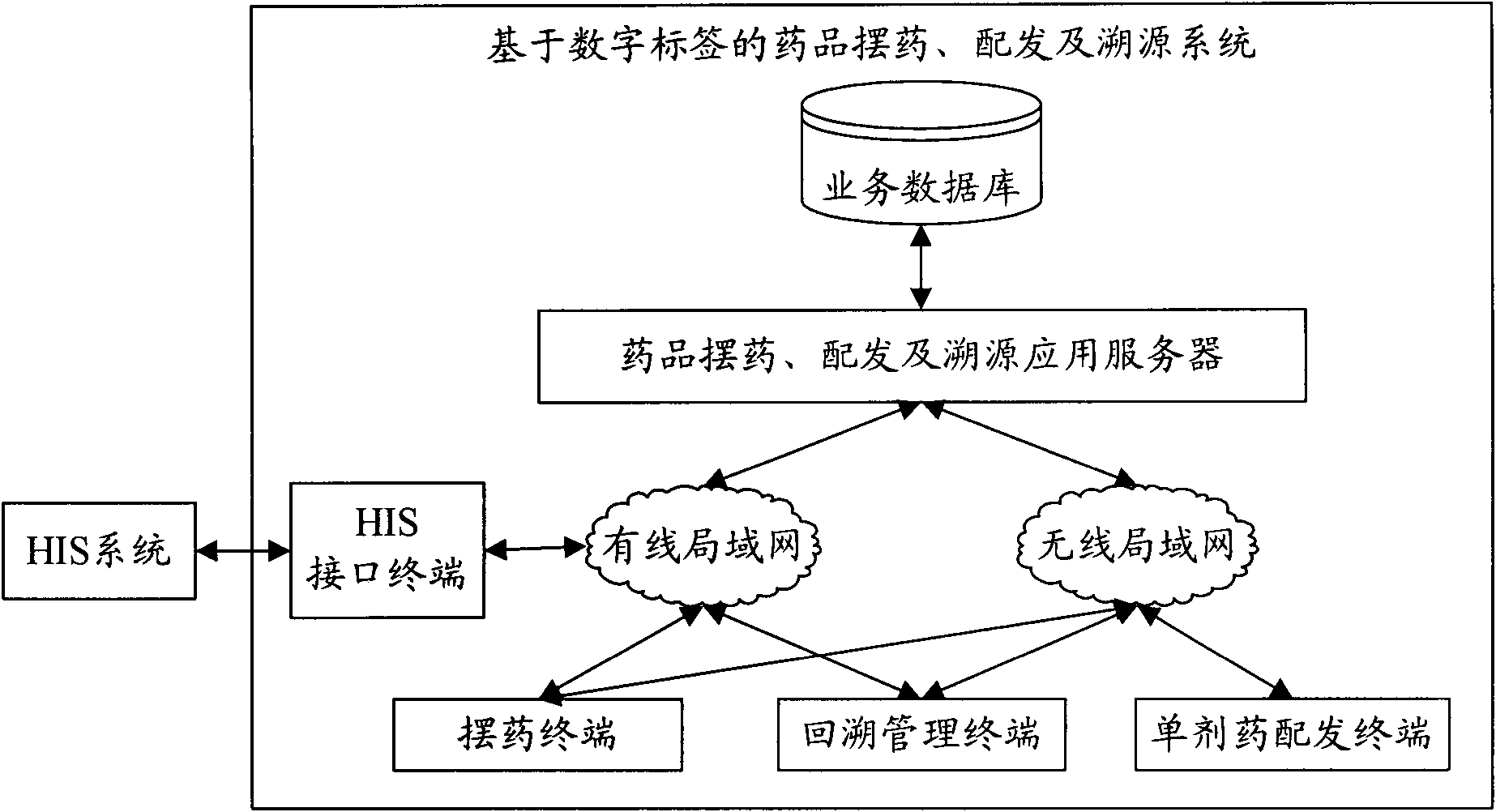
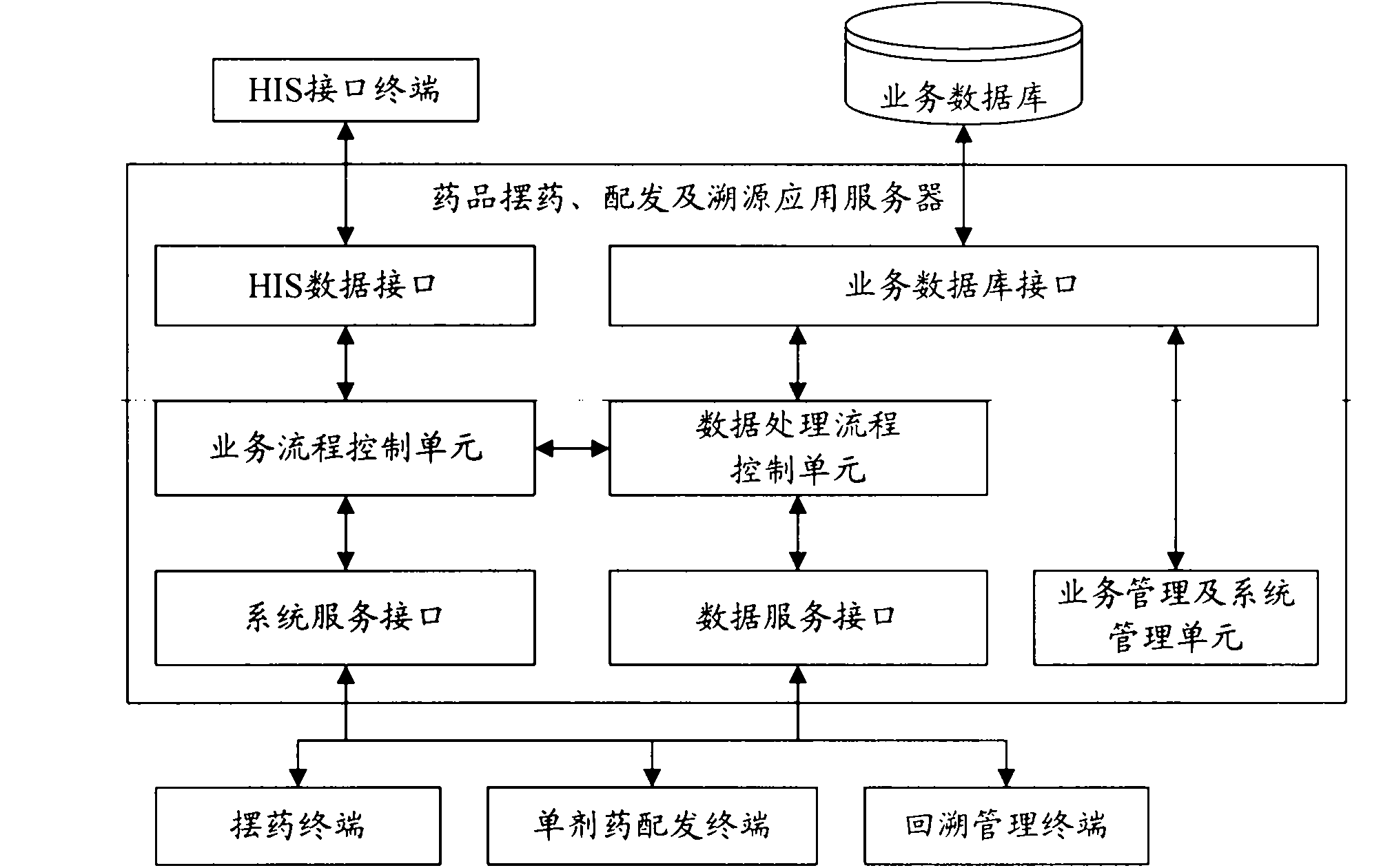
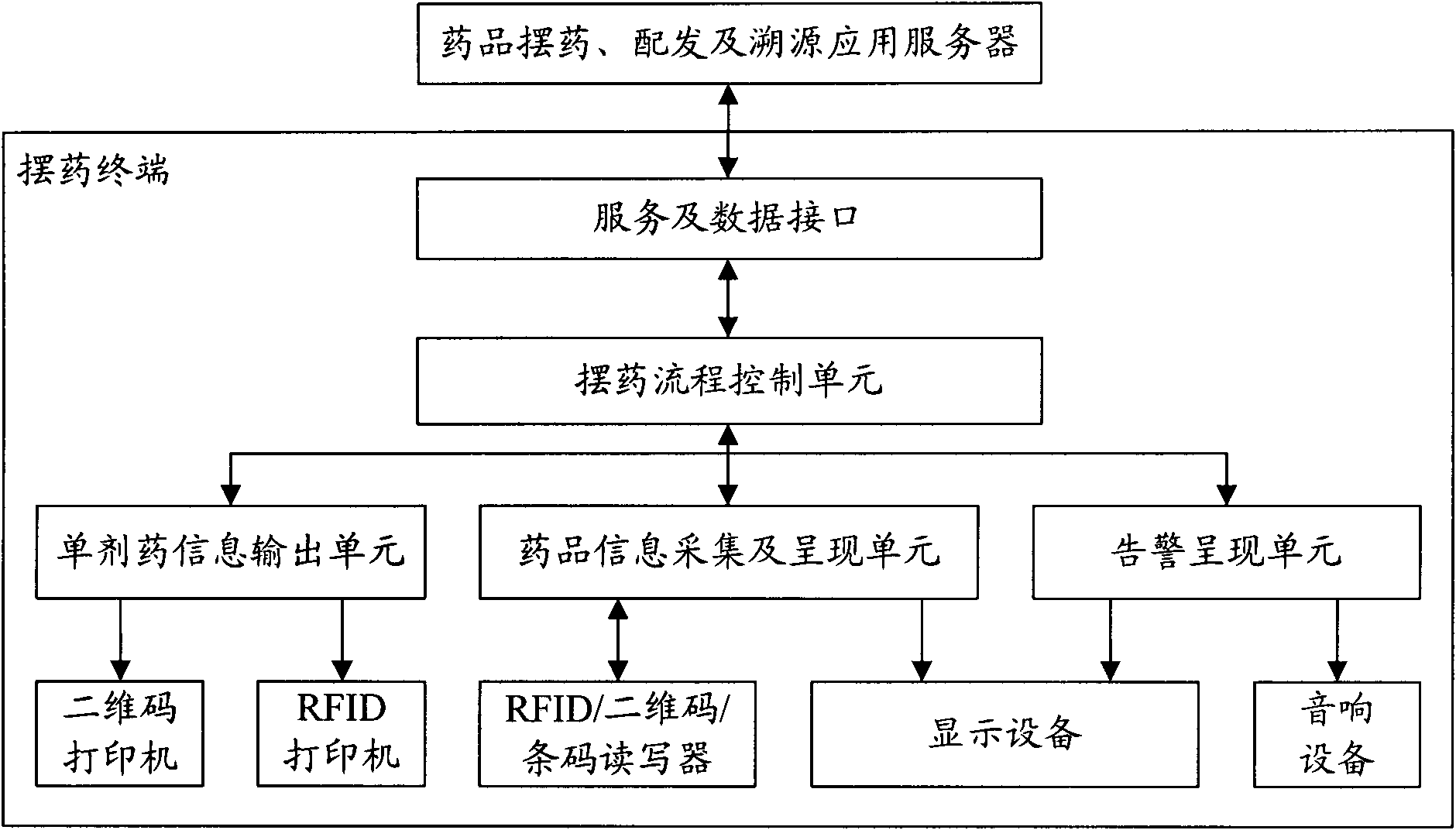
System and method for drug placement, distribution and source tracing based on digital tags
InactiveCN101923606AEnsure medication safetyPrevent medication errorsCo-operative working arrangementsSpecial data processing applicationsPharmacyApplication server
The invention relates to a system and a method for drug placement, distribution and source tracing based on digital tags. The system is formed by connecting the following devices in a wired local network and a wireless local network inside a hospital: a drug placement, distribution and source tracing application server connected with a service database, a plurality of drug placing terminals, a single drug distributing terminal, a source tracing management terminal and an HIS (Hospital Information System) interface terminal interacting information with an HIS system. The system is a set of information system for drug placement, distribution and source tracing constructed by adopting a computer technology, an RFID (Radio Frequency Identification Devices) technology and a two-dimensional code technology. The information system is used for supporting hospital staff to complete two operating steps of drug placement in pharmacies and drug distribution in sick rooms during the drug circulation inside the hospital based on the digital tags, providing scientific and visualized examination means and tools and monitoring and checking each key link during drug placement and distribution. The system can realize the safe control mechanism of drugs for treating patients during the circulation inside the hospital and can reduce the accident potential caused by human mistakes.
Owner:WUXI BUPT SENSING TECH & IND ACADEMY +2
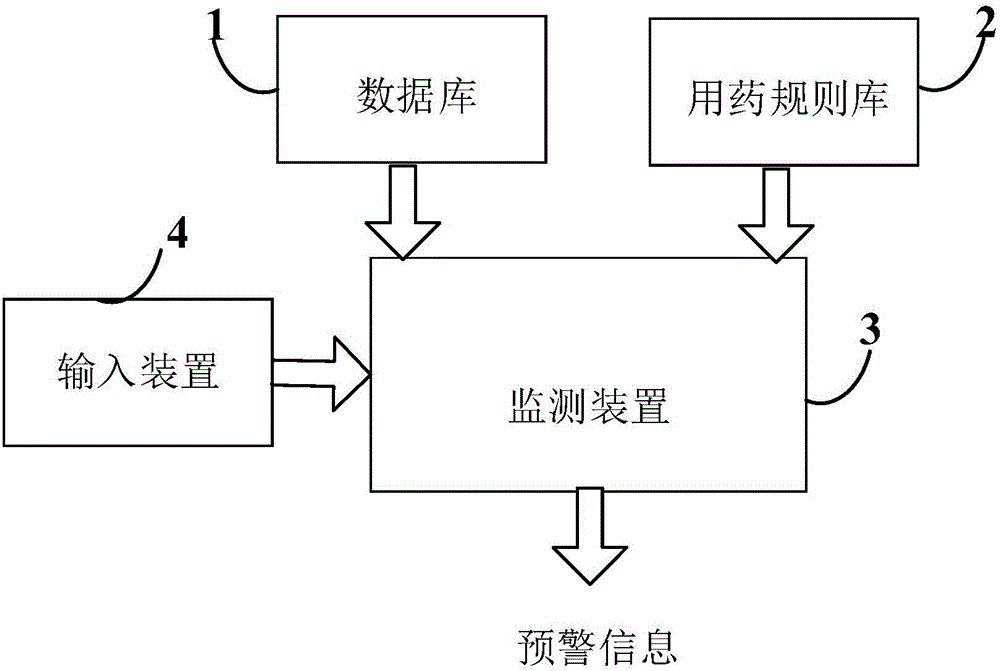
Safe medication monitoring system and monitoring method thereof
InactiveCN106126935AEnsure medication safetyImprove work efficiencyComputer-assisted medicine prescription/deliverySpecial data processing applicationsDiseaseMedication information
The invention provides a safe medication monitoring system and a monitoring method thereof. The system comprises: a database, a medication rule base, and a monitoring device. The database is used to store data information related to past medical history of a to-be-examined patient. The data information comprises: basic information of the to-be-examined patient, and disease information, examination results, medication information, allergic history, and treatment information of the patient in past medical history. The medication rule base is used to store safe medication rules. The monitoring device determines whether medicine in a medication prescription is safe for the to-be-examined patient according to the database and the data stored in the medication rule base, and generates early warning information when the medicine is determined to be not safe. The system and the method monitor medicine in a given medication prescription in time, so as to ensure safe medication.
Owner:BEIJING QUALITY & ZEAL INFORMATION TECH CO LTD
Butyl phthalide raw material drug product and preparation method thereof
ActiveCN105130934AGuaranteed clinical efficacyEnsure medication safetyOrganic active ingredientsNervous disorderBiomedical engineeringClinical efficacy
The present invention provides a butyl phthalide raw material drug product with the butyl phthalide content not less than 99.0%; the raw material drug is stable in quality, and can ensure the clinical curative effect and drug safety of a butyl phthalide preparation.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
Ibuprofen injection and preparation method thereof
InactiveCN102085179ALow priceImprove securityOrganic active ingredientsAntipyreticSodium bicarbonateSodium acetate
The invention relates to an ibuprofen injection, which comprises ibuprofen and one or a plurality of alkaline cosolvents pharmaceutically acceptable selected from anhydrides or hydrates of sodium carbonate, sodium bicarbonate,sodium citrate, sodium citrate, sodium phosphate, disodium dihydrogen pyrophosphate, sodium tartrate and sodium acetate. The invention also relates to a preparation method of the ibuprofen injection.
Owner:罗军
Recombinant melittin and application thereof
InactiveCN102229664AGood antibacterial effectNo toxicityAntibacterial agentsPeptide/protein ingredientsDiseaseFallopian Tube Diseases
The invention discloses a recombinant melittin and an application thereof, and belongs to the technical field of genetic engineering. An amino acid sequence of the recombinant melittin protein is shown in the formula SEQ ID NO.1. The recombinant melittin protein sequence can be utilized for a preparation of gene medicines utilized for preventing and treating fallopian tubal diseases of breeding hens, in other words, the recombinant melittin protein sequence is connected with a carrier pVAX1 to form a recombinant melittin expression carrier. In addition, a CMV promoter of the carrier pVAX1 is replaced by a chicken ovalbumin gene end 5' regulatory sequence which is an ov sequence, thus the recombinant melittin can be expressed specifically in chicken fallopian tubal tissue. The recombinant melittin and the gene medicines have the advantages of good effects of treating fallopian tubal diseases of breeding hens, safety, no toxicity and broad application prospect.
Owner:SOUTH CHINA AGRI UNIV
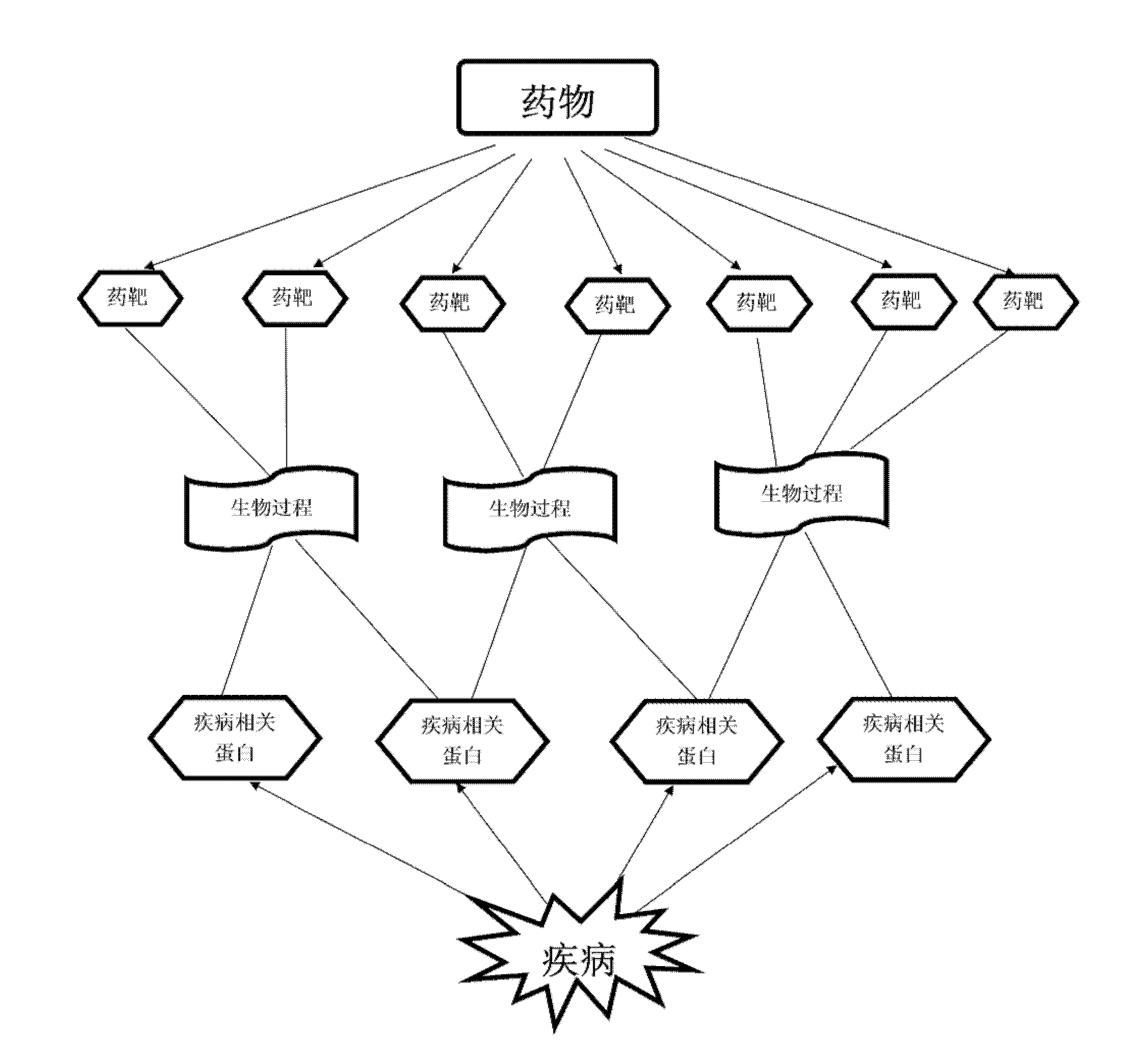
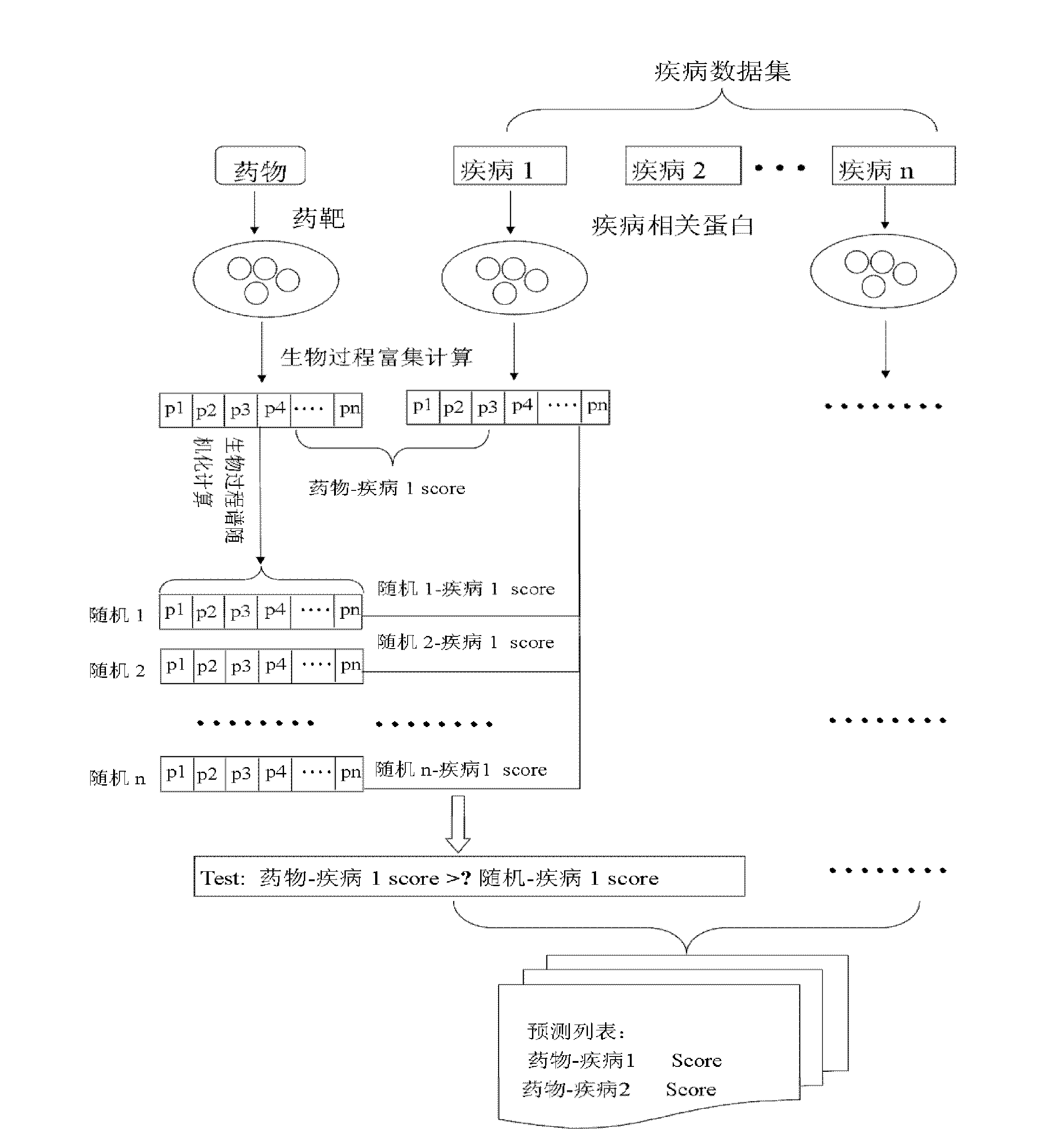
Method for predicating novel curative effects of medicament based on biological process and application of method
InactiveCN102507883AFunctional Space ExpansionEnsure medication safetyTesting medicinal preparationsDrugSide effect
The invention relates to a method for predicating novel curative effects of a medicament based on a biological process, which is disease-oriented and used to predicate a potential novel treating function of the medicament based on a medicament-disease relevance of a biological process spectrum. The method comprises the following steps of: (1) constructing a disease biological process spectrum and a medicament biological process spectrum; (2) calculating medicament-disease relevance scores on the layer of the biological process spectrum; (3) judging the significance of the relevance scores; and (4) screening a medicament-disease relation with the significance and carrying out further experimental demonstration on functions of the medicament by a user according to a sequencing result of the relevance scores. The invention further relates to an application of the method to the predication of the novel curative effects of the medicament. The predication method provided by the invention is ingenious in design, can be used for more comprehensively evaluating the medicament-disease relation, has important meanings of looking for novel medicaments for treating diseases, finding potential toxic or side effects of the medicaments, expanding a treating function space of the traditional medicaments, developing new uses for approved medicaments and the like, and is suitable for being popularized and applied on a large scale.
Owner:SHANGHAI CENT FOR BIOINFORMATION TECH
Azacitidine freeze-drying powder injection and preparation method thereof
InactiveCN101632643ANot prone to oxidationLong storage timePowder deliveryOrganic active ingredientsVitamin CMANNITOL/SORBITOL
The invention discloses a medicinal azacitidine freeze-drying powder injection for treating myelodysplastic syndrome and a preparation method thereof. The prescription of the azacitidine freeze-drying powder injection comprises azacitidine, mannitol and vitamin C. The invention solves the problem of rapid impurity increase caused by different crystal forms formed in the processes of rapidly freezing and drying the prior powder injection by optimizing the prescription and improving the preparation method.
Owner:HANGZHOU XIANDA MEDICINE TECH
Method for preparing deproteinized extract of calf blood and freeze-dried powder thereof
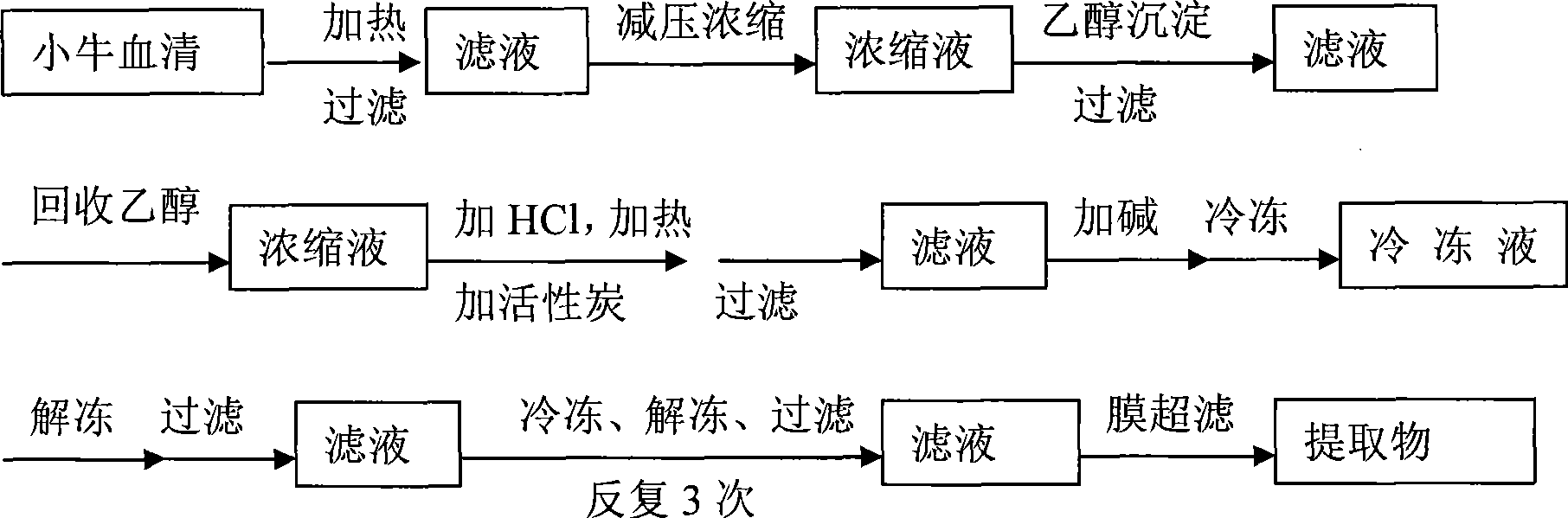
ActiveCN101433553AHigh activityThe process is simple and convenientPowder deliveryDigestive systemFreeze-dryingCosmetic appearance
The invention aims to provide a method for preparing deproteinized calf blood extract and freeze-dried powder thereof. The method has simple process and is easy for mass production, the obtained extract has high bioactivity and good product purity, the freeze-dried powder is subjected to gradient temperature rise and drying, and the finished product has good appearance and is easy to dissolve. The deproteinized calf blood extract is prepared by the method comprising the following steps: heating collected blood serum and deproteinizing the blood serum, filtering the blood serum and then condensing the filtrate, removing protein by ethanol; then adding active carbon and acid into the filtrate to hydrolyze and decolor the filtrate; filtering the filtrate and then adjusting pH value to a proper range, freezing the filtrate and removing protein repeatedly; and finally using fibrous membrane to hyperfiltrate high molecular substance to obtain the deproteinized calf blood extract, then adding excipient and water for injection into the extract, pre-freezing the extract, and heating the extract in gradient and drying the extract in vacuum to obtain finished products.
Owner:吉林康乃尔药业有限公司
Preparation of cefixime cephalosporin and fine purification method
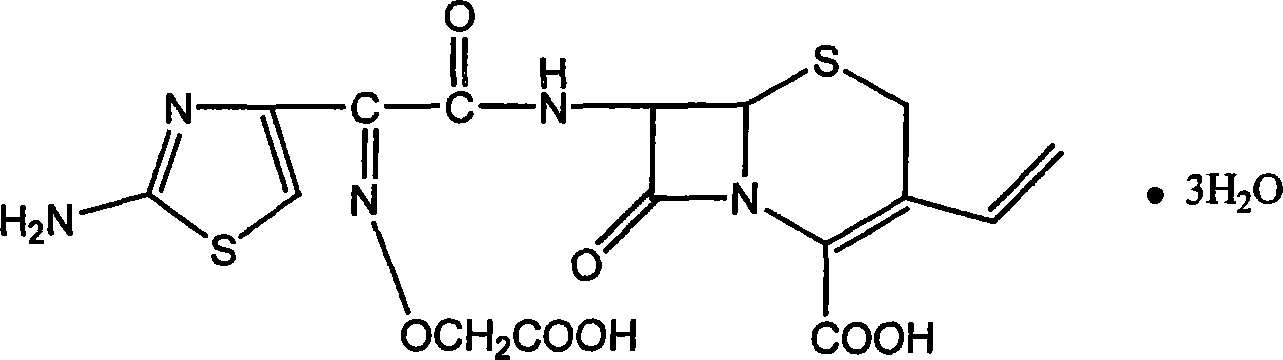
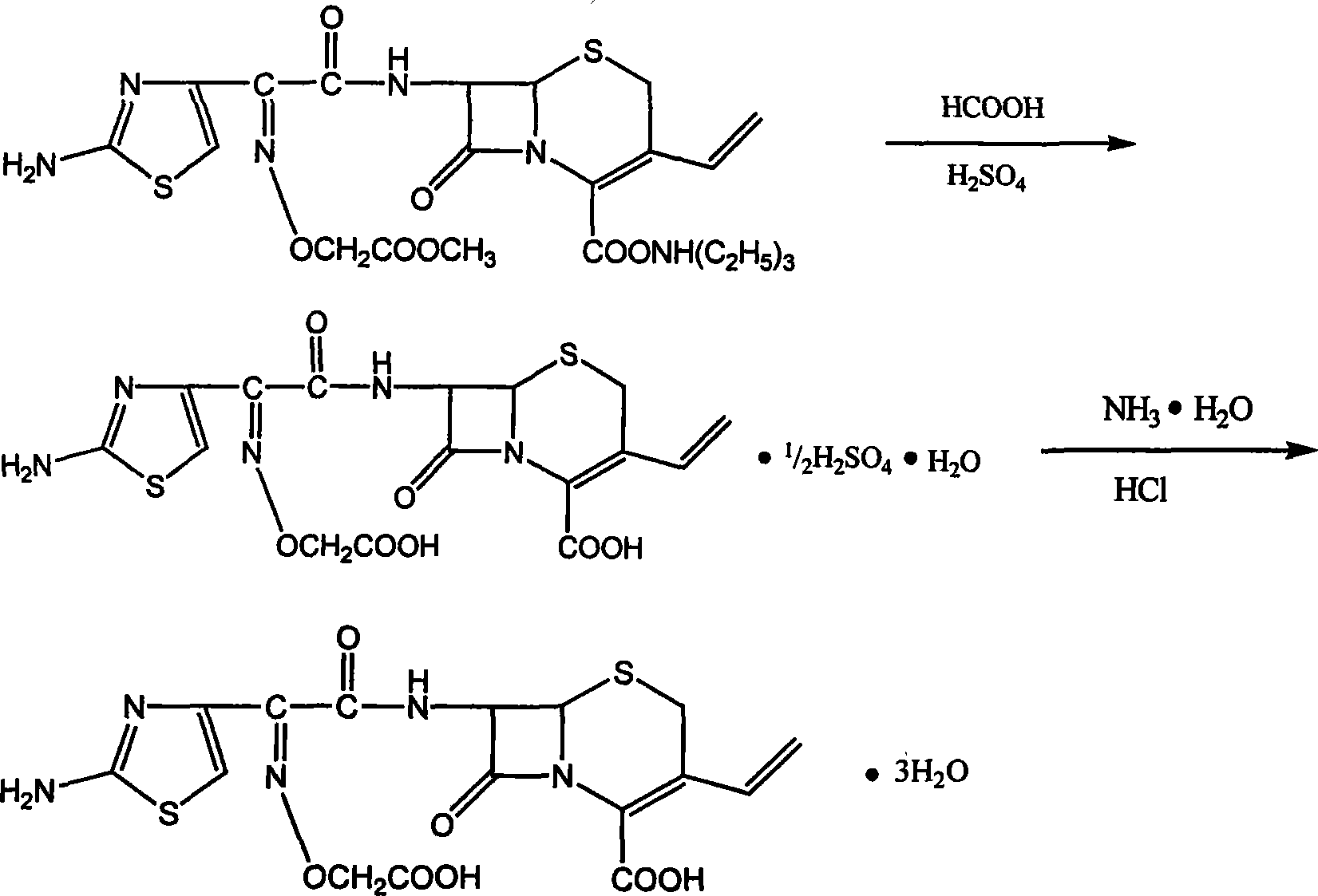
InactiveCN101220040AImprove product qualityEasy to operateOrganic chemistryIce waterPurification methods
The invention relates to a preparation and refining method for cefixime, which is conducted as in the following technical steps: the first step, cefixime methyl triethyl amine salt is dissolved in an organic solvent, filtered and washed after reaction with formic acid anhydrous and 98 percent of concentrated sulfuric acid below 0 DEG C, and dried in vacuum at 50 DEG C to obtain cefixime monohydrate monosulphate; the second step, the cefixime monohydrate monosulphate is dissolved in deionized water below 0 DEG C, the pH value is adjusted by ammonia, after being discolored and filtrated by activated carbon, the filtrate is added with ethanol and then 4mol / L of hydrochloride is used for adjusting the pH value, and after filtering, washing by icing water and drying at 30 DEG C in vacuum, cefixime trihydrate acid products are obtained. The method has the advantages of simple operation, little pollution, low manufacturing cost, good quality of prepared cefixime, great convenience for industrialization manufacturing and substantial economic benefits. Simultaneously, as the product of cefixime is presented as white powder with content over 98 percent, guarantees are provided for safe medication in the quality of medicines.
Owner:四川方向海瑞实业有限责任公司
Medicine composition containing ceftin cyclodextrin clathrate, and its preparing method
InactiveCN101002782AImprove water solubilityEasy to dissolveAntibacterial agentsOrganic active ingredientsSolubilityAlpha-Cyclodextrin
An inclusion compound of cefuroxime axetil with high solubility, stability and activity contains cefuroxime axetil and the pharmacologically acceptable cyclodextrin chosen from alpha-cyclodextrin, beta-cyclodextrin, gamma-cyclodextrin and their derivatives. Its preparing process is also disclosed.
Owner:海南和德通医药科技信息咨询有限公司
Gene medicine conveying system and method of preparing the same
InactiveCN101138636AAvoid damageHigh encapsulation efficiencyPowder deliveryGenetic material ingredientsAdjuvantAdditive ingredient
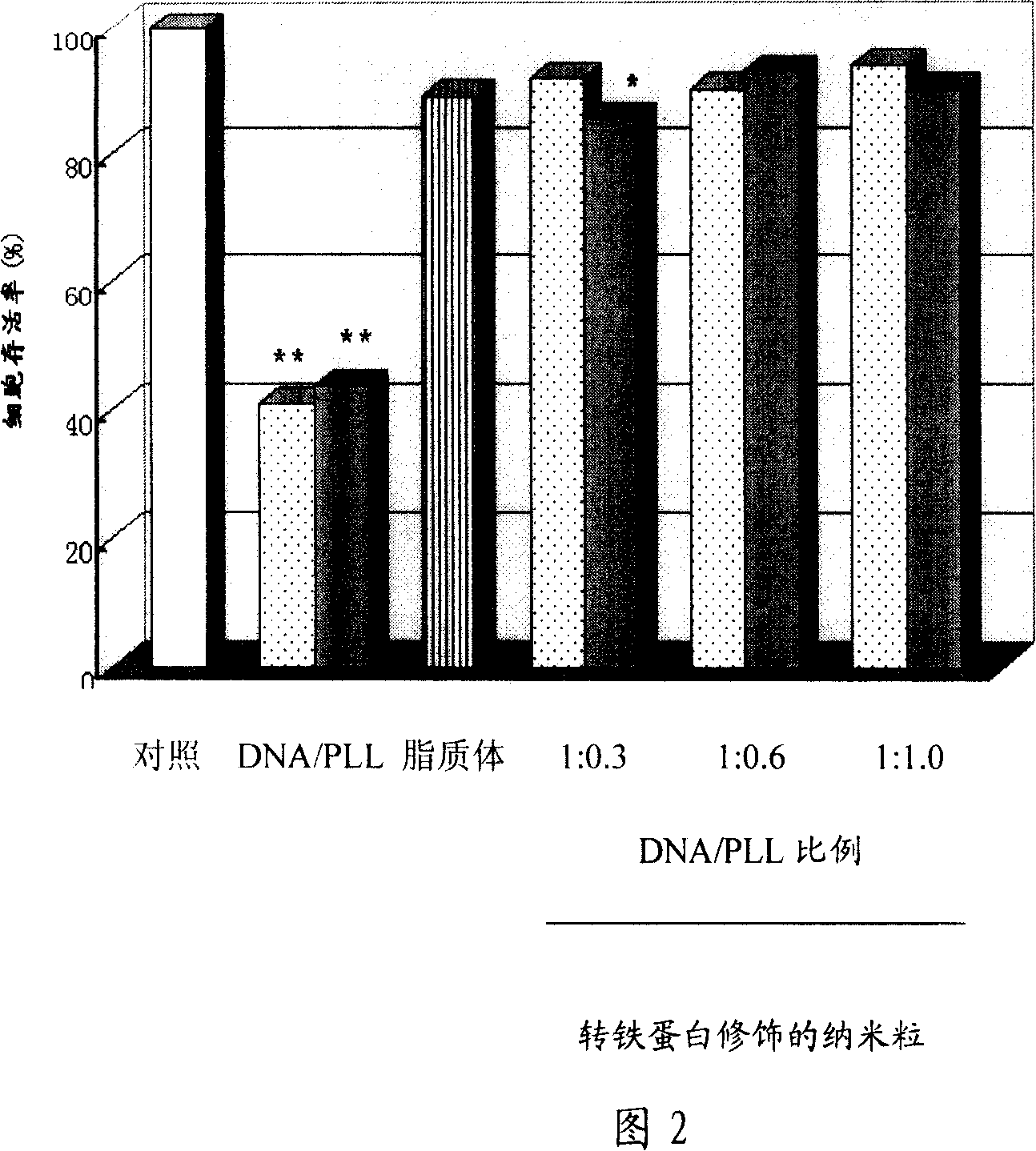
The present invention relates to a transportation system for the genetic medicine and the preparation method. The transportation system is a composite consisting of the genetic medicine, the cationic peptide or the polymer and any selected adjuvant ingredient. The composite is packaged in a nano particle of polymer material modified by the PEG. The ligand modification is conducted in the nano particle surface. The preparation method comprises the following steps. Step one, the genetic medicine, the cationic peptide or the polymer and any selected adjuvant ingredient are mixed in order to prepare the composite solution; step two, the organic solution of the polymer material is added into the composite solution and dispersed into the water phase, therefore the W / O / W-typed multiple emulsion is prepared, followed by separation of the collected nano particles; step three, the nano particle surface modified by the ligand and the collected nano particles are separated after modification. The stability of the genetic medicine in the preparation process and the encapsulation efficiency are both improved in the system. The surface modification not only prolongs the plasma half-life and improves the targeting property in the transportation system, but also enhances the transfection efficiency and greatly reduces the cytotoxicity in the transportation system, which therefore guarantees the safety of the medicine application.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Headspace gas chromatography method for determining residual quantity of organic solvent in glycine bulk drug
InactiveCN108614058AEffective quality controlEasy to operateComponent separationNitrogen gasCapillary column
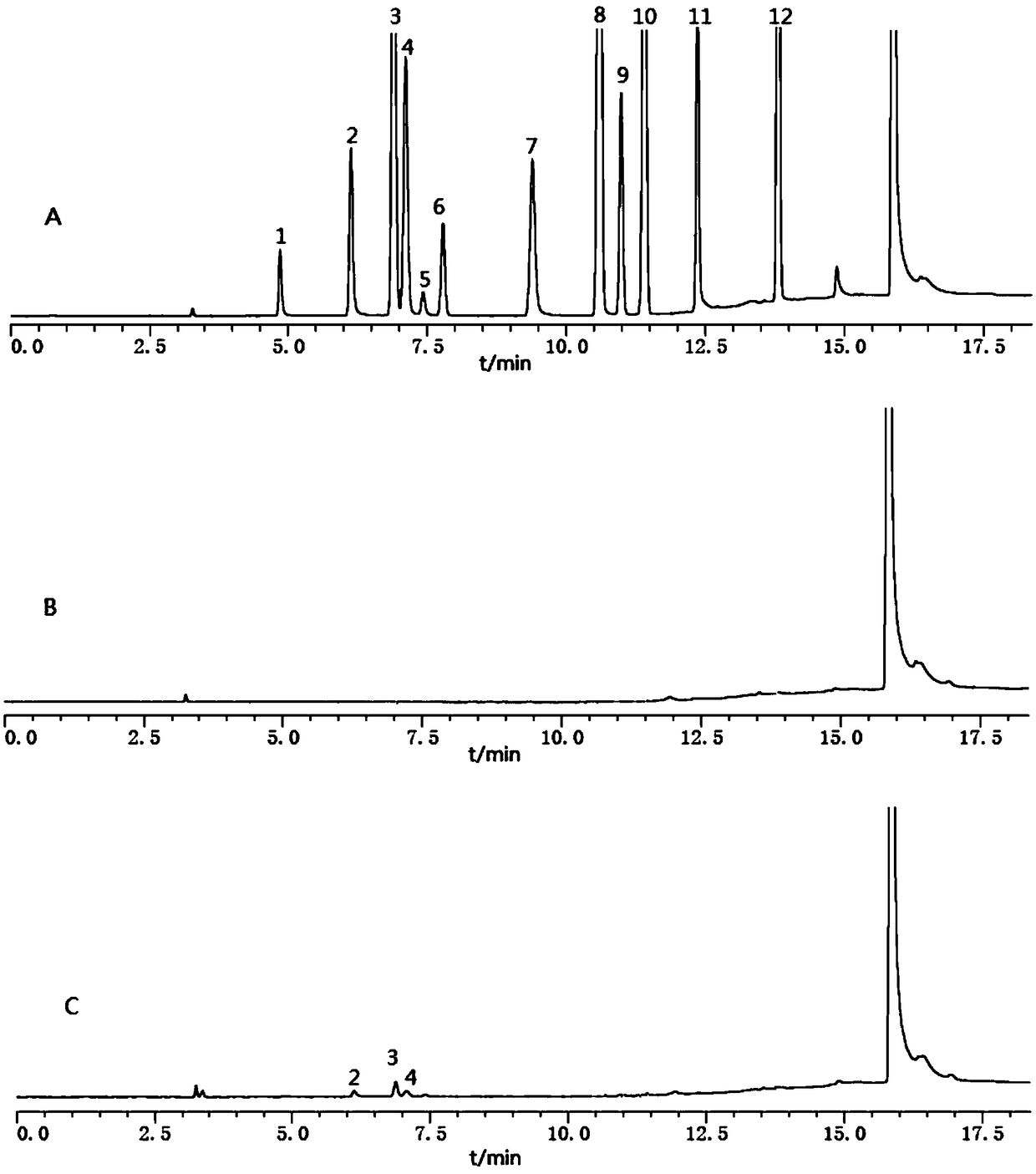
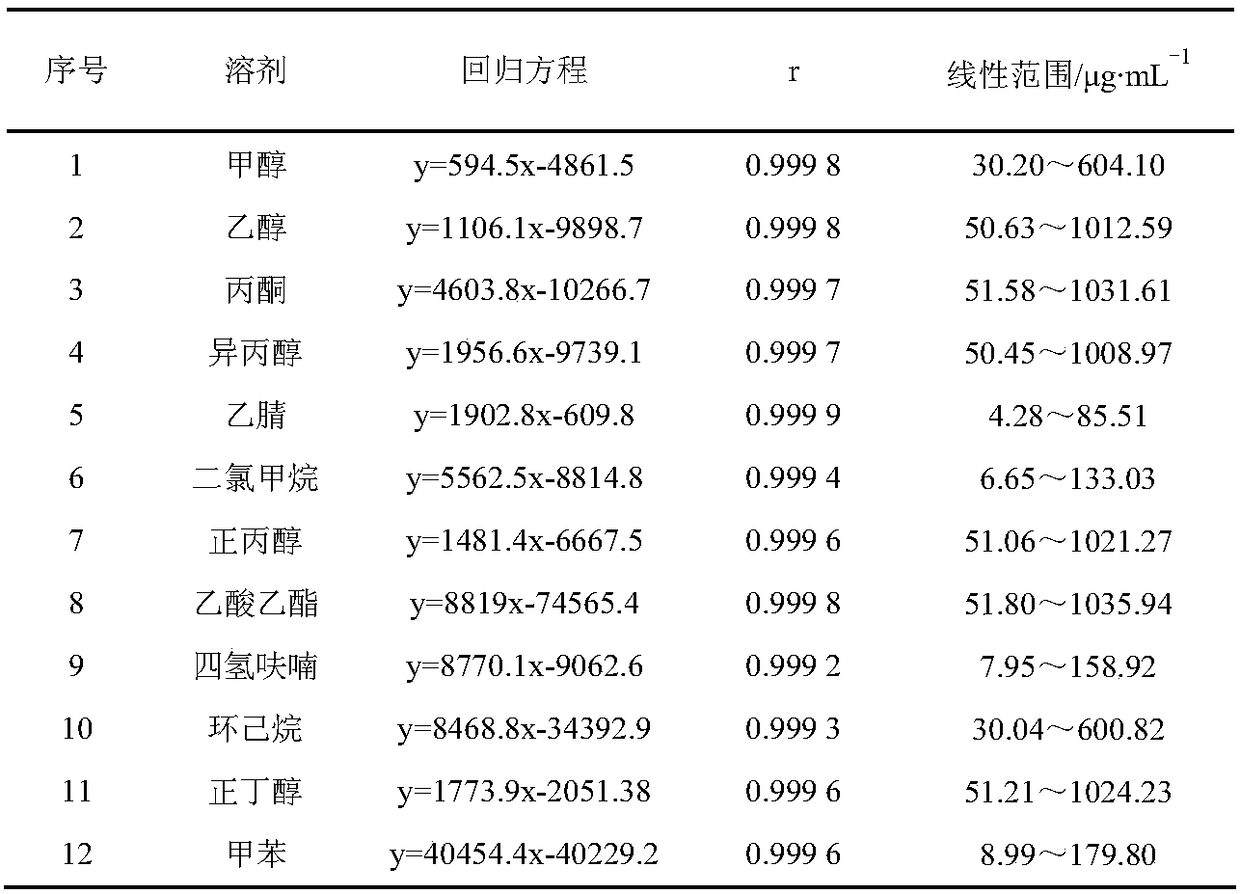
The invention discloses a headspace gas chromatography method for determining the residual quantity of an organic solvent in a glycine bulk drug. The method is implemented under conditions that a DB-624 capillary column and a hydrogen flame ionization detector are used, sample inlet temperature is 150 DEG C, the detector temperature is 250 DEG C, high purity nitrogen is taken as carrier gas with flow rate being 2.2 mL / min, the split ratio is 10:1, headspace heating temperature is 80 DEG C, and equilibrium time is 35 min; besides, glycine is easily soluble in water and the 12 solvents are soluble in water to a certain degree, so that an aqueous solvent is adopted. The method can be used for simultaneously determining the residual quantity of the 12 organic solvents including methanol, ethanol, acetone, isopropanol, acetonitrile, dichloromethane, n-propanol, ethyl acetate, tetrahydrofuran, cyclohexane, n-butanol and toluene in the glycine bulk drug, has the characteristics of being easyto operate, quick, sensitive and accurate in result, and provides reference for effectively controlling the quality of the glycine raw material and ensuring medication safety.
Owner:广西壮族自治区食品药品检验所
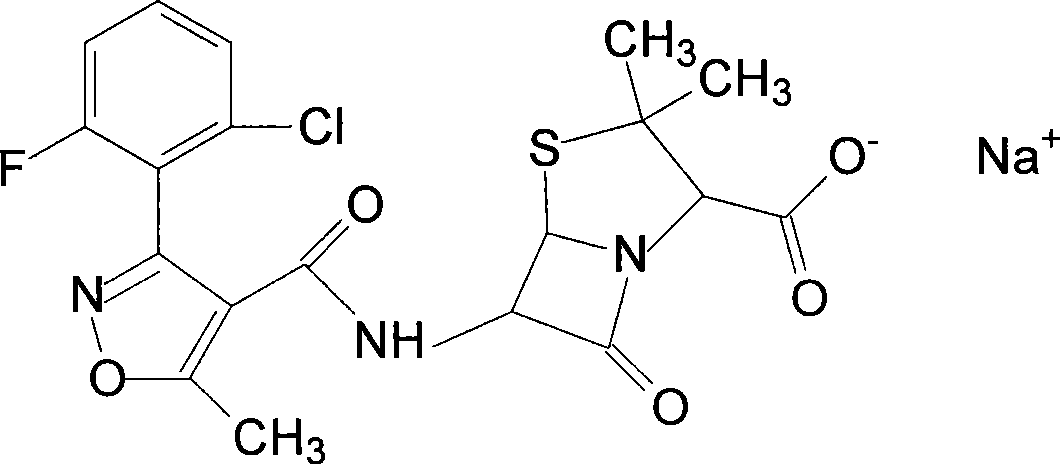
Flucloxacillin sodium compound and preparation thereof
InactiveCN101475578AHigh purityImprove product qualityAntibacterial agentsOrganic chemistryPurification methodsFlucloxacillin sodium
The invention discloses a flucloxacillin sodium compound and a method for preparing the same. The method comprises that: firstly, N'N-dibenzylethylene diamine salt and flucloxacillin acid are mixed and reacted to form a salt; and secondly, high-purity flucloxacillin sodium is obtained through the displacement of a cation exchange resin. The invention adopts a purification method which is simple and easy to operate, thereby greatly improving the purity of the flucloxacillin sodium compound to a certain degree, removing a large amount of high molecular polymers, stabilizing the product quality of related preparations and ensuring the safety of clinic medication use.
Owner:海南本创医药科技有限公司
Electronic prescription circulation method, server and system
InactiveCN107958694AEnsure medication safetyEasy access to medicineDrug and medicationsMedical educationMedication information
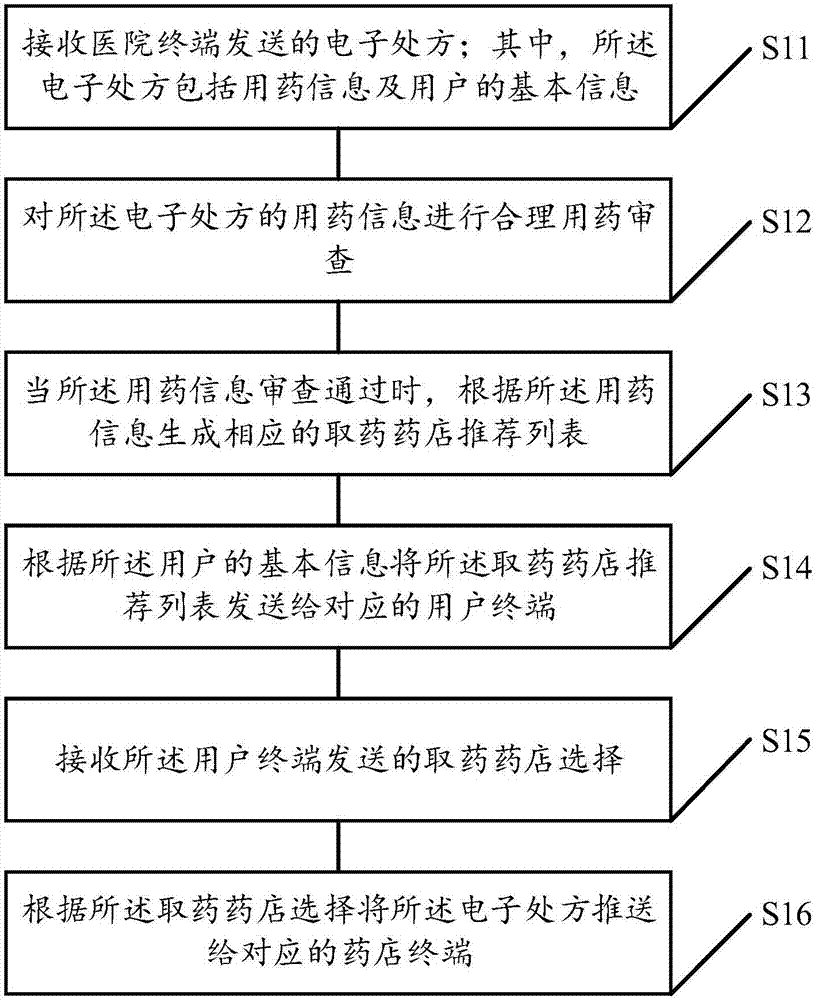
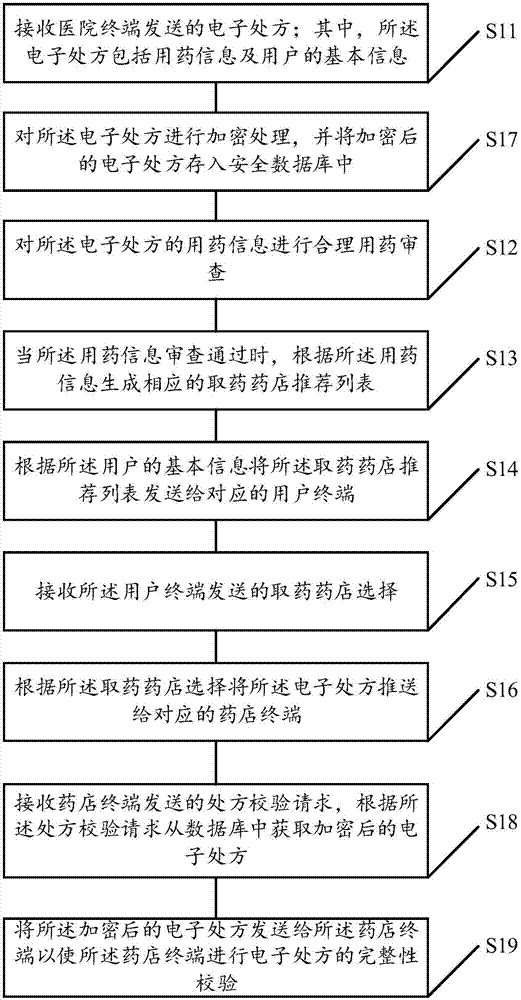
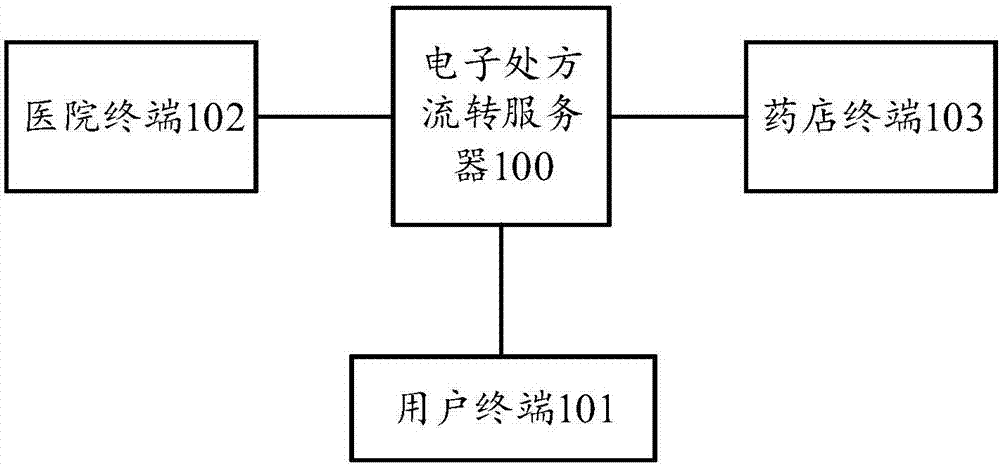
The invention discloses an electronic prescription circulation method which comprises a step of receiving an electronic prescription sent by a hospital terminal, wherein the electronic prescription comprises medication information and basic information of a user, a step of carrying out reasonable medication examination on the medication information of the electronic prescription, a step of generating a corresponding drug taking pharmacy recommendation list according to the medication information when the medication information examination is approved, a step of sending the drug taking pharmacyrecommendation list to a corresponding user terminal according to the basic information of the user, a step of receiving drug taking pharmacy selection sent by the user terminal, and a step of pushing the electronic prescription to a corresponding pharmacy terminal according to the drug taking pharmacy selection. The invention also provides an electronic prescription circulation server and system, the electronization of a medical prescription is realized, the user can conveniently view the prescription in real time and carry and store the prescription, intelligent pharmacy recommendation is achieved, and the user can conveniently take medicines.
Owner:广东易健通信息科技有限公司
Medicine recommendation method and equipment
PendingCN110289068AEnsure medication safetyImprove medication experienceDrug and medicationsDrug referencesDiseaseMedicine allergy
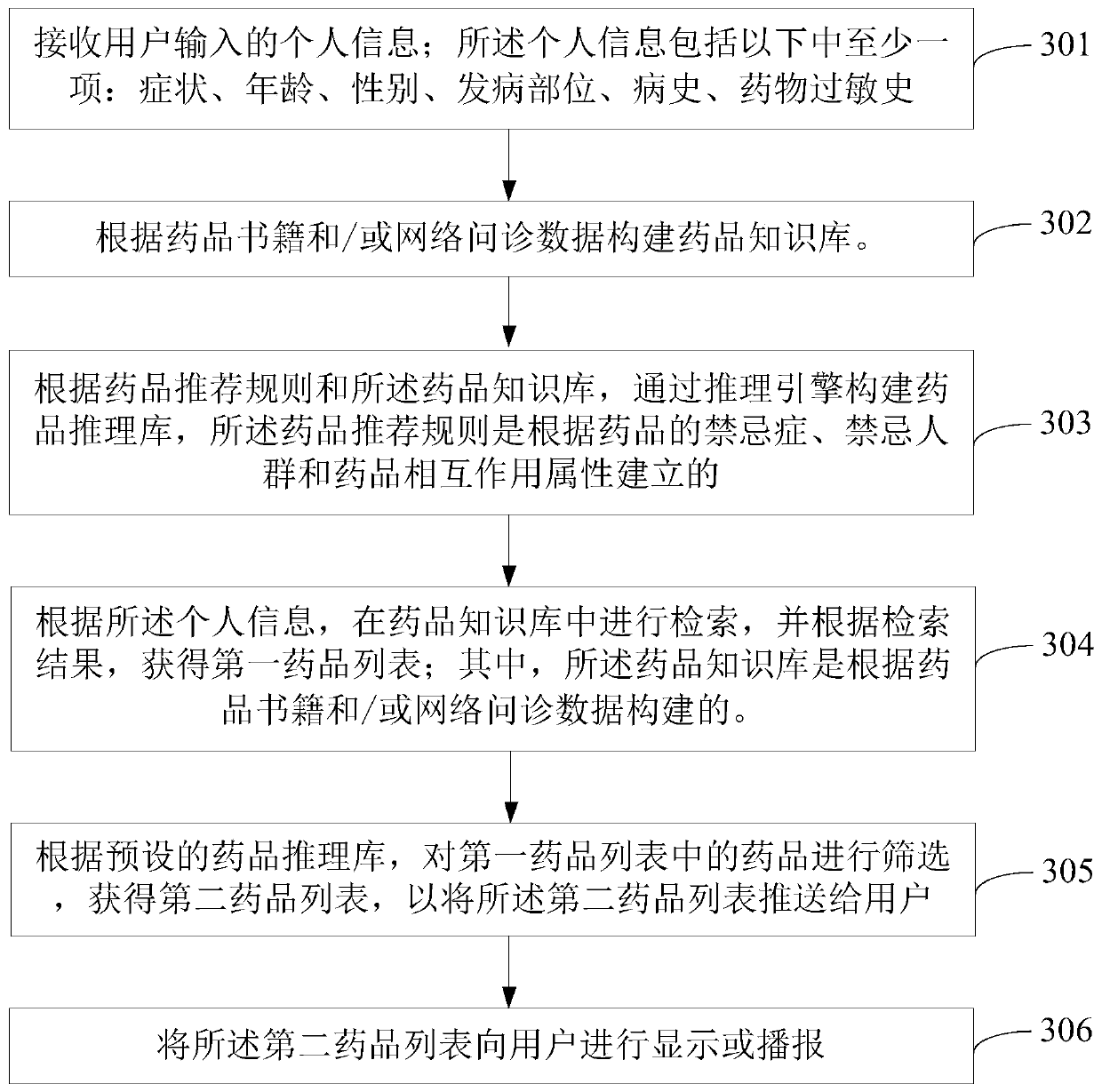
The embodiment of the invention provides a medicine recommendation method and device. The method comprises the steps of receiving personal information input by a user, wherein the personal information comprises at least one of symptoms, ages, genders, disease sites, medical history and medicine allergy history; searching a medicine knowledge base according to the personal information, and obtaining a first medicine list according to a search result, wherein the medicine knowledge base is constructed according to medicine books and / or network inquiry data; screening the medicines in the first medicine list according to a preset medicine inference library to obtain a second medicine list, so as to push the second medicine list to the user, wherein the medicine inference library is constructed through an inference engine according to medicine recommendation rules and the medicine knowledge base. According to the embodiment of the invention, proper medicines can be accurately recommended to the user on the premise of ensuring the medicine use safety of the user, so that the medicine use experience of the user is improved.
Owner:BEIJING BAIDU NETCOM SCI & TECH CO LTD
Butylphthalide medicine active composition and preparation method of butylphthalide medicine active composition
ActiveCN102716121AComply with medicinal requirementsQuality improvementOrganic active ingredientsOrganic chemistryButylphthalidePharmaceutical drug
The invention provides a butylphthalide medicine active composition, which comprises the following ingredients: first ingredients: the butylphthalide content is higher than or equal to 98.0 percent; second ingredients: the second ingredients are one kind of materials or several kinds of materials selected from methylene phthalide, ethylene phthalide, propylene phthalide, butane phthalide, amylene phthalide, phthalide, methyl phthalide, ethyl phthalide, propyl phthalide and amyl phthalide, in addition, the content of the second ingredients is higher than 0 but is lower than or equal to 2.0 percent, when the second ingredients comprise any one kind of materials from methylene phthalide, ethylene phthalide, propylene phthalide, butane phthalide and amylene phthalide, the content of any one kind of included ingredients does not exceed 0.5 percent, and when the second ingredients comprise any one kind of materials from phthalide, methyl phthalide, ethyl phthalide, propyl phthalide and amyl phthalide, the content of any one kind of included ingredients does not exceed 1.0 percent. The quality of the medicine active composition is stable, and the clinic curative effect and the medication safety of the butylphthalide preparation can be ensured.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Method of preparing total flavones of tropaeolum for injections
ActiveCN101234147AIn line with injection standardsNo pollution in the processAntibacterial agentsAntipyreticAdditive ingredientPolyamide
The invention discloses a preparation method of a Flos Trollii Flavonoid for injection. The extract of the Flos Trollii Flavonoid substantially comprises orientoside, vitexin, etc., flavone ingredients, the flavonoid content of which is more than 70 percent. The invention discloses the preparation method of the extract, which is mainly characterized in that the extract is acquired through refined treatment with twice column chromatography; the adsorption materials adopted are two among macroporous resin, polyamide or silica gel, reverse phase silica gel and gelatin and so on; the extract is prepared with modern technology of extraction, concentration and refining; the method is simple and easy to operate, improves the effective component purity of the raw medicinal materials and reduces the dosage and adverse reactions.
Owner:上海中创医药科技有限公司
Preparation method and detection method of roflumilast material
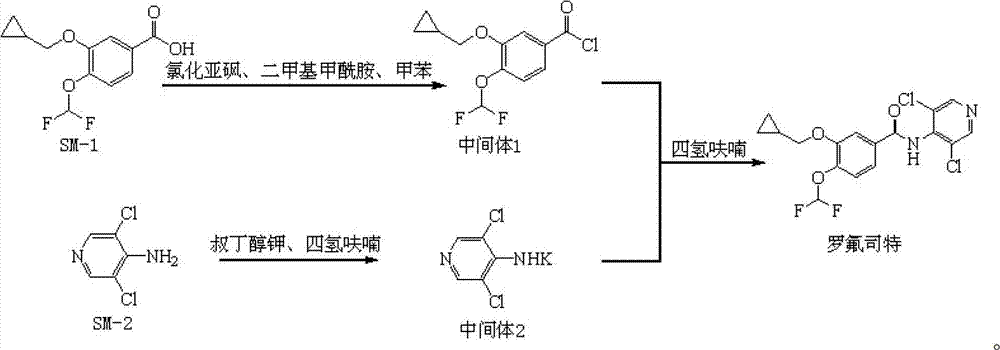
ActiveCN102964297ASimple preparation processReduce adverse reactionsOrganic chemistryMaterial analysis by observing effect on chemical indicatorBenzoic acidDisease
The invention discloses a preparation method and a detection method of a roflumilast material. The preparation method comprises the following steps: mixing 3-cyclopropyl methoxy group-4-difluoro methoxy group benzoic acid SM-1, thionyl chloride, dimethyl formamide with toluene, and carrying out an acylating chlorination reaction to obtain a midbody 1; mixing 3,5-dichloro-4-aminopyridine SM-2, tetrahydrofuran with potassium tert-butoxide and carrying out a salt forming reaction to obtain tetrahydrofuran solution of a midbody 2; and then mixing the midbody 1 and the midbody 2 with tetrahydrofuran, carrying out amidation to obtain a crude product of roflumilast, and refining the crude product of roflumilast to prepare the roflumilast material. Aiming to overcome the shortage of the prior art, the preparation process of the roflumilast material is optimized, so that the curative effect for treating diseases such as chronic obstructive pulmonary disease (COPD) is more remarkable; and besides, a systematic, complete and effective composition identifying and content measuring method is provided, so that the quality of the medicine can be effectively controlled, and the clinical effect is ensured.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Open-throat sword spray used for treating throat diseases and preparation method thereof
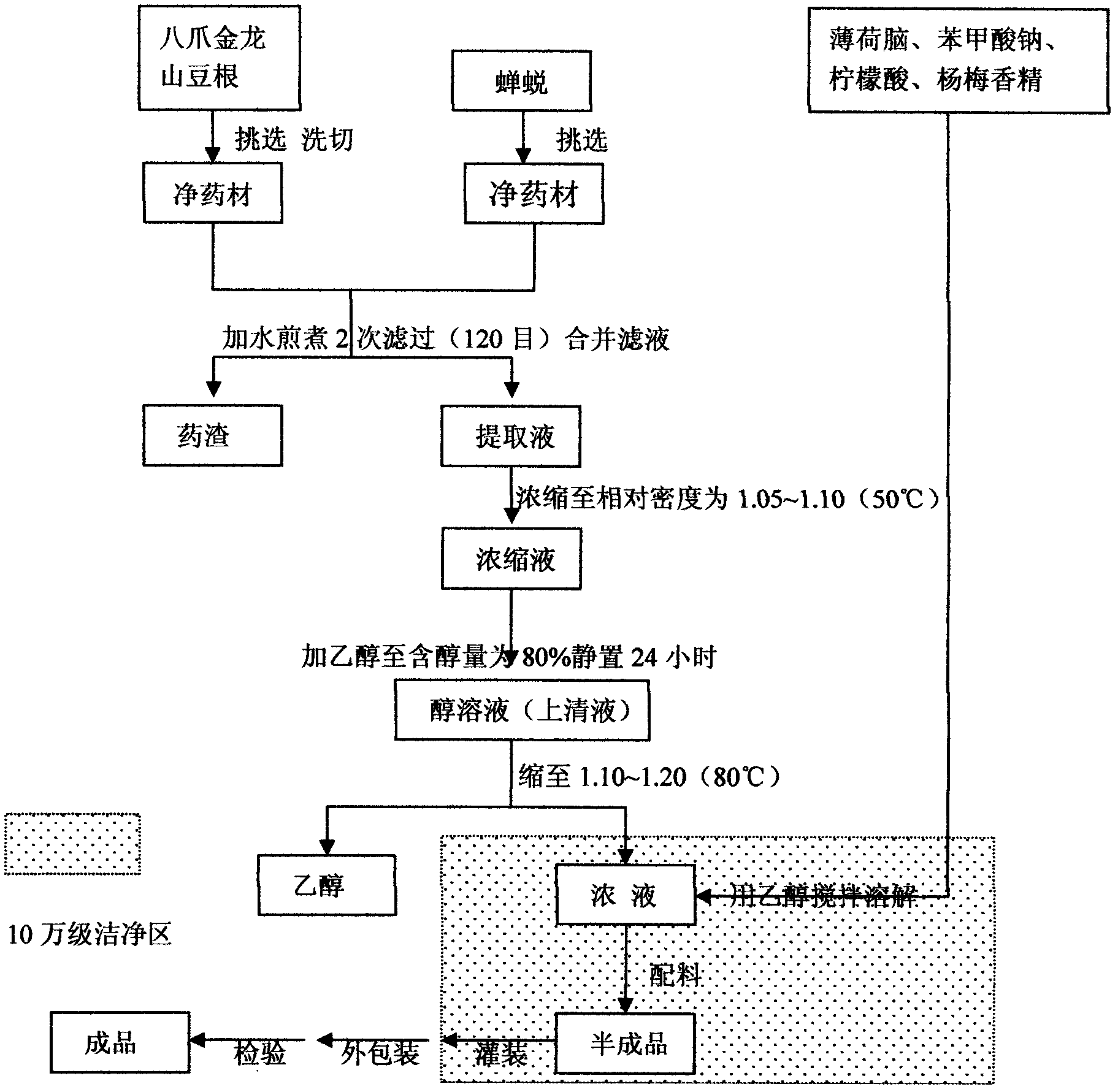
InactiveCN103040927AIncrease productionOptimal soaking timeAnthropod material medical ingredientsAerosol deliveryDrying mouthOral ulcers
The invention relates to an open-throat sword spray used for treating throat diseases and a preparation method thereof, and relates to a drug which has the effects of clearing heat and removing toxicity and relieve swelling and pains, and is used for treating diseases such as acute laryngopharyngitis, chronic laryngopharyngitis, amygdalitis, swollen sore throat, stomatitis, dental ulcer, dry mouth and bitter mouth, swollen gums, recurrent oral ulcer and the like, and particularly relates to an oral preparation prepared by using four traditional Chinese medicinal materials containing ardisia crenata sims, radix sophorae tonkinensis, cicada slough and menthol as raw materials. Compared with a conventional method, the method has optimized extraction process parameters, increased transfer rate of active ingredients and increased yield. Besides, the method has more stable and reliable quality, better effect curative effects and better security.
Owner:贵州三力制药股份有限公司
Medicine composition containing cefateram cyclodextrin capsule and its preparing method
InactiveCN100998595AImprove water solubilityEasy to dissolveAntibacterial agentsOrganic active ingredientsDrugIrritation
A composite medicine with high solubility, stability and activity and low hemolytic irritation contains cefteram ester and the pharmacologically acceptable dextrin chosen from beta-cyclodextrin and its derivatives. Its preparing process is also disclosed.
Owner:海南和德通医药科技信息咨询有限公司
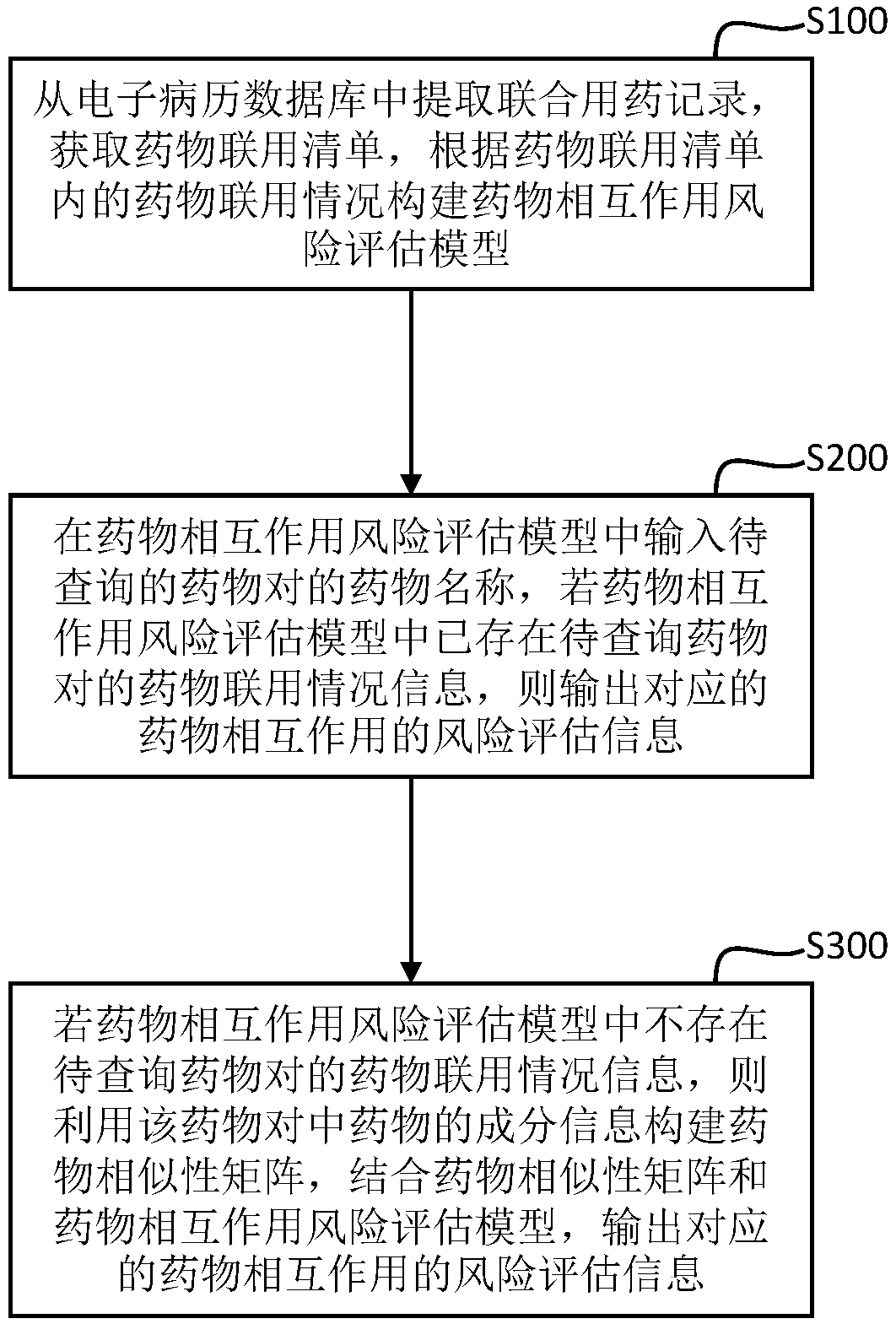
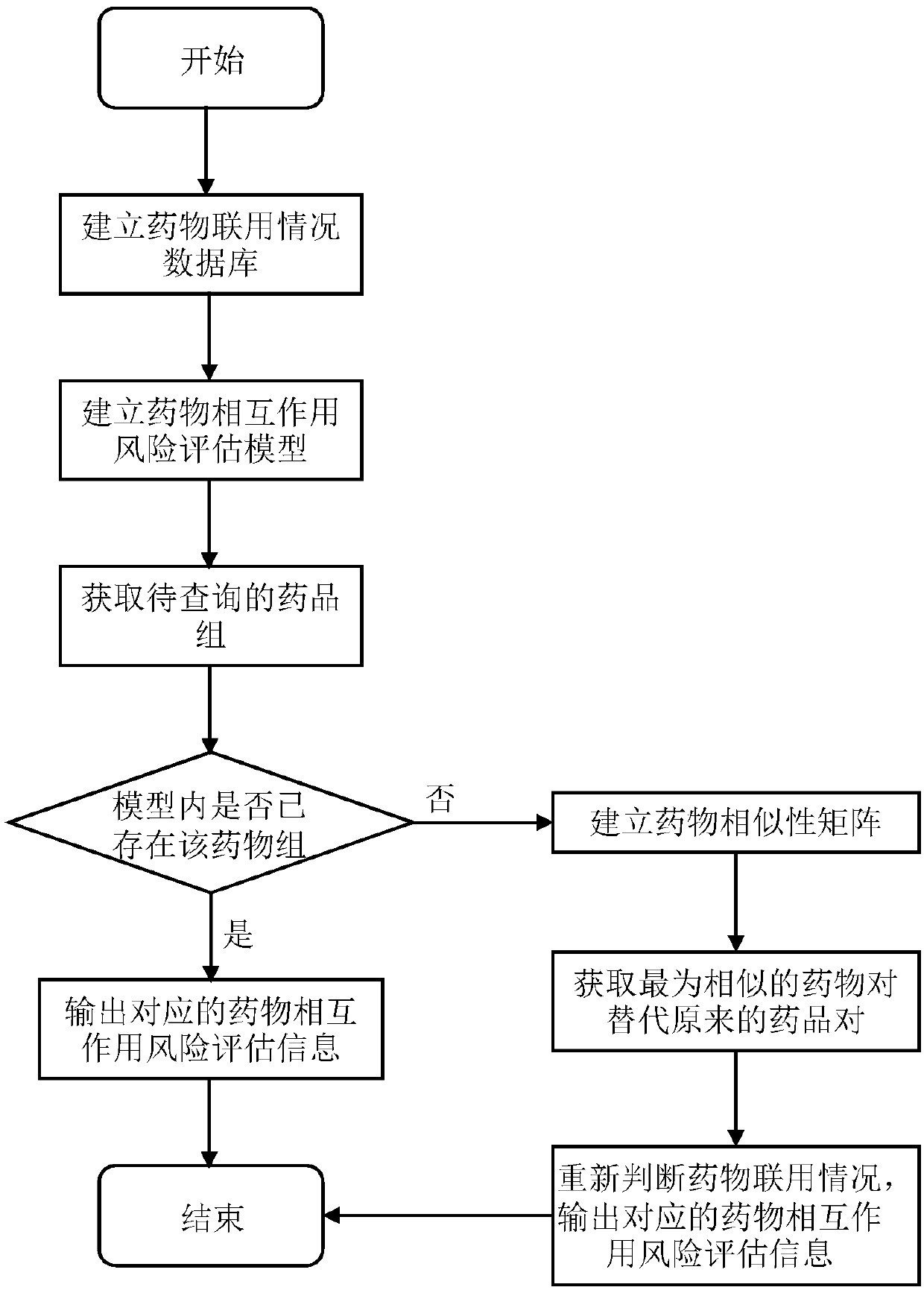
Drug interaction modeling and risk evaluation method, terminal equipment and storage medium
ActiveCN108630322ALow incidence of adverse reactionsEnsure medication safetyMedical data miningDrug referencesDrug groupMedical emergency
Owner:XIAMEN UNIV
Muscular amino acid and peptide nucleoside powder injection and its preparation method
InactiveCN1522757AImprove stabilityHigh preparation process yieldPowder deliveryPeptide/protein ingredientsMedicineFreeze-drying
The present invention relates to a sarcopeptidoglucoside powder injection and its preparation method. It is formed from polypeptide, hydroxanthine and excipient. Said invention adopts the modern biological extraction technique to prepare sarcopeptidoglucoside solution, and utilizes the modern low-temp. freeze-drying preparation technique to obtain the invented sterile freeze-dried powder injection. When it is used, it can be dissolved by injection water or infusion fluid, then can be injected. Said invention has good stability, long storage time and high safety, etc.
Owner:黑龙江江世药业有限公司
Composition containing clopidogrel bisulfate crystal particles
ActiveCN101851247ASolve the sticking problemAvoid mass generationOrganic active ingredientsOrganic chemistryCrystalline particleClopidogrel Bisulfate
The invention discloses clopidogrel bisulfate crystal particles with the median particle diameter of at least 120 microns. The invention also discloses a method for preparing the crystal particles, a pharmaceutical composition containing the crystal particles and a preparation method of the pharmaceutical composition.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Detection method for atractylodes macrocephala koidz medicinal materials
InactiveCN102680631ATrue reflection of qualityEnsure medication safetyComponent separationHplc fingerprintMedicine
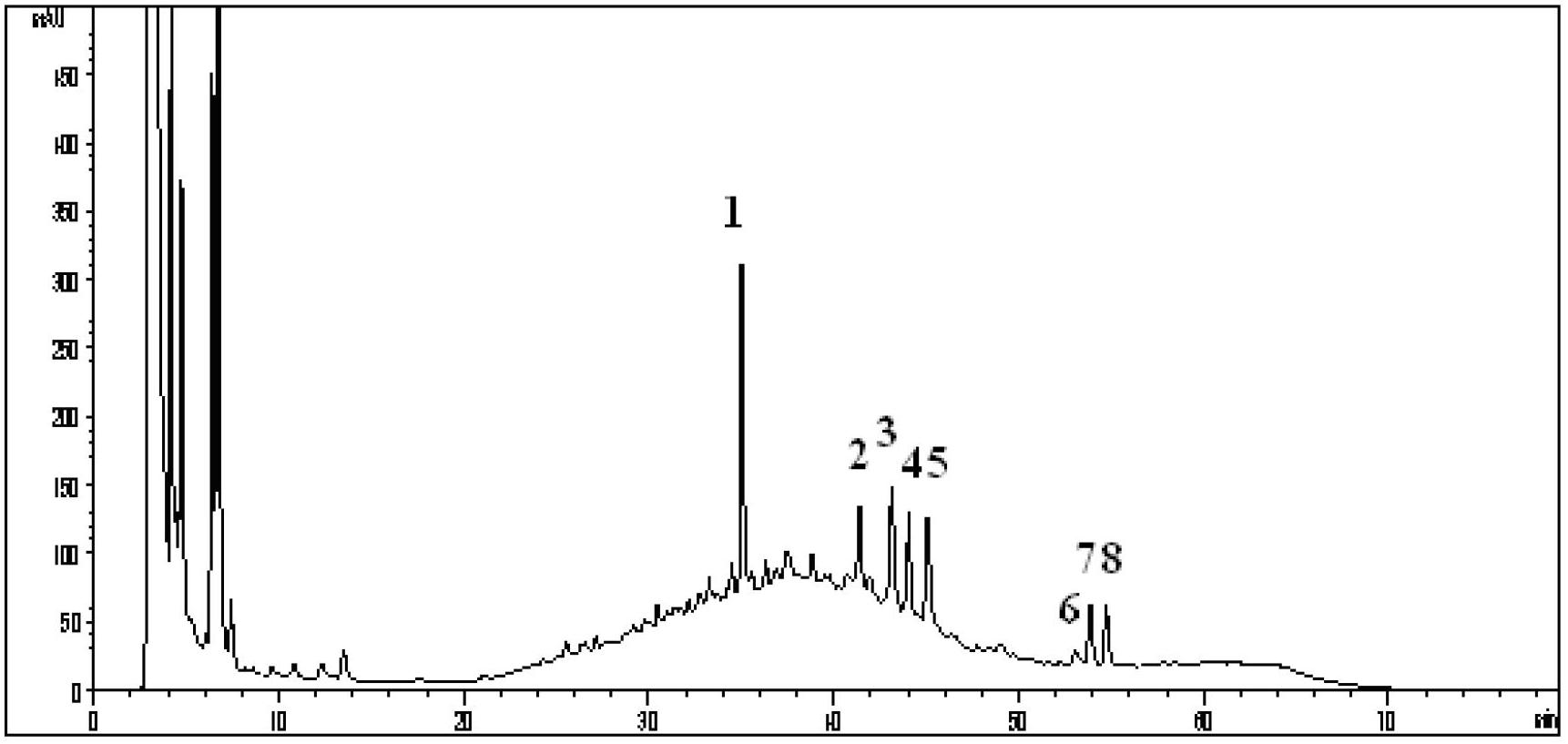
The invention discloses a detection method for atractylodes macrocephala koidz medicinal materials. The method adopts HPLC (high performance liquid chromatography) fingerprint spectrum for detection and includes the operation steps of: (1) preparing sample solution; (2) preparing reference solution; and (3) respectively and precisely absorbing the sample solution and the reference solution to inject into a liquid chromatograph so as to elute by taking an acetonitrile-water system as a flowing phase and detect at wavelengths of 248+ / -5nm. After the atractylodes macrocephala koidz medicinal materials are decocted with water, and then the HPLC fingerprint spectrum is applied for detection, so that quality of the atractylodes macrocephala koidz medicinal materials can be reflected more truly. Moreover, chromatographic conditions such as the flowing phase are selected specifically, so that chromatogram baselines are stable and convenient to integrate, resolution of characteristic peaks is good, and similarity among different medicinal materials is high. The detection method for the atractylodes macrocephala koidz medicinal materials can be effectively used for quality control of atractylodes macrocephala koidz and provides a guarantee of medication security of the atractylodes macrocephala koidz medicinal materials.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Preparation technology of sarcosine peptide glycoside injection liquid
InactiveCN1824294ARaise the sterilization temperatureGuaranteed absolute sterilityNervous disorderPeptide/protein ingredientsPhosphateDrug product
A process for preparing the injection of carnosine features that the chitosan and oligose are used for adsorbing the modified protein and fat to improve the purity of product, and the 6-aminopurine phosphate is used as the protector for increasing the sterilizing temp and time to 121 deg.C and 30 min, so having no bacterial pollution.
Owner:长春白求恩制药有限公司
Physician, nursing and pharmacy integrated omnibearing reasonable safety transfusion early-warning monitoring method
InactiveCN108777163AReduce unreasonable phenomenaEnsure medication safetyMedical communicationDrug and medicationsPharmacyMedical prescription
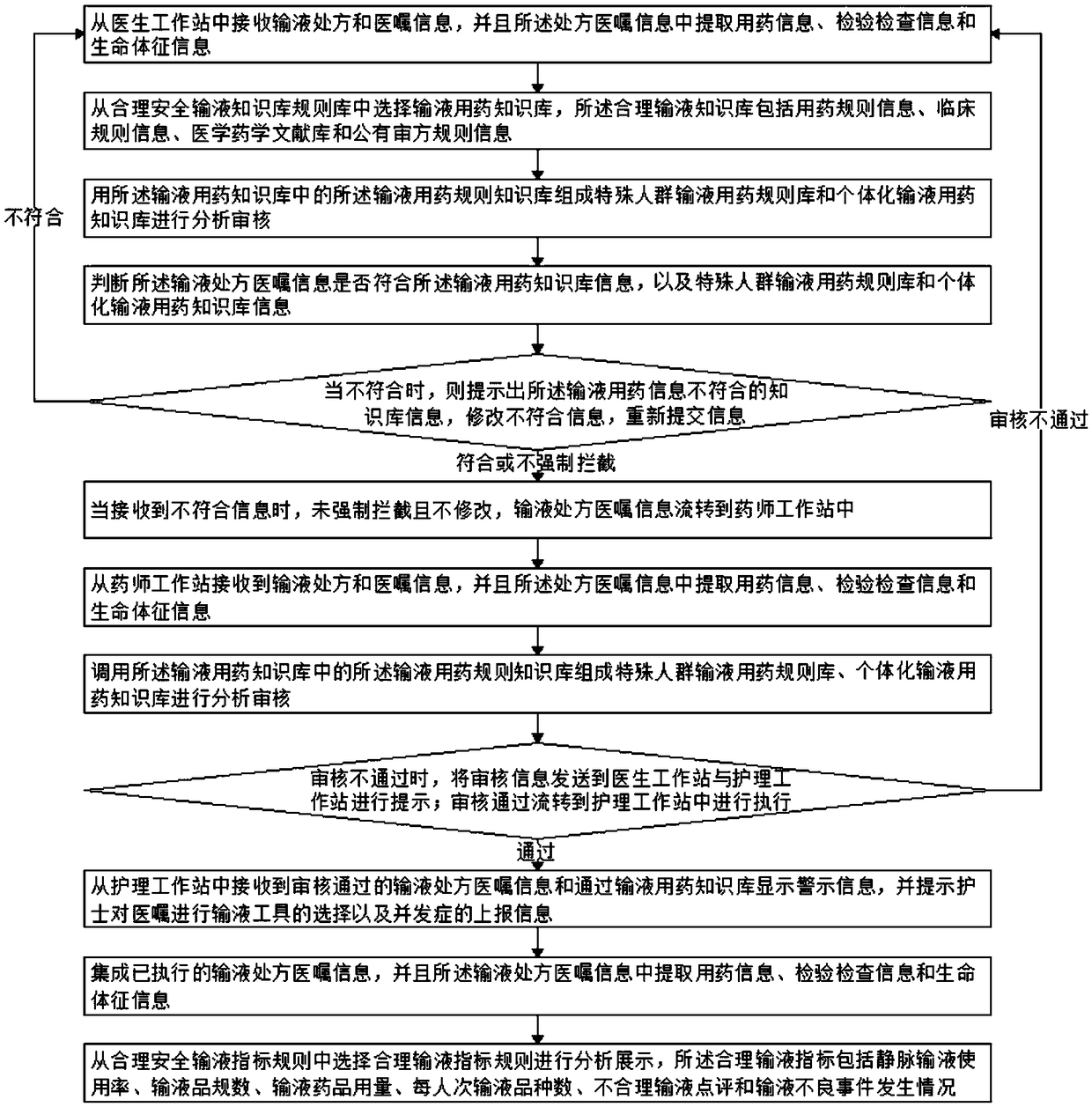
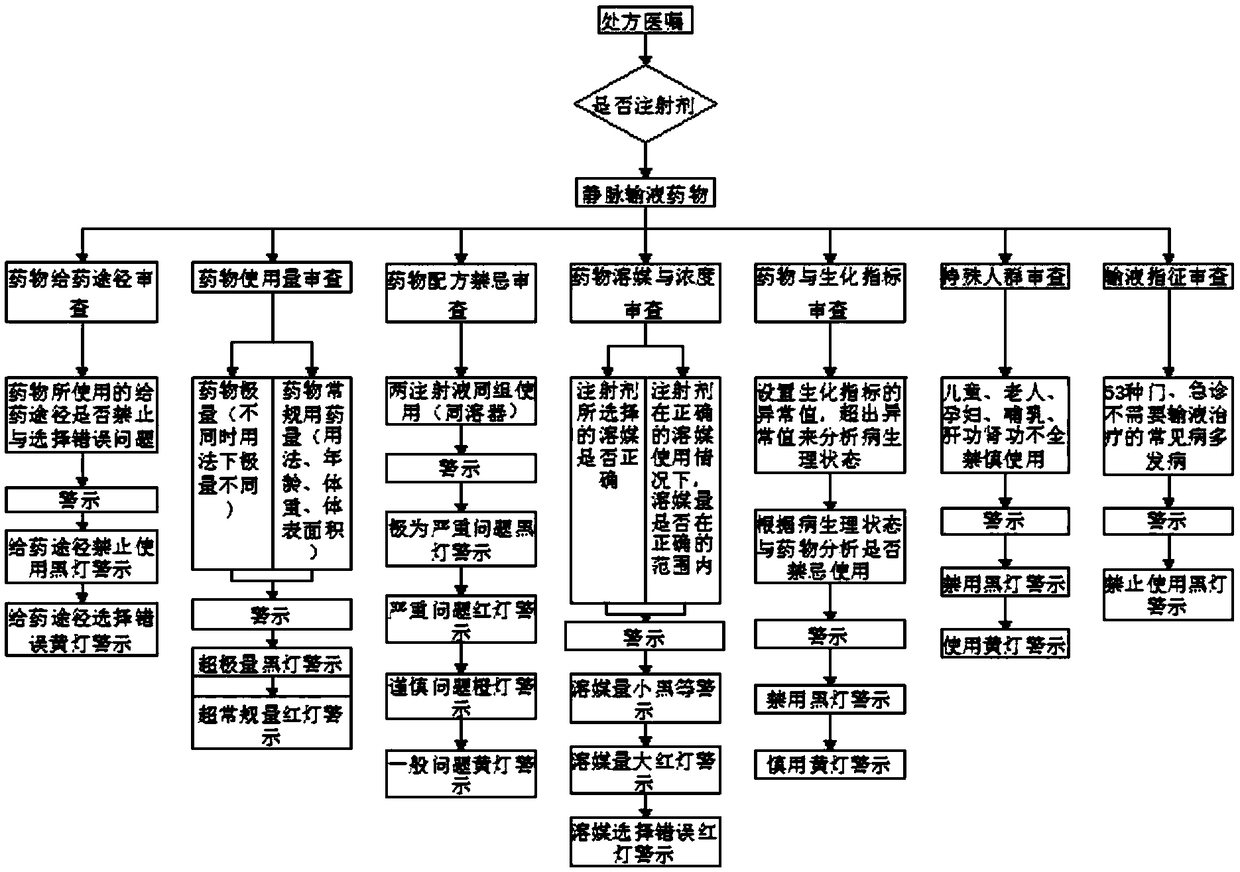
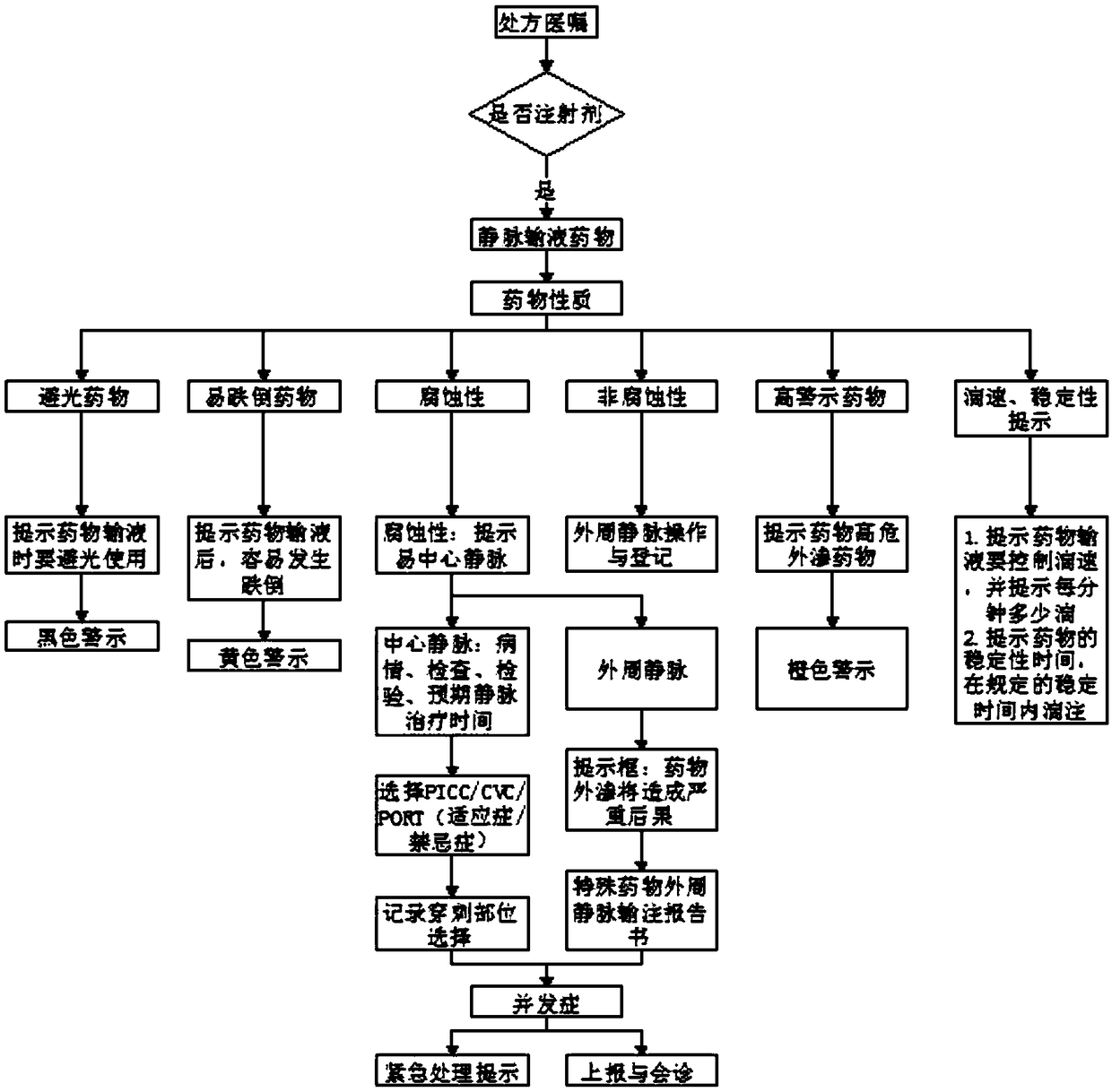
The invention relates to the technical field of transfusion monitoring, and discloses a physician, nursing and pharmacy integrated omnibearing reasonable safety transfusion early-warning monitoring method. The method comprises the following flow: receiving a transfusion prescription and doctor advice information from a doctor work station, extracting pharmacy information, examination information and vital sign information from the prescription and doctor advice information; selecting a transfusion pharmacy knowledge base from a reasonable safety transfusion knowledge base rule base, wherein the reasonable transfusion pharmacy knowledge base comprises pharmacy rule information, clinic rule information, a medicine and pharmacy literature database and public prescription evaluation rule information. The method provided by the invention achieves the automatic screening and evaluation of the infusion reaction risks through the biochemical indexes of a patient, achieves the safety checking of a tool, achieves the marking and prompt of dripping speed, light avoiding and transfusion stable time, helps a nurse carry out the medication safety monitoring and take measures, and guarantees themedication safety.
Owner:广州天恒信息科技有限公司 +1
Preparation method of sodium cantharidate
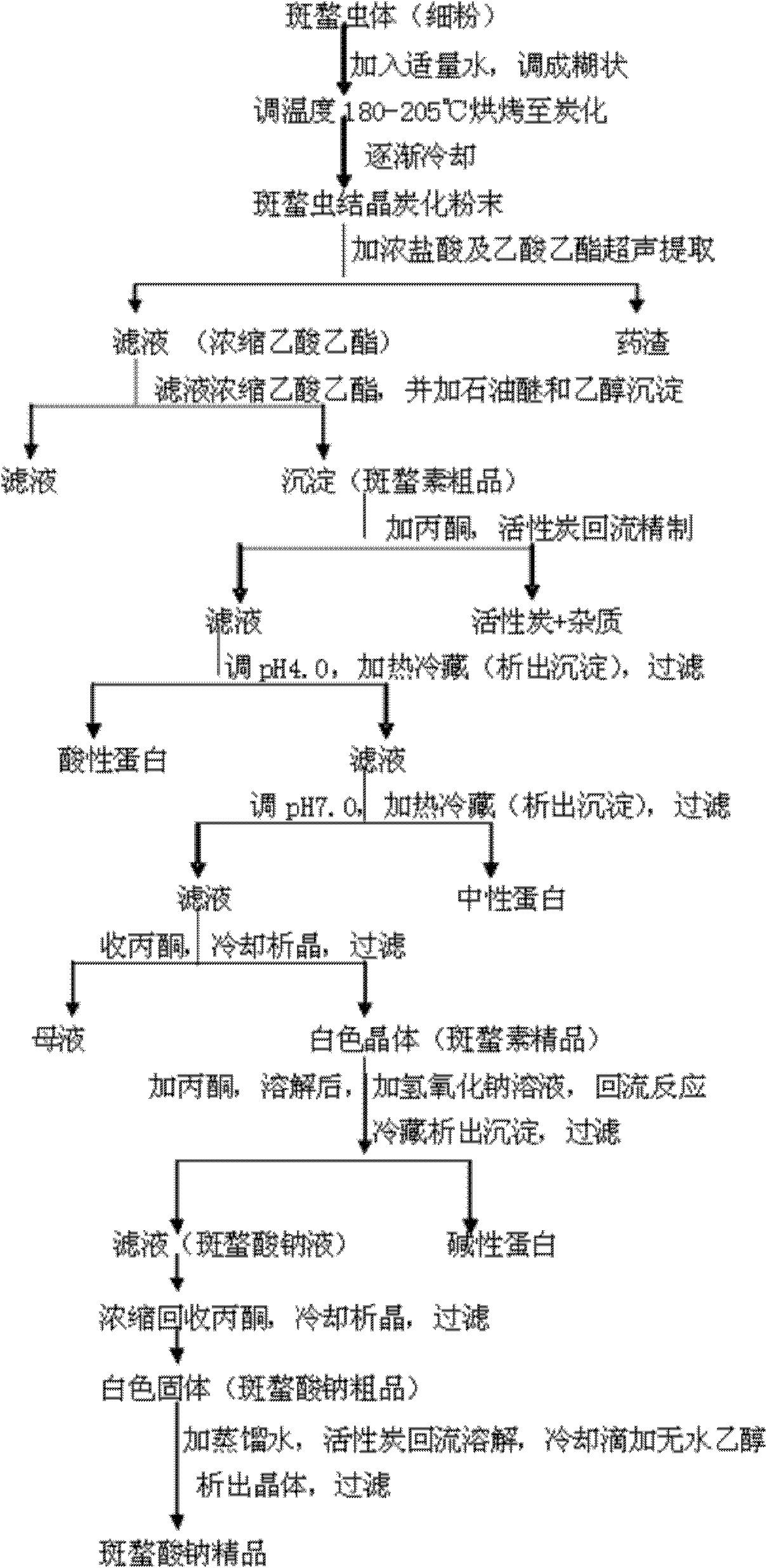
ActiveCN102146086ASimple extraction processSimple manufacturing processOrganic chemistryCarbonizationCantharidin
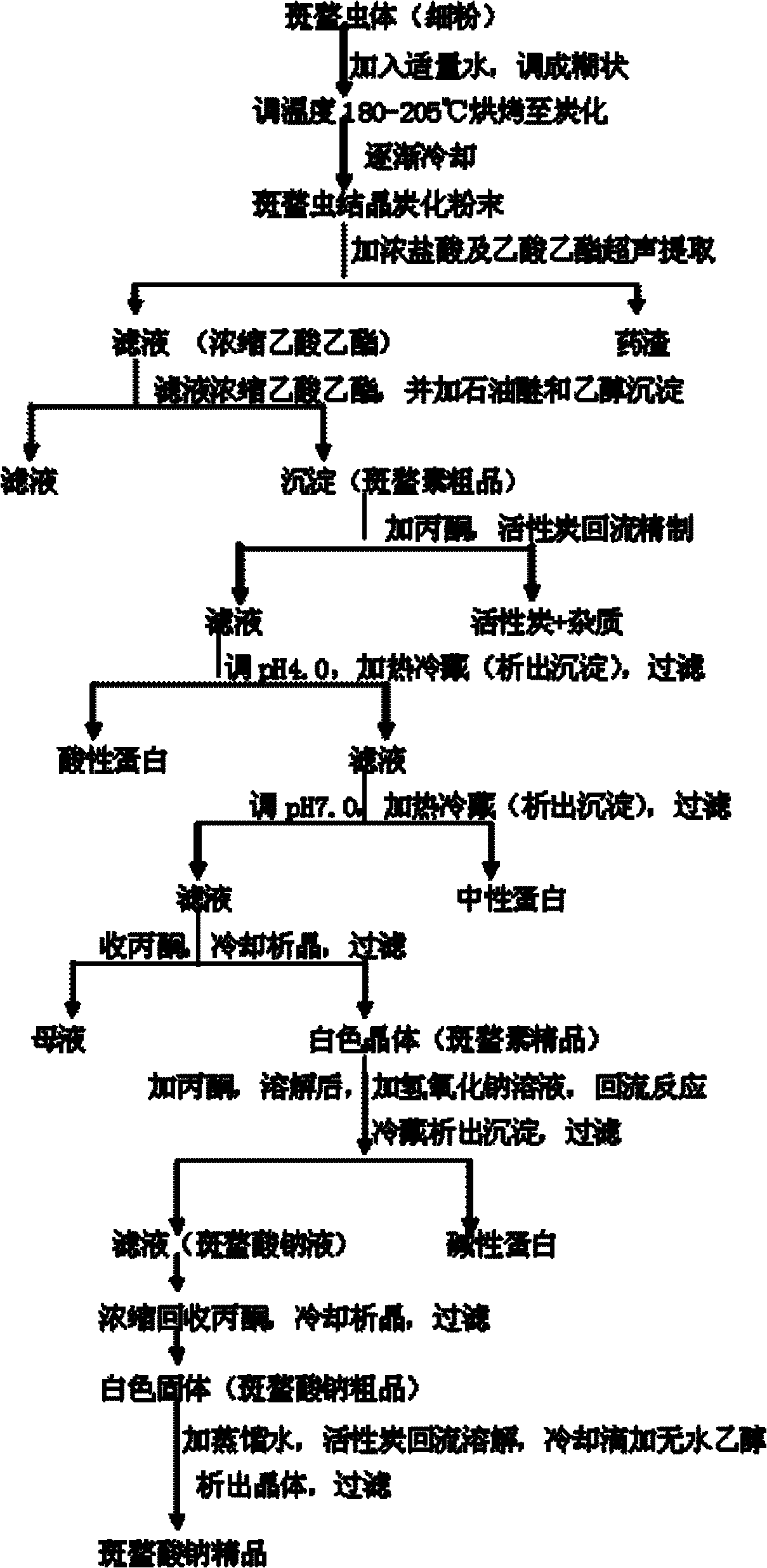
The invention discloses a preparation method of sodium cantharidate. The method comprises the following processing steps: performing high temperature carbonization of cantharis, extracting cantharidin, degreasing cantharidin and precipitating, refining cantharidin, synthetizing sodium cantharidate, refining sodium cantharidate, etc. By adopting the preparation method, the extracting time of cantharidin can be greatly shortened, lipidic and peptdic proteins and impurities in crude cantharidin and refined cantharidin can be completely removed, and the yield and purity of cantharidin can be increased. Therefore, sodium cantharidate with higher purity and yield can be obtained, the quality and safety of sodium cantharidate can be further increased and the quality reliability of sodium cantharidate can be increased when used in an injection.
Owner:贵州君之堂制药有限公司
Checking device for drug information of patient
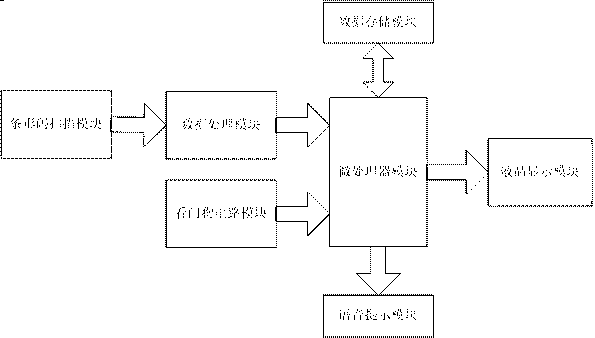
InactiveCN103279911AEnsure medication safetyData processing applicationsSensing by electromagnetic radiationLiquid-crystal displayBarcode
The invention relates to a checking device for drug information of a patient. The checking device for the drug information of the patient comprises a microprocessor module and a bar code scanning module. The bar code scanning module is connected with the microprocessor module by a data processing module, and the microprocessor module is also connected with a data storage module and a liquid crystal display module. According to the checking device for the drug information of the patient provided by the invention, the problem that a pharmacist can easily make mistakes when getting drugs in the prior art is solved. The prescribing information of the patient is scanned through bar codes, and types and specifications of the drugs are determined by the bar codes of the drugs. The two kinds of information are checked; when not matched, the two kinds of information are displayed by a display screen, and a voice prompt is accompanied to ensure medication safety.
Owner:MAYHAP IND SUZHOU
Characteristic DNA sequence for dendrobium officinale test-tube plantlet and application thereof
ActiveCN101235377AImprove accuracyStrong specificityMicrobiological testing/measurementFermentationPlantletSeedling
The invention relates to a character DNA sequence of dendrobium officinale tube seedling and the application. The invention discloses a character DNA sequence of dendrobium officinale tube seedling, which is the DNA sequence of SEQ ID NO: 1or the complementary sequence. The invention also discloses a usage for utilizing specificity probe of dendrobium officinale tube seedling in identifying dendrobium officinale tube seedling and an identifying method. The character DNA sequence of dendrobium officinale tube seedling which is used to identify dendrobium officinale tube seedling and dendrobium officinale has high accuracy and excellent specificity, the identification technique is easy to master.
Owner:上海上药神象健康药业有限公司
Membranous milkvetch root extract, as well as preparation and application methods thereof
InactiveCN102641326AEnsure medication safetyQuality controllableRespiratory disorderPlant ingredientsWater contentCalycosin
The invention provides a membranous milkvetch root extract, as well as preparation and application methods thereof. The extract is extracted by taking traditional Chinese medicine membranous milkvetch root as a raw material, and the extract contains the following components in percentage by weight: 0.66%-0.73% of calycosin, 0.36%-0.42% of onocol and 34.6%-36.9% of total saponins from membranous milkvetch root. The preparation method of the extract disclosed by the invention takes the content of the calycosin, the content of the onocol, the content of the total saponins from the membranous milkvetch root, the water content, the residue on ignition and the content of heavy metals in the extract as indexes for optimization. According to the preparation method provided by the invention, the quality of the membranous milkvetch root extract can be better controlled, the safety in medication can be ensured, and membranous milkvetch root medicaments with controllable quality and clear material base can be deeply developed for complying with the requirements of traditional Chinese medicine modernization.
Owner:CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com