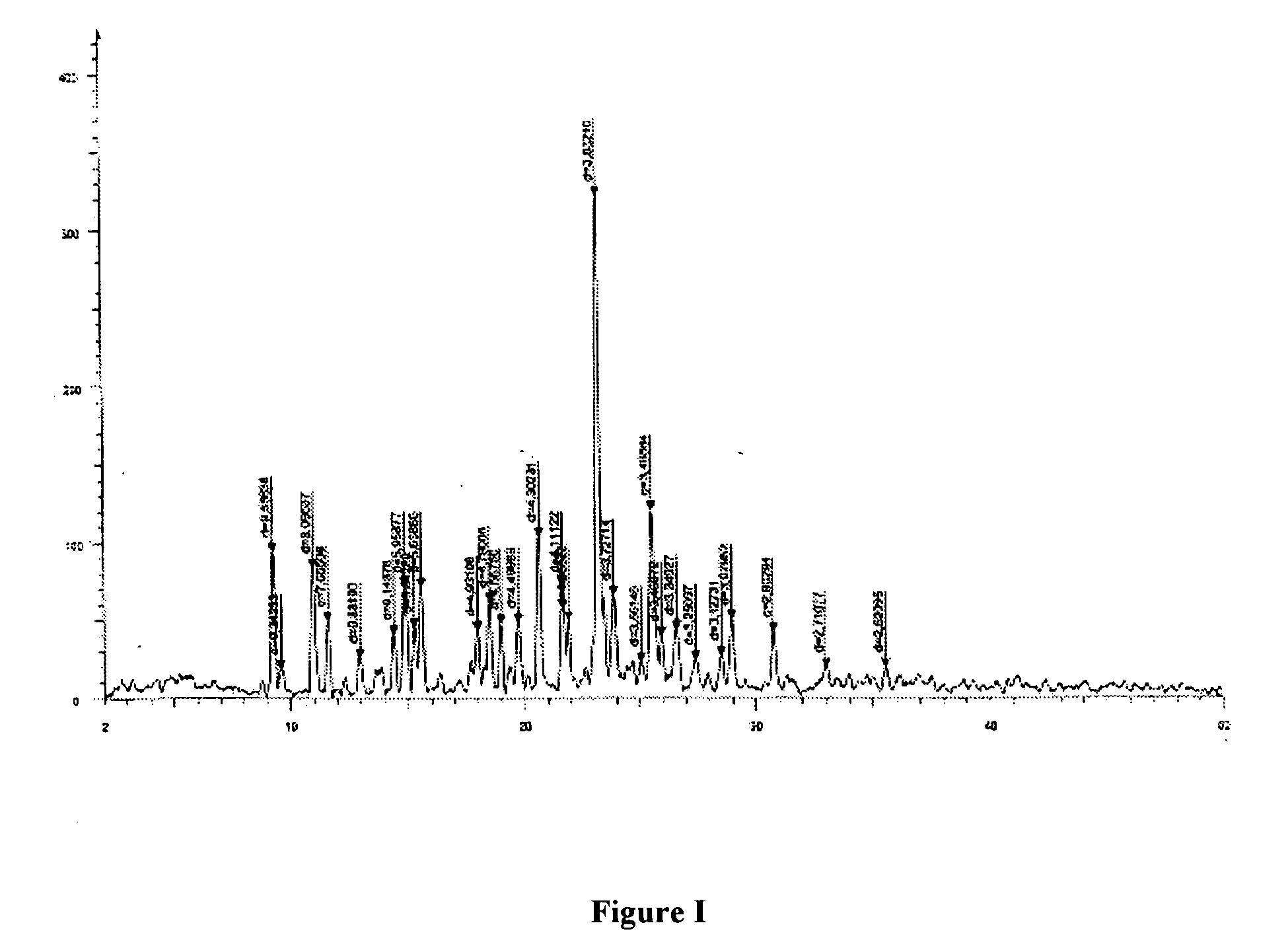
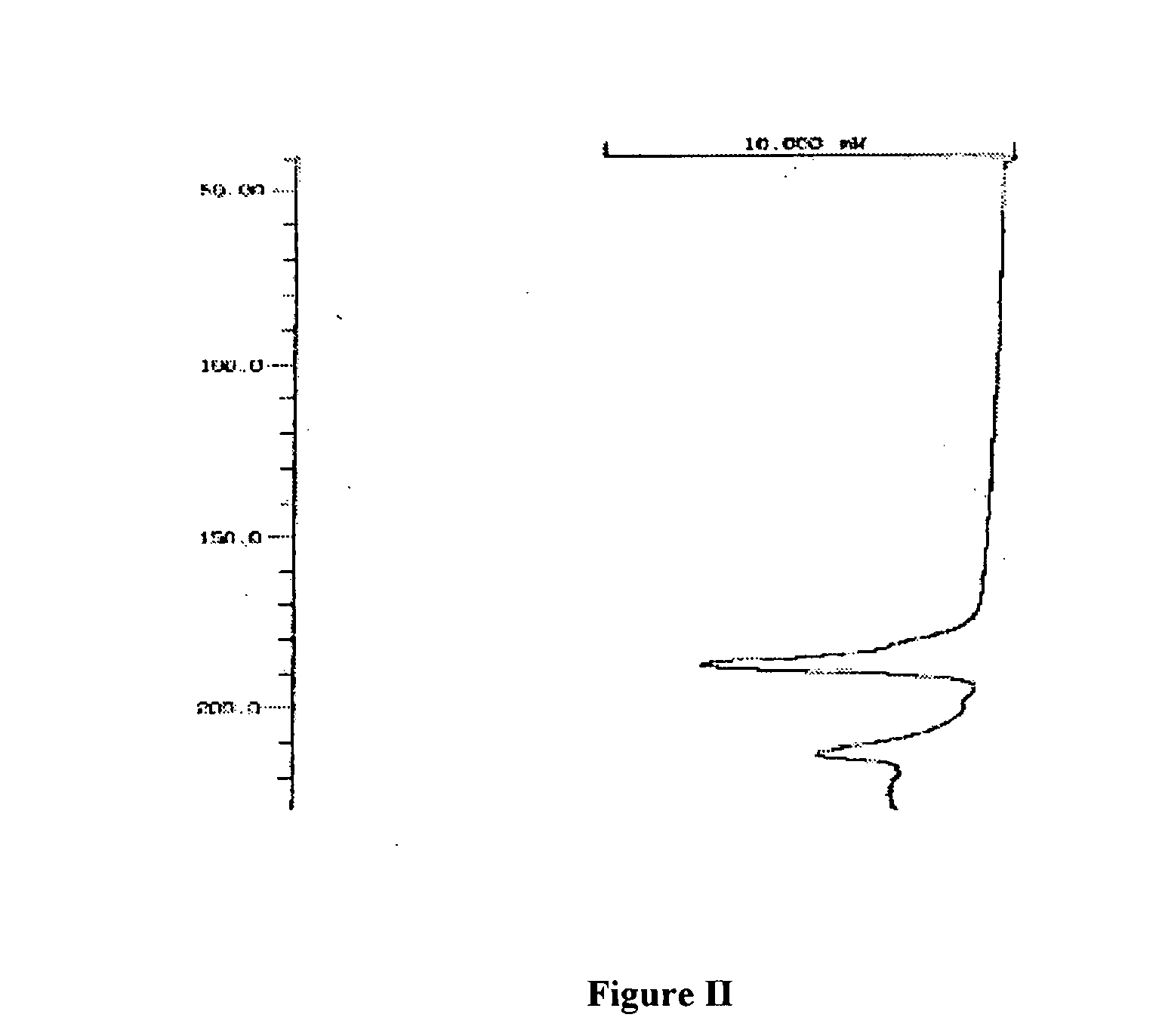
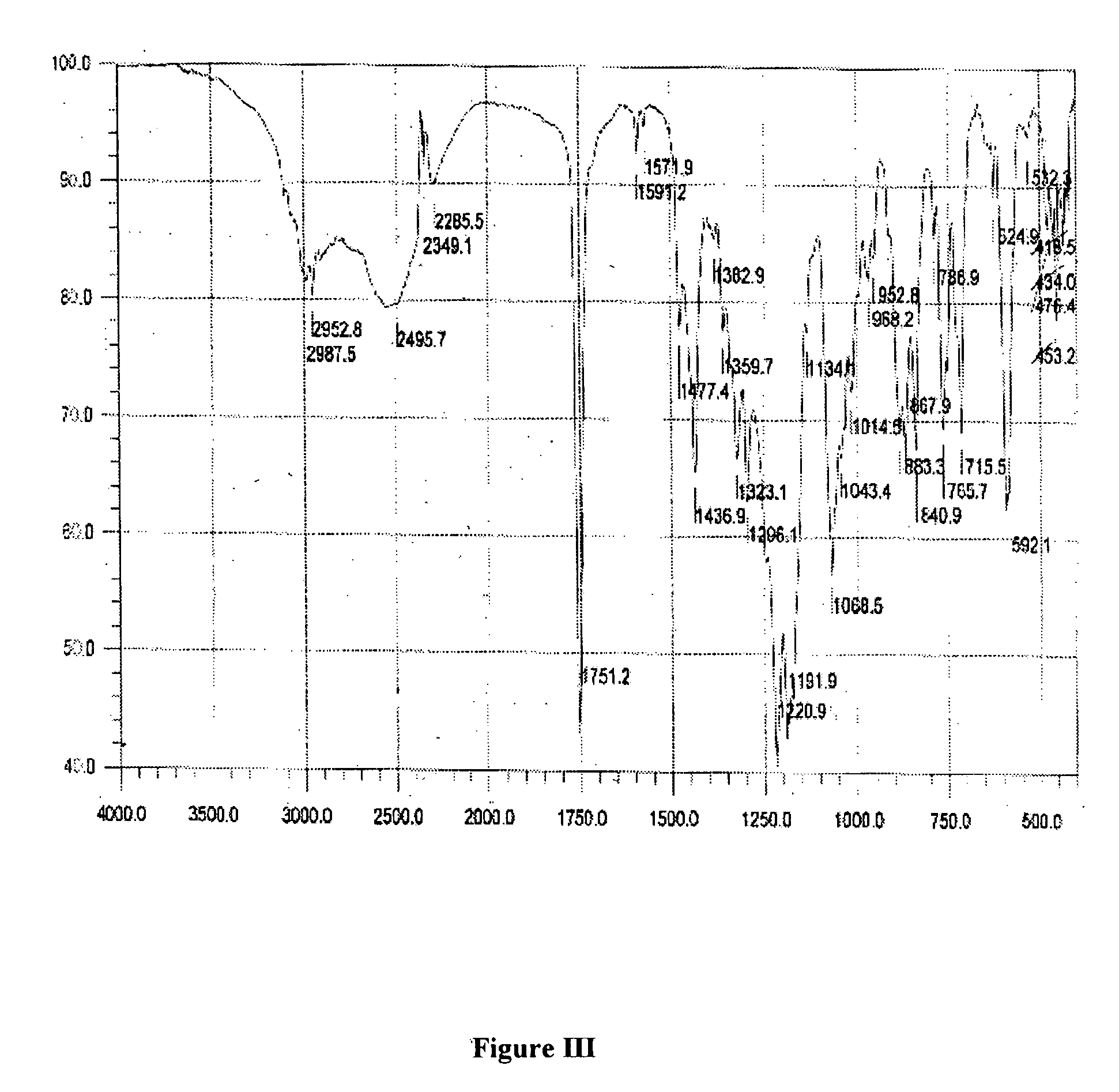
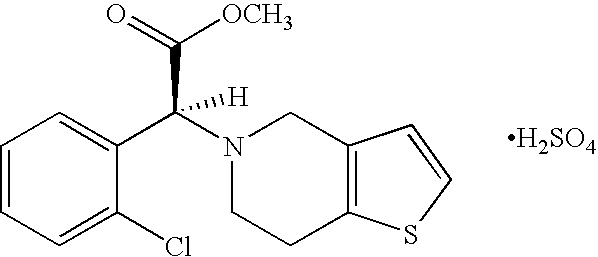
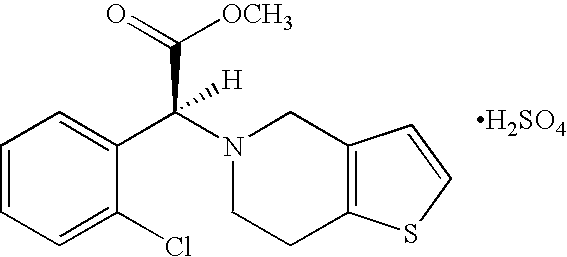
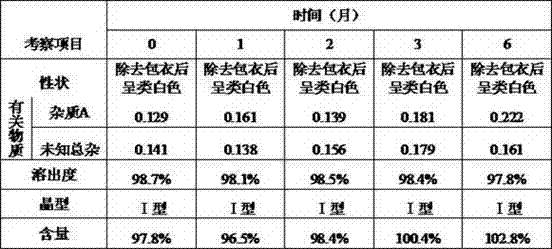
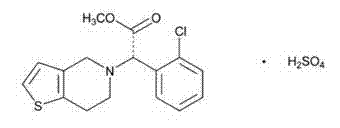
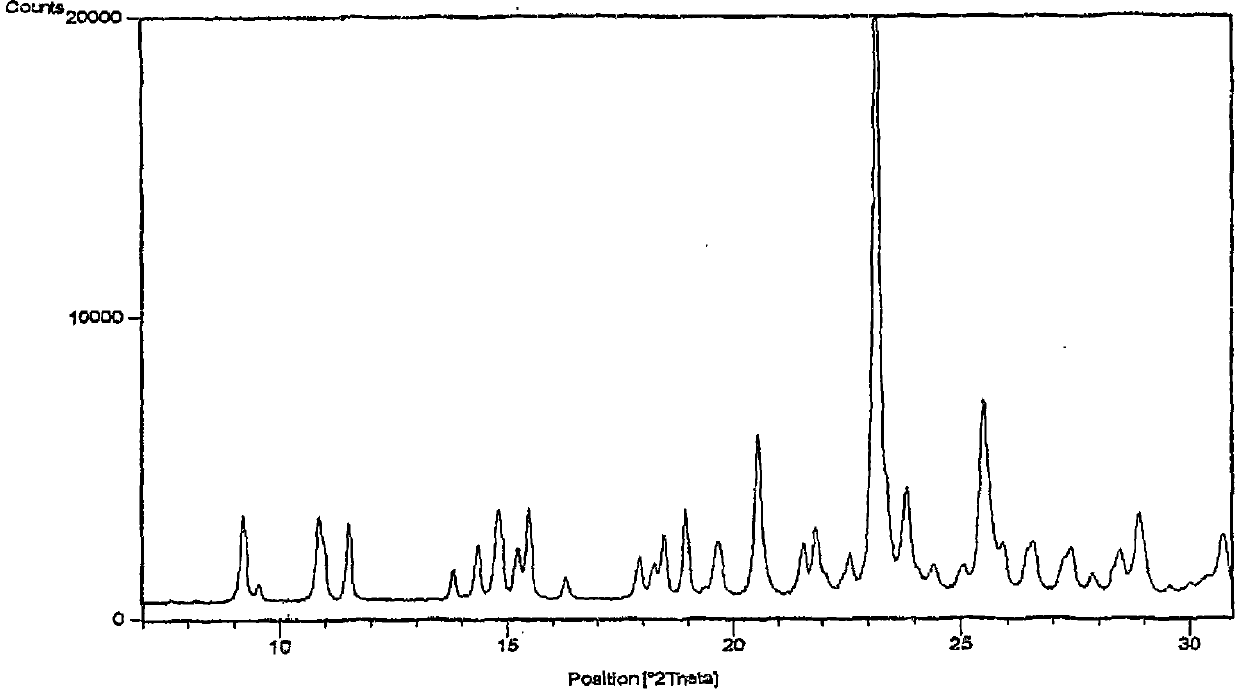
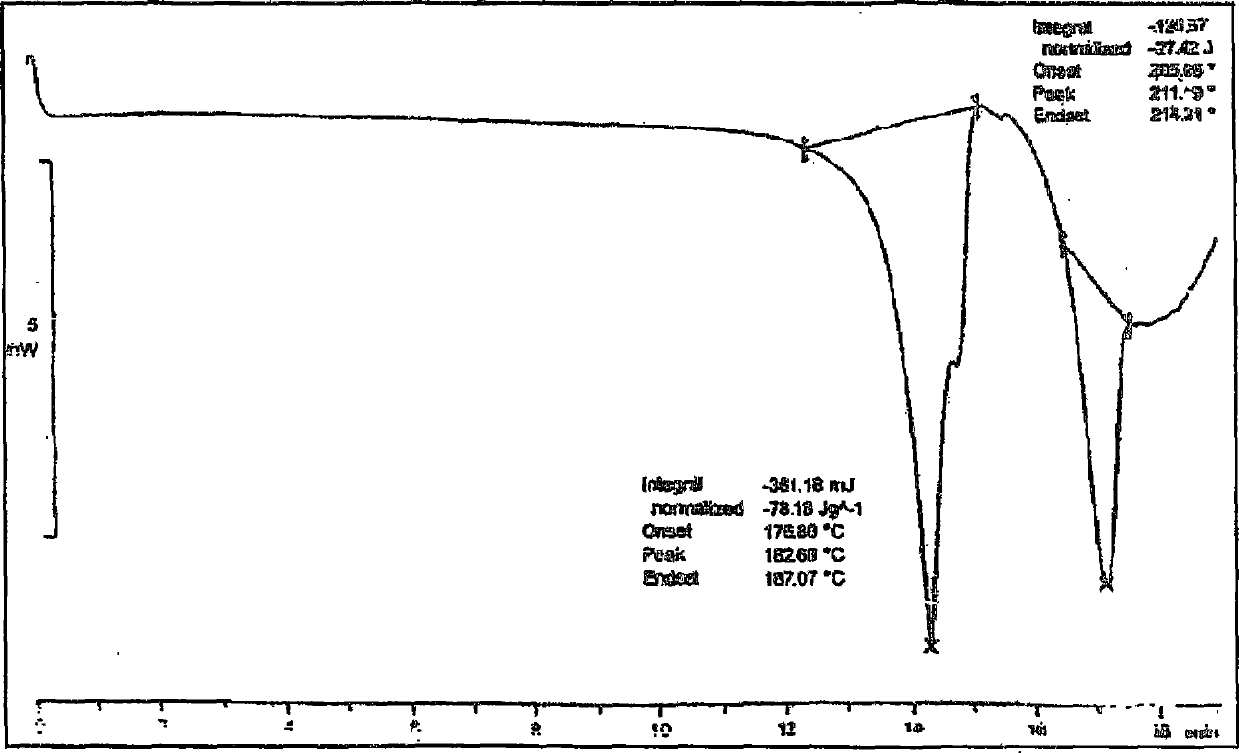
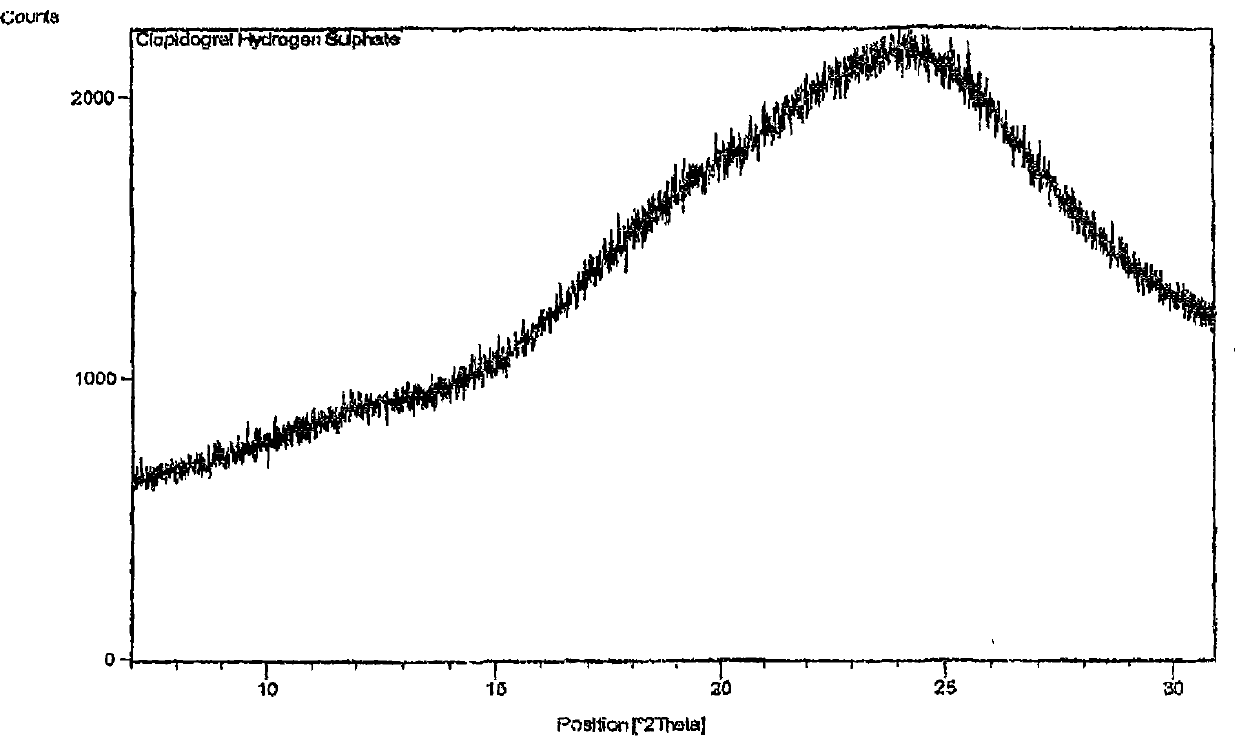
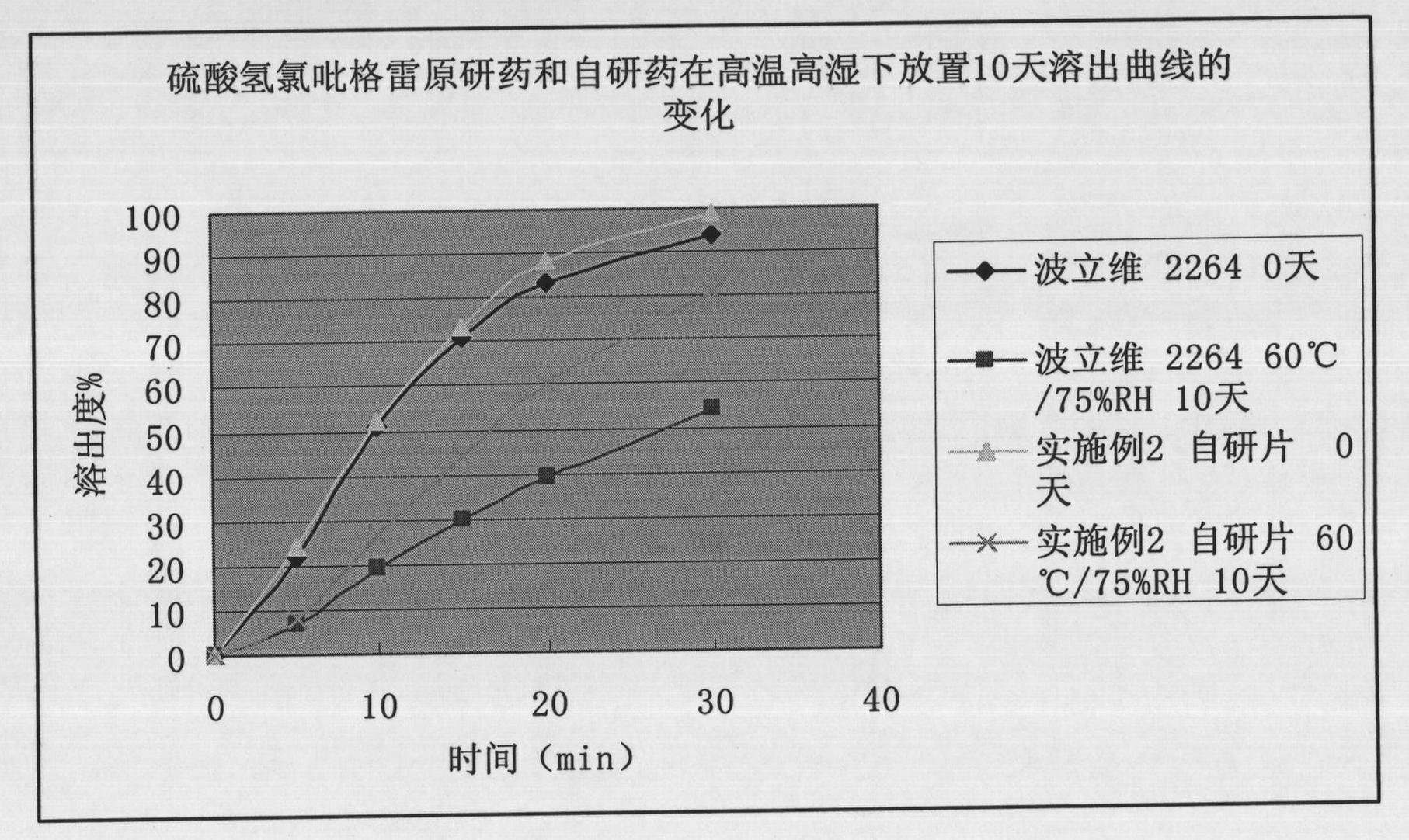
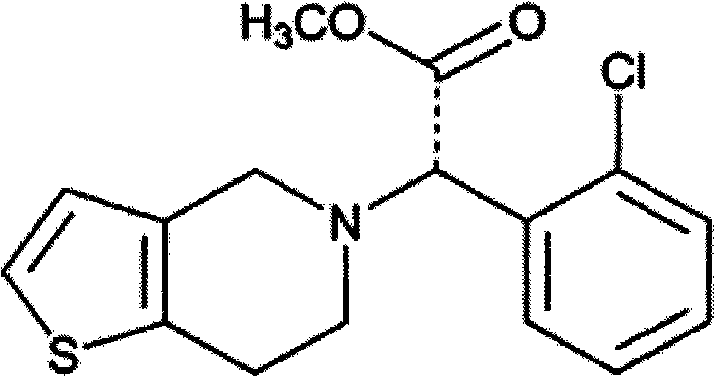
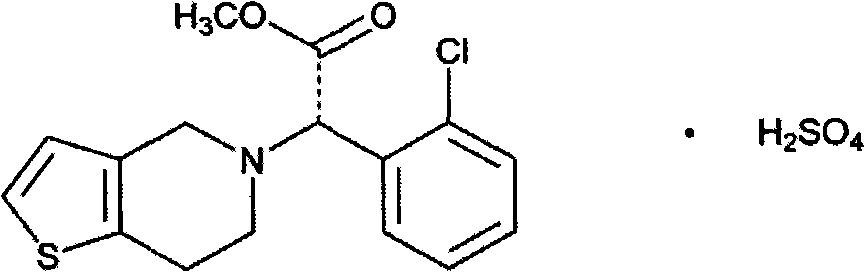
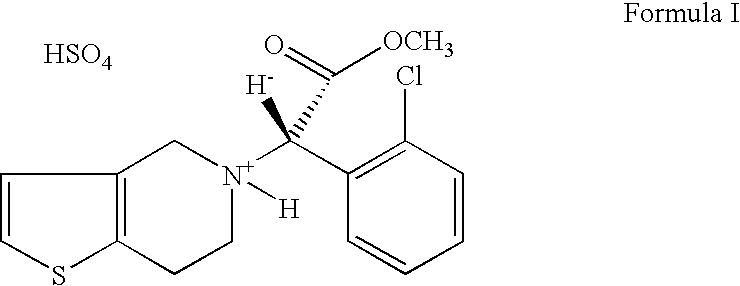
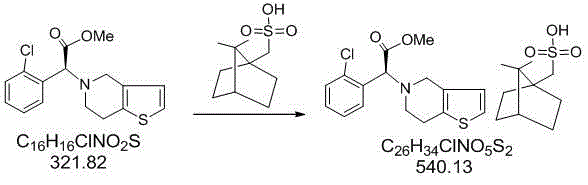
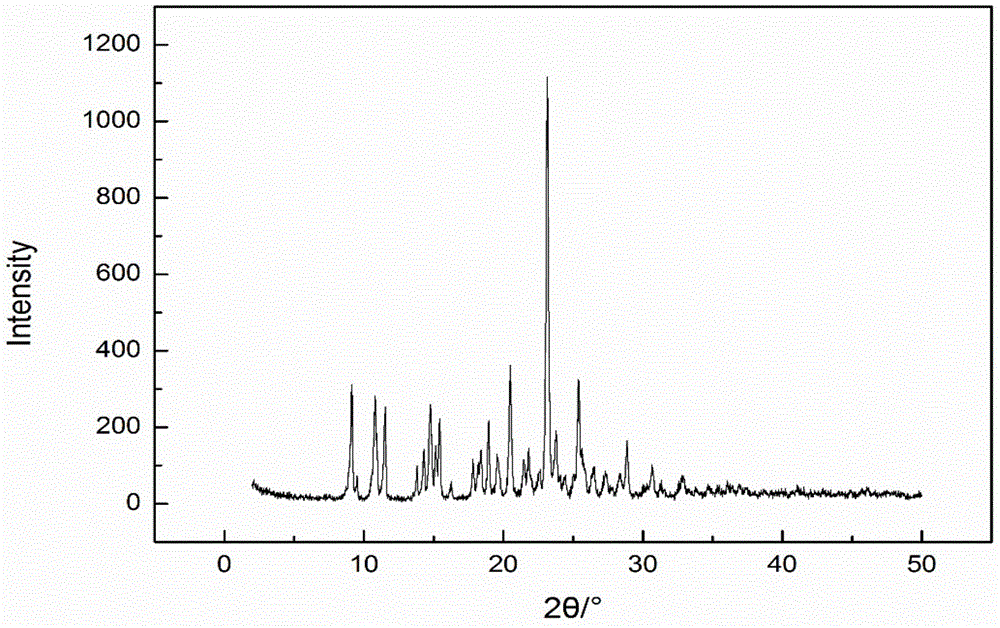
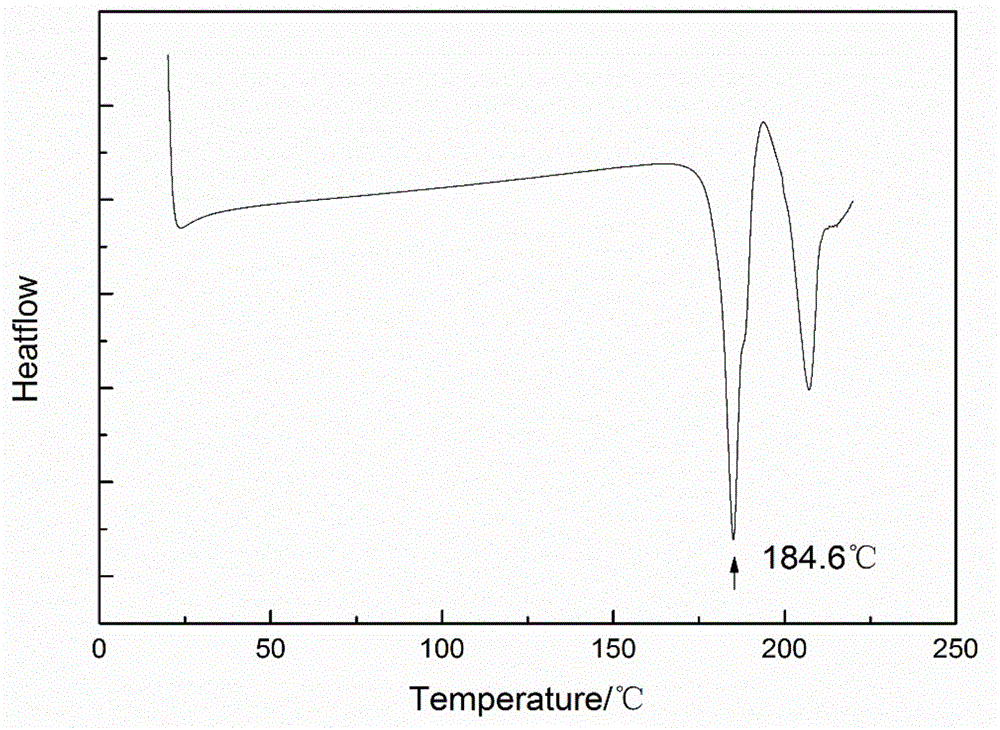
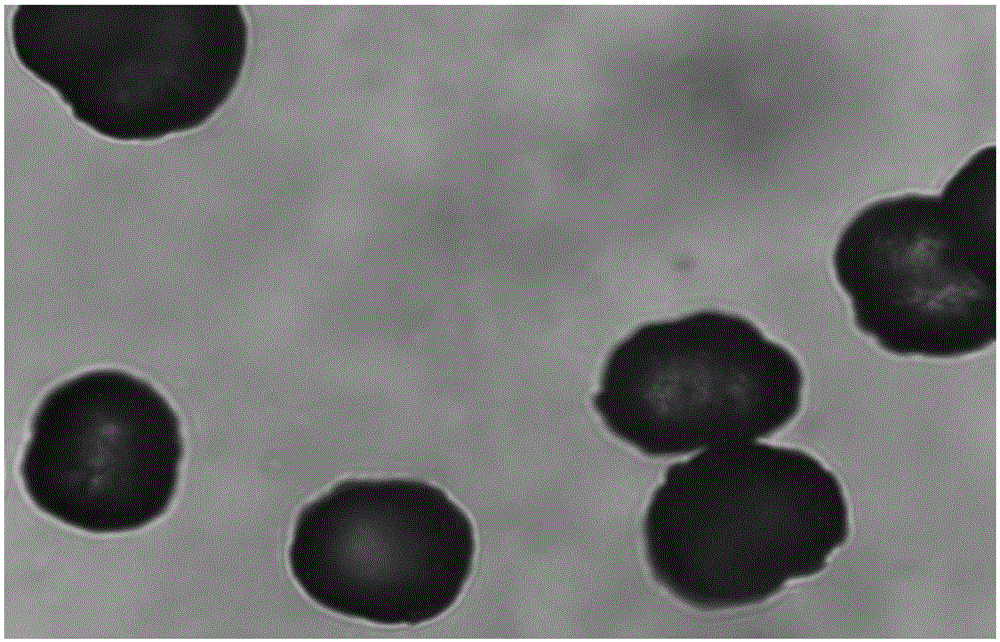
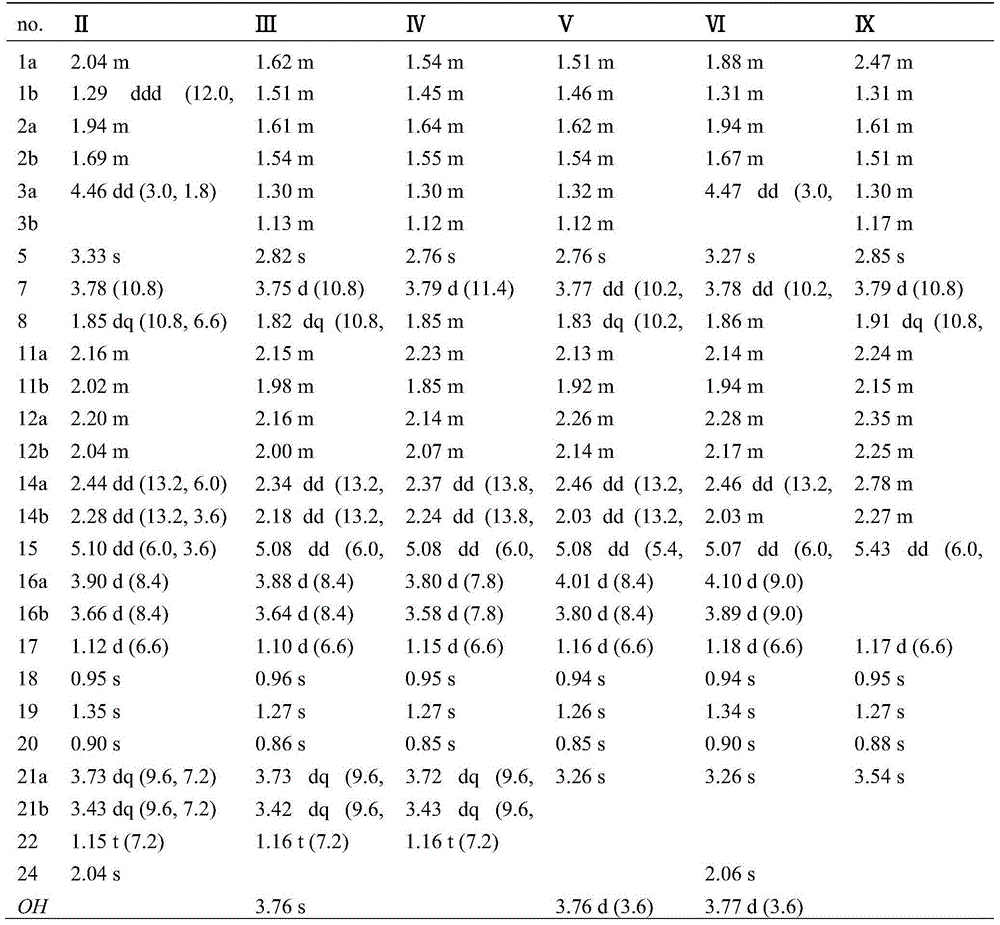
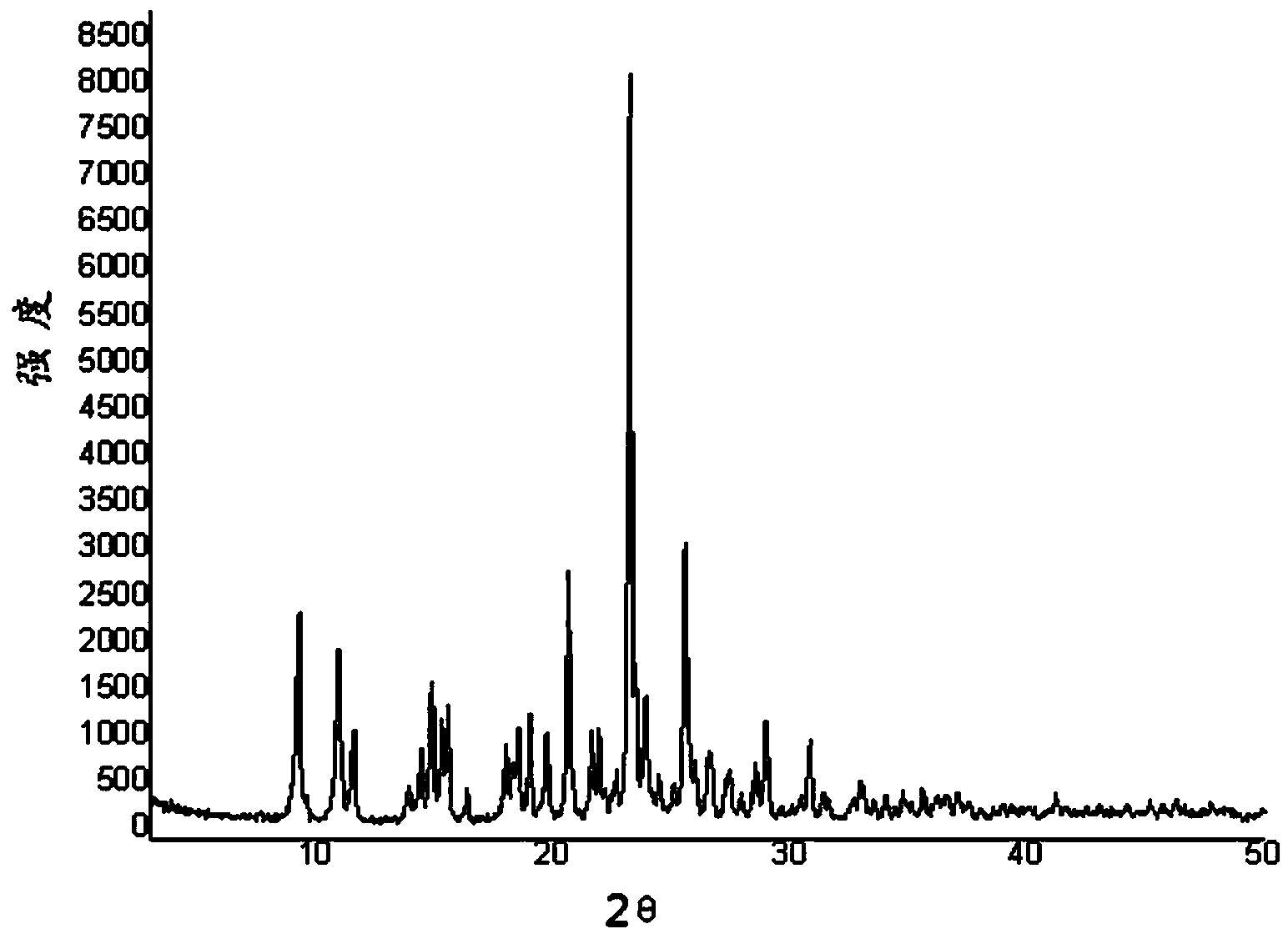
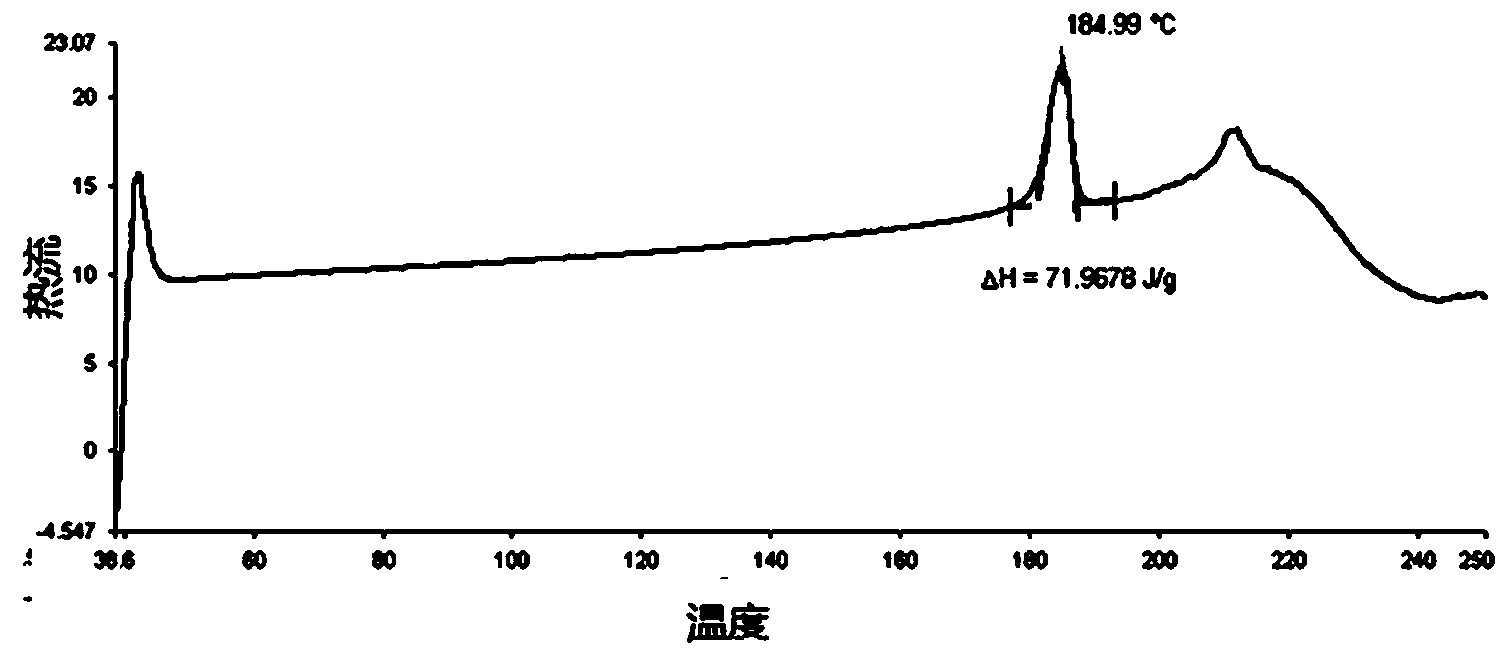
Patents
Literature
119 results about "Clopidogrel Bisulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A thienopyridine with antiplatelet activity. Clopidogrel bisulfate irreversibly alters the platelet receptor for adenosine diphosphate (ADP), thereby blocking the binding of ADP to its receptor, inhibiting ADP-mediated activation of the glycoprotein complex GPIIb/IIIa, and inhibiting fibrinogen binding to platelets and platelet adhesion and aggregation. (NCI04)
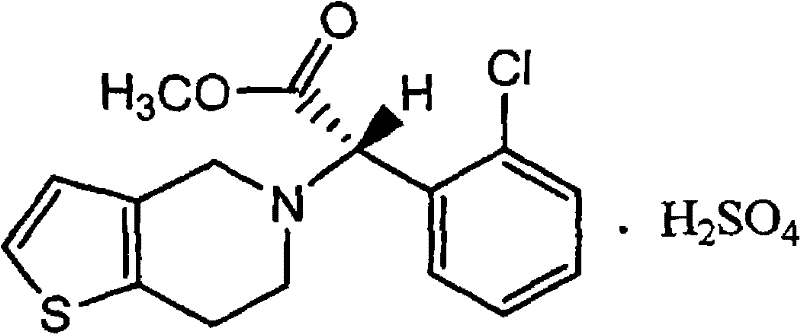
Clopidogrel bisulfate tablet formulation
Pharmaceutical tablets comprising clopidogrel bisulfate and a lubricant selected from zinc stearate, stearic acid, and sodium stearyl fumarate.
Owner:SHERMAN BERNARD CHARLES
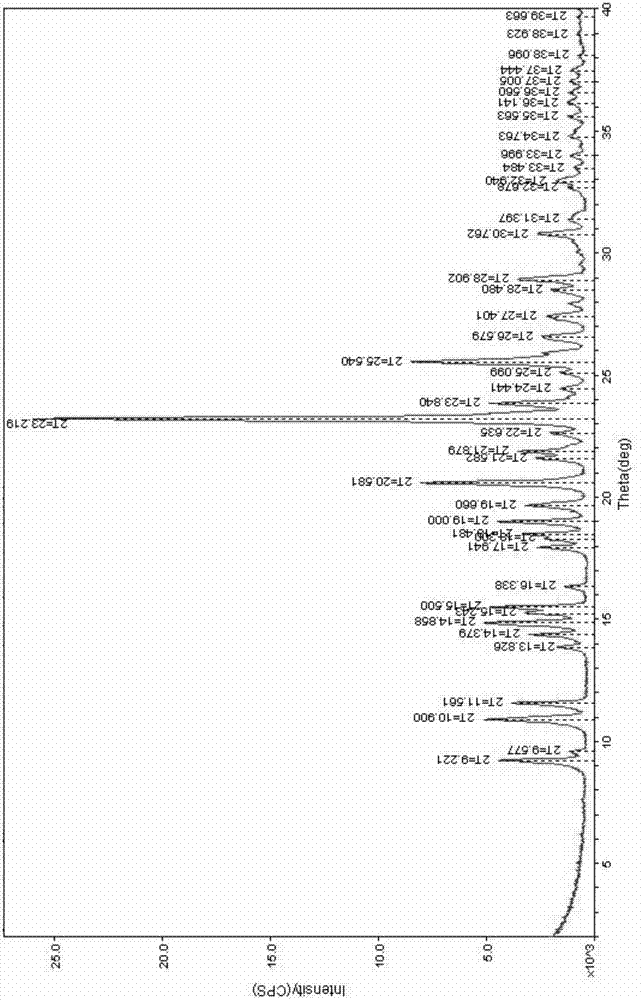
Novel process for preparation of clopidogrel bisulfate polymorph - Form I
A process for making Clopidogrel Bisulfate Form I which comprises dissolving Clopidogrel Bisulfate Form II in a solublizing solvent at room temperature to form a solution; adding an anti-solvent to the said solution till turbid; stirring the said turbid solution; collecting the precipitated solid and drying the final solid product, form I.
Owner:SAWANT KAMLESH DIGAMBAR +2
Polymorphs and amorphous form of (s)-(+)-clopidogrel bisulfate
Owner:CADILA HEALTHCARE LTD
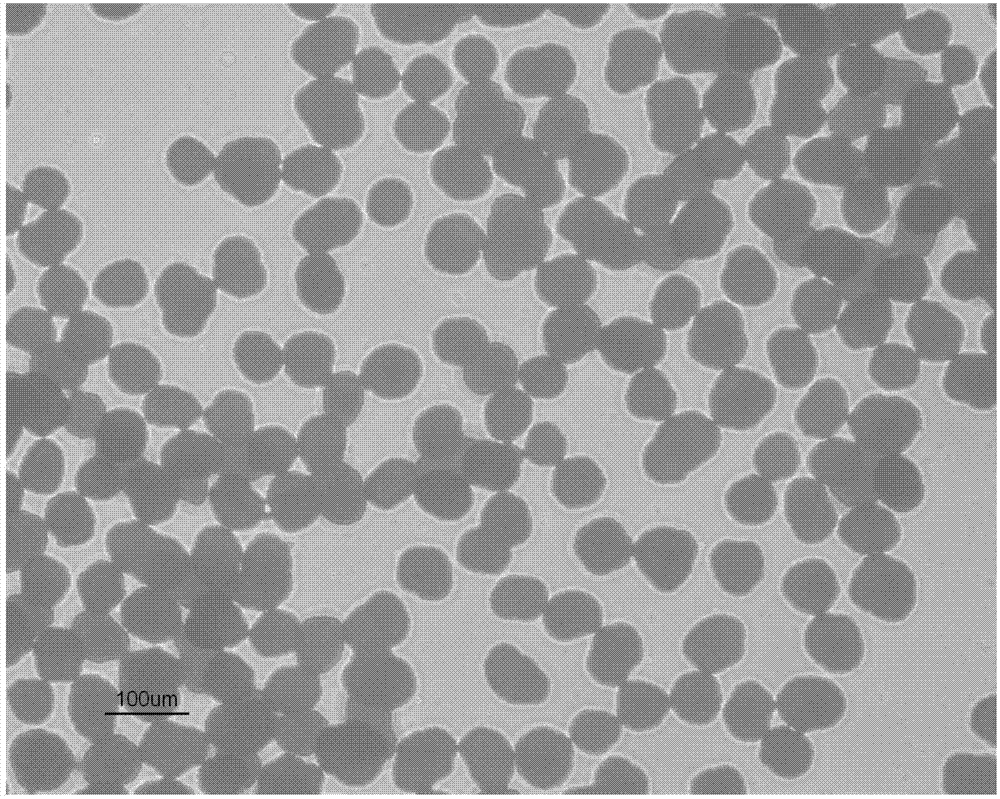
Spherical particles of clopidogrel bisulfate, pharmaceutical composition including same, and method for manufacturing same
InactiveCN103717207AFull strengthEasy to compressOrganic active ingredientsPowder deliveryDrug productUltimate tensile strength
The present invention relates to spherical particles of clopidogrel bisulfate having a 10% volume particle diameter (d0.1) of 30 [mu]m or more and a 50% volume particle diameter (d0.5) in the range of 50 to 200 [mu]m, and to a pharmaceutical composition including same. The spherical particles of the present invention not only significantly improve unfavorable properties in a conventional pharmaceutical preparation of clopidogrel bisulfate, i.e. defective compression and flow and a strong surface electrostatic force, but can also significantly reduce tableting impediments such as weight deviations and sticking during preparation by means of a direct powder compression technique, and the danger of crystalline conversion. Thus, the composition has improved physiochemical stability and, due to anti-clotting effects of the composition, can be effectively used as a therapeutic agent for arteriosclerosis, stroke, myocardial infarction, and atherosclerosis.
Owner:SAMJIN PHARMA +1
Process for synthesizing I-clopidogrel hydrogen sulfate
The invention provides two methods for synthesizing Clopidogrel sulfate of single I crystal system, wherein the first method starts from indefinite form Clopidogrel sulfate, and the second method starts from Clopidogrel salts.
Owner:SHANGHAI INST OF TECH
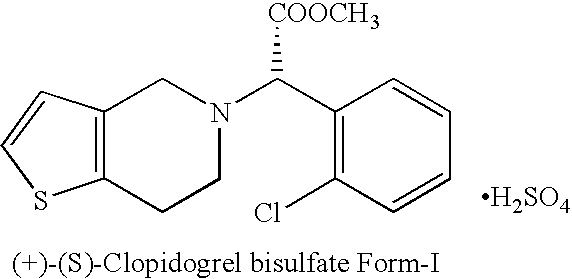
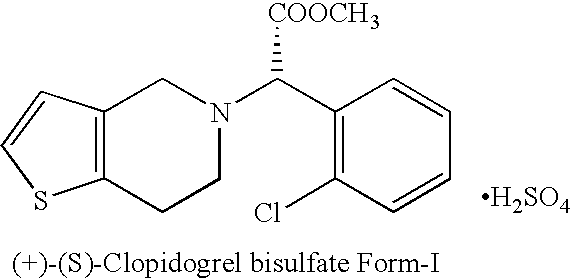
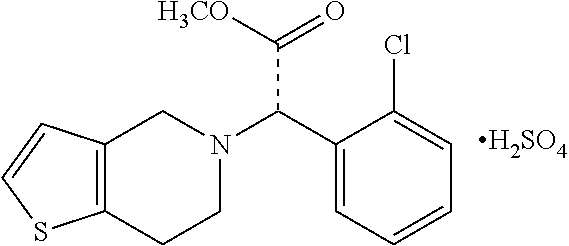
Process for the manufacture of (+)-(s)-clopidogrel bisulfate form-1
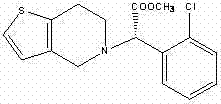
The present invention relates to a novel process for the manufacture of blood-platelet aggregation inhibiting agent. In particular, the present invention is directed to a process for the manufacture of Methyl-(+)-(S)-α-(2-chlorophenyl)-6,7-dihydrothieno [3,2-c]pyridine-S-(4H)acetate bisulfate Form-I.
Owner:WOCKHARDT LTD
Improved preparation method of II-type clopidogrel hydrogen sulfate crystal
InactiveCN103524528AInhibit side effectsGood lookingOrganic chemistryPhysical chemistryCombinatorial chemistry
The invention discloses an improved preparation method of a II-type clopidogrel hydrogen sulfate crystal, which is an improvement of the existing II-type clopidogrel hydrogen sulfate crystal form preparation technique. The method greatly enhances the yield on the premise of ensuring the product appearance (the average yield is up to 92%), and prepares the product with better appearance on the premise of obviously enhancing the product yield.
Owner:吉林省博大伟业制药有限公司
Clopidogrel bisulfate tablet and preparation method thereof
ActiveCN102058550AImprove stabilitySolve problems in large-scale preparationOrganic active ingredientsPharmaceutical non-active ingredientsMedicineMedical prescription
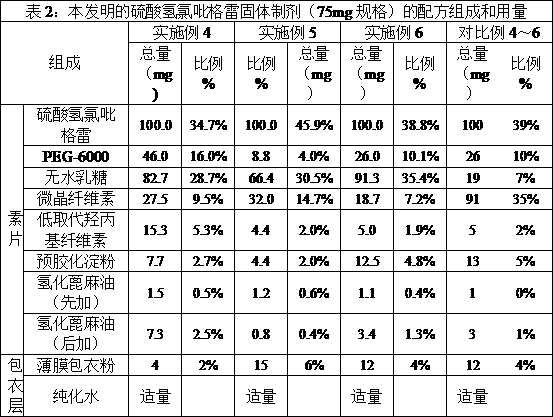
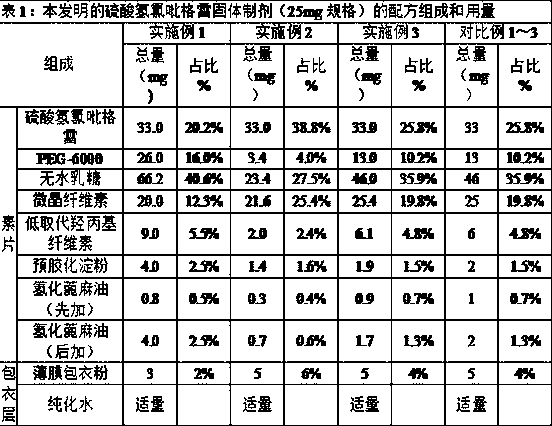
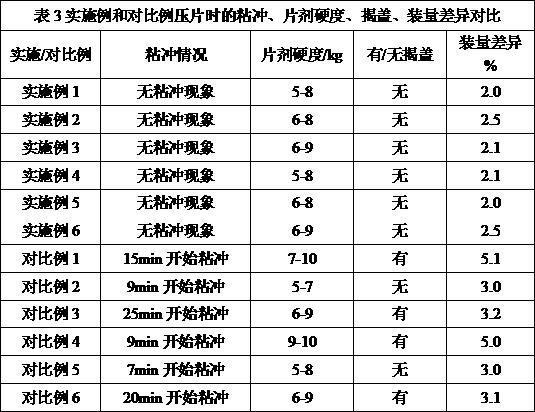
The invention relates to a clopidogrel bisulfate tablet and a preparation method thereof. Each clopidogrel bisulfate tablet comprises 25-75 mg of clopidogrel bisulfate measured in the term of clopidogre, fillers, disintegrants, solubilizers, flow aids and lubricants. In the invention, the method of adding granular microcrystalline cellulose and aerosil through airflow crushing disposal to a prescription is adopted, therefore, the process of dry granulating is simple and feasible, reproducibility is good, prepared products have high stability, and the requirement of continuous large-scale production can be satisfied.
Owner:JIANGSU YABANG QIANGSHENG PHARMA
Process for preparing I-clopidogrel hydrogen sulfate
InactiveCN1690060AConvenient and stable preparationStable in natureOrganic chemistryCrystal systemInfrared
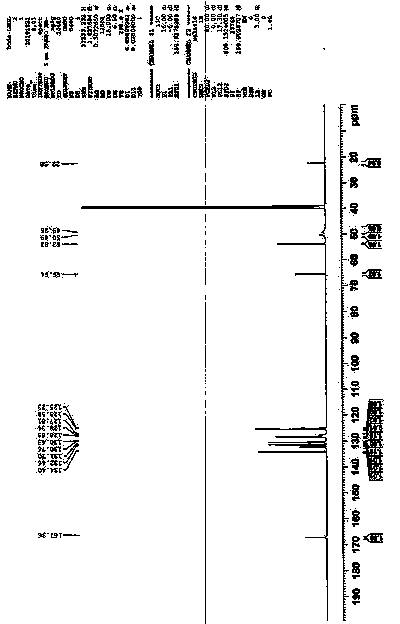
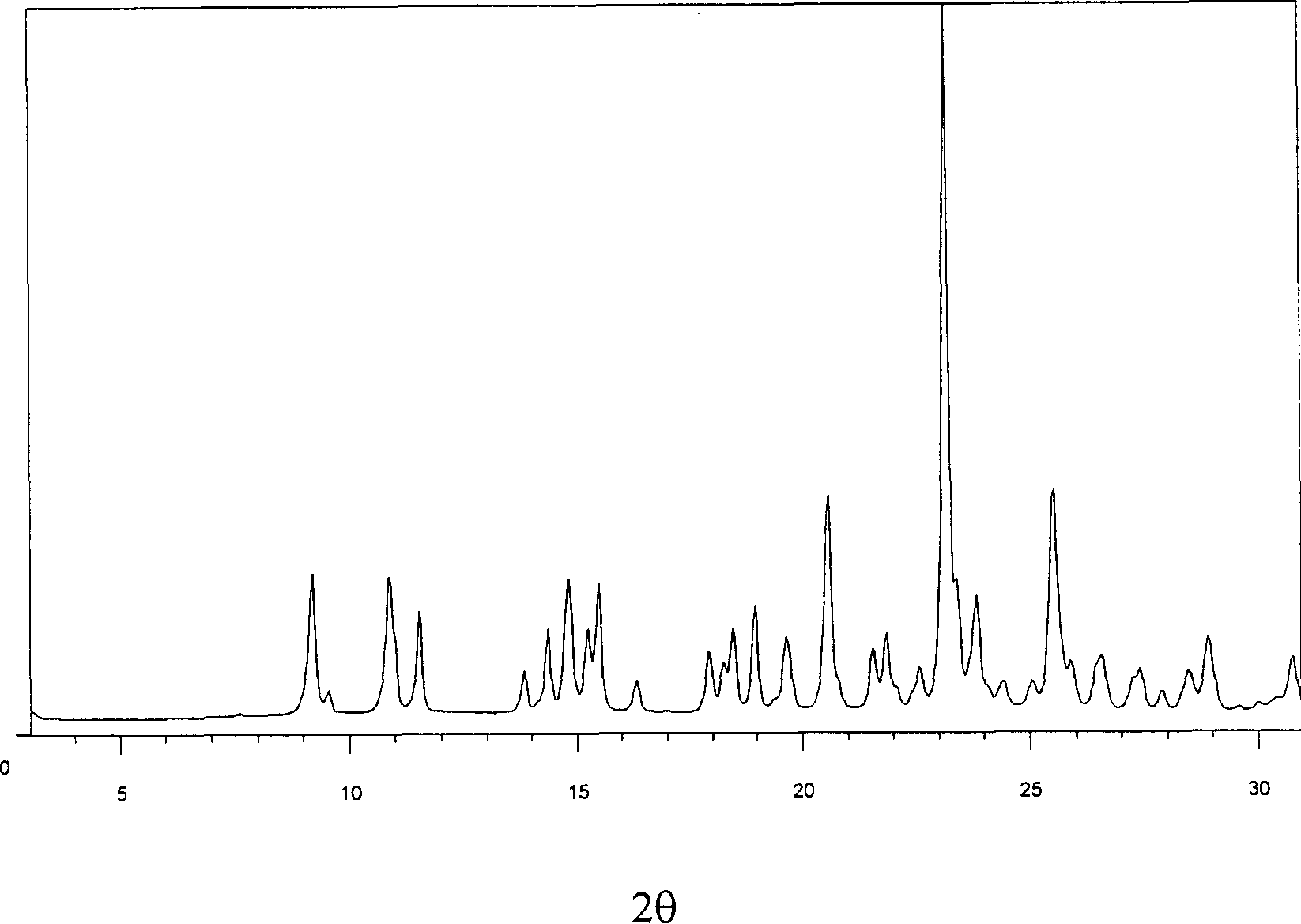
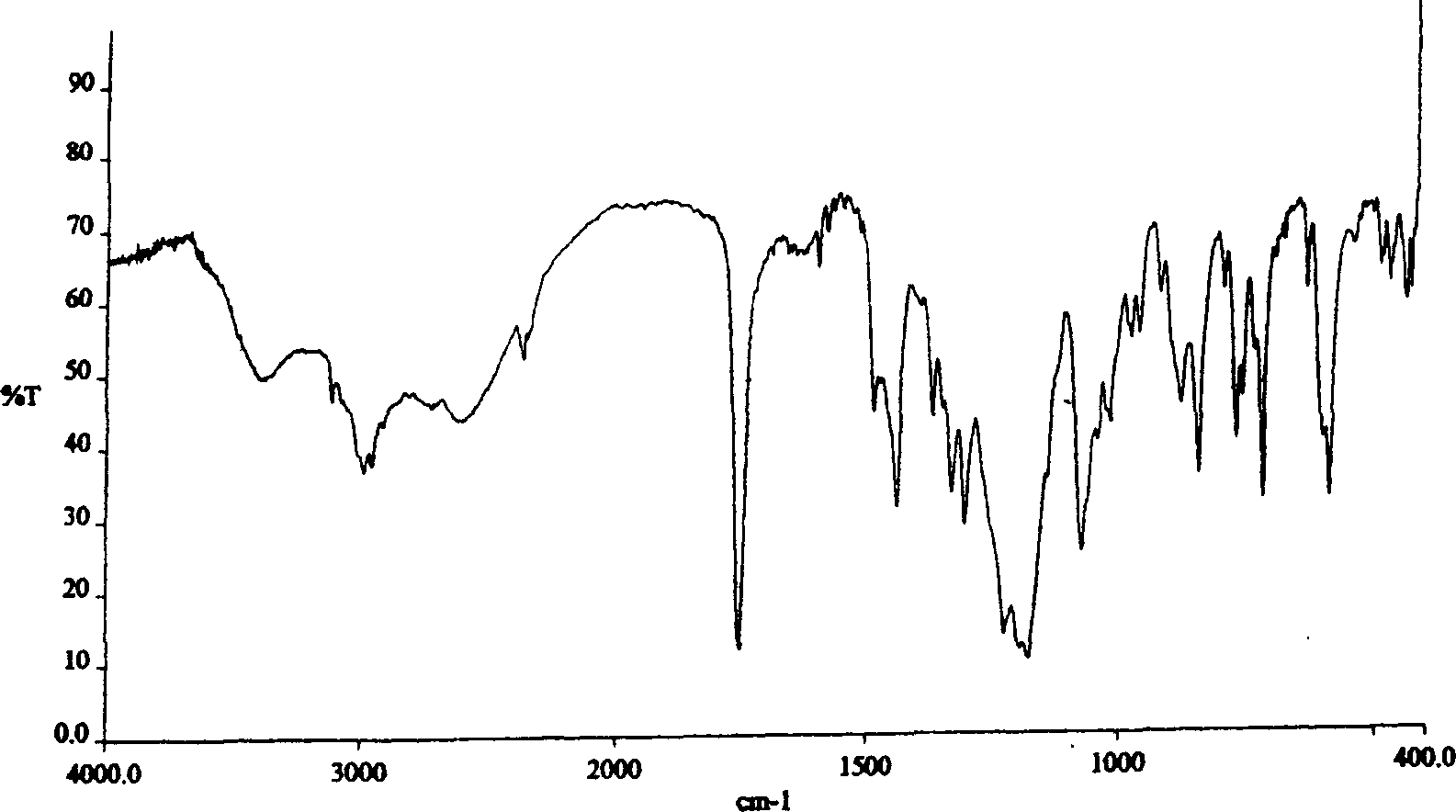
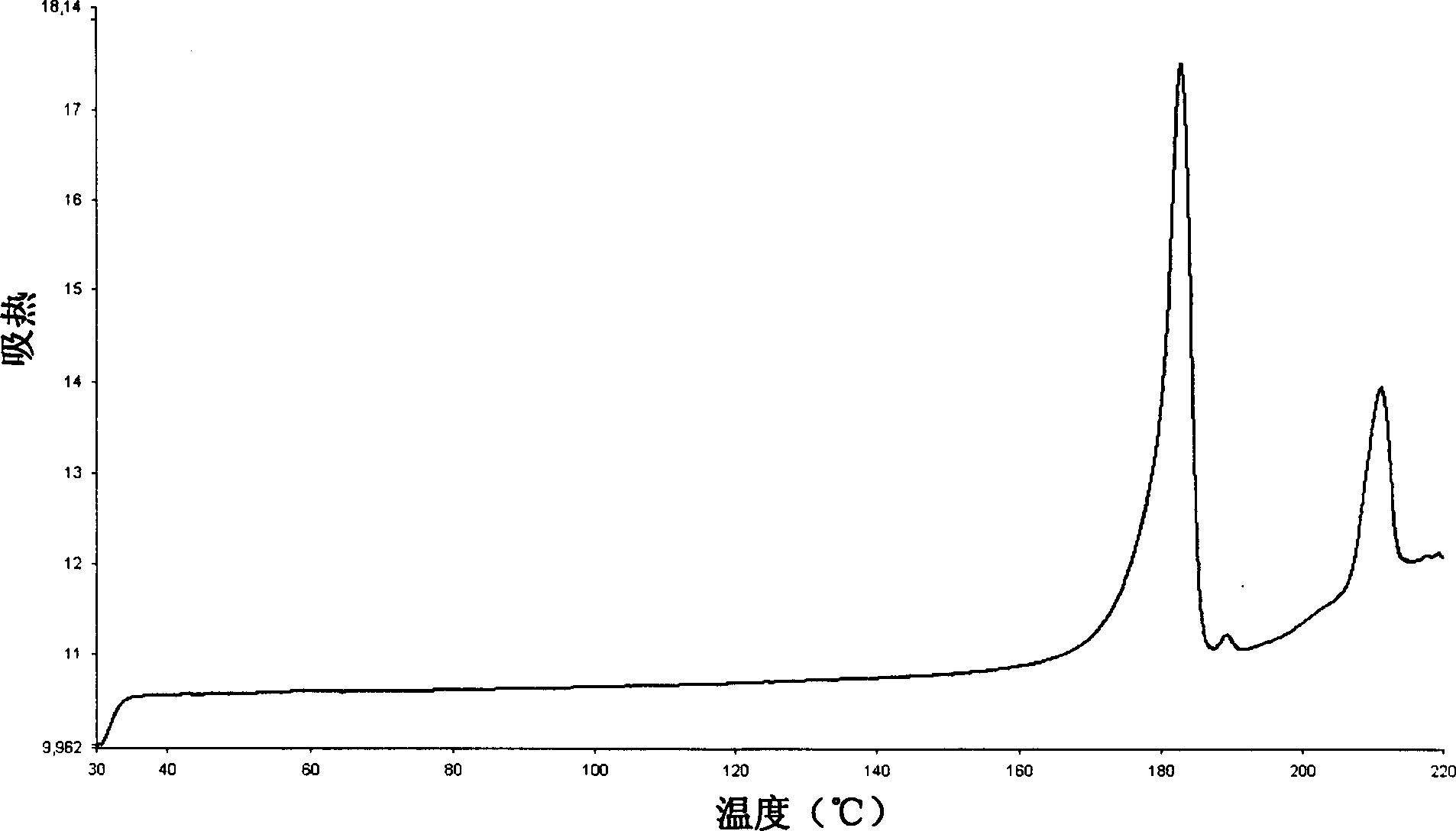
The invention relates to a method for producing sulfuric acid chlorogray of styleó±, containing the following steps: under the protection of inert gas and cooled with iced water, adding aqueous solution with sodium carbonate / potassium into organic solvent comprising chlorogray salt until the pH is higher than 9; and demixing the liquid, extracting the water layer with the same organic solvent, after combining with organic phase, drying and concentrating it to get free chlorogray alkali; then dissolving it in solvent, dropping in stoichiometric sulfate liquor, and the chlorogray formed, with the temperature controlled between minus 20 Deg.C to 5 Deg.C; then filtering the mixed solution, vacuum drying it, and then the sulfuric acid chlorogray of styleó±can be collected. The sulfuric acid chlorogray produced in such way is proved to beó±morph crystal system confirmed by X-ray diffraction, infrared spectrum and DSC spectrogram, and the fusible point is 181~186 Deg.C, and the specific rotation is 52.0í½55.0 deg. (c=1, carbinol). The method can produce sulfuric acid chlorogray of styleó±steadly.
Owner:上海开特生物科技有限公司
Processes for preparing different forms of (s)-(+)- clopidogrel bisulfate
InactiveUS20070082924A1Easy to handleBiocideOrganic chemistryMedicinal chemistryClopidogrel Bisulfate
Owner:CADILA HEALTHCARE LTD
Clopidogrel bisulfate medicine composition and preparation method
ActiveCN102512415AGood reproducibilityQuality improvementOrganic active ingredientsPill deliveryPharmaceutical SubstancesOrganic chemistry
The invention relates to a clopidogrel bisulfate medicine composition and a preparation method. The composition contains clopidogrel bisulfate, as well as proper filler, disintegrant and lubricant, wherein the content of the clopidogrel bisulfate is 25-60%, the content of the filler is 35-65%, the content of the disintegrant is 2.5-8%, and the content of the lubricant is 2-6%. Additionally, the invention further provides a preparation method for preparing tablets from the composition. Each component in the composition disclosed by the invention is good in compatibility, low in price and easy to get; and the preparation for tablets is simple in process and without the need to add new equipment, capable of efficiently solving the problem of sticking appearing during preparation process, andsuitable for industrialized production.
Owner:SHANDONG QIDU PHARMA
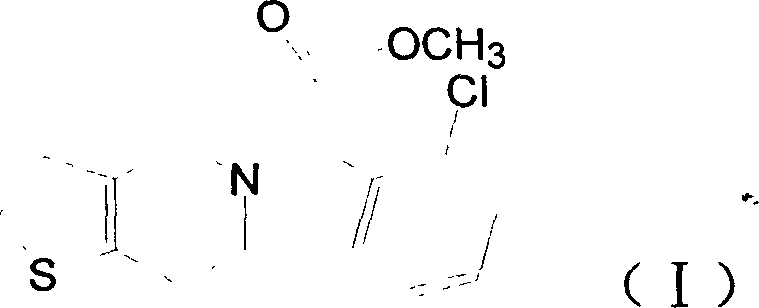
Process for the preparation of polymorphic forms of clopidogrel hydrogen sulfate
The present invention relates to a novel process for the preparation of polymorphic forms of clopidogrel hydrogen sulfate, namely methyl (+)-(S)-a-(o-chlorophenyl)-6,7-dihydrotliieno [3,2-c] pyridine-5(4H)-acetate hydrogen sulfate of formula (I). Particularly the present invention relates to the process for the preparation of form (I) and amorphous clopidogrel hydrogen sulfate.
Owner:IND SWIFT LAB
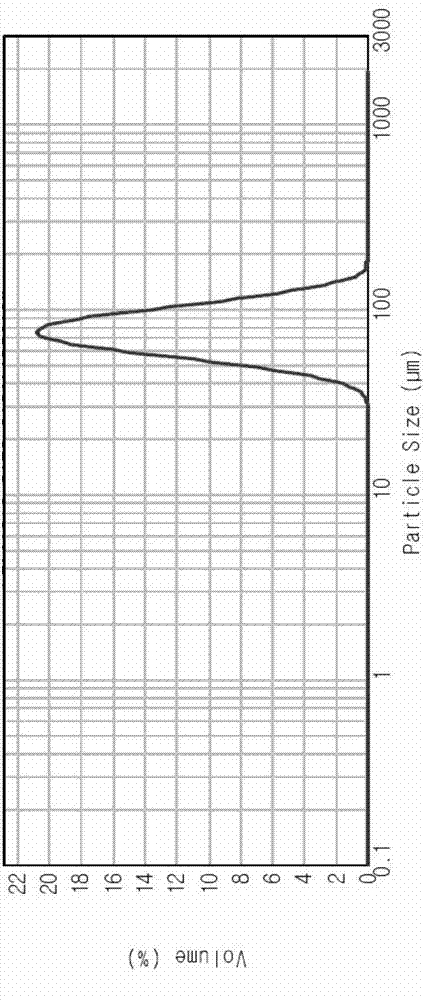
Composition containing clopidogrel bisulfate crystal particles
ActiveCN101851247ASolve the sticking problemAvoid mass generationOrganic active ingredientsOrganic chemistryCrystalline particleClopidogrel Bisulfate
The invention discloses clopidogrel bisulfate crystal particles with the median particle diameter of at least 120 microns. The invention also discloses a method for preparing the crystal particles, a pharmaceutical composition containing the crystal particles and a preparation method of the pharmaceutical composition.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Formulations of Clopidogrel Bisulphate
InactiveUS20090060996A1Improve stabilityImprove solubilityBiocidePill deliveryClopidogrel bisulphateDissolution
This invention relates to pharmaceutical tablet formulations of clopidogrel bisulphate which include glyceryl dibehenate as lubricant. The tablets are found to be very stable and to exhibit suitable dissolution characteristics.
Owner:ACTAVIS GRP PTC EHF
Preparation method for preparing high-purity II type (+)-(s)-clopidogrel bisulfate
The invention provides a preparation method for preparing high-purity II type (+)-(S)-clopidogrel bisulfate. The preparation method comprises the following steps of: dissolving I type (+)-(S)-clopidogrel bisulfate in C1-C3 lower alcohol, adding a poor solvent, stirring and precipitating little amount of crystal, filtering and removing; and continuously stirring the mother liquor, crystallizing, filtering, vacuum-drying and obtaining the high-purity II type (+)-(S)-clopidogrel bisulfate. The product produced by the method has high purity and high yield, the technique condition is mild, and themethod is applicable industrial production.
Owner:ESTEVE HUAYI PHARMA
Stabilized L-Arginine platelet aggregation inhibitory compositions and processes for making same
Linking Magnesium ions to Nitric Oxide precursor L-Arginine, chemically 2-amino-5-guanidino valeric acid, with a platelet aggregation inhibitor compound such as, but not limited to acetylsalicylic acid or clopidogrel bisulfate, unexpectedly results in a pharmaceutically stabilized compositions with extended shelf life to be taken orally to provide gradual release vasodilatory and anti-platelet aggregation pharmacological activity with reduced potential for producing gastrointestinal lesions. L-Arginine releases ADNO (Arginine derived Nitric Oxide) in the coronary artery epithelium as EDRF (endothelium dependent relaxing factor) to dilate the arteries to promote blood flow to the myocardium, and the platelet aggregation inhibitor such acetylsalicylic acid or clopidogrel and others of this class of drugs inhibits or antagonizes the aggregation adhesion of platelets in the blood stream. Aggregated or clumped blood platelets contribute to arterial stenosis due to formation of atherosclerotic plaques that occlude coronary and other circulatory arteries. In addition to coronary arteries, atherosclerotic plaques can occlude and stenose carotid arteries and femoral arteries due to aggregated or clumped blood platelets, and the subject of this patent discovery will also be of cardiovascular health benefit respectively in preventing carotid cerebrovascular accidents and femoral artery leg circulation disease.
Owner:KAPLAN LEONARD L
Novel method for synthesizing related substance B of clopidogrel bisulfate
ActiveCN101591346ASave raw materialsHigh yieldOrganic chemistryBlood disorderAcid hydrolysisPlatelet
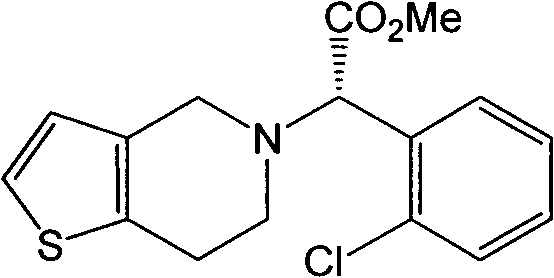
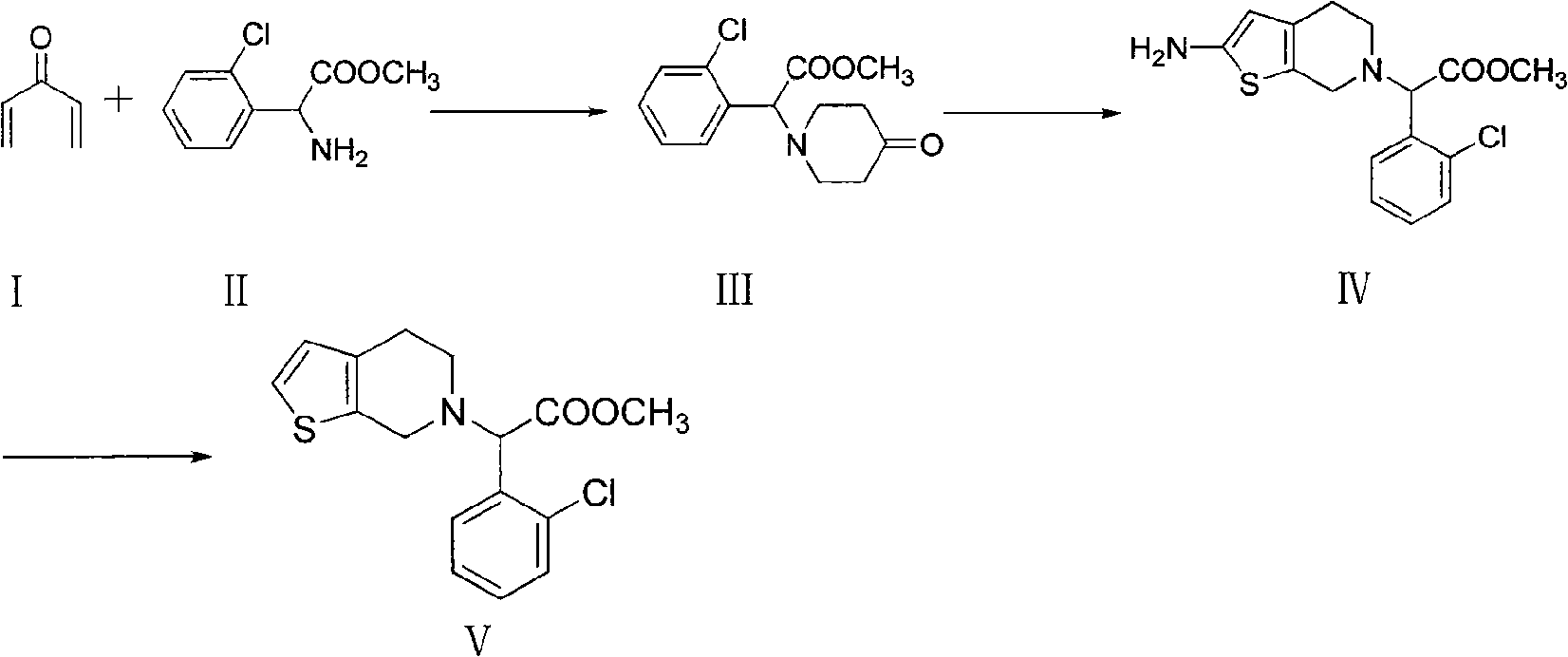
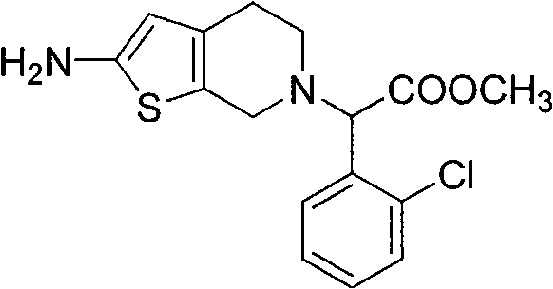
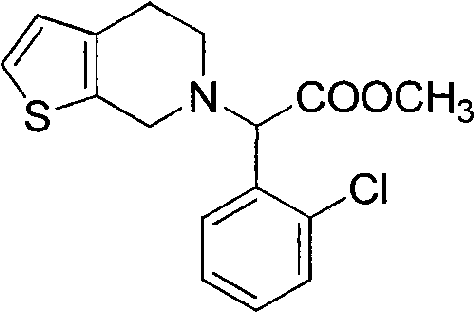
The invention relates to a method for preparing a related substance B in a platelet aggregation inhibition medicament-clopidogrel bisulfate. The method comprises the steps of: adopting dienone and L-2-chlorophenylglycine methyl ester to obtain tetrahydropyridone through a closed-loop reaction; then obtaining a 2-aminothienopyridine derivative through a Gewald reaction; and finally obtaining a target product through diazotization and acid hydrolysis. The process takes the synthesis of a pyridine ring first and then the synthesis of a thiophene ring as a route, obtains the target product with higher yield through a classical reaction, and provides novel thought for the synthesis of the related substance B of the clopidogrel bisulfate.
Owner:北京华禧联合科技发展有限公司
Preparation method of clopidogrel hydrogen sulfate I
InactiveCN102070648AHigh purityOvercome the external conditions of synthesisOrganic chemistryOrganic solventDrug compound
The invention relates to a preparation method of a drug compound, in particular to a preparation method of clopidogrel hydrogen sulfate I. The method provided by the invention comprises the following steps: 1, mixing clopidogrel salt with an organic solvent and water, adding an acid binding agent to react to generate free alkali of clopidogrel, extracting the water layer with the organic solvent, combining the organic phases and carrying out concentration; and 2. adding the organic solvent into the free alkali of clopidogrel obtained after concentration, dropwise adding sulfuric acid into the solution for reaction based on the molar ratio of the sulfuric acid to the free alkali of clopidogrel, filtering the reactant and drying the filtrate to obtain the crystal form of clopidogrel hydrogen sulfate I, wherein the molar ratio is (0.8-1.05):1.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Crystallizing method for obtaining I crystal form (+)-(S)-clopidogrel bisulfate
InactiveCN102050829AThe crystallization process is easy to controlHigh yieldOrganic chemistryAlcoholSolvent
The invention discloses a crystallizing method for obtaining I crystal form (+)-(S)-clopidogrel bisulfate. The method disclosed by the invention comprises the following steps: dissolving (+)-(S)-clopidogrel free alkali in an alcohol solvent, and dropwise adding an alcohol solution of concentrated sulfuric acid, thereby obtaining the I crystal form (+)-(S)-clopidogrel bisulfate by controlling the dropwise adding temperature, mixing time and recrystallization temperature.
Owner:北京华禧联合科技发展有限公司
Synthesis method of high-purity I-type (+)-(S)-clopidogrel hydrogen sulfate
The invention discloses a synthesis method of high-purity I-type (+)-(S)-clopidogrel hydrogen sulfate, which comprises the following steps: 1) mixing an acetone solution of L(-)-camphorsulfonic acid and an acetone solution of (+)-(S)-clopidogrel crude product to carry out salification reaction for 6-10, filtering after the reaction finishes, and treating the filter cake to obtain (+)-(S)-clopidogrel L(-)-camphorsulfonate; 2) dissolving the product obtained in the step 1) in a mixture of dichloromethane or ethyl acetate and water, regulating the pH value to 7-8, stirring for 10-60 minutes, standing to stratify, and treating the organic layer to obtain a (+)-(S)-clopidogrel pure product; and 3) dissolving the product obtained in the step 2) in an organic solvent, adding a crystal seed, stirring, adding an organic solvent solution of sulfuric acid, heating to 50-60 DEG C, stirring for 1-3 hours, cooling to room temperature, filtering, and treating the filter cake to obtain the high-purity I-type (+)-(S)-clopidogrel hydrogen sulfate. The method has the advantages of simple technique, high product yield and high product purity, and is suitable for industrial production.
Owner:SHANGHAI MODERN HASEN SHANGQIU PHARMA
Production technique of clopidogrel hydrogen sulfate
The invention relates to a production technique of clopidogrel hydrogen sulfate, which comprises the following steps: (1) preparation of amide hydrochloride, (2) preparation of levo-camphorsulfonate, (3) preparation of clopidogrel hydrogen sulfate crude product and (4) preparation of I-type clopidogrel hydrogen sulfate. The technique is simple, reduces the synthesis steps, lowers the consumption of toxic solvents, enhances the drug safety, facilitates the recovery and treatment of the waste liquid and lowers the production cost. The purity of the clopidogrel hydrogen sulfate obtained by the method is high, and the yield is 6.5% higher than other methods.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Pharmaceutical composition containing spherical clopidogrel bisulfate I crystal form and preparation method of pharmaceutical composition
ActiveCN105012298AComply with clinical drug requirementsConducive to the realization of powder direct compression processOrganic active ingredientsOrganic chemistryClopidogrel BisulfateCrystal
The invention provides pharmaceutical composition containing spherical clopidogrel bisulfate I crystal form and a preparation method of the pharmaceutical composition. Direct powder compressing can be achieved favorably by the powder property of the pharmaceutical composition, and sticking during tablet compressing is avoided. The invention further provides a clopidogrel bisulfate tablet using direct powder compressing and a preparation method of the tablet. The tablet meets the requirements of clinical medication.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD +2
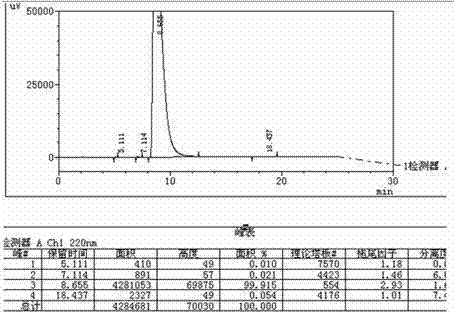
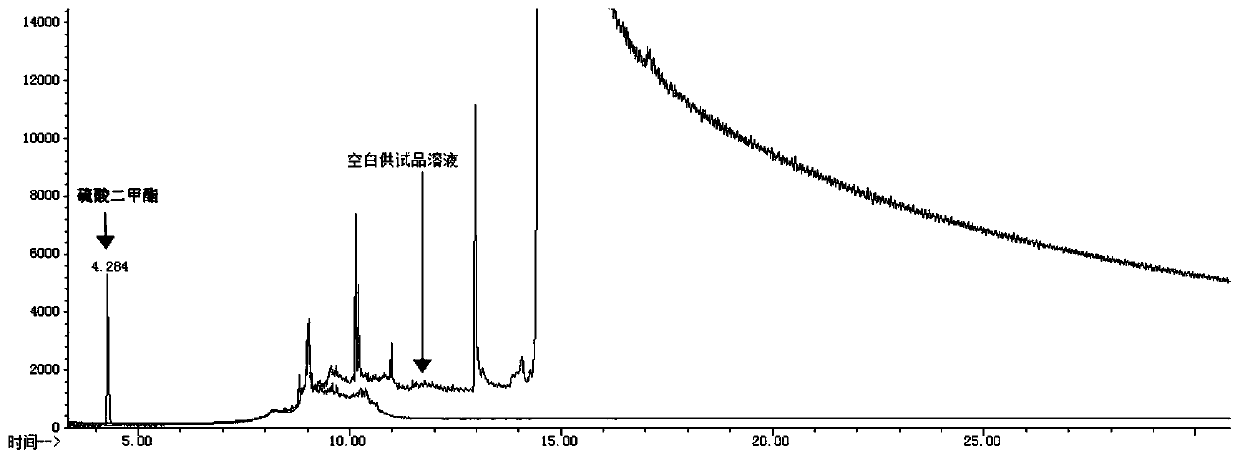
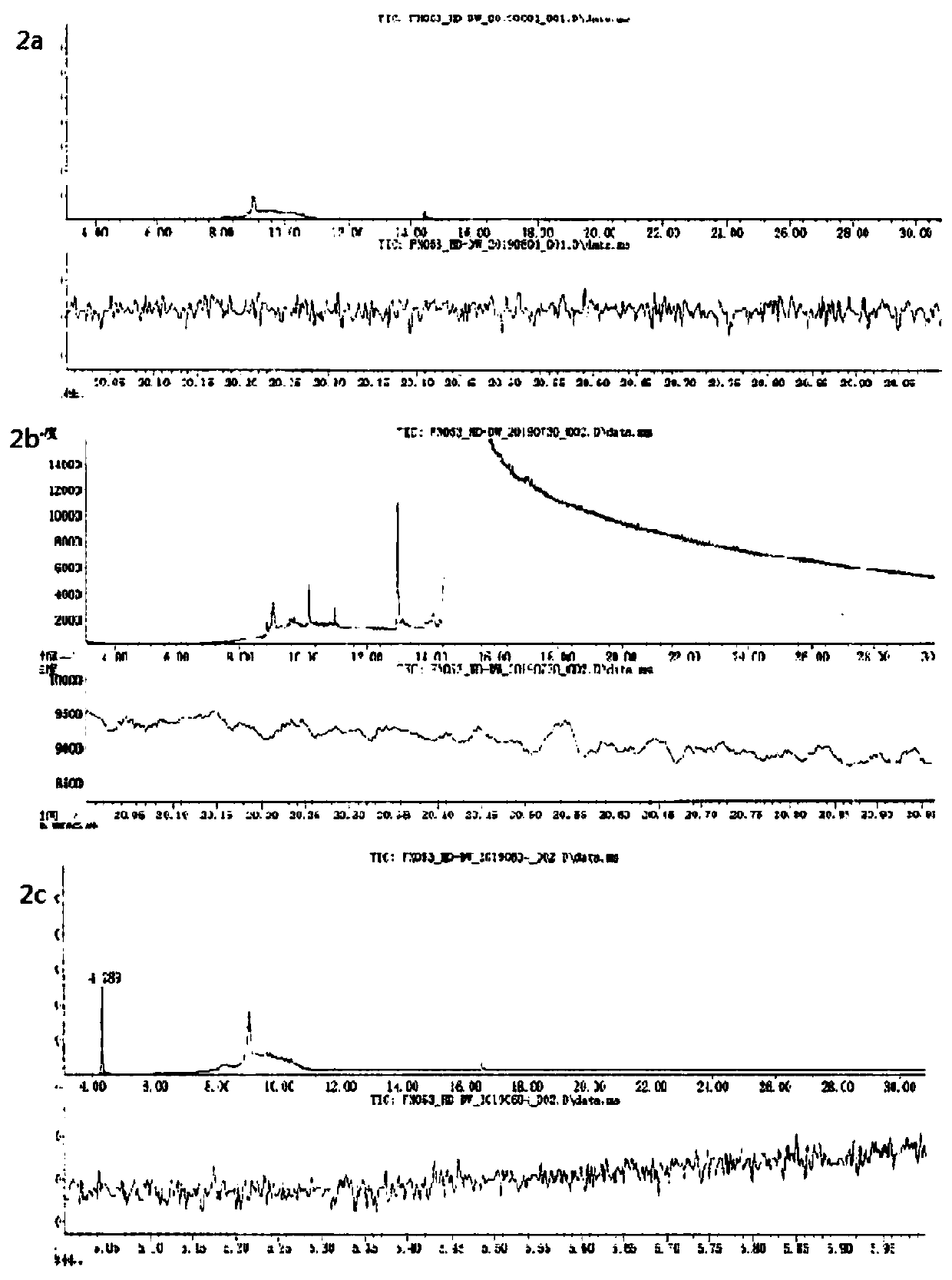
Method for determining content of dimethyl sulfate in clopidogrel hydrogen sulfate
InactiveCN111579689AHigh detection sensitivityImprove stabilityComponent separationPhysical chemistryMass spectrometry
The invention discloses a method for determining the content of dimethyl sulfate in clopidogrel hydrogen sulfate, and belongs to the technical field of medicines. The method adopts a gas chromatograph-mass spectrometer to determine the content of dimethyl sulfate in clopidogrel hydrogen sulfate, and comprises the following steps: firstly, preparing a test solution, and storing the prepared solution in an environment below 0 DEG C for later use; then setting detection conditions of a gas chromatograph-mass spectrometer; after the sample introduction condition is met, starting sample introduction, completing the detection process, and rcording a chromatogram. The method for detecting the dimethyl sulfate in the clopidogrel hydrogen sulfate is high in detection sensitivity, the linear range of the dimethyl sulfate is within the range of 5%-500% of the limit concentration, and the linear relation between the concentration and the peak area is good. According to recovery rate, repeatabilityand sample injection precision tests, the precision and accuracy of the method are good. A reference substance solution stability test shows that the reference substance solution has good stability after being placed at 0 DEG C or below for 6 hours.
Owner:JIANGSU LIANHUAN PHARMA
Type I clopidogrel hydrogen sulfate salt preparation method
The invention discloses a type I clopidogrel hydrogen sulfate salt preparation method which comprises the following steps: (1) mixing clopidogrel salt with an organic solvent, adding water, adding an acid binding agent or an acid binding agent water solution to the solvent, stirring for reaction to generate clopidogrel free alkali, standing for layering, extracting aqueous phase with the same organic solvent, combining the organic phase, washing with water, recovering the solvent to dry; and (2) adding an organic solvent into the concentrated clopidogrel free alkali, stirring to fully dissolve the clopidogrel free alkali, cooling, adding seed crystal, according to the molar ratio of sulfuric acid to clopidogrel free alkali of 0.9-1.05:1, dropwise adding sulfuric acid solution into the solution, standing, filtering, and drying to obtain the type I clopidogrel hydrogen sulfate. The type I clopidogrel hydrogen sulfate obtained by the method has the advantages of high purity and stable property.
Owner:YABAO PHARMA GRP CO LTD
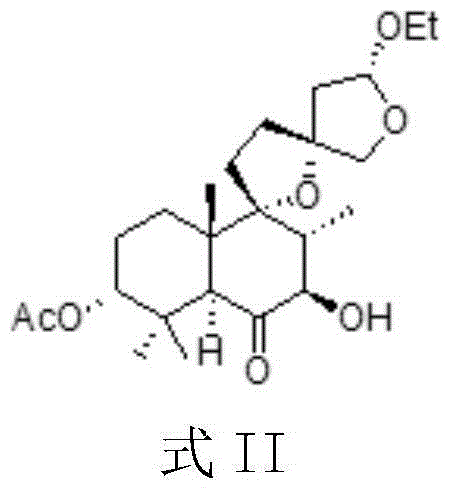
Crystal form of diterpenoid compound
ActiveCN104086559A"Clopidogrel bisulfate" works wellGood effectOrganic chemistryBlood disorderSpace groupLeonurus japonicus
The invention provides a crystal form of a compound as shown in the formula II. The crystal form belongs to an orthorhombic crystal system; the space group is P212121; the crystal cell parameters are as shown in the specification, wherein alpha is equal to 90.00 DEG, beta is equal to 99.00 DEG, and gamma is equal to 90.00 DEG; Z is equal to 4; the crystal cell volume is as shown in the specification. The invention also provides a preparation method and application of the crystal form. The compound with the crystal form in the formula II, which is separated from leonurus japonicus, has a certain inhibiting effect on platelet aggregation and has a better effect than clopidogrel bisulfate as an anti-platelet aggregation drug under the same concentration (0.1mM), so that a new choice is provided for developing a novel natural anti-platelet aggregation drug.
Owner:CHENGDU FIRST PHARMACEDTICAL CO LTD
Method for preparing crystalline form I of clopidogrel bisulfate
The invention provides a method for preparing a crystalline form I of clopidogrel bisulfate. The method is characterized by comprising a step of dissolving raw materials of a clopidogrel bisulfate crystal form product into an organic solvent methanol or ethanol; a step of adding, in a temperature range of -30 DEG C to 30 DEG C, the dissolved and clarified solution into a mixed solution of an acid and an anti-solvent; a step of stirring uniformly and centrifuging for 3-60 min; and a step of drying the centrifugation product, after the centrifugation product is washed with the anti-solvent, to obtain the stable crystalline form I powder of the clopidogrel bisulfate. The product of the method is an aggregation of tetragonal-structured crystals, and has good stability. The method has simple operation, suitability for industrial production, capability of environmental protection, high product purity and high yield.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
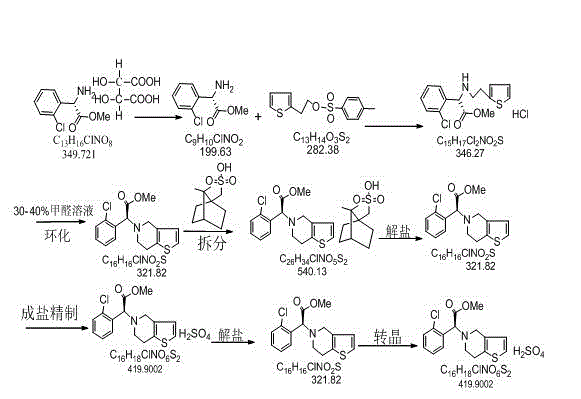
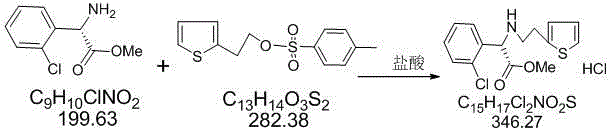
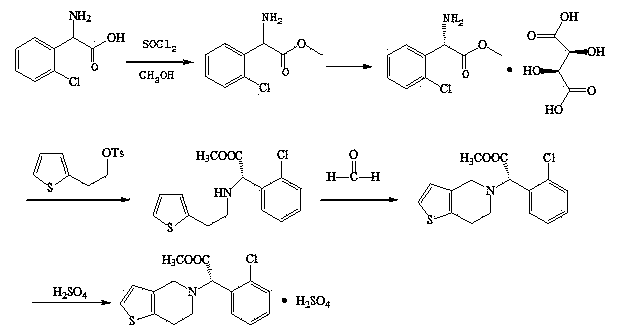
Preparation method of clopidogrel and intermediate thereof
ActiveCN103509037AHigh yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationChlorobenzeneBenzenesulfinic acid
The invention relates to a preparation method of clopidogrel and an intermediate of clopidogrel. The preparation method comprises the following steps: using L-2-chlorophenylglycine as a starting raw material, firstly taking reaction to L-2-chlorophenylglycine with methanol to generate L-2-chlorophenylglycine methyl ester, then using L-(+)-tartaric acid to split L-2-chlorophenylglycine methyl ester to obtain S-(+)-chlorophenylglycine methyl ester and L-(+)-tartrate, taking reaction to the split product with 2-(2-thienyl)-ethanol-p-toluenesulfonate to obtain 2-chlorophenyl-2-thienylethylamine methyl acetate hydrochloride, taking reaction with formaldehyde to close ring to obtain clopidogrel, and carrying out acidification with sulfuric acid to obtain clopidogrel hydrosulfate. The provided synthetic method is simple in route, high in yield, low in cost, and simple to operate. The method for preparing clopidogrel and the intermediate of clopidogrel is easy to industrially produce. During the test process, the method is free from special, toxic and harmful reagents, and beneficial to environment protection. The synthetic process can be operated at normal temperature; the reaction conditions are mild; the processing equipment is simple; the method is easy to realize industrialized production.
Owner:SHANDONG LUYAO PHARMA
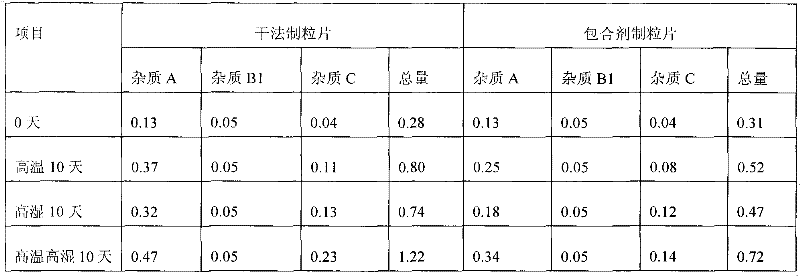
Preparation method of crystalline clopidogrel bisulfate tablets
ActiveCN102240269AIncreased fluidity and compressibilityImprove stabilityOrganic active ingredientsPill deliveryDistillationRoom temperature
The invention provides a preparation method of crystalline clopidogrel bisulfate tablets, comprising the following steps of: mixing a medicinal solvent and an inclusion agent with stirring, dissolving by heating to the temperature of 35-90 DEG C, staying cool to room temperature; adding the crystalline clopidogrel bisulfate with uniformly stirring; drying the medicinal solvent by distillation, sieving to obtain clopidogrel bisulfate inclusion particles; uniformly mixing the obtained clopidogrel bisulfate inclusion particles and a pharmaceutic adjuvant at the weight part ratio of 1:0.02-10, followed by tabletting, wherein the addition amount of the inclusion agent is 0.05-0.2 times the weight of clopidogrel bisulfate and the addition amount of the medicinal solvent is 0.5-10 times the weight of clopidogrel bisulfate. According to the clopidogrel bisulfate tablets provided by the invention, the crystal form is not changed; related substances are not increased, the fluidity and compressibility of the raw materials are enhanced, and the stability of the tablets is raised; in addition, the preparation method of the tablets is more suitable for large-scale industrial production.
Owner:天津泰普制药有限公司
Preparation Method Of The Solid Formulation Of Clopidogrel Bisulfate
InactiveUS20130011473A1Reduce adhesionReduce exposed surface areaBiocidePowder deliveryMedicineDiluent
A solid formulation of clopidogrel bisulfate and its preparation method are disclosed. The formulation comprises clopidogrel bisulfate as active ingredient, colloidal silicon dioxide as anti-adherent / coating and the carriers selected from diluent, binder, glidant, disintegrant and / or lubricant.
Owner:SHANGHAI ANBISON LAB
Preparation method of clopidogrel bisulfate solid preparation
ActiveCN110339178AReduce the probability of stickingIncrease dissolution rateOrganic active ingredientsDrageesSulfateDissolution
The invention relates to a preparation method of a clopidogrel bisulfate solid preparation. The method discloses a two-step granulation process of melt-granulating and then dry-pressing granulating for clopidogrel bisulfate, and the obtained granules have good compressibility and fluidity, and are not easy to stick to a die, so that the problems of tablet sticking, easy revealing or splitting which are common in the preparation of oral tablets of clopidogrel bisulfate is solved. The preparation method can ensure the rapid dissolution of the drug in the preparation, and the long-term retentionof the sample does not increase the impurities. The preparation process of the present invention is highly practical and can be used for large-scale preparation of clopidogrel sulfate bulk drugs withdifferent physical properties.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com