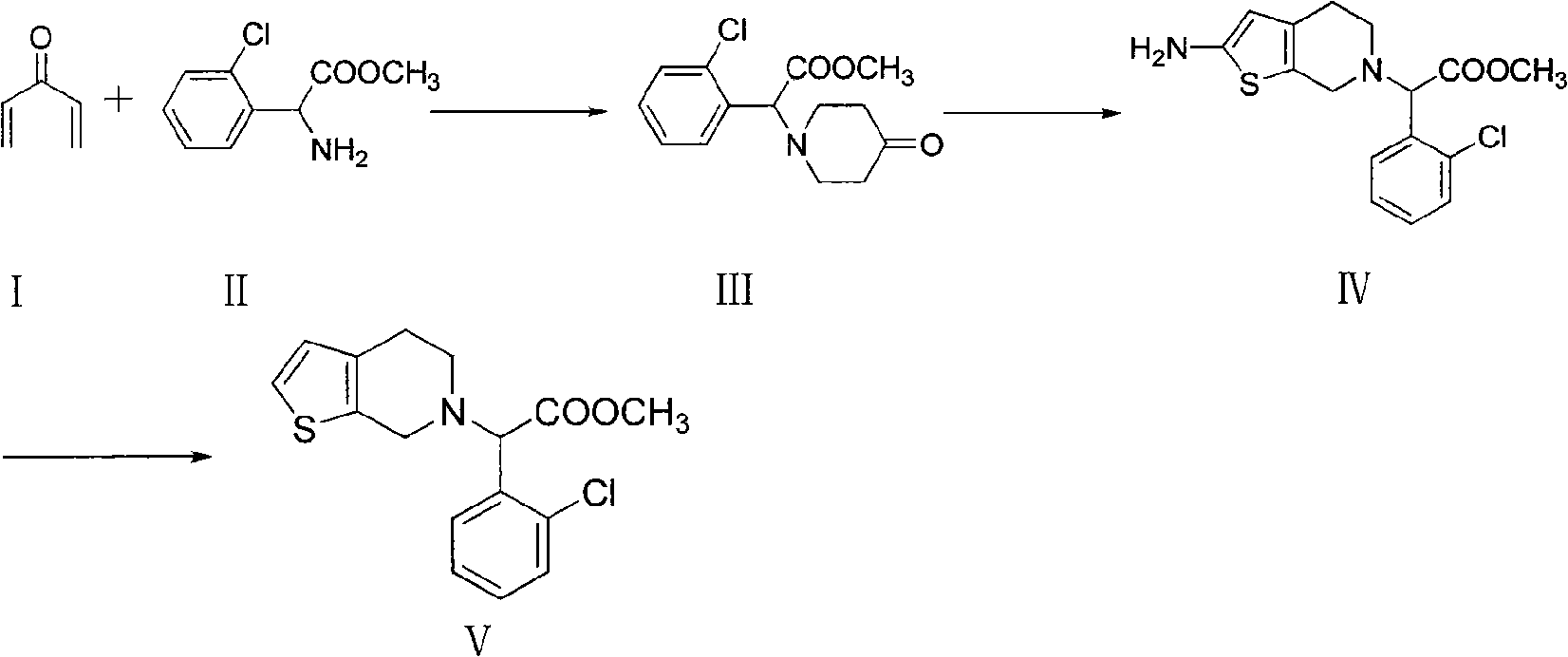
Novel method for synthesizing related substance B of clopidogrel bisulfate
A technology of clopidogrel hydrogen sulfate and related substances, applied in the new synthesis field of clopidogrel hydrogen sulfate related substance B, can solve the problems of high price and high cost of raw materials, and achieves the advantages of industrialized production, improvement of total yield, and cost reduction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Synthesis of methyl 2-(2-chlorophenyl)-2-(4-tetrahydropyridon-1-yl)acetate (III).
[0021] Add 8.21g (0.1mol) of I and 20g (0.1mol) of II into 200ml of DMF (such as other solvents mentioned above) and stir to dissolve, keeping a certain temperature. Afterwards, the reaction solution was slowly poured into ice water, and the oily matter was stirred to solidify, filtered, and recrystallized from ethanol to obtain a white solid powder. It was filtered and dried to obtain 22.6g III with a yield of 80% and a melting point of 152-154°C. 1H NMR spectrum data (chemical shift δ): 2.64(t, 4H, N-CH 2 ), 2.55(t, 4H, CH 2 -CO), 3.68(s, 3H, CH 3 ), 4.74(s, 1H, N-CH), 7.37, 7.43, 7.44, 7.51(m, 4H, Ph-H).
Embodiment 2
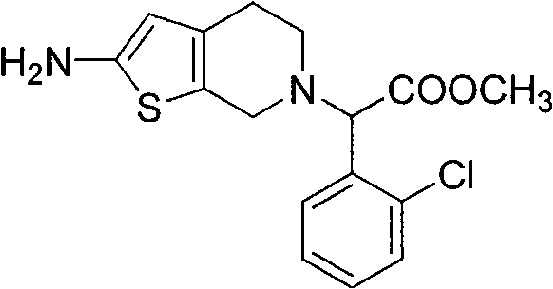
[0022] Example 2: 2-(2-amino-4,5 dihydrothiophene[2,3-c]pyridin-6(7H)-yl)-2-(2-chlorophenyl)methyl acetate (IV) synthesis.
[0023] Dissolve 22.5g (0.08mol) III, 2.56g (0.08mol) of sulfur powder, 9.05g (0.08mol) of ethyl cyanoacetate in 200ml of ethanol, add dropwise 15.75ml (0.18mol) of morpholine, and keep the reaction solution at a certain temperature The mixture was reacted at room temperature, filtered to obtain a solid, and recrystallized from ethanol to obtain 17.5 g of light yellow crystals, with a yield of 65% and a melting point of 195-197°C. 1H NMR spectrum data (chemical shift δ) is: 2.69~2.75(tt, 4H, CH 2 -CH 2 ), 3.62 (s, 2H, CH 2 ), 3.68 (s, 3H, CH 3 ), 4.74(s, 1H, CH), 5.55(s, 1H, CH), 6.99(s, 2H, NH 2 ), 7.51, 7.59, 7.65, 7.68 (m, 4H, Ph-H).
Embodiment 3
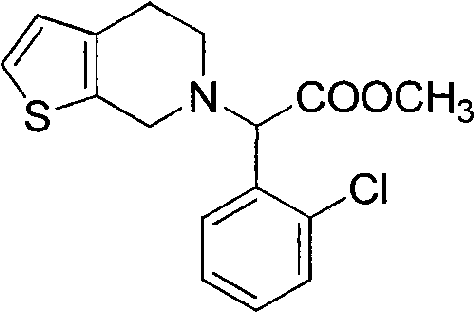
[0024] Example 3: Synthesis of methyl 2-(2-chlorophenyl)-2-(4,5-dihydrothieno[2,3-c]pyridin-6(7H)-yl)acetate (V).
[0025] Add 17.5g (0.052mol) III, hydrochloric acid into ice water, add sodium nitrite dropwise, keep at -5°C ~ 0°C, stir for reaction, then add hypophosphorous acid, stir at room temperature, add dichloromethane, add concentrated ammonia water dropwise, adjust pH to 8, liquid separation, concentration of the organic layer to obtain an oily substance, after adding acetone, concentrated sulfuric acid was added dropwise under ice bath conditions, stirred until a large amount of solid appeared, filtered, and dried to obtain 15.9 g of white solid as the target product V, the yield was 73 %. 1H NMR spectrum data (chemical shift δ): 2.69~2.75(tt, 4H, CH 2 -CH 2 ), 3.62 (s, 2H, CH 2 ), 3.68 (s, 3H, CH 3 ), 4.74 (s, 1H, CH), 5.55 (s, 1H, CH), 5.86, 7.38 (d, 2H, C 4 h 2 S), 7.52, 7.60, 7.67, 7.69 (m, 4H, Ph-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com