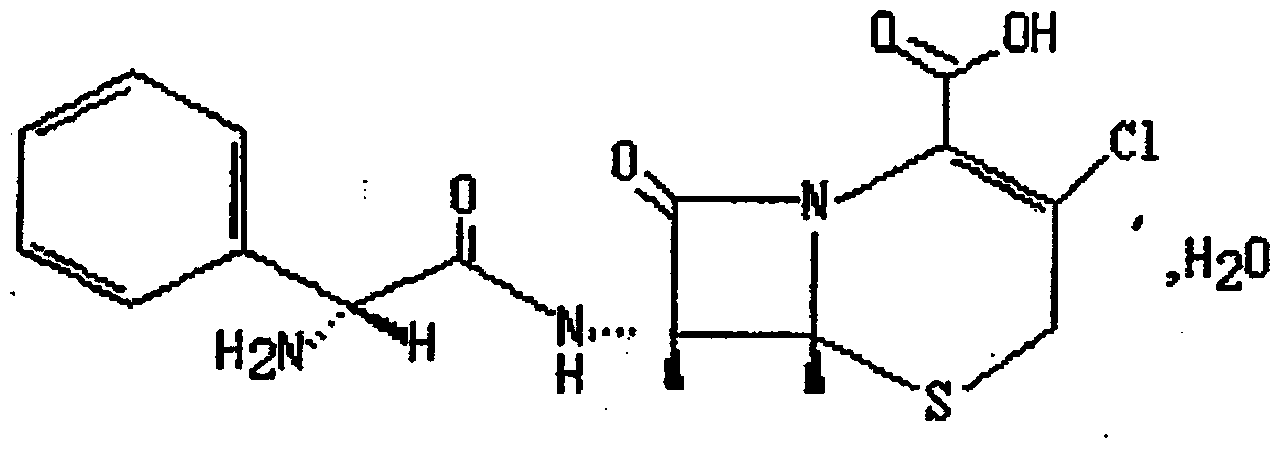
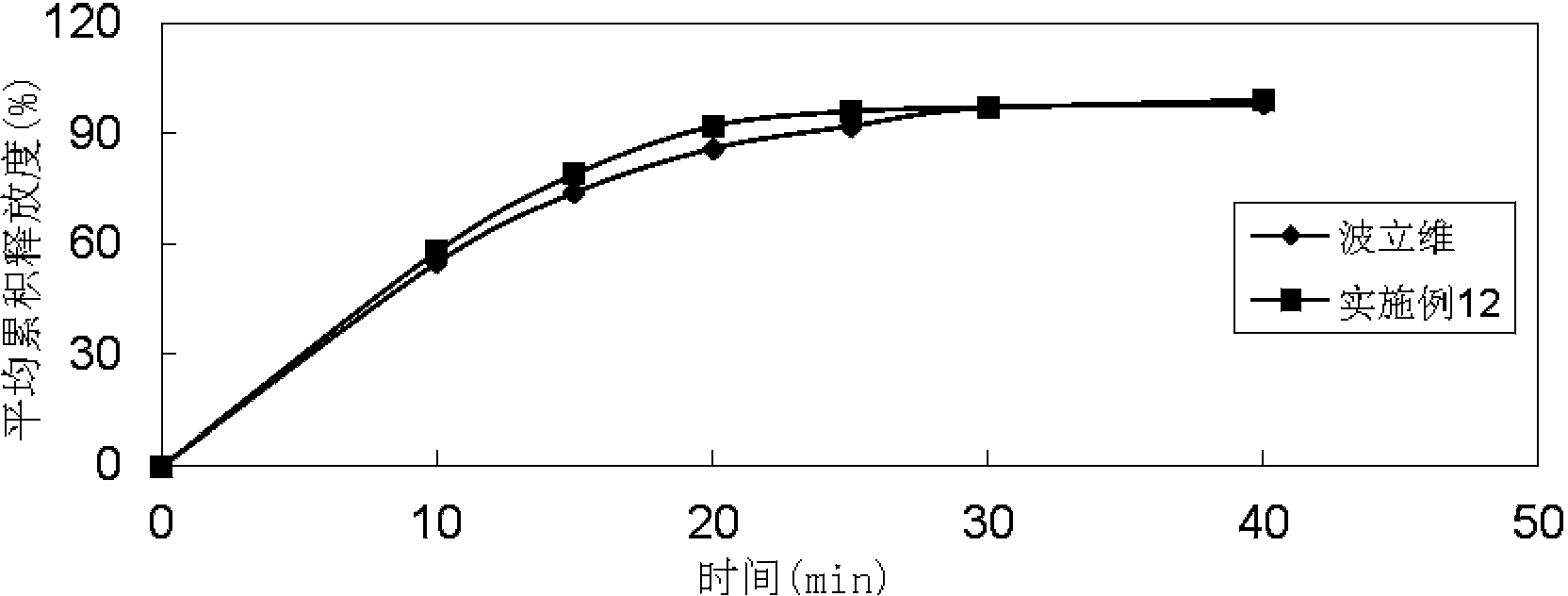
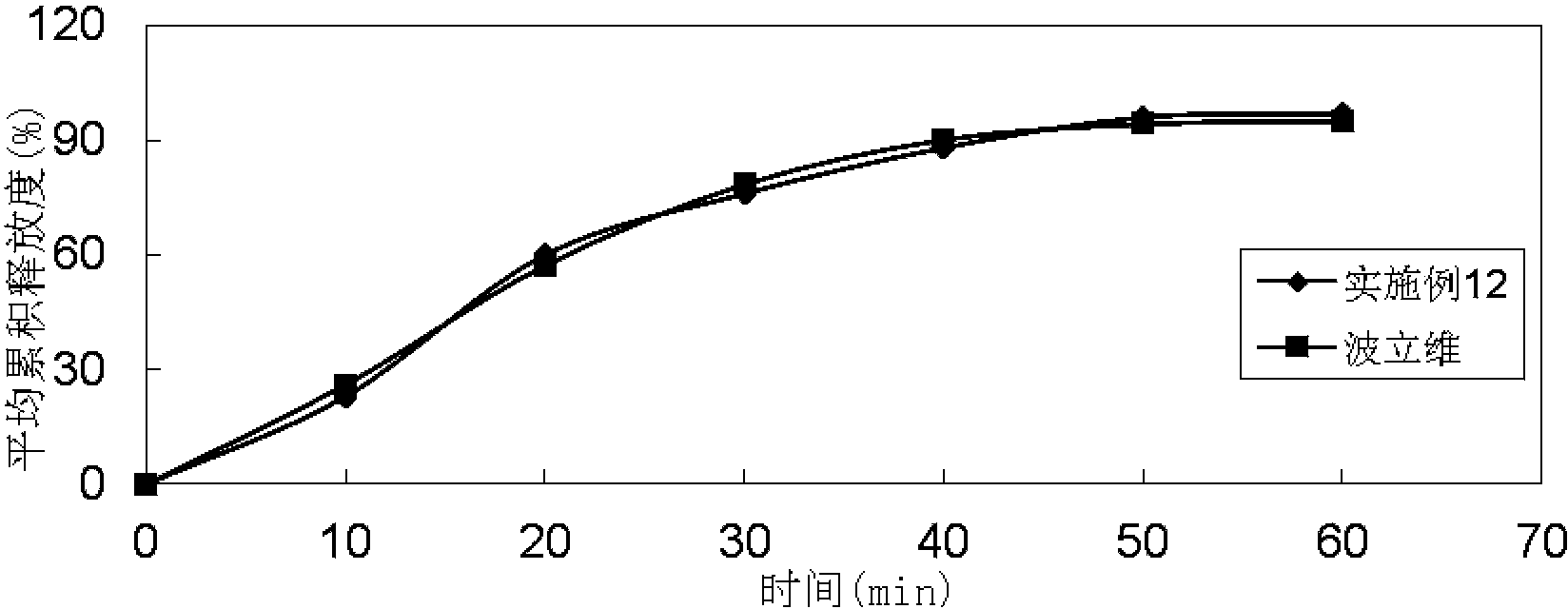
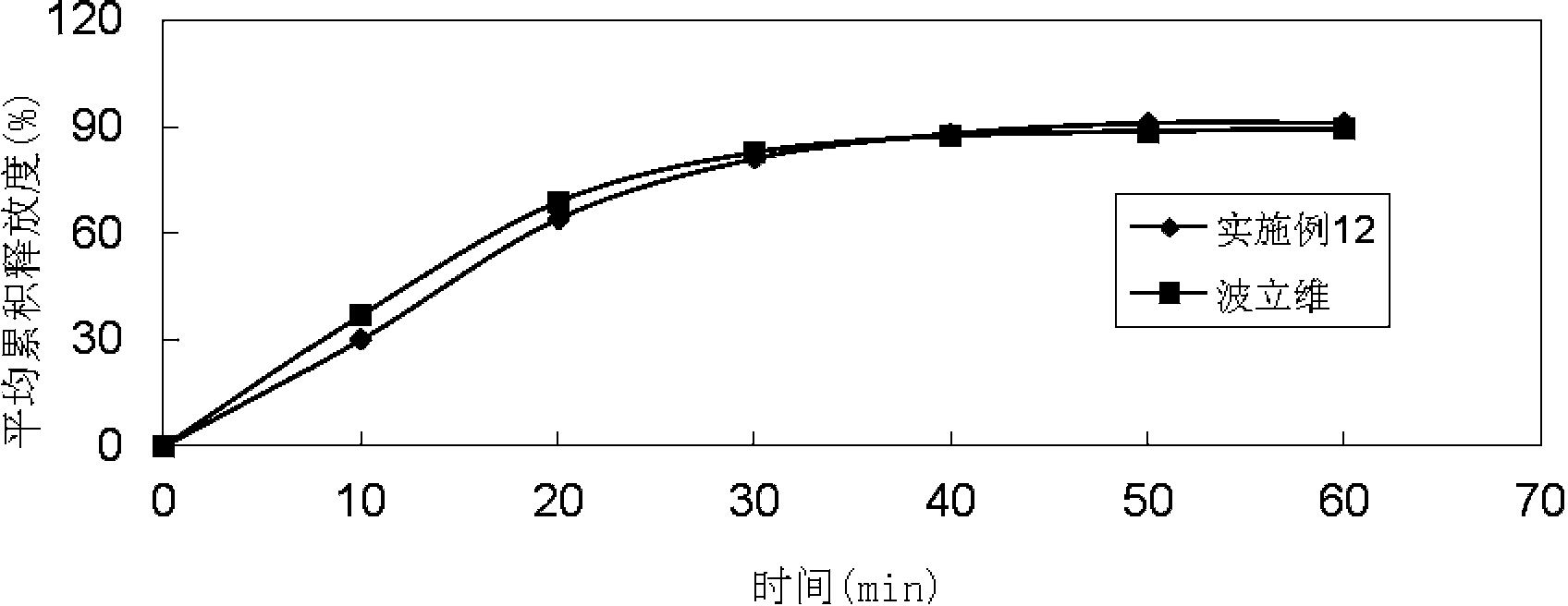
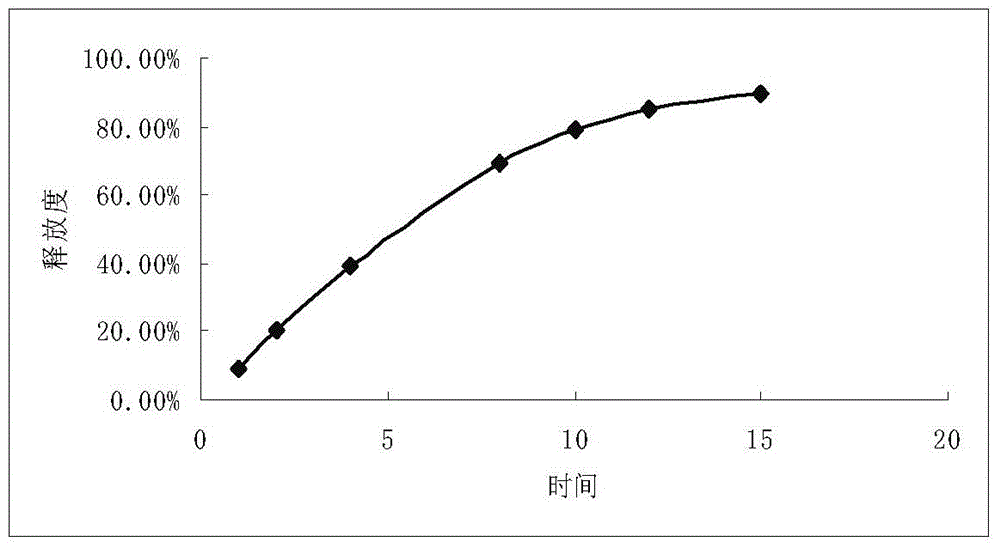
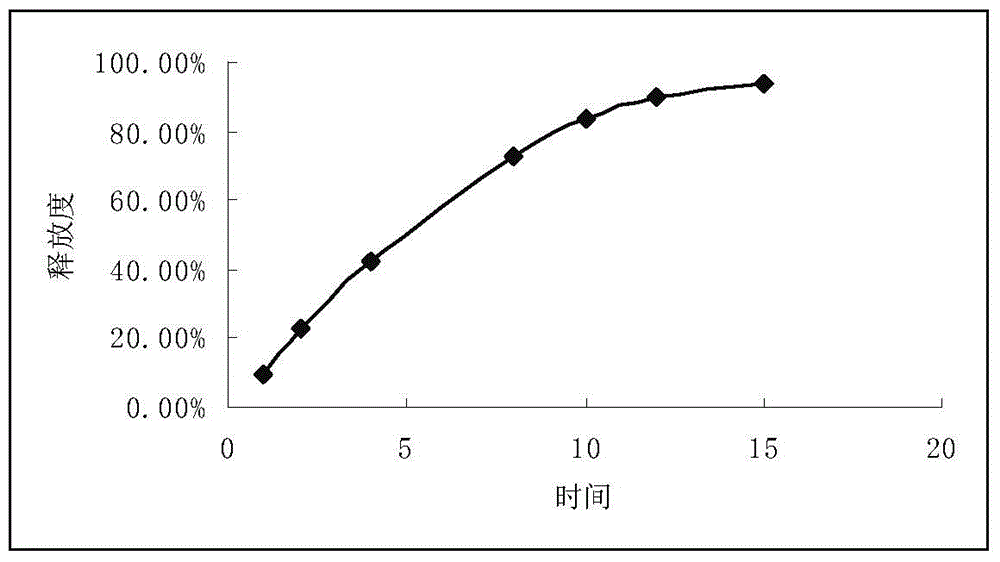
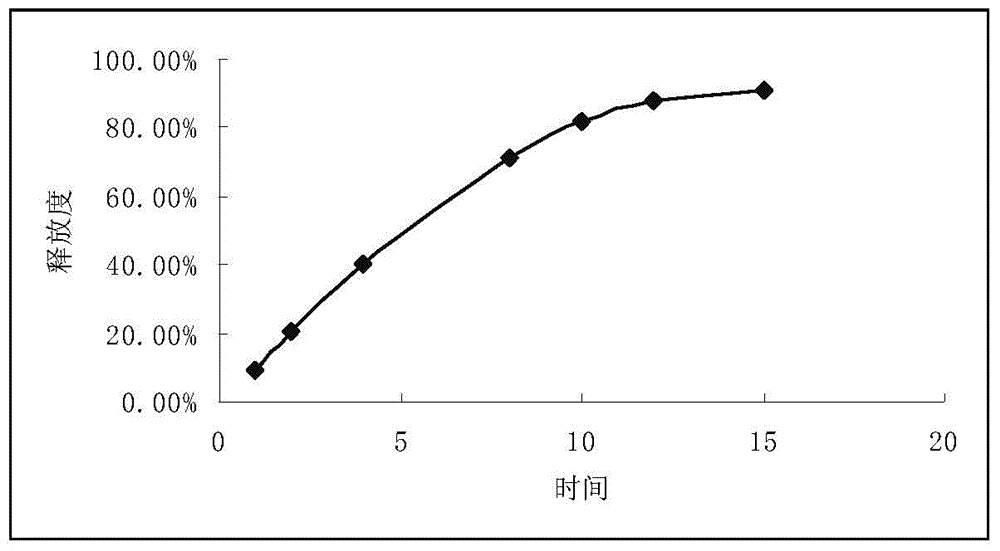
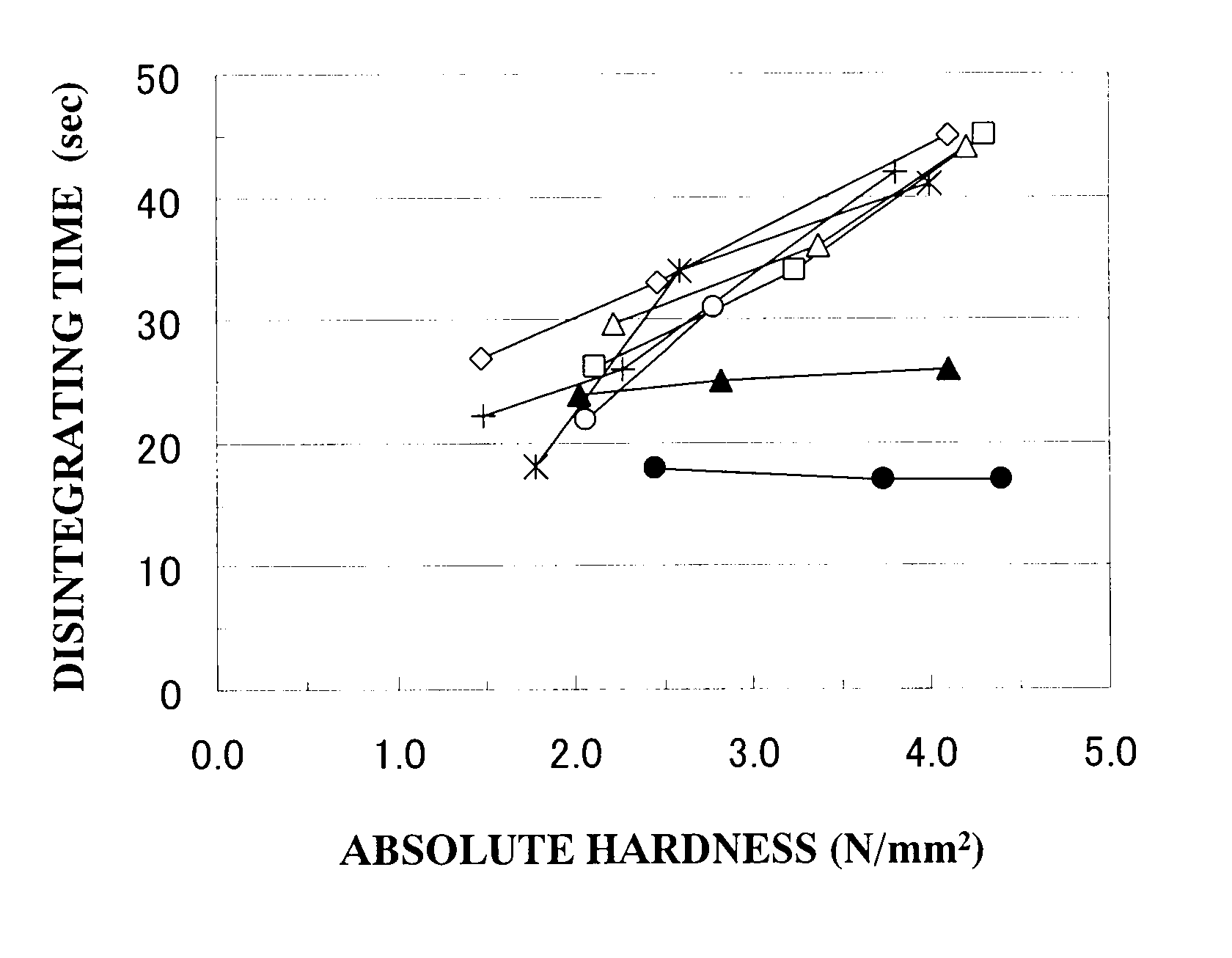
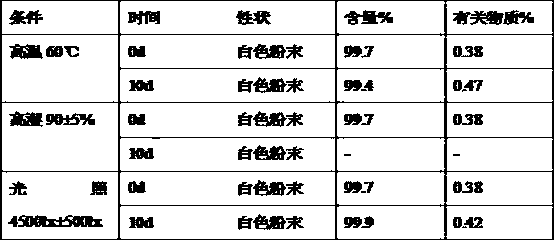
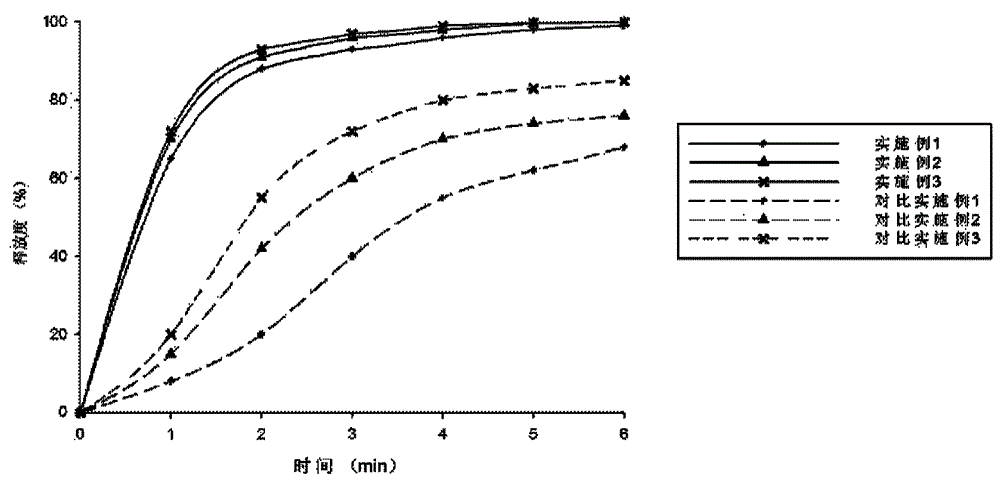
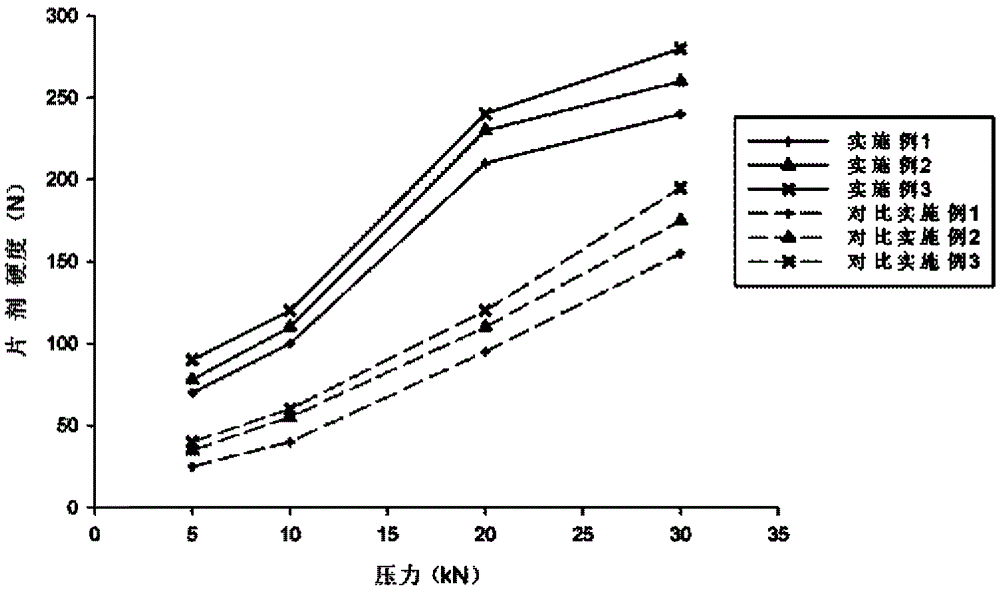
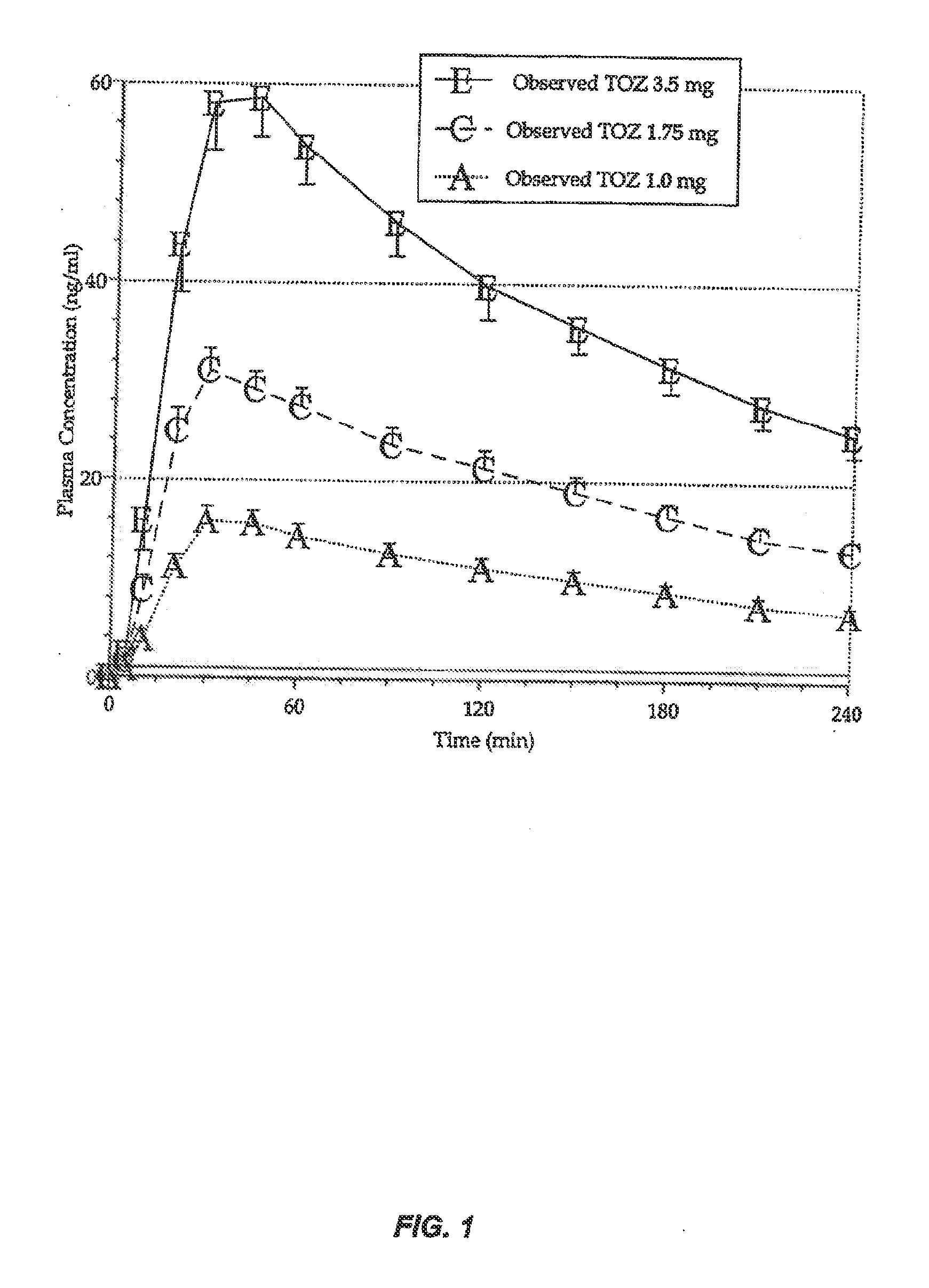
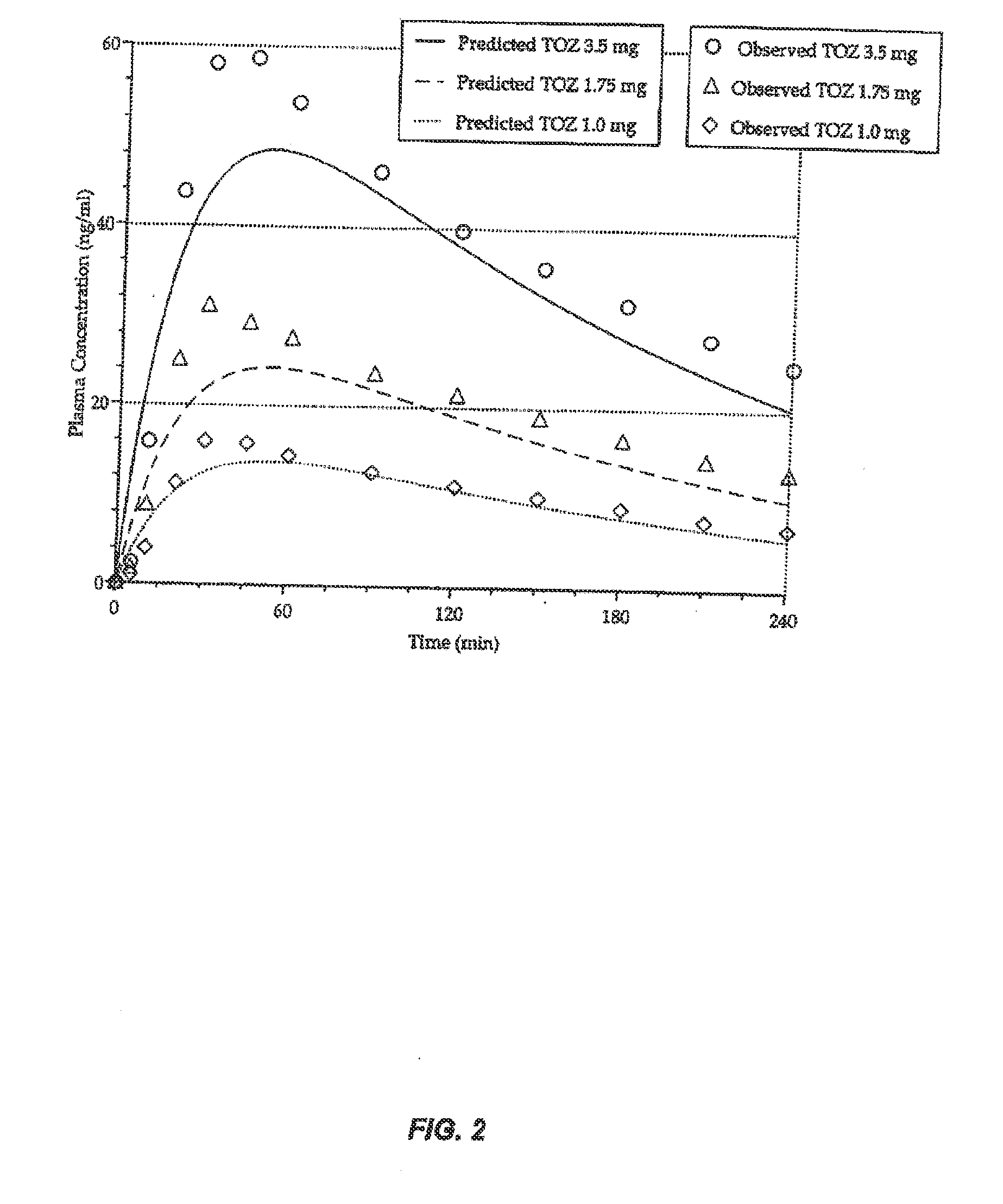
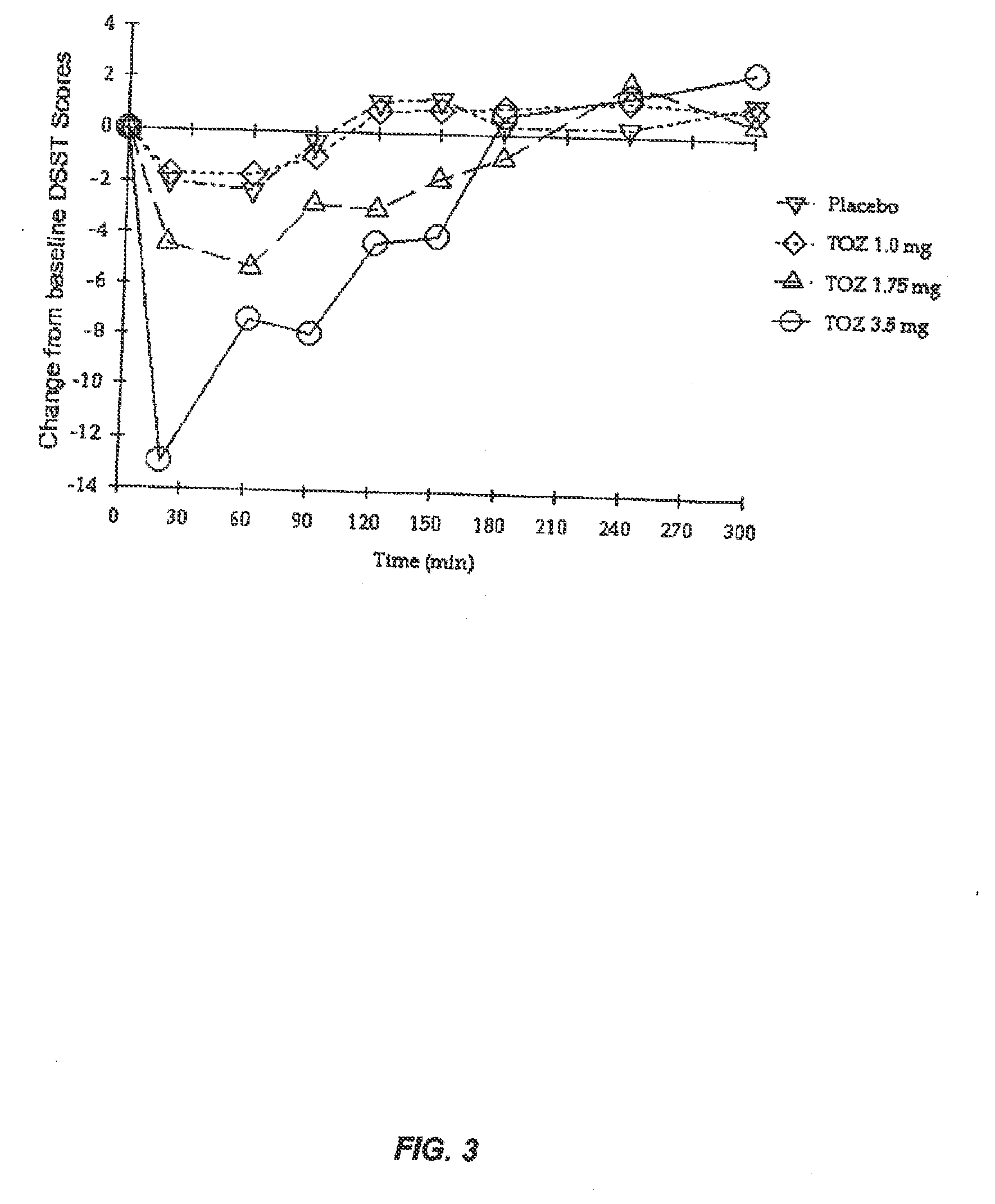
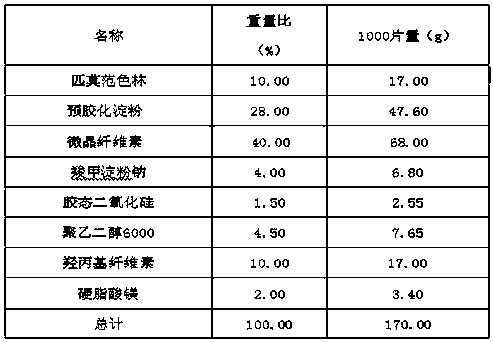
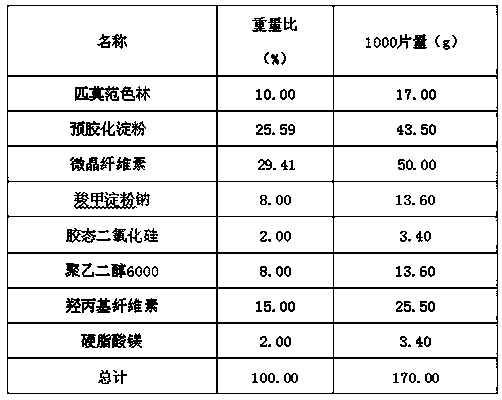
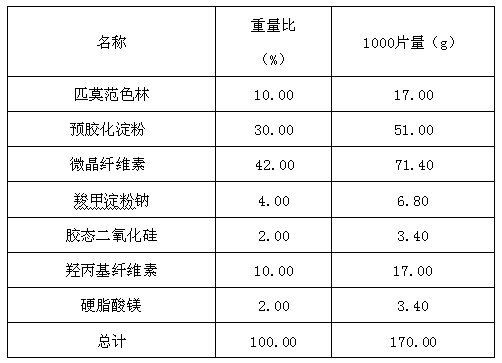
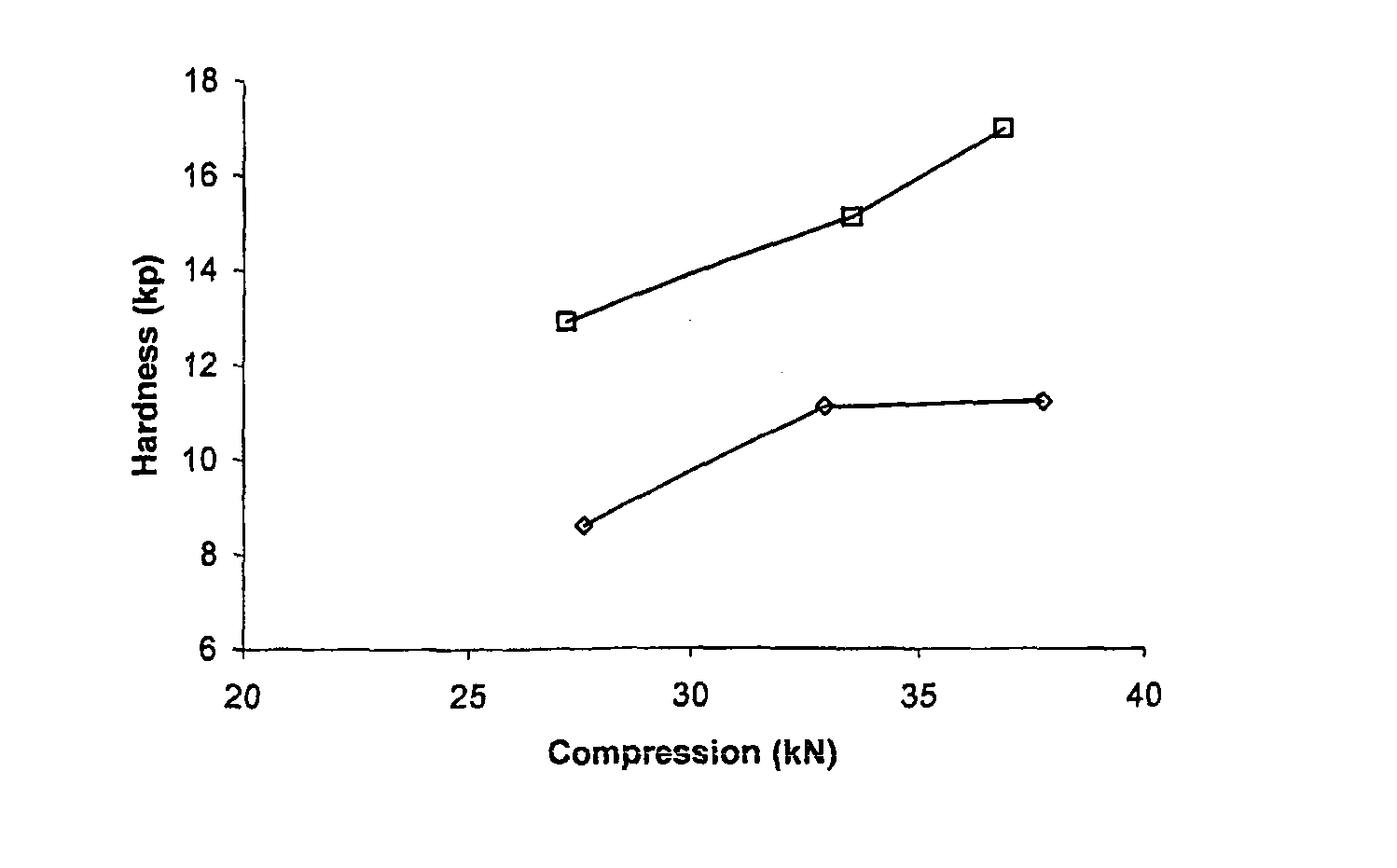
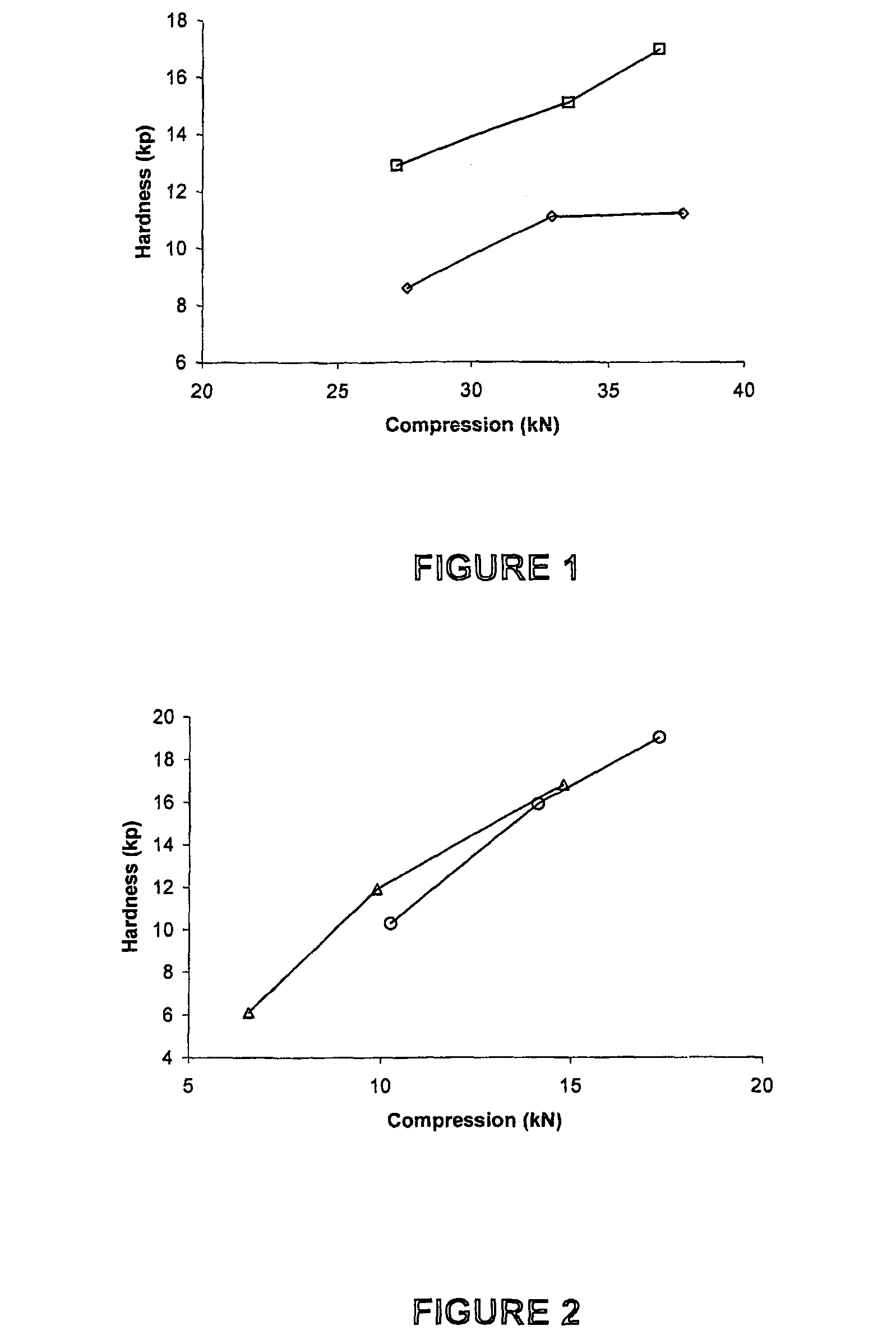
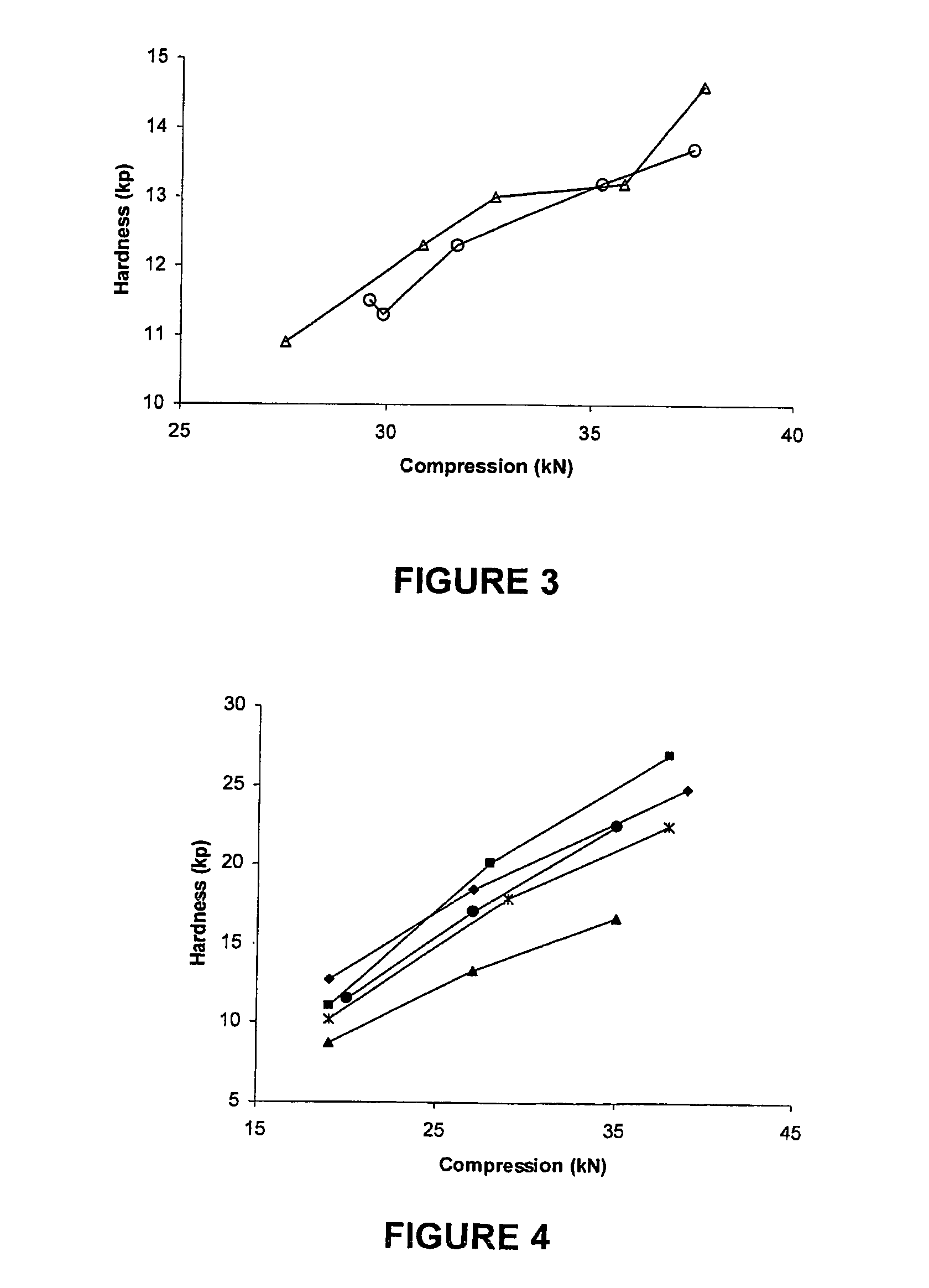
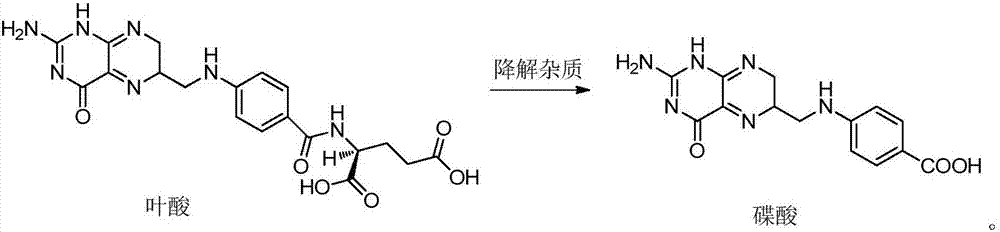
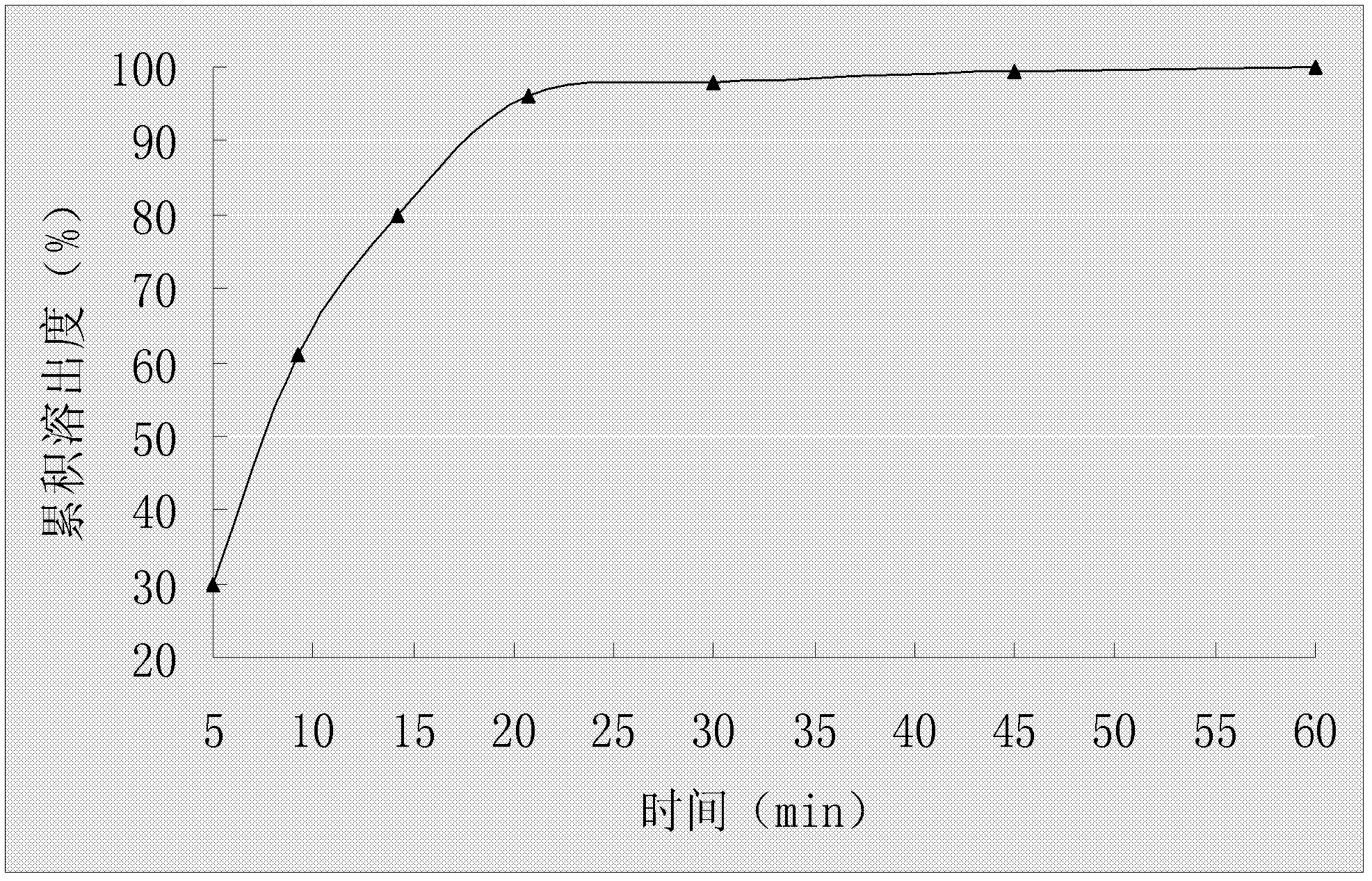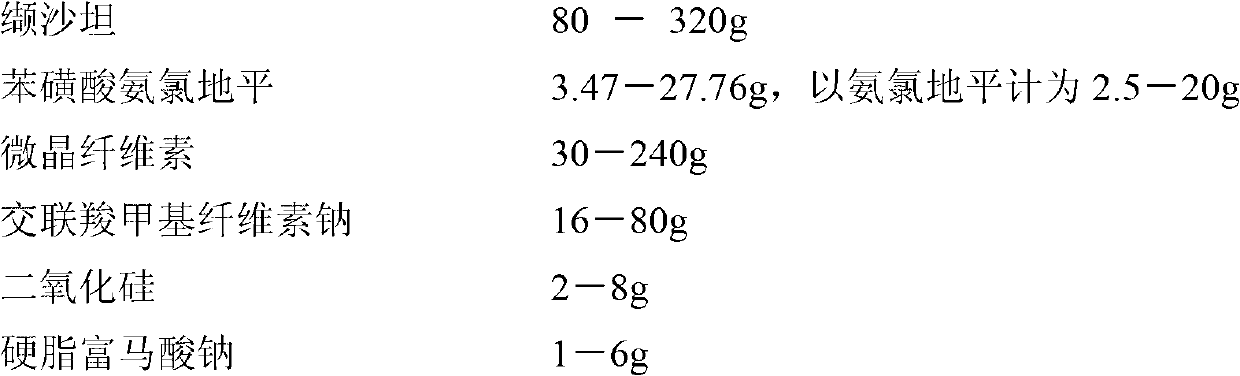Patents
Literature
133 results about "Sodium stearyl fumarate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clopidogrel bisulfate tablet formulation
Pharmaceutical tablets comprising clopidogrel bisulfate and a lubricant selected from zinc stearate, stearic acid, and sodium stearyl fumarate.
Owner:SHERMAN BERNARD CHARLES
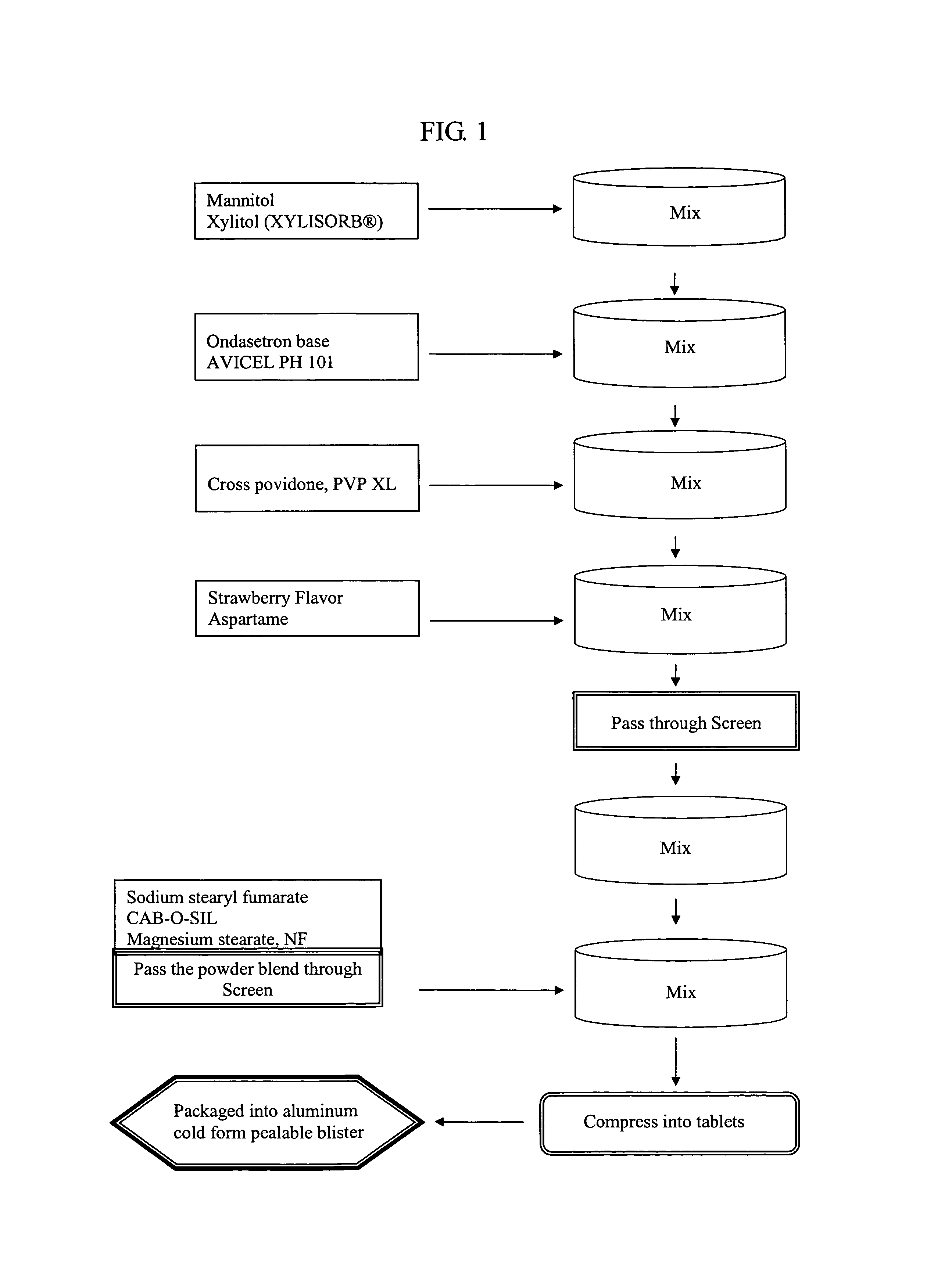
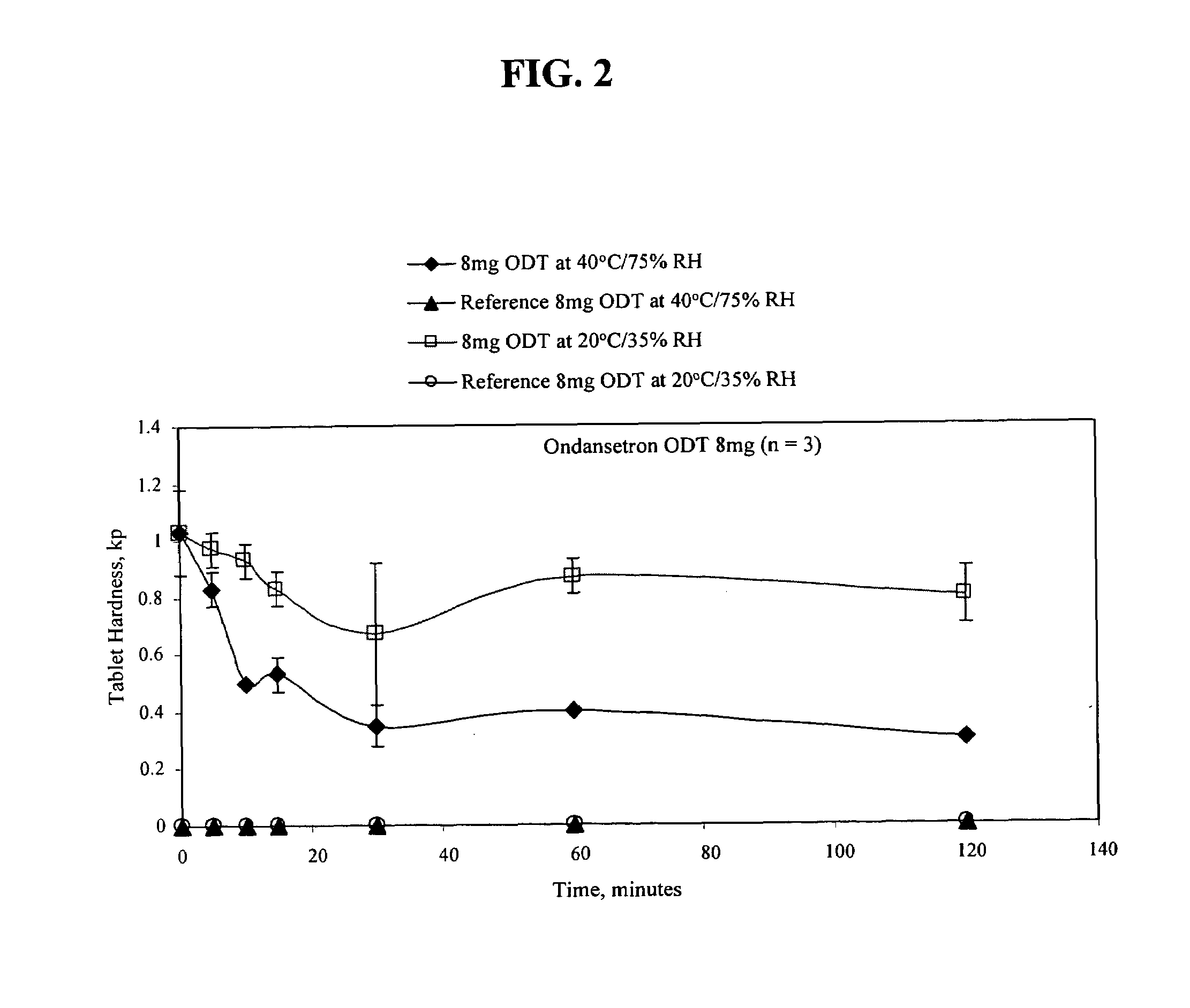
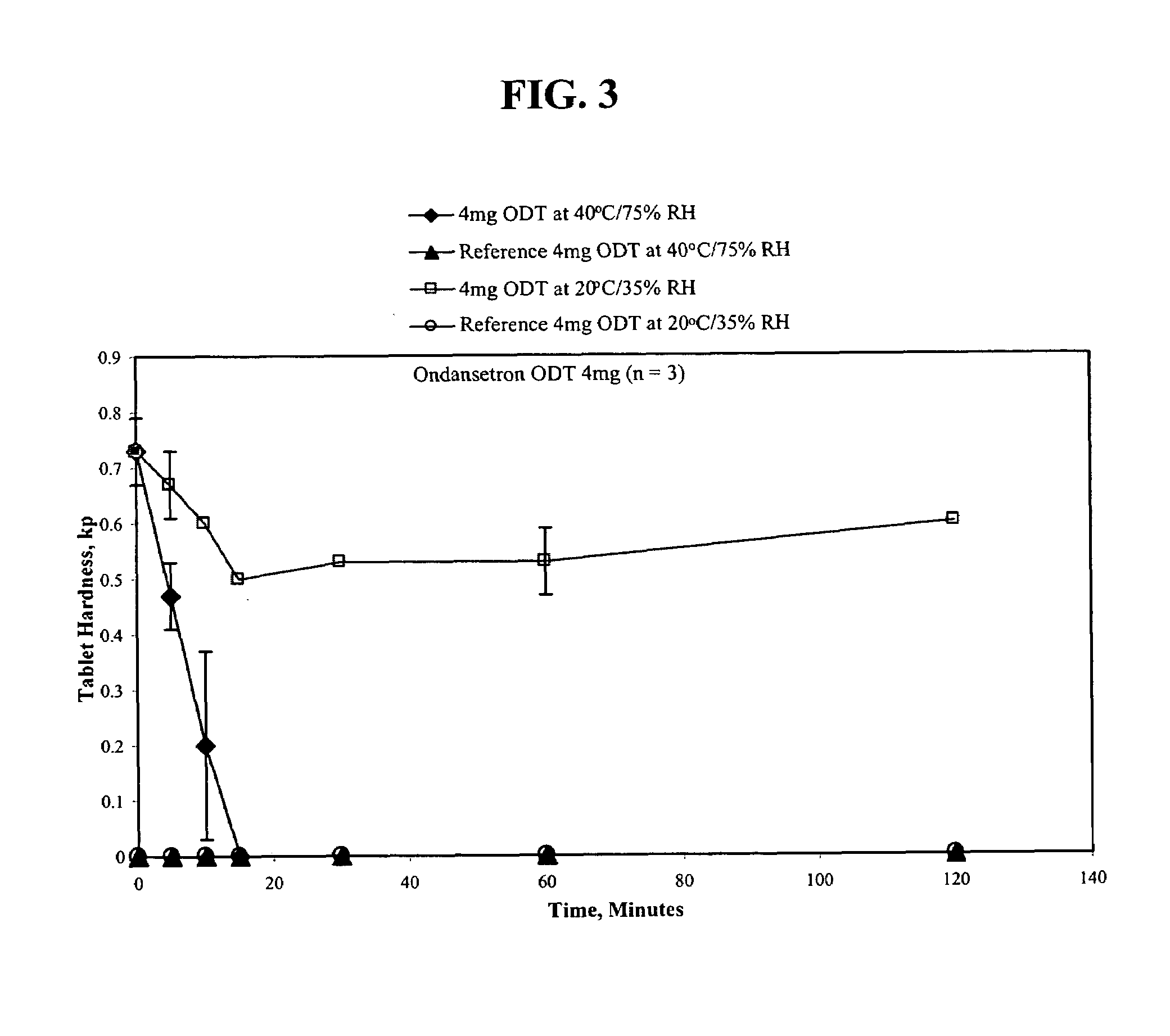
Ondansetron orally disintegrating tablets
InactiveUS7390503B1Safe and effective absorptionImprove bioavailabilityPowder deliveryPill deliveryWater dispersibleOrally disintegrating tablet
An ondansetron solid orally disintegrating dosage form for oral administration having at least one first water-dispersible component or water-insoluble cellulose derivative, a component having a —CHOH functional group, a disintegrating agent and at least one lubricant is provided. The dosage form can comprise ondansetron, a hydrophilic polymer such as microcrystalline cellulose, a component having a —CHOH functional group such as mannitol or xylitol and a disintegrating agent such as crospovidone. The lubricant may be a mixture of magnesium stearate, sodium stearyl fumarate and colloidal silicon dioxide. The present invention provides a non-effervescent tablet comprising the ondansetron dosage form. Another aspect of the invention is the treatment of emesis such as nausea and vomiting caused by cancer chemotherapy and radiation by the administration of the ondansetron formulation of the present composition. Finally, a process of forming an ondansetron disintegrating tablet using the ondansetron dosage form is disclosed.
Owner:BARR LAB
Lenalidomide composition tablets and preparation method thereof
ActiveCN105534981AThe preparation process is stableStable manufacturing processOrganic active ingredientsOrganic chemistryBeta-CyclodextrinsSodium stearyl fumarate
The invention discloses lenalidomide composition tablets and a preparation method thereof. The tablets comprise components in percentage by weight as follows: 10%-18% of lenalidomide crystals, 28%-40% of lactose, 28%-40% of microcrystalline cellulose, 1%-5% of konjac glucomannan, 10%-15% of beta-cyclodextrin, 5%-10% of sodium carboxymethyl starch, 0.5%-1.5% of sodium stearyl fumarate and 0.5%-1.5% of powdered cellulose. The preparation technology of the lenalidomide composition tablets is stable, simple and easy to operate, technological parameters are reliable, and the production cycle is shorter; experimental research shows that the lenalidomide raw material is stable in crystal form, higher in purity and capable of meeting the quality requirement of final products; the synthesis technology is concise, stable, feasible and applicable to mass production; the quality is controllable, the stability is better, and the lenalidomide raw material is unchanged under conditions of affecting factors such as the high temperature, high humidity and 10 days of illumination and is stable after being stored for 6 months under the acceleration condition.
Owner:DEYANG HUAKANG PHARMA
Film coating
InactiveUS20040058001A1Improve propertiesNegates needOrganic active ingredientsDigestive systemControlled releaseMethylmethacrylates
A film coating composition suitable for use in coating pharmaceutical formulations comprising a) an acrylic polymer dispersion, e.g. an ethylacrylate / methylmethacrylate copolymer such as Eudragit NE30D, b) a surfactant, c) sodium stearyl fumarate, and d) a water-containing liquid useful for the achievement of controlled release from pharmaceutical formulations such as tablets, pellets, etc.
Owner:ASTRAZENECA AB
Solid pharmaceutical composition containing rivaroxaban
ActiveCN105232488AGood dissolution effectReduce usageOrganic active ingredientsOrganic chemistryRivaroxabanMedical prescription
The invention relates to a pharmaceutical composition containing rivaroxaban, in particular to a solid pharmaceutical composition containing rivaroxaban and a preparation method of the solid pharmaceutical composition. The solid pharmaceutical composition containing the rivaroxaban can be further prepared into a film-coated tablet by a specific preparation technology to serve as a specific administration mode. The solid pharmaceutical composition containing the rivaroxaban and the preparation method of the solid pharmaceutical composition have the advantages that by application of the specific preparation technology, using surfactants in a preparation is avoided and dissolution rate of the tablet is increased effectively; by means of using sodium stearyl fumarate in the composition, marked increase of degraded impurities I during long-term storage of the tablet is avoided effectively; through stability accelerating research, the film-coated tablet containing the rivaroxaban, prepared by the prescription and technological steps, is stable and controllable in quality.
Owner:SHANGHAI LINKCHEM TECH CO LTD
Formulations of amlodipine maleate
InactiveUS20050019395A1Reduce productionCertain stabilityBiocidePill deliveryMedicineAmlodipine Maleate
The present invention provides improved, more stable formulations of amlodipine maleate where the formulations comprise from none to a minimal amount of magnesium. Such stable formulations show decreased production of the impurity amlodipine aspartate. Accordingly, the present invention provides formulations of amlodipine maleate comprising lubricants such as sodium stearyl fumarate, dimeticone, macrogol 6000, hydrogenated castor oil, and stearic acid. Methods of making and using the improved formulations are also provided.
Owner:TEVA PHARM USA INC
Tablet containing clopidogrel hydrogen sulfate and preparation method thereof
ActiveCN102302465ASolve the problem of accelerated clopidogrel degradationImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsVegetable oilPolyethylene glycol
The invention discloses a tablet containing clopidogrel hydrogen sulfate. The tablet comprises clopidogrel hydrogen sulfate micropills which are prepared into tablets through coating; the micropills are prepared by clopidogrel hydrogen sulfate, a diluent and an adhesion agent; the diluent is mannitol or / and microcrystalline cellulose; the adhesion agent is an anhydrous ethanol solution of hydroxypropylcellulose or povidone; and an lubricating agent is one or more of magnesium stearate, zinc stearate, talc, polyethylene glycol 6000, stearic acid, sodium stearyl fumarate, sodium lauryl sulfate and hydrogenated vegetable oil.
Owner:ZHEJIANG ANGLIKANG PHARMA
Film coating
A film coating composition suitable for use in coating pharmaceutical formulations comprising a) an acrylic polymer dispersion, e.g. an ethylacrylate / methylmethacrylate copolymer such as Eudragit NE30D, b) a surfactant, c) sodium stearyl fumarate, and d) a water-containing liquid useful for the achievement of controlled release from pharmaceutical formulations such as tablets, pellets, etc.
Owner:ASTRAZENECA AB
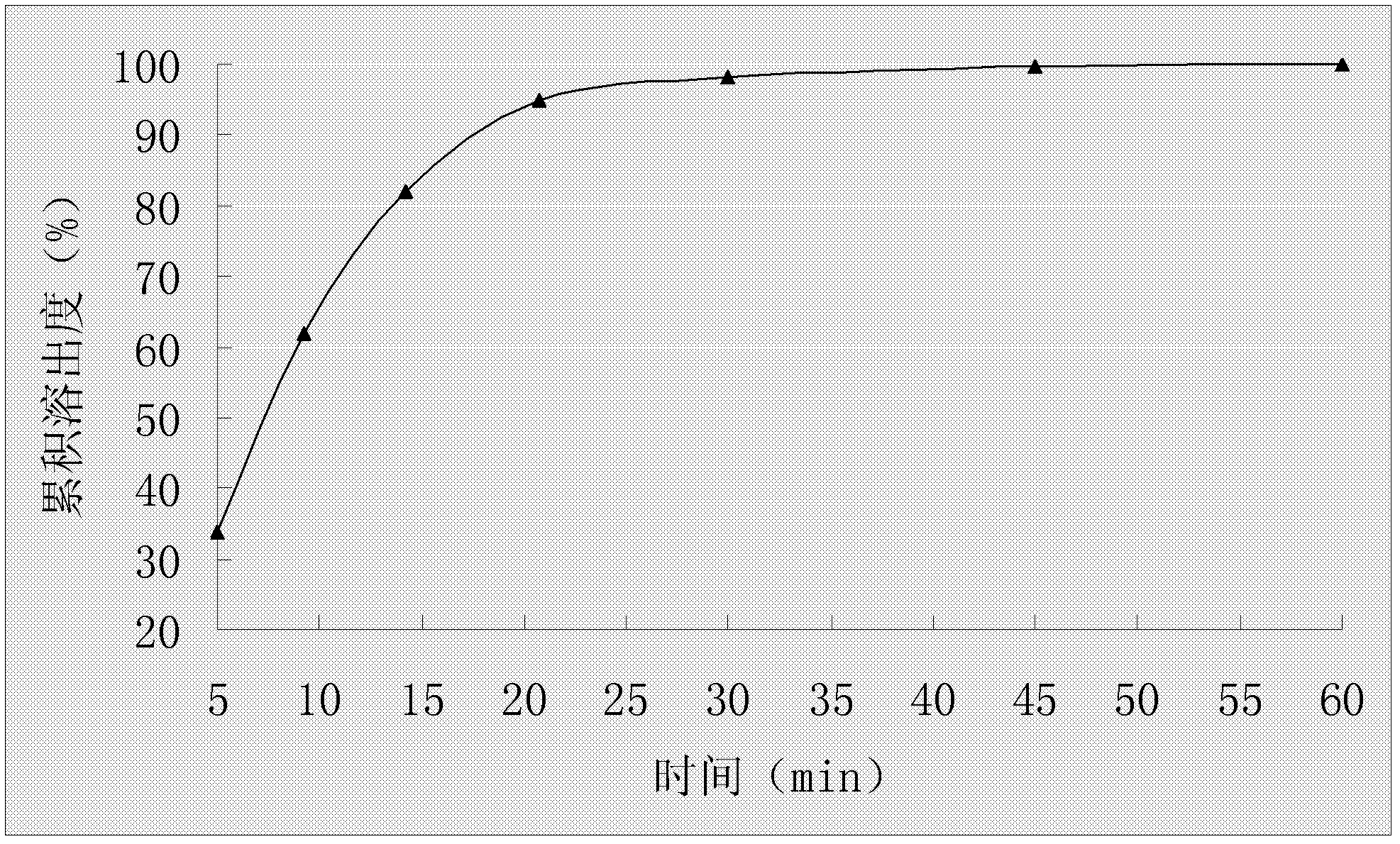
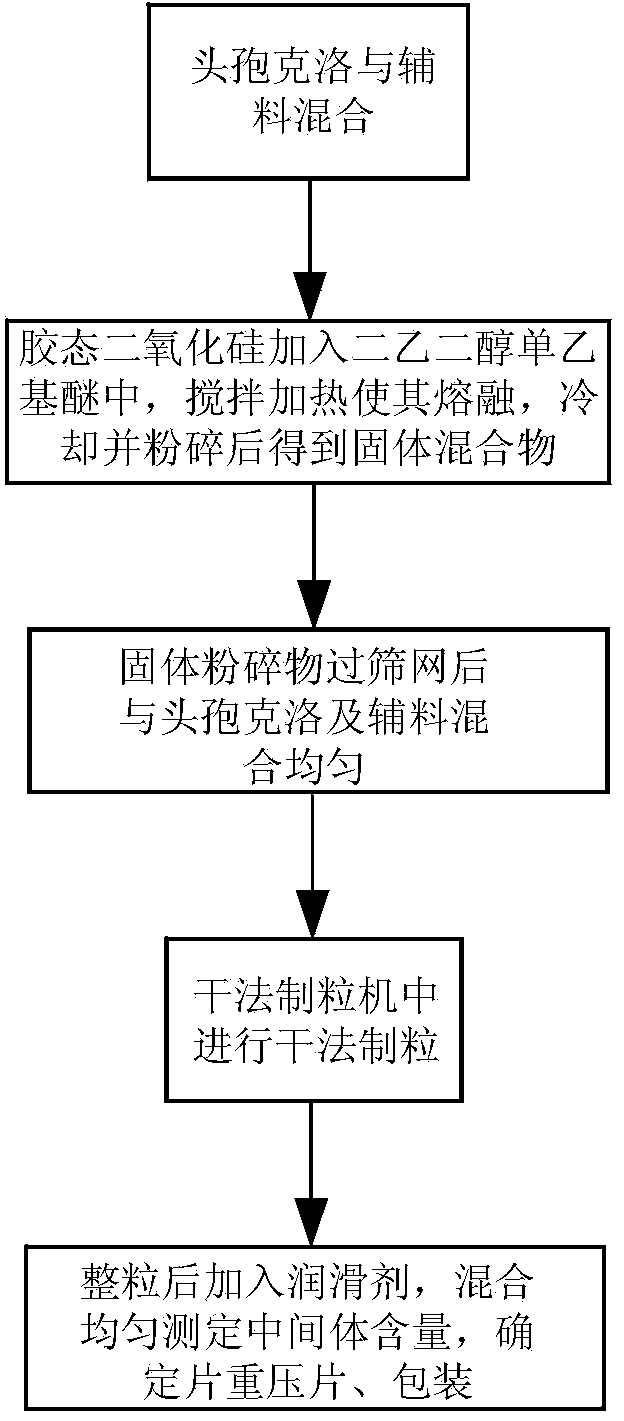
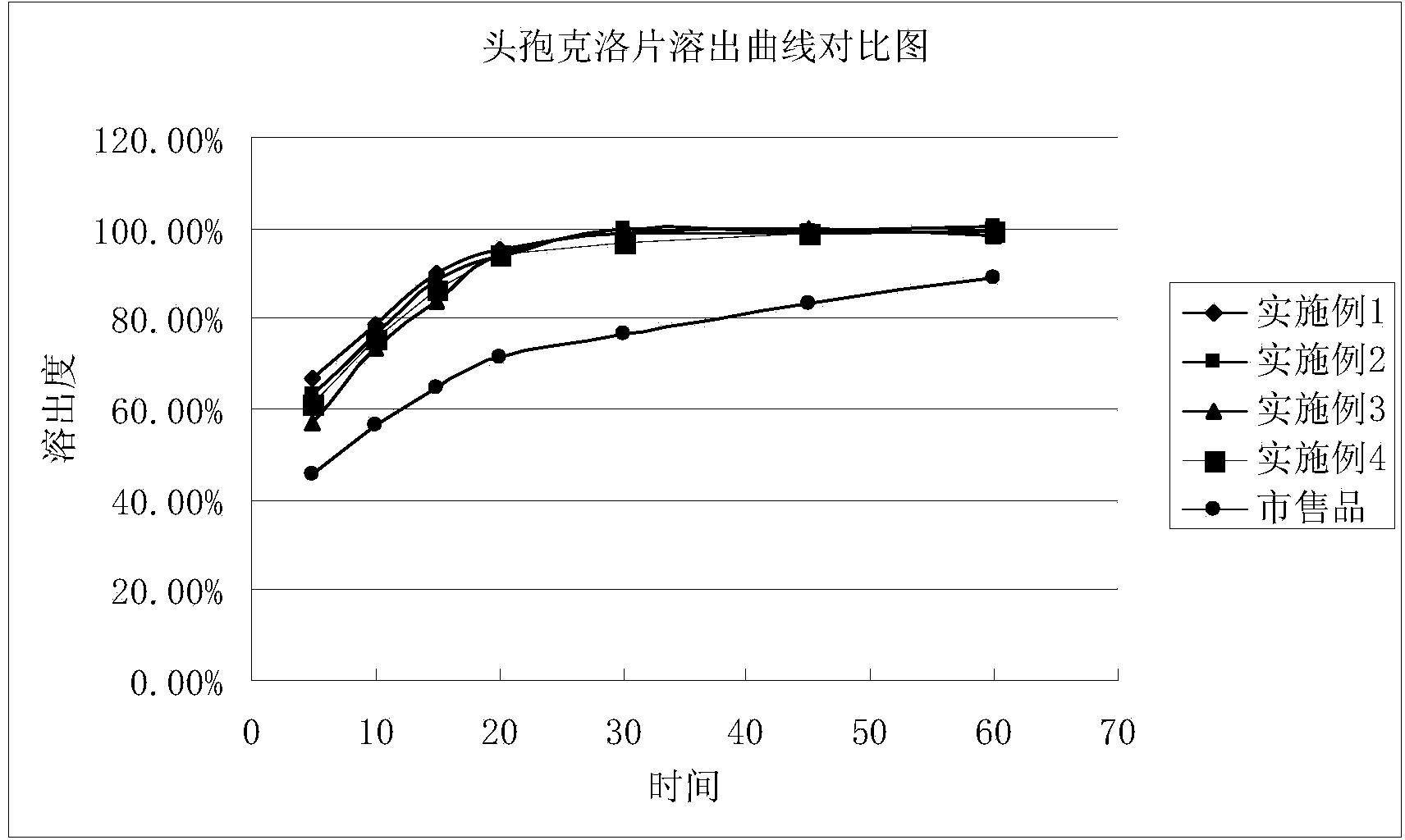
Stable cefaclor tablet composition and preparation method thereof
ActiveCN103623412AImprove solubilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsDiethylene glycol monoethyl etherBioavailability
The invention discloses a cefaclor tablet pharmaceutical tablet composition which comprises cefaclor, lactose, microcrystalline cellulose, crosslinked povidone, diethylene glycol monoethyl ether, colloidal silicon dioxide and glyceryl behenate or sodium stearyl fumarate. The preparation method comprises the following steps: evenly mixing the cefaclor with the pharmaceutical auxiliary materials; adding the colloidal silicon dioxide into the diethylene glycol monoethyl ether, heating while stirring for melting, and cooling to obtain a solid mixture; screening, and evenly mixing the cefaclor auxiliary materials; granulating by a dry process; and after finishing the granules, adding the lubricant, evenly mixing, measuring the intermediate content, determining the tablet weight, tabletting and packaging. The invention provides a legal and reasonable cefaclor table composition and a preparation method thereof, thereby obtaining the cefaclor tablet which has the advantages of stabler and more controllable quality, higher bioavailability and simpler technique.
Owner:SHANGHAI NEW ASIATIC PHARMA MINHANG
Method for drying biological cellulose hydrogel
ActiveCN102875847AImprove mechanical propertiesReduce chippingDrying using combination processesDrying solid materials without heatCelluloseThermal insulation
The invention relates to a method for drying biological cellulose hydrogel. The method comprises the following steps of: soaking part of dehydrated biological cellulose hydrogel in a surfactant-containing solution, and then drying. The surfactant is one or more of glycerin fatty acid ester, sucrose fatty acid ester, soyabean lecithin, acetin, tartaric acid glyceride, diacetyl tartaric acid glyceride, citrate, polyglycerol fatty acid ester, stearoyl citrate, stearyl tartrate, sodium stearyl lactate, calcium stearyl lactate, sodium stearyl fumarate and sorbitan fatty acid ester. By the method for drying the biological cellulose hydrogel, the damage to the spatial net structure of biological cellulose in the drying process can be reduced, and the cracking in the drying, packaging and conveying processes is reduced, and the thermal insulation property of a biological cellulose dried product can be improved.
Owner:HAINAN GUANGYU BIOTECH
Clopidogrel tablet and preparation method thereof
InactiveCN103877056AAvoid the phenomenon of melting and aggravating stickingGood compatibilityOrganic active ingredientsInorganic non-active ingredientsCoated tabletsMANNITOL/SORBITOL
The invention discloses a clopidogrel tablet and a preparation method thereof. The clopidogrel tablet is a coated tablet which consists of a clopidogrel table body and a coating material, wherein the clopidogrel table body comprises the following ingredients in parts by weight of clopidogrel free alkali: 75 parts of pharmaceutically acceptable salt of clopidogrel, 6-9 parts of sodium stearyl fumarate, 3-6 parts of talcum powder, 90-135 parts of diluent, 2-6 parts of bonding agent and 7-17 parts of disintegrating agent, wherein the diluent is made of combined mannitol and microcrystalline cellulose in a weight ratio of (2:1)-(1:1); the weight of the coating material accounts for 2.7-3.3% of the weight of the clopidogrel tablet. By adopting the clopidogrel tablet, not only is the problem of sticking in the tabletting process solved, but also the mixed lubricant and clopidogrel have good compatibility, the phenomena that active ingredients are degraded or the configuration is inversed are avoided, and the clopidogrel tablet is high in stability, good in quality, and high in similarity in dissolving behavior to that of an original triturate plavix.
Owner:WUHAN SUNRISE BIO PHARMA SCI & TECH
Propafenone hydrochloride sustained-release capsule and preparation method thereof
InactiveCN103908443AEvenly dispersedRelease stabilityOrganic active ingredientsPharmaceutical delivery mechanismSucroseSustained Release Capsule
The invention relates to a propafenone hydrochloride sustained-release capsule and a preparation method thereof. The propafenone hydrochloride sustained-release capsule is prepared by filling sustained-release micro-tablets with the diameter of 2-3 mm, and has good dosage dispersion and uniformity. The micro tablets are composed of propafenone hydrochloride, a framework material, a lubricant and a diluent. The framework material is one or more of methyl cellulose, ethyl cellulose, hydroxypropyl methylcellulose and povidone; and a usage amount of the framework material is 1%-10%. The lubricant is one or more of sodium stearyl fumarate, magnesium stearate, micro-powder silica gel, talcum powder, calcium stearate and polyethylene glycol; and a usage amount of the lubricant is 0.1%-1.5%. The diluent is one or more of sucrose, mannitol, microcrystalline cellulose, lactose and polyethylene glycol; and a usage amount of the diluent is 2%-12%. According to the propafenone hydrochloride sustained-release capsule, the usage amount of a main drug is 77%-98%; and propafenone level in blood plasma reaches the maximum value 3-8 hours after drug delivery. The propafenone hydrochloride sustained-release capsule is stable in drug release and has a good sustained-release effect. An object of the invention is to provide a preparation method of the propafenone hydrochloride sustained-release capsule. The preparation method is simple in process, high in yield, good in stability and suitable for large-scale production.
Owner:LP PHARM (XIAMEN) CO LTD
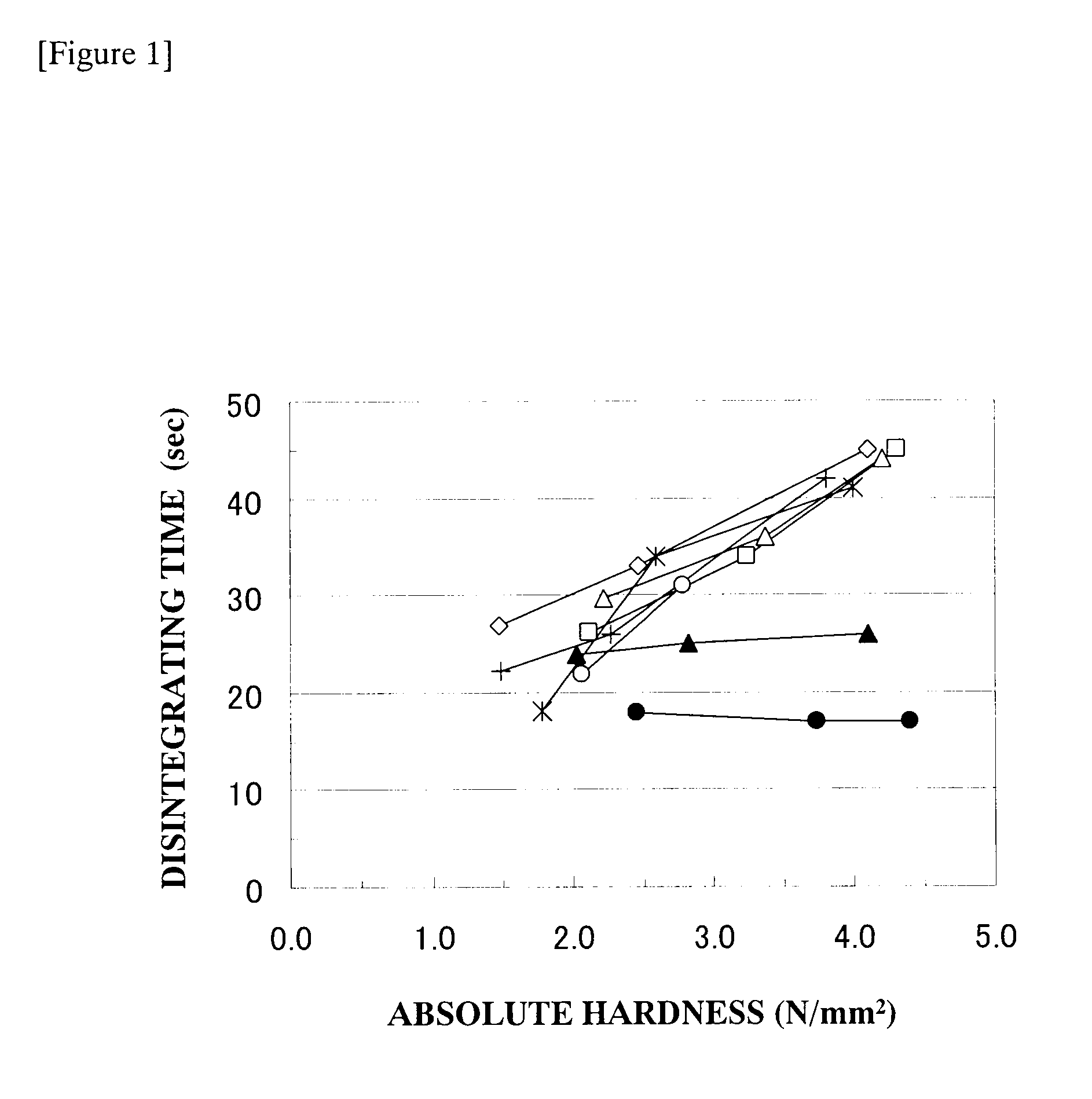
Oral disintegrating tablet
InactiveUS20100098756A1Good effectAppropriate strengthBiocidePharmaceutical non-active ingredientsCrospovidonesD-mannitol
An oral disintegrating tablet containing (1) D-mannitol, (2) an active ingredient, (3) one or more disintegrating agents selected from the group consisting of crospovidone and carmellose, and (4) one or more lubricants selected from the group consisting of sodium stearyl fumarate and sucrose esters of fatty acids. The oral disintegrating tablet of the present invention has some excellent properties of (1) allowing easy production in a common facility without necessitating a specialized pharmaceutical technique, (2) having an appropriate strength that does not breakdown in the process of distribution, (3) having a fast disintegrating ability in the oral cavity, and (4) also having excellent ingestion feel such as greatly reduced bitterness or gritty feel; therefore, the tablet can be suitably used as a dosage form that is suitable for aged individuals, children, and seriously ill patients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
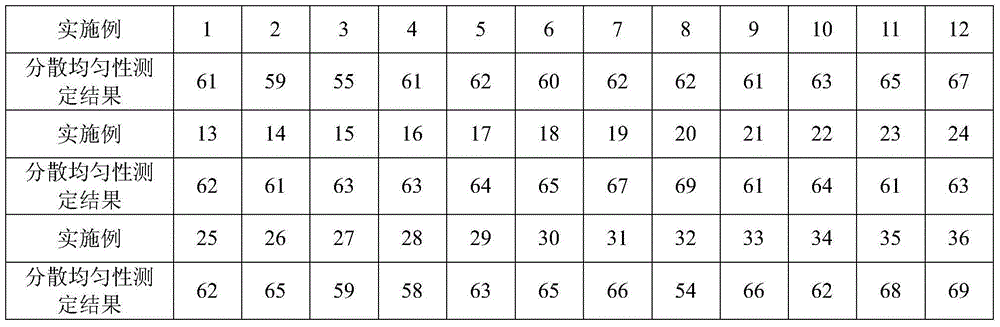
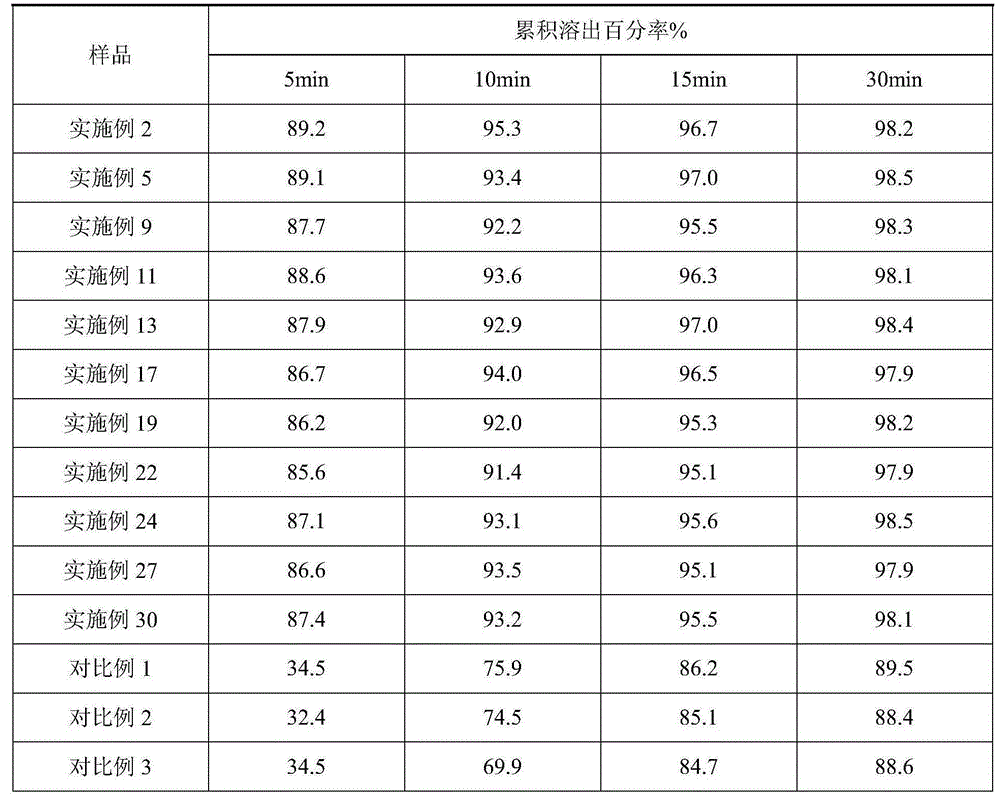
Roflumilast dispersible tablet composition and preparation method thereof
ActiveCN104644575AImprove complianceImprove dispersion uniformityPill deliveryPharmaceutical non-active ingredientsDissolutionSodium stearyl fumarate
The invention provides a roflumilast dispersible tablet composition, which is characterized by being composed of: roflumilast, copovidone, sodium stearyl fumarate and pharmaceutically-acceptable auxiliary materials. The roflumilast dispersible tablet composition prepared through the method in the invention is good in dispersion uniformity. A common tablet needs more than 5 min to disintegrate completely, while the roflumilast dispersible tablet can be disintegrated completely only within 70 s and can pass through a 2# sieve, wherein the time is far less than 3 min specified in Chinese pharmacopoeia. The roflumilast dispersible tablet is greatly increased in disintegration speed and meanwhile is greatly increased in dissolution rate compared with the common tablets in the prior art. The dissolution rate can reach 85% at 5 min and furthermore can reach 95% at 15 min. The roflumilast dispersible tablet composition is greatly increased in bio-utilization rate.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Preparation method of common auxiliary material
ActiveCN104177260AOrganic compound preparationOrganic chemistry methodsReaction temperaturePharmaceutical formulation
The invention relates to a preparation method of a common pharmaceutical preparation auxiliary material sodium stearyl fumarate. An organic alkaline catalyst is utilized to lower the reaction temperature, thereby obtaining the high-quality product at high yield. By selecting the reaction conditions, the product satisfying the quality requirements can be prepared, and satisfies the requirements for pharmaceutical preparations. The method is simple to operate, has the advantages of high safety and low cost, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
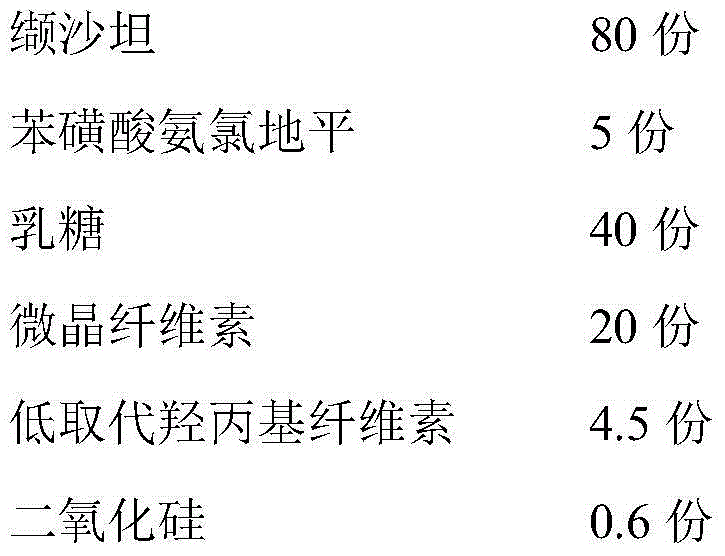
Valsartan amlodipine pharmaceutical composition and preparation method thereof
InactiveCN104367574APromote dissolutionImprove stabilityPharmaceutical non-active ingredientsCoatingsValsartanMedicine
The invention relates to a valsartan amlodipine pharmaceutical composition and a preparation method thereof. The pharmaceutical composition is composed of the following formula: 5 parts by weight of amlodipine besylate, 80 parts by weight of valsartan, 20-50 parts by weight of lactose, 10-40 parts by weight of microcrystalline cellulose, 3-6 parts by weight of low substituted hydroxypropyl cellulose, 0.3-2 parts by weight of silica and 0.5-5 parts by weight of sodium stearyl fumarate. The pharmaceutical composition is prepared from a direct powder compression process. The invention adopts a small amount of disintegrating agent to reach dissolution of more than 90%, and the composition has the advantages of good stability and fast disintegration. The preparation method provided by the invention has simple production process, reduces the corresponding investment in equipment factory, and saves the production cost. The tablet produced by the direct powder compression has faster disintegration, and helps to improve the dissolution of the drug; and through test, the tablet prepared by the method of the invention has dissolution above 90% in 15 min.
Owner:JIANGXI SHIMEI PHARM CO LTD
Orally disintegrating tablet formulations of mirtazapine and process for preparing the same
InactiveUS20110257159A1High mechanical strengthPleasant mouth-feelBiocideNervous disorderOrally disintegrating tabletSilicon dioxide
Silicon dioxide free orally disintegrating tablet formulations of mirtazapine or a pharmaceutically acceptable salt thereof having crospovidone and sodium stearyl fumarate and one or more pharmaceutically acceptable excipients and a process for preparing such a formulation.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
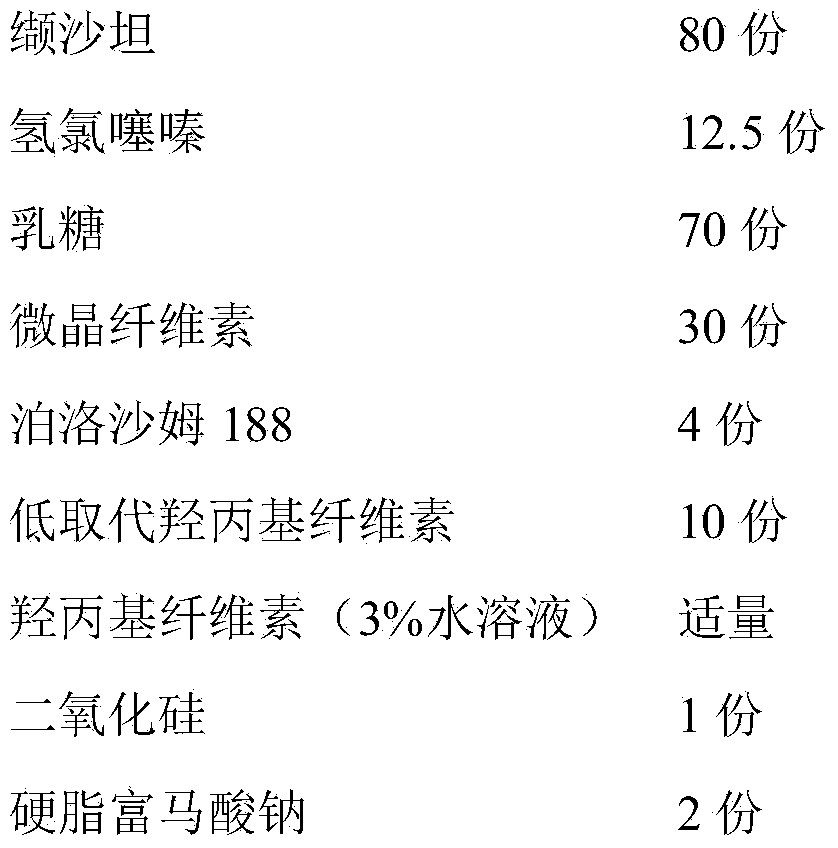
Valsartan/hydrochlorothiazide pharmaceutical composition and preparation method thereof
InactiveCN104274468AImprove liquidityDisintegrates quicklyPill deliveryPharmaceutical non-active ingredientsMedicineDissolution
The invention relates to a valsartan / hydrochlorothiazide pharmaceutical composition and a preparation method thereof. The pharmaceutical composition is composed of the following components in parts by weight: 80 parts of valsartan, 12.5 parts of hydrochlorothiazide, 20-80 parts of lactose, 20-80 parts of microcrystalline cellulose, 2-10 parts of poloxamer, 4-20 parts of low substituted hydroxypropyl cellulose, a moderate amount of hydroxypropyl cellulose, 0.4-2 parts of silicon dioxide, and 0.5-5 parts of sodium stearyl fumarate. The pharmaceutical composition is prepared through the processes of carrying out micronization on the components in advance, controlling the particle sizes of raw materials, adding a moderate amount of cosolvent, granulating by using a common wet method, and tabletting. The pharmaceutical composition is simple in production process, effectively improves the dissolution rate of drugs, and has the advantages of fast disintegration, quick dissolution, good stability, and the like.
Owner:JIANGXI SHIMEI PHARM CO LTD
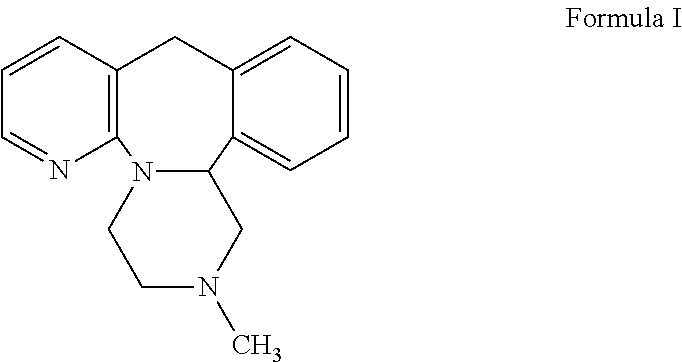
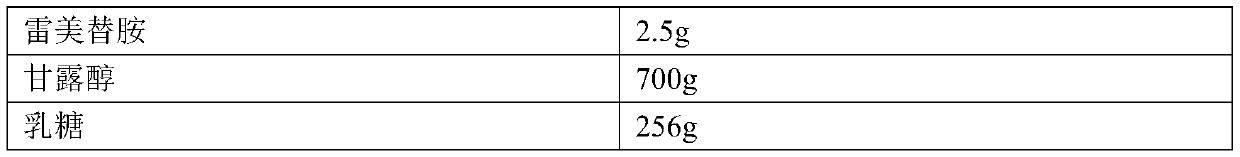
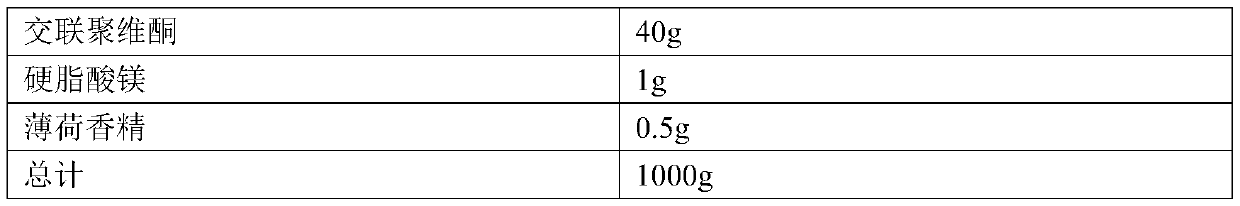
Ramelteon sublingual tablet and preparation method thereof
InactiveCN110433142AGreat tasteGrind evenlyOrganic active ingredientsNervous disorderCross-linkSucrose
The invention belongs to the field of pharmaceutical preparations, and relates to ramelteon sublingual tablets and a preparation method thereof. The sublingual tablet contains effective amounts of ramelteon, a filler, a disintegrant, a lubricant, and a flavoring agent, and the proportion of a main drug is 0.1 to 0.5%; the filler is selected from one or a combination of mannitol, lactose, sucrose and xylitol; the disintegrant is selected from one or the combination of crospovidone, cross linked sodium carboxymethyl cellulose, and low-substituted hydroxypropyl cellulose; and the lubricant is selected from one or a combination of magnesium stearate, aerosil, and sodium stearyl fumarate; and the flavoring agent is mint flavor. The invention also provides the preparation method of the ramelteonsublingual tablets, that is, ramelteon and the filler are ground and mixed by a ball mill, and mixed with the disintegrating agent, the lubricant, and the flavoring agent, and the materials are pressed to prepare tablets. The ramelteon sublingual tablet of the invention can avoid the first pass effect of the liver and improve the bioavailability.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
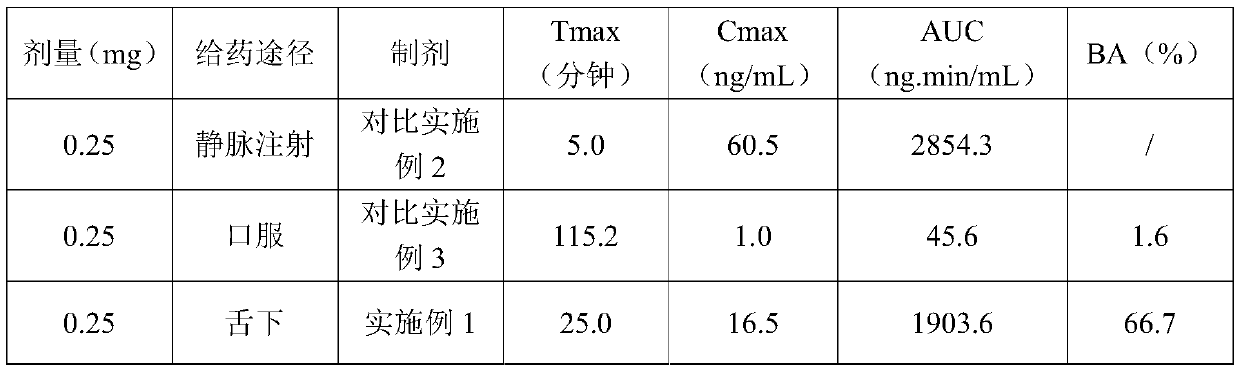
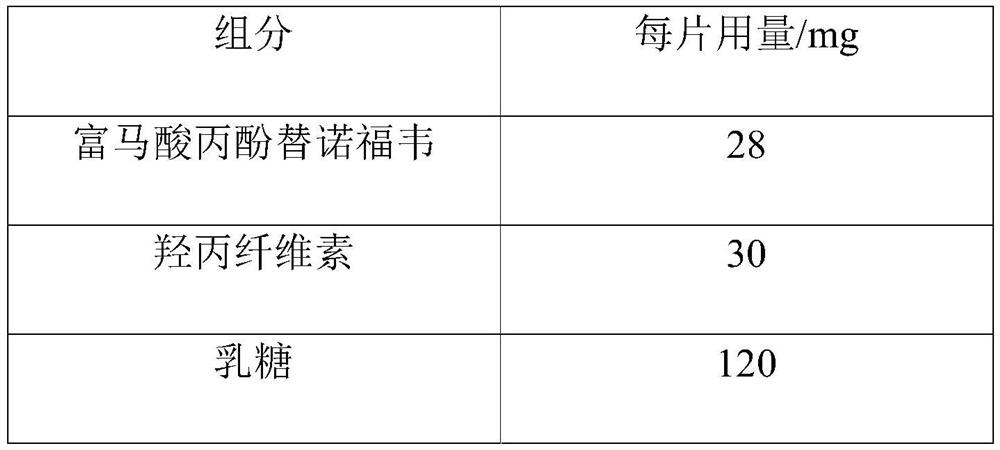
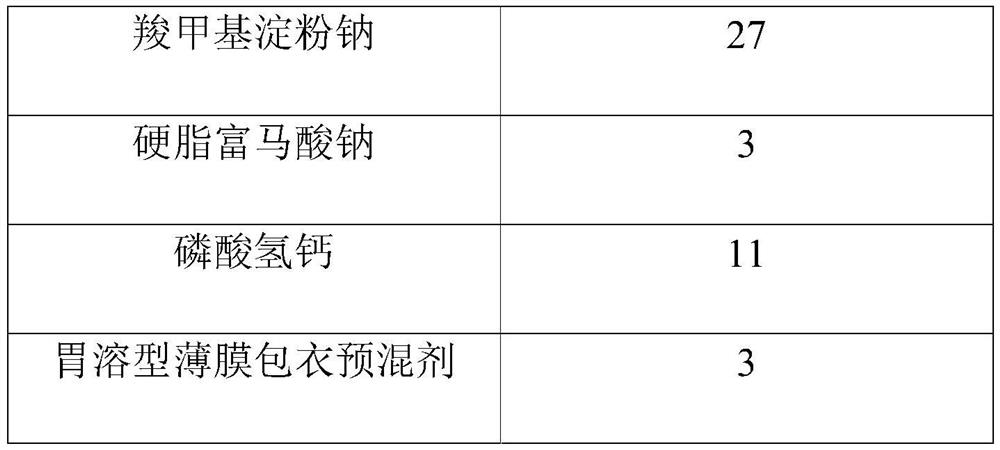
Tenofovir alafenamide fumarate tablet, preparation method thereof and detection method of related substances
ActiveCN112336695AImprove roundnessNarrow particle size distributionOrganic active ingredientsComponent separationDrugs preparationsTenofovir alafenamide
The invention relates to the technical field of pharmaceutical preparations, and particularly discloses a tenofovir alafenamide fumarate tablet and a preparation method thereof. The tenofovir alafenamide fumarate tablet comprises the following components in percentage by weight: 12%-14% of tenofovir alafenamide fumarate, 10%-15% of a cross-linking agent, 45%-58% of a diluent, 5%-12% of a disintegrating agent, 1%-5% of sodium stearyl fumarate and 5%-15% of calcium hydrophosphate. The tenofovir alafenamide fumarate tablet is prepared from the raw materials and auxiliary materials with a fluidized bed granulation method. The tenofovir alafenamide fumarate tablet prepared by steps of selecting the auxiliary materials and matching the optimized auxiliary material proportion with the fluidized bed granulation process is high in in-vitro dissolution rate, low in impurity content and good in stability, the safety of clinical application is improved, the tenofovir alafenamide fumarate tablet can be consistent with an original ground product in four dissolution media, besides, the preparation process is simple, and the tablet is suitable industrial production.
Owner:NORTH CHINA PHARMA HUAKUN HEBEI BIOTECH
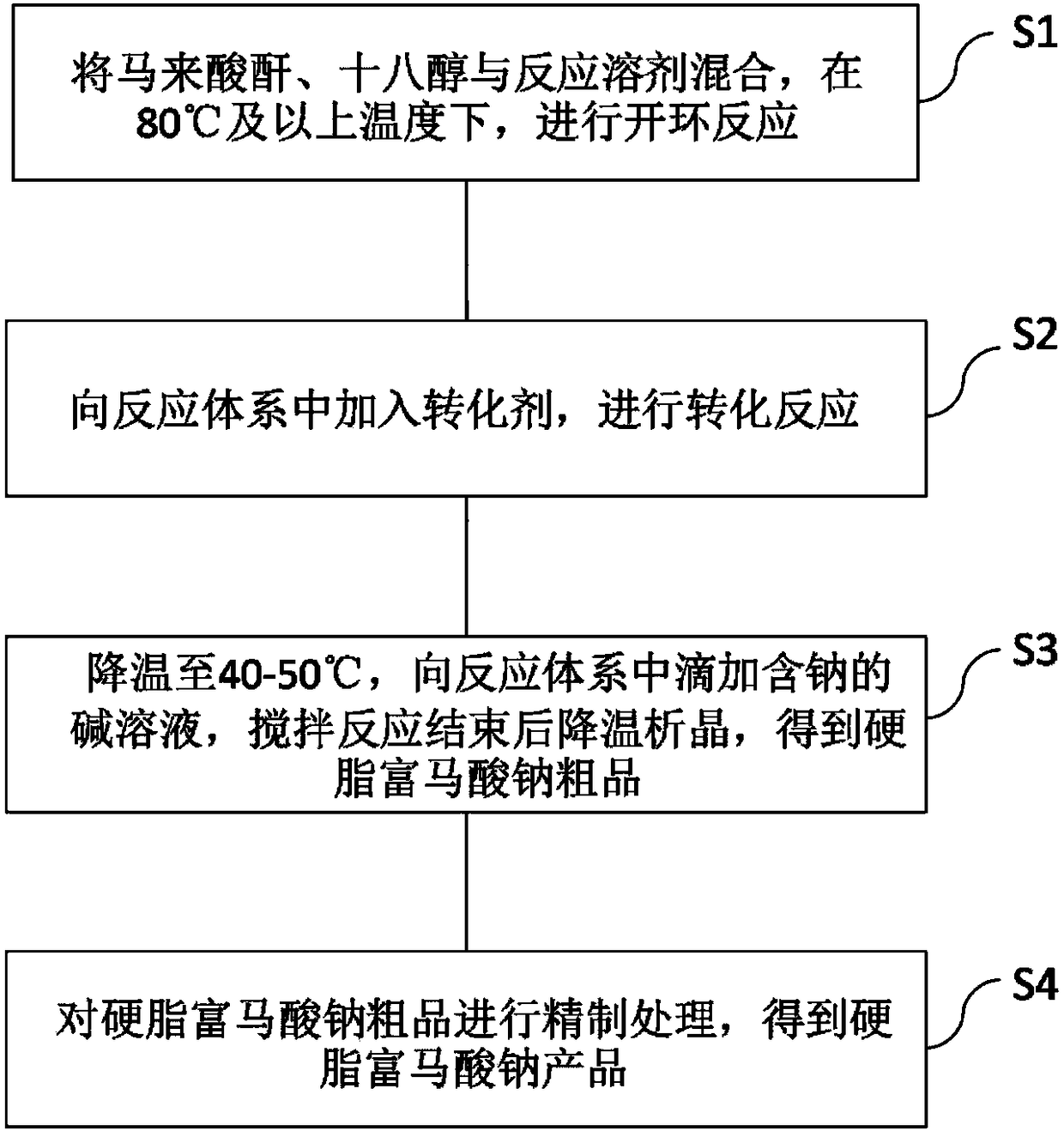
Sodium stearyl fumarate auxiliary material and preparation method thereof
ActiveCN108586250AReduce the use effectReduce problem sizeOrganic compound preparationCarboxylic acid esters separation/purificationSolventSodium stearyl fumarate
The invention provides a sodium stearyl fumarate auxiliary material and a preparation method thereof. The preparation method comprises the following steps: S1, mixing maleic anhydride, octadecanol anda reaction solvent, heating, and carrying out a ring-opening reaction; S2, adding a converting agent to carry out a conversion reaction; S3, cooling, dropping a sodium-containing alkali solution, stirring, and cooling to separate a crystal after the reaction is completed so as to obtain a crude product; S4, carrying out refining treatment, so as to obtain a sodium stearyl fumarate product. Sodiumstearyl fumarate is prepared by using a one-pot method, middle products do not need to be separated, preparation processes and aftertreatment steps can be greatly simplified, and reaction conditionsare gentle; moreover, the total yield is remarkably increased, the purity is remarkably improved, and the sodium stearyl fumarate auxiliary material is suitable for being used as a medicine and food auxiliary material and is applicable to industrial production.
Owner:苏州东南药业股份有限公司
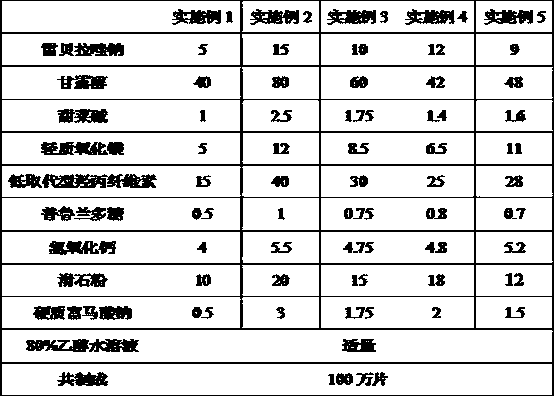
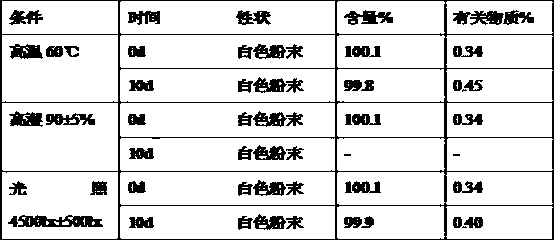
Rebeprazole sodium plain tablets, rebeprazole sodium enteric tablets and preparation method of rebeprazole sodium enteric tablets
ActiveCN110507628AHigh hardnessImprove stabilityOrganic active ingredientsDigestive systemCellulosePullulan
The invention discloses rebeprazole sodium plain tablets. The rebeprazole sodium plain tablets comprise the following components in parts by weight of 5-15 parts of rebeprazole sodium, 40-80 parts ofmannitol, 1-2.5 parts of betaine, 5-12 parts of light weight magnesium oxide, 15-40 parts of low substituted hydroxypropy cellulose, 0.5-1 part of pullulan polysaccharide, 4-5.5 parts of calcium hydroxide, 10-20 parts of talcum powder and 0.5-3 parts of sodium stearyl fumarate. The invention provides rebeprazole sodium enteric tablets containing the rebeprazole sodium plain tablets and a preparation method of the rebeprazole sodium enteric tablets. The preparation method comprises the following steps of S1, preparing the rebeprazole sodium plain tablets; S2, performing protective layer coating; S3, performing isolating layer coating; and S4, performing enteric layer coating. Rebeprazole sodium components and coating thereof are improved, dissolution and stability of products are promoted,and the technical problem that tablet core dissolution of the rebeprazole sodium coating tablets or enteric tablets is poor, so that the bioavailability of the rebeprazole sodium is low can be solved.
Owner:双鹤药业(海南)有限责任公司
Premixed auxiliary material for preparing orally disintegrating tablet through direct compression
ActiveCN105012955AFacilitated releaseImprove the lubrication effectPharmaceutical non-active ingredientsPill deliveryHydrogen phosphateOrally disintegrating tablet
The invention provides a premixed auxiliary material for preparing an orally disintegrating tablet through direct compression. The auxiliary material is composed of the following auxiliary materials such as mannitol, trehalose, lactose, anhydrous sodium hydrogen phosphate, crosslinked carboxymethylcellulose sodium, microcrystalline cellulose, polacrilin potassium, polyvinylpyrrolidone and sodium stearyl fumarate. The premixed auxiliary material is obtained by re-crystallizing, grinding and screening by using a method according to respective parameters. The premixed auxiliary material is particularly suitable for dosage forms such as the orally disintegrating tablet and a buccal tablet.
Owner:HUNAN ER KANG PHARMA
Pharmaceutical composition containing valsartan and amlodipine besylate and preparation method
InactiveCN102988364AImprove product qualityImprove uniformityPharmaceutical non-active ingredientsDrageesValsartanAmlodipine besilate
The invention relates to a pharmaceutical composition containing valsartan and amlodipine besylate and a preparation method. The composition takes valsartan and amlodipine besylate as raw materials, and comprises pharmaceutically acceptable excipients of microcrystalline cellulose, crosslinked sodium carboxymethyl cellulose, silica and sodium stearyl fumarate. The preparation method comprises following steps: carrying out dry granulation on the raw materials and part of the excipients firstly, and adding the sodium stearyl fumarate to carry out tabletting and coating to be produced into film-coated tablets. The preparation method is characterized in that the dry granulation is adopted to avoid the influence of moisture on main ingredients during wet granulation, and the sodium stearyl fumarate serves as a lubricant instead of commonly used magnesium stearate to prevent the influence of the magnesium stearate on the main ingredients, therefore on the premise of disintegrating and dissolving the product better, the product quality is further stabilized and the drug safety is enhanced.
Owner:天津飞鹰玉川药业有限公司
Compositions and methods for treating middle-of-the-night insomnia
The present invention provides compositions having a therapeutically effective amount of zolpidem, carbonate buffer, bicarbonate buffer, and a mixture comprising large and fine particles of silicon dioxide. Compositions having a therapeutically effective amount of zolpidem, carbonate buffer, bicarbonate buffer, and sodium stearyl fumarate are also described.
Owner:SINGH NIKHILESH +1
Pimavanserin tablet and preparation method thereof
InactiveCN109568278ADissolution stabilityReduce static electricityOrganic active ingredientsNervous disorderLow-substituted hydroxypropylcellulosePolyethylene glycol
The invention belongs to the field of pharmaceutic preparation, and particularly relates to a pimavanserin tablet and a preparation method thereof. The pimavanserin tablet is composed of active components of pimavanserin, colloidal silicon dioxide, polyethylene glycol, a filling agent selected from dextrin, corn starch, pregelatinized starch, cellulose microciystalline, mannitol and lactose, a disintegrating agent selected from carboxymethyl starch sodium, croscarmellose sodium, low-substituted hydroxypropyl cellulose and crospovidone, and a lubricating agent selected from magnesium stearate,talcum powder, and sodium stearyl fumarate. The pimavanserin tablet has good stability, and can significantly improve drug dissolution and bioavailability.
Owner:BEIJING VENTUREPHARM BIOTECH
Tabletting process
ActiveUS8715724B2Easy to chargePreventing and reducing accumulationBiocideLiquid surface applicatorsMedicineBULK ACTIVE INGREDIENT
A process for producing a compressed solid dosage form containing an active ingredient. The process includes a step of preparing core elements containing the active ingredient. Optionally the core elements are coated with a pharmaceutically acceptable coating layer to form coated pellets. The core elements or pellets are treated with an anti-static agent and compressed with suitable excipients to form the compressed solid dosage form. Preferred anti static agents are starch, microcrystalline cellulose, kaolin, bentonite, silicates, silicon dioxide, cellulose, stearic acid, sodium stearyl fumarate and glyceryl behenate.
Owner:MAYNE PHARMA INT
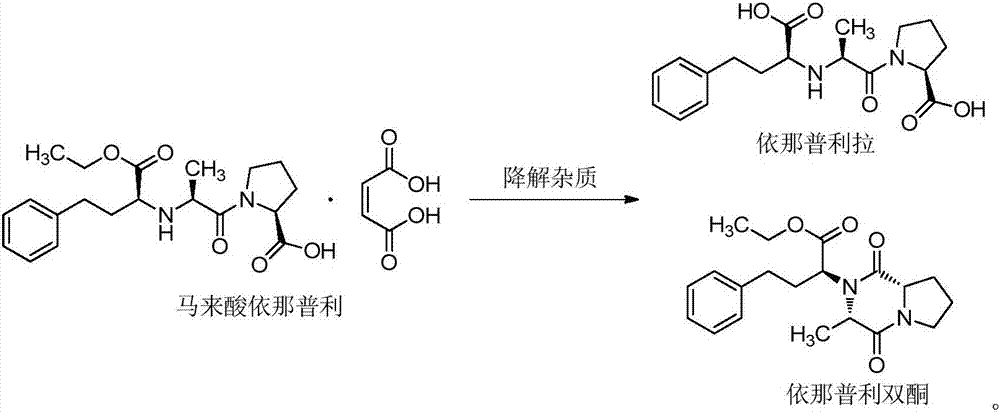
Pharmaceutical composition of enalapril maleate folic acid and preparation method thereof
InactiveCN106963938AGood compatibilityImprove stabilityOrganic active ingredientsDipeptide ingredientsCross-linkMannitol
The invention provides a pharmaceutical composition of enalapril maleate folic acid. The composition comprises enalapril maleate, folic acid, filler, a disintegrant, a binder and a lubricant. The composition is characterized in that the filler is selected from composition, lactose anhydrous or mannitol, the disintegrant is selected from one or more of cross-linked sodium carboxymethylcellulose or low substituted hydroxypropyl cellulose, the binder is selected from povidone K30, hydroxypropyl methyl cellulose E5, hydroxypropyl cellulose SL-FP or hydroxypropyl methylcellulose 603, and the lubricant is selected from talcum powder, sodium stearyl fumarate or glyceryl behenate. The excipients and raw materials selected by the invention have good compatibility, the preparation obtained by dry granulation has good stability, can ensure safe use of drugs in clinical practice, and the technological operation is simple and is suitable for industrial production.
Owner:NANJING YOKO PHARMA +2
Outer packaging film for instant noodle bowl
InactiveCN105860219AAffect qualityImprove mechanical propertiesLow-density polyethylenePolyvinyl alcohol
The invention discloses an outer packaging film for an instant noodle bowl and relates to the technical field of plastic films. The outer packaging film is prepared from, by weight, 40-50 parts of low-density polyethylene, 15-20 parts of polypropylene, 10-15 parts of medical stone powder, 8-12 parts of hydrogenated castor oil, 6-10 parts of microcrystalline cellulose, 6-10 parts of polyvinyl alcohol, 5-8 parts of casein-soybean glue, 4-6 parts of flexibilizer, 4-6 parts of sodium polyacrylate, 3-5 parts of carnauba wax, 3-5 parts of lauric acid monoglyceride, 2-3 parts of sorbitol, 2-3 parts of stearyl citrate and 1-2 parts of sodium stearyl fumarate. The packaging film is remarkable in mechanical performance, high in using safety, capable of externally packaging the instant noodle bowl after being stretched and excellent in sealing performance and weather resistance, the phenomenon that the quality of instant noodles is affected due to the fact that water and oxygen in air penetrate through the packaging film in the storage and shelf life is prevented, printing ink can be effectively attached to the packaging film, and therefore product information such as production dates and batches can be conveniently displayed.
Owner:ANHUI CHAOHU SOUTH MEMBRANE IND
Preparation method for telmisartan tablets
InactiveCN111700866APrescription process optimizationHigh similarityOrganic active ingredientsPharmaceutical non-active ingredientsAqueous ethanolMannitol
The invention relates to a preparation method for telmisartan tablets. The preparation method comprises the following steps: S1, weighing telmisartan, sodium hydroxide and meglumine to prepare an aqueous solution or an aqueous ethanol; S2, adding weighed povidone and sieved mannitol into a fluidized bed to make the povidone and mannitol in a fluidized state, spraying the mixed solution prepared instep S1, performing spray granulation, and performing granule finishing after drying; and S3, adding the finished granules to the weighed magnesium stearate and sodium stearyl fumarate, and performing tabletting after uniform mixing to obtain plain tablets, wherein the hardness of the plain tablets is controlled to be 7-11kgf. The preparation method optimizes the prescription technique of the telmisartan tablets, and improves the similarity of multiple dissolution curves of own products and reference preparations.
Owner:CHONGQING CONQUER PHARML
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com