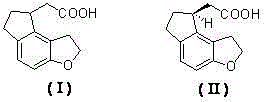Patents
Literature
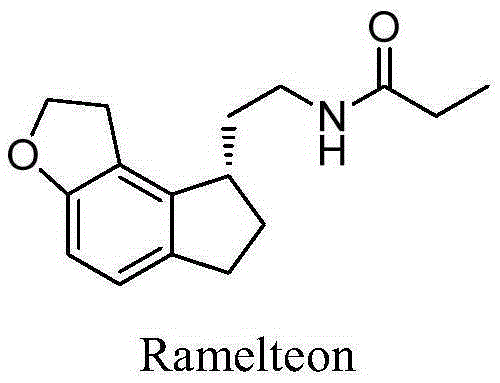
57 results about "Ramelteon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
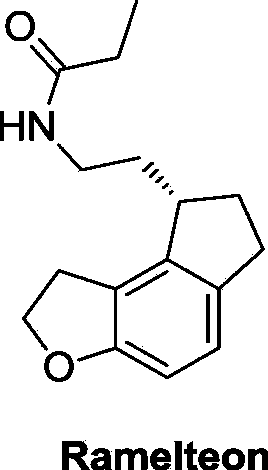
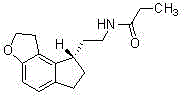
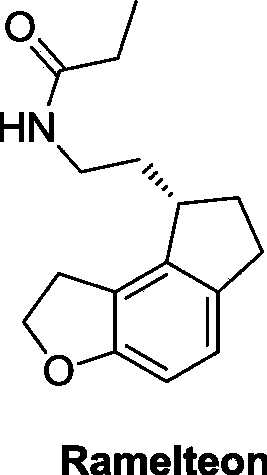
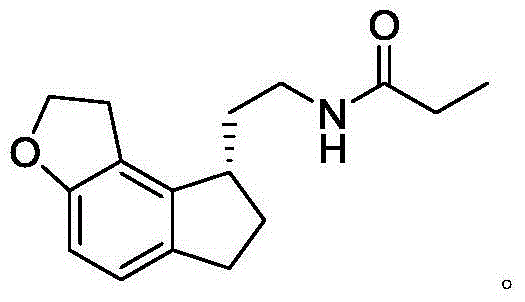
This medication is used to treat sleeplessness (insomnia).
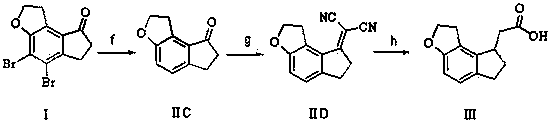
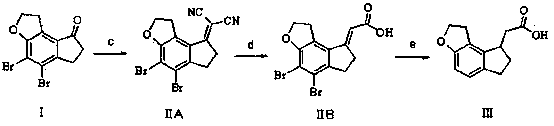
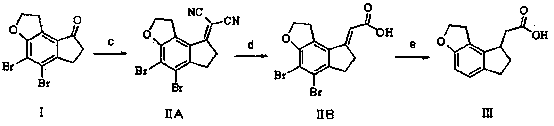
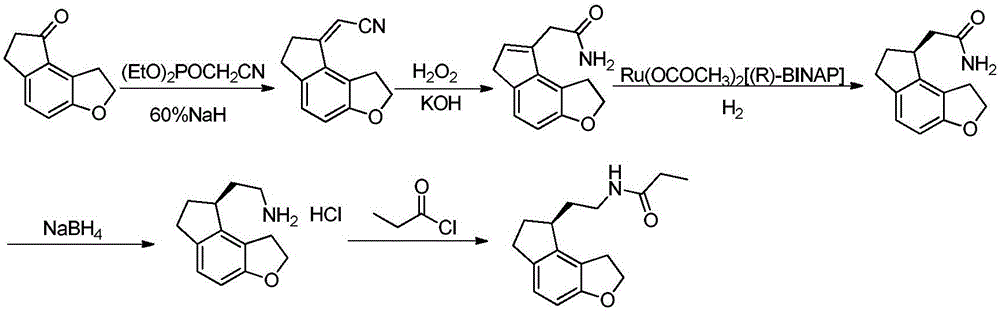
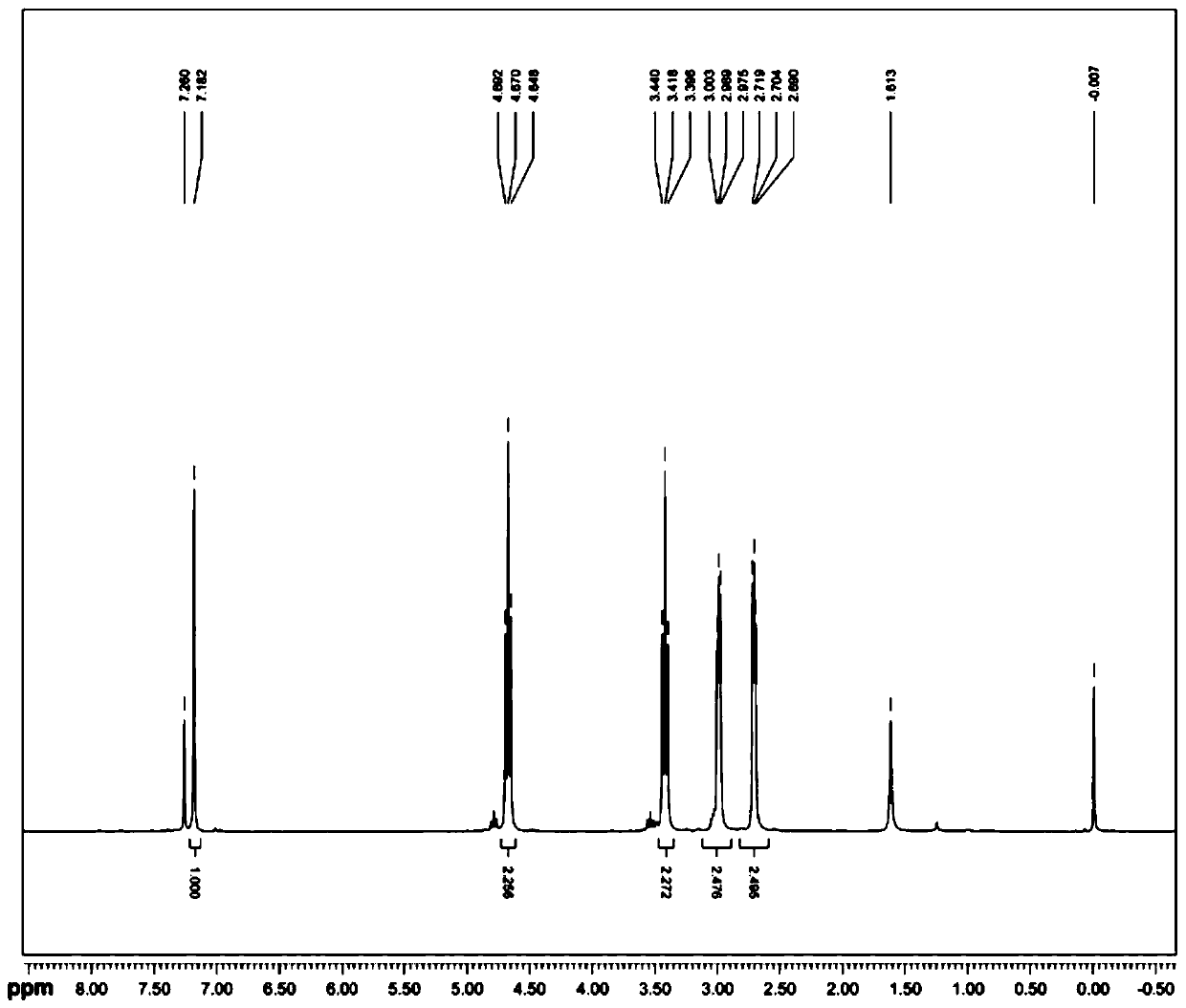
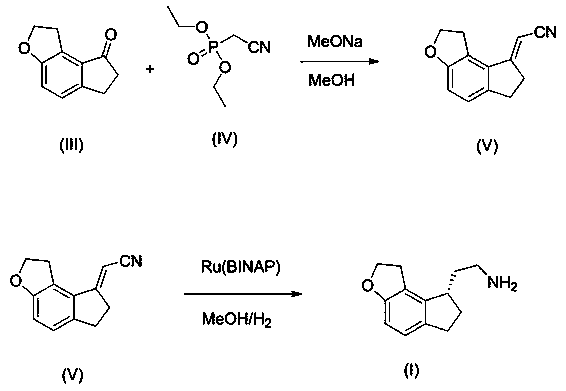
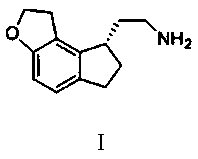
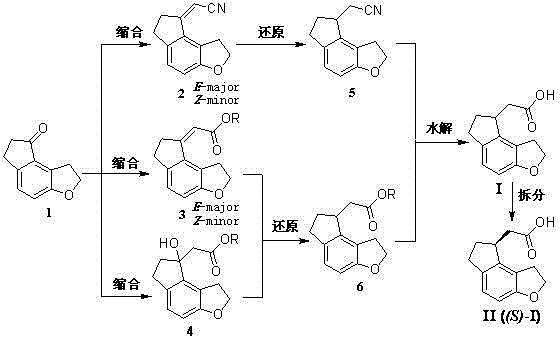
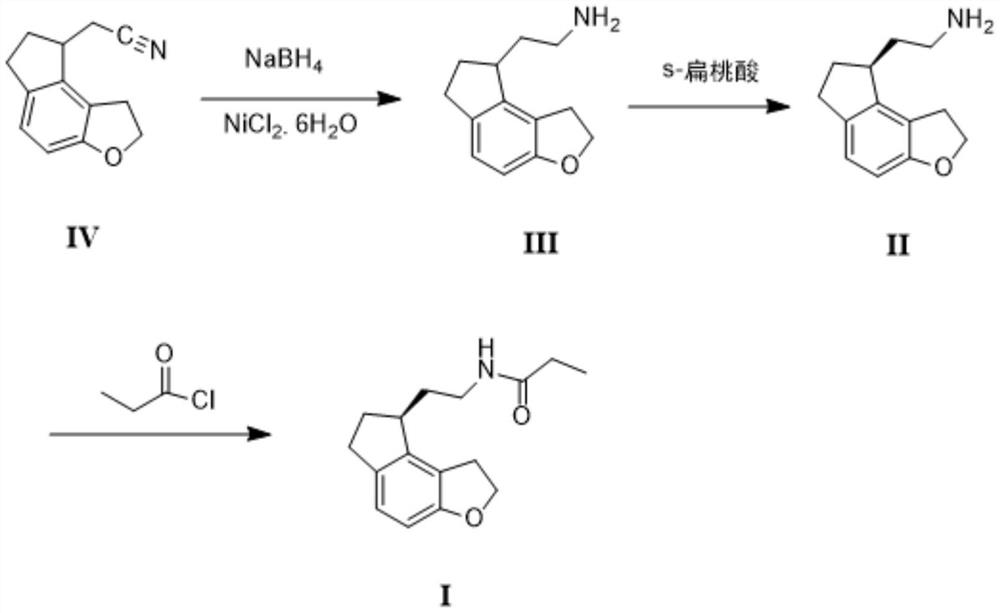
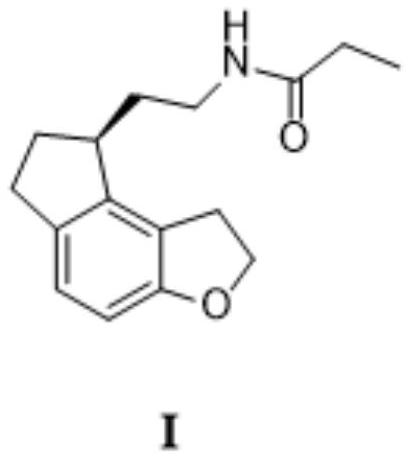
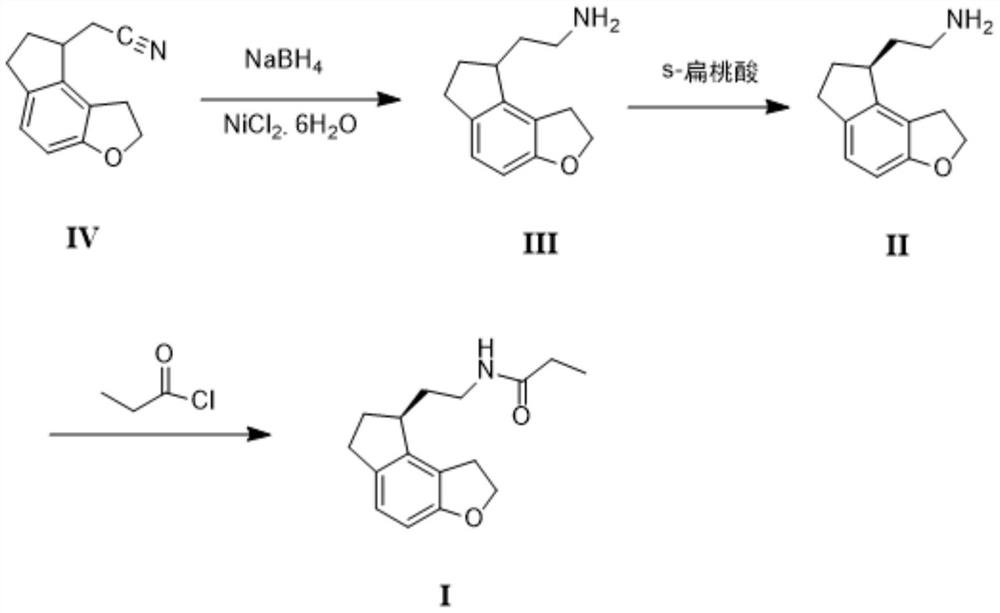
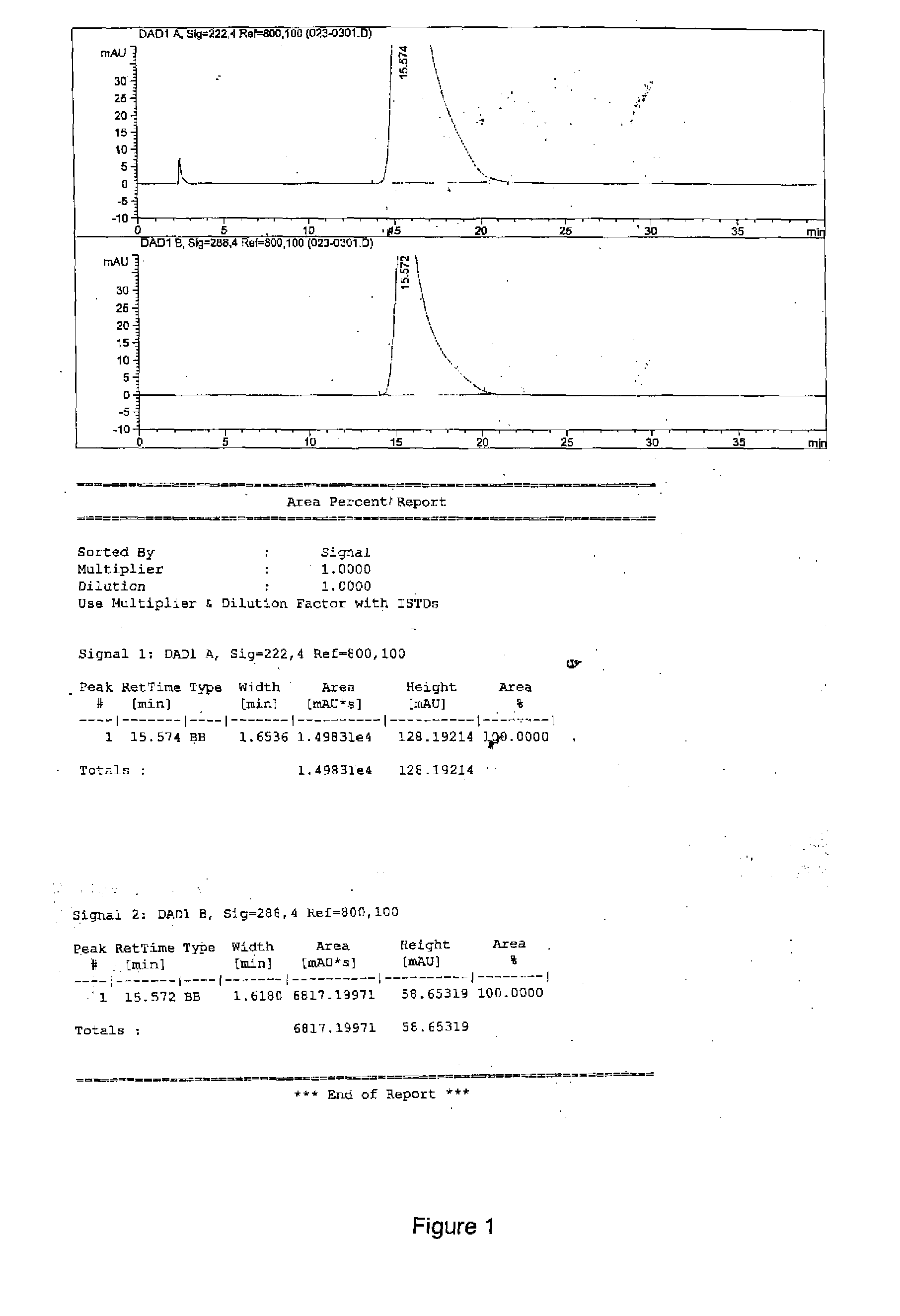
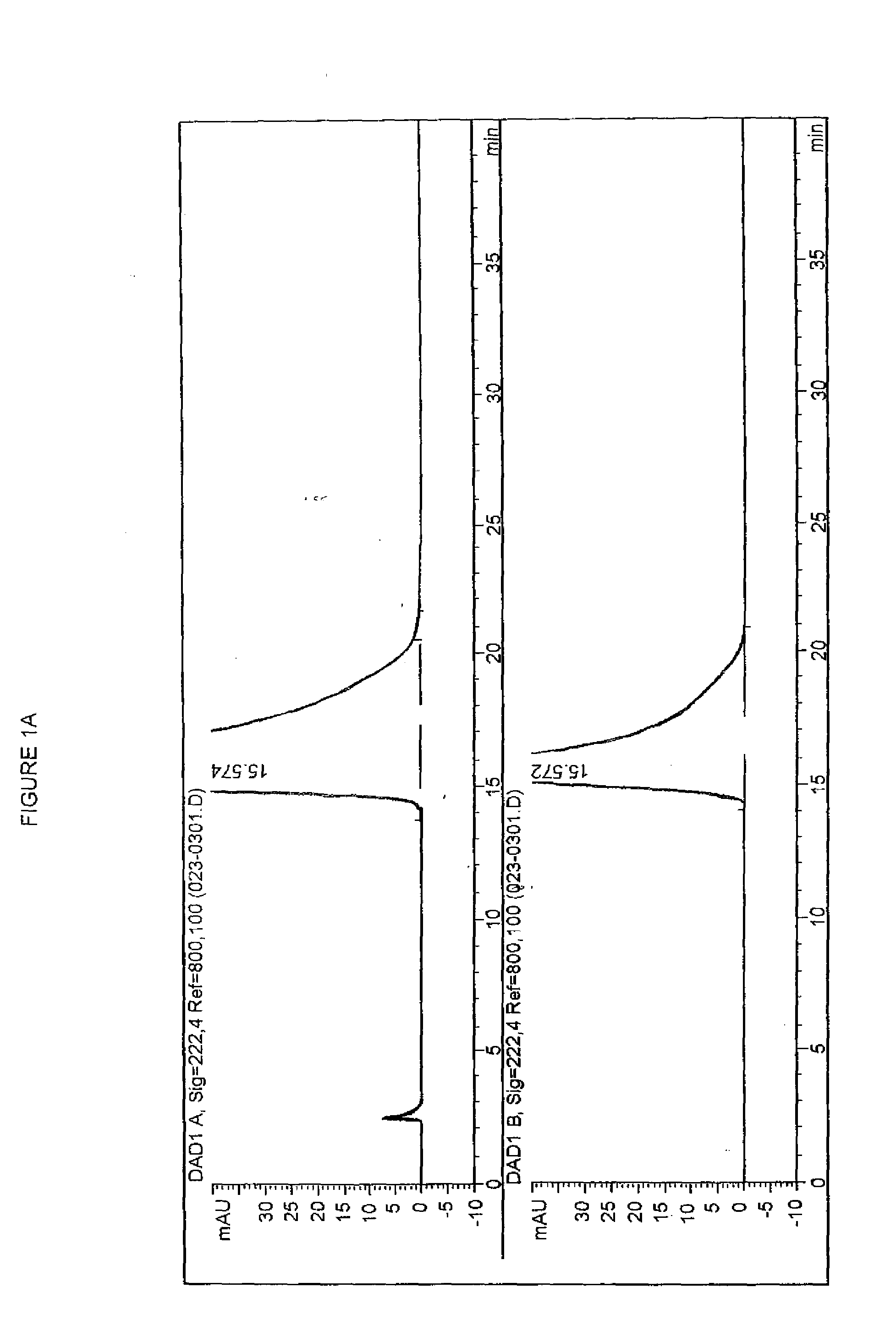
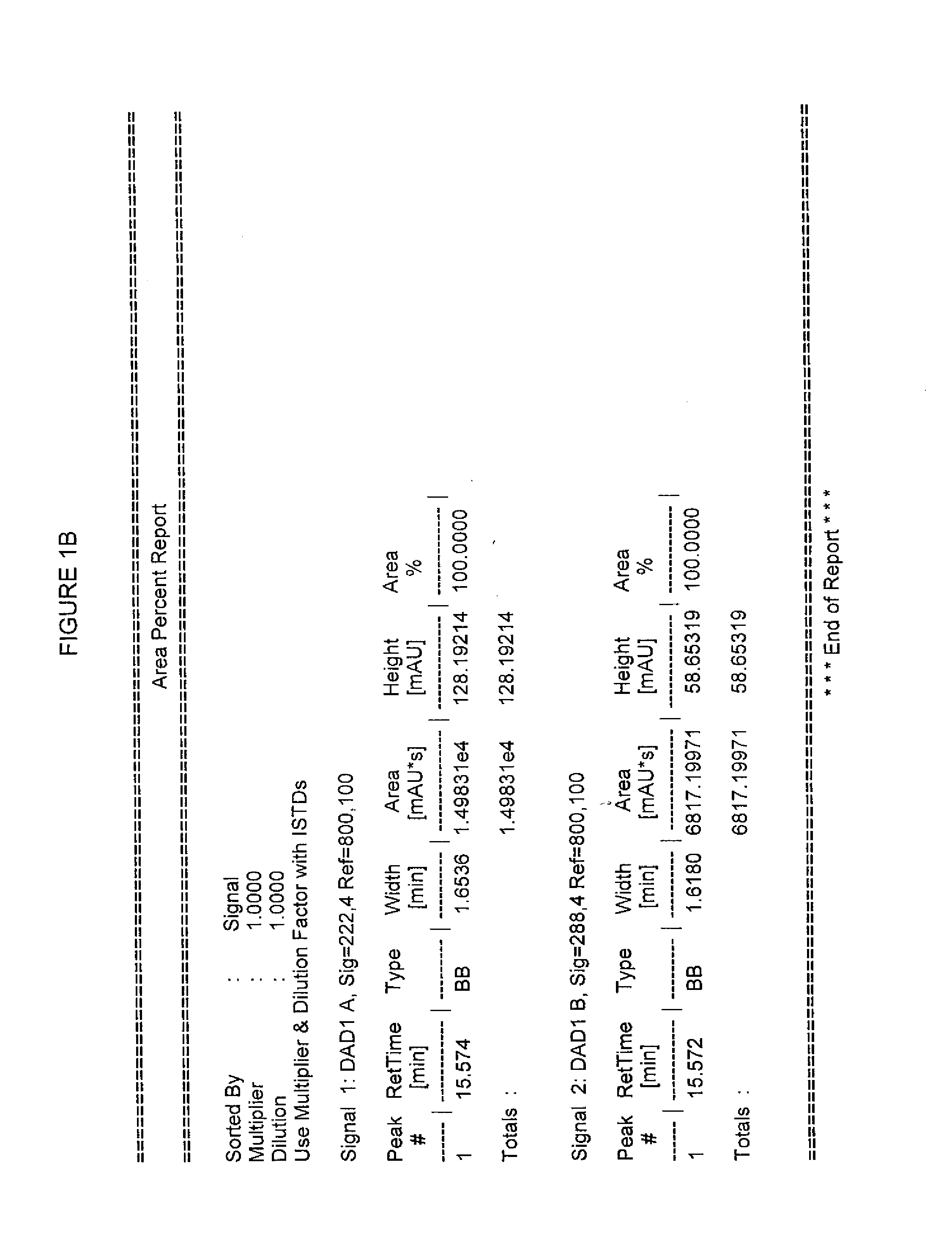
2-(1,6,7,8-tetrahydrogen-2H-indeno-[5,4-b] furan-8-group) acetonitrile, preparation method and applciation
InactiveCN101824012AHigh yieldHigh purityOrganic active ingredientsNervous disorderFuranAcetonitrile
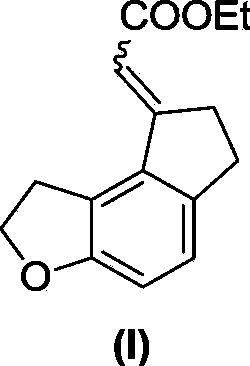
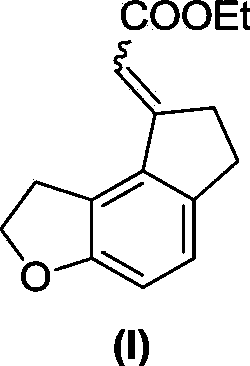
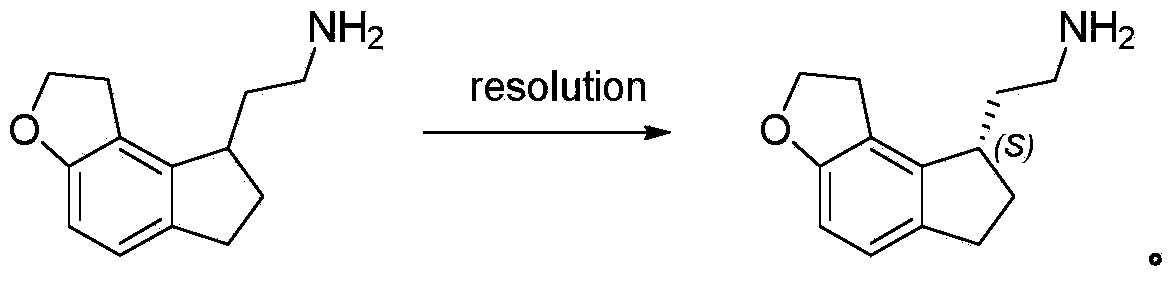
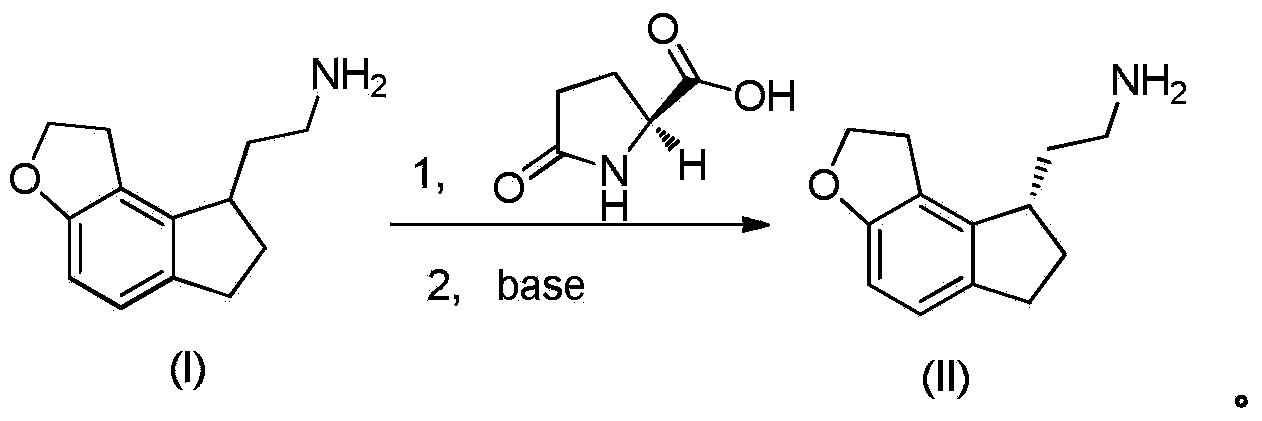
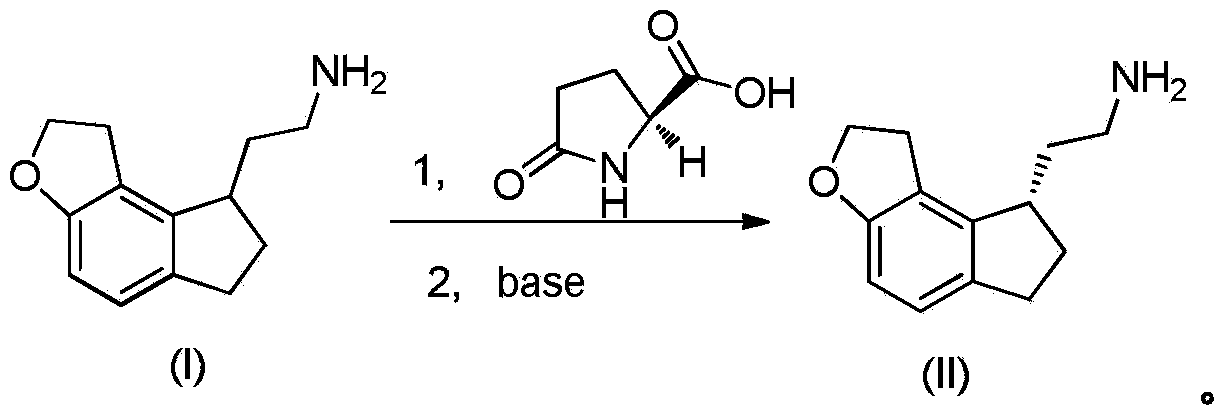
The invention discloses 2-(1,6,7,8-tetrahydrogen-2H-indeno-[5,4-b] furan-8-group) acetonitrile (I), an optical isomer (II) thereof, a preparation method of the compound and application of the compound in preparing ramelteon which is a medicament for treating insomnia.
Owner:SICHUAN UNIV
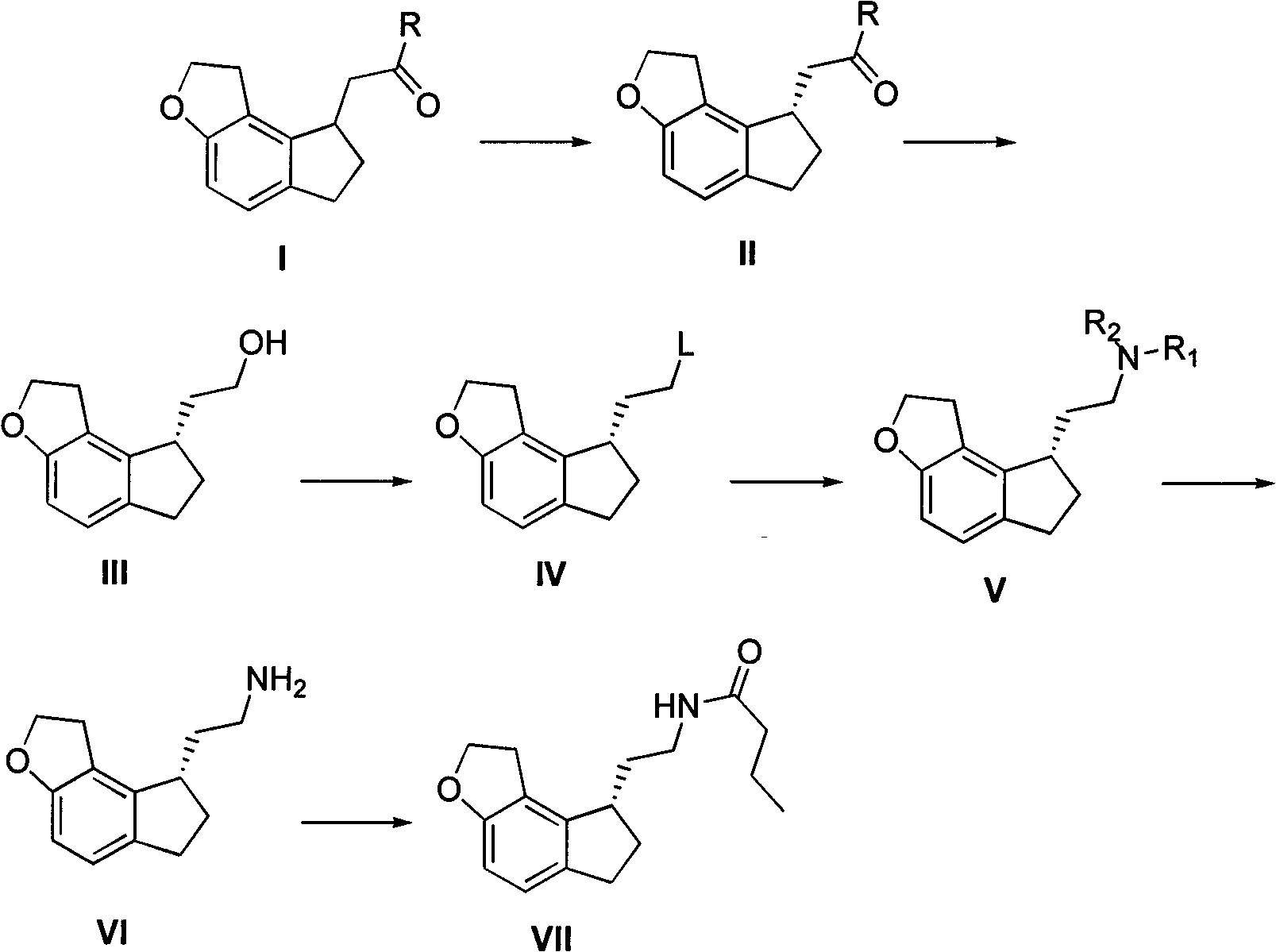
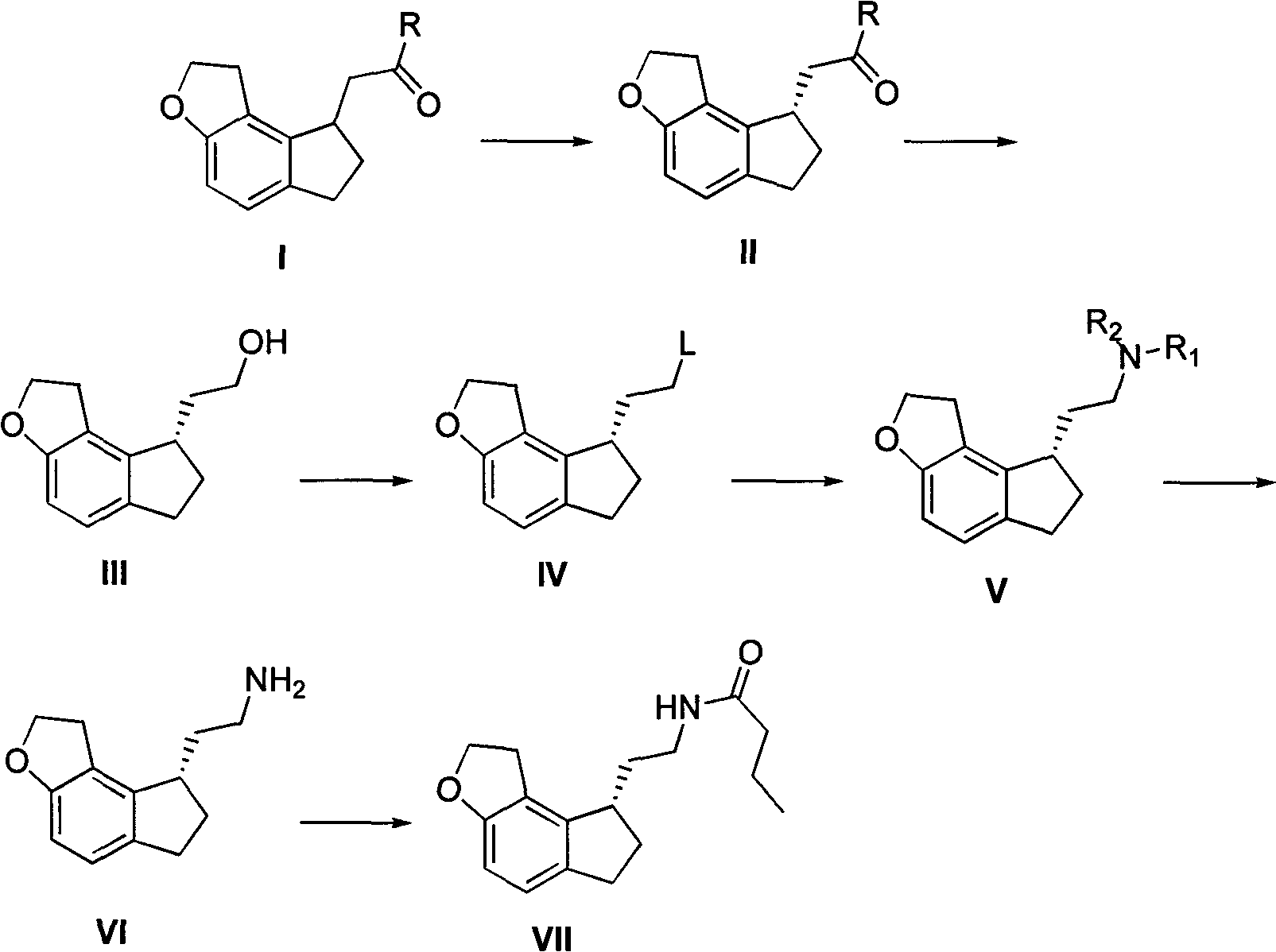
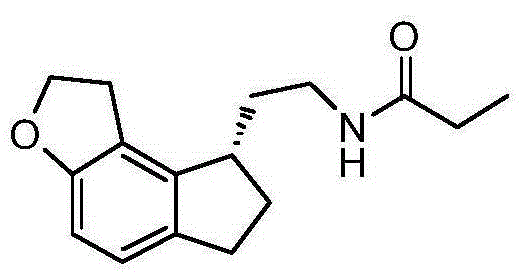
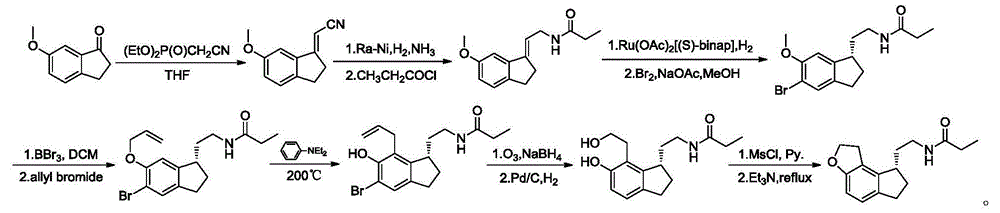
Preparation method and intermediate of ramelteon
InactiveCN102924410AReduce dosageRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionPropionyl chlorideOrganic solvent
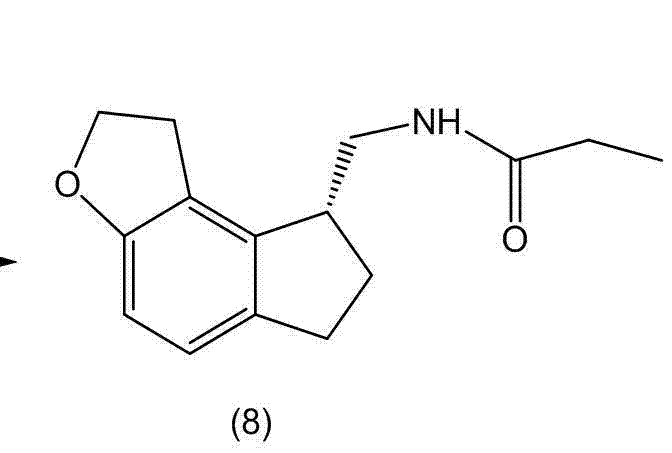
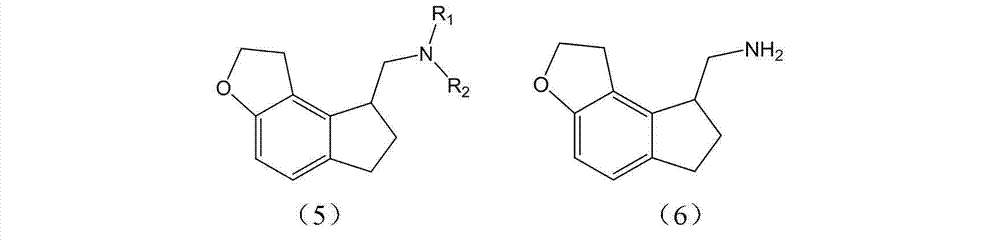
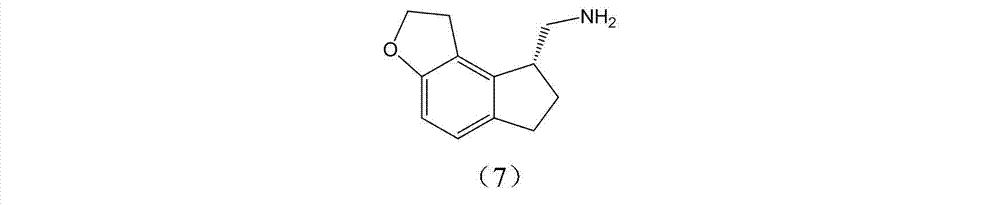
The invention discloses a preparation method and an intermediate of ramelteon. The preparation method comprises the following steps: 1. deprotecting a compound (5) in an organic solvent to obtain a compound (6); 2. after condensing the compound (6) and a chiral resolution reagent, crystallizing to obtain an S type condensate crystal, and hydrolyzing under alkaline conditions to obtain an S type optical isomer compound (7) of the compound (6); and 3. reacting the compound (7) with propionyl chloride under alkaline conditions to generate the ramelteon (8).
Owner:CHINA RESOURCES SAIKE PHARMA
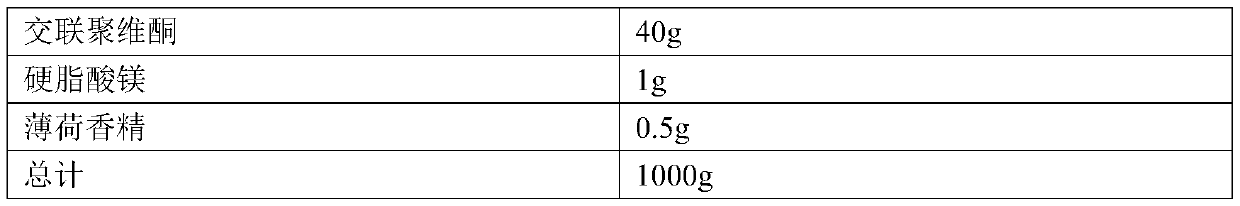
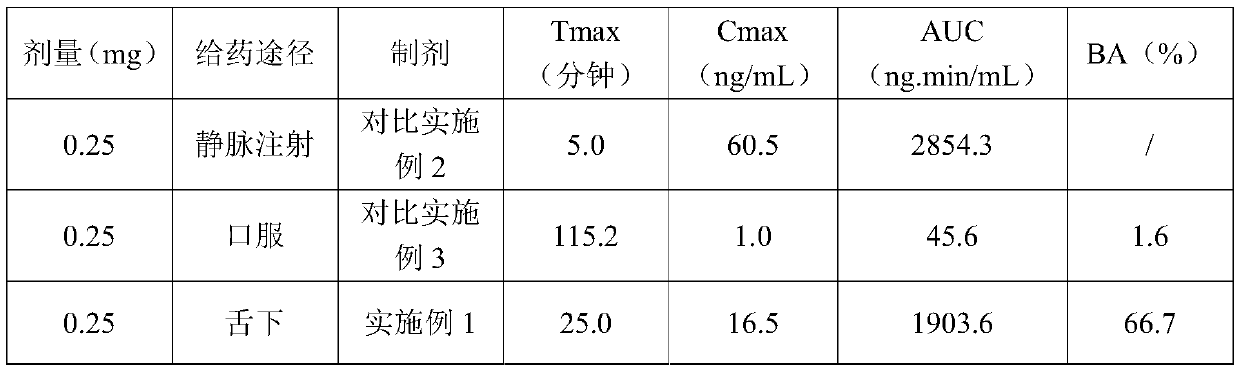
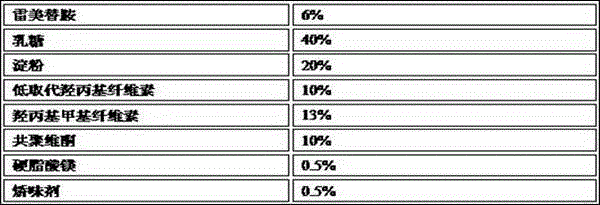
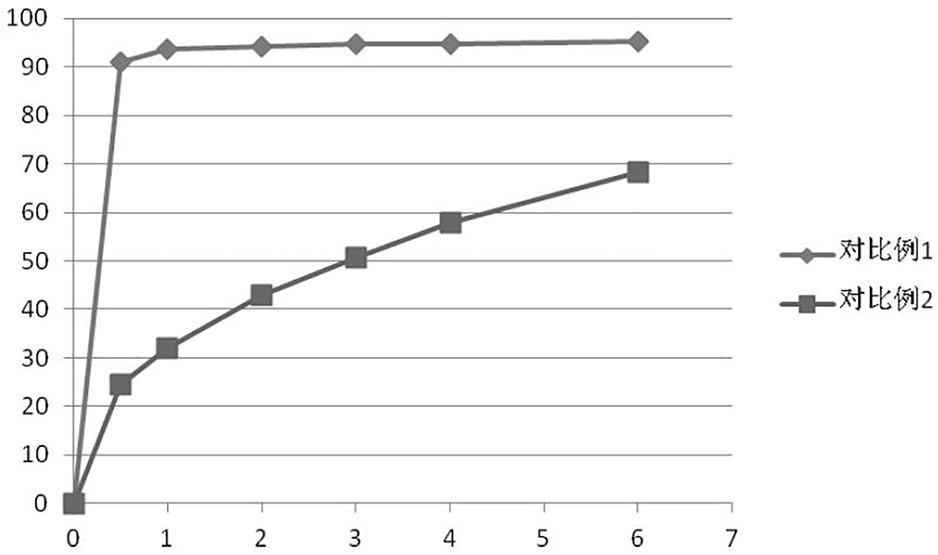
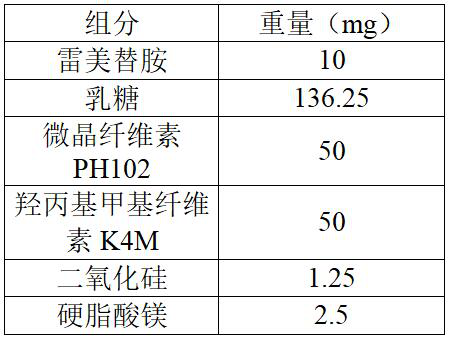
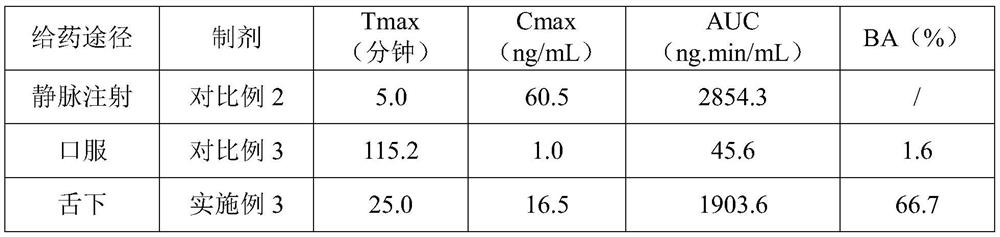
Ramelteon sublingual tablet and preparation method thereof
InactiveCN110433142AGreat tasteGrind evenlyOrganic active ingredientsNervous disorderCross-linkSucrose
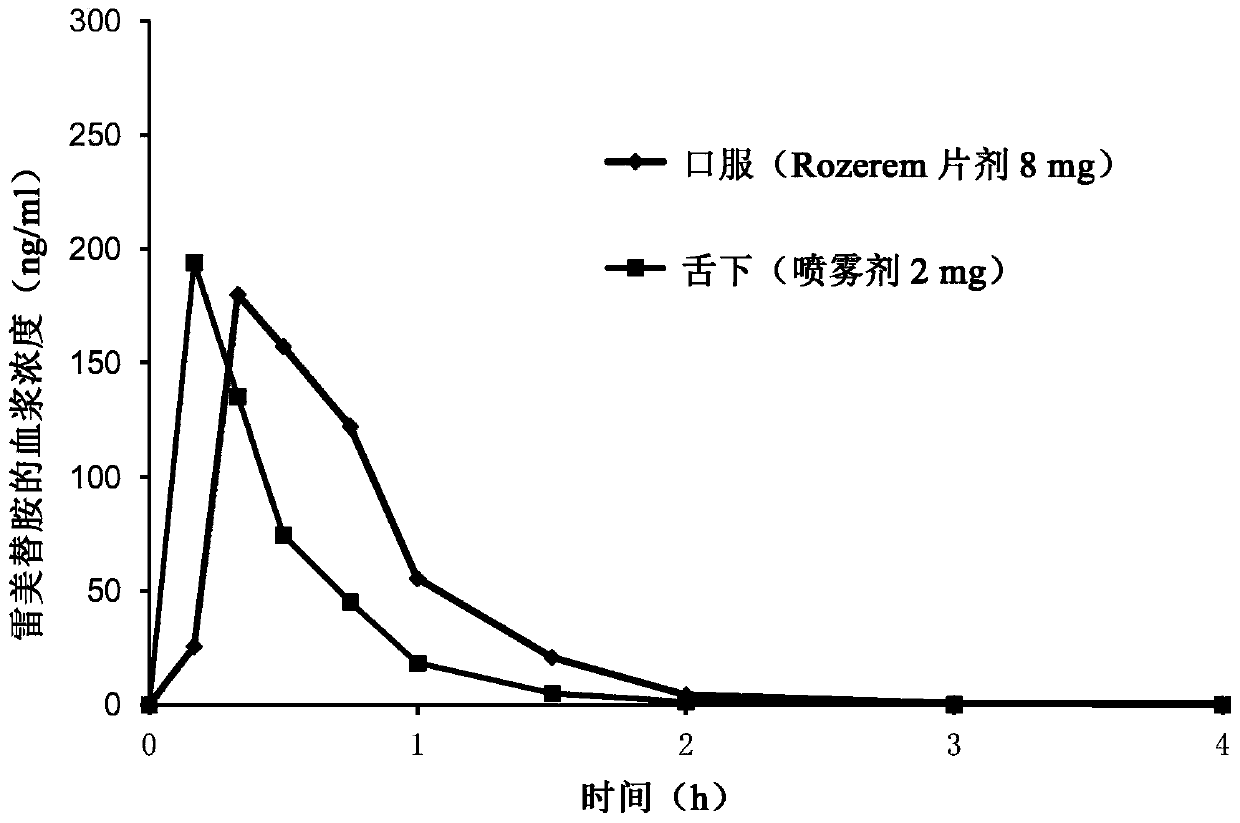
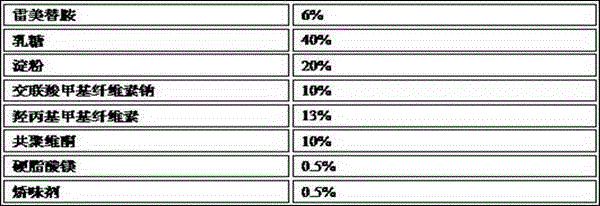
The invention belongs to the field of pharmaceutical preparations, and relates to ramelteon sublingual tablets and a preparation method thereof. The sublingual tablet contains effective amounts of ramelteon, a filler, a disintegrant, a lubricant, and a flavoring agent, and the proportion of a main drug is 0.1 to 0.5%; the filler is selected from one or a combination of mannitol, lactose, sucrose and xylitol; the disintegrant is selected from one or the combination of crospovidone, cross linked sodium carboxymethyl cellulose, and low-substituted hydroxypropyl cellulose; and the lubricant is selected from one or a combination of magnesium stearate, aerosil, and sodium stearyl fumarate; and the flavoring agent is mint flavor. The invention also provides the preparation method of the ramelteonsublingual tablets, that is, ramelteon and the filler are ground and mixed by a ball mill, and mixed with the disintegrating agent, the lubricant, and the flavoring agent, and the materials are pressed to prepare tablets. The ramelteon sublingual tablet of the invention can avoid the first pass effect of the liver and improve the bioavailability.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Preparation method of ramelteon intermediate
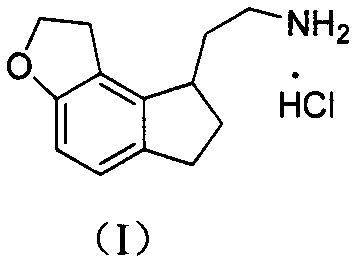
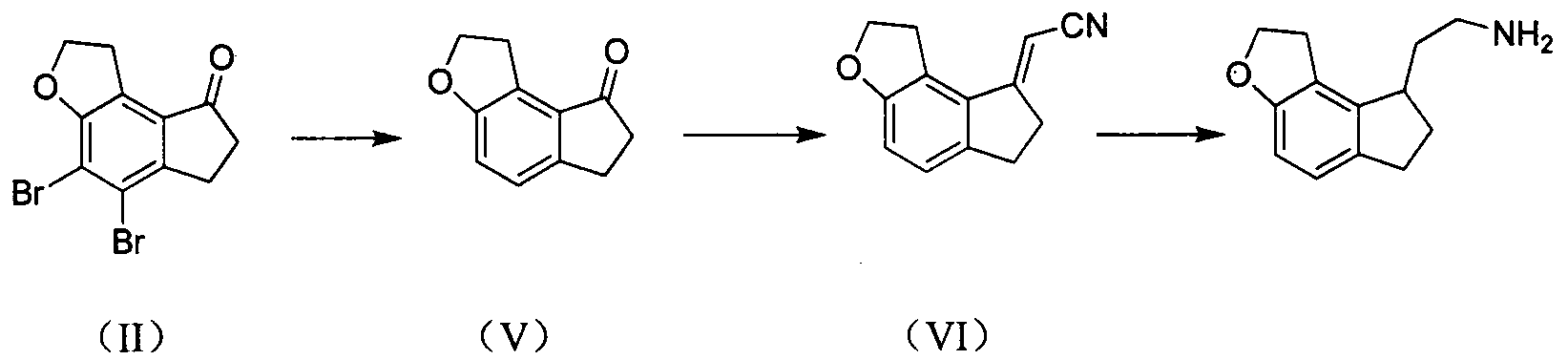
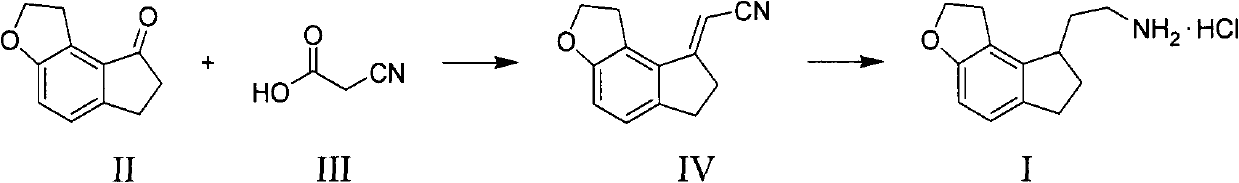
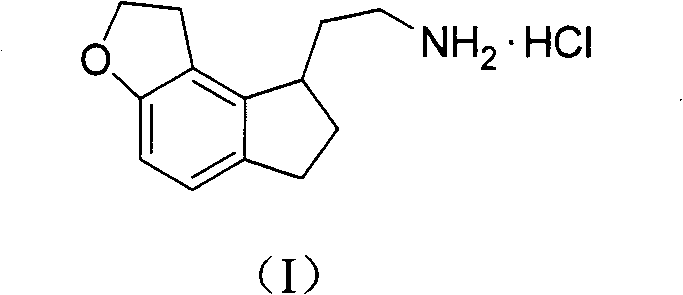
The invention discloses a novel preparation method of a ramelteon intermediate 2-(1, 2, 6, 7-tetrahydro-8H-indeno[5, 4-b] furan-8-yl) ethylamine hydrochloride (I). The method is characterized by comprising the following steps: dehydrating and condensing and heating and decarboxylating 4, 5-dibromo-1, 2, 6, 7-tetrahydro-8H-indeno[5, 4-b] furan-8-one (II) and cyanoacetic acid (III) under the effect of a catalyst to obtain a compound (IV); then, hydrogenating the (IV) and salifying to obtain the ramelteon intermediate hydrochloride (I). The invention provides a novel preparation of the ramelteon intermediate hydrochloride (I). According to the method, cyanoacetic acid (II) which is cheap and easily available is used, so that the method is simplified in synthetic step, high in efficiency and simple and convenient to operate, and the method is a preparation method which is more suitable for industrialized production.
Owner:CHINA PHARM UNIV
Novel method for preparing ramelteon key intermediate
The invention discloses a novel method for preparing a ramelteon key intermediate 2-(1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-yl)ethylamine hydrochloride (I). The method is characterized by comprising the following steps of: performing dehydration condensation and heated decarboxylation on 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (II) and cyanoacetic acid (III) under the action of a catalyst to obtain a compound (IV); and directly hydrogenating and salifying the compound (IV) without separating and purifying to obtain the ramelteon key intermediate 2-(1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-yl)ethylamine hydrochloride (I). The invention provides the novel method for preparing the ramelteon key intermediate hydrochloride (I). In the method, the cheap and readily available cyanoacetic acid (II) is used; and the method is high in yield and low in cost, is easy and convenient to operate, is environment-friendly and is suitable for industrialized production.
Owner:CHINA PHARM UNIV
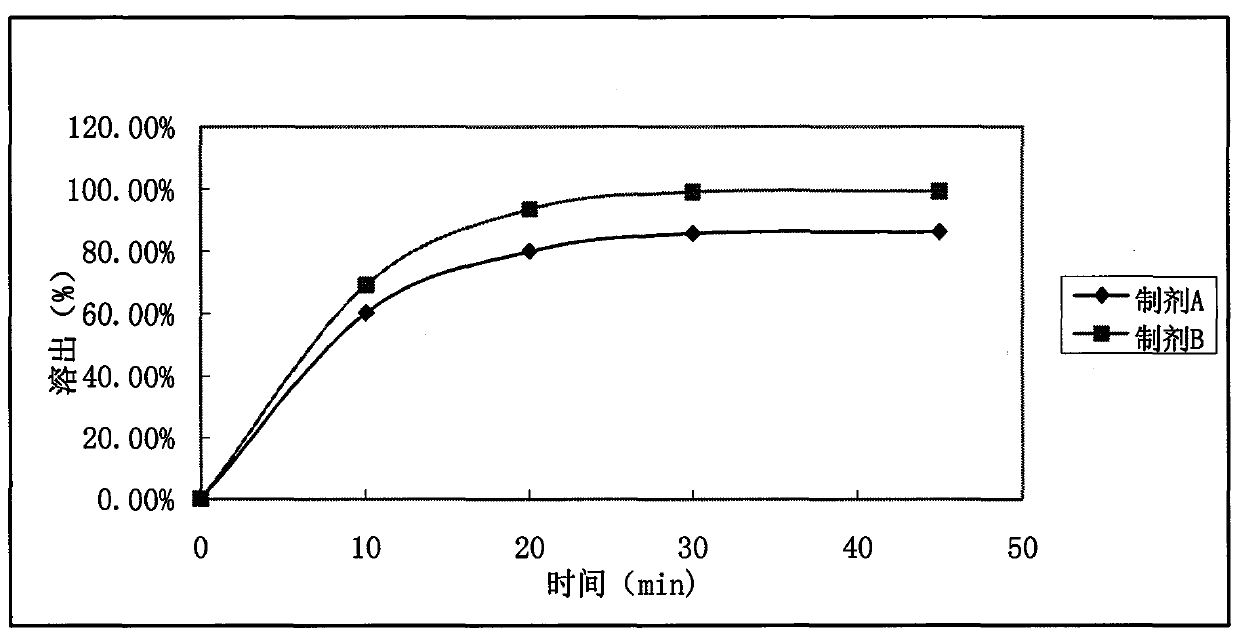
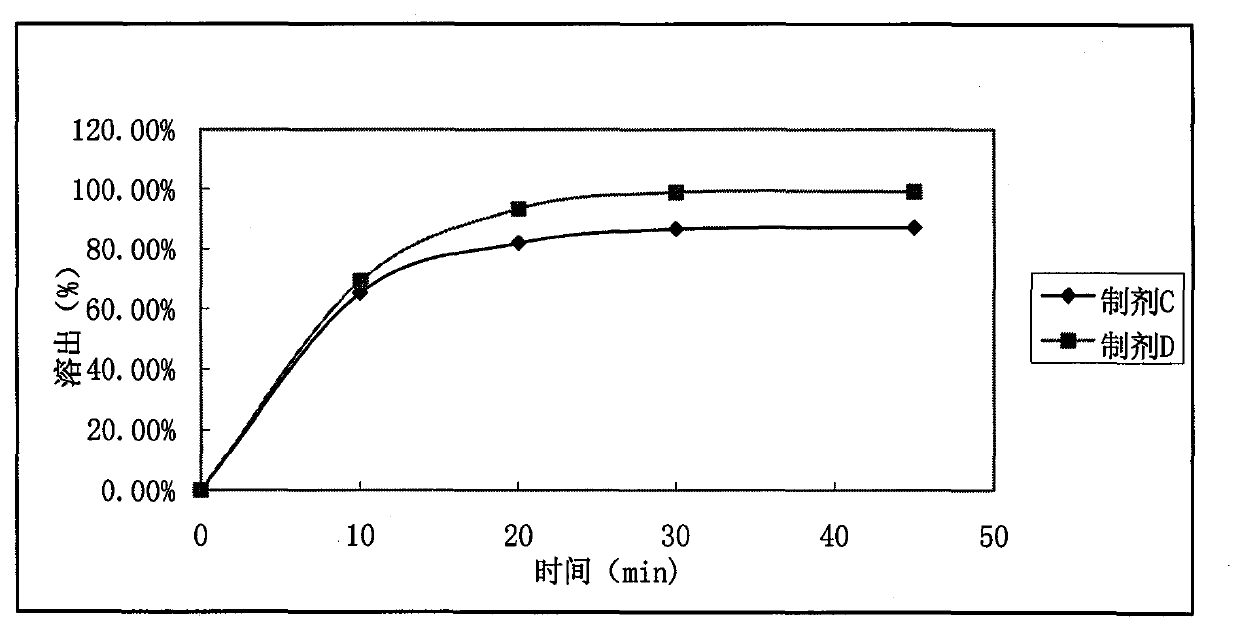
Tablet containing ramelteon and copovidone
ActiveCN103989643AExcellent adhesionImprove bioavailabilityOrganic active ingredientsNervous disorderMagnesium stearateStearic acid
The invention relates the technical field of medicinal preparations and discloses a tablet containing ramelteon and copovidone. Through use of copovidone, the tablet solves the problem of low bioavailability of the ramelteon preparation prepared by the prior art. Through common preparation methods, ramelteon, copovidone and other required components are mixed according to a preset ratio and the mixture is pressed to form the tablet. A preparation method of the tablet comprises the following steps of mixing ramelteon, copovidone and a needed excipient in a granulator to obtain a uniform mixture, adding a needed binder into the granulator, carrying out granulation, taking out the granules, carrying out drying, carrying out screening to obtain powder having required granule sizes, adding magnesium stearate and a required disintegrating agent into the powder of which the granule sizes are adjusted and carrying out mixing to obtain particles to be tabletted. The tablet provided by the invention has greatly improved in-vitro bioavailability.
Owner:WUHAN LEADPHARM TECH CO LTD
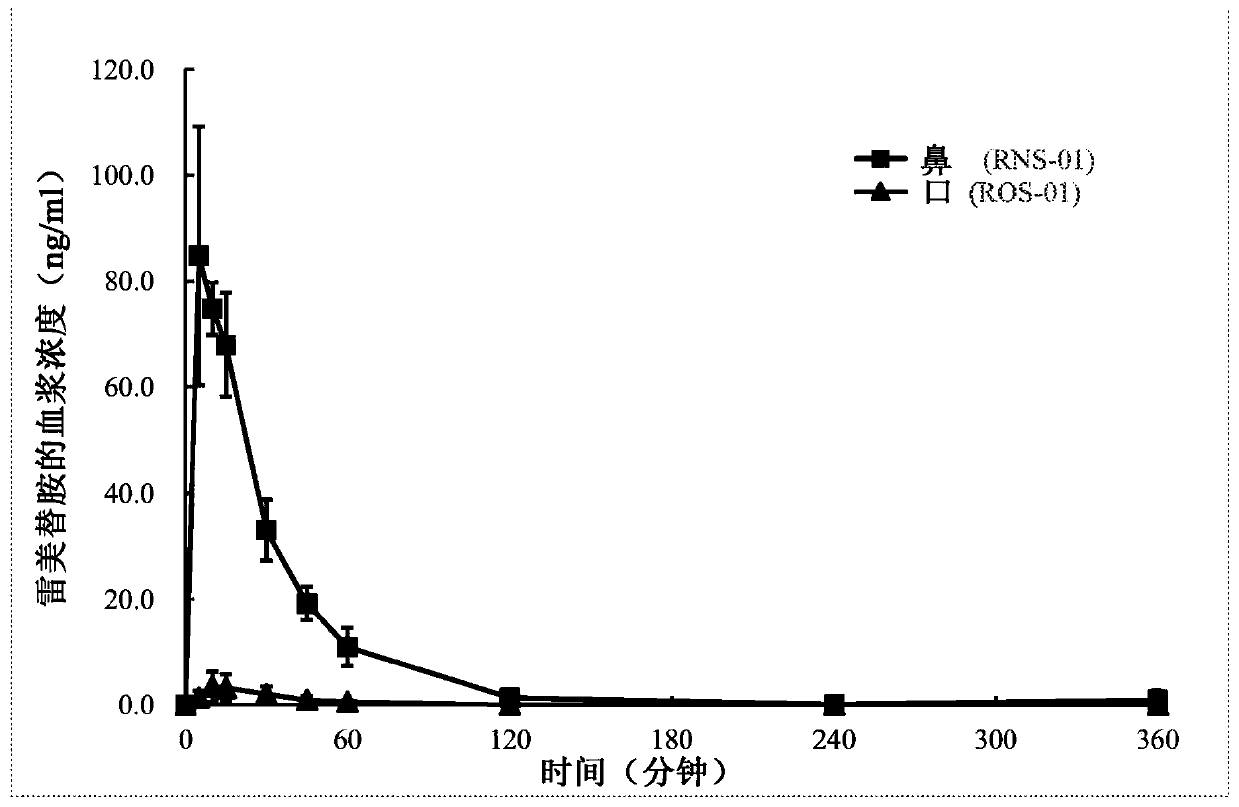
Pharmaceutical compositions of ramelteon and methods of use thereof
Pharmaceutical compositions of ramelteon for use in the treatment of insomnia or jet lag by transmucosal administration. More specifically, the pharmaceutical composition is formulated into nasal spray or nasal drop for intranasal administration, or formulated into sublingual spray for sublingual administration, or formulated into a solid dosage for sublingual administration.
Owner:MAXINASE LIFE SCI LTD
Preparation method for key intermediate of ramelteon
The invention discloses a preparation method for a key intermediate of ramelteon (a compound represented by formula (I)). The preparation method includes the steps as follows: dissolving a compound represented by formula 1, a compound represented by formula 2, a metal catalyst, an alkali, norbornene and a ligand in an organic solvent for carrying out a reaction to obtain the compound represented by formula (I). The synthetic route of the invention is a convergent synthesis which is different from a linear synthesis in a conventional synthetic route. The synthetic route of the invention is short in linearity and is high in yield. The reaction equation of the invention is represented by a formula as follows.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Preparation method of Ramelteon intermediate
ActiveCN103570651ASimple stepsThe reaction mechanism is matureOrganic chemistryFuranProcess engineering
The invention relates to a preparation method of a Ramelteon intermediate. The Ramelteon intermediate is prepared by taking 4,5-dibromo-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one as a raw material, and then subjecting the raw material to a series of simple reactions so as to obtain the Ramelteon intermediate. The preparation method of the Ramelteon intermediate has the advantages of high yield, high product purity, simple operation, low cost, and suitability for mass production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
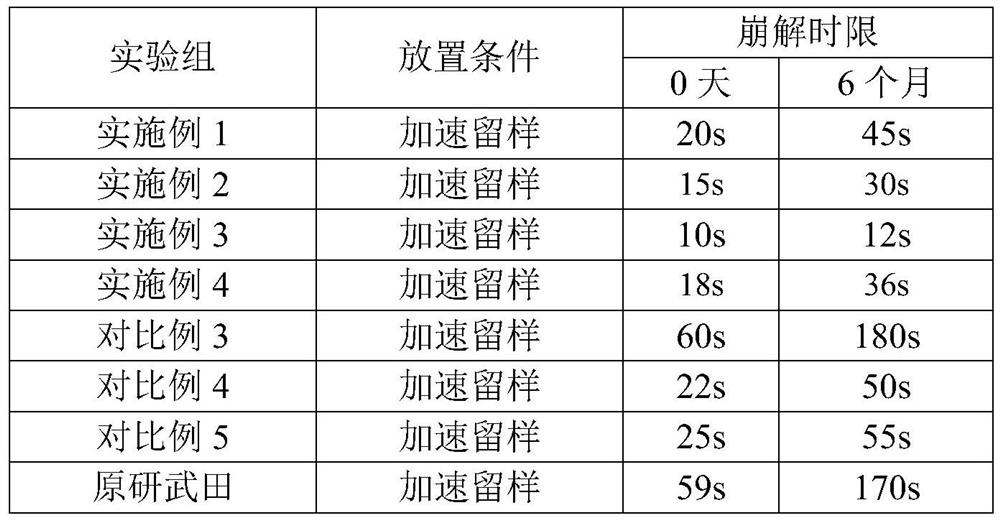
Ramelteon oral disintegrating tablets and preparation method thereof
The invention belongs to the technical field of medicines and relates to ramelteon oral disintegrating tablets and a preparation method thereof. When being disintegrated, the ramelteon oral disintegrating tablets provided by the invention are wholly disintegrated within 60 seconds according to Chinese Pharmacopoeia Standards and have no hard cores. Raw drugs are sensitive to humidity so that dry-process granulation is used for replacing traditional wet-process granulation. The ramelteon oral disintegrating tablets provided by the invention are prepared from ramelteon and auxiliary components including a disintegrating agent, a filling agent, a binding agent, a sweetness flavoring agent and the like, and a dry-process granulation process is adopted. The ramelteon oral disintegrating tablets have the overall effects of high disintegrating speed, simple process steps, low cost, convenience for taking, good mouth feel, stable quality, reliable curative effect and the like; after the ramelteon oral disintegrating tablets are orally taken, the ramelteon oral disintegrating tablets are rapidly dispersed into fine particles or powder in an oral cavity, so that the ramelteon oral disintegrating tablets are particularly suitable for patients who cannot easily orally take the tablets and have insomnia; when the preparation reaches gastrointestinal tracts, the preparation exists in a form of fine particles, and thus the dissolving rate of the medicine is accelerated; and the medicine is widely distributed in the gastrointestinal tracts and a plurality of absorption points exist, so that the bioavailability can be improved.
Owner:BEIJING VENTUREPHARM BIOTECH
Method for preparing ramelteon intermediate by racemization
The invention discloses a racemization method of (R)-2-(1,6,7,8-tetrahydro-2H-indeno(5,4-b)furan-8-yl)acetic acid (R-I). The method comprises the following steps: 1) in an organic solvent and under the action of a radical initiator and a catalyst, R-I undergoes oxydehydrogenation to be converted to a compound II; and 2) in an organic solvent, the compound II undergoes hydrogenation reduction in the presence of a hydrogenation catalyst so as to obtain racemate-I. By the preparation method, use of expensive catalysts is avoided. In addition, cost is low and post-treatment is simple. Waste is changed into valuable things, and discharge of ''three wastes (waste gas, waste water and industrial residue)'' is reduced. The method is beneficial to environment protection. Yield of the product is high; the insufficiency of low yield caused by the resolution reaction in the Route Three is made up. The method can be used for synthesis of ramelteon and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Porcine oocyte in-vitro maturation culture solution as well as preparation method and application thereof
ActiveCN110684723AReducing ROS levelsIncreased Diffusion IndexCell culture active agentsGerm cellsPolyvinyl alcoholPancreatic hormone
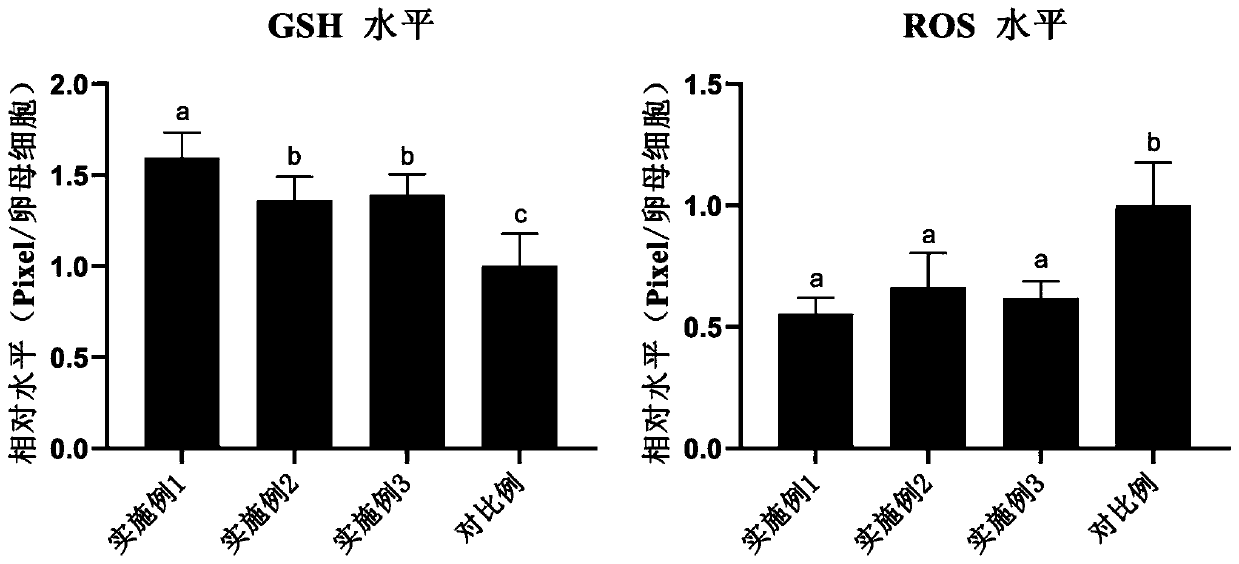
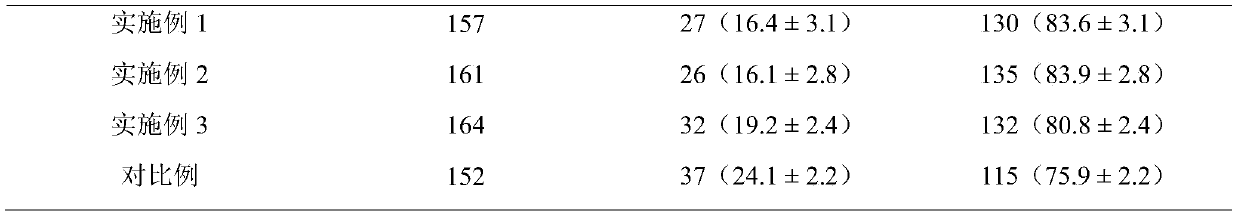
The invention discloses a porcine oocyte in-vitro maturation culture solution as well as a preparation method and application thereof and belongs to the technical field of oocyte in-vitro maturation culture. In order to solve the problems of low porcine oocyte in-vitro maturation rate and development rate at present, the invention provides the porcine oocyte in-vitro maturation culture solution aswell as the preparation method and application thereof. The culture solution comprises a base culture solution TCM-199, penicillin, streptomycin, NaHCO3, 4-hydroxyethylpiperazine ethane sulfonic acid, polyvinyl alcohol, sodium pyruvate, insulin, cysteine, an epidermal growth factor, a porcine follicular fluid, pregnant mare serum gonadotropin, human chorionic gonadotropin and ramelteon. The culture solution can increase the oocyte in-vitro maturation quality, the cumulus cell diffusion index, the glutathione level, the parthenogenetic embryo blastocyst rate and blastocyst total cell number, the in-vitro fertilization embryo cleavage rate, the blastocyst rate and blastocyst total cell number and decrease the ROS level of oocytes.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Preparation method of high-purity ramelteon
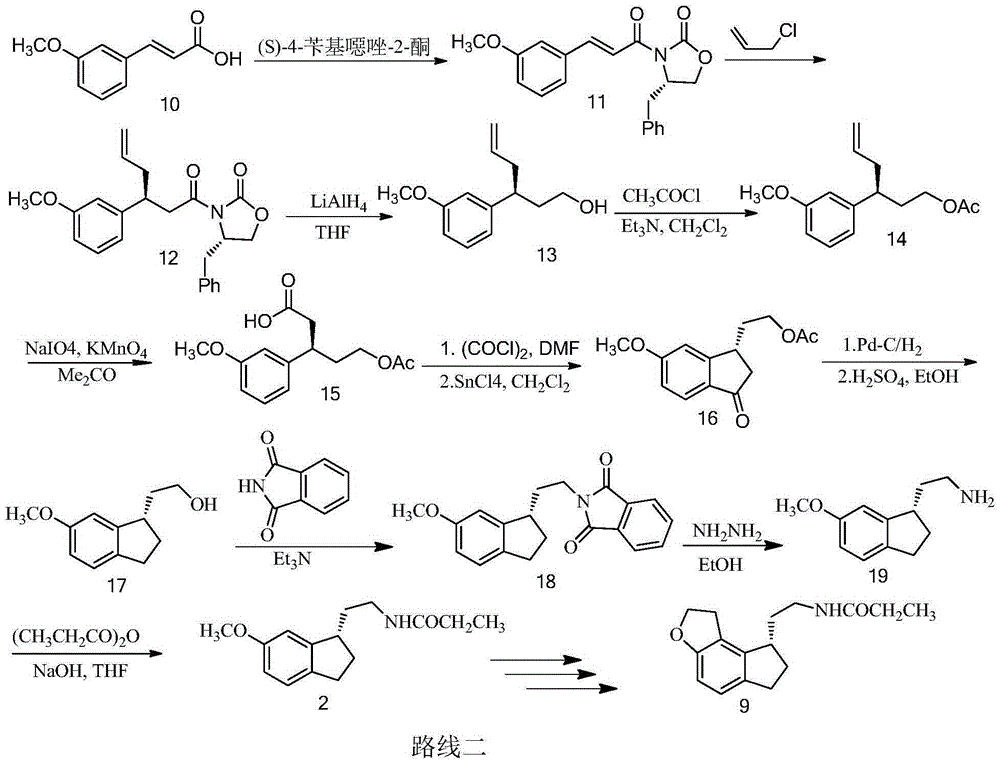
The invention discloses a preparation method of high-purity ramelteon. The preparation method comprises the following steps: taking 1,2,6,7-tetrahydro-8H-indene[5,4-b]furan-8-ketone as a starting material; carrying out reduction and amino protection through wittig-horner reaction; carrying out amino deprotection under an acidic condition; carrying out hydrogenation reaction; then carrying out chiral resolution and acrylation reaction, thus obtaining the ramelteon. The ramelteon obtained through the invention is high in product purity and higher in yield; and formation of impurities is inhibited.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Method for synthesizing Ramelteon
ActiveCN102321056AMechanism research is thoroughSingle reaction siteOrganic chemistryRamelteonStereochemistry
The invention relates to a method for synthesizing Ramelteon. In the method, a compound I is selected as a starting substance, and Ramelteon is obtained by substitution, chiral separation, reduction, substitution, nucleophilic reaction and other reactions. Compared with the prior art, the method provided by the invention has the advantages that the entire process is environment-friendly, and the yield and purity of the product are high.
Owner:宁波人健药业集团股份有限公司
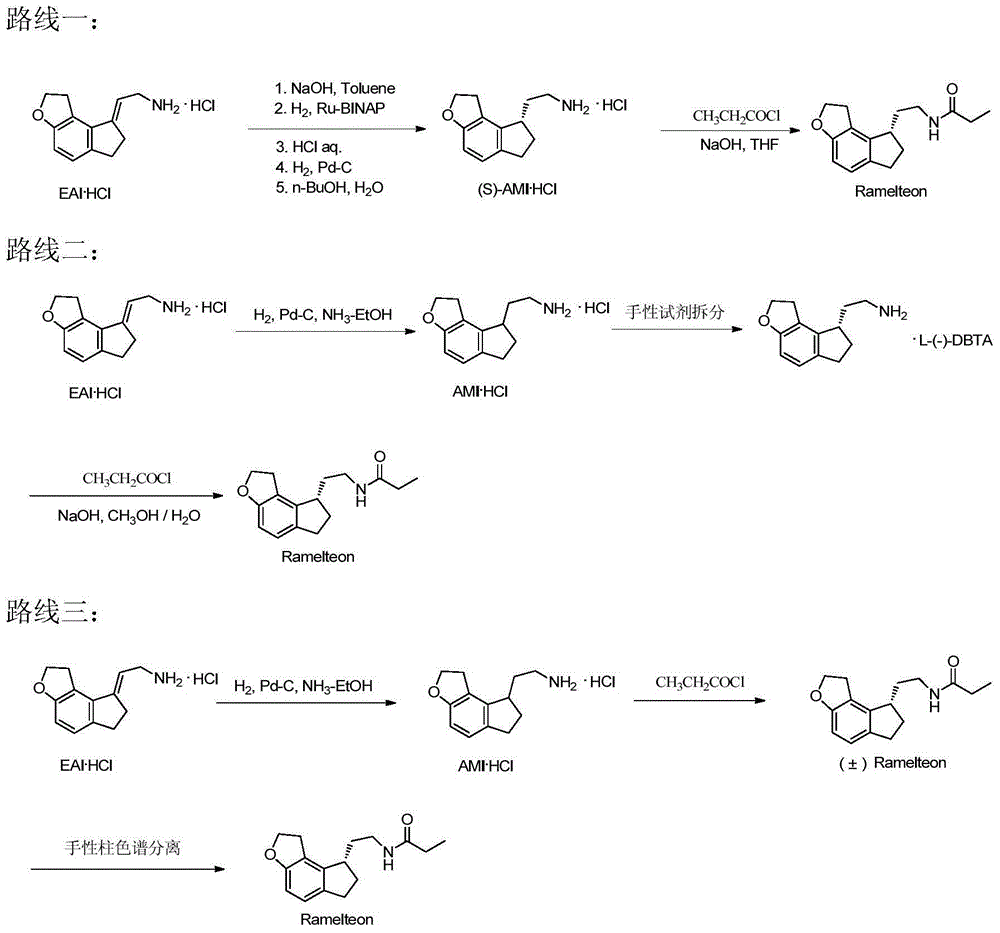
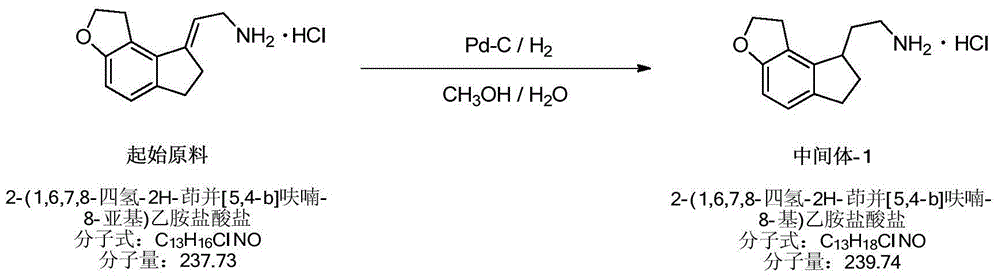
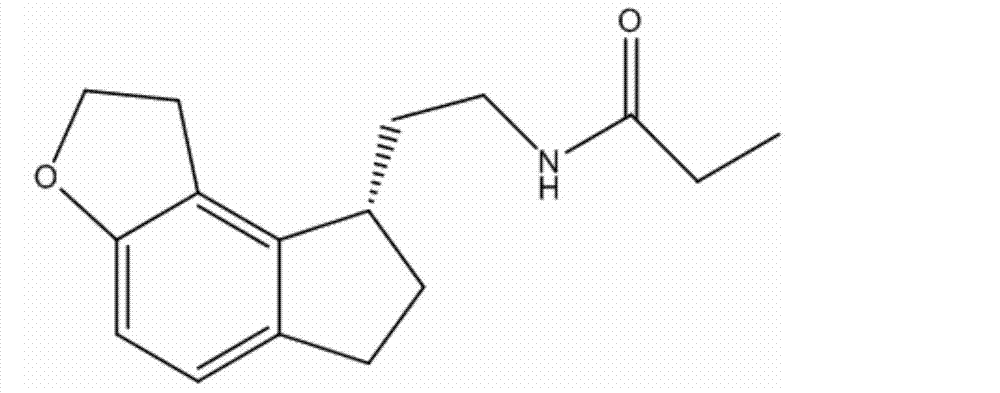
Preparation method of Ramelteon
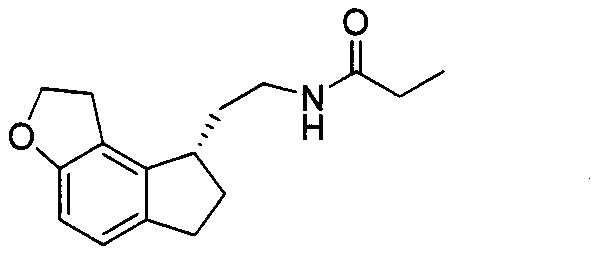
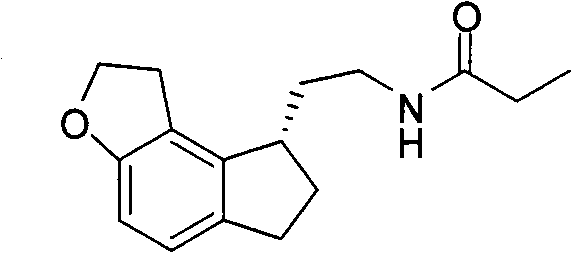
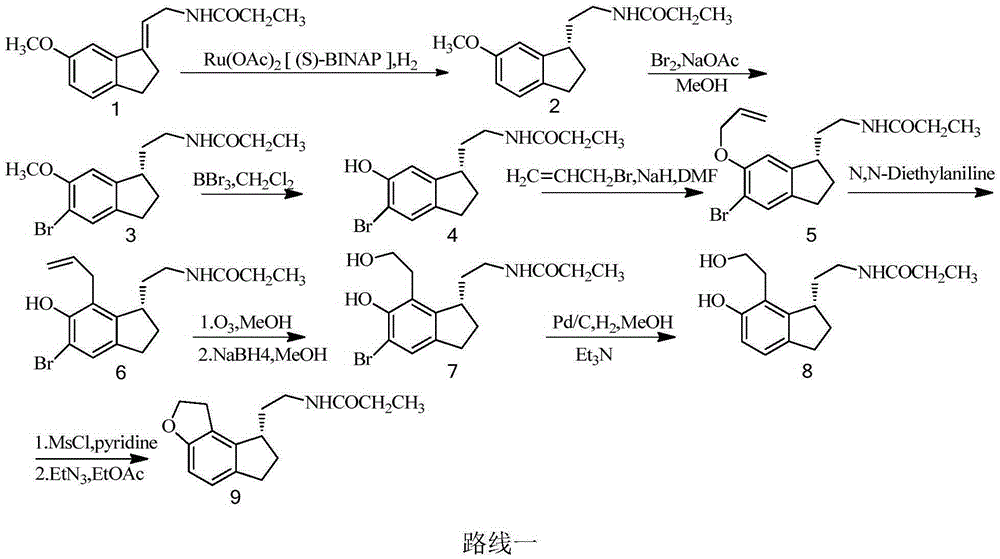
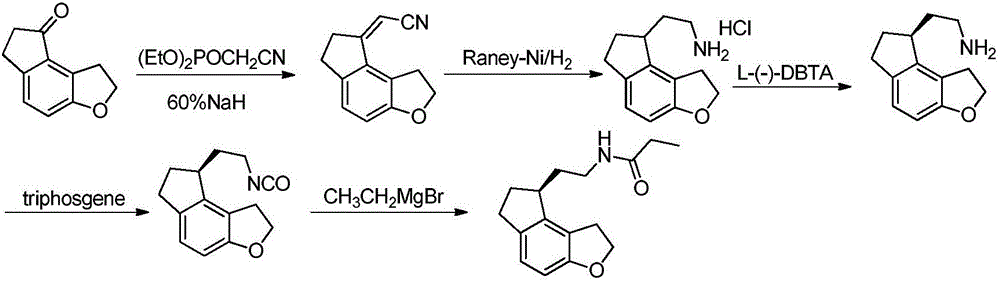
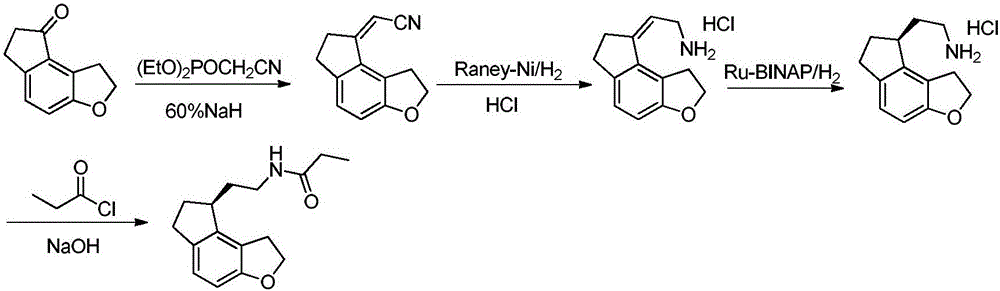
The invention relates to a preparation method of Ramelteon. The method mainly comprises three reaction steps of hydrogenation, chiral resolution and acylation reaction. According to the synthesis of the Ramelteon, 2-(1,6,7,8-tetralin-2H-indeno[5,4-b]furan-8-subunit) ethylamine hydrochloride serves as a start raw material, Pd-C serves as a catalyst, and 2-(1,6,7,8-tetralin-2H-indeno[5,4-b]furan-8-base) ethylamine hydrochloride, namely an midbody-1, is acquired through catalytic hydrogenation; chiral resolution is conducted on the midbody-1 through dibenzoyl-L-tartrate, so that (S)-2-(1,6,7,8-tetralin-2H-indeno[5,4-b]furan-8-base) ethylamine dibenzoyl-L-tartrate, namely a midbody-2, is acquired; an acylation reaction is conducted on the midbody-2 and propionyl chloride, so that a crude product of the Ramelteon is acquired, and a finished product of the Ramelteon is acquired after the crude product is refined and qualified.
Owner:JUMPCAN PHARMA GRP
Preparation method of ramelteon intermediates
The invention relates to a preparation method of ramelteon intermediates, the preparation method comprises the following steps: adding diethyl cyanomethylphosphonate into an organic solvent, mixing, adding an alkali in batches, dropping a solution of compounds I for reaction, extracting, washing and drying, thus obtaining a compound II; adding an organic solvent, aluminum chloride and lithium aluminium hydride into a reaction vessel, dropping a solution of the compound II, first reacting at a low temperature, then reacting at the room temperature, adjusting the pH of the reaction solution to 8-9, filtering, drying a filter liquor to obtain a solid compound, dissolving the solid compound in dichloromethane (DCM), then adding HCl / EtOH, adjusting the pH to be acidic, filtering and drying to obtain a compound III, and thus respectively obtaining a compound IV, a compound V and a compound VI by high pressure hydrogenation under the effects of different catalysts. The preparation method has the advantages of simple process, high yield, low cost and high purity of target products, and is conducive to the mass production of a ramelteon key intermediate.
Owner:安徽联创生物医药股份有限公司
Preparation method of ramelteon impurity
ActiveCN110776485ASafe restore conditionOrganic chemistryBulk chemical productionChemical synthesisAluminium chloride
The invention relates to a preparation method of a ramelteon impurity, and belongs to the technical field of chemical synthesis. The preparation method comprises the following steps: (1) reacting a compound I with oxalyl chloride to generate an acyl chloride compound, adding aluminum trichloride, and carrying out a Friedel-Crafts reaction to generate a compound II; and (2) directionally generatinga compound III from the compound II under the action of Raney cobalt and hydrazine hydrate. The preparation method provides a novel and safe reduction condition for direct synthesis of the monosubstituted impurity, and the product does can be directly used as an impurity reference substance without purification, and also can be directly subjected to a subsequent impurity transfer experiment.
Owner:SHANDONG ZOUPING DAZHAN NEW MATERIALS
Ramelteon composition and tablet thereof
ActiveCN104224741AImprove stabilityReduce oxidative degradationOrganic active ingredientsNervous disorderMedicineDissolution
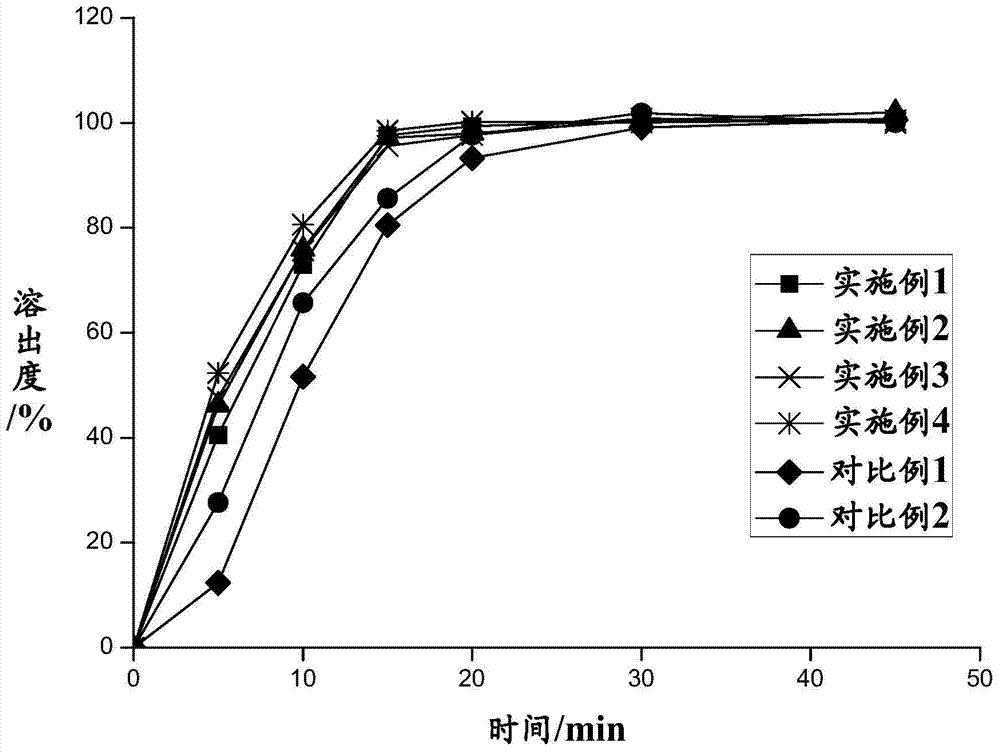
The invention provides a Ramelteon composition. The Ramelteon is composed of the following components, by weight part, 5-8 parts of Ramelteon and 0.5-3 parts of polyvidone. The invention also discloses a Ramelteon tablet. The Ramelteon tablet is composed of the following components including, by weight part, 5-8 parts of Ramelteon, 0.5-3 parts of polyvidone, 80-110 parts of filling agent, 3-10 parts of disintegrating agent and 0.5-1.5 parts of lubricant. The Ramelteon tablet is high in dissolution rate and stability and is prepared through a conventional method, which is simple in process, easy to operate, low in requirements on micronizing equipment, low in production cost and applicable to industrial mass production.
Owner:BEIJING COLLAB PHARMA
Preparation method of ramelteon
The invention belongs to the field of medicinal chemistry, and mainly relates to a (S)-2-(1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-yl)-1-ethylamine compound represented by a formula I. The method has the advantages that the operation is simple, the steps are reduced compared with the previous method, the route is shortened, and the used solvent is single in variety, convenient to recycle and high in yield, wherein the formula I is defined in the specification.
Owner:BEIJING VENTUREPHARM BIOTECH
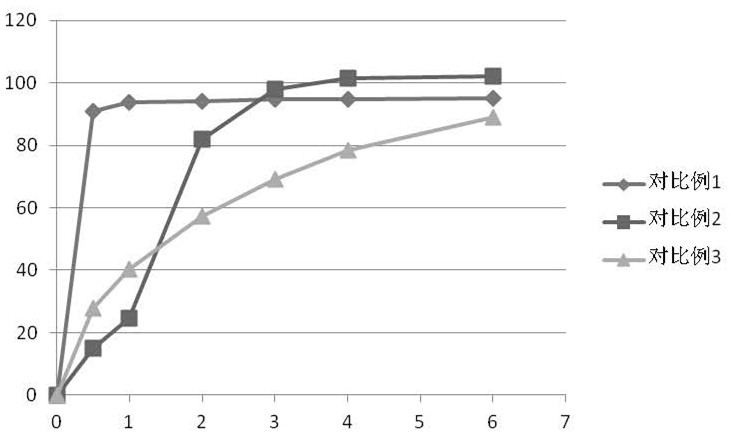
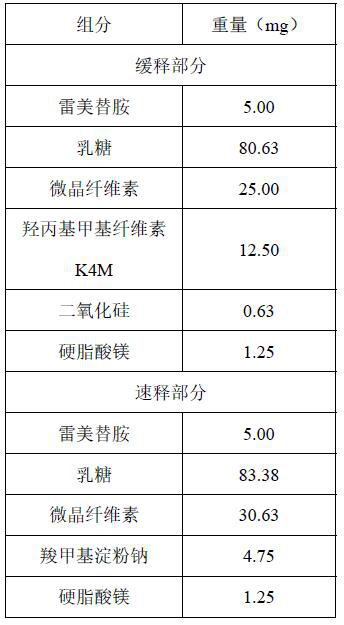
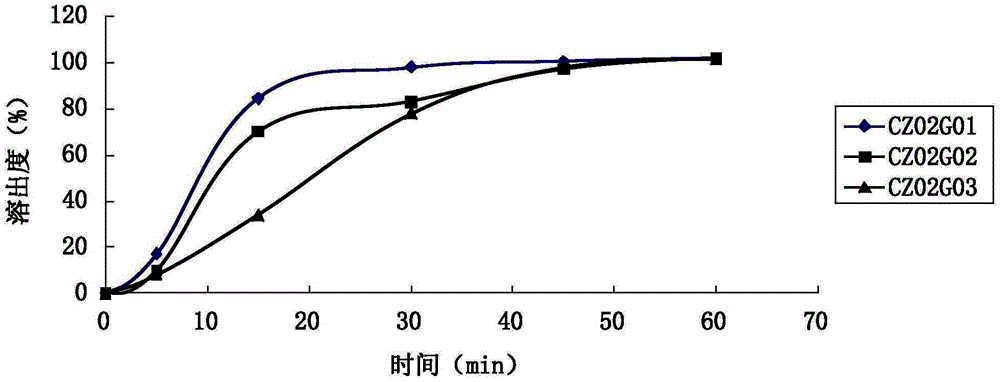
Ramelteon sustained release preparation and preparation method thereof
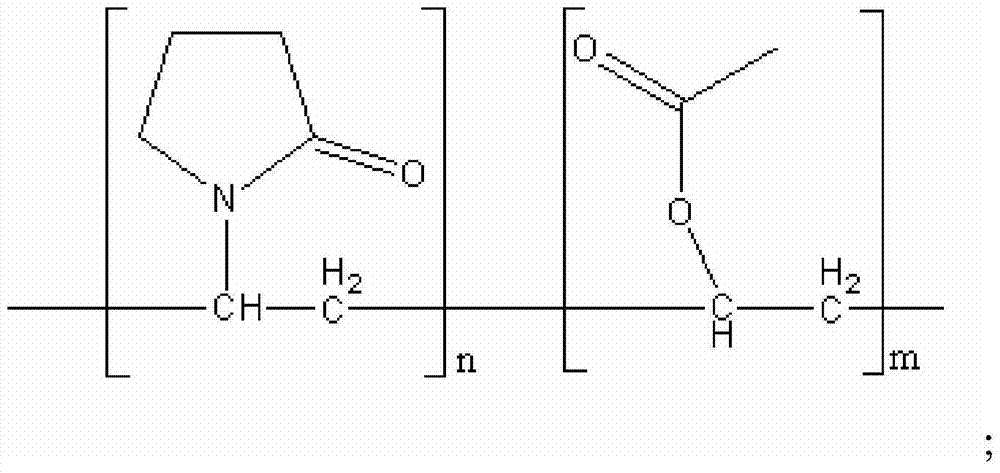
ActiveCN113274364ASustained releaseAdequate sleep timeOrganic active ingredientsNervous disorderSucrosePyrrolidinones
The invention provides a Ramelteon sustained release preparation. The dosage form of the Ramelteon sustained release preparation is a tablet, a granule or a capsule. The Ramelteon sustained-release preparation comprises the following raw materials in parts by weight: 1 to 30 parts of Ramelteon, 50 to 250 parts of a filling agent, 5 to 50 parts of a sustained-release material and 0.2 to 10 parts of a lubricating agent; the sustained-release material is selected from at least one of hydroxypropyl methylcellulose, polyvinylpyrrolidone, hydroxypropyl cellulose, sodium alginate, calcium alginate, guar gum and xanthan gum; and the filling agent is selected from at least one of lactose, mannitol, sorbitol, cane sugar, microcrystalline cellulose and calcium hydrophosphate. The Ramelteon sustained release preparation can maintain enough drug concentration in a long time, prolongs the drug action time, and is completely dissolved out.
Owner:广东科泰鼎润药业科技有限公司
Ramelteon sublingual tablets and preparation method thereof
ActiveCN112190555APromote absorptionImprove bioavailabilityOrganic active ingredientsNervous disorderBiochemistryFirst pass effect
The invention relates to ramelteon sublingual tablets. The ramelteon sublingual tablets comprise the following materials in percentage by weight of 0.1%-0.5% of ramelteon, 95%-97% of a filler, 3%-5% of a disintegrating agent, 0.05%-0.5% of a lubricating agent and 0.05% of a corrigent. The ramelteon sublingual tablets provided by the invention are good in taste, good in stability, good in content uniformity, short in disintegration time limit, and capable of avoiding the first-pass effect of the liver and greatly improving the compliance of a patient; and compared with ramelteon oral tablets, the ramelteon sublingual tablets are higher in relative bioavailability and are greatly superior to ramelteon sublingual tablets which are researched and developed originally.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Resolution method of ramelteon intermediate
InactiveCN104327021AEasy to buyMany stepsOptically-active compound separationOrganic racemisationHydrogenOrganic solvent
The invention provides a resolution method of a ramelteon intermediate, which comprises the following steps: (1) carrying out salification reaction on a compound (I) 2,2-(1,6,7,8-tetrahydro-2H-indeno[5,4-B]furyl-8-yl)ethylamine and a resolution reagent L-pyroglutamic acid in a methanol-containing organic solvent at 20-100 DEG C to obtain a ramelteon intermediate compound (II) crude product; and (2) recrystallizing the ramelteon intermediate compound (II) crude product in an organic solvent, and dissociating under the action of alkali to obtain the target product ramelteon intermediate compound (II). The reaction route of the method is disclosed in the specification.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
Tricyclic acetate compound with optical activity, and preparation method and application thereof
The invention belongs to the field of medicinal chemistry, and relates to salt formed by 2-(1,6,7,8-tetralin-2H-indeno-[5,4-b]furan-8-yl) acetic acid with optical activity and chiral amine, a preparation method for the salt and application of the salt to the preparation of ramelteon for treating insomnia and an optical isomer of the ramelteon.
Owner:SICHUAN UNIV
Preparation method of ramelteon
PendingCN112500380ALow priceHigh chiral resolution efficiencyOrganic chemistry methodsChemical compoundPhysical chemistry
The invention discloses a preparation method of ramelteon, which comprises the following steps: by using a compound IV as a starting material, carrying out reduction, chiral resolution and acylation reaction to obtain the ramelteon. The product II obtained by using a chiral resolving agent has very high chiral purity and yield, the resolution yield reaches 45% or above, and the highest resolutionyield in the existing literature technology is about 30%; the finally obtained ramelteon product is high in purity, repeated recrystallization purification is not needed, the total yield reaches 42% or above, the synthesis steps are short, and the operation process is simple.
Owner:SHANDONG LUYAO PHARMA
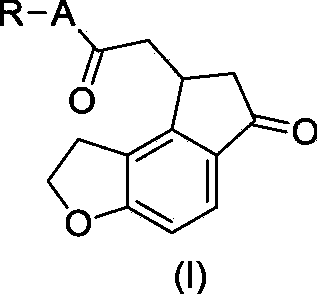
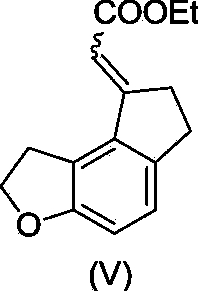
Critical intermediates used for preparing ramelteon, preparing method thereof and applications thereof
ActiveCN103880795AStarting materials are cheap and readily availableMild reaction conditionsOrganic chemistryHydrogenChemical compound
The invention discloses critical intermediates (with the structure formula (I) ) used for preparing ramelteon. In the formula (I), A is O or S; R is hydrogen, methyl, ethyl, n-propyl, isopropyl, cyclopropyl, n-butyl, isobutyl, tert-butyl, cyclobutyl, n-pentyl, isopentyl, cyclopentyl, phenyl, benzyl or p-methoxybenzyl; and when chiral carbon exists, the chemical compounds in the formula (I) are racemate or optically active compounds. When the A in the formula (I) is O and the R in the formula (I) is ethyl, the chemical compound is the chemical compound shown as the structure formula (II). In addition, the invention further discloses a preparing method of the chemical compound shown as the formula (II) and applications of the formula (II) in preparation of the ramelteon used for treating insomnia.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
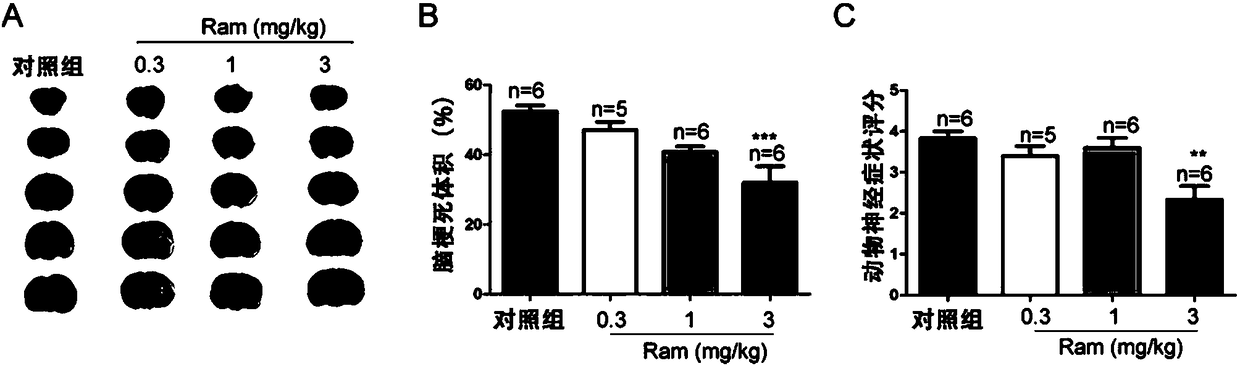
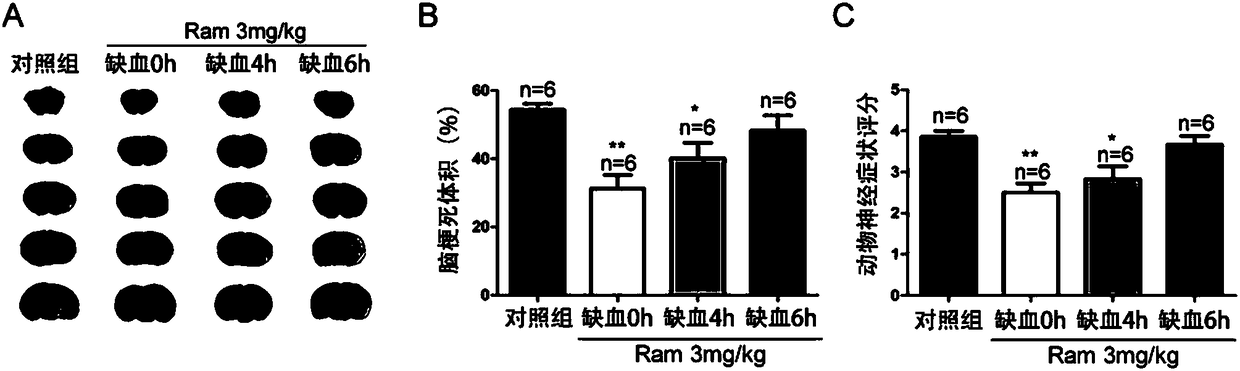
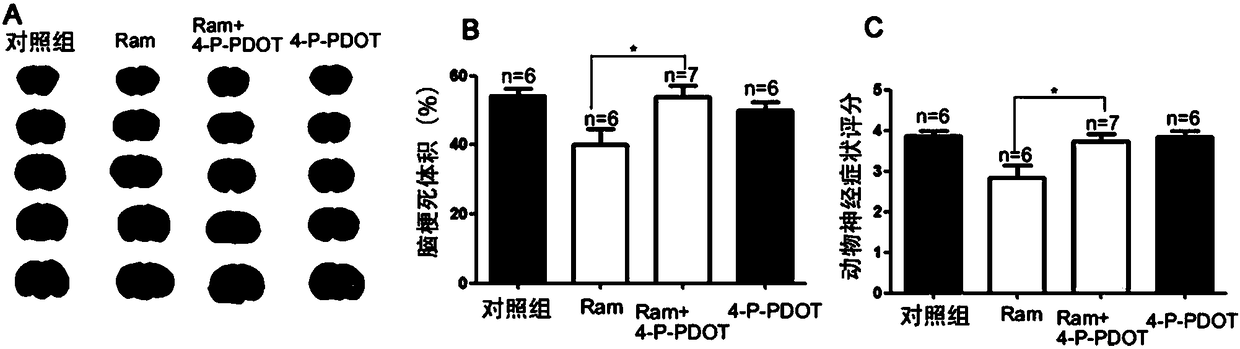
Application of ramelteon in preparation of drug for treating ischemic brain injury
InactiveCN108542902AOrganic active ingredientsNervous disorderInflammatory factorsCentral sulcus artery
The invention discloses application of ramelteon in preparation of a drug for treating ischemic brain injury. Ramelteon can alleviate acute focal cerebral ischemic injury and neurological dysfunctioncaused by permanent middle cerebral artery embolization in mice, inhibit pathological inflammatory response, and promote neural functional recovery in the late stage of ischemia. The use of ramelteonis achieved primarily by the following mechanism: ramelteon can inhibit cell autophagy during cerebral ischemia and reduces autophagic death of neurons by stimulating the MT2 melatonin receptor; in addition, ramelteon can also inhibit the release of inflammatory factors secondary to cerebral ischemia, reduce inflammatory cell damage, and ultimately exert a therapeutic effect against cerebral ischemia. The application provided by the invention has the beneficial effects of providing a new use of ramelteon, and providing a basis for screening new drugs.
Owner:ZHEJIANG UNIV
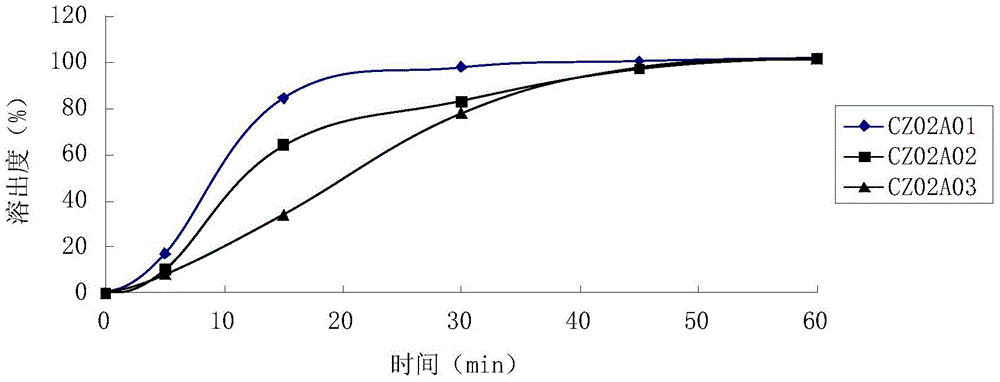
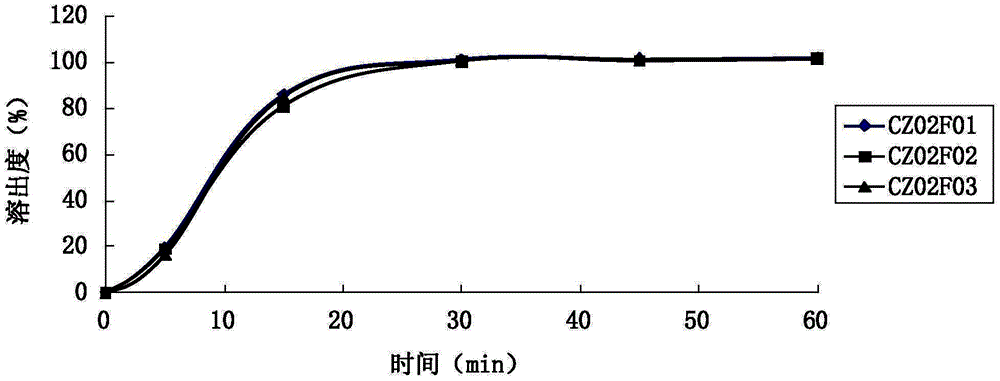
Ramelteon quick-release and slow-release double-release preparation and preparation method thereof
ActiveCN113274365AFast releaseAdequate sleep timeOrganic active ingredientsNervous disorderCelluloseImmediate release
The invention provides a Ramelteon quick-release and slow-release dual-release preparation. The Ramelteon quick-release and slow-release dual-release preparation comprises a quick-release part and a slow-release part; and the dosage form of the Ramelteon quick-release and slow-release double-release preparation is a double-layer tablet, a granule or a capsule, wherein the quick release part comprises the following raw materials in parts by weight: 1 to 30 parts of Ramelteon, 20 to 160 parts of a first filling agent, 0.5 to 10 parts of a disintegrating agent and 0.1 to 5 parts of a first lubricating agent; the slow-release part comprises the following raw materials in parts by weight: 1 to 30 parts of Ramelteon, 20 to 160 parts of a second filling agent, 5 to 30 parts of a sustained-release material and 0.2 to 10 parts of a second lubricating agent; the slow-release material is selected from at least one of hydroxypropyl methylcellulose, polyvinylpyrrolidone, hydroxypropyl cellulose, sodium alginate, calcium alginate, guar gum and xanthan gum. The preparation has a relatively high release speed in the initial stage, and meanwhile, the action time of the medicine can be prolonged.
Owner:海南慧谷药业有限公司
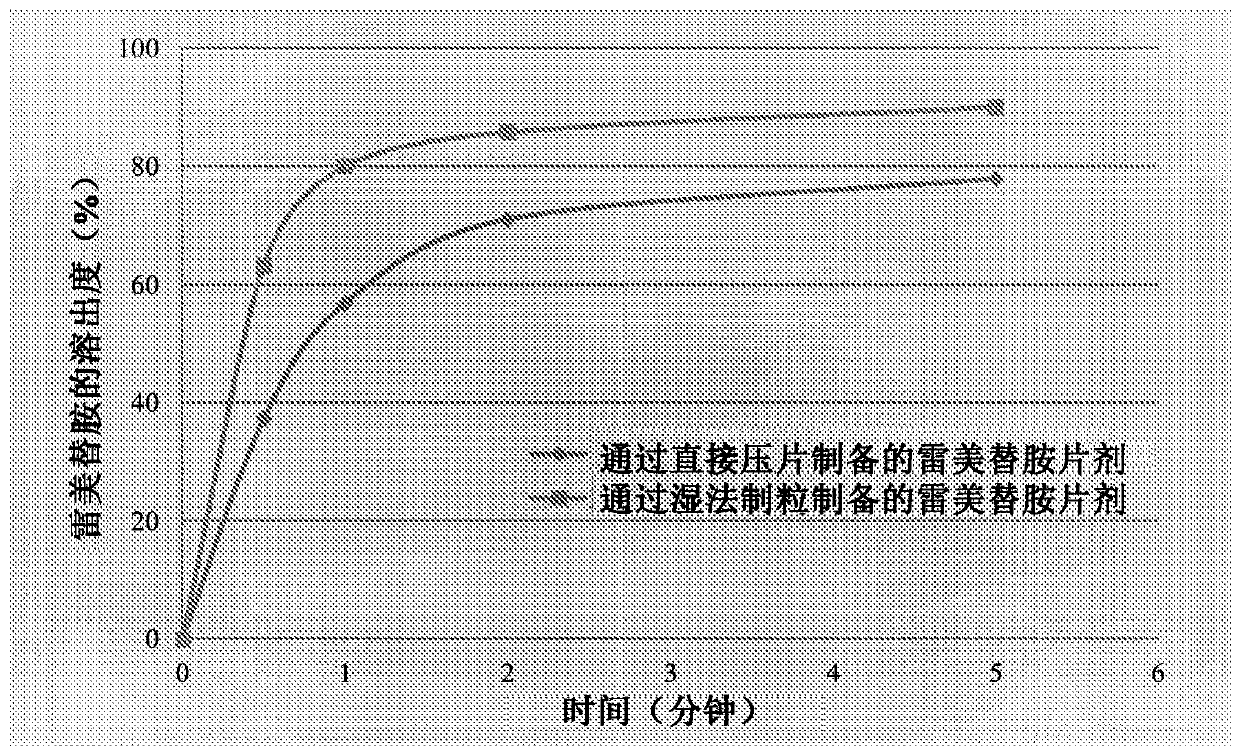
Ramelteon tablets and preparation process thereof
The invention belongs to the field of medicinal preparations, and discloses ramelteon tablets and a preparation process thereof. According to the tablets, a polyvidone solution is used as an adhesive solution for granulating, and the weight ratio of polyvidone to ramelteon is 1:15-1:1. The preparation process effectively inhibits the changes of dissolution behavior of active ingredients in insoluble medicaments.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
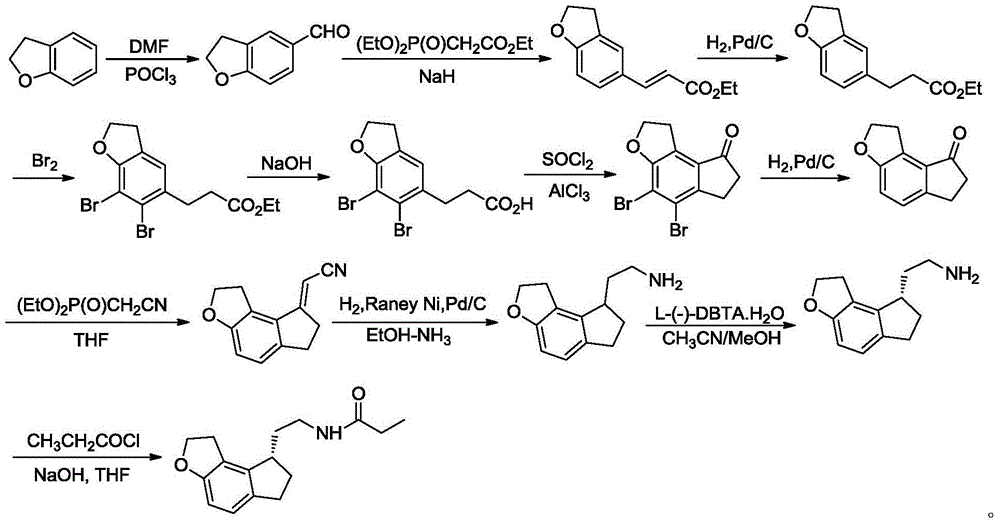
Synthetic preparation method for key intermediate of ramelteon
The invention discloses a synthetic preparation method for a key intermediate of ramelteon 1,2,6,7-tretrahydro-8H-indeno[5,4-b]furan-8-one. According to the preparation method, by taking 2,3-dihydrobenzofuran-4-formaldehyde as an initial raw material, the key intermediate of ramelteon 1,2,6,7-tretrahydro-8H-indeno[5,4-b]furan-8-one is synthesized through Grignard reaction, oxidizing reaction and Nazarov cyclization reaction. The preparation method has the advantages of being high in reaction selectivity, few in side effects, high in total yield and quality, environmental-friendly, simple and convenient in process operation, high in stability and controllability and the like, and is suitable for industrial large-scaled production.
Owner:CHANGZHOU YABANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![2-(1,6,7,8-tetrahydrogen-2H-indeno-[5,4-b] furan-8-group) acetonitrile, preparation method and applciation 2-(1,6,7,8-tetrahydrogen-2H-indeno-[5,4-b] furan-8-group) acetonitrile, preparation method and applciation](https://images-eureka.patsnap.com/patent_img/f2281036-02a4-4bf0-84a8-2382d493fc3f/B200910058469XD0000011.PNG)
![2-(1,6,7,8-tetrahydrogen-2H-indeno-[5,4-b] furan-8-group) acetonitrile, preparation method and applciation 2-(1,6,7,8-tetrahydrogen-2H-indeno-[5,4-b] furan-8-group) acetonitrile, preparation method and applciation](https://images-eureka.patsnap.com/patent_img/f2281036-02a4-4bf0-84a8-2382d493fc3f/B200910058469XD0000012.PNG)
![2-(1,6,7,8-tetrahydrogen-2H-indeno-[5,4-b] furan-8-group) acetonitrile, preparation method and applciation 2-(1,6,7,8-tetrahydrogen-2H-indeno-[5,4-b] furan-8-group) acetonitrile, preparation method and applciation](https://images-eureka.patsnap.com/patent_img/f2281036-02a4-4bf0-84a8-2382d493fc3f/B200910058469XD0000021.PNG)